Abstract
Cyclooxygenase-2 (COX-2/PTGS2) mediates hyperoxia-induced impairment of lung development in newborn animals and is increased in the lungs of human infants with bronchopulmonary dysplasia (BPD). COX-2 catalyzes the production of cytoprotective prostaglandins, such as prostacyclin (PGI2), as well as proinflammatory mediators, such as thromboxane A2. Our objective was to determine whether iloprost, a synthetic analog of PGI2, would attenuate hyperoxia effects in the newborn mouse lung. To test this hypothesis, newborn C57BL/6 mice along with their dams were exposed to normoxia (21% O2) or hyperoxia (85% O2) from 4 to 14 days of age in combination with daily intraperitoneal injections of either iloprost 200 µg·kg−1·day−1, nimesulide (selective COX-2 antagonist) 100 mg·kg−1·day−1, or vehicle. Alveolar development was estimated by radial alveolar counts and mean linear intercepts. Lung function was determined on a flexiVent, and multiple cytokines and myeloperoxidase (MPO) were quantitated in lung homogenates. Lung vascular and microvascular morphometry was performed, and right ventricle/left ventricle ratios were determined. We determined that iloprost (but not nimesulide) administration attenuated hyperoxia-induced inhibition of alveolar development and microvascular density in newborn mice. Iloprost and nimesulide both attenuated hyperoxia-induced, increased lung resistance but did not improve lung compliance that was reduced by hyperoxia. Iloprost and nimesulide reduced hyperoxia-induced increases in MPO and some cytokines (IL-1β and TNF-α) but not others (IL-6 and KC/Gro). There were no changes in pulmonary arterial wall thickness or right ventricle/left ventricle ratios. We conclude that iloprost improves lung development and reduces lung inflammation in a newborn mouse model of BPD.
Keywords: infant, newborn; lung development; oxidative stress; prostacyclin
INTRODUCTION
Bronchopulmonary dysplasia (BPD), a major cause of morbidity and mortality in premature infants with persistent abnormalities in lung function in survivors, is characterized by impaired alveolar septation in combination with varying degrees of lung fibrosis and abnormal vascular remodeling (2, 49). An important component in the pathogenesis of BPD is injury to the developing lung, attributable to hyperoxic exposure (3, 9).
Previous studies have shown that cyclooxygenase-2 [COX2; also known as prostaglandin-endoperoxide synthase 2 (PTGS2)] expression and many proinflammatory mediators are increased in the lung of newborn mice exposed to hyperoxia (13). COX-2 is a key regulatory enzyme involved in the production of prostaglandins and other prostanoids and catalyzes the production of cytoprotective prostaglandins, such as prostacyclin (PGI2), as well as proinflammatory thromboxanes, such as thromboxane A2 (TxA2) (35). Choo-wing et al. (17) showed that COX-2 inhibition using inhibitors such as celecoxib attenuated hyperoxia-induced inhibition of newborn lung alveolarization in C57BL6/J mice. However, Britt et al. (13) found that while C3H/HeN mice lacking COX-2 (COX-2−/−) and mice with COX-2 inhibition had fewer lung macrophages, the lack or inhibition of COX-2 not only did not prevent hyperoxia-induced lung developmental deficits but actually increased neonatal mortality. Such differences may be due to differences in the animal model, including variances in the mouse strain as well as timing of initiation and duration of hyperoxia exposure (8), as it is possible that COX-2 inhibition or absence in one model but not in the other may reduce beneficial PGI2 production more than it inhibits proinflammatory modulators.
PGI2 is produced via COX-2 and PGI2 synthase and is made by many cell types, including endothelial cells, epithelial cells, fibroblasts, and leukocytes (33). Fibrotic lung disease after bleomycin exposure is modulated by COX-2-dependent prostanoid production, and mice lacking COX-2 or the PGI2 receptor (IP) are more susceptible to bleomycin-induced fibrosis, indicating a role for COX-2 and its product PGI2 in attenuating lung fibrosis (33). PGI2 and its analogs have also been shown to benefit murine models of chronic obstructive pulmonary disease (30). Importantly, PGI2 and its analogs (e.g., epoprostenol, iloprost, and treprostinil) are already approved and available for human clinical use in either adults (44) or children (21) with pulmonary hypertension. However, there is a lack of data on PGI2 and its analogs for the prevention or management of BPD in the absence of pulmonary hypertension. Hence, our objective in this study was to determine if the use of iloprost would attenuate hyperoxia-induced inhibition of lung development and function in a newborn mouse model and to compare the effects of iloprost to that of a selective COX-2 antagonist (nimesulide).
METHODS
All procedures were approved by the Institutional Animal Care and Use Committee and were consistent with the Public Health Service policy on Humane Care and Use of Laboratory Animals (Office of Laboratory Animal Welfare, 2002). Timed pregnant C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME).
Animal model.
Newborn C57BL/6 mice along with their dams were exposed to normoxia (21% O2; control group) or hyperoxia (85% O2) from 4 to 14 days of age in sealed a Plexiglas chamber with continuous oxygen monitoring, as previously described (41). Nursing dams were switched between hyperoxia and room air-exposed litters every 24 h to prevent damage to their lungs and to provide equal nutrition to each litter. Daily animal maintenance was carried out with exposure of the animals to room air for <10 min per day. A standard mouse pellet diet and water were provided ad libitum.
Treatment with iloprost and nimesulide.
Newborn mouse pups were treated with iloprost (PGI2 analog, 200 µg/kg) (R&D Systems, Minneapolis, MN), nimesulide (a selective COX-2 antagonist; 100 mg/kg), or vehicle (saline) daily with intraperitoneal injection during the period of exposure to hyperoxia or normoxia. The dosage of iloprost (57) and nimesulide (24) were based on data from the existing literature and dose-ranging studies (1/10th to 10 times the dose used) that indicated that this dose was well tolerated without mortality and with marked reduction of proinflammatory cytokines IL-1β and TNF-α mRNA (see below). In addition, we performed pharmacokinetic analysis by HPLC of lung homogenates from P7 mice following exposure to these doses of either iloprost or nimesulide. Nimesulide concentrations rose rapidly, peaked at 12 h, and were lower at 24 h (average: <100 ng/g at time 0; 2,800 ng/g at 20 min; 3,000 ng/g at 1 h, 3,800 ng/g at 3 h; 7,900 ng/g at 6 h; 15,000 ng/g at 12 h; and 1,600 ng/g at 24 h). On the other hand, iloprost concentrations were above detectable levels (>0.2 ng/g) only at the 20 min time point (15 ng/g), consistent with the half-life of iloprost in humans of 20–30 min (iloprost drug label at United States Food and Drug Administration: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021779s006lbl.pdf). Because of this short half-life, iloprost is often administered by means of frequent (every 2–4 h) inhalation in humans. However, Zhu et al. (57) (single dose) and Lammi et al. (30) (5 times/wk) have demonstrated that even infrequent dosing of iloprost exerts beneficial effects. In addition, newborn mice have lower survival with frequent manipulation and intraperitoneal injections that would be required for dosing more frequently than once per day. Hence, we first evaluated once-daily dosing of both nimesulide and iloprost in this preclinical study on newborn mice.
Lung mechanics.
After completion of hyperoxia or air exposure, P14 mice were sedated with ketamine/xylazine, and pulmonary mechanics (compliance and total lung resistance) were evaluated on a flexiVent using a 24G Angiocath inserted via a tracheostomy as described previously (22).
Lung morphometry.
Lung alveolar morphometry was performed on inflation-fixed lungs as described previously (22), with measurements of mean linear intercepts (MLIs) being performed on 6 random fields, excluding large airways or blood vessels, and radial alveolar counts (RACs) on 6 random distal airways per hematoxylin-eosin-stained section of the entire left lung (apex to base) per animal.
Lung microvasculature was assessed using immunohistochemical staining for endomucin using a monoclonal primary antibody (MAB2624; EMD Millipore, Burlington, MA) at 1:100 × 1 h, with a Rat HRP-DAB Secondary Kit (CTS017; R&D systems) followed by image analysis quantitation as we have previously described (6, 39, 41). Lung vascular morphometry was done as described previously (1, 5), with the wall thickness (WT) of 20 consecutive pulmonary arteries per section expressed as a percentage of the vessel diameter. Right ventricular hypertrophy was estimated by measurement of the right ventricle (RV) and left ventricle (LV) free WT ratio just inferior to the mitral leaflet attachment as described earlier (1, 5), which is more accurate than the Fulton index of RV/(LV + Septum) weight ratio in newborn mice.
All morphometric analysis was carried out by a single examiner masked to the group assignment.
Analysis of cytokines by real-time RT-PCR and multiplex ELISA.
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) from homogenized mouse lung. Quantitative real-time PCR for multiple cytokines (IL-1β, IL-6, KC/Gro, IL-10, and TNF-α) was performed using Taqman probes purchased from Applied Biosystems (Foster City, CA), as described previously (5, 6). All gene expression levels were normalized to 18S RNA. Homogenized mouse lung was also evaluated for protein amounts of multiple cytokines using the V-PLEX Proinflammatory Panel 1 (mouse) Immunoassay Kit custom made to include only the five cytokines (IL-1β, IL-6, KC/Gro, IL-10, and TNF-α) and analyzed using the MESO Quickplex SQ 120 (Meso Scale Diagnostics, Rockville, MD).
Analysis of myeloperoxidase.
Lungs were homogenized in fresh chilled tissue protein extraction reagent buffer plus proteinase inhibitors. Samples were centrifuged at 13,000 g, and myeloperoxidase (MPO) activity in the supernatant was determined at 412 nm, using a commercially available MPO activity colorimetric assay kit (BioVision, Milpitas, CA).
Analysis of collagen, elastin, and airway mucosal thickness.
Verhoeff-van Gieson’s staining was done to evaluate collagen and elastin deposition and distribution in a qualitative and semiquantitative manner [scoring of perivascular, peribronchial, and alveolar septal staining in a 1 (none) to 5 (dense) range in 6 random ×400 sections]. Bronchiolar WT (mucosa + underlying collagen/elastin layer) was measured in ×400 sections using image analysis software (MetaMorph v.6.2r4; Universal Imaging).
Analysis of 6-keto-PGF1α.
To evaluate overall production of PGI2, we measured its metabolite 6-keto-PGF1α in lung homogenate supernatants from P14 mice using a commercially available ELISA (Cayman Chemical, Ann Arbor, MI).
RESULTS
Hyperoxia inhibited alveolar septation, which was prevented by iloprost but not nimesulide.
Mice exposed to hyperoxia-vehicle had enlarged air spaces and fewer alveolar septa compared with air-vehicle (Fig. 1). Air-nimesulide and hyperoxia-nimesulide mice had lung histology similar to their corresponding vehicle controls, indicating that nimesulide did not impact alveolar development significantly. In contrast, hyperoxia-iloprost mice had smaller alveoli/saccules with more septations compared with hyperoxia-vehicle or hyperoxia-nimesulide and were similar to air-vehicle, indicating attenuation of hyperoxia-induced inhibition of septation by iloprost (Fig. 1). The inhibition of alveolar development in the hyperoxia-vehicle mice was demonstrated by an increase in the MLI and a reduction in the RAC compared with air-vehicle mice (Fig. 1). The air-nimesulide and hyperoxia-nimesulide mice had MLIs and RACs similar to their corresponding vehicle controls. Hyperoxia-iloprost mice had a significant reduction in MLI and an increase in RAC compared with hyperoxia-vehicle mice and had an MLI and RAC not different than air-vehicle mice (Fig. 1).
Fig. 1.
Iloprost attenuates hyperoxia-induced inhibition of neonatal mouse lung development. Alveolar development in C57BL/6 mouse pups at 14 days of age. Representative photomicrographs of hematoxylin-eosin-stained sections of lungs from C57BL/6 mouse pups (A–F) given either vehicle (A and D), nimesulide (B and E), or iloprost (C and F) during air (21% O2: A–C) or hyperoxia (85% O2: D–F) exposure from P4–P14 (×100; calibration bars = 250 µm). In mouse pups given vehicle (A and D), alveolar size is larger in hyperoxia-exposed (D) compared with air-exposed mice (A), indicating delay in septation. Administration of iloprost (F) but not nimesulide (E) significantly attenuates the hyperoxia-induced increase in alveolar size but does not change alveoli of air-exposed animals given either nimesulide (B) or iloprost (C). Mean linear intercept (G) and radial alveolar count (H) at 14 days of age in C57BL/6 mouse pups given either vehicle, nimesulide, or iloprost while being exposed to air or hyperoxia (mean ± SE; n = 6 mice/group; *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding vehicle).
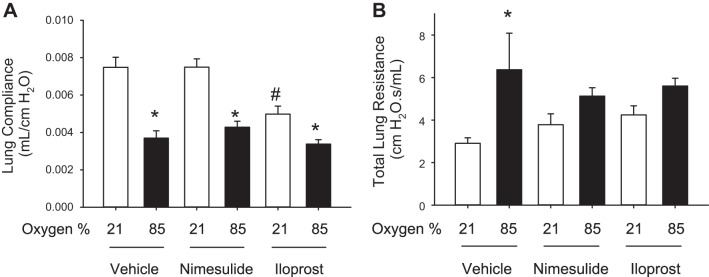
Hyperoxia-induced decreases in lung compliance were not affected by nimesulide or iloprost, although hyperoxia-induced increases in total lung resistance were reduced by both nimesulide and iloprost.
Hyperoxia-vehicle mice had decreased lung compliance and increased total lung resistance compared with air-vehicle mice (Fig. 2).
Fig. 2.
Hyperoxia-induced reduction in lung compliance is not altered by nimesulide or iloprost, but hyperoxia-induced increases in lung resistance are attenuated by both nimesulide and iloprost. Iloprost reduces lung compliance even in normoxia. A: lung compliance was reduced by hyperoxia, and neither nimesulide nor iloprost prevented this reduction in lung compliance. Iloprost administration was associated with a reduction in lung compliance even in normoxia. B: total lung resistance was increased in hyperoxia-vehicle mice, and both nimesulide and iloprost reduced resistance to the level not significantly different from that seen in air-vehicle mice (mean ± SE; n = 6 mice/group; *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding vehicle).
Administration of nimesulide did not alter lung compliance compared with corresponding vehicle controls, with a marked decrease in lung compliance noted with hyperoxia. Lung resistance in air-nimesulide was similar to air-vehicle, although resistance in hyperoxia-nimesulide was significantly decreased compared with hyperoxia-vehicle and was statistically not different from air-nimesulide (Fig. 2).
Iloprost decreased compliance in air compared with air-vehicle mice, whereas compliance in hyperoxia was further diminished and was similar to hyperoxia-vehicle mice (Fig. 2). Lung resistance in air-iloprost was similar to air-vehicle, and resistance in hyperoxia-iloprost was significantly decreased compared with hyperoxia-vehicle and was statistically not different from air-iloprost (Fig. 2). Lung volumes were very similar in all groups of mice, ranging from 0.29 to 0.35 ml/mouse, and no differences were seen between groups in lung volume at P14 [mean (SD); air-vehicle: 0.32 (0.02); air-nimesulide: 0.33 (0.02); air-iloprost 0.30 (0.01); hyperoxia-vehicle: 0.30 (0.01); hyperoxia-nimesulide: 0.31 (0.01); hyperoxia-iloprost: 0.30 (0.01); P, not significant]. Results were not different when morphometry was adjusted for lung volumes.
Hyperoxia reduced lung microvascular density, which was prevented by iloprost but not nimesulide.
Mice exposed to hyperoxia and given vehicle had a reduction in endomucin staining consistent with reduced capillary density compared with air-vehicle mice (Fig. 3). Air-nimesulide and air-iloprost mice had microvasculature similar to vehicle-exposed mice, indicating that nimesulide and iloprost do not impact microvascular development significantly in normoxia. In contrast, hyperoxia-exposed mice given iloprost had higher microvascular density compared with hyperoxia-exposed mice given either vehicle or nimesulide, and the microvascular density in this group of mice was not statistically different from air-vehicle mice (Fig. 3).
Fig. 3.
Iloprost attenuates hyperoxia-induced inhibition of microvascular density in newborn mouse lung. Microvascular density as evaluated by endomucin staining in C57BL/6 mouse pups at 14 days of age. Representative photomicrographs of endomucin stained sections of lungs from C57BL/6 mouse pups (A–F) given either vehicle (B and E), nimesulide (A and D), or iloprost (C and F) during air (21% O2: A–C) or hyperoxia (85% O2: D–F) exposure from P4–P14 (×400; calibration bars = 50 µm). In mouse pups given vehicle (B and E), microvasculature is more sparse in hyperoxia-exposed (E) compared with air-exposed mice (B), indicating reduction in capillary density. Administration of iloprost (F) but not nimesulide (D) significantly attenuates the hyperoxia-induced decrease in microvascular density. Air-exposed animals given either nimesulide (A) or iloprost (C) are similar to air-exposed animals given vehicle (B). Endomucin-stained area at 14 days of age in C57BL/6 mouse pups (G) given either vehicle, nimesulide, or iloprost while being exposed to air or hyperoxia (mean ± SE; n = 4 mice/group; *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding vehicle).
Hyperoxia, nimesulide, or iloprost did not change pulmonary arterial remodeling or right ventricular hypertrophy.
The WT% in all groups of mice was comparable [median (25th–75th centiles): air-vehicle: 13.3% (10–17); air-nimesulide: 13.1% (11–16); air-iloprost: 13.4% (11–17); hyperoxia-vehicle: 13.5% (10–19); hyperoxia-nimesulide: 13.8% (9–20); hyperoxia-iloprost: 13.6% (10–18); P NS].
The RV/LV ratio was also not statistically different [mean (SD): air-vehicle: 0.33 (0.03); air-nimesulide: 0.335 (0.06); air-iloprost: 0.32 (0.03); hyperoxia-vehicle: 0.35 (0.06); hyperoxia-nimesulide: 0.345 (0.06); hyperoxia-iloprost: 0.32 (0.05); P NS).
Hyperoxia-induced increases in IL-1β and TNF-α mRNA and protein were prevented by both nimesulide and iloprost.
Hyperoxia-vehicle mice had increased IL-1β, IL-6, KC/Gro, IL-10, and TNF-α mRNA in lung homogenates compared with air-vehicle mice (Fig. 4). Administration of nimesulide and iloprost did not alter IL-1β and TNF-α mRNA compared with corresponding air-vehicle controls but attenuated the increase in both cytokines following hyperoxia exposure (Fig. 4). However, nimesulide and iloprost did not reduce the hyperoxia-induced increases in IL-6, KC/Gro, or IL-10.
Fig. 4.
Hyperoxia-induced increases in IL-1β and TNF-α mRNA and protein are reduced by both nimesulide and iloprost. mRNA of IL-1β (A), IL-6 (B), KC/Gro (C), IL-10 (D), and TNF-α (E) was measured by real-time quantitative RT-PCR, and protein concentrations of IL-1β (F), IL-6 (G), KC/Gro (H), IL-10 (I), and TNF-α (J) were measured by multiplex immunoassay in homogenized lungs from mice exposed to air or hypoxia from birth to 7 days and showed that hyperoxia increased both IL-1β and TNF-α and that these increases were markedly reduced by both nimesulide as well as iloprost (n = 6 mice/group; mean ± SE, *P < 0.05 vs. corresponding air at the same time point; #P < 0.05 vs. corresponding saline).
Hyperoxia induced increases in Il-1β, Il-6, KC/Gro, and TNF-α protein in lung homogenates and reduced IL-10 protein. The hyperoxia-induced increases in IL-1β and TNF-α were prevented by nimesulide and iloprost, but the changes in the other cytokines (IL-6, KC/Gro, and IL-10) were not affected by either nimesulide or iloprost (Fig. 4).
Hyperoxia-induced increases in myeloperoxidase were prevented by both nimesulide and iloprost.
Hyperoxia significantly increased MPO activity in lung homogenates, and while nimesulide and iloprost did not alter MPO in air-exposed mouse lungs, they prevented the hyperoxia-induced increase in MPO (Fig. 5).
Fig. 5.

Hyperoxia-induced increases in myeloperoxidase (MPO) were prevented by both nimesulide and iloprost. MPO activity was measured in lung homogenates of mice given either vehicle, nimesulide, or iloprost during air (21% O2) or hyperoxia (85% O2) exposure from P4-P14. MPO activity was increased by hyperoxia in vehicle-exposed mice. Nimesulide and iloprost exposure did not alter MPO concentrations in normoxia compared with air-vehicle and prevented the hyperoxia-induced increases in MPO (n = 4 mice/group; mean ± SE, *P < 0.05 vs. corresponding air at same time point; #P < 0.05 vs. corresponding saline).
Hyperoxia, nimesulide, or iloprost did not change collagen staining or bronchiolar mucosal thickness, whereas hyperoxia-induced reductions in alveolar septal elastin were partially improved by nimesulide and prevented by iloprost.
Collagen staining was noted primarily in perivascular and peribronchial regions and diffusely to a lesser magnitude in the interstitium. No obvious differences in interstitial or perivascular/peribronchial collagen staining were noted at P14 (Fig. 6). The bronchiolar WTs were also not statistically different [mean (SD) (microns): air-vehicle: 6.3 (0.5); air-nimesulide: 7.2 (1.5); air-iloprost: 6.9 (1.4); hyperoxia-vehicle: 7.6 (1.5), hyperoxia-nimesulide: 7.1 (1.8), hyperoxia-iloprost: 7.3 (1.5); P NS] and neither were airway external diameters (data not shown) (Fig. 6).
Fig. 6.
Collagen and elastin staining. Verhoeff-van Gieson’s staining was done to evaluate collagen (pink) and elastin (black) deposition and distribution in lung sections imaged at lower magnification (×100: A–F; calibration bars = 100 µm) and higher magnification (×400: G–L; calibration bars = 50 µm). Collagen staining was noted primarily in perivascular and peribronchial regions and diffusely in the interstitium. Elastin was noted primarily in pulmonary arterial walls (internal and external elastic laminae), lining the airways, and in the tips/free margins of alveolar septa. Hyperoxia-induced reductions in alveolar septal elastin were partially improved by nimesulide and prevented by iloprost. Br, bronchiole; PA, pulmonary artery.
Elastin was noted primarily in pulmonary arterial walls (internal and external elastic laminae), lining the airways, and in the tips/free margins of alveolar septa. Exposure to hyperoxia or to nimesulide or iloprost did not lead to major changes in vascular or airway elastin. However, hyperoxia markedly reduced elastin in alveolar septa. This hyperoxia-induced reduction in alveolar septal elastin was partially improved by nimesulide and prevented by iloprost (Fig. 5).
Hyperoxia increased 6-keto-PGF1α, a marker of PGI2 synthesis, and this increase was prevented by nimesulide but not altered by iloprost, which also increased 6-keto-PGF1α during normoxia.
Hyperoxia increased 6-keto-PGF1α, a marker of PGI2 synthesis, by more than 2.5-fold (Fig. 7). Nimesulide did not significantly change 6-keto-PGF1α during normoxia but prevented the hyperoxia-induced increase. Iloprost increased 6-keto-PGF1α during normoxia almost twofold compared with air-vehicle, and the concentration in lung homogenates during hyperoxia exposure was similar to the hyperoxia-vehicle (i.e., the hyperoxia-induced increase was not significantly altered by iloprost) (Fig. 7).
Fig. 7.

6-keto-GF1α in mouse lung homogenates is increased by hyperoxia and iloprost, but the hyperoxia-induced increase is prevented by nimesulide. 6-keto-PGF1α was measured in lung homogenates of mice given either vehicle, nimesulide, or iloprost during air (21% O2) or hyperoxia (85% O2) exposure from P4-P14. 6-keto-PGF1α activity was increased by hyperoxia in vehicle-exposed mice. Nimesulide did not alter 6-keto-PGF1α concentrations in normoxia but prevented the hyperoxia-induced increases. Iloprost increased 6-keto-PGF1α concentrations in normoxia, and hyperoxia-induced increases were similar to those in hyperoxia-vehicle mice (n = 4 mice/group; mean ± SE, *P < 0.05 vs. corresponding air at the same time point; #P < 0.05 vs. corresponding saline).
DISCUSSION
Improvement in alveolar and microvascular development associated with attenuation of lung resistance by administration of iloprost, a PGI2 analog, in the murine hyperoxia model of BPD was the major finding of this study. Reductions in MPO and specific proinflammatory cytokines (IL-1β and TNF-α) with iloprost and nimesulide were also observed.
COX-1 is present constitutively, whereas COX-2 is expressed primarily after inflammatory insult (35). COX-2 is rapidly and robustly expressed in response to many proinflammatory stimuli in pulmonary disorders (46). It has been suggested that inhibiting COX-2 expression to repress airway inflammation may be too blunt an approach because prostanoids have diverse actions and can exert anti-inflammatory functions in addition to proinflammatory actions (46). COX-2 catalyzes production of proinflammatory thromboxanes, such as TxA2, as well as anti-inflammatory prostaglandins, such as PGI2 (35).
COX-2 has more intense bronchial staining in the fetal as compared with term infant lung (31), suggesting a potential role in development. COX-2 is localized in epithelial cells in alveoli and in ciliated epithelial cells in bronchi. In fetuses, COX-2 is seen in most alveolar epithelial cells, whereas it is more scattered with advancing gestational age (31). The role of COX-2 in BPD is not settled. COX-2 does not seem to be highly differentially expressed in human lungs with established BPD compared with gestational age-matched controls without BPD (11). However, COX-2 expression is increased in the lung of newborn mice (13, 25, 31) as well as preterm rabbits (47) exposed to hyperoxia.
Choo-wing et al. (17) showed that COX-2 inhibitors, such as celecoxib, attenuated hyperoxia-induced inhibition of newborn lung alveolarization in C57BL6/J mice, but Britt et al. (13) observed that although COX-2−/− C3H/HeN mice and mice with COX-2 inhibition had fewer lung macrophages, there was no reduction in hyperoxia-induced lung development effects. Our study was similar to that of Britt et al. (21) in that although we observed marked reductions in some proinflammatory cytokines, there was no appreciable improvement in hyperoxia-induced inhibition of alveolar septation with nimesulide. It is possible that differences in the details of the animal model or COX-2 inhibition could account for these differences.
The mechanisms by which iloprost improves alveolarization, microvascular density, and lung resistance in the newborn mouse model of chronic hyperoxia exposure are unclear. It is possible that iloprost, by reducing proinflammatory cytokines, such as IL-1β and TNF-α, reduces phagocyte recruitment (MPO) and reduces lung injury. Hyperoxia activates the inflammasome, causing IL-1β release and inflammation (28). Overexpression of IL-1β leads to a phenotype similar to BPD (14), and IL-1 receptor antagonist prevents BPD in newborn mice (40), indicating a key role for IL-1β in newborn mouse models of BPD. TNF-α is a potent proinflammatory cytokine associated with human BPD (23). Hyperoxia not only increases lung TNF-α (7, 32) but also prolongs TNF-α-mediated activation of NFκB (53). Antagonism of TNF-α improves alveolar development in hyperoxic neonatal rats (42), indicating that TNF-α is also important in murine models of BPD. Certainly, hyperoxia and iloprost may also modulate other pro- and anti-inflammatory mediators in addition to the five we evaluated in this study. However, nimesulide did not improve lung development despite similar reductions in IL-1β and TNF-α. The differing effects of nimesulide and iloprost on 6-keto-PGF1α, an indicator of PGI2 synthesis, yield a clue as to potential mechanisms. Hyperoxia increased 6-keto-PGF1α (suggesting increased PGI2 synthesis in hyperoxia as a compensatory phenomenon), and this increase was prevented by nimesulide (indicating reduction of PGI2 synthesis), whereas it was maintained by iloprost (which also increased 6-keto-PGF1α in normoxia). These data suggest that modulation of PGI2 synthesis or metabolism may account for some effects of iloprost.
Other effects of iloprost may be due to reduced oxidative injury. PGI2 analogs (such as beraprost) reduce lipid peroxidation and prevent depletion of cellular antioxidants in lipopolysaccharide-induced injury to alveolar epithelial cells (52). Beraprost also inhibits cigarette smoke extract-induced emphysema in rats by attenuating apoptosis, inhibiting proteolytic enzymes, and augmenting antioxidants in addition to reducing inflammatory cytokines (15). The role of PGI2 in preventing oxidative injury is evident in studies using mice lacking the PGI2 receptor (IP−/−), wherein PGI2 produced endogenously during cardiac ischemia-reperfusion had protective effects independent of its effects on platelets and neutrophils in IP+/+ but not in IP−/− mice (54). PGI2 is also a vasodilator that inhibits injury-induced vascular muscle proliferation and platelet activation and specifically inhibits the response to TxA2 (16). A single injection of a sustained-release PGI2 analog suppresses airway inflammation and remodeling in an asthma model (27). Similar inhibition of bronchial smooth muscle may result in the improved lung resistance noted in our model.
We did not observe any evidence of pulmonary arterial thickening or right ventricular hypertrophy in this newborn mouse model of chronic hyperoxia exposure, despite hyperoxia-induced reductions in microvascular density, unlike our previous experience with the newborn mouse model of chronic hypoxia exposure (1, 5, 6). This is consistent with findings by other investigators, e.g., Nakanishi et al. (38), who did not find any neomuscularization of peripheral pulmonary arteries or right ventricular hypertrophy in newborn mice exposed to chronic hyperoxia. However, some investigators using similar but not identical mouse models (20, 45, 50) have noted right ventricular hypertrophy and vascular remodeling. In neonatal rats, Masood et al. (34) showed that selective COX-2 inhibitors prevent hyperoxia-induced vascular pruning. Unlike rats, mice do not develop marked pulmonary vascular pruning or remodeling, which makes differences harder to identify (3). The newborn mouse model is similar to that of human BPD, dependent upon the timing of postnatal initiation of supplemental oxygen exposure, the concentration of supplemental oxygen, and duration of exposure (8) wherein pulmonary hypertension is noted only in a small fraction (18%, about one-fifth) of infants who are born extremely preterm (10). The current concept of “New BPD” is of an arrest of alveolarization and abnormal microvasculature without marked vascular remodeling or pulmonary hypertension, except in a small proportion of infants (4).
We noted reduced lung compliance with iloprost even in normoxia, although the reasons for this finding were not obvious on histology. In humans, nonspecific interstitial pneumonitis has occasionally been noted (26, 37) which may reduce lung compliance, although we did not find any interstitial pneumonia in mice exposed to iloprost. Human trials (48) as well as animal studies (18) have shown that nebulized iloprost improves gas exchange without detrimental effects on pulmonary mechanics or systemic hemodynamics. While inhaled and intravenous PGI2 have slightly different effects (12, 43), the overall effects and benefits regarding gas exchange and pulmonary hypertension are similar.
A major strength of our study is the evaluation of both lung structure and mechanics using multiple parameters in addition to cytokines, MPO, and 6-keto-PGF1α in a newborn mouse model. This enabled us to identify a potential drawback of the use of iloprost (impairment of lung compliance). There are limitations of this model as chronic hyperoxia exposure, while reproducible and useful, may not closely simulate impaired lung development observed in human BPD that follows mechanical ventilation of a surfactant-deficient preterm infant lung with relatively high oxygen concentrations (3).
PGI2 analogs are increasingly used for management of pulmonary hypertension in children (21, 36) as well as neonates (29, 55). Iloprost has been used in extremely preterm infants, primarily for the management of pulmonary hypertension (19, 56), although no studies have evaluated its utility for the prevention of BPD. Prenatal treatment using a low-release PGI2 agonist enhances pulmonary vascular and alveolar development in a rat model of fetal lung hypoplasia (51). Our study suggests that iloprost may be a potential therapeutic candidate for preventing or attenuating BPD, although effects on lung compliance will need to be carefully monitored. Future dose-ranging studies of PGI2 analogs (e.g., iloprost, beraprost, and treprostinil) in larger preterm animal models are required to determine optimal dose and dosing intervals as well as mode of administration (inhaled vs. subcutaneous vs. intravenous) and effects on longer-term pulmonary and other organ outcomes.
GRANTS
This study was supported by National Heart, Blood, and Lung Institute Grants R01-HL-092906 and U01-HL-122626 (to N. Ambalavanan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.O., C.V.L., V.B., and N.A. conceived and designed research; N.O., B.H., and N.A. performed experiments; N.O., B.H., and N.A. analyzed data; C.V.L. and N.A. interpreted results of experiments; N.A. prepared figures; N.O. drafted manuscript; N.O., C.V.L., B.H., V.B., and N.A. edited and revised manuscript; N.O., C.V.L., B.H., V.B., and N.A. approved final version of manuscript.
REFERENCES
- 1.Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res 57: 631–636, 2005. doi: 10.1203/01.PDR.0000159512.55862.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambalavanan N, Carlo WA. Bronchopulmonary dysplasia: new insights. Clin Perinatol 31: 613–628, 2004. doi: 10.1016/j.clp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanan N, Morty RE. Searching for better animal models of BPD: a perspective. Am J Physiol Lung Cell Mol Physiol 311: L924–L927, 2016. doi: 10.1152/ajplung.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Mourani P. Pulmonary hypertension in bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 100: 240–246, 2014. doi: 10.1002/bdra.23241. [DOI] [PubMed] [Google Scholar]
- 5.Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 295: L86–L95, 2008. doi: 10.1152/ajplung.00534.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambalavanan N, Nicola T, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Role of matrix metalloproteinase-2 in newborn mouse lungs under hypoxic conditions. Pediatr Res 63: 26–32, 2008. doi: 10.1203/PDR.0b013e31815b690d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Ari J, Makhoul IR, Dorio RJ, Buckley S, Warburton D, Walker SM. Cytokine response during hyperoxia: sequential production of pulmonary tumor necrosis factor and interleukin-6 in neonatal rats. Isr Med Assoc J 2: 365–369, 2000. [PubMed] [Google Scholar]
- 8.Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol 307: L936–L947, 2014. doi: 10.1152/ajplung.00159.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhandari A, Bhandari V. Pathogenesis, pathology and pathophysiology of pulmonary sequelae of bronchopulmonary dysplasia in premature infants. Front Biosci 8: e370–e380, 2003. doi: 10.2741/1060. [DOI] [PubMed] [Google Scholar]
- 10.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 129: e682–e689, 2012. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya S, Go D, Krenitsky DL, Huyck HL, Solleti SK, Lunger VA, Metlay L, Srisuma S, Wert SE, Mariani TJ, Pryhuber GS. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med 186: 349–358, 2012. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bratel T, Lagerstrand L, Brodin LA, Nowak J, Randmaa I. Ventilation-perfusion relationships in pulmonary arterial hypertension: effect of intravenous and inhaled prostacyclin treatment. Respir Physiol Neurobiol 158: 59–69, 2007. doi: 10.1016/j.resp.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Britt RD Jr, Velten M, Tipple TE, Nelin LD, Rogers LK. Cyclooxygenase-2 in newborn hyperoxic lung injury. Free Radic Biol Med 61: 502–511, 2013. doi: 10.1016/j.freeradbiomed.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bry K, Whitsett JA, Lappalainen U. IL-1beta disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol 36: 32–42, 2007. doi: 10.1165/rcmb.2006-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Hanaoka M, Chen P, Droma Y, Voelkel NF, Kubo K. Protective effect of beraprost sodium, a stable prostacyclin analog, in the development of cigarette smoke extract-induced emphysema. Am J Physiol Lung Cell Mol Physiol 296: L648–L656, 2009. doi: 10.1152/ajplung.90270.2008. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science 296: 539–541, 2002. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 17.Choo-Wing R, Syed MA, Harijith A, Bowen B, Pryhuber G, Janér C, Andersson S, Homer RJ, Bhandari V. Hyperoxia and interferon-γ-induced injury in developing lungs occur via cyclooxygenase-2 and the endoplasmic reticulum stress-dependent pathway. Am J Respir Cell Mol Biol 48: 749–757, 2013. doi: 10.1165/rcmb.2012-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dembinski R, Brackhahn W, Henzler D, Rott A, Bensberg R, Kuhlen R, Rossaint R. Cardiopulmonary effects of iloprost in experimental acute lung injury. Eur Respir J 25: 81–87, 2005. doi: 10.1183/09031936.04.10085504. [DOI] [PubMed] [Google Scholar]
- 19.Eifinger F, Sreeram N, Mehler K, Huenseler C, Kribs A, Roth B. Aerosolized iloprost in the treatment of pulmonary hypertension in extremely preterm infants: a pilot study. Klin Padiatr 220: 66–69, 2008. doi: 10.1055/s-2007-984370. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Gonzalez A, Alex Mitsialis S, Liu X, Kourembanas S. Vasculoprotective effects of heme oxygenase-1 in a murine model of hyperoxia-induced bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 302: L775–L784, 2012. doi: 10.1152/ajplung.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansmann G, Apitz C. Treatment of children with pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 102, Suppl 2: ii67–ii85, 2016. doi: 10.1136/heartjnl-2015-309103. [DOI] [PubMed] [Google Scholar]
- 22.James ML, Ross AC, Nicola T, Steele C, Ambalavanan N. VARA attenuates hyperoxia-induced impaired alveolar development and lung function in newborn mice. Am J Physiol Lung Cell Mol Physiol 304: L803–L812, 2013. doi: 10.1152/ajplung.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jónsson B, Tullus K, Brauner A, Lu Y, Noack G. Early increase of TNF alpha and IL-6 in tracheobronchial aspirate fluid indicator of subsequent chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed 77: F198–F201, 1997. doi: 10.1136/fn.77.3.F198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kale VM, Hsiao CJ, Boelsterli UA. Nimesulide-induced electrophile stress activates Nrf2 in human hepatocytes and mice but is not sufficient to induce hepatotoxicity in Nrf2-deficient mice. Chem Res Toxicol 23: 967–976, 2010. doi: 10.1021/tx100063z. [DOI] [PubMed] [Google Scholar]
- 25.Kazzi SN, Kim UO, Quasney MW, Buhimschi I. Polymorphism of tumor necrosis factor-alpha and risk and severity of bronchopulmonary dysplasia among very low birth weight infants. Pediatrics 114: e243–e248, 2004. doi: 10.1542/peds.114.2.e243. [DOI] [PubMed] [Google Scholar]
- 26.Kesten S, Dainauskas J, McLaughlin V, Rich S. Development of nonspecific interstitial pneumonitis associated with long-term treatment of primary pulmonary hypertension with prostacyclin. Chest 116: 566–569, 1999. doi: 10.1378/chest.116.2.566. [DOI] [PubMed] [Google Scholar]
- 27.Kimura Y, Koya T, Kagamu H, Shima K, Sakamoto H, Kawakami H, Hoshino Y, Furukawa T, Sakagami T, Hasegawa T, Narita M, Suzuki E, Narita I. A single injection of a sustained-release prostacyclin analog (ONO-1301MS) suppresses airway inflammation and remodeling in a chronic house dust mite-induced asthma model. Eur J Pharmacol 721: 80–85, 2013. doi: 10.1016/j.ejphar.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 28.Kolliputi N, Shaik RS, Waxman AB. The inflammasome mediates hyperoxia-induced alveolar cell permeability. J Immunol 184: 5819–5826, 2010. doi: 10.4049/jimmunol.0902766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakshminrusimha S, Mathew B, Leach CL. Pharmacologic strategies in neonatal pulmonary hypertension other than nitric oxide. Semin Perinatol 40: 160–173, 2016. doi: 10.1053/j.semperi.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lammi MR, Ghonim MA, Pyakurel K, Naura AS, Ibba SV, Davis CJ, Okpechi SC, Happel KI, deBoisblanc BP, Shellito J, Boulares AH. Treatment with intranasal iloprost reduces disease manifestations in a murine model of previously established COPD. Am J Physiol Lung Cell Mol Physiol 310: L630–L638, 2016. doi: 10.1152/ajplung.00297.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lassus P, Wolff H, Andersson S. Cyclooxygenase-2 in human perinatal lung. Pediatr Res 47: 602–605, 2000. doi: 10.1203/00006450-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Lindsay L, Oliver SJ, Freeman SL, Josien R, Krauss A, Kaplan G. Modulation of hyperoxia-induced TNF-alpha expression in the newborn rat lung by thalidomide and dexamethasone. Inflammation 24: 347–356, 2000. doi: 10.1023/A:1007096931078. [DOI] [PubMed] [Google Scholar]
- 33.Lovgren AK, Jania LA, Hartney JM, Parsons KK, Audoly LP, Fitzgerald GA, Tilley SL, Koller BH. COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 291: L144–L156, 2006. doi: 10.1152/ajplung.00492.2005. [DOI] [PubMed] [Google Scholar]
- 34.Masood A, Yi M, Lau M, Belcastro R, Li J, Kantores C, Pace-Asciak CR, Jankov RP, Tanswell AK. Cyclooxygenase-2 inhibition partially protects against 60% O2 -mediated lung injury in neonatal rats. Pediatr Pulmonol 49: 991–1002, 2014. doi: 10.1002/ppul.22921. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell JA, Warner TD. Cyclo-oxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br J Pharmacol 128: 1121–1132, 1999. doi: 10.1038/sj.bjp.0702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno-Galdó A, Torrent-Vernetta A, de Mir Messa I, Rovira Amigo S, Gran Piña F, Gartner S, Albert Brotons D. Use of inhaled iloprost in children with pulmonary hypertension. Pediatr Pulmonol 50: 370–379, 2015. doi: 10.1002/ppul.23044. [DOI] [PubMed] [Google Scholar]
- 37.Morimatsu H, Goto K, Matsusaki T, Katayama H, Matsubara H, Ohe T, Morita K. Rapid development of severe interstitial pneumonia caused by epoprostenol in a patient with primary pulmonary hypertension. Anesth Analg 99: 1205–1207, 2004. doi: 10.1213/01.ANE.0000130615.28893.52. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD Jr. TGF-beta-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 293: L151–L161, 2007. doi: 10.1152/ajplung.00389.2006. [DOI] [PubMed] [Google Scholar]
- 39.Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen YF, Ambalavanan N. Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 296: L738–L750, 2009. doi: 10.1152/ajplung.90603.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nold MF, Mangan NE, Rudloff I, Cho SX, Shariatian N, Samarasinghe TD, Skuza EM, Pedersen J, Veldman A, Berger PJ, Nold-Petry CA. Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proc Natl Acad Sci USA 110: 14384–14389, 2013. doi: 10.1073/pnas.1306859110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olave N, Lal CV, Halloran B, Pandit K, Cuna AC, Faye-Petersen OM, Kelly DR, Nicola T, Benos PV, Kaminski N, Ambalavanan N. Regulation of alveolar septation by microRNA-489. Am J Physiol Lung Cell Mol Physiol 310: L476–L487, 2016. doi: 10.1152/ajplung.00145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oncel MY, Yurttutan S, Alyamac Dizdar E, Gokce IK, Gonul II, Topal T, Canpolat FE, Dilmen U. Beneficial effect of etanercept on hyperoxic lung injury model in neonatal Rats. J Invest Surg 29: 1–5, 2016. doi: 10.3109/08941939.2015.1034898. [DOI] [PubMed] [Google Scholar]
- 43.Opitz CF, Wensel R, Bettmann M, Schaffarczyk R, Linscheid M, Hetzer R, Ewert R. Assessment of the vasodilator response in primary pulmonary hypertension. Comparing prostacyclin and iloprost administered by either infusion or inhalation. Eur Heart J 24: 356–365, 2003. doi: 10.1016/S0195-668X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- 44.Perrin S, Chaumais MC, O’Connell C, Amar D, Savale L, Jaïs X, Montani D, Humbert M, Simonneau G, Sitbon O. New pharmacotherapy options for pulmonary arterial hypertension. Expert Opin Pharmacother 16: 2113–2131, 2015. doi: 10.1517/14656566.2015.1074177. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds CL, Zhang S, Shrestha AK, Barrios R, Shivanna B. Phenotypic assessment of pulmonary hypertension using high-resolution echocardiography is feasible in neonatal mice with experimental bronchopulmonary dysplasia and pulmonary hypertension: a step toward preventing chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 11: 1597–1605, 2016. doi: 10.2147/COPD.S109510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rumzhum NN, Ammit AJ. Cyclooxygenase 2: its regulation, role and impact in airway inflammation. Clin Exp Allergy 46: 397–410, 2016. doi: 10.1111/cea.12697. [DOI] [PubMed] [Google Scholar]
- 47.Salaets T, Richter J, Brady P, Jimenez J, Nagatomo T, Deprest J, Toelen J. Transcriptome analysis of the preterm rabbit lung after seven days of hyperoxic exposure. PLoS One 10: e0136569, 2015. doi: 10.1371/journal.pone.0136569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawheny E, Ellis AL, Kinasewitz GT. Iloprost improves gas exchange in patients with pulmonary hypertension and ARDS. Chest 144: 55–62, 2013. doi: 10.1378/chest.12-2296. [DOI] [PubMed] [Google Scholar]
- 49.Surate Solaligue DE, Rodríguez-Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 313: L1101–L1153, 2017. doi: 10.1152/ajplung.00343.2017. [DOI] [PubMed] [Google Scholar]
- 50.Sureshbabu A, Syed M, Das P, Janér C, Pryhuber G, Rahman A, Andersson S, Homer RJ, Bhandari V. Inhibition of regulatory-associated protein of mechanistic target of rapamycin prevents hyperoxia-induced lung injury by enhancing autophagy and reducing apoptosis in neonatal mice. Am J Respir Cell Mol Biol 55: 722–735, 2016. doi: 10.1165/rcmb.2015-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Umeda S, Miyagawa S, Fukushima S, Oda N, Saito A, Sakai Y, Sawa Y, Okuyama H. Enhanced pulmonary vascular and alveolar development via prenatal administration of a slow-release synthetic prostacyclin agonist in rat fetal lung hypoplasia. PLoS One 11: e0161334, 2016. doi: 10.1371/journal.pone.0161334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vicil S, Erdoğan S. Beraprost sodium, a prostacyclin (PGI) analogue, ameliorates lipopolysaccharide-induced cellular injury in lung alveolar epithelial cells. Turk J Med Sci 45: 284–290, 2015. doi: 10.3906/sag-1401-108. [DOI] [PubMed] [Google Scholar]
- 53.Wong HR, Odoms KK, Denenberg AG, Allen GL, Shanley TP. Hyperoxia prolongs tumor necrosis factor-alpha-mediated activation of NF-kappaB: role of IkappaB kinase. Shock 17: 274–279, 2002. doi: 10.1097/00024382-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Xiao CY, Yuhki K, Hara A, Fujino T, Kuriyama S, Yamada T, Takayama K, Takahata O, Karibe H, Taniguchi T, Narumiya S, Ushikubi F. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation 109: 2462–2468, 2004. doi: 10.1161/01.CIR.0000128046.54681.97. [DOI] [PubMed] [Google Scholar]
- 55.Xu Z, Zhu L, Liu X, Gong X, Gattrell W, Liu J. Iloprost for children with pulmonary hypertension after surgery to correct congenital heart disease. Pediatr Pulmonol 50: 588–595, 2015. doi: 10.1002/ppul.23032. [DOI] [PubMed] [Google Scholar]
- 56.Yilmaz O, Kahveci H, Zeybek C, Ciftel M, Kilic O. Inhaled iloprost in preterm infants with severe respiratory distress syndrome and pulmonary hypertension. Am J Perinatol 31: 321–326, 2014. doi: 10.1055/s-0033-1348949. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Y, Liu Y, Zhou W, Xiang R, Jiang L, Huang K, Xiao Y, Guo Z, Gao J. A prostacyclin analogue, iloprost, protects from bleomycin-induced pulmonary fibrosis in mice. Respir Res 11: 34, 2010. doi: 10.1186/1465-9921-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]