Abstract
Induction of the chaperone heat shock protein 72 (HSP72) through heat treatment (HT), exercise, or overexpression improves glucose tolerance and mitochondrial function in skeletal muscle. Less is known about HSP72 function in the liver where lipid accumulation can result in insulin resistance and nonalcoholic fatty liver disease (NAFLD). The purpose of this study was 1) to determine whether weekly in vivo HT induces hepatic HSP72 and improves glucose tolerance in rats fed a high-fat diet (HFD) and 2) to determine the ability of HSP72 to protect against lipid accumulation and mitochondrial dysfunction in primary hepatocytes. Male Wistar rats were fed an HFD for 15 wk and were given weekly HT (41°C, 20 min) or sham treatments (37°C, 20 min) for the final 7 wk. Glucose tolerance and insulin sensitivity were assessed, along with HSP72 induction and triglyceride storage, in the skeletal muscle and liver. The effect of an acute loss of HSP72 in primary hepatocytes was examined via siRNA. Weekly in vivo HT improved glucose tolerance, elevated muscle and hepatic HSP72 protein content, and reduced muscle triglyceride storage. In primary hepatocytes, mitochondrial morphology was changed, and fatty acid oxidation was reduced in small interfering HSP72 (siHSP72)-treated hepatocytes. Lipid accumulation following palmitate treatment was increased in siHSP72-treated hepatocytes. These data suggest that HT may improve systemic metabolism via induction of hepatic HSP72. Additionally, acute loss of HSP72 in primary hepatocytes impacts mitochondrial health as well as fat oxidation and storage. These findings suggest therapies targeting HSP72 in the liver may prevent NAFLD.
Keywords: heat shock proteins, liver metabolism, mitochondria, nonalcoholic fatty liver disease, steatosis
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is characterized by an excessive accumulation of lipids in the liver that ultimately contribute to the development of hepatic insulin resistance, hyperinsulinemia, hyperglycemia, and type 2 diabetes (39). Mitochondrial function and fat oxidation are critical to liver function, and past evidence indicates increased lipid accumulation because of reduced mitochondrial function contributes to the pathobiology of metabolic disease (26, 57). However, the mechanisms that alter susceptibility for hepatic steatosis are poorly understood.
The heat shock response is a defense system to combat cellular stress (2) composed of a family of heat shock proteins (HSPs; 11, 41, 42). Best known for their chaperone functions, HSPs also have more recently identified roles in regulation of metabolism (8, 24, 27). Most of the work identifying the role of HSP72 in metabolism has focused on the skeletal muscle; however, very little is known about the role of HSP72 in liver metabolism. Kurucz et al. (32) first demonstrated that HSP72 expression was markedly decreased in skeletal muscle of patients with insulin resistance and with type 2 diabetes. Subsequent studies from our laboratory and others showed that heat treatment (HT), transgenic overexpression of HSP72, and pharmacological induction of HSP72 effectively prevent high-fat diet (HFD)-induced glucose intolerance and skeletal muscle insulin resistance (1, 8, 21–25, 27, 36). HSP72 induction and HT also enhance skeletal muscle mitochondrial integrity, content, and function (7, 8, 15, 24, 27, 37, 60). As a result, HSP72 has a well-established role in regulating glucose homeostasis, insulin sensitivity, and oxidative capacity in skeletal muscle.
Three past studies have indicated a potential role for HSP72 in liver metabolism. First, the small molecule drug matrine, used for treatment of chronic viral infections and tumors in the liver, activates hepatic HSP72, improves glucose tolerance, and reduces triglyceride (TAG) storage in vivo (65). Second, hepatic induction of HSP72 by administration of the compound geranylgeranylacetone ameliorates hepatic insulin resistance and reduces proinflammatory signaling (1). Finally, a correlation was identified between decreased HSP72 protein levels in the livers of patients with obesity with progression of insulin resistance and NAFLD (14). However, to date no studies have examined the direct effect of reduced HSP72 on hepatic lipid metabolism.
The purpose of the present study was twofold. First, to determine whether in vivo HT intervention would induce hepatic HSP72 expression and improve glucose tolerance in rats following 8 wk of high-fat feeding. Previously we had noted that 12 wk of weekly HTs in conjunction with 12 wk of high-fat feeding protected against the development of insulin resistance (24); however, studies have not investigated the ability of HT to be used as an intervention in rodents fed an HFD. Therefore, we introduced our HTs as an intervention halfway through our HFD feeding cycle. Our second aim was to examine the impact of direct HSP72 modulation on primary hepatocytes in vitro. Given that the liver has high mitochondrial density and fast mitochondrial turnover (40), it is likely that HSP72 plays an important role in maintaining hepatic mitochondrial function. We hypothesize that HSP72 is important in maintaining hepatic fatty acid oxidation (FAO), therefore preventing lipid storage. If our hypothesis is correct, then targeting HSP72 could be an effective strategy for reducing hepatic insulin resistance, NAFLD, and type 2 diabetes.
METHODS
Experimental animals and in vivo HT.
Eight-week-old male Wistar rats (~150–180 g each) were purchased from Charles River Laboratories (Wilmington, MA) and housed in a temperature-controlled facility (22 ± 2°C) with 12:12-h light/dark cycles. Animals were allowed ad libitum access to water and food. Rats were fed a modified Kraegen high-fat diet (60% kcal from fat, 20% kcal from carbohydrates, and 20% kcal from protein) for 15 wk (24, 58). The diet contains the following: 254 g/kg casein, 85 g/kg sucrose, 169 g/kg cornstarch, 11.7 g/kg vitamin mix, 1.3 g/kg choline chloride, 67 g/kg mineral mix, 51 g/kg bran, 3 g/kg methionine, 19 g/kg gelatin, 121 g/kg corn oil, and 218 g/kg lard. During the last 7 wk of the HFD, rats received either weekly in vivo HT or sham treatment (ST, n = 9/group). All animals were anesthetized with an intraperitoneal injection of pentobarbital sodium (5 mg/100 g body wt) before ST or HT. HT consisted of lower body immersion in a 42°C water bath to gradually increase body temperature to between 41°C and 41.5°C where it was maintained for 20 min. ST consisted of immersion in a 37°C water bath and maintaining body temperature at 37°C for 20 min (24, 55). Body temperature was monitored by a Thermoworks Microtherma 2 (American Fork, UT) rectal thermometer. After treatment, 0.5 ml of 0.9% saline was injected intraperitoneally to aid in recovery. Forty-eight hours following the final HT or ST and following a 10-h overnight fast, animals were again anesthetized with pentobarbital sodium, and tissues were dissected for experimental procedures. All protocols and procedures were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center.
Glucose tolerance testing and other blood measures.
One week before euthanasia, and 48 h following HT or ST, rats underwent an intraperitoneal glucose tolerance test. After an overnight fast, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (5 mg/100 g body wt) and injected with a glucose load of 2 g/kg body weight. Tail blood was removed every 30 min and assessed for blood glucose using a glucometer and the manufacturer’s test strips (Accu-Chek Active, Roche Diagnostics, Indianapolis, IN). Blood was allowed to clot for 30 min on ice and spun at 3,000 g for 60 min at 4°C, and serum was drawn off and frozen at −80°C. Serum was analyzed for concentration of insulin using an ELISA (Alpco, Salem, NH). Serum TAGs and nonesterified fatty acids were also determined by colorimetric assays using the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI and Wako Diagnostics, Richmond, VA, respectively).
Calculation of homeostatic model assessment-insulin resistance.
Fasting glucose and insulin values obtained on the day of the glucose tolerance test were utilized to calculate insulin resistance through homeostatic model assessment-insulin resistance [(fasting glucose × fasting insulin)/2,430] where glucose levels are in milligram per deciliter and insulin levels are in microunits per milliliter as done previously (5, 38).
Glucose transport.
Insulin-stimulated glucose transport into extensor digitorum longus (EDL) was determined as done previously (21, 24, 25, 64). After dissection, muscle strips were placed in vials in a shaking incubator (35°C) for 60 min containing Krebs-Henseleit bicarbonate (KHB) buffer with 8 mM glucose and 32 mM mannitol. Noninsulin-treated muscles stayed in the same vials for 30 additional min. Insulin-treated muscles were transferred to new vials for 30 min in the same buffer with the addition of insulin (1 mU/ml) at 35°C. Muscle strips were then transferred to new vials containing 2 ml of KHB and 40 mM mannitol, with or without insulin (1 mU/ml) for 10 min at 29°C. Muscle strips were again transferred to new vials containing 2 ml of KHB and 4 mM 2-[1,2-3H]deoxyglucose (1.5 µCi/ml) and 36 mM [14C]mannitol (0.2 µCi/ml), with or without insulin (1 mU/ml) for 20 min. During all incubation steps, muscle strips were exposed to a gas phase of 95% O2-5% CO2 at 29°C. Finally, muscle strips were blotted, clamp-frozen at −80°C, and processed for determination of intracellular and extracellular accumulation of 2-deoxyglucose.
Adipose tissue imaging.
Epididymal white adipose tissue (eWAT) was fixed overnight in 4% paraformaldehyde, placed in 70% ethanol for 48–72 h, processed, and paraffin embedded. Ten-micrometer sections were places on slides and subsequently stained with hematoxylin-eosin (H&E). Images were taken on a Nikon 80i microscope (Melville, NY) and were quantified using ImageJ.
Hepatocyte primary cell culture.
Hepatocytes from C57Bl6/J mice (~8–20 wk of age) were isolated by collagenase perfusion. We utilized a different model from our in vivo experiment to highlight the importance of the heat shock response in multiple models. Animals were anesthetized with 1–2 ml of isoflurane. The hepatic portal vein was cannulated, and the liver was infused with a perfusion buffer (1× HBSS calcium and magnesium free, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 mM HEPES) at 8.2 ml/min. The portal vein and diaphragm were cut, and the superior vena cava was clamped. After 10 min with the first perfusion buffer, the buffer was changed to a second perfusion buffer (1× HBSS with Ca2+/Mg2+, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 mM HEPES, 0.025 mg/ml collagenase; Roche Liberase, Basel, Switzerland). Collagenase digestion continued for ~7–10 min until signs of digestion were observed. The perfusion pump was stopped, and the liver was excised. The liver was placed in a 100-ml sterile beaker containing 20–30 ml of a cold third buffer (1× HBSS Ca2+/Mg2+-free, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 mM HEPES, 100 nM insulin). The liver was chopped with scissors and forceps and filtered through cell filters beginning with 100 µm, then 70 µm, and then 30 µm. Cells were collected into a 50-ml conical tube. Cells were centrifuged in a volume of 50 ml at 50 g for 5 min at 4°C. The medium was aspirated, and the wash step was repeated two more times using the cold third buffer. After the third wash, the cells were resuspended in Williams’ Medium E (Sigma, St. Louis, MO), and viability was determined using trypan blue. Hepatocytes were plated on collagen-coated plates (rat tail collagen type I, Corning, Corning, NY) in hepatocyte growth medium (Williams’ E, 10% FBS, 4 mM l-glutamine, 100 U penicillin/100 mg streptomycin, 2 ng/ml rat EGF, 100 nM insulin, 100 nM dexamethasone, 0.1% BSA, 10 mM sodium pyruvate). For FAO experiments hepatocytes were plated into a 12-well plate (2 × 105 cells/well). For all other experiments, hepatocytes were plated in 6-well plates (1 × 106 cells/well). For lipid exposure experiments, cells were treated with 250 µM palmitate for 24 h or with the vehicle with no palmitate. Palmitate was conjugated to 1% BSA in hepatocyte growth medium. Further cell experiments are described below.
siRNA transfection.
Hepatocytes were transfected with 15 nM HSP72 siRNA (NM_212504; Sigma) or MISSION siRNA Universal Negative Control #1 using Mission siRNA transfection reagent (Sigma) according to the manufacturer’s protocol in hepatocyte growth media. Hepatocyte transfection lasted 24–48 h. To confirm knockdown in all experiments, hepatocytes were treated with siRNA, were heat treated, and were harvested 24 h following HT to perform a Western blot.
HT in primary hepatocytes.
Hepatocytes were heat treated (42°C) or sham treated (37°C) in a water bath for 30 min, and the growth medium was changed immediately after. Experiments continued 24 h following HT.
Lipid droplet detection.
Along with siRNA or HT, primary hepatocytes from a mouse liver were treated with palmitate or vehicle as described above. Cells were then treated with 3.8 µM Bodipy 493/503 (cat. no. D-3922, Thermo Fisher Scientific, Rockford, IL) for 15 min in serum-free Williams’ E medium and then washed with 1× PBS. Cells were then imaged live using confocal imaging. Images were acquired with the Nikon A1 Confocal Live Cell Scanning Microscope. Signal intensity was determined using ImageJ.
TAG content.
Triacylglycerol content from various tissues was evaluated as done previously (52, 61). Intramuscular triacylglycerol concentration was determined based on the methods by Frayn and Maycock (19). The tibialis anterior, consisting of mostly glycolytic muscle fibers (13), was homogenized in 3 ml of 2:1 chloroform-methanol, transferred to 13 × 100-mm borosilicate glass tubes, vortexed, and incubated overnight at 4°C. The following day, 3 ml of 4 mmol/l MgCl2 were added to each tube, vortexed, and centrifuged at 1,000 g for 1 h at 4°C. The bottom organic layer (1.5 ml) was drawn off and placed into clean borosilicate glass tubes, allowed to dry overnight, reconstituted with 500 µl of ethanolic KOH, and heated at 75°C for 20 min. Following heating, 1 ml of 0.15 mmol/l MgSO4 was added to each tube, centrifuged at 1,000 g for 1 h at 4°C, and supernatant removed and assayed for TAG concentration using a commercially available colorimetric assay (cat. nos. T-2449 and F-6428, Sigma). Liver was processed similarly except that after drying overnight, samples were reconstituted in butanol/Triton X-110 and assayed directly afterwards (52).
Liver triacylglycerol concentration was also detected from primary hepatocytes plated from a mouse liver. After the treatments, cells were harvested using lysis buffer (0.03% SDS in PBS), followed by addition of 1 ml of 2:1 chloroform-methanol to each tube of lysate. The tubes were centrifuged for 1 h (1,000 g, 4°C), and the organic layer was transferred to a clean Eppendorf tube. Tubes were then transferred to a fume hood for 48–72 h to evaporate the organic phase, and then each sample was reconstituted in 75 µl of butanol/Triton X-114 (3:2). Total TAGs were measured using the Triglyceride Reagent and Glycerol Free Reagent kit (cat. nos. T-2449 and F-6428, Sigma). Optical density was evaluated at 540 nm.
Mitochondrial quality measurement.
Hepatocytes from a mouse liver were treated with 100 nM MitoTracker Green FM (cat. no. M-7514, Thermo Fisher) in Williams’ E Medium, a green fluorescent mitochondrial stain that localizes to mitochondria regardless of mitochondrial membrane potential. Cells were also stained with 600 nM tetramethylrhodamine, ethyl ester (TMRE; cat. no. T-669, Thermo Fisher) in Williams’ E Media, which is a red florescent stain sequestered by active mitochondria. Cells were washed with warm PBS, stained with both stains for 30 min, and then washed again with warm PBS. Cells were fixed with 3.7% paraformaldehyde and imaged using confocal microscopy. Images were acquired with the Nikon A1 Confocal Live Cell Scanning Microscope. Signal intensity was determined using Image J. The TMRE/MitoTracker ratio was calculated to evaluate the ratio of functional, live mitochondria to nonfunctional mitochondria. This allowed us to evaluate mitochondrial quality between our groups.
FAO.
FAO was determined in primary hepatocytes based on previous protocols (45). Primary hepatocytes in 12-well plates were serum starved and then washed with warm PBS. Cells were then incubated in FAO reaction medium containing DMEM-low glucose (Invitrogen, Waltham, MA), 0.5 µCi/ml [1-14C]palmitate, 100 µM palmitate, 0.25% BSA, 1 mM carnitine, and 12.5 mM HEPES (pH ~7.4) at 37°C for 3 h in triplicate. To identify carnitine palmitoyltransferase-1 (CPT-1)-mediated FAO, some wells were treated with the CPT-1 inhibitor etomoxir (100 µM). CPT-1-mediated FAO (also known as mitochondrial FAO) is calculated as the difference between total FAO and FAO in the presence of etomoxir. FAO in the presence of etomoxir is also known as nonmitochondrial FAO. After 3 h, the medium from each well was collected, and an aliquot of medium was put into the sealed trapping device. The 14CO2 was driven from the medium aliquot by addition of perchloric acid and trapped in NaOH, which was collected and analyzed by liquid scintillation counting for determination of complete FAO to CO2. The acidified media was collected, refrigerated, and centrifuged (16,000 g, 4°C). An aliquot was analyzed by liquid scintillation counting for determination of the acid-soluble metabolites (ASMs) of FAO. ASMs are radiolabeled fatty acids that have not been completely oxidized to CO2, thus ASMs represent incomplete FAO. The cells were rinsed three times with ice-cold KHB and lysed with SDS lysis buffer. The protein concentration of the lysate was determined by bicinchoninic acid assay.
Transmission electron microscopy.
Hepatocytes from a mouse liver were fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer. The hepatocytes were rinsed twice for 10 min each with cacodylate buffer and were then postfixed in 1% osmium tetroxide buffer for 1 h. The cells were rinsed 3 times for 10 min each with distilled water. The cells were dehydrated in a graded series of ethanol as follows: 50%, 70%, 80%, 95%, 100%, 100%, 10 min each. The cells were placed into propylene oxide 2Xs for 20 min each then transferred to a half/half mixture of propylene oxide/Embed 812 medium mixture resin (Electron Microscopy Sciences, Fort Washington, PA), and samples were left to infiltrate overnight. The cells were placed into 100 Embed 812 resin for 1 h. They were then placed into BEEM capsules size 00 and cured overnight in a 65°C oven. The individual sample blocks were sectioned using a Diatome diamond knife on a Leica UC-7 ultramicrotome at 80-nm thick and picked up on 200 mesh copper grids. Samples were examined using a Jeol JEM-1400 transmission electron microscope operated at 100 kv.
Western blotting.
Muscles and liver were processed for Western blotting by methods previously described (24, 25, 55). Briefly, muscle and liver tissue were homogenized in a 12:1 (volume-to-weight) ratio of ice-cold cell extraction buffer containing 10 mM Tris·HCl (pH 7.4); 100 mM NaCl; 1 mM each of EDTA, EGTA, NaF, and phenylmethylsulfonyl fluoride; 2 mM Na3VO4; 20 mM Na4P2O7; 1% Triton X-100; 10% glycerol; 0.1% SDS; 0.5% deoxycholate; and 250 µl/5 ml protease inhibitor cocktail. Homogenates were rotated for 30 min at 4°C and then centrifuged for 20 min at 3,000 revolutions/min at 4°C, and the supernatant was removed.
In cell culture experiments before harvesting, hepatocytes were serum starved for 6 h. Cells for Western blotting were rinsed with PBS, scraped, collected, and pelleted. The cell pellets were lysed in radioimmunoprecipitation assay buffer [50 mM Tris·HCl pH 7.4, 1% Nonidet P-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM NaF, 1 mM NA3VO4, and protease inhibitors (Thermo Fisher)] and were centrifuged at 12,000 revolutions/min × 12 min 4°C. The protein content of the supernatant for tissue homogenates and cell culture determined by Bradford assay and lysates were stored at −80°C until analysis.
Samples were diluted in HES buffer and Laemmli buffer containing 100 mM DTT (Thermo Fisher) based on protein concentration to generate samples containing equal concentration of protein. Samples were heated in a boiling water bath 5 min. For assessment of mitochondrial complexes, samples were diluted in HEPES/EDTA/sucrose (HES) buffer and nonreducing lane marker buffer not containing DTT (Thermo Fisher) and were not boiled. Protein (20–80 µg) was separated on SDS-PAGE gels, followed by a wet transfer to a nitrocellulose membrane for 1.5–4 h at 200–250 mA. Membranes were blocked in TBS, 0.1% Tween 20 (TBST), and 5% nonfat dry milk or 5% BSA followed by incubation with the appropriate primary antibodies. Following three brief washes with TBST, blots were incubated with an appropriate horseradish peroxidase-conjugated secondary antibody in TBST 1% nonfat dry milk or BSA at a concentration of 1:10,000 for 1 h at room temperature. Blots were then washed twice with TBST and once with TBS, dried, and visualized by ECL. Bands were quantified using ImageJ or Image Laboratory densitometry. Blots from liver and muscle were then stripped for 15–20 min at 55°C in buffer containing 62.5 mM Tris·HCl, 2% SDS, and 100 mM 2-mercaptoethanol and reprobed for α-tubulin or β-actin as a loading control. Ponceau (Sigma) was used as a loading control from cell culture studies.
Antibodies.
HSP72 primary antibody (cat. no. SPA-810) and cytochrome c (cat. no. AAM-175) were purchased from Enzo Life Sciences (Farmingdale, NY). Peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α; cat. no. 516557) was purchased from Cal-Biochem (Darmstadt, Germany). MitoProfile Total OXPHOS Rodent WB Antibody Cocktail (cat. no. 110413) was purchased from MitoSciences (Eugene, OR). Bradford protein quantification reagent was purchased from Bio-Rad (Hercules, CA). Secondary antibodies used included goat anti-mouse (cat. no. 170-5047, Bio-Rad), donkey anti-rabbit (cat. no. 711-035-152, Jackson ImmunoResearch, West Grove, PA), and goat anti-rabbit (cat. no. sc-2004, Santa Cruz Biotechnology, Dallas, TX). ECL reagents were purchased from Thermo Fisher.
Statistical analysis.
Results are presented as means ± SE. Statistical significance was set at P < 0.05. Analysis was performed using Sigma Plot for Windows, version 12.0 (Systat Software, Chicago, IL). Data were compared by two-way ANOVA for glucose uptake and hepatocyte TAG content and two-way repeated measures ANOVA for glucose and insulin curves from glucose tolerance tests. Fisher least-significant difference post hoc analyses were performed where appropriate, as noted. All other data were compared by unpaired t-tests. Cohen’s d effect size was calculated and reported when trends were observed. Where raw values did meet the assumptions of equal variance or normal distribution, values were logarithmically, square root, or reciprocally transformed.
RESULTS
At the end of the initial 8-wk period of high-fat feeding, body weight (Sham: 498.8 ± 19.1 g, Heat: 492.1 ± 15.7 g) and daily food intake (Sham: 19.2 ± 0.8 g/day, Heat: 19.4 ± 1.1 g/day) were similar before beginning HT or ST. Glucose tolerance was also similar after 8 wk of a HFD as seen by fasting glucose (Sham: 141.4 ± 2.7 mg/dl, Heat: 139.7 ± 5.1 mg/dl), fasting insulin (Sham: 1.6 ± 0.2 ng/ml and Heat: 2.1 ± 0.4 ng/ml), glucose area under the curve (AUC; Sham: 27,026.7 ± 2,206.4 mg/dl and Heat: 26,870.0 ± 2,493.0 mg/dl), and insulin AUC (Sham: 645.7 ± 37.6 ng/ml and Heat: 683.2 ± 61.4 ng/ml).
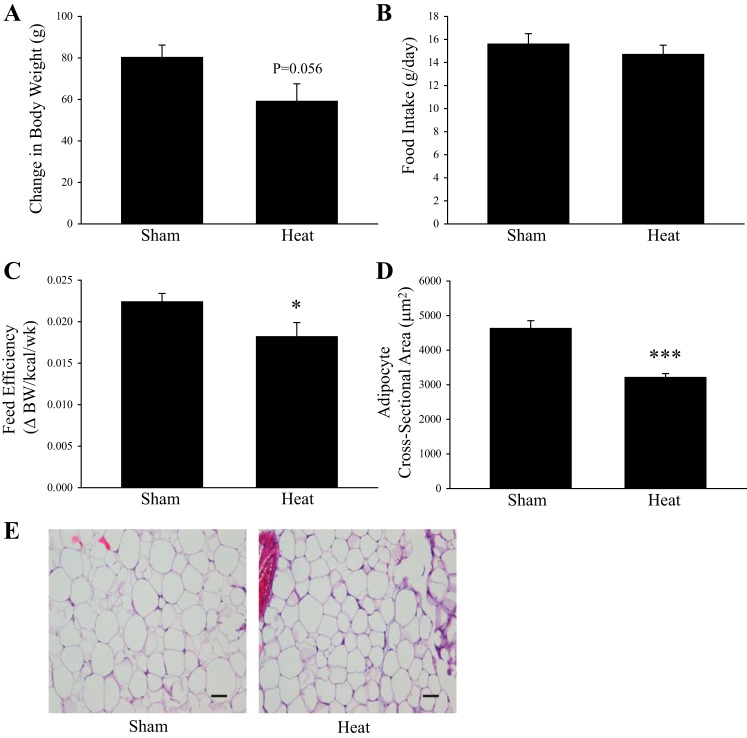
During the 7-wk HT or ST period there was a trend for HT rats to gain less weight on the HFD compared with ST animals (P = 0.056, effect size = 0.965, Fig. 1A). Food intake was not significantly different following HT (Fig. 1B). Feed efficiency, calculated as the change in body weight divided by the kilocalories consumed during this period, was 17% lower in the HT rats compared with ST (P < 0.05, Fig. 1C). Coinciding with modest changes in body weight, adipocyte size in the eWAT was 31% lower in HT rats compared with ST (P < 0.001, Fig. 1, D and E). There was no significant difference in eWAT mass when normalized to body weight between ST (2.9 ± 0.23%) and HT (2.6 ± 0.16%).
Fig. 1.
Body weight-related changes following weekly heat treatment in rats fed a high-fat diet (HFD). Change in (Δ) body weight (A), food intake (B), feed efficiency (C), and cross-sectional area (D) of adipocytes from epididymal white adipose tissue (eWAT) in rats fed an HFD for 15 wk and receiving weekly in vivo sham treatment (ST) (37°C, 20 min) or heat treatment (HT) (41°C, 20 min) during the last 7 wk of the HFD. Representative images of 10-µm thick sections of eWAT stained with hematoxylin-eosin (H&E) (E). Bar represents 50 µm. *P < 0.05, ***P < 0.001, significant differences between groups determined by unpaired t-test. Values are means ± SE; n = 8–9 animals/group.
HT, glucose tolerance, and insulin sensitivity.
Whole body insulin resistance has been consistently shown in rats after just 3–6 wk of high-fat feeding (30, 48, 58, 59). Fasting blood glucose and insulin concentration were not significantly different between ST and HT rats (Fig. 2, A and B). However, following an intraperitoneal injection of glucose 1 wk before the final sham treatment/heat treatment, and 48 h after the last sham treatment/heat treatment, HT rats had significantly lower blood glucose concentrations compared with ST (P < 0.01, Fig. 2C), as well as 26% lower glucose AUC values (P < 0.01, Fig. 2D). Insulin concentration over time (Fig. 2E), and insulin AUC values (Fig. 2F) were not significantly different between ST and HT rodents as well as homeostatic model assessment-insulin resistance (Fig. 2G), a method for assessing insulin resistance. Serum TAGs were not significantly different between ST (120.7 ± 11.3 mg/dl) and HT rats (114.3 ± 17.1 mg/dl) along with serum nonesterified fatty acid concentrations (ST: 0.469 ± 0.04 mM, HT: 0.421 ± 0.04 mM). We also evaluated skeletal muscle insulin sensitivity in the EDL muscle of ST and HT rodents. Both insulin (P < 0.01) and HT (P < 0.05) demonstrated significant main effects of increasing glucose uptake in the EDL (Fig. 2H).
Fig. 2.
Heat treatment (HT) improves glucose tolerance and insulin-stimulated glucose uptake in rats fed a high-fat diet (HFD). Fasting blood glucose (A) and serum insulin concentration (B) in rats fed an HFD for 15 wk and receiving weekly in vivo sham treatment (ST) (37°C, 20 min) or heat treatment (HT) (41°C, 20 min) during the last 7 wk of the HFD. Blood glucose (C) and insulin concentrations (E) in response to an intraperitoneal glucose injection and area under the curve (AUC) of glucose (D) and AUC of insulin (F). ST animals are represented by open circles, whereas HT animals are represented by closed circles (C, E). **P < 0.01, significant difference as determined by an unpaired t-test. For the blood glucose concentrations, a significant treatment × time interaction was observed (P < 0.01) whereby glucose concentrations were significantly lower in HT than ST animals as determined by a two-way repeated measure ANOVA (RM ANOVA). *P < 0.05, significant time point differences as determined by Fisher’s post hoc analysis. HOMA-IR was not significantly different between groups (G). Insulin-stimulated glucose uptake in the extensor digitorum longus (EDL) muscle in ST and HT rats (H). Values are means ± SE; n = 6–9 animals/group. ##P < 0.01, significant main effect of insulin and †P < 0.05, significant main effect of HT as determined by two-way ANOVA.
Liver and skeletal muscle responses to HT.
Induction of HSP72 by HT was determined in skeletal muscle and the liver. HSP72 levels were 315% greater in the EDL of HT rats compared with ST (P < 0.001, Fig. 3A). Hepatic HSP72 protein expression was 54% greater following HT compared with ST (P < 0.01, Fig. 3C). In addition to HSP72 induction, we also evaluated TAG storage in muscle and liver. We observed that in the tibialis anterior, a primarily glycolytic muscle like the EDL (13), TAG content was 20% lower in HT rats compared with ST (P < 0.05, Fig. 3B). Hepatic TAG content was not significantly different between groups; however, TAG content was 49% lower in HT animals and trended toward significance (P = 0.07, effect size = 2.7, Fig. 3D).
Fig. 3.
Heat treatment (HT) induces heat shock protein 72 (HSP72) and decreases triglyceride content in muscle and liver. Sham-treated (ST) and HT rats were fed a high-fat diet (HFD) for 15 wk and received weekly in vivo ST (37°C, 20 min) or HT (41°C, 20 min) during the last 7 wk of the HFD. HSP72 protein expression in the extensor digitorum longus (EDL) (first two bands are sham; second two bands are heat; A), triglyceride content in tibialis anterior (B), hepatic HSP72 expression (first two bands are sham; second two bands are heat; C), and hepatic triglyceride content were compared between ST and HT rats (D). Values are means ± SE; n = 8–9 animals/group. *P < 0.05, **P < 0.01, ***P < 0.001, significant differences between treatment groups determined by unpaired t-test.
Loss of HSP72 in primary hepatocytes disrupts mitochondrial integrity.
Because of the lack of knowledge regarding the role of HSP72 in the liver, we next investigated the effect of direct HSP72 loss on hepatocyte metabolism. Exposure to siRNA for HSP72 in primary hepatocytes resulted in a 58% knockdown of protein expression following HT (P < 0.01, Fig. 4A). Control and HSP72 siRNA-treated hepatocytes were examined by transmission electron microscopy to evaluate differences in mitochondrial morphology. Mitochondria treated with short interfering HSP72 (siHSP72) were larger and swollen compared with control siRNA-treated cells (Fig. 4B). Hepatocytes treated with control siRNA or siHSP72 were also stained with TMRE, a potentiometric dye taken up only by functional mitochondria (Fig. 4, C and F, images in red labeled A and D) and MitoTracker green, a dye taken up by both functional and nonfunctional mitochondria (Fig. 4, D and F, images in green labeled B and E). There was not a significant difference in TMRE/MitoTracker green staining between groups. However, it trended toward a reduced (64%) ratio in siHSP72 compared with control siRNA-treated cells (P = 0.09, effect size = 1.83, Fig. 4, E and F, merged images C and F). Together, this demonstrates a trend toward reduction in functional mitochondria and a change in mitochondrial morphology in hepatocytes lacking HSP72.
Fig. 4.
Heat shock protein 72 (HSP72) knockdown in primary hepatocytes disrupts mitochondrial integrity. Primary hepatocytes from C57/Bl6 mice were transfected with HSP72 siRNA or control siRNA (siCTL) and heat-treated 24 h later (A). A 58% knockdown of HSP72 protein expression was observed 24 h following heat treatment (HT) (first two bands are control siRNA; second two bands are siHSP72). Values are means ± SE; n = 3 wells. **P < 0.01, significant difference between treatment groups determined by an unpaired t-test. Primary hepatocytes in the presence or absence of HSP72 were imaged through transmission electron microscopy at ×5,000 (B). Representative images are shown. Primary hepatocytes treated with control siRNA or siHSP72 were stained with tetramethylrhodamine, ethyl ester (TMRE) (C and F; left images in red), which stains only mitochondria with intact membrane potentials and MitoTracker Green (center images in green) to stain all mitochondria (D and F). MitoTracker/TMRE images were also compared between groups (E and F; right merged images). Representative confocal images taken at ×20 are shown, along with quantification of signal intensity done using ImageJ. Intensity was analyzed by unpaired t-tests.
Mitochondrial protein expression was then evaluated to determine if reductions to mitochondrial integrity were paralleled by reductions in mitochondrial protein expression. There was no difference in protein expression of PGC-1α and electron transport chain complexes between control siRNA-treated and HSP72 siRNA-treated hepatocytes (Fig. 5, A, C, and D). However, cytochrome c protein expression increased by 20% with a loss of HSP72 (P < 0.05, Fig. 5B). These data suggest that an acute loss of HSP72 does not lead to a reduction in mitochondrial protein expression or biogenesis and may lead to a compensation by increasing protein expression of cytochrome c.
Fig. 5.
Effect of HSP72 reduction on mitochondrial protein expression. Primary hepatocytes from C57/Bl6 mice were transfected with HSP72 siRNA or control siRNA. Protein expression levels of peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) (first two bands are control siRNA; second two bands are siHSP72; A) Cytochrome c (first two bands are control siRNA; second two bands are siHSP72; B) and electron transport chain (ETC) Complexes I–V (C and D). Values are means ± SE; n = 3–4. *P < 0.05, significant difference between treatment groups as determined by unpaired t-test.
A loss of HSP72 in primary hepatocytes reduces FAO.
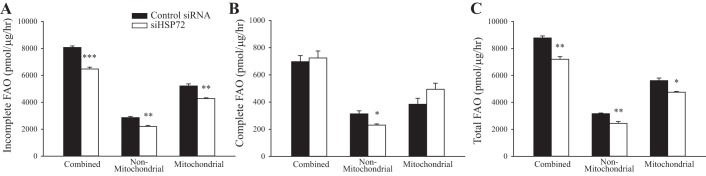
To determine if mitochondrial function was altered by an acute loss in HSP72 expression, FAO of [14C]palmitate was assessed in control siRNA and siHSP72-treated primary hepatocytes. Incomplete FAO (Fig. 6A), complete FAO through the tricarboxylic acid (TCA) cycle to CO2 (Fig. 6B), and total FAO (incomplete + complete FAO to CO2, Fig. 6C) were evaluated. A separate set of hepatocytes was also treated with etomoxir, an inhibitor of CPT-1, which allows for determination of differences between mitochondrial FAO and nonmitochondrial FAO mediated by other organelles such as peroxisomes.
Fig. 6.
Knockdown of heat shock protein 72 (HSP72) alters primary hepatocyte fatty acid oxidation. 14C-radiolabeled fatty acid oxidation (FAO) of palmitate (100 µM) in primary hepatocytes was evaluated in the presence and absence of the carnitine palmitoyltransferase-1 (CPT-1) inhibitor etomoxir (100 µM) allowing for the evaluation of mitochondrial and nonmitochondrial mediated FAO separately and together (combined FAO). Incomplete FAO, complete FAO to CO2, and total FAO were determined. A: incomplete, combined FAO, nonmitochondrial incomplete FAO and mitochondrial incomplete FAO in control siRNA and siHSP72- treated hepatocytes. B: complete, combined FAO to CO2, nonmitochondrial complete FAO to CO2, and mitochondrial complete FAO. C: total FAO (determined by adding incomplete and complete FAO to CO2), nonmitochondrial total FAO, and mitochondrial total FAO. Values are means ± SE; n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, significant differences determined by unpaired t-test between control siRNA and siHSP72 groups.
A loss of HSP72 resulted in a 20% reduction of incomplete FAO (P < 0.001, Fig. 6A) and a 19% reduction in total FAO (P < 0.01, Fig. 6C). Complete FAO to CO2 was not different between groups (Fig. 6B). Mitochondrial incomplete FAO and nonmitochondrial incomplete FAO were reduced by 19%, (P < 0.01) and 23% (P < 0.01), respectively, with a loss of HSP72. Reductions in mitochondrial total FAO (16% P < 0.05) and nonmitochondrial total FAO (22%, P < 0.01) were also observed in siHSP72-treated hepatocytes. Additionally, siHSP72 treatment resulted in a 26% decrease in nonmitochondrial complete FAO (P < 0.05). No significant difference in mitochondrial complete FAO was observed with a reduction in HSP72. Collectively, these data suggest that a loss of HSP72 may decrease total FAO in primary hepatocytes because of decreased mitochondrial and nonmitochondrial incomplete FAO.
HSP72 expression in primary hepatocytes modulates lipid storage.
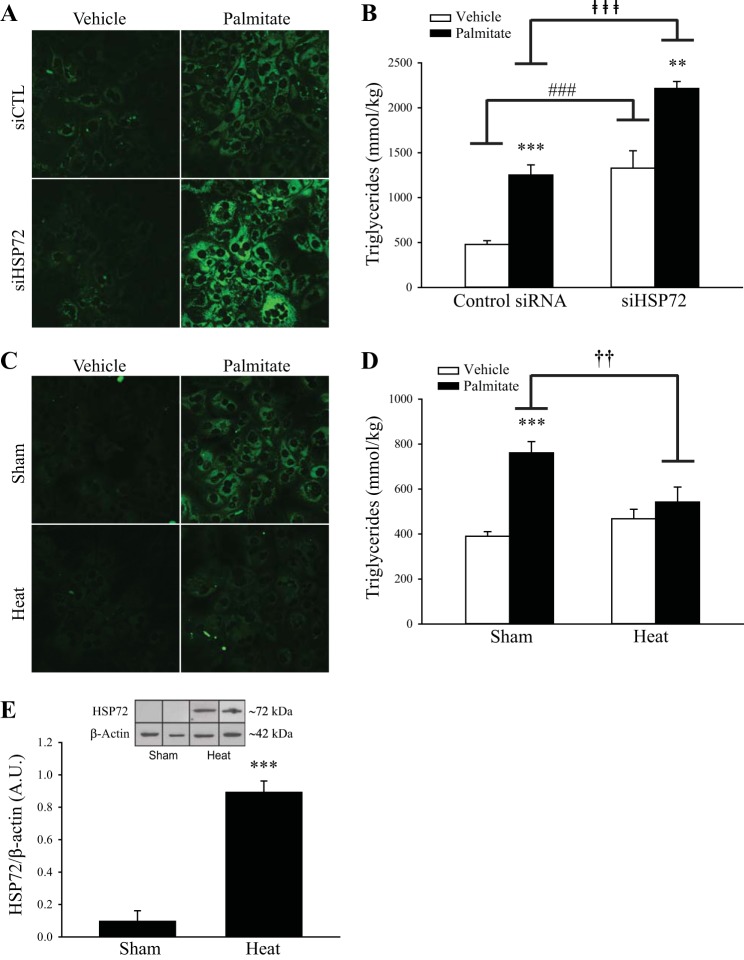
The impact of altering HSP72 protein expression on lipid accumulation was then evaluated in primary hepatocytes, and a significant interaction was found (P < 0.05). Palmitate treatment increased TAG 161% in control siRNA-treated hepatocytes (P < 0.001, Fig. 7, A and B). When compared with control siRNA-treated hepatocytes, siHSP72-treated hepatocytes had 77% greater lipid accumulation following 24 h of palmitate treatment (250 µM; P < 0.001, Fig. 7, A and B, closed bars). In addition, nonpalmitate, siHSP72-treated hepatocytes demonstrated a 177% increase in lipid accumulation compared with control (P < 0.001, Fig. 7B, open bars).
Fig. 7.
Heat shock protein 72 (HSP72) modulates lipid storage in primary hepatocytes. A: primary hepatocytes from C57/Bl6 mice were first treated with control siRNA or siRNA for HSP72 for 24 h and then with 250 µM palmitate or ehicle for 24 h. Cells were then stained with Bodipy for 20 min before imaging at ×40 to assess triacylglycerol content. B: liver triglyceride (TAG) content was also determined biochemically through a colorimetric assay. C: in separate experiments, cells were treated with palmitate (250 µM) or vehicle for 24 h and then exposed to sham treatment (ST) (37°C) or heat treatment (HT) (42°C) for 30 min. After these treatments, cells were stained with Bodipy and imaged at ×40. D: biochemical liver TAG was also determined. n = 6 wells. A significant condition (siRNA, HT) × treatment (vehicle, palmitate) interaction was observed in both siRNA (P < 0.05) and HT (P < 0.01) TAG assay experiments as determined by two-way ANOVA. Fisher’s post hoc analyses were performed to identify differences between groups. **P < 0.01 and ** P < 0.001, significant differences between vehicle and palmitate treatment within control siRNA, siHSP72 and sham groups. ###P < 0.001 and ⱡⱡⱡP < 0.001, significant differences between siRNA-treated groups. ††P < 0.01, significant difference between sham and heat-treated groups. E: HSP72 induction in hepatocytes 24 h following ST (37°C) or HT (42°C) for 30 min (first two bands are sham; second two bands are heat). ***P < 0.001, a significant difference determined by unpaired t-test between ST and HT groups. n = 3. Values are means ± SE.
A separate set of hepatocytes was exposed to ST (37°C) or HT (42°C) for 30 min to increase HSP72 protein expression. HT was performed after 24 h of palmitate treatment. A significant interaction was found between these treatments (P < 0.01). Palmitate treatment increased hepatic TAG by 95% in ST hepatocytes (P < 0.001, Fig. 7, C and D). However, HT blunted lipid accumulation in primary hepatocytes. TAG accumulation was 29% lower in HT hepatocytes compared with ST hepatocytes following 24 h palmitate exposure (P < 0.01, Fig. 7, C and D, black bars). HSP72 protein content was significantly increased in hepatocytes as a result of the HT (P < 0.001, Fig. 7E).
DISCUSSION
NAFLD is the liver component of metabolic disease and is highly prevalent (47). HSPs are known to play important roles in skeletal muscle metabolism, but their potential roles in liver metabolism are unclear. In this study, we demonstrated that in vivo HT induces hepatic HSP72 expression. We also found that HSP72 is important in maintaining mitochondrial morphology and FAO, as well as preventing lipid storage in primary hepatocytes (Fig. 8).
Fig. 8.
Hepatic heat shock protein 72 (HSP72), lipid accumulation and whole body metabolic homeostasis. HSP72 was found by Di Naso et al. (14) to be reduced with the progression of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). Based on the results of this study, we propose that reduced HSP72 that is observed with NAFLD progression results in reduced fatty acid oxidation (FAO), impaired mitochondrial integrity and increased lipid storage. This may contribute to impaired whole body insulin sensitivity and glucose tolerance that occurs with metabolic disease.
In addition to identifying liver-specific outcomes in this study, to our knowledge these are the first findings to indicate the ability of HT to reverse the damaging metabolic effects of a prior HFD. Previously, we showed that 12 wk of weekly in vivo HT prevented HFD-induced whole body and skeletal muscle insulin resistance (24). In the present study, we expand upon these findings by showing that after 8 wk of high-fat feeding, weekly in vivo HT improved whole body glucose tolerance, reduced adipocyte size, increased skeletal muscle glucose uptake, and reduced skeletal muscle TAG storage compared with ST. Importantly, activity levels and whole body energy expenditure were not monitored and could be a confounding factor in heat-mediated metabolic improvements. The effect of heat on these outcomes should be explored in future work and would further clarify what whole effects HT has in vivo.
As excess lipid accumulation in the liver is a hallmark of NAFLD, reductions in TAG storage represent a potential treatment modality that has not been widely explored. Past work by our laboratory and others has identified increased hepatic HSP72 protein expression with heat and exercise (3, 20, 24, 56); however, these studies did not investigate liver steatosis. Heat stress was shown to reduce TAG storage, inflammation, and increased insulin responsiveness in the liver of high-fat-fed mice and db/db mice (43, 44); however, HSP levels were not measured. Studies have also identified pharmacological compounds that increase hepatic HSP72 and improve hepatic metabolism; however, it is unknown if these were direct effects (1, 65). In the current study, we observed that hepatic HSP72 protein expression is induced by weekly HT in rats fed an HFD. In our in vitro studies, we aimed to identify if HSP72 has a role in the protection against hepatic lipid accumulation. We found that with an acute loss of HSP72, lipid accumulation was increased following palmitate exposure. However, HT effectively prevented lipid accumulation compared with ST. These promising results from our in vitro studies have identified the possible role of HSP72 in preventing hepatic lipid accumulation.
Identifying the effects of HSP72 loss on mitochondrial integrity and FAO is important because of the strong mitochondrial component in the development of NAFLD (26, 46). We found with a loss of HSP72 in hepatocytes, palmitate FAO was reduced along with mitochondrial integrity as shown by changes in morphology. Drew et al. (15) found that the mitochondrial degradation pathway, mitophagy, was impaired and contributed to the accumulation of damaged mitochondria in skeletal muscle of whole body HSP72 knockout animals. It is possible that impaired mitophagy occurs with a loss of HSP72 in the liver, and future studies will be needed to investigate this interaction. Rodent studies have also demonstrated that impaired FAO contributes to the development of hepatic steatosis and insulin resistance (51, 53, 66). However, there is no evidence that decreased FAO contributes to the advancement of NAFLD in humans despite the observation of impaired ATP production and increased reactive oxygen species (6, 9, 50, 57). It is possible that reduced HSP72 regulation of FAO contributes to increased lipid storage; however, future research must identify the significance of this finding in the context of NAFLD or nonalcoholic steatohepatitis (NASH) development in humans.
Fatty acids are mainly oxidized in the mitochondria through the subsequent reactions of β-oxidation. Acetyl-CoA is produced, which then may enter the TCA cycle and be completely oxidized to CO2, known as complete FAO (16). Incomplete oxidation of fatty acids can also occur and form ASMs such as acetyl-CoA, ketone bodies, and TCA cycle intermediates. In this study, we observed reduced total mitochondrial FAO with a loss of HSP72 in primary hepatocytes, which was driven by reductions in incomplete hepatic FAO. About two-thirds of the fat that comes to the liver is converted into ketones (63), therefore the majority of FAO is directed to ketogenesis from incomplete FAO (49). Cotter et al. (10) showed that ketogenesis can be a mechanism of lipid disposal even in nonfasted conditions, and dysfunction in this pathway promotes the development of NAFLD into NASH. It is possible that HSP72 could be involved in regulation of ketogenic pathways and that the subsequent blunting of ketogenesis is connected to steatosis; however, more research is needed to determine this hypothesis.
One limitation of this study is the fact that we did not investigate more aspects of lipid handling such as lipid secretion as VLDL, which is an important mechanism for the liver to export lipid from hepatocytes (17). Effects of HSP72 loss on lipid transport and TAG synthesis would be other important aspects of lipid handling to investigate. Preliminary studies in our laboratory showed no difference in fatty acid transporters CD36 and fatty acid-binding protein 1 or the TAG synthesis proteins diglyceride acyltransferase 1 and diglyceride acyltransferase 2 with a loss of HSP72 (data not shown). Future work investigating a range of time points may help pinpoint when the changes in signaling and protein expression may occur. In our experiments, we utilized both mice and rats to answer our research questions. Including both species demonstrates that the HSP response is conserved across models and has similar metabolic benefits despite species-specific differences in metabolic profiles (29).
Perspectives and Significance
Our in vitro results mainly focused on the effects of basal reductions of HSP72 on hepatic metabolism and suggest an impaired response to metabolic challenges with reduced HSP72 protein expression. This is an important area of investigation because of reductions in basal HSP72 in both skeletal muscle with the progression of diabetes (32) and in the liver with the progression of NAFLD (14). Future studies investigating the ability of HT to improve metabolic function with hepatic HSP72 insufficiency would provide greater insight to HSP72-mediated mechanisms and whether redundant heat-related mechanisms exist.
Based on the findings from Di Naso et al. (14) that showed decreased HSP72 with the advancement of NAFLD, this protein may not only be important in the prevention of steatosis but also in the advancement of NAFLD. In ~20% of individuals, NAFLD eventually develops into a more severe liver disease called NASH. It is possible that a loss of HSP72 induction contributes to the transition of NAFLD into NASH (14). Hepatic HSP72 protein content could be a potential marker of NAFLD stage, although this would be difficult to measure in vivo. HSPs can also be released extracellularly into circulation, and it has been suggested that extracellular HSP72 levels could be a potential maker of metabolic disease stage (4, 18, 28, 34, 35, 62). As suggested by Di Naso et al. (14) and others, future work needs to identify if intracellular as well as extracellular HSP72 could serve as a specific marker of liver disease stage (12, 31, 54).
To our knowledge, this is the first study to directly reduce hepatic HSP72 levels and to identify the importance of HSP72 in FAO and the prevention of steatosis. To identify the importance of hepatic HSP72 mechanisms in whole-body metabolic homeostasis fully, the development of a liver-specific HSP72 knockout model will be necessary in future investigation of these mechanisms. Our findings highlight the need for continued investigation into NAFLD treatments that activate the heat shock response.
GRANTS
The project was supported by National Institutes of Health (NIH) Grant AG-031575 and the Molecular Regulation of Cell Development and Differentiation Center of Biomedical Research Excellence (COBRE) Grant P30-GM-122731 awarded to P. C. Geiger. R. S. Rogers was supported by a Kansas University Medical Center Biomedical Research Training Program, and A. E. Archer was supported by the Madison and Lila Self Graduate Fellowship.
This core is supported in part by grants from the National Institute of General Medical Sciences (nos. P20-GM-103549 and P30-GM-118247). The Electron Microscopy Research Laboratory (EMRL) is supported, in part, by NIH COBRE Grant 9P200-GM-0104936. The Jeol JEM-1400 transmission electron microscope used in the study was purchased with funds from NIH Grant S10-RR-027564.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.E.A., R.S.R., A.T.V.S., E.M.M., C.S.M., J.P.T., and P.C.G. conceived and designed research; A.E.A., R.S.R., A.T.V.S., and J.L.W. performed experiments; A.E.A., R.S.R., and E.M.M. analyzed data; A.E.A., R.S.R., A.T.V.S., E.M.M., C.S.M., J.P.T., and P.C.G. interpreted results of experiments; A.E.A. and R.S.R. prepared figures; A.E.A. drafted manuscript; A.E.A., R.S.R., A.T.V.S., E.M.M., C.S.M., J.P.T., and P.C.G. edited and revised manuscript; A.E.A., R.S.R., A.T.V.S., J.L.W., E.M.M., C.S.M., J.P.T., and P.C.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Cell Isolation Core at the University of Kansas Medical Center. We also acknowledge the University of Kansas Medical Center Electron Microscopy Research Laboratory facility for assistance with the transmission electron microscope. The authors also thank Jill Morris, Grace Meers, Camron Myers, Isabelle Ellington, Vandita Garimella, and Kathleen White for technical support.
REFERENCES
- 1.Adachi H, Kondo T, Ogawa R, Sasaki K, Morino-Koga S, Sakakida M, Kawashima J, Motoshima H, Furukawa N, Tsuruzoe K, Miyamura N, Kai H, Araki E. An acylic polyisoprenoid derivative, geranylgeranylacetone protects against visceral adiposity and insulin resistance in high-fat-fed mice. Am J Physiol Endocrinol Metab 299: E764–E771, 2010. doi: 10.1152/ajpendo.00075.2010. [DOI] [PubMed] [Google Scholar]
- 2.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11: 545–555, 2010. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atalay M, Oksala NKJ, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hänninen O, Sen CK. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol (1985) 97: 605–611, 2004. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- 4.Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem 278: 21601–21606, 2003. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- 5.Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab 295: E1269–E1276, 2008. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD Jr. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol 31: 430–434, 1999. doi: 10.1016/S0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen HW, Chen SC, Tsai JL, Yang RC. Previous hyperthermic treatment increases mitochondria oxidative enzyme activity and exercise capacity in rats. Kaohsiung J Med Sci 15: 572–580, 1999. [PubMed] [Google Scholar]
- 8.Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA 105: 1739–1744, 2008. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA 282: 1659–1664, 1999. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 10.Cotter DG, Ercal B, Huang X, Leid JM, d’Avignon DA, Graham MJ, Dietzen DJ, Brunt EM, Patti GJ, Crawford PA. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest 124: 5175–5190, 2014. doi: 10.1172/JCI76388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotto JJ, Kline M, Morimoto RI. Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation. Evidence for a multistep pathway of regulation. J Biol Chem 271: 3355–3358, 1996. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- 12.De Maio A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones 16: 235–249, 2011. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol (1985) 80: 261–270, 1996. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 14.Di Naso FC, Porto RR, Fillmann HS, Maggioni L, Padoin AV, Ramos RJ, Mottin CC, Bittencourt A, Marroni NA, de Bittencourt PI JR. Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity (Silver Spring) 23: 120–129, 2015. doi: 10.1002/oby.20919. [DOI] [PubMed] [Google Scholar]
- 15.Drew BG, Ribas V, Le JA, Henstridge DC, Phun J, Zhou Z, Soleymani T, Daraei P, Sitz D, Vergnes L, Wanagat J, Reue K, Febbraio MA, Hevener AL. HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes 63: 1488–1505, 2014. doi: 10.2337/db13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton S. Control of mitochondrial β-oxidation flux. Prog Lipid Res 41: 197–239, 2002. doi: 10.1016/S0163-7827(01)00024-8. [DOI] [PubMed] [Google Scholar]
- 17.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 134: 424–431, 2008. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol 544: 957–962, 2002. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J Lipid Res 21: 139–144, 1980. [PubMed] [Google Scholar]
- 20.González B, Manso R. Induction, modification and accumulation of HSP70s in the rat liver after acute exercise: early and late responses. J Physiol 556: 369–385, 2004. doi: 10.1113/jphysiol.2003.058420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupte AA, Bomhoff GL, Geiger PC. Age-related differences in skeletal muscle insulin signaling: the role of stress kinases and heat shock proteins. J Appl Physiol (1985) 105: 839–848, 2008. doi: 10.1152/japplphysiol.00148.2008. [DOI] [PubMed] [Google Scholar]
- 23.Gupte AA, Bomhoff GL, Morris JK, Gorres BK, Geiger PC. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J Appl Physiol (1985) 106: 1425–1434, 2009. doi: 10.1152/japplphysiol.91210.2008. [DOI] [PubMed] [Google Scholar]
- 24.Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes 58: 567–578, 2009. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol (1985) 110: 451–457, 2011. doi: 10.1152/japplphysiol.00849.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gusdon AM, Song KX, Qu S. Nonalcoholic Fatty liver disease: pathogenesis and therapeutics from a mitochondria-centric perspective. Oxid Med Cell Longev 2014: 637027, 2014. doi: 10.1155/2014/637027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, Connor T, Watt MJ, Carpenter K, Hargreaves M, McGee SL, Hevener AL, Febbraio MA. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 63: 1881–1894, 2014. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun 324: 511–517, 2004. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 29.Kowalski GM, Bruce CR. The regulation of glucose metabolism: implications and considerations for the assessment of glucose homeostasis in rodents. Am J Physiol Endocrinol Metab 307: E859–E871, 2014. doi: 10.1152/ajpendo.00165.2014. [DOI] [PubMed] [Google Scholar]
- 30.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 40: 1397–1403, 1991. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 31.Krause M, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, Homem de Bittencourt PI JR. The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediators Inflamm 2015: 249205, 2015. doi: 10.1155/2015/249205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurucz I, Morva A, Vaag A, Eriksson K-F, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 51: 1102–1109, 2002. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- 34.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem 280: 23349–23355, 2005. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 35.Lancaster GI, Møller K, Nielsen B, Secher NH, Febbraio MA, Nybo L. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones 9: 276–280, 2004. doi: 10.1379/CSC-18R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Literati-Nagy B, Kulcsar E, Literati-Nagy Z, Buday B, Peterfai E, Horvath T, Tory K, Kolonics A, Fleming A, Mandl J, Koranyi L. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res 41: 374–380, 2009. doi: 10.1055/s-0028-1128142. [DOI] [PubMed] [Google Scholar]
- 37.Liu C-T, Brooks GA. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J Appl Physiol (1985) 112: 354–361, 2012. doi: 10.1152/japplphysiol.00989.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 39.Mayo Clinic Nonalcoholic fatty liver disease (Online). https://www.mayoclinic.org/diseases-conditions/nonalcoholic-fatty-liver-disease/symptoms-causes/syc-20354567 [10 October 2017].
- 40.Miwa S, Lawless C, von Zglinicki T. Mitochondrial turnover in liver is fast in vivo and is accelerated by dietary restriction: application of a simple dynamic model. Aging Cell 7: 920–923, 2008. doi: 10.1111/j.1474-9726.2008.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science 259: 1409–1410, 1993. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- 42.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12: 3788–3796, 1998. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 43.Morino-Koga S, Yano S, Kondo T, Shimauchi Y, Matsuyama S, Okamoto Y, Suico MA, Koga T, Sato T, Shuto T, Arima H, Wada I, Araki E, Kai H. Insulin receptor activation through its accumulation in lipid rafts by mild electrical stress. J Cell Physiol 228: 439–446, 2013. doi: 10.1002/jcp.24149. [DOI] [PubMed] [Google Scholar]
- 44.Morino S, Kondo T, Sasaki K, Adachi H, Suico MA, Sekimoto E, Matsuda T, Shuto T, Araki E, Kai H. Mild electrical stimulation with heat shock ameliorates insulin resistance via enhanced insulin signaling. PLoS One 3: e4068, 2008. doi: 10.1371/journal.pone.0004068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol 303: G979–G992, 2012. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris EM, Rector RS, Thyfault JP, Ibdah JA. Mitochondria and redox signaling in steatohepatitis. Antioxid Redox Signal 15: 485–504, 2011. doi: 10.1089/ars.2010.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Institute of Diabetes and Digestive and Kidney Diseases Nonalcoholic fatty liver disease & NASH (Online). https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash [1 November 2017]. [PubMed]
- 48.Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes 46: 1768–1774, 1997. doi: 10.2337/diab.46.11.1768. [DOI] [PubMed] [Google Scholar]
- 49.Patterson RE, Kalavalapalli S, Williams CM, Nautiyal M, Mathew JT, Martinez J, Reinhard MK, McDougall DJ, Rocca JR, Yost RA, Cusi K, Garrett TJ, Sunny NE. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am J Physiol Endocrinol Metab 310: E484–E494, 2016. doi: 10.1152/ajpendo.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Carreras M, Del Hoyo P, Martín MA, Rubio JC, Martín A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 38: 999–1007, 2003. doi: 10.1002/hep.1840380426. [DOI] [PubMed] [Google Scholar]
- 51.Rector RS, Morris EM, Ridenhour S, Meers GM, Hsu FF, Turk J, Ibdah JA. Selective hepatic insulin resistance in a murine model heterozygous for a mitochondrial trifunctional protein defect. Hepatology 57: 2213–2223, 2013. doi: 10.1002/hep.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 53.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodrigues-Krause J, Krause M, O’Hagan C, De Vito G, Boreham C, Murphy C, Newsholme P, Colleran G. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones 17: 293–302, 2012. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers RS, Beaudoin MS, Wheatley JL, Wright DC, Geiger PC. Heat shock proteins: in vivo heat treatments reveal adipose tissue depot-specific effects. J Appl Physiol (1985) 118: 98–106, 2015. doi: 10.1152/japplphysiol.00286.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salo DC, Donovan CM, Davies KJ. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med 11: 239–246, 1991. doi: 10.1016/0891-5849(91)90119-N. [DOI] [PubMed] [Google Scholar]
- 57.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 58.Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW. Fat feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. Am J Physiol Endocrinol Metab 251: E576–E583, 1986. doi: 10.1152/ajpendo.1986.251.5.E576. [DOI] [PubMed] [Google Scholar]
- 59.Sutherland LN, Capozzi LC, Turchinsky NJ, Bell RC, Wright DC. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: potential mechanisms and the relationship to glucose intolerance. Am J Physiol Endocrinol Metab 295: E1076–E1083, 2008. doi: 10.1152/ajpendo.90408.2008. [DOI] [PubMed] [Google Scholar]
- 60.Tamura Y, Kitaoka Y, Matsunaga Y, Hoshino D, Hatta H. Daily heat stress treatment rescues denervation-activated mitochondrial clearance and atrophy in skeletal muscle. J Physiol 593: 2707–2720, 2015. doi: 10.1113/JP270093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitham M, Fortes MB. Heat shock protein 72: release and biological significance during exercise. Front Biosci 13: 1328–1339, 2008. doi: 10.2741/2765. [DOI] [PubMed] [Google Scholar]
- 63.Williamson JR, Browning ET, Scholz R. Control mechanisms of gluconeogenesis and ketogenesis. I. Effects of oleate on gluconeogenesis in perfused rat liver. J Biol Chem 244: 4607–4616, 1969. [PubMed] [Google Scholar]
- 64.Young DA, Uhl JJ, Cartee GD, Holloszy JO. Activation of glucose transport in muscle by prolonged exposure to insulin. Effects of glucose and insulin concentrations. J Biol Chem 261: 16049–16053, 1986. [PubMed] [Google Scholar]
- 65.Zeng XY, Wang H, Bai F, Zhou X, Li SP, Ren LP, Sun RQ, Xue CC, Jiang HL, Hu LH, Ye JM. Identification of matrine as a promising novel drug for hepatic steatosis and glucose intolerance with HSP72 as an upstream target. Br J Pharmacol 172: 4303–4318, 2015. doi: 10.1111/bph.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang D, Liu Z-X, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA 104: 17075–17080, 2007. doi: 10.1073/pnas.0707060104. [DOI] [PMC free article] [PubMed] [Google Scholar]