Abstract
The electronic cigarette (e-cig) has been suggested as a safer alternative to tobacco cigarettes. However, the health effects of e-cigs on the airways have not been fully investigated. Nicotine, the primary chemical constituent of the e-cig aerosol, has been shown to stimulate vagal bronchopulmonary C-fiber sensory nerves, which upon activation can elicit vigorous pulmonary defense reflexes, including airway constriction. In this study, we investigated the bronchomotor response to e-cig inhalation challenge in anesthetized guinea pigs and the mechanisms involved in regulating these responses. Our results showed that delivery of a single puff of e-cig aerosol into the lung triggered immediately a transient bronchoconstriction that sustained for >2 min. The increase in airway resistance was almost completely abolished by a pretreatment with either intravenous injection of atropine or inhalation of aerosolized lidocaine, suggesting that the bronchoconstriction was elicited by cholinergic reflex mechanism and stimulation of airway sensory nerves was probably involved. Indeed, electrophysiological recording further confirmed that inhalation of e-cig aerosol exerted a pronounced stimulatory effect on vagal bronchopulmonary C-fibers. These effects on airway resistance and bronchopulmonary C-fiber activity were absent when the e-cig aerosol containing zero nicotine was inhaled, indicating a critical role of nicotine. Furthermore, a pretreatment with nicotinic acetylcholine receptor antagonists by inhalation completely prevented the airway constriction evoked by e-cig aerosol inhalation. In conclusion, inhalation of a single puff of e-cig aerosol caused a transient bronchoconstriction that was mediated through cholinergic reflex and triggered by a stimulatory effect of nicotine on vagal bronchopulmonary C-fiber afferents.
Keywords: airway, bronchoconstriction, C-fiber, electronic cigarette, nicotine
INTRODUCTION
Although the toxicity of cigarettes is commonly ascribed to their combustion byproducts and their addictive nature mostly attributed to their nicotine content, nicotine is also responsible for causing airway irritation and cough (23, 24). Electronic cigarettes (e-cigs) are battery-powered atomizing/vaporizing devices that deliver nicotine contained in the e-liquid in aerosolized and vaporized forms for inhalation. Over the course of the last decade with presumption of being a safe substitute for cigarettes, they are being advertised as a safe method for nicotine replacement therapy and have gained increasing popularity since their introduction (9, 32, 36). However, it has been reported that e-cig use not only may not help with smoking cessation, but it may result in dual usage of both e-cig and cigarettes (37, 41). Furthermore, although e-cigs may contain lower concentrations of combustion byproducts than tobacco cigarettes (15), the pathophysiological effects of nicotine and other chemical constituents delivered by the e-cig aerosol on airway function remain mostly unknown (29, 34).
Sensory signals arising from the lung and airways are conducted exclusively in vagus nerves and their branches (10, 28). A majority (>70%) of these vagal bronchopulmonary afferents conduct action potentials in nonmyelinated (C) fibers (19), and a characteristic feature of these C-fiber afferents is their exquisite sensitivity to chemical irritants (10, 28, 31). It is well documented that these vagal bronchopulmonary C-fiber afferents play an important role in regulating airway functions under both healthy and disease conditions (10, 28). Stimulation of these C-fiber afferents can also elicit powerful centrally mediated pulmonary defense reflex responses, such as cough and bronchoconstriction (10, 27, 28). Studies conducted in our laboratory have reported the first evidence that vagal pulmonary C-fibers are stimulated by inhalation of a single puff of cigarette smoke in anesthetized dogs, and nicotine contained in the tobacco smoke is primarily responsible for this action (25). Our follow-up studies have further demonstrated in both experimental animals and human subjects that this action results from an activation of nicotinic acetylcholine receptor (nAChR) expressed on these airway sensory nerves (23–26).
In light of this background information and existing important questions regarding the potential pathophysiological effects of e-cigs in the airways, we carried out this study to investigate: 1) the acute airway responses to inhalation of a single puff of e-cig aerosol, 2) the role of nicotine delivered by the e-cig aerosol, and 3) the mechanisms involved in regulating these responses.
MATERIALS AND METHODS
The procedures described below were performed in accordance with the recommendations of the “Guide for the Care and Use of Laboratory Animals,” published by the National Institutes of Health, and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
Animal preparation.
Young, male, and pathogen-free Hartley guinea pigs were anesthetized with intraperitoneal injections of α-chloralose (100 mg/kg) and urethane (500 mg/kg) dissolved in a 2% borax solution, and one-tenth of supplemental doses of the same anesthetics were administered intravenously whenever necessary to maintain abolition of the corneal and withdrawal reflexes. After the trachea was cannulated, the guinea pig was placed in a supine position and ventilated with a respirator (Ugo Basile model 7025; Monvalle VA, Italy) at a constant frequency of 60 breaths/min and a tidal volume (VT) of 7–8 ml/kg; the latter was adjusted in each animal to maintain the end-tidal CO2 concentration (cat. no. 1260, Novametrix, Wakefield, MA) between 4.5 and 5.5%. The right jugular vein and the right carotid artery were cannulated for intravenous injections and for arterial blood pressure (ABP) measurement, respectively. A catheter for measuring intrapleural pressure was inserted into the right intrapleural space via a surgical incision between the 5th and 6th ribs; this incision was sealed air-tight with Vetbond (3M, Maplewood, MN), and pneumothorax was then corrected by hyperinflation (3 × VT). A heating pad was placed under the animal to maintain body temperature at ~36°C. At the end of experiment, animals were euthanized by intravenous injection of KCl (3 M; 2 ml).
Measurements of lung mechanics.
Transpulmonary pressure was measured as the difference between tracheal pressure and intrapleural pressure with a differential pressure transducer (cat. no. MP 45-28, Validyne, Northridge, CA). Respiratory flow was measured with a heated pneumotachograph and a differential pressure transducer (cat. no. MP 45-14, Validyne). All signals were recorded and analyzed by a computer and a data acquisition system (cat. no. TS-100, Biocybernetics, Taipei, Taiwan) to calculate total pulmonary resistance (RL) and dynamic lung compliance (Cdyn) on a breath-by-breath basis.
E-cig aerosol inhalation challenge.
Aerosol of e-cigs was generated by an atomizer (Subtank Mini, KangerTech, Shenzhen, China) with a 0.5-Ω coil and a power control device (TC-75 KBOX Mini, KangerTech) set at 50 W and 5 V. Except mentioned otherwise, the brand of Old Kentucky (Shenzhen, China) e-liquid containing 12 mg/ml was used in this study. Aerosol was drawn at the flowrate of 20 ml/s, mixed with room air in 1:1 ratio (50% dilution), and one single breath of this diluted aerosol at the volume of 2 × VT was immediately delivered into the lung via the tracheal cannula, held for 2 s, and then exhaled; the actual volume of e-cig aerosol delivered into the lung was estimated to be ~60% after deducting the dead space in the inspiratory circuit. These parameters were selected in this study to mimic the average puff profile in human e-cig users (12, 39, 42) and extrapolated to the body size of guinea pigs. Five minutes before and 10 min after each challenge, the lung was hyperinflated (3 × VT) twice to maintain a constant volume history of the lung (33). At least 20 min elapsed between 2 tests for recovery. Baseline and peak airway responses (RL and Cdyn) were calculated by averaging 20 breaths before and 10 consecutive breaths of highest responses occurring within the first minute after the inhalation challenge, respectively.
Electrophysiological recording of bronchopulmonary C-fiber activity.
Vagal bronchial and pulmonary C-fiber afferent activities were recorded by using the “single fiber” recording technique as described in detail in our previous report (25). Briefly, a midsternal thoracotomy was performed, and the lungs were ventilated with a respirator with the tip of the expiratory line placed 3 cm under water to maintain a near-normal resting lung volume. The right cervical vagus nerve was sectioned and the caudal end was immersed in a pool of mineral oil and placed on a small dissecting platform. A thin filament was teased away from the desheathed nerve trunk and placed on a miniature monopolar electrode to record its afferent fiber activity (FA). Action potentials were amplified by a preamplifier (cat. no. P-511-K, Grass, Warwick, RI) and monitored by an audio monitor (cat. no. AM-8-RS, Grass). The thin filament was further split until the FA arising from a single unit was electrically isolated. Vagal bronchopulmonary C-fibers usually exhibited no or only a sparse baseline activity (<1 impulse/s) during eupneic ventilation but could be activated mildly by hyperinflation of the lungs (>3 × VT). Both pulmonary and bronchial C-fibers were identified by their intense responses to a bolus injection of capsaicin (1–2 µg/kg) into the right atrium, and they were distinguished by the latency of their responses: pulmonary C-fibers were activated within 1 s after the capsaicin injection, whereas bronchial C-fibers displayed a longer latency (>5 s). The receptor location was further identified by its responses to gentle pressing of the lung surface and external walls of the airways with a blunt-ended glass rod at the end of the experiment. All signals were recorded on a thermal writer and analyzed by a computer and a data acquisition system (TS-100, Biocybernetics) at a 3,000-Hz sampling rate and expressed in 1-s intervals.
Experimental protocols.
The following seven series of experiments were carried out: study 1, experiments were carried out to determine the effects of e-cig aerosol inhalation challenge on RL and Cdyn and to evaluate how these effects were affected by increasing the dilution of e-cig aerosol with air; study 2, to investigate the role of nicotine, changes in RL and Cdyn in response to inhalation of e-cig aerosol generated from e-liquids containing 0 and 12 mg/ml nicotine were compared in the same group of animals; the sequence was alternated between animals to achieve a balance design; study 3, to determine if e-cig aerosol-induced bronchoconstriction was mediated through cholinergic reflex, airway responses to e-cig inhalation challenge were compared between before and 15 min after a pretreatment with atropine sulfate (a muscarinic acetylcholine receptor antagonist; 0.1 mg/kg iv); study 4, to investigate the role of vagal bronchopulmonary sensory nerves, airway responses to e-cig inhalation challenge were compared between before and 2 min after a pretreatment with inhalation of lidocaine aerosol (a local anesthetic; 4% concentration, 90-s duration); study 5, to determine the involvement of nAChRs in the activation of airway sensory nerves, airway responses to e-cig inhalation challenge were compared between before and 5 min after a pretreatment with four breaths of aerosolized mecamylamine (2% concentration) or hexamethonium (0.5%); both are nonselective antagonists of nAChR; study 6, to determine if the bronchoconstrictive effect of inhaled e-cig aerosol varied between different brands of e-cig, airway responses to five other brands of commercial e-liquids containing the same nicotine concentration (12 mg/ml) were compared: Blu (Charlotte, NC), eVo (Nicopure Laboratories, Trinity, FL), JC (Johnson Creek, Hartland, WI), NJOY King (NJOY, Scottsdale, AZ), and V2 Cig (VMR Products, Miami, FL). These different brands of e-liquids were selected based upon their popularity and availability (4); and study 7, to investigate if inhaled e-cig aerosol stimulated vagal bronchopulmonary C-fibers and whether this effect was dependent upon nicotine, neural activity conducted in these afferents were continuously recorded before, during, and after inhalation challenge with e-cig aerosol generated from e-liquids containing 0 and 12 or 18 mg/ml of nicotine.
Statistical analysis.
A one-way or two-way analysis of variance (ANOVA) was used for the statistical analysis. When the two-way ANOVA showed a significant interaction, pair-wise comparisons were made with a post hoc analysis (Fisher’s least significant difference). Data were reported as means ± SE. A P value less than 0.05 was considered significant.
Materials.
Atropine sulfate (2 mg/ml), lidocaine (4%), mecamylamine (4%), and hexamethonium (2%) were prepared in isotonic saline. E-liquids were purchased from local retailers and drug stores. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
RESULTS
Seven study series were carried out in a total of 35 guinea pigs (average body weight: 383.9 ± 6.4 g). About 40% (15/35) of these animals were used in two study series.
Study 1.
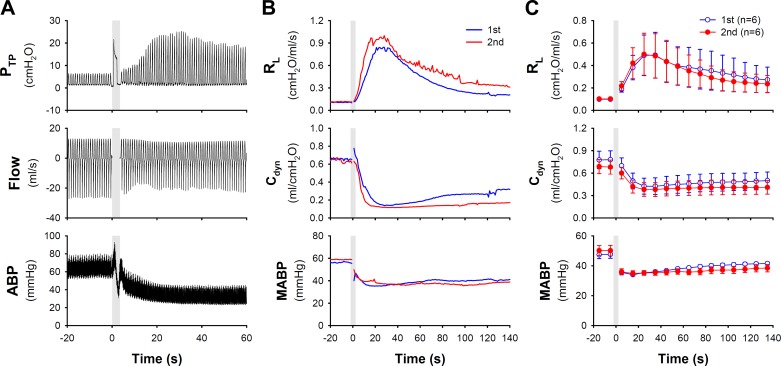
Delivery of e-cig aerosol (nicotine concentration: 12 mg/ml; 50% dilution) triggered an immediate increase in transpulmonary pressure and a decrease in ABP (Fig. 1A). It caused a pronounced increase in RL (ΔRL), which reached a peak (Δ = 434 ± 217% of the baseline) in 20–40 s and then gradually returned to baseline 3–5 min later (Fig. 1, B and C). It also induced a decrease in Cdyn (Δ = −48 ± 11% of the baseline), which occurred rapidly but sustained. The reduced Cdyn could be completely reversed when the lung was hyperinflated (3 × VT) at the end of the 10-min recording period, suggesting that the sustaining decrease in Cdyn was, in large part, caused by lung atelectasis. The degree and pattern of increase in RL and decrease in Cdyn were reproducible when the same e-cig inhalation challenge was repeated in the same animals 20 min later (Fig. 1, B and C).
Fig. 1.
Airway constriction evoked by e-cig aerosol inhalation challenge in anesthetized guinea pigs. A: experimental record illustrating the changes of transpulmonary pressure (PTP), flowrate and arterial blood pressure (ABP) after inhalation of one single puff of e-cig aerosol (diluted to 50% concentration by mixing with air in 1:1 ratio; total puff volume 6.0 ml; e-liquid nicotine concentration 12 mg/ml) in a guinea pig (379 g). Shaded bar depicted the delivery of e-cig aerosol into the lung. B: to test the reproducibility of airway responses, two inhalation challenges of the same e-cig aerosol were administered 20 min apart in the same guinea pig. C: group data illustrating the reproducibility of the airway and cardiovascular responses to two e-cig inhalation challenges separated by 20 min in six guinea pigs. Data represent means ± SE. Cdyn, dynamic lung compliance; MABP, mean ABP; RL, total pulmonary resistance.
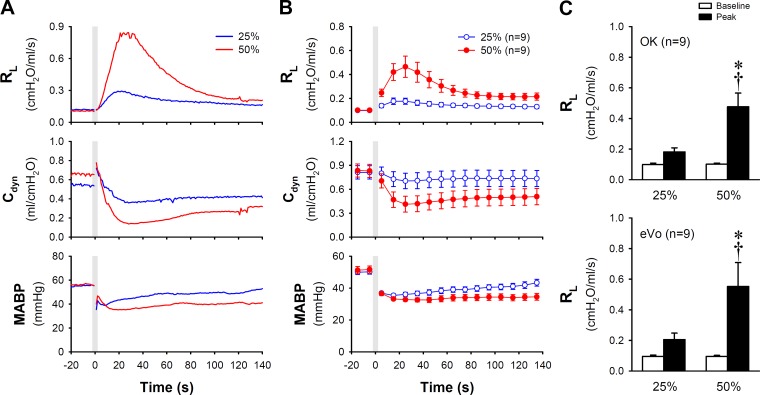
The ΔRL in response to inhalation of e-cig aerosol was proportionally decreased when the aerosol was further diluted with air in the same animals (Fig. 2). ΔRL in response to e-cig aerosol with 50% dilution (aerosol mixed with air in 1:1 ratio) and 25% dilution (1:3 ratio) were 0.375 ± 0.088 and 0.082 ± 0.019 cmH2O·ml−1·s−1, respectively (P < 0.05, n = 9 animals; Fig. 2C top). A similar difference in the airway responses was also observed in the same group of guinea pigs when e-cig aerosol was generated from another brand (eVo) of e-liquid and delivered in the same manner as described above (P < 0.05, n = 9; Fig. 2C bottom).
Fig. 2.
Airway responses to inhalation of e-cig aerosol diminished by dilution with air. A: representative experimental traces comparing the responses of RL, Cdyn, and MABP to inhalation challenge of e-cig aerosol mixing with air in 1:1 (50%) and 1:3 (25%) ratios at a total puff volume of 6.5 ml in an anesthetized guinea pig (425 g). B: histogram illustrating the group data of airway responses in nine guinea pigs. C: top: group data (n = 9 animals) illustrating the responses of RL calculated from that in B; the baseline (open bar) and the peak response (closed bar) were calculated by averaging 20 consecutive breaths immediately before and 10 consecutive breaths of peak RL within 60 s after the challenge, respectively. OK, Old Kentucky (e-cig brand). Bottom: group data obtained from the same group of animals when e-cig aerosol was generated from another brand (eVo) of e-liquid (n = 9) and delivered in the same manner. Data represent means ± SE. *Significantly different from the corresponding baseline (P < 0.05); †Significantly different from the corresponding data point in the 25%-dilution group (P < 0.05). Cdyn, dynamic lung compliance; MABP, mean arterial blood pressure; RL, total pulmonary resistance.
Study 2.
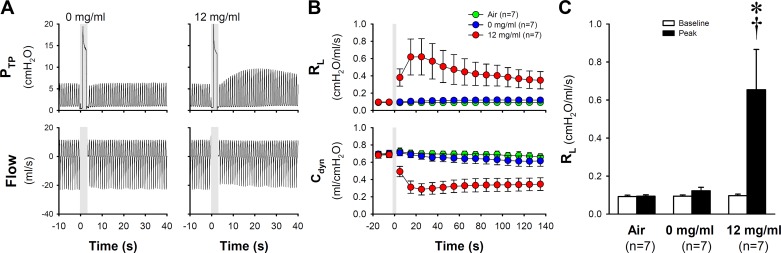
Our results clearly showed that the bronchoconstrictive effect of e-cig aerosol was dependent on the nicotine contained in the e-liquid: inhalation of e-cig aerosol generated from e-liquids containing 12 mg/ml nicotine induced airway constriction; RL increased from baseline of 0.096 ± 0.008 to a peak of 0.651 ± 0.232 cmH2O·ml−1·s−1 (P < 0.05, n = 7; Fig. 3). In a sharp contrast, inhalation of aerosol generated by e-liquid of 0 mg/ml nicotine did not cause significant bronchoconstriction, in spite of a small but consistent increase in RL in all the animals tested; baseline and peak RL were 0.094 ± 0.007 and 0.122 ± 0.018 cmH2O·ml−1·s−1, respectively (P > 0.05, n = 7; Fig. 3). Delivery of air alone in the same manner also failed to induce any changes in RL (baseline and peak RL were 0.092 ± 0.007 and 0.094 ± 0.007 cmH2O·ml−1·s−1, respectively; P > 0.05, n = 7; Fig. 3).
Fig. 3.
The role of nicotine in regulating the airway response to e-cig aerosol inhalation challenge. A: experimental records illustrating the responses to e-cig aerosol generated from e-liquid containing two different nicotine concentrations (0 and 12 mg/ml) in a guinea pig (389 g). Shaded bars depicted the delivery of a single puff of e-cig aerosol (diluted to 50% by mixing with air in 1:1 ratio and total puff volume 6.0 ml). B: histogram of group data illustrating the responses of RL and Cdyn to air and e-cig aerosol (0 and 12 mg/ml) inhalation challenges in seven guinea pigs. C: group data of the peak responses calculated from B illustrating the effects of air and e-cig aerosol inhalation challenges on RL. For calculations of baseline and peak responses in C, see the legend of Fig. 2. Data represent means ± SE (n = 7). *Significantly different from corresponding baseline (P < 0.05); †Significantly different from the corresponding data point in air group (P < 0.05). Cdyn, dynamic lung compliance; PTP; transpulmonary pressure; RL, total pulmonary resistance.
To evaluate further a potential bronchoconstrictive effect of nonnicotine constituents contained in the e-cig aerosol, airway responses to inhalation of undiluted (100%) e-cig aerosol containing 0 mg/ml nicotine were measured in six of these animals; it caused a significant increase in RL, which reached a peak of 113.1 ± 2.2% of the baseline (P < 0.05; n = 6).
Study 3.
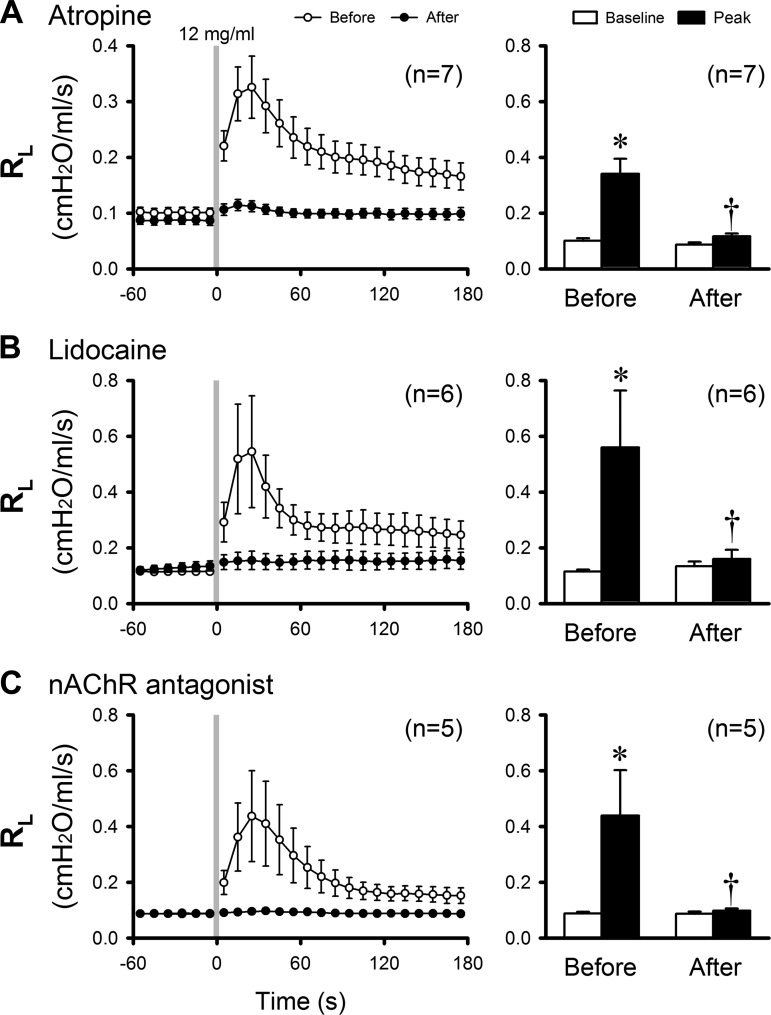
Airway responses to inhalation of e-cig aerosol (12 mg/kg nicotine) were compared between before and 15 min after a pretreatment with atropine sulfate (0.1 mg/kg iv); the ΔRL evoked by the e-cig aerosol inhalation was significantly reduced from 0.240 ± 0.056 cmH2O·ml−1·s−1 before to 0.030 ± 0.005 cmH2O·ml−1·s−1 after atropine (P < 0.05, n = 7; Fig. 4A). Similar results were also obtained in the same group of guinea pigs when e-cig aerosol was generated from another brand of e-liquid (eVo) containing the same concentration of nicotine (12 mg/ml; P < 0.05, n = 7)
Fig. 4.
Effects of pretreatment with atropine (A; n = 7), lidocaine (B; n = 6), and nAChR (C) antagonists (n = 5) on the airway responses to inhalation challenges of e-cig aerosol (diluted with air to 50% and total puff volume 2 × VT; e-liquid nicotine concentration 12 mg/ml) in anesthetized guinea pigs. Left: histogram illustrating the group responses. Right: group data of the peak responses calculated from that in left; see the legend of Fig. 2 for calculations of baseline and peak responses. *P < 0.05, significantly different from baseline; †P < 0.05, significantly different from the control (before treatment) responses. Data represent means ± SE. nAChR,nicotinic acetylcholine receptor; VT; tidal volume.
Study 4.
To determine the role of airway sensory nerves in eliciting the cholinergic reflex, airway responses to inhalation of e-cig aerosol (12 mg/kg nicotine) were compared between before and 2 min after a pretreatment with inhalation of aerosolized lidocaine (4% for 90 s). The ΔRL evoked by the e-cig aerosol was almost completely eliminated: 0.445 ± 0.199 before and 0.027 ± 0.015 cmH2O·ml−1·s−1 after the lidocaine pretreatment (P < 0.05, n = 6; Fig. 4B). A similar blocking effect of lidocaine was also observed in the same group of animals when e-cig aerosol was generated from eVo e-liquid containing the same concentration of nicotine (12 mg/ml; P < 0.05, n = 6).
Study 5.
Airway responses to e-cig aerosol inhalation challenge (12 mg/ml nicotine) was determined before and 5 min after a pretreatment with antagonists of the nAChR: mecamylamine (2%, 4 breaths) or hexamethonium (0.5%, 4 breaths) aerosol. Because the blocking effects of mecamylamine and hexamethonium were similar, the data from these two pretreatments were pooled for statistical analysis. The ΔRL induced by inhalation of e-cig aerosol was completely abolished: ΔRL was 0.351 ± 0.159 before and 0.011 ± 0.003 cmH2O·ml−1·s−1 after the pretreatment with the nAChR antagonist (P < 0.05, n = 5; Fig. 4C). In addition, the same pretreatment with nAChR antagonists did not block the bronchoconstriction and bradycardia evoked by electrical stimulation (5 V, 10 Hz, 1-ms duration for 10 s) applied by a bipolar stimulating electrode on the distal end of one sectioned cervical vagus nerve in all five guinea pigs tested.
Study 6.
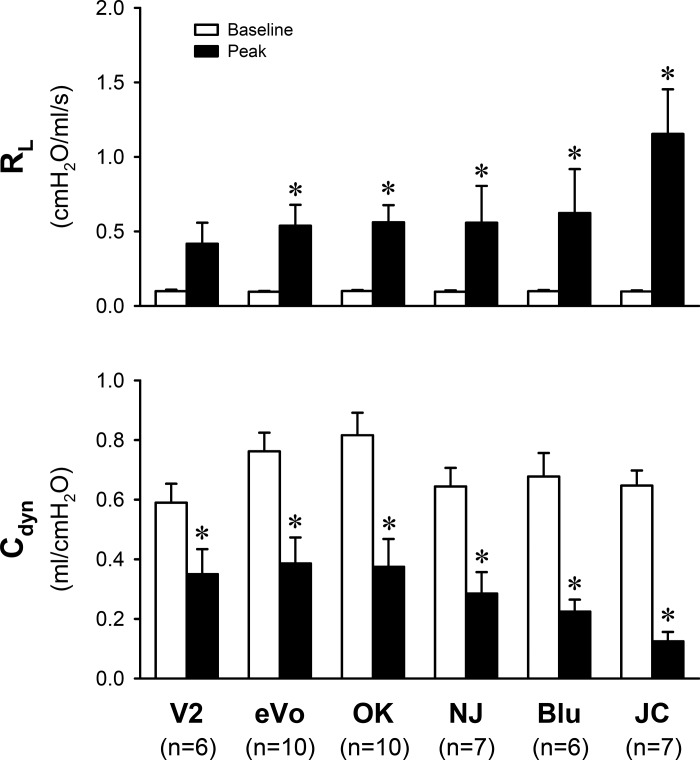
Each of the six different brands of e-liquids contained 12 mg/ml nicotine. Aerosols were generated by the same atomizer and power control device as described earlier. Inhalation challenges of the aerosols generated from all six e-liquids caused an increase in RL (ΔRL) and a decrease in Cdyn (ΔCdyn), but the magnitudes of these changes varied substantially among them: ΔRL ranged from 0.318 ± 0.135 to 1.057 ± 0.296 cmH2O·ml−1·s−1, and ΔCdyn ranged from −0.240 ± 0.056 to −0.523 ± 0.048 ml/cmH2O (Fig. 5).
Fig. 5.
Difference in the bronchoconstrictive effect of inhalation of e-cig aerosol generated from six different brands of e-liquids containing the same nicotine concentration (12 mg/ml). In NJ and JC, e-liquids of two different nicotine concentrations were mixed to reach the desired concentration (12 mg/ml). See the legend of Fig. 2 for calculations of baseline and peak responses. Data represent means ± SE *Significantly different (P < 0.05) from baseline. Cdyn, dynamic lung compliance; JC, Johnson Creek; NJ, NJOY; OK, Old Kentucky; RL, total pulmonary resistance.
Study 7.
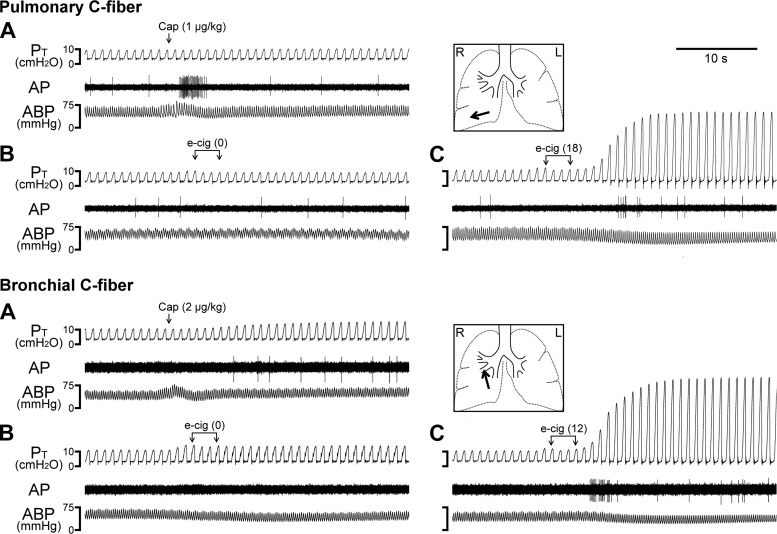
To avoid the interruption of the ventilation in open-chest animals during the e-cig inhalation challenge, three consecutive breaths of e-cig aerosols were delivered by the respirator via the tracheal cannula; the actual volume of e-Cig aerosol delivered into the lung was estimated to be ~1.8 × VT after deducting the dead space in the inspiratory circuit. Our results showed that inhalation of e-cig aerosol consistently stimulated vagal bronchopulmonary C-fibers (e.g., Fig. 6). A representative example recorded from a vagal bronchial C-fiber is shown in the bottom of Fig. 6; the receptor was characterized by its mild response to lung hyperinflation and its distinct response to a bolus injection of capsaicin (2 µg/kg) into the right atrium after a delay of 7–8 s. Indeed, the receptor location was identified in the right middle hilar bronchus (arrow) after the experiment. Within 5 s after the delivery of the e-cig aerosol containing 18 mg/ml nicotine into the lung, a burst of intense neural activity was evoked, which lasted for >20 s and was accompanied by an increase in tracheal pressure and a mild decease in ABP (bottom of Fig. 6). In a sharp contrast, e-cig aerosol containing 0 mg/ml nicotine did not generate any effect on the same C-fiber receptor (bottom of Fig. 6). The intense and sustaining bronchoconstriction generated by the e-cig aerosol inhalation was consistent with our observations in studies 1–3, which was most likely mediated through cholinergic reflex elicited by the vagal afferent discharge arising from the contralateral lung via its intact vagus nerve.
Fig. 6.
Experimental records illustrating the stimulatory effect of e-cig inhalation challenge on vagal pulmonary and bronchial C-fibers in two open-chest, anesthetized guinea pigs. Arrows in A marked the bolus injection of capsaicin (Cap; 1 µg/kg, top; 2 µg/kg, bottom) into the right atrium via a venous catheter. Brackets in B and C depicted the time when three consecutive breaths of aerosol generated from e-liquid containing 0, 12, and 18 mg/ml nicotine [e-cig (0), e-cig (12), and e-cig (18)], respectively, were delivered into the tracheal cannula. To avoid the change in resting lung volume in the open-chest preparation, aerosol was delivered by the respirator; the actual volume of e-cig aerosol delivered into the lung was estimated to be ~1.8 × VT after deducting the dead space in the inspiratory circuit. Inset: the locations of the receptor fields were identified (red arrows) in the right lower lobe (top) and right middle hilar bronchus (bottom) by gentle pressing of airway external wall and lung surface with a blunt-ended glass rod after the experiment. Body weights: 373 g (top) and 296 g (bottom). AP, action potential; ABP, arterial blood pressure; L, left; PT, tracheal pressure; R, right.
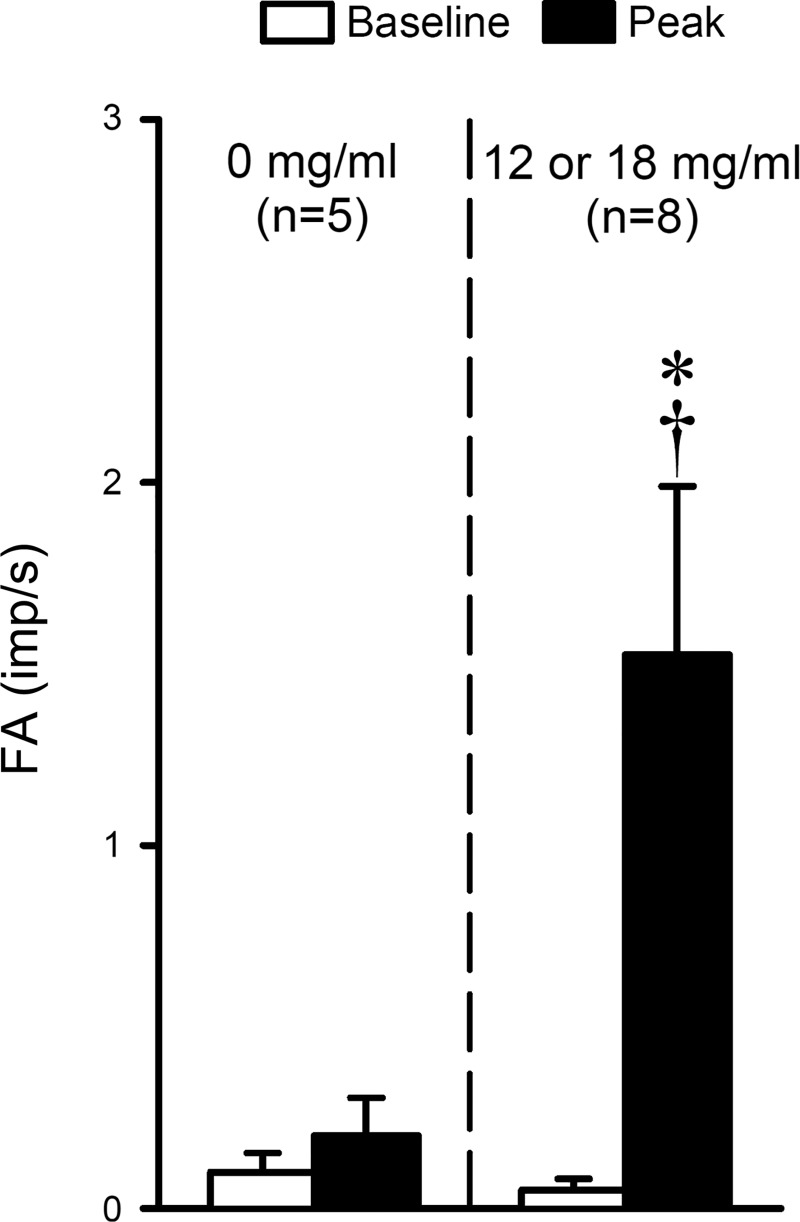
Group data showed that inhalation of aerosol generated from e-liquid containing 12–18 mg/ml nicotine increased the FA sharply from 0.05 ± 0.03 impulses/s (imp/s) at baseline (20-s average) to a peak response (10-s average of peak FA occurring within the first 30 s after the inhalation challenge) of 1.53 ± 0.46 imp/s (P < 0.05, n = 8; Fig. 7). In contrast, delivery of e-cig aerosol containing 0 mg/ml nicotine did not significantly change the fiber activity (baseline: 0.10 ± 0.05 imp/s; peak: 0.20 ± 0.10 imp/s; P > 0.05, n = 5; Fig. 7).
Fig. 7.
Stimulatory effect of e-cig inhalation challenge on vagal bronchopulmonary C-fibers. Fiber activity (FA) during baseline was calculated as the 20-s average of FA before inhalation, and the peak response as the 10-s average of peak FA occurring within the first 30 s after the inhalation challenge. E-cig aerosols were generated from e-liquids containing two different concentrations of nicotine (0 and 12 or 18 mg/ml) and delivered into the lung in the same manner as described in the legend of Fig. 6. FA was expressed in impulses/s (imp/s). Data represent means ± SE obtained from eight fibers recorded in seven guinea pigs; responses to e-cig aerosol of 0 mg/ml nicotine were not tested in three of these fibers. *Significantly different (P < 0.05) from the corresponding baseline; †Significantly different (P < 0.05) from the corresponding data point in response to 0 mg/ml nicotine.
DISCUSSION
Delivery of a single puff of e-cig aerosol into the lung in a manner mimicking the human e-cig vaping pattern triggered an acute bronchoconstriction that sustained for >2 min and was reproducible in the same animals (Figs. 1–3). This airway constrictive response was generated primarily by nicotine contained in the e-cig aerosol (Fig. 3) and attenuated when the e-cig aerosol was diluted with air (Fig. 2). The increase in RL was almost completely abolished by a pretreatment with either intravenous injection of atropine or inhalation of aerosolized lidocaine (Fig. 4), suggesting that the bronchoconstriction was mediated through a cholinergic reflex mechanism and a stimulation of airway sensory nerves was probably involved. Indeed, our results obtained from electrophysiological recording experiments further confirmed that inhalation of e-cig aerosol exerted a pronounced stimulatory effect on vagal bronchopulmonary C-fibers (Figs. 6 and 7), lending additional support to our hypothesis.
In the single-fiber recording experiments, we have made the first direct observation that inhaled e-cig aerosol stimulated both bronchial and pulmonary vagal C-fiber afferents (Figs. 6 and 7). These C-fiber afferents represent the majority of the vagal sensory nerves innervating the respiratory tract (10, 19), and play an important role in regulating airway defense functions in both normal and pathophysiological conditions (10, 28, 31). These sensory endings exhibit extensive axonal arborization that either extends into the space between epithelial cells or forms network-like plexus immediately beneath the basement membrane of epithelium in various species, including humans (2, 22, 46). One of the characteristic physiological properties of these sensory endings is their exquisite sensitivity to inhaled chemical irritants (10, 28). It is extensively documented that stimulation of these C-fiber sensory endings can elicit a number of vigorous protective reflex responses, including cough, mucus secretion and bronchoconstriction; most of these reflexes are mediated through the cholinergic pathways (10, 28). When bronchial and pulmonary C-fiber sensory endings were activated by inhaled e-cig aerosol in this study, action potentials arising from these endings were conducted through vagal afferents to the brainstem, and reflexly elicited the airway smooth muscle contraction via the release of acetylcholine form post-ganglion parasympathetic neurons. Thus, a pretreatment with atropine sulfate, a muscarinic acetylcholine receptor antagonist, almost completely abolished the increase in RL evoked by e-cig inhalation challenge (Fig. 4A). As illustrated in Fig. 4B, the pretreatment with inhalation of lidocaine aerosol was equally effective in ablation of the reflex bronchoconstriction because of its blocking effect on the C-fiber discharges; lidocaine could have also blocked the conduction in the postganglion parasympathetic fibers (20), but this possibility was dismissed because aerosolized lidocaine delivered at this dose did not block the bronchoconstriction caused by electrical stimulation of cervical vagus nerve (the same protocol as in study 5) in one animal tested in our preliminary experiment. Intense and/or sustained stimulation of these C-fiber afferents can also trigger the release of tachykinins and calcitonin gene-related peptide synthesized and stored in these neurons from their sensory terminals (3, 13, 40). These neuropeptides can in turn act on a number of target cells in the airways (e.g., smooth muscles, cholinergic ganglia, mucous glands, immune cells), and elicit the local “axon reflexes” such as bronchoconstriction, protein extravasation, and inflammatory cell chemotaxis, leading to the development of “neurogenic inflammation” in the airways (3). Although we did not investigate the potential involvement of tachykinins in the bronchoconstriction induced by e-cig inhalation, their effects generated by inhaling a single puff of e-cig aerosol at this nicotine concentration appeared to be negligible in this study because only a minimal response remained after the atropine pretreatment. However, considering the well-documented knowledge that the relative contribution by tachykininergic mechanism to the bronchoconstriction increases as the intensity and duration of C-fiber stimulation are increased (7), it is quite possible that neurogenic inflammation resulting from tachykinin release may occur if the number of successive puffs is increased, as during the normal e-cig vaping in humans. This possibility of more severe pathophysiological effects in the airways associated with a larger quantity of e-cig consumption requires further investigation.
Both stimulatory effects of the e-cig aerosol inhalation on bronchopulmonary C-fiber activity and airway resistance were almost undetectable when the e-liquid containing zero nicotine was administered (Figs. 3, 6, and 7). Furthermore, after the pretreatment with inhalation of either mecamylamine or hexamethonium aerosol, the airway constriction evoked by e-cig aerosol inhalation was completely abolished (Fig. 4C). Both these observations indicated that nicotine delivered in the e-cig aerosol was responsible for the activation of these C-fiber sensory endings in the airways and lung. The possibility that the blocking effect of the nAChR antagonist may have also affected the transmission at the airway cholinergic ganglia can be ruled out based upon the fact that the same nAChR antagonist pretreatment did not block the bronchoconstriction and bradycardia evoked by electrical stimulation of cervical vagus nerve, indicating that a minimal systemic effect was induced by the nAChR antagonists administered at these doses. Indeed, the expression of nAChRs at the vagal bronchopulmonary C-fiber sensory neurons has been demonstrated in our previous studies (17, 24, 47). For example, patch-clamp electrophysiological recording experiments showed that nicotine evoked an inward current in isolated bronchopulmonary sensory neurons, which was blocked completely by hexamethonium (17). Furthermore, the RT-PCR analysis has shown the expression of mRNA encoding for several nAChR subunits in bronchopulmonary sensory neurons (17). All these observations supported our conclusion that activation of nAChRs is primarily responsible for the stimulatory effect of e-cig inhalation on bronchopulmonary C-fiber afferents.
Non-nicotine constituents contained in the e-cig aerosol and vapor have been reported to exert a number of adverse actions in the airways and lung (18, 29, 30, 34), in addition to the potent stimulatory effects caused by nicotine. Some of these constituents such as acrolein, formaldehyde, acetaldehyde, propylene glycol, and reactive oxygen species are known for their irritant properties (1, 5, 6, 11, 43, 44); for example, acrolein and formaldehyde have been shown to activate the transient receptor potential ankyrin 1 receptor (5, 43), a ligand-gated nonselective cation channel, which is abundantly expressed in the bronchopulmonary C-fibers (35). Activation of transient receptor potential ankyrin 1 receptor is known to elicit bronchoconstriction and coughing (1, 6, 14). In this study, although the e-cig aerosol (50% dilution) containing 0 mg/ml nicotine caused a small but consistent increase in RL in all seven guinea pigs tested, the increase was not statistically significant (Fig. 3C). However, when airway responses to inhalation of undiluted (100%) e-cig aerosol containing 0 mg/ml nicotine were tested in six guinea pigs, it triggered a distinct increase in RL, revealing a potential bronchoconstrictive effect of the nonnicotine constituents.
Both the increase in RL and decrease in Cdyn evoked by inhalation of e-cig aerosol generated from six different brands of e-liquid appeared to vary more substantially than expected in the same group of animals (Fig. 5), despite that they contained the same concentration of nicotine and the e-cig aerosol was generated and delivered in the same manner. To avoid a possible tachyphylaxis in the response to nicotine, the order of inhalation challenges with these different brands of e-liquids was altered, but the variability of the responses persisted among them. These results are in support of the notion that the development of standardized e-cig device and e-liquid for research use with more consistent delivery and established profile of chemical constituents will be required if the research data, such as those obtained in this study, are shared or compared between different laboratories.
In view of the facts, including the perception of e-cig as a safe substitute for cigarettes (9, 38), the current trend in increased ownership of e-cig (32, 36), and their popularity among youth (21, 41), it is expected that the use of e-cigs among patients with lung diseases will continue to increase (41). More importantly, the paucity of definitive data and scientific evidence in regard to the safety of e-cig use in patients with lung disease renders recommendations for or against use of e-cig a challenging task for physicians (9, 38, 41). Although our findings in this study will need to be confirmed in the study performed in human subjects, a recent study has reported that inhalation of a single e-cig caused acute airway constriction and reduced exhaled nitric oxide concentration in healthy human smokers (45). Furthermore, it is now recognized that activation of cholinergic mechanism may have more profound and sustaining pathophysiological effects on airways than that previously considered (8, 16). As such, these repetitive, albeit transient, bronchoconstriction triggered by e-cig inhalation and mediated through cholinergic pathway may lead to long-term deteriorating effects in the airways of chronic e-cig users. Further investigations will be required to test this hypothesis.
GRANTS
This study was supported in part by National Institutes of Health Grants UL1-TR-001998 (to M. Khosravi and L.-Y. Lee), AI-123832 (to L.-Y. Lee), and HL-96914 (to L.-Y. Lee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.K., R.L.L., and L.-Y.L. conceived and designed research; M.K., R.L.L., and L.-Y.L. performed experiments; R.L.L. analyzed data; M.K., R.L.L., and L.-Y.L. interpreted results of experiments; R.L.L. and L.-Y.L. prepared figures; M.K., R.L.L., and L.-Y.L. drafted manuscript; M.K., R.L.L., and L.-Y.L. edited and revised manuscript; M.K., R.L.L., and L.-Y.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Thomas Kelly, Arit Harvanko, and Dr. Kristin Ashford for advice in this study, and Dr. An-Hsuan Lin for technical assistance.
REFERENCES
- 1.Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118: 2574–2582, 2008. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baluk P, Nadel JA, McDonald DM. Substance P-immunoreactive sensory axons in the rat respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol 319: 586–598, 1992. doi: 10.1002/cne.903190408. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ. Neurogenic inflammation in the airways. Respir Physiol 125: 145–154, 2001. doi: 10.1016/S0034-5687(00)00210-3. [DOI] [PubMed] [Google Scholar]
- 4.Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One 10: e0117222, 2015. doi: 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc 7: 269–277, 2010. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118: 1899–1910, 2008. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchan P, Adcock JJ. Capsaicin-induced bronchoconstriction in the guinea-pig: contribution of vagal cholinergic reflexes, local axon reflexes and their modulation by BW443C81. Br J Pharmacol 105: 448–452, 1992. doi: 10.1111/j.1476-5381.1992.tb14273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buels KS, Fryer AD. Muscarinic receptor antagonists: effects on pulmonary function. Handb Exp Pharmacol 208: 317–341, 2012. doi: 10.1007/978-3-642-23274-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caponnetto P, Russo C, Bruno CM, Alamo A, Amaradio MD, Polosa R. Electronic cigarette: a possible substitute for cigarette dependence. Monaldi Arch Chest Dis 79: 12–19, 2013. doi: 10.4081/monaldi.2013.104. [DOI] [PubMed] [Google Scholar]
- 10.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- 11.Conklin DJ. Acute cardiopulmonary toxicity of inhaled aldehydes: role of TRPA1. Ann N Y Acad Sci 1374: 59–67, 2016. doi: 10.1111/nyas.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham A, Slayford S, Vas C, Gee J, Costigan S, Prasad K. Development, validation and application of a device to measure e-cigarette users’ puffing topography. Sci Rep 6: 35071, 2016. doi: 10.1038/srep35071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Swert KO, Joos GF. Extending the understanding of sensory neuropeptides. Eur J Pharmacol 533: 171–181, 2006. doi: 10.1016/j.ejphar.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 14.Geppetti P, Patacchini R, Nassini R, Materazzi S. Cough: the emerging role of the TRPA1 channel. Lung 188, Suppl 1: S63–S68, 2010. doi: 10.1007/s00408-009-9201-3. [DOI] [PubMed] [Google Scholar]
- 15.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P III, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23: 133–139, 2014. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 7: 73, 2006. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Q, Ni D, Lee LY. Expression of neuronal nicotinic acetylcholine receptors in rat vagal pulmonary sensory neurons. Respir Physiol Neurobiol 161: 87–91, 2008. doi: 10.1016/j.resp.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higham A, Rattray NJ, Dewhurst JA, Trivedi DK, Fowler SJ, Goodacre R, Singh D. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respir Res 17: 56, 2016. doi: 10.1186/s12931-016-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst 5: 165–176, 1982. doi: 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- 20.Kesler BS, Canning BJ. Regulation of baseline cholinergic tone in guinea-pig airway smooth muscle. J Physiol 518: 843–855, 1999. doi: 10.1111/j.1469-7793.1999.0843p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob Res 17: 219–227, 2015. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komatsu T, Yamamoto M, Shimokata K, Nagura H. Distribution of substance P-immunoreactive and calcitonin gene-related peptide-immunoreactive nerves in normal human lungs. Int Arch Allergy Appl Immunol 95: 23–28, 1991. doi: 10.1159/000235449. [DOI] [PubMed] [Google Scholar]
- 23.Lee LY, Gerhardstein DC, Wang AL, Burki NK. Nicotine is responsible for airway irritation evoked by cigarette smoke inhalation in men. J Appl Pysiol (1985) 75: 1955–1961, 1993. doi: 10.1152/jappl.1993.75.5.1955. [DOI] [PubMed] [Google Scholar]
- 24.Lee LY, Gu Q. Cough sensors. IV. Nicotinic membrane receptors on cough sensors. Handb Exp Pharmacol 187: 77–98, 2009. doi: 10.1007/978-3-540-79842-2_5. [DOI] [PubMed] [Google Scholar]
- 25.Lee LY, Kou YR, Frazier DT, Beck ER, Pisarri TE, Coleridge HM, Coleridge JC. Stimulation of vagal pulmonary C-fibers by a single breath of cigarette smoke in dogs. J Appl Physiol (1985) 66: 2032–2038, 1989. doi: 10.1152/jappl.1989.66.5.2032. [DOI] [PubMed] [Google Scholar]
- 26.Lee LY, Lin RL, Khosravi M, Xu F. Reflex bronchoconstriction evoked by inhaled nicotine aerosol in guinea pigs: role of the nicotinic acetylcholine receptor. J Appl Physiol (1985) 125: 117–123, 2018. doi: 10.1152/japplphysiol.01039.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001. doi: 10.1016/S0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 28.Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol 4: 287–324, 2014. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 29.Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, Done D, Kurtzman R, Ossip DJ, Robinson R, McIntosh S, Rahman I. Environmental health hazards of e-cigarettes and their components: oxidants and copper in e-cigarette aerosols. Environ Pollut 198: 100–107, 2015. doi: 10.1016/j.envpol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10: e0116732, 2015. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev 96: 975–1024, 2016. doi: 10.1152/physrev.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 17: 1195–1202, 2015. doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- 33.Mead J, Collier C. Relation of volume history of lungs to respiratory mechanics in anesthetized dogs. J Appl Physiol 14: 669–678, 1959. doi: 10.1152/jappl.1959.14.5.669. [DOI] [Google Scholar]
- 34.Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I. Inflammatory and Oxidative Responses Induced by Exposure to Commonly Used e-Cigarette Flavoring Chemicals and Flavored e-Liquids without Nicotine. Front Physiol 8: 1130, 2018. doi: 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586: 1595–1604, 2008. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Center for Health Statistics National Health Interview Survey. Atlanta, GA: CDC, 2014. [Google Scholar]
- 37.Pechacek TF, Nayak P, Gregory KR, Weaver SR, Eriksen MP. the potential that electronic nicotine delivery systems can be a disruptive technology: results from a national survey. Nicotine Tob Res 18: 1989–1997, 2016. doi: 10.1093/ntr/ntw102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polosa R, Campagna D, Sands MF. Counseling patients with asthma and allergy about electronic cigarettes: an evidence-based approach. Ann Allergy Asthma Imunol mmunology 116: 106–111, 2016. doi: 10.1016/j.anai.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Robinson RJ, Hensel EC, Morabito PN, Roundtree KA. Electronic cigarette topography in the natural environment. PLoS One 10: e0129296, 2015. doi: 10.1371/journal.pone.0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solway J, Leff AR. Sensory neuropeptides and airway function. J Appl Physiol (1985) 71: 2077–2087, 1991. doi: 10.1152/jappl.1991.71.6.2077. [DOI] [PubMed] [Google Scholar]
- 41.Soneji SS, Sung HY, Primack BA, Pierce JP, Sargent JD. Quantifying population-level health benefits and harms of e-cigarette use in the United States. PLoS One 13: e0193328, 2018. doi: 10.1371/journal.pone.0193328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N, Shihadeh A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res 17: 150–157, 2015. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respir Cell Mol Biol 40: 756–762, 2009. doi: 10.1165/rcmb.2008-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respir Physiol Neurobiol 178: 406–413, 2011. doi: 10.1016/j.resp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141: 1400–1406, 2012. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV. Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 141: 1533–1543, 2006. doi: 10.1016/j.neuroscience.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Yang W, Zhang G, Gu Q, Lee LY. Calcium transient evoked by nicotine in isolated rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 292: L54–L61, 2007. doi: 10.1152/ajplung.00182.2006. [DOI] [PubMed] [Google Scholar]