Abstract
Pregnancy is associated with significant anatomic and functional changes to the cardiopulmonary system. Using pregnant C57BL/6 mice, we characterized changes in pulmonary structure and function during pregnancy in healthy animals and following infection with influenza A virus (IAV). We hypothesized that pregnancy-associated alterations in pulmonary physiology would contribute to the more severe outcome of IAV infection. Nonpregnant and pregnant females (at embryonic day 10.5) were either mock-infected or infected with 2009 H1N1 IAV for assessment of pulmonary function, structure, and inflammation at 8 days postinoculation. There were baseline differences in pulmonary function, with pregnant females having greater lung compliance, total lung capacity, and fixed lung volume than nonpregnant females. Following IAV infection, both pregnant and nonpregnant females exhibited reduced circulating progesterone, which in nonpregnant females was associated with increased pulmonary resistance and decreased lung compliance, minute ventilation, and oxygen diffusing capacity compared with uninfected nonpregnant females. In pregnant females, reduced concentrations of progesterone were associated with adverse pregnancy outcomes, but measures of pulmonary function were preserved following IAV infection and were not significantly different from uninfected pregnant mice. Following IAV infection, infectious virus titers and total numbers of pulmonary leukocytes were similar between pregnant and nonpregnant females, but the histological density of pulmonary inflammation was reduced in pregnant animals. These data suggest that pregnancy in mice is associated with significant alterations in pulmonary physiology but that these changes served to preserve lung function during IAV infection. Pregnancy-associated alterations in pulmonary physiology may serve to protect females during severe influenza.
Keywords: acute lung injury, progesterone, pulmonary inflammation, pulmonary structure, respiratory infection
INTRODUCTION
Pregnancy in humans is associated with numerous alterations in normal physiology, including anatomic and functional changes of the cardiopulmonary system that are essential for meeting the metabolic demands of the mother and fetus. In women, expansion of the uterus with a growing fetus causes anterior displacement of the diaphragm (24), but a compensatory increase in the chest diameter leaves the inspiratory and vital capacities largely unchanged (3). Elevated levels of estrogens during pregnancy induce hyperemia and hypersecretion of respiratory epithelial cells, which can lead to edema and obstruction of the upper airways (12), and rising progesterone levels increase respiratory drive and the partial pressure of oxygen (30), which accommodates the 20% increase in maternal oxygen consumption at term pregnancy (9). Furthermore, plasma volume and cardiac output increase, and systemic vascular resistance decreases to maintain perfusion of the fetus (12). Knowledge of these physiological adaptations is essential for distinguishing normal clinical dyspnea during pregnancy from pathological respiratory conditions.
Pregnant women demonstrate differential susceptibility to infectious and noninfectious respiratory disease. For example, infection with either seasonal or pandemic strains of influenza A viruses (IAVs) during pregnancy is associated with increased disease severity compared with the general population. It is estimated that pregnant women, especially those in their third trimester, are three to seven times more likely to be hospitalized and up to twice as likely to die from IAV-associated disease (18, 35, 39, 47). Pregnant women are also more susceptible to infection with varicella zoster virus, and before the licensing of a vaccine, it was estimated that more than one in four adult cases of varicella pneumonia were in pregnant women (6). In contrast, the severity of asthma during pregnancy is dichotomous, with two-thirds of women demonstrating unchanged or slightly increased disease severity during pregnancy and the other one-third of women experiencing significant clinical improvement (44). Cigarette smoking during pregnancy exacerbates alterations of maternal airway function (4), but the implications of this on secondary infection remain unknown.
Mice are among the most common experimental models of both infectious and noninfectious respiratory disease pathogenesis, including studies in pregnancy (2, 5, 22, 23, 28, 31, 37); little is known, however, about pulmonary functional changes during pregnancy, or how pregnancy-associated pulmonary alterations might influence the pathogenesis of respiratory diseases in mice. Furthermore, the correspondence between pulmonary functional changes in pregnant mice and women has not been discussed. We sought to characterize the pulmonary physiology of pregnant and nonpregnant C57BL/6 mice at baseline and following infection with IAV to determine how pregnancy might affect the functional response of the lungs to an infectious respiratory insult.
MATERIALS AND METHODS
Animals.
Adult (7–9 wk old) male (for breeding only) and female C57BL/6 mice were purchased from Charles River Laboratories (Frederick, MD). Mice were mated in trios (2 females per male) for 24 h. Pregnancy was confirmed by weight gain (>8 g) measured 9 days following mating, as previously described (13). At the time of infection, all females were between 8 and 14 wk of age. Following infection, nonpregnant female mice were housed five animals per cage, and pregnant mice were housed one animal per cage. All mice were housed under standard BSL-2 housing conditions with food and water ad libitum. Euthanasia was performed by anesthetic (ketamine-xylazine) overdose followed by exsanguination by cardiac puncture using a 25-gauge needle and 1-ml syringe. All animal procedures were approved by The Johns Hopkins University Animal Care and Use Committee under animal protocol M015H236.
Virus infection.
Pregnant [embryonic day (E)10] and nonpregnant female mice were infected with mouse-adapted IAVs A/California/04/09 (ma2009; H1N1) generated by Dr. Andrew Pekosz from a published sequence (10). For the infections, mice were anesthetized with a ketamine (100 mg/kg) and xylazine (10 mg/kg) cocktail and inoculated intranasally with 101 TCID50 units of ma2009 virus suspended in 30 μl of DMEM or mock-infected with DMEM alone. Mice were monitored daily for changes in body mass. Pregnancies were determined to be aborted when the percentage of maternal body weight gain was less than 50% of the corresponding mock-infected pregnant controls, and aborted pregnancies were confirmed by laparotomy following euthanasia.
Quantification of tissue viral burden.
Lungs were homogenized in 500 μl of sterile PBS, and infectious viral burden was measured from supernatants by a TCID50 assay, as previously described (49). Briefly, tenfold dilutions of lung homogenate supernatants were plated onto a monolayer of Madin-Darby canine kidney (MDCK) cells in replicates of six for 6 days at 32°C. Cells were stained with naphthol blue black (Sigma Aldrich) and scored for cytopathic effects. The TCID50 titer was calculated according to the Reed-Muench method.
Progesterone measurements.
Steroid hormones were extracted from 250 to 500 μl of serum using a liquid-liquid extraction method. Briefly, serum samples were mixed with methyl-tert-butyl ether at a 5:1 solvent-to-sample ratio. The solvent layer was allowed to separate for 5 min and then transferred into a clean tube and evaporated to dryness in a fume hood overnight. The extracted hormone was resuspended in at least 500 μl of assay buffer and vortexed. The concentration of progesterone was quantified by competitive immunoassay (Enzo Life Sciences), according to the manufacturer’s instructions.
Flow cytometry.
Lungs were excised and single-cell suspensions generated following red blood cell lysis. The total numbers of viable cells were determined using a hemocytometer and trypan blue (Invitrogen) exclusion, and the cells were resuspended at 1 × 106 cells/ml in RPMI 1640 (Cellgro) supplemented with 10% fetal bovine serum (Fisher Scientific) and 1% penicillin-streptomycin. The viability of cells was determined by use of a fixable Live/Dead aqua viability dye (Invitrogen), and Fc receptors were blocked using anti-CD16/32 (BD Biosciences). Leukocyte populations were stained with the following antibodies (eBiosciences): CD45-PE-Cy7 (Clone 30-F11), CD3-APC (Clone 17A2), CD4-PerCP-Cy5.5 (Clone RM4.5), CD8-AF700 (Clone 53–6.7), CD11b-PerCP-Cy5.5 (Clone M1/70), CD11c-APC (Clone (N418), and Ly6G-FITC (Clone Gr-1). Data were acquired using a Fortessa fluorescence-activated cell sorter (with FACSDiva software) and analyzed using FlowJo (v. 10) software (Tree Star). Total cell counts were determined based on the percentages of live cells in the live cell gate multiplied by the total live cell counts acquired before staining by the trypan blue exclusion counts obtained on a hemocytometer.
Histopathology.
Following euthanasia, the left bronchus was ligated, and the left lung lobe was removed. The right lung lobes were then inflated with zinc-buffered formalin (Z-fix, Anatech) delivered for 2 min at a fixed pressure (25 cmH2O). The trachea was tied under pressure, and the lungs were dissected free and placed in fixative for 48 h. Fixed lung volumes were then measured by water displacement, as previously described (27), and tissues were subsequently embedded in paraffin, cut into 5-μm sections, and mounted on glass slides. Slides were stained with hematoxylin and eosin (H&E) and used to evaluate lung inflammation. Histopathological scoring was performed by a single blinded observer using a 0–3 scale (0, no inflammation; 1, mild inflammation; 2, moderate inflammation; 3, severe inflammation) for bronchiolitis, perivasculitis, alveolitis, and edema, as previously described (11). The sum of these parameters represents the cumulative inflammation score. Images were taken using a Nikon Eclipse E800 camera.
Pulmonary function phenotyping.
Pulmonary function analyses were performed in nonpregnant and pregnant mice 8 days postinoculation (dpi). Respiratory rate, tidal volume, and minute ventilation were determined by barometric whole body plethysmography performed on unanesthetized mice, as previously described (32). Briefly, mice were placed in a closed chamber with 100% humidity that was connected to a pressure transducer. Pressure fluctuations that occurred during the breathing cycle were recorded over time along with the temperature inside the chamber. Measurements of respiratory rate, tidal volume, and minute ventilation were calculated from the resulting tracings based on 100% humidity in the chamber and the temperature difference between that within the chamber and the mouse. Mice were then anesthetized with a ketamine-xylazine cocktail (100 and 10 mg/kg, respectively), and a tracheostomy was performed for cannulation with an 18-gauge stub needle. Following tracheotomy, 0.8 ml of a gas mixture containing 0.3% neon, 0.3% CO in room air was quickly injected through the cannula into the lungs, held for 9 s, and then quickly withdrawn for measurement of lung diffusing capacity (DFCO) by gas chromatography (Inficon, Micro GC model 3000A), as previously described (26). The DFCO for each mouse was calculated as 1 − (CO9/COc)/(Ne9/Nec) (c = calibration gas, 9 = gas from the 9-s exhaled sample). Following measurement of DFCO, mice were mechanically ventilated with oxygen using a Flexivent system (Scireq) at a rate of 150 breaths/minute and a tidal volume of 0.25 ml with a PEEP of 3 cmH2O. Total respiratory resistance and compliance were measured 1 min following a deep inspiration to 30 cmH2O. The lungs were then degassed with oxygen absorption, and quasi-static pressure-volume measurements were immediately taken in situ using a system detailed in previous studies (25). The total lung capacity and residual volume were calculated from the resulting pressure-volume curves.
Statistical analyses.
Longitudinal morbidity and abortion data were analyzed with repeated-measures ANOVA and χ2-tests, respectively. Litter size, virus titers, flow cytometry, histopathology, and fixed lung volume data were analyzed using Student’s t-test. Pulmonary function data were analyzed using either a t-test or two-way ANOVA with Bonferroni post hoc correction. Mean differences were considered statistically significant if P < 0.05. All statistical analyses were performed using GraphPad Prism 7 software.
RESULTS
Baseline pulmonary physiology is altered during pregnancy in C57BL/6 mice.
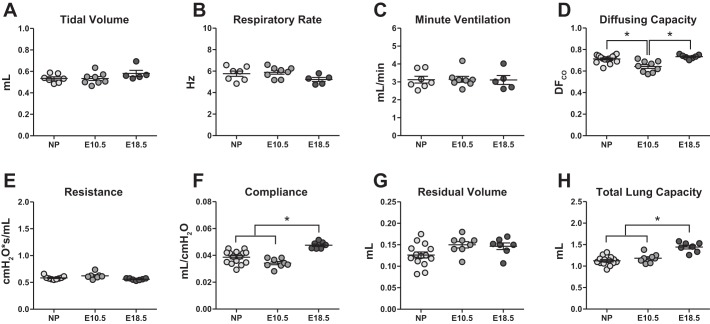
Baseline differences in pulmonary physiology and function exist between pregnant and nonpregnant women and are hypothesized to contribute to altered respiratory disease pathogenesis during pregnancy (3, 9, 12, 24, 30). Owing to the increased oxygen demands of the growing fetus, pregnancy is associated with ‘physiological hyperventilation’ and respiratory alkalosis that occur consequent to an increase in tidal volume and minute ventilation (12). To determine whether similar baseline differences in pulmonary physiology exist between nonpregnant and pregnant mice, we performed in vivo pulmonary function testing (PFT) on nonpregnant and pregnant C57BL/6 mice. PFT of pregnant mice was performed at both embryonic day (E)10.5 and E18.5, which correspond to midgestation and term pregnancy, respectively, in C57BL/6 mice (34). Whole body plethysmography was used to measure quiet tidal breathing in unanesthetized animals. In contrast to observations in humans, there were no differences in tidal volume, respiratory rate, or minute ventilation between nonpregnant and pregnant mice (Fig. 1, A–C). There was a small, but significant, reduction in pulmonary diffusing capacity in pregnant females at E10.5 compared with either nonpregnant females or pregnant females at E18.5 (Fig. 1D). During normal pregnancy in women, hormone-mediated relaxation of tracheobronchial smooth muscles causes a decrease in pulmonary resistance at term, but there are no reported changes in either static or dynamic lung compliance (29). In mice, there were no differences in pulmonary resistance (Fig. 1E), but pregnant females at E18.5 demonstrated significantly greater dynamic lung compliance compared with nonpregnant females and pregnant females at E10.5 (Fig. 1F). Due to the anterior displacement of the diaphragm during late pregnancy, lung volumes undergo major changes. Specifically, in women, the expiratory reserve volume and residual volume (RV) decrease progressively, but relaxation of the chest wall and compensatory lateral expansion preserves total lung capacity (TLC) (29). Although the RV was not significantly different between nonpregnant and pregnant mice (Fig. 1G), the TLC was significantly greater in pregnant animals at E18.5 compared with either those at E10.5 or nonpregnant females (Fig. 1H). Moreover, the heightened lung compliance in pregnant mice at E18.5 was positively correlated with a greater TLC (Pearson correlation, R2 = 0.8536, P < 0.05), despite measurements of normal tidal breathing remaining unchanged.
Fig. 1.
Baseline pulmonary function is altered during pregnancy in C57BL/6 mice. Pulmonary function testing was performed on nonpregnant (n = 7–15) and pregnant mice at embryonic day (E)10.5 (n = 7–9) or E18.5 (n = 5–7). Whole body plethysmography was used to measure tidal volume (A), respiratory rate (B), and minute ventilation (C) from unanesthetized animals. Following anesthesia and tracheal cannulation, pulmonary diffusing capacity (D) was measured by gas chromatography, and dynamic resistance (E) and compliance (F) were measured using a Flexivent system with forced oscillation technique. Following euthanasia, the residual volume (G) and total lung capacity (H) were calculated from pressure volume curves. Bars represent means ± SE. All data sets were analyzed using one-way ANOVAs with Bonferroni post hoc correction, where *P < 0.05.
Sublethal IAV infection of pregnant mice results in severe maternal disease and adverse pregnancy outcomes.
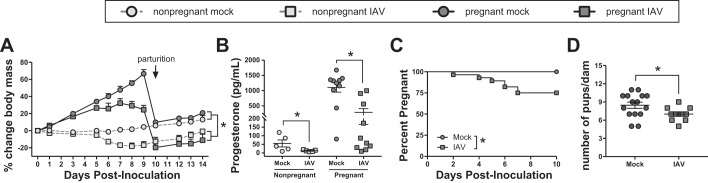
IAV infection during pregnancy is associated with heightened disease severity in women (18, 35, 39, 47), and severe disease can lead to obstetric complications, including spontaneous abortion, preterm delivery, and neonatal death (45). To evaluate whether pregnant mice mimic similar disease outcomes, pregnant (E10.5) and nonpregnant female mice were infected with a sublethal dose of mouse-adapted 2009 (ma2009) H1N1 IAV. Over the course of IAV infection, the average body mass of both nonpregnant and pregnant females was significantly reduced compared with their respective mock-infected controls (Fig. 2A). Eight days postinoculation (dpi), corresponding to E18.5 in pregnant females and with peak clinical disease in both pregnant and nonpregnant females (Fig. 2A), serum progesterone concentrations were significantly reduced in both nonpregnant and pregnant IAV-infected females compared with their respective mock-infected controls (Fig. 2B). A significant number of pregnant mice (7/28, 25%) aborted their pregnancies within 7 days of IAV infection, whereas there were no spontaneous abortions in the mock-infected pregnant mice (Fig. 2C). Of the dams that carried their pregnancies to term, the size of litters born from IAV-infected dams was significantly reduced compared with those born from uninfected dams (Fig. 2D). There was, however, no significant correlation between serum progesterone levels and litter size (Spearman correlation, r = 0.3925, P = 0.1482), and there was no difference in the average neonatal body mass at postnatal day (PND)0 (mock-infected = 1.48 ± 0.07; IAV-infected = 1.31 ± 0.13).
Fig. 2.
Influenza A virus (IAV) infection of pregnant mice results in severe maternal disease and adverse pregnancy outcomes. A: nonpregnant (n = 10) and pregnant [embryonic day (E)10.5; n = 10–15] mice were infected with a sublethal dose of IAV or vehicle (mock) (n = 6–8/treatment) and monitored daily for changes in body mass. B: serum progesterone was quantified at 8 days postinoculation (dpi), corresponding to E18.5 in pregnant females. In the pregnant dams, spontaneous abortion was monitored over the course of infection (C), and litter size was quantified for each dam that carried her pregnancy to term (D). Bars represent means ± SE. Data were analyzed using repeated-measures ANOVA (A), two-way ANOVA (B), χ2-test (C), and Student’s t-test (D), where *P < 0.05.
Pulmonary inflammatory responses to IAV infection are similar between pregnant and nonpregnant mice.
To determine whether pregnancy altered virus replication or pulmonary inflammation during IAV infection, total pulmonary leukocytes and tissue viral burden were quantified by flow cytometry and TCID50 assay, respectively. Eight days postinfection, there were no differences in infectious virus titers between pregnant and nonpregnant females (Fig. 3A), and by 14 dpi, infectious virus was cleared from the lungs in all females (data not shown). Moreover, at 8 dpi, there were no differences in the total number of CD45+ leukocytes in the lungs of pregnant and nonpregnant mice (Fig. 3B). Further stratification of leukocyte populations revealed no differences in either total numbers or relative proportions (i.e., percentage of CD45+ cells) of specific immune cell subsets, including CD11c+ dendritic cells and interstitial macrophages, Ly6G+ neutrophils, SiglecF+ alveolar macrophages and eosinophils as well as CD4+ and CD8+ T cells (Table 1). To determine the character and distribution of lung pathology in pregnant and nonpregnant mice during IAV infection, we performed histopathological scoring of H&E-stained lung sections for markers of pulmonary inflammation. Eight days postinfection, histological evidence of pulmonary inflammation and edema was reduced in pregnant compared with nonpregnant females (Fig. 3, C and D), but the fixed lung volumes were increased in pregnant compared with nonpregnant mice (Fig. 3E). These data suggest that sublethal IAV infection of pregnant mice mimics aspects of the human disease phenotype, including maternal morbidity and adverse pregnancy outcomes. Moreover, despite similar numbers of total CD45+ immune cells in the lungs of nonpregnant and pregnant mice, the visual density of pulmonary inflammation was reduced in pregnant mice, likely due to increased lung compliance and greater expansion of airspaces during fixation.
Fig. 3.
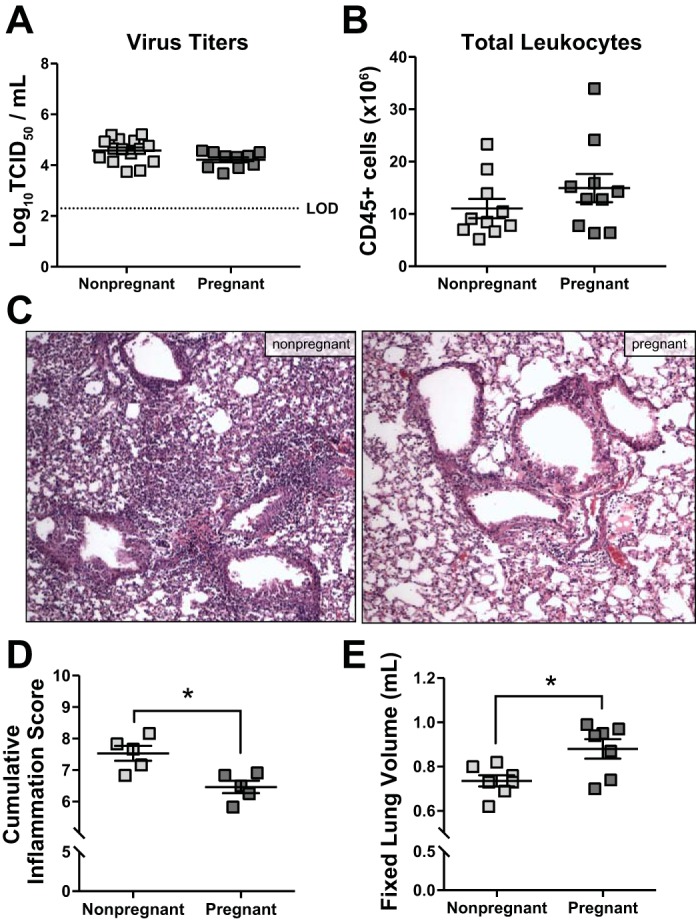
Pulmonary inflammatory responses to influenza A virus (IAV) infection are similar between pregnant and nonpregnant female mice. Nonpregnant (n = 5–15) and pregnant [embryonic day (E)10.5; n = 5–10] mice were inoculated with a sublethal dose of IAV or vehicle (mock). Females were euthanized 8 days postinoculation (dpi), corresponding to E18.5 in pregnant dams. Infectious virus (A), determined by 50% tissue culture infectious dose (TCID50), and total numbers of CD45+ inflammatory cells (B), determined by flow cytometry, were quantified from the lungs. Histopathology was performed on fixed lung tissue, and representative images from H&E-stained sections were taken at ×20 magnification (C). D: cumulative pulmonary inflammation was quantified based on a 0–3 scale for bronchiolitis, perivasculitis, alveolitis, and edema, and the sums of each score are presented. E: fixed lung volume was determined from inflated lungs by water displacement. Bars represent means ± SE. The stippled line in A represents the limit of detection (LOD). All data sets were analyzed using Student’s t-test, where *P < 0.05.
Table 1.
Total numbers and proportions of CD45+ cells in lungs of nonpregnant and pregnant female mice 8 days after infection with IAV
| Total Cells (×105) ± SE |
%CD45+ Cells ± SE |
||||||
|---|---|---|---|---|---|---|---|
| Cell Population(s) | Surface Marker(s) | Nonpregnant | Pregnant | P Value | Nonpregnant | Pregnant | P Value |
| Myeloid cells | CD11b+ | 65.20 ± 11.16 | 80.52 ± 15.34 | 0.528 | 62.74 ± 1.12 | 62.20 ± 1.90 | 0.855 |
| Dendritic cells and interstitial macrophages | CD11b+CD11c+ | 27.44 ± 4.98 | 33.32 ± 6.41 | 0.571 | 26.32 ± 1.63 | 26.12 ± 2.34 | 0.956 |
| Neutrophils | CD11b+Ly6G+ | 4.35 ± 0.77 | 5.35 ± 1.02 | 0.543 | 4.17 ± 0.18 | 4.15 ± 0.25 | 0.963 |
| Alveolar macrophages and eosinophils | CD11b+SiglecF+ | 0.27 ± 0.04 | 0.34 ± 0.06 | 0.468 | 0.27 ± 0.02 | 0.26 ± 0.01 | 0.919 |
| T cells | CD3+ | 31.48 ± 4.34 | 43.68 ± 8.95 | 0.346 | 31.90 ± 4.27 | 33.42 ± 3.16 | 0.821 |
| CD4+ T cells | CD3+CD4+ | 10.58 ± 1.77 | 15.05 ± 3.70 | 0.400 | 10.19 ± 0.56 | 11.23 ± 1.10 | 0.511 |
| CD8+ T cells | CD3+CD8+ | 16.99 ± 2.63 | 24.08 ± 5.36 | 0.360 | 17.83 ± 3.41 | 18.65 ± 2.68 | 0.881 |
Values are means ± SE. IAV, influenza A virus.
Following IAV infection, pulmonary function is preserved in pregnant compared with nonpregnant mice.
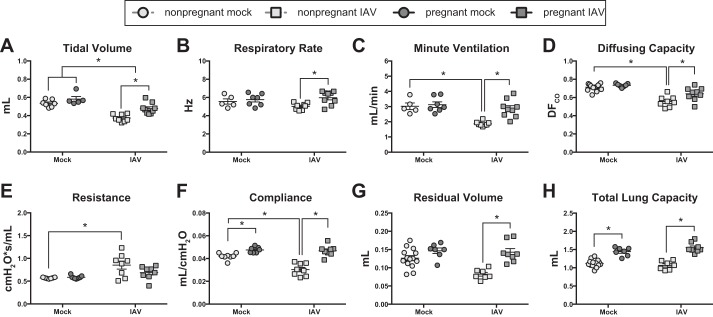
Inflammation-mediated pulmonary tissue damage following IAV infection was observed in nonpregnant, and to a lesser extent in pregnant, female mice (Fig. 3C), which could lead to pulmonary edema, obstruction of air spaces, turbulent airflow, and increased pulmonary resistance (21). To determine the functional effects of IAV infection on pulmonary physiology in pregnant compared with nonpregnant mice, PFT was performed 8 dpi, corresponding to peak clinical disease (Fig. 2A). Consistent with previous reports in nonpregnant mice (43), IAV infection of nonpregnant females resulted in reduced tidal volumes, stable respiratory rates, and corresponding reductions in minute ventilation (Fig. 4, A–C). Also similar to previous studies (51), IAV infection of nonpregnant females resulted in increased pulmonary resistance (Fig. 4D). In nonpregnant mice, the pulmonary inflammation following IAV infection (Fig. 3D) corresponded to a decrease in lung compliance and diffusing capacity (Fig. 4, E and F). The RV and TLC in IAV-infected nonpregnant mice, however, remained unchanged compared with uninfected nonpregnant females (Fig. 4, G and H).
Fig. 4.
Following influenza A virus (IAV) infection, pulmonary function is preserved in pregnant vs. nonpregnant mice. Nonpregnant (n = 5–11) and pregnant [embryonic day (E)10.5] (n = 5–9) mice were infected with a sublethal dose of IAV or vehicle (mock). Eight days postinoculation (dpi), corresponding to E18.5 in pregnant dams, pulmonary function testing was performed. Whole body plethysmography was used to measure tidal volume (A), respiratory rate (B), and minute ventilation (C) from unanesthetized animals. Following anesthesia and tracheal cannulation, pulmonary diffusing capacity (D) was measured by gas chromatography, and dynamic resistance (E) and compliance (F) were measured using a Flexivent system with forced oscillation technique. Following euthanasia, the residual volume (G) and total lung capacity (H) were calculated from pressure volume curves. Bars represent the means ± SE. All data sets were analyzed using a two-way ANOVA with Bonferroni post hoc correction, where *P < 0.05.
To test the hypothesis that IAV infection differentially impacts pulmonary function in pregnant vs. nonpregnant mice, PFT measures obtained 8 dpi (i.e., corresponding to E18.5 in pregnant mice) were compared. Similarly to nonpregnant females, IAV infection of pregnant mice caused a decrease in tidal volume, but there was no effect of infection on either respiratory rate or minute ventilation, and all tidal breathing measurements were significantly greater in pregnant compared with nonpregnant females (Fig. 4, A–C). In contrast to nonpregnant females, pregnant mice did not experience a significant change in pulmonary resistance, compliance, or diffusing capacity following IAV infection (Fig. 4, D–F). The RV and TLC of pregnant mice 8 dpi also remained unchanged from baseline and were significantly greater than infected, nonpregnant females (Fig. 4, G and H). Together, these data suggest that sublethal IAV infection results in more significant impairment to pulmonary function in nonpreganant, compared with pregnant, C57BL/6 mice.
DISCUSSION
Pregnancy in women is associated with significant changes to pulmonary physiology that may impact the pathogenesis of respiratory disease. Because mechanistic studies cannot ethically be performed in pregnant women, mice are a common animal model of respiratory disease and acute lung injury caused by both infectious and noninfectious insults. Despite numerous studies of respiratory disease pathogenesis in pregnant mouse models (2, 5, 22, 23, 31, 37), pulmonary functional changes during pregnancy have not been described. This study provides a comprehensive characterization of pulmonary functional changes during pregnancy in C57BL/6 mice and describes the functional responses to an acute respiratory pathogen. Our data reveal unique pregnancy-associated changes in mice to pulmonary function that may influence the pathogenesis of respiratory disease. Furthermore, following a sublethal IAV infection, our data provide evidence that pregnancy in mice protects against respiratory impairment associated with IAV-induced lung injury. On tne basis of these observations, we conclude that pregnancy-associated pulmonary functional changes in mice do not contribute to heightened maternal disease or adverse pregnancy outcomes during IAV infection. This study improves our understanding of respiratory physiology in mouse models, which has broad relevance to translational pulmonary research.
A significant conclusion from this study is that pregnancy in mice is associated with unique changes in pulmonary physiology that have not been previously reported in pregnant women. While some of these differences may reflect the limitations of available techniques for measuring pulmonary function in humans, others may expose important species-specific physiological differences. In both women (15) and mice (16), pregnancy is associated with significant expansion of both circulating blood volume and red blood cell mass to accommodate fetal oxygen demands. Whereas pregnant women compensate for this by increasing minute ventilation and oxygen consumption (20), we observed no comparable increase in tidal volume, respiratory rate, or minute ventilation in pregnant compared with nonpregnant mice. Additionally, there was no difference in pulmonary diffusing capacity between pregnant and nonpregnant females, suggesting that increased ventilation may not be required to meet fetal oxygen demands in mice. There was a transient decrease in pulmonary diffusing capacity at midgestation (i.e., E10.5) in mice that was not evident at term (i.e., E18.5). The decrease in diffusing capacity at E10.5 was mild and unlikely to have clinical significance, as IAV infection of pregnant females at E10.5 resulted in no change to diffusing capacity, as measured at peak disease (i.e., 8 dpi, E18.5).
Additionally, in pregnant women, the developing fetus displaces the diaphragm, thereby altering the chest cavity and lung shape. Third-trimester pregnancy is associated with a decrease in forced vital capacity and expiratory volume but unchanged dynamic respiratory compliance and TLC due to lateral expansion of the thorax (36) (29). In contrast, term (i.e., E18.5) pregnancy in mice was associated with a significant increase in lung compliance and TLC, regardless of IAV infection status. Moreover, these physiological changes were unique to late pregnancy, as they were not observed in pregnant animals at E10.5. Although a change in TLC during pregnancy has not been reported in humans, it may reflect differences in measurement techniques used to obtain corresponding values. More specifically, human TLC is typically calculated based on gas dilution or plethysmography (7), both of which require voluntary muscular effort, which may be decreased during pregnancy; in contrast, TLC in mice is the lung volume measured from anesthetized animals at fixed high pressure. The reasons why lung compliance and TLC increase during pregnancy in mice are unclear, but these observations might reflect some intrinsic temporal change in lung structure that permits greater expansive capacity. Lung compliance in vivo is influenced by the quantity and composition of pulmonary surfactant, which functions to reduce alveolar surface tension (17); therefore, it is possible that late pregnancy is associated with either increased production or altered composition of surfactant. Lung compliance in vivo may also be influenced indirectly by chest wall compliance. The heightened lung compliance in late-pregnant mice, however, was confirmed by the increase in fixed lung volume that was measured ex vivo, when the chest wall was not present. While additional studies are needed to determine the mechanism and functional significance of increased lung compliance in late-pregnant mice, these observations reveal a unique physiological variable that may differentially influence respiratory function in pregnant mice compared with pregnant women.
Studies aimed at understanding the physiological determinants of heightened IAV-associated disease during pregnancy have focused primarily on the immunological responses to IAV infection, with many studies documenting that pregnancy alters local and systemic immune responses to IAV infection in both humans (8, 38, 46) and mice (2, 5, 22, 23, 28, 31, 37). Pregnancy in women is associated with alterations in immune responses that promote fetal tolerance, and disruption of pregnancy-associated immune modulation can lead to pregnancy complications (33). The impact of these immunological shifts on inflammatory responses occurring outside the reproductive tract, however, remains ill defined. In the context of IAV infection of the respiratory tract, it has been hypothesized that heightened helper T cell (Th) type 2 (Th2) and regulatory T cell responses combined with reduced Th1 and Th17 cell responses during pregnancy may contribute to increased disease susceptibility, but there is a paucity of data to support this mechanistic link (19, 39).
Data from IAV infection studies in pregnant mice have been conflicting and inconclusive, and a causal relationship between immunological skewing and heightened IAV-associated disease has not been established. Furthermore, the differences in mouse strain, IAV strain and dose, and the day of infection during pregnancy make it difficult to compare the results across different studies. For example, whereas studies of high-dose pandemic (A/Cal/04/2009) H1N1 IAV infection in BALB/c mice have shown that pregnancy increases total numbers of pulmonary immune cells (31), other studies that used lower-dose (A/PR/8/1935 or A/Cal/04/2009) H1N1 IAV inocula in C57BL/6 mice report either no change (37) or reduced pulmonary inflammation in pregnant compared with nonpregnant, animals (5). Also incongruent across studies are the patterns of proinflammatory cytokines and chemokines, including IL-6, TNFα, and CCL2, that are induced as part of the classic ‘cytokine storm’ following IAV infection; whereas some studies (2, 31) demonstrate heightened cytokine induction in pregnant animals, others (5, 37) report the opposite phenotype. Similarly, while some studies have reported that pregnant mice (both BALB/c and C57BL/6 strains) support greater IAV replication than nonpregnant mice (2, 5, 22, 23, 28, 37), others have reported similar viral replication kinetics (31). In our study, infection of pregnant C57BL/6 mice with a sublethal dose of pandemic (A/Cal/04/2009) H1N1 IAV resulted in no difference in virus replication or clearance compared with nonpregnant females. We also found no difference in either the total number of pulmonary leukocytes or in any of the innate and adaptive immune cell subsets in pregnant compared with nonpregnant mice. Because analyses in this study are limited to a single timepoint (i.e., 8 dpi), we cannot rule out the possibility that differences in the kinetics of virus replication, inflammatory cell recruitment, or pulmonary repair may exist between pregnant and nonpregnant mice.
More recent studies of both seasonal and pandemic IAV infection of both nonpregnant (11) and pregnant mice (5, 28) have revealed a link between circulating progesterone concentrations and pulmonary disease. In nonpregnant mice, physiological concentrations of progesterone protect female mice during IAV infection by enhancing pulmonary epithelial repair and function (11). During pregnancy in both women and mice, progesterone levels increase dramatically, and high circulating progesterone is essential for pregnancy maintenance. Reduced circulating progesterone in pregnant women has been associated with obstetric complications, including preeclampsia in women (50), and reduced serum and placental progesterone following IAV infection of pregnant mice has been hypothesized to contribute to adverse pregnancy outcomes, including spontaneous abortion, preterm birth, reduced litter size, and fetuses that are small for their gestational age (22, 23, 28). Consistent with these reports, we observed a reduction in serum progesterone following IAV infection of both nonpregnant and pregnant females, which corresponded to spontaneous abortion and reduced litter size in pregnant mice. In nonpregnant females, reduced progesterone may be associated with disruption of the estrous cycle and prolongation of diestrus, as has been previously reported following IAV infection of intact female mice (40). Also, consistent with previous studies in nonpregnant female mice (11), reduced serum progesterone at 8 dpi was associated with a decrease in pulmonary function. In pregnant females, however, although progesterone levels were significantly reduced from baseline, they were still maintained at or above physiological levels, and thus pulmonary function was preserved. It is unclear whether reduced progesterone is a cause of spontaneous abortion and fetal loss, as there was not a significant correlation between serum progesterone and litter size. Future studies are warranted to determine the mechanisms of pregnancy disruption during IAV infection as well as the utility of exogenous progesterone treatment to improve pregnancy outcomes.
In humans, acute, uncomplicated IAV infection has been reported to cause restrictive ventilatory defects and associated alterations in tidal breathing patterns (21). More severe infections resulting in viral pneumonia can additionally cause a decrease in pulmonary diffusing capacity, which is correlated with feelings of ‘well-being,’ and can be used to monitor long-term recovery (14). Pneumonia is a significant cause of maternal and fetal morbidity and mortality following IAV infection during pregnancy, and compared with the general population, pregnant women experience more severe complications, including respiratory failure (1, 41). As in other high-risk groups, the development of pneumonia and acute respiratory distress syndrome (ARDS) are the among the most common complications of IAV infection in pregnant women (48). Further, among pregnant women with ARDS, underlying IAV infection is a significant risk factor for mortality (42), but the mechanisms underlying the predisposition during pregnancy remain unknown.
Similar to nonpregnant humans, IAV infection of nonpregnant female mice resulted in a significant reduction in tidal volume and minute ventilation, and a significant increase in pulmonary resistance during peak disease (i.e., 8 dpi). Nonpregnant female mice also experienced a significant decrease in pulmonary diffusing capacity, which averaged 17% during peak disease. In contrast, IAV infection of pregnant mice resulted in no change to either minute ventilation or pulmonary resistance, and pulmonary diffusing capacity in pregnant mice was relatively preserved, with an average 9% reduction at peak disease. Together, these data suggest that pregnant mice maintain pulmonary function, including efficient oxygen exchange, better than nonpregnant mice following IAV infection. Many factors can impede oxygen exchange in the lungs, including pulmonary inflammation and edema, which effectively reduces the functional surface area of the alveolar epithelium. In our study, histological evaluation of the lungs following IAV infection showed reduced evidence of pulmonary inflammation and edema in pregnant compared with nonpregnant females. Quantification of total pulmonary leukocytes, however, revealed similar frequencies of total CD45+ cells in both pregnant and nonpregnant females, suggesting that the total number of immune cells in the lungs is the same. The discrepancy between the histopathological inflammatory changes and quantification of total cell numbers may be explained by the differences in lung compliance, total lung capacity, and fixed lung volume. Because of the greater lung compliance in pregnant mice, the lungs are able to expand to greater volumes when infused to the same pressure. The result of this is greater total lung capacity and fixed lung volume in pregnant compared with nonpregnant females, owing to greater expansion of the airspaces rather than a greater volume of lung tissue. Consequently, the cellular density on fixed tissue sections may appear reduced, despite similar total CD45+ cell numbers. Although it is unclear whether cellular density or total CD45+ cell numbers in the lungs are more important for predicting influenza severity, we speculate that the greater expansive capacity of the lungs in pregnant females may serve to increase the available surface area for oxygen exchange to improve pulmonary function.
IAV infection of pregnant women is associated with heightened morbidity and mortality compared with the general population, and hospitalization rates and deaths from IAV infection in pregnant women are greatest during the third trimester (18, 35, 39, 47), but the mechanisms that mediate this increased disease severity remain elusive. In the present study, we have shown that pregnant mice also experience significant morbidity and body weight loss during IAV infection despite relatively preserved pulmonary function. Moreover, our data show no evidence of altered pulmonary immune responses during pregnancy, and pregnant mice retained sufficient antiviral responses to recognize and clear a sublethal IAV infection, suggesting that the lung-specific physiological and immunological responses to IAV infection do not underlie the heightened disease severity that occurs during pregnancy. Instead, these and others’ (28) data suggest that the physiological responses to IAV infection during pregnancy may be compartmentalized, and adverse maternal and fetal outcomes may be mediated by systemic and tissue-specific responses, which occur independent of the immunologic and functional responses in the lungs. Although our data suggest that pregnancy preserves pulmonary function in the context of IAV infection, additional studies are warranted to determine whether pregnancy is similarly protective in the context of other infectious or noninfectious respiratory insults. Moreover, longitudinal analysis of pulmonary function over the course of gestation and postpartum period, as well as consideration of other variables, including age, parity, and IAV exposure history, may enhance translation of these data to pregnant women.
GRANTS
This work was supported by the NIH/NIAID Center of Excellence in Influenza Research and Surveillance contract HHS N272201400007C (S. L. Klein) and NIH T32 OD011089 Training Veterinarians for Careers in Biomedical Research (M. S. Vermillion).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S.V. and S.L.K. conceived and designed research; M.S.V., A.N., L.G.v.S., and J.M.L. performed experiments; M.S.V. analyzed data; M.S.V., W.M., and S.L.K. interpreted results of experiments; M.S.V. prepared figures; M.S.V. and S.L.K. drafted manuscript; M.S.V., A.N., L.G.v.S., W.M., and S.L.K. edited and revised manuscript; M.S.V., A.N., L.G.v.S., W.M., and S.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Klein, Davis, and Pekosz laboratories for detailed discussions about these data. We thank Cory Brayton and the Johns Hopkins Mouse Phenotyping Core for assistance with the development of the pulmonary histopathology scoring system.
REFERENCES
- 1.Berkowitz K, LaSala A. Risk factors associated with the increasing prevalence of pneumonia during pregnancy. Am J Obstet Gynecol 163: 981–985, 1990. doi: 10.1016/0002-9378(90)91109-P. [DOI] [PubMed] [Google Scholar]
- 2.Chan KH, Zhang AJ, To KK, Chan CC, Poon VK, Guo K, Ng F, Zhang QW, Leung VH, Cheung AN, Lau CC, Woo PC, Tse H, Wu W, Chen H, Zheng BJ, Yuen KY. Wild type and mutant 2009 pandemic influenza A (H1N1) viruses cause more severe disease and higher mortality in pregnant BALB/c mice. PLoS One 5: e13757, 2010. doi: 10.1371/journal.pone.0013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contreras G, Gutiérrez M, Beroíza T, Fantín A, Oddó H, Villarroel L, Cruz E, Lisboa C. Ventilatory drive and respiratory muscle function in pregnancy. Am Rev Respir Dis 144: 837–841, 1991. doi: 10.1164/ajrccm/144.4.837. [DOI] [PubMed] [Google Scholar]
- 4.Das TK, Moutquin JM, Parent JG. Effect of cigarette smoking on maternal airway function during pregnancy. Am J Obstet Gynecol 165: 675–679, 1991. doi: 10.1016/0002-9378(91)90307-D. [DOI] [PubMed] [Google Scholar]
- 5.Engels G, Hierweger AM, Hoffmann J, Thieme R, Thiele S, Bertram S, Dreier C, Resa-Infante P, Jacobsen H, Thiele K, Alawi M, Indenbirken D, Grundhoff A, Siebels S, Fischer N, Stojanovska V, Muzzio D, Jensen F, Karimi K, Mittrücker HW, Arck PC, Gabriel G. Pregnancy-related immune adaptation promotes the emergence of highly virulent H1N1 influenza virus strains in allogenically pregnant mice. Cell Host Microbe 21: 321–333, 2017. doi: 10.1016/j.chom.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Esmonde TF, Herdman G, Anderson G. Chickenpox pneumonia: an association with pregnancy. Thorax 44: 812–815, 1989. doi: 10.1136/thx.44.10.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flesch JD, Dine CJ. Lung volumes: measurement, clinical use, and coding. Chest 142: 506–510, 2012. doi: 10.1378/chest.11-2964. [DOI] [PubMed] [Google Scholar]
- 8.Forbes RL, Wark PA, Murphy VE, Gibson PG. Pregnant women have attenuated innate interferon responses to 2009 pandemic influenza A virus subtype H1N1. J Infect Dis 206: 646–653, 2012. doi: 10.1093/infdis/jis377. [DOI] [PubMed] [Google Scholar]
- 9.Grindheim G, Toska K, Estensen ME, Rosseland LA. Changes in pulmonary function during pregnancy: a longitudinal cohort study. BJOG 119: 94–101, 2012. doi: 10.1111/j.1471-0528.2011.03158.x. [DOI] [PubMed] [Google Scholar]
- 10.Hale BG, Steel J, Manicassamy B, Medina RA, Ye J, Hickman D, Lowen AC, Perez DR, García-Sastre A. Mutations in the NS1 C-terminal tail do not enhance replication or virulence of the 2009 pandemic H1N1 influenza A virus. J Gen Virol 91: 1737–1742, 2010. doi: 10.1099/vir.0.020925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall OJ, Limjunyawong N, Vermillion MS, Robinson DP, Wohlgemuth N, Pekosz A, Mitzner W, Klein SL. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog 12: e1005840, 2016. doi: 10.1371/journal.ppat.1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegewald MJ, Crapo RO. Respiratory physiology in pregnancy. Clin Chest Med 32: 1–13, 2011. doi: 10.1016/j.ccm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Heyne GW, Plisch EH, Melberg CG, Sandgren EP, Peter JA, Lipinski RJ. A simple and reliable method for early pregnancy detection in inbred mice. J Am Assoc Lab Anim Sci 54: 368–371, 2015. [PMC free article] [PubMed] [Google Scholar]
- 14.Horner GJ, Gray FD Jr. Effect of uncomplicated, presumptive influenza on the diffusing capacity of the lung. Am Rev Respir Dis 108: 866–869, 1973. [DOI] [PubMed] [Google Scholar]
- 15.Hytten F. Blood volume changes in normal pregnancy. Clin Haematol 14: 601–612, 1985. [PubMed] [Google Scholar]
- 16.Inra CN, Zhou BO, Acar M, Murphy MM, Richardson J, Zhao Z, Morrison SJ. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 527: 466–471, 2015. doi: 10.1038/nature15530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inselman LS, Chander A, Spitzer AR. Diminished lung compliance and elevated surfactant lipids and proteins in nutritionally obese young rats. Lung 182: 101–117, 2004. doi: 10.1007/s00408-003-1048-4. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ, Novel Influenza AP; Novel Influenza A (H1N1) Pregnancy Working Group . H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 374: 451–458, 2009. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis 12: 1638–1643, 2006. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen D, Webb KA, Davies GA, O’Donnell DE. Mechanical ventilatory constraints during incremental cycle exercise in human pregnancy: implications for respiratory sensation. J Physiol 586: 4735–4750, 2008. doi: 10.1113/jphysiol.2008.158154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johanson WG Jr, Pierce AK, Sanford JP. Pulmonary function in uncomplicated influenza. Am Rev Respir Dis 100: 141–146, 1969. [DOI] [PubMed] [Google Scholar]
- 22.Kim HM, Kang YM, Song BM, Kim HS, Seo SH. The 2009 pandemic H1N1 influenza virus is more pathogenic in pregnant mice than seasonal H1N1 influenza virus. Viral Immunol 25: 402–410, 2012. doi: 10.1089/vim.2012.0007. [DOI] [PubMed] [Google Scholar]
- 23.Kim JC, Kim HM, Kang YM, Ku KB, Park EH, Yum J, Kim JA, Kang YK, Lee JS, Kim HS, Seo SH. Severe pathogenesis of influenza B virus in pregnant mice. Virology 448: 74–81, 2014. doi: 10.1016/j.virol.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Kolarzyk E, Szot WM, Lyszczarz J. Lung function and breathing regulation parameters during pregnancy. Arch Gynecol Obstet 272: 53–58, 2005. doi: 10.1007/s00404-004-0691-1. [DOI] [PubMed] [Google Scholar]
- 25.Limjunyawong N, Fallica J, Horton MR, Mitzner W. Measurement of the pressure-volume curve in mouse lungs. J Vis Exp 95: 52376, 2015. doi: 10.3791/52376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limjunyawong N, Fallica J, Ramakrishnan A, Datta K, Gabrielson M, Horton M, Mitzner W. Phenotyping mouse pulmonary function in vivo with the lung diffusing capacity. J Vis Exp 95: e52216, 2015. doi: 10.3791/52216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limjunyawong N, Mock J, Mitzner W. Instillation and fixation methods useful in mouse lung cancer research. J Vis Exp 102: e52964, 2015. doi: 10.3791/52964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Littauer EQ, Esser ES, Antao OQ, Vassilieva EV, Compans RW, Skountzou I. H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLoS Pathog 13: e1006757, 2017. doi: 10.1371/journal.ppat.1006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LoMauro A, Aliverti A. Respiratory physiology of pregnancy: physiology masterclass. Breathe (Sheff) 11: 297–301, 2015. doi: 10.1183/20734735.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyons HA. Centrally acting hormones and respiration. Pharmacol Ther B 2: 743–751, 1976. [DOI] [PubMed] [Google Scholar]
- 31.Marcelin G, Aldridge JR, Duan S, Ghoneim HE, Rehg J, Marjuki H, Boon AC, McCullers JA, Webby RJ. Fatal outcome of pandemic H1N1 2009 influenza virus infection is associated with immunopathology and impaired lung repair, not enhanced viral burden, in pregnant mice. J Virol 85: 11208–11219, 2011. doi: 10.1128/JVI.00654-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitzner W, Wagner E. Results in parenchymal strips vs. the intact inflated lung. J Appl Physiol (1985) 84: 2198–2199, 1998. doi: 10.1152/jappl.1998.84.6.2198. [DOI] [PubMed] [Google Scholar]
- 33.Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 17: 469–482, 2017. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 34.Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, Donahue LR. Mouse gestation length is genetically determined. PLoS One 5: e12418, 2010. doi: 10.1371/journal.pone.0012418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 148: 1094–1102, 1998. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 36.Pastro LD, Lemos M, Fernandes FL, Saldiva SR, Vieira SE, Romanholo BM, Saldiva PH, Francisco RP. Longitudinal study of lung function in pregnant women: influence of parity and smoking. Clinics (Sao Paulo) 72: 595–599, 2017. doi: 10.6061/clinics/2017(10)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pazos MA, Kraus TA, Muñoz-Fontela C, Moran TM. Estrogen mediates innate and adaptive immune alterations to influenza infection in pregnant mice. PLoS One 7: e40502, 2012. doi: 10.1371/journal.pone.0040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Periolo N, Avaro M, Czech A, Russo M, Benedetti E, Pontoriero A, Campos A, Peralta LM, Baumeister E. Pregnant women infected with pandemic influenza A(H1N1)pdm09 virus showed differential immune response correlated with disease severity. J Clin Virol 64: 52–58, 2015. doi: 10.1016/j.jcv.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 62: 263–271, 2012. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7: e1002149, 2011. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues J, Niederman MS. Pneumonia complicating pregnancy. Clin Chest Med 13: 679–691, 1992. [PubMed] [Google Scholar]
- 42.Rush B, Martinka P, Kilb B, McDermid RC, Boyd JH, Celi LA. Acute respiratory distress syndrome in pregnant women. Obstet Gynecol 129: 530–535, 2017. doi: 10.1097/AOG.0000000000001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders CJ, Johnson B, Frevert CW, Thomas PG. Intranasal influenza infection of mice and methods to evaluate progression and outcome. Methods Mol Biol 1031: 177–188, 2013. doi: 10.1007/978-1-62703-481-4_20. [DOI] [PubMed] [Google Scholar]
- 44.Schatz M, Harden K, Forsythe A, Chilingar L, Hoffman C, Sperling W, Zeiger RS. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol 81: 509–517, 1988. doi: 10.1016/0091-6749(88)90187-X. [DOI] [PubMed] [Google Scholar]
- 45.Uchide N, Ohyama K, Bessho T, Takeichi M, Toyoda H. Possible roles of proinflammatory and chemoattractive cytokines produced by human fetal membrane cells in the pathology of adverse pregnancy outcomes associated with influenza virus infection. Mediators Inflamm 2012: 270670, 2012. doi: 10.1155/2012/270670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanders RL, Gibson PG, Murphy VE, Wark PA. Plasmacytoid dendritic cells and CD8 T cells from pregnant women show altered phenotype and function following H1N1/09 infection. J Infect Dis 208: 1062–1070, 2013. doi: 10.1093/infdis/jit296. [DOI] [PubMed] [Google Scholar]
- 47.Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, Carlino LO, Owen R, Paterson B, Pelletier L, Vachon J, Gonzalez C, Hongjie Y, Zijian F, Chuang SK, Au A, Buda S, Krause G, Haas W, Bonmarin I, Taniguichi K, Nakajima K, Shobayashi T, Takayama Y, Sunagawa T, Heraud JM, Orelle A, Palacios E, van der Sande MA, Wielders CC, Hunt D, Cutter J, Lee VJ, Thomas J, Santa-Olalla P, Sierra-Moros MJ, Hanshaoworakul W, Ungchusak K, Pebody R, Jain S, Mounts AW; WHO Working Group for Risk Factors for Severe H1N1pdm Infection . Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med 8: e1001053, 2011. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Riel D, Mittrücker HW, Engels G, Klingel K, Markert UR, Gabriel G. Influenza pathogenicity during pregnancy in women and animal models. Semin Immunopathol 38: 719–726, 2016. doi: 10.1007/s00281-016-0580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.vom Steeg LG, Vermillion MS, Hall OJ, Alam O, McFarland R, Chen H, Zirkin B, Klein SL. Age and testosterone mediate influenza pathogenesis in male mice. Am J Physiol Lung Cell Mol Physiol 311: L1234–L1244, 2016. doi: 10.1152/ajplung.00352.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wan J, Hu Z, Zeng K, Yin Y, Zhao M, Chen M, Chen Q. The reduction in circulating levels of estrogen and progesterone in women with preeclampsia. Pregnancy Hypertens 11: 18–25, 2018. doi: 10.1016/j.preghy.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Zosky GR, Cannizzaro V, Hantos Z, Sly PD. Protective mechanical ventilation does not exacerbate lung function impairment or lung inflammation following influenza A infection. J Appl Physiol (1985) 107: 1472–1478, 2009. doi: 10.1152/japplphysiol.00393.2009. [DOI] [PubMed] [Google Scholar]