Abstract
Systemic insulin resistance and glucose intolerance occur with as little as 3 days of a high-fat diet (HFD) in mice and humans; the mechanisms that initiate acute insulin resistance are unknown. Most laboratories house mice at 22°C, which is below their thermoneutral temperature (~30°C). Cold stress has been shown to increase white adipose tissue (WAT) browning, alter lipid trafficking, and impair immune function, whereas energy intake and expenditure decrease with increasing ambient temperature; importantly, dysregulation of these parameters has been strongly linked to obesity-induced insulin resistance. Therefore, we compared acute changes in glucose metabolism and the metabolic phenotype in lean mice in response to a control diet or HFD housed at standard vivarium (22°C) and thermoneutral (30°C) temperatures. Glucose intolerance occurred following 1 or 5 days of HFD and was independent of housing temperature or adiposity; however, the reduction in tissue-specific glucose clearance with HFD diverged by temperature with reduced brown adipose tissue (BAT) glucose uptake at 22°C but reduced soleus glucose uptake at 30°C. Fasting glucose, food intake, and energy expenditure were significantly lower at 30°C, independent of diet. Additionally, markers of browning in both BAT and inguinal subcutaneous WAT, but not perigonadal epididymal WAT, decreased at 30°C. Together, we find housing temperature has a significant impact on the cellular pathways that regulate glucose tolerance in response to an acute HFD exposure. Thus, even short-term changes in housing temperature should be highly considered in interpretation of metabolic studies in mice.
Keywords: adipose tissue browning, energy expenditure, glucose metabolism, thermoneutral housing
INTRODUCTION
Obesity-induced insulin resistance is thought to occur secondary to the release of proinflammatory cytokines and chemokines, which may be secreted as an adaptive response to maintain adipose tissue function in the presence of expanding or hypoxic adipocytes (32). Adipose tissue-derived cytokines and chemokines have been shown to regulate insulin action in an autocrine, paracrine, and endocrine manner (29). However, the mechanism(s) that regulate and initiate the inflammatory response in obesity are not clearly understood. High-fat diet (HFD)-induced glucose intolerance and insulin resistance have been observed in both lean rodents (21, 22, 24, 43) and humans (6) in as little as three days of high-fat feeding. Interestingly, Lee and colleagues demonstrated in mice that inflammation is not necessary for the 3-day HFD-induced glucose intolerance and insulin resistance (24), despite a marked increase in proinflammatory gene expression in white adipose tissue (WAT). Recent studies have identified a relationship between housing temperature and inflammatory response (14); in the study by Lee et al. (24), mice were housed well below the murine thermoneutral zone (29°C–34°C), which may confound these results. For example, Tian et al. (39) demonstrated that long-term (9–10 wk) thermoneutral housing of mice accelerates perivascular WAT inflammation in response to HFD. Similarly, Giles et al. (14) have shown that thermoneutral housing as compared with conventional housing exacerbates lipopolysaccharide-induced inflammation.
In most laboratories, mice are housed at room temperature, between 20°C and 26°C, which is recommended within the Guide for the Care and Use of Laboratory Animals (27). Mice housed at routine vivarium temperatures have been shown to have less adipose tissue inflammation relative to mice housed at thermoneutral temperatures and have also been shown to have greater energy intake and expenditure (11). Together, these results may influence net energy balance and potentially lead to unpredictable outcomes in metabolic data. Considering the laboratory mouse is used as a preferred model system for the study of many metabolic diseases, because of the relative ease of creating genetic perturbations, it is imperative to understand the differences between mouse and human thermal physiology to ensure the applicability of preclinical findings. Housing mice below their thermoneutral zone has been referred to as a subthermoneutral stress (14), which is not common in human dwellings of daily life.
Chronic subthermoneutral stress has been shown to promote nonshivering thermogenesis, which is achieved through mitochondria-rich brown adipose tissue (BAT; 11). BAT contains uncoupling protein-1 (UCP-1), which serves to mediate heat generation by the uncoupling of oxidative phosphorylation from ATP synthesis (16). Chronic cold exposure not only influences inflammation and energetics but has been shown to upregulate BAT mass, Ucp1 gene expression in BAT, and induce “browning” of WAT through increased Ucp1 gene expression and an increase in multilocular cells within WAT (28). BAT activity has been estimated to account for 2.7%–5% of the basal metabolic rate in humans, which could cumulatively promote more than 4 kg of fat loss per year (41, 42). Furthermore, increased BAT mass has been shown to regulate glucose homeostasis and improve insulin sensitivity in a mass-dependent manner (34).
Currently, most studies comparing behavioral, metabolic, and inflammatory responses to varying ambient temperatures have been long-term (≥8 wk). An acute response to changes in housing temperature on metabolic variables in the laboratory mouse has not been examined. Therefore, the purpose of this study was to investigate the effect of short-term thermoneutral housing (30°C) compared with standard vivarium housing temperature (22°C) on glucose tolerance, metabolism, and behavior (e.g., food intake, activity) in response to HFD.
METHODS
Experimental design.
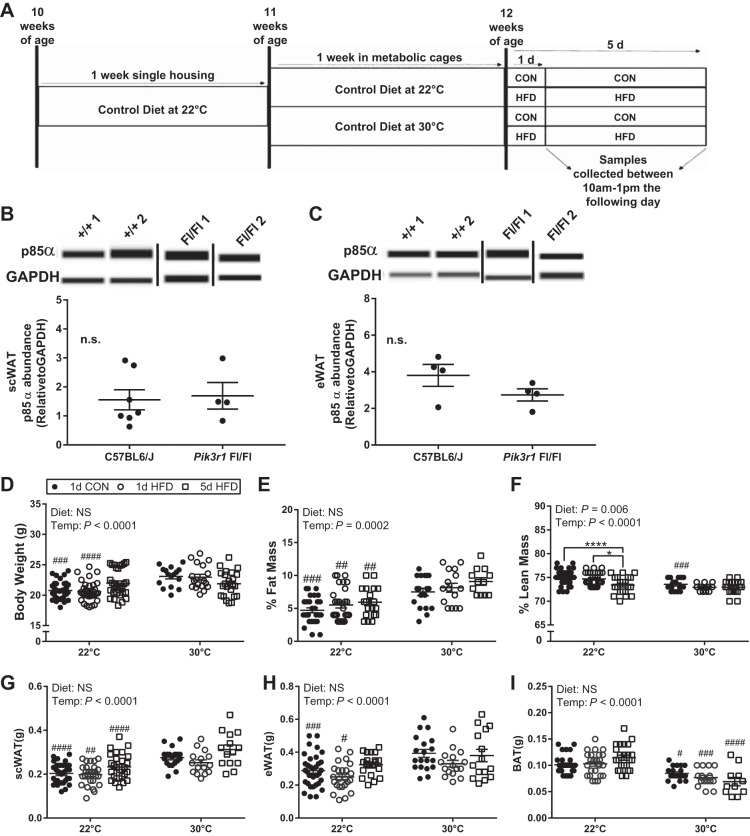
All experiments were approved by Institutional Animal Care and Use Committee at the University of Oregon. Mice with a mixed B6/129S background and homozygous for loxP sites flanking exon 8 of the Pik3r1 gene (Pik3r1tm1Lca/J; no. 012871, The Jackson Laboratory, Bar Harbor, ME) were used in these studies. p85α abundance is not different in mice with the targeted allele compared with wild type in the absence of recombinant cre (Fig. 1, A and B) as has been shown previously (1, 25) and was used in thermoneutral studies to complement ongoing work in this mouse line. All mice were housed at standard vivarium temperature (TS; 22°C) with a 12:12-h light/dark cycle (light cycle: 0600–1800; dark cycle: 1800–0600) and fed chow diet with 13% fat (Picolab Rodent Diet 20, LabDiet, St. Louis, MO) until 10 wk of age. At 10 wk of age, male mice were singly housed in standard-sized cages and placed within an environmental cabinet set at TS and fed a control diet (CON: 10% calories from fat; cat. no. D12450H, Research Diets, New Brunswick, NJ) for 1 wk. The 12-h light/dark cycle remained the same throughout the study. After the cage acclimation period, mice remained on CON but were randomly assigned to murine thermoneutral temperature (TN; 30°C) or remained at TS for 1 wk. At 12 wk of age, mice either remained on CON or were switched to an HFD (45% calories from fat; cat. no. D12451, Research Diets) for 1 day (1 full light/dark cycle) or 5 days (5 full light/dark cycles). Both the CON and HFDs were matched for sucrose (17%). At the end of the diet period, mice were euthanized by cervical dislocation following isoflurane anesthesia between 1000 and 1300, and tissues [inguinal subcutaneous WAT (scWAT), interscapular BAT, and perigonadal epididymal WAT (eWAT)] were rapidly dissected, snap-frozen in liquid nitrogen, and stored at −80°C until analysis.
Fig. 1.
Body weight and adiposity are increased in wild-type (WT) mice housed at murine thermoneutral temperature (Temp) (30°C) vs. standard vivarium temperature (22°C) independent of diet. Illustration of the study design (A). p85α abundance in subcutaneous white adipose tissue (scWAT) from Pik3r1flox/flox mice and C57BL6/J mice (B). p85α abundance in epididymal WAT (eWAT) from Pik3r1flox/flox mice and C57BL6/J mice (C). Body weight and body composition before and after 1-day control (CON), 1-day high-fat diet (HFD), and 5-day HFD treatment at standard vivarium temperature (n = 35 mice/group) and murine thermoneutral temperature (n = 20/group; D). Inguinal scWAT (E), eWAT (F), and brown adipose tissue (BAT) (G) weight following 1 or 5 days of CON and HFD feeding at 22°C (n = 35/group) and 30°C (n = 20/group). Data were analyzed by a two-way ANOVA (diet × temperature) with a Sidak multiple comparison test. P values for main effects (P ≤ 0.01) are listed in each graph. #Significant findings with multiple comparison test for temperature (#P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001); and *significant findings with multiple comparison test for diet (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). NS, not significant.
Body composition.
Body composition (lean tissue, fat, and free fluid) was measured by time domain (TD)-NMR using the Minispec LF50 mouse NMR (Bruker BioSpin, Billerica, MA) in anesthetized mice before and after the 1- and 5-day period. Percentages were calculated from body weight measured on a standard scale (Mettlor-Toledo, Columbus, OH) using the Minispec Software.
Food intake and total energy expenditure.
Using a metabolic monitoring system (Promethion, Sable Systems, Las Vegas, NV), we measured cage behavior (food intake, water intake, and activity), oxygen, carbon dioxide, and water vapor during the 12:12-h light/dark cycles (light cycle: 0600–1800; dark cycle: 1800–0600) following 1 and 5 full light and dark cycles. The metabolic cages were kept inside an environmental cabinet allowing temperature control throughout the study. Metascreen software (Sable Systems) was used for data collection and data were processed with ExpeData (Sable Systems) proprietary macros. Energy expenditure (kcal/h) was calculated relative to body weight (kg). Food intake (g/day) was converted to kcal/day and made relative to body weight (kg).
Oral glucose tolerance test.
Glucose tolerance tests were performed in conscious mice following the 1-day and 5-day diet treatments. Mice were fasted for 4 h (0600–1000) before testing to normalize glucose and insulin levels. Tails were nicked with a sterile scissors, and ~2–5 μl of blood were analyzed with a handheld glucometer (Contour, Ascensia Diabetes Care, Parsippany, NJ). After the fasting glucose measurements, the animals were given 5 g dextrose/kg body wt by oral gavage. Glucose was measured from the tail blood at 10, 20, 30, 45, 60, 75, 90, and 120 min. Glucose area under the curve was calculated from baseline glucose values using Graphpad Prism 7.0 (GraphPad Software, La Jolla, CA).
Insulin tolerance test.
Insulin tolerance tests were performed in conscious mice following 1- and 5-day diet treatments. Mice were fasted for 4 h (0600–1000) before testing to normalize glucose and insulin levels. Tails were nicked with sterile scissors, and ~2–5 μl of blood were analyzed with a handheld glucometer (Ascensia Diabetes Care). After the fasting glucose measurements, the animals received an intraperitoneal injection of 0.25 U insulin (Humulin-R, Eli Lilly and Company, Indianapolis, IN) per kg body wt. Glucose was measured from tail blood again at 10, 20, 30, 40, 50, and 60 min.
RNA isolation and quantitative real-time PCR.
Inguinal scWAT, perigonadal eWAT, and interscapular BAT were homogenized using the Bead Ruptor Elite, Bead Mill Homogenizer (OMNI International, Kennesaw, GA) at 5 m/s for 30 s × 2, in 100 mg/1 ml Qiazol (Qiagen, Valencia, CA). Total RNA was isolated using a Pure Link RNA Kit (Invitrogen, Eugene, OR) according to the manufacturer’s protocol and quantified with a nanodrop spectrophotometer. cDNA was synthesized from total RNA using iScript reverse transcriptase (Bio-Rad Laboratories, Hercules, CA), and gene expression was measured by quantitative real-time PCR in a CFX384 instrument (Bio-Rad Laboratories). Gene expression was calculated as the 2-ΔΔCT with target genes normalized to β-actin and an internal reference sample (CON at 22°C). Mouse PCR primers (Bio-Rad Laboratories) were used for Actb, Ucp1, Prdm16, Cidea, Cox8b, Ppargc1a, Adipoq, Leptin, Il1b, Il4, Il6, Il10, Il13, Ifng, and Ccl2.
Immunoblotting.
scWAT, eWAT, and BAT samples were homogenized with a BDC-2002 homogenizer (Compact Digital, Caframo Laboratory Solutions, Ontario, Canada) at 100 mg tissue/300 μl of homogenization buffer (20 mM Tris·HCl pH 7.4, 150 mM NaCl, 1% IGEPAL CA-630, 20 mM sodium fluoride, 2 mM EDTA pH 8.0, 2.5 mM sodium pyrophosphate, 20 mM β-glycerophosphate, 10% glycerol with added protease inhibitors [1 mg/ml aprotinin, 1 mg/ml leupeptin, 1 mg/ml pepstatin, 6 mg/ml Pefabloc SC, and Phosphatase Inhibitor Cocktail 2 and 3 (Sigma-Aldrich, San Jose, CA)]. Samples were solubilized at 4°C for 1 h with rotation and spun at 4°C at a speed of 12,000 g for 15 min. Protein abundance was measured by bicinchoninic acid assay (Thermo Fisher Scientific, Eugene, OR). Target proteins were measured in tissue homogenates (1 mg/ml) by capillary electrophoresis using a Wes instrument (Protein Simple, Biotechne) with rabbit polyclonal antibodies for PRDM16 (1:25, cat. no. PA5-20872), UCP-1 (1:50, cat. no. PA1-24894; Thermo Fisher Scientific), or phosphatidylinositol 3-kinase p85 (1:25, cat. no. ABS-233, EMD Millipore, Burlington, MA) and with GAPDH (1:1,000, cat. no. 25778, Santa Cruz Biotechnology, Santa Cruz, CA) as a loading control. Data were quantitated using Compass Software (Protein Simple, Biotechne) with target proteins expressed relative to the loading control.
In vivo deoxy-d-2-[3H]glucose uptake.
To measure tissue-specific glucose uptake after 1 day of diet treatment, mice were fasted for 4 h (0600–1000) and injected intraperitoneally with 2 g/kg cold glucose spiked with 0.5 µCi/g deoxy-d-2-[3H]glucose (Perkin Elmer, Waltham, MA). Tissues were harvested 30 min after the injection, weighed, and snap-frozen in liquid nitrogen. Tissues were processed in 1 ml homogenizing buffer with glass on glass kontes tubes using a BDC-2002 homogenizer (Caframo LabSolutions, Caframo, Georgian Bluffs, Ontario, Canada). Fifty microliters of homogenate were counted on a scintillation counter. A bicinchoninic acid assay (Pierce, Thermo Fisher Scientific, Waltham, MA) was run on individual tissue lysates and counts per minute were made relative to tissue weight.
Statistical analysis.
Data were analyzed by a two-way analysis of variance (ANOVA) for main effects of diet and temperature except for the oral glucose tolerance test, which used a repeated measures two-way ANOVA for main effects of group and temperature. A Sidak’s multiple comparisons post hoc test was performed when significant main effect differences were detect using a P value of ≤0.01. All data are presented as means ± SE. Statistics were calculated using Graph Pad Prism version 7 (Graph Pad Software, La Jolla, CA). No differences were detected between 1 and 5 days CON at each temperature for all physiological measures (data not shown). Herein, 1-day CON data are reported.
RESULTS
Short-term thermoneutral housing combined with acute HFD increases body weight and adiposity in male mice.
Body weight was increased in male mice housed at 30°C for 8 days compared with 22°C with CON or HFD; however, these temperature-mediated differences in body weight were ameliorated by a longer (5 days) HFD exposure (Fig. 1D). Short-term TN as compared with TS housing also altered body composition. Percent fat mass increased independent of diet type (CON vs. HFD) or duration of HFD (Fig. 1E). The percent lean mass was reduced in 30°C versus 22°C with 1-day CON and HFD but similar to body weight, temperature-mediated differences in lean mass were absent with 5-day HFD. At 22°C, 5-day HFD reduced the percent lean mass compared with 1-day CON and 1-day HFD (Fig. 1F). The increase in percent fat mass with TN housing is driven, in part, by an increase in scWAT and eWAT mass with 1-day CON and 1-day HFD (Fig. 1, G and H). eWAT mass was not different between TS and TN housing with 5-day HFD (Fig. 1I). BAT mass was reduced in CON (100 ± 3 mg vs. 85 ± 3 mg), 1-day HFD (100 ± 5 mg vs. 77 ± 4 mg), and 5-day HFD (120 ± 5 mg vs. 69 ± 8 mg) with TN housing (Fig. 1G).
Short-term thermoneutral housing alters the mouse behavior and the metabolic and circadian response to HFD.
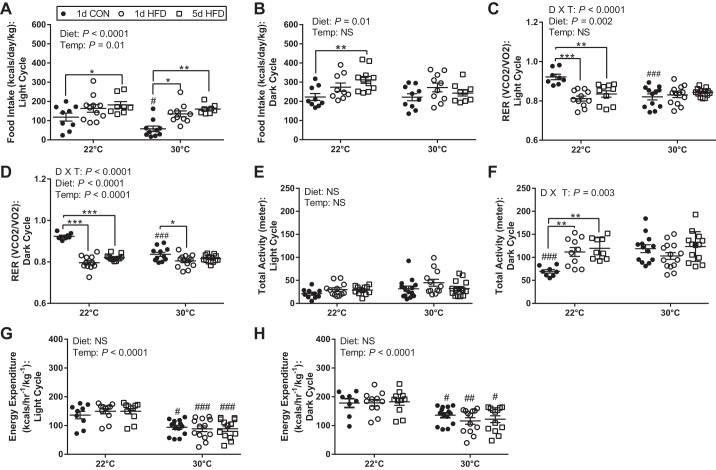
Mouse feeding behavior was changed by both diet and housing temperature. Specifically, TN suppressed food intake on 1-day CON (118 ± 21 vs. 57 ± 14 kcal) but not 1-day and 5-day HFD, during the light cycle compared with 22°C (Fig. 2A). Additionally, HFD increased light cycle food intake at both temperatures and was greater with 5-day HFD (22°C: 182 ± 18 vs. 118 ± 21 kcal; 30°C: 160 ± 9 vs. 57 ± 14 kcal) compared with 1-day CON (Fig. 2A). During the dark cycle, HFD led to a stepwise increase in daily food intake (kcal/kg) only at TS (CON: 223 ± 18 kcal; 1-day HFD: 274 ± 22 kcal; 5-day HFD: 312 ± 18 kcal) and was significantly greater after 5 days with HFD relative to CON (Fig. 2B). No difference in food intake between diet groups was observed at 30°C (Fig. 2B). There was a significant interaction of diet and housing temperature on measurements of respiratory exchange ratio (RER) in both the light and dark cycles (Fig. 2, C and D). RER was higher in mice on the 1-day CON at 22°C versus 30°C in both the light (0.92 vs. 0.82) and dark (0.92 vs. 0.84) cycle. At TS housing, RER was lower with HFD as anticipated during the light and dark cycle (Fig. 2, C and D). In contrast, RER was not different between mice fed CON versus HFD at TN housing during the light cycle, and the difference in dark cycle RER observed at 22°C with HFD was diminished at 30°C (Fig. 2, C and D). There were no differences in total activity during the light cycle by diet or temperature (Fig. 2E); however, there was a significant interaction of diet and housing temperature on measurements of total activity during the dark cycle (Fig. 2F). At 22°C, HFD (1 and 5 days) increased dark cycle activity (1-day HFD: 112 ± 10 m; 5-day HFD: 120 ± 8 m) compared with CON (70 ± 4 m). In contrast, dark cycle activity was not influenced by diet in mice housed at 30°C; activity of mice on CON was as great as mice on HFD (CON: 119 ± 8 m; 1-day HFD: 103 ± 7 m; 5-day HFD: 124 ± 9 m). There was no additive effect of HFD on activity of mice at TN (Fig. 2F). Energy expenditure (EE) was lower with 30°C versus 22°C independent of diet throughout the circadian cycle (Fig. 2, G and H).
Fig. 2.
Housing temperature (Temp) alters the metabolic phenotype of mice on control (CON) and high-fat diet (HFD). Food intake assessed during the light (A) and dark cycles (B) during 1-day CON, 1-day HFD, and 5-day HFD at 22°C (n = 10/group) and 30°C (n = 15/group). Respiratory exchange ratio (RER) assessed during the light (C) and dark cycles (D) during 1-day CON, 1-day HFD, and 5-day HFD at 22°C (n = 10/group) and 30°C (n = 15/group). Total activity assessed during the light (E) and dark (F) cycles during 1-day CON, 1-day HFD, and 5-day HFD at 22°C (n = 10/group) and 30°C (n = 15/group). Energy expenditure assessed during the light (G) and dark cycles (H) during 1-day CON, 1-day HFD, and 5-day HFD at 22°C (n = 10/group) and 30°C (n = 15/group). V̇co2, volume of carbon dioxide produced; V̇o2, volume of oxygen consumed. Data reported as means ± SE. Data were analyzed by a two-way ANOVA [diet (D) × temperature (T)] or interactions with a Sidak multiple comparison test. P values for main effects (P ≤ 0.01) are listed in each graph. #Significant findings with multiple comparison test for temperature (#P < 0.05, ##P < 0.01, ###P < 0.001, ####P 0.0001); and *significant findings with multiple comparison test for diet (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). NS, not significant.
Thermoneutral housing alters glucose metabolism and insulin sensitivity in response to an HFD.
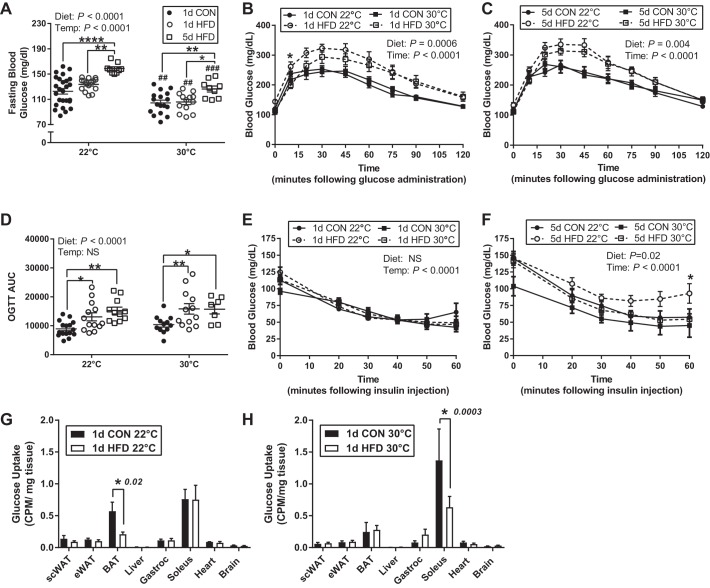
Fasting glucose increased with 5-day HFD compared with CON and 1-day HFD within each housing temperature; however, fasting glucose was lower in each diet group at 30°C housing (Fig. 3A). Glucose tolerance was reduced following 1 and 5 days of HFD feeding and was only influenced by temperature at the 10-min timepoint at 22°C following 1-day HFD (Fig. 3, B–D). There were no differences between all CON groups (data not shown) and all HFD groups (data not shown). There was no effect of diet or temperature on glucose disposal in response to an insulin bolus after 1 day; however, a 5-day HFD at 22°C but not 30°C significantly decreased insulin sensitivity (Fig. 3, E and F). Examining tissue-specific effects of temperature on glucose metabolism, we found that housing temperature shifted the distribution of glucose uptake during insulin-stimulated condition such that glucose uptake was highest in soleus muscle at 30°C whereas BAT had the greatest uptake at 22°C in mice on CON. With 1-day HFD, we found tissue-specific reductions in glucose disposal by temperature. Specifically, 1-day HFD decreased BAT glucose disposal by 63% at 22°C housing but not 30°C (Fig. 3, G and H). In contrast, at 30°C housing but not 22°C, soleus glucose disposal was reduced by 54% with 1-day HFD feeding (Fig. 3, G and H).
Fig. 3.
Short-term thermoneutral housing alters on glucose homeostasis and insulin sensitivity in response to a high-fat diet (HFD). Fasting (4 h) glucose following 1-day control (CON), 1-day HFD, and 5-day HFD treatment at standard vivarium temperature (Temp) (22°C; n = 20/group) and murine thermoneutral temperature (30°C; n = 15/group; A). Oral glucose tolerance test (OGTT) and glucose area under the curve (AUC) (D) following 1 (B) or 5 days (C) of CON and HFD treatment at standard vivarium temperature (n = 17/group) and murine thermoneutral temperature (n = 15/group). Insulin tolerance tests following 1 (E) or 5 days (F) of CON and HFD at 22°C (n = 8/group) and 30°C (n = 7/group) housing temperatures. In vivo 2-deoxy-d-[3H]glucose uptake following 1 day of CON or HFD at 22°C (n = 5/group; G) and 30°C (n = 5/group; H) housing temperatures. CPM, counts/min; eWAT, epididymal white adipose tissue; scWAT, subcutaneous white adipose tissue BAT, brown adipose tissue; gastroc, gastrocnemius. Data reported as means ± SE. Data were analyzed by a two-way ANOVA (diet × temperature) with a Sidak multiple comparison test. P values for main effects (P ≤ 0.01) are listed in each graph. #Significant findings with multiple comparison test for temperature (#P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001); and *significant findings with multiple comparison test for diet (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Thermonetural housing reduces markers of adipose tissue thermogenesis in BAT.
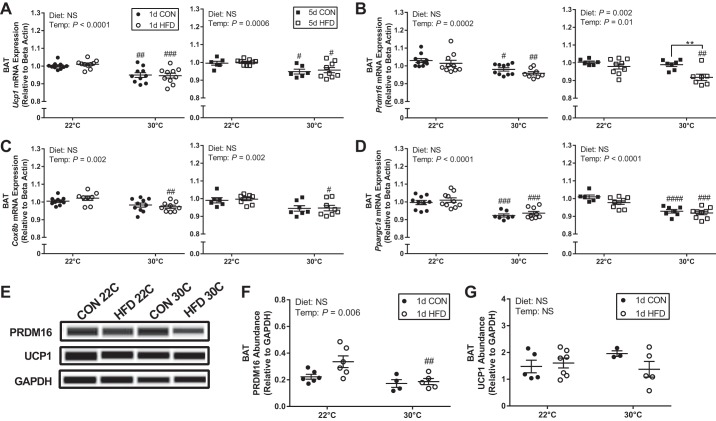
In addition to the decrease in BAT mass, TN housing also decreased gene expression of key regulators of thermogenesis. Specifically, there was a main effect of temperature to decrease Ucp1, Prdm16 (PR domain containing 16), Cox8b (cytochrome c oxidase subunit 8B), and Ppargc1a (pparg coactivator 1 α) gene expression after 8 and 12 days at 30°C compared with constant 22°C housing (Fig. 4, A–D). For Ucp1 and Ppargc1a (Fig. 4, A and D), the decrease in expression with 30°C was independent of diet type (CON vs. HFD) and time on diet (1 vs. 5 days). Prdm16 expression was decreased at 30°C after 1 day, independent of diet; however, it was only decreased with HFD and not CON with longer exposure at 30°C (Fig. 4B), suggesting a transient effect of temperature on Prdm16 expression in mice fed CON. Cox8b expression was only decreased at 30°C with HFD independent of time on diet (Fig. 4C). As these are relatively small changes in gene expression, we sought to determine whether the differences were sufficient to alter protein abundance. PRDM16 protein abundance was decreased at 30°C following 1-day HFD treatment (Fig. 4, E and F), whereas no difference was detected for UCP-1 (Fig. 4, E and G).
Fig. 4.
Short-term thermoneutral housing reduces gene expression markers of thermogenesis in brown adipose tissue (BAT). Ucp1 (A), Prdm16 (B), Cox8b (C), and Ppargc1a (D) following 1-day control (CON), 1-day high-fat diet (HFD), 5-day CON, and 5-day HFD treatment at standard vivarium temperature (22°C) and murine thermoneutral temperature (30°C). Immunoblots (E) and quantification of PRDM16 (F) and uncoupling protein 1 (UCP-1) (G) abundance in BAT. Data reported as means ± SE. Data were analyzed by a two-way ANOVA (diet × temperature) with a Sidak multiple comparison test. P values for main effects (P ≤ 0.01) are listed in each graph. #Significant findings with multiple comparison test for temperature (#P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001); and *significant findings with multiple comparison test for diet (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). NS, not significant.
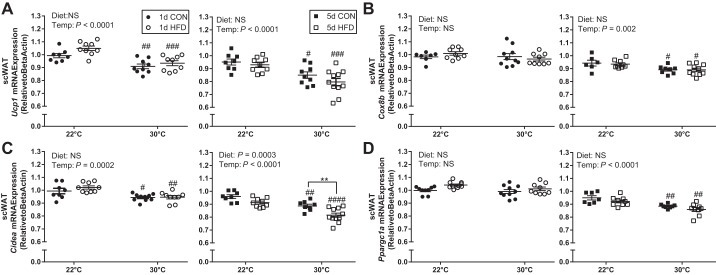
Diet and thermoneutral housing reduce some markers of adipose tissue browning in inguinal scWAT but not eWAT.
In scWAT, there was a main effect of temperature to decrease Ucp1, Cox8b, and Ppargc1a expression with TN compared with TS housing. Specifically, Ucp1 expression was decreased at 30°C compared with 22°C independent of diet type (CON vs. HFD) and time on diet (1 vs. 5 days). Cox8b (Fig. 5B), Ppargc1a (Fig. 5D), and Prdm16 (data not shown) gene expression was decreased at 30°C compared with 22°C only after longer time (12 days) in TN conditions independent of diet type. Similar to the changes in Ucp1 expression, Cidea (cell death-inducing DNA fragmentation factor, α-subunit-like a) expression was decreased at 30°C independent of diet type (CON vs. HFD) or length of time at TN conditions (8 and 12 days); additionally, 5-day HFD feeding further reduced Cidea expression compared with 5-day CON at 30°C (Fig. 5C). Thermoneutral housing did not decrease Ucp1, Prdm16, Cidea, Cox8b, or Ppargc1a gene expression in visceral eWAT tissue following 1 or 5 days of diet (data not shown).
Fig. 5.
Short-term thermoneutral housing reduces activation of browning genes in inguinal subcutaneous white adipose tissue (scWAT). Ucp1 (A), Cox8b (B), Cidea (C), and Ppargc1a (D) following 1-day control (CON), 1-day high-fat diet (HFD), 5-day CON, and 5-day HFD treatment at standard vivarium temperature (Temp) (22°C) and murine thermoneutral temperature (30°C). Data reported as mean ± SE. Data were analyzed by a two-way ANOVA (diet × temperature) with a Sidak multiple comparison test. P values for main effects (P ≤ 0.01) are listed in each graph. #Significant findings with multiple comparison test for temperature (#P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001); and *significant findings with multiple comparison test for diet (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). NS, not significant.
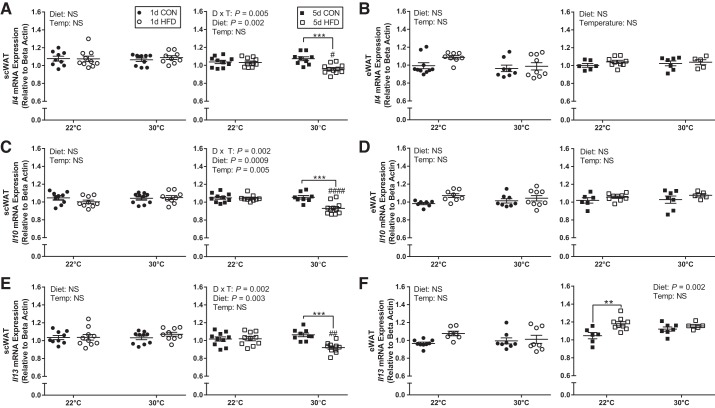
Thermal neutral housing augments the effect of HFD to reduce anti-inflammatory gene expression in inguinal scWAT.
With 1 day of HFD compared with CON, there was no change in the gene expression of interleukin (Il) 4, Il10, and Il13 anti-inflammatory markers at either temperature in both scWAT and eWAT depots (Fig. 6, A–F). However, with longer diet exposure there was a significant interaction between diet and temperature to reduce the gene expression of these key anti-inflammatory markers in scWAT. Specifically, Il4, Il10, and Il13 were reduced with 5 days of HFD feeding only at 30°C and not at 22°C (Fig. 6, A, C, D). In contrast, there was no change in the gene expression of Interleukin (Il) 4, Il10, and Il13 with temperature in eWAT following 5-day diet treatment (Fig. 6, B, D, F). Il13 expression was increased in eWAT with 5-day HFD compared with CON at 22°C but not at 30°C (Fig. 6F).
Fig. 6.
Short-term thermoneutral housing reduces inguinal subcutaneous white adipose tissue (scWAT) anti-inflammatory gene expression in response to HFD. scWAT (A) and epididymal WAT (eWAT) (B) Il4 gene expression, scWAT (C) and eWAT (D) Il10 gene expression, and scWAT (E) and eWAT (F) Il13 gene expression following 1-day control (CON), 1-day high-fat diet (HFD), 5-day CON, and 5-day HFD treatment at standard vivarium temperature (Temp) (22°C) and murine thermoneutral temperature (30°C). D × T, diet × temperature. Data reported as means ± SE. #Significant findings with multiple comparison test for temperature (#P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001); and *significant findings with multiple comparison test for diet (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
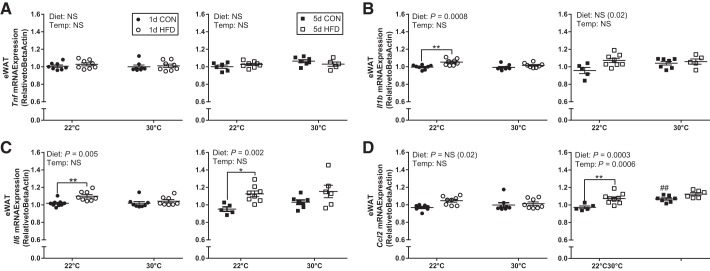
Thermal neutral housing attenuates the effect of a HFD to increase proinflammatory gene expression in eWAT.
Overall, expression of some proinflammatory genes was elevated by short-term HFD in eWAT at TS housing, but this effect was lost with TN housing. Specifically, there was no effect of diet (1 or 5 days) or temperature on Tnf (Tumor necrosis factor) expression in eWAT (Fig. 7A). There was a transient effect of HFD to increase Il1b with 1 day at 22°C but not at 30°C, which was diminished by longer exposure to HFD in eWAT (Fig. 7B). Il6 expression increased following 1 and 5 days of HFD at 22°C and not 30°C in eWAT (Fig. 7C). Finally, Ccl2 [chemokine (C-C motif) ligand 2] expression was significantly higher at 30°C compared with 22°C with longer exposure to TN housing; exposure to 5-day HFD in mice at 22°C increased Ccl2 expression to levels to mice at 30°C in eWAT (Fig. 7D). No differences were detected in Tnf, Il1b, Il6, or Ccl2 gene expression in scWAT with short-term HFD or housing temperature (data not shown).
Fig. 7.
Standard vivarium housing temperature (Temp) (22°C) but not thermoneutral housing increases epididymal white adipose tissue (eWAT) proinflammatory gene expression in response to an high-fat diet (HFD). eWAT Tnf (A), Il1b (B), Il6 (C), and Ccl2 (D) gene expression following 1-day CON, 1-day HFD, 5-day control (CON), and 5-day HFD at standard vivarium temperature (22°C) and murine thermoneutral temperature (30°C). Data reported as means ± SE. #Significant findings with multiple comparison test for temperature (#P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001); and *significant findings with multiple comparison test for diet (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
DISCUSSION
The most salient findings of this study are that TN housing altered the metabolic and cellular response to acute HFD in key pathways that have been implicated in HFD-induced insulin resistance. To our knowledge, this is the first study to assess the effect of TN housing on in vivo glucose metabolism, adipose tissue browning, and adipose tissue inflammatory gene expression after such a brief time domain (8 or 12 days total: 7-day acclimation + 1- or 5-day diet treatment).
Short-term TN housing had a significant and rapid impact on the phenotype and metabolism of the mice. Specifically, TN housing influenced food intake, activity, energy expenditure (EE), and RER resulting in altered fat accretion, adipose tissue distribution, and substrate utilization. We found that TN housing decreased food intake during the light cycle relative to mice housed at TS, particularly in mice on CON. This increase in food intake may be necessary to support the higher EE measured in TS versus TN. These observations are in agreement with previous reports (9, 14, 15) showing an inverse relationship between housing temperature and food intake in chronic conditions. For example, in a 20-wk study that utilized a similar experimental design, mice housed at 30°C had increased WAT (scWAT and eWAT) mass and decreased BAT mass relative to mice housed at 22°C (9). Our data show that these changes in adipose tissue (scWAT, eWAT, and BAT) mass in the response to housing temperature occur very rapidly with as little as an 8-day acclimation. We also found that RER was higher at 22°C housing relative to 30°C across both the light and dark cycles with CON feeding, which supports previous studies showing higher carbohydrate oxidation in mice on a standard chow diet housed at lower temperatures for longer periods of time (10, 12, 14, 15). We did not observe temperature differences in RER during HFD feeding likely reflecting the greater influence of the diet composition (i.e., higher fat) on metabolism and the overall decrease in glucose tolerance. We also found that BAT glucose uptake at 22°C during CON feeding was much higher relative to CON fed mice at 30°C. At the cellular level, increased glucose utilization by BAT at 22°C is supported by higher gene expression of Ucp1, Ppargc1a, Prdm16, and PRDM16 protein abundance, which combined promote mitochondrial metabolism that is favorable for heat generation through uncoupled respiration (4). Although 1-day exposure to 4°C has shown to be sufficient to decrease BAT UCP-1 protein abundance relative to 22°C (19), we did not see a difference in the present study with 8-day acclimation likely because of the large variability in the measurement. Taken together, these data demonstrate that short-term thermoneutral housing is sufficient to alter energy intake, adipose tissue weight, substrate utilization, and energy expenditure.
Fasting glucose is primarily regulated by hepatic glycogen stores (30) and is influenced by circulating catecholamines. Subthermoneutral stress increases sympathetic nervous system activity and increases circulating norepinephrine (9). Although we do not have data regarding norepinephrine turnover, we speculate that the increase in fasting glucose measured at TS housing may be because of elevated norepinephrine in response to subthermoneutral stress that results in increased hepatic glycogenolysis (17). After 5 days of HFD, we find that fasting glucose increased in mice housed at both 22°C and 30°C but was higher in all conditions (CON and HFD) in mice at 22°C versus 30°C. Although we observed differences by housing temperature in several factors that have been shown to influence glucose tolerance, including energy expenditure (44), BAT mass (34), and upregulation of UCP-1 in BAT and scWAT (35), no differences were detected in glucose tolerance between mice housed at 22°C and 30°C. Additionally, we found a similar decrease in glucose tolerance with 1- and 5-day HFD feeding at both housing temperatures. To our knowledge, this is the first rodent study to identify mild glucose intolerance following 1 day of HFD feeding. The magnitude of change in glucose area under the curve with 5 days of HFD and was not greater than at 1 day. Interestingly, when Cui and colleagues (9) pair-fed mice housed at 22°C to match the reduced intake of mice at 30°C, mice housed at 22°C displayed improvements in glucose tolerance and insulin sensitivity. Thus, we may have seen temperature-based differences in glucose tolerance across, if we had implemented pair-feeding.
Although systemic glucose tolerance was similar by temperature, we did find tissue-specific differences in glucose clearance indicating that the cellular mechanisms that lead to reduced glucose tolerance with HFD acutely are different depending on housing temperature. Specifically, HFD-feeding reduced insulin-mediated BAT glucose uptake in mice at 22°C but reduced soleus muscle glucose uptake in mice at 30°C. The increase in BAT glucose uptake at TS likely reflects the greater energy demand of BAT in mice housed at 22°C versus 30°C, which is reflected by the decrease in BAT mass and EE in mice housed at TN. These findings have significant implications for studies examining mechanisms that initiate insulin resistance in obesity as it suggests that mice living under conditions of chronic subthermoneutral stress respond differently to HFD challenges than those housed in TN conditions.
Expansion of scWAT, even in the face of obesity, has been suggested to promote insulin sensitivity in both rodents and humans (20, 23, 26, 31, 33, 37). Implantation of scWAT, but not visceral WAT, into the abdominal cavity of mice improves whole body metabolism (18, 40). In further support that scWAT and eWAT depots are cell autonomous, Stanford and colleagues (35) demonstrated that transplantation of scWAT, but not eWAT, from exercise-trained mice into the visceral or subcutaneous cavity of sedentary and HFD-fed mice significantly improved glucose tolerance and insulin sensitivity in recipient mice. The same study also observed an exercise-induced increase in scWAT Ucp1 and Prdm16 expression, which was not seen in eWAT (35). Moreover, inguinal scWAT has been shown to be more susceptible to browning than eWAT (2, 5, 7, 8, 16). These data agree with our findings that scWAT responds more rapidly than eWAT to subthermoneutral stress by increasing markers of browning (Ucp1, Cox8b, Cidea, and Ppargc1a). An inverse relationship between periventricular WAT browning and inflammation at 30°C housing was recently shown by Tian and colleagues (39). The study found that although inflammation augmented the development of atherosclerosis, it did not influence insulin resistance relative to 22°C. Thermoneutral housing has also been shown to exacerbate nonalcoholic fatty liver disease and the response to proinflammatory stimuli by influencing toll-like receptor 4 responsiveness (14, 36). Knockout of toll-like receptor 4 in adipose tissue has been shown to protect against whole-body insulin resistance following an acute lipid challenge (38); thus, a connection exists between housing temperature, inflammation, and whole-body glucose metabolism. We also observed subtle differences in some inflammatory gene markers following 1- and more so 5-day HFD feeding that was depot- and temperature-dependent. Specifically, there was a decrease in anti-inflammatory gene expression (Il4, Il10, and Il13) in scWAT only in mice housed at 30°C after 5-day HFD with no increase in proinflammatory gene expression. Together, these data suggest that a reduction in the anti-inflammatory cytokines in scWAT proceeds or initiates the shift to a proinflammatory state that is typically associated with obesity. In eWAT, there were again subtle increases in some proinflammatory markers (Il1b, Il6, and Ccl2) with HFD but only in mice housed at 22°C and not 30°C. The lack of an increase in eWAT inflammatory markers in mice housed at 30°C may be attributed to the overall greater eWAT and scWAT mass and perhaps greater potential for expansion. WAT expansion has been shown to reduce adipose tissue inflammation in obesity (20).
An increase in BAT mass has been proposed to be a protective mechanism against subthermoneutral stress, because of the capacity of BAT to generate heat through uncoupled respiration, via UCP-1 (4). In a mass-dependent manner, BAT has also been associated with an increase in energy expenditure and improvements in insulin sensitivity (35). In contrast, loss of UCP-1 exacerbates HFD-induced impairments in glucose metabolism (45) and results in obesity even in chow-fed rodents (13). To our knowledge, we are the first to identify that changes in energy expenditure, BAT mass, and downregulation of BAT browning genes occur as rapidly as within 8 days of TN housing. BAT has also been proposed to be a site of diet-induced thermogenesis (3), as a potential protective mechanism to enhance energy expenditure in the face of long-term abundant energy intake. Here, we observed reduced BAT Ucp1 expression, decreased EE, and increased adiposity in mice after short-term housing at 30°C relative to 22°C that was associated with greater insulin resistance when challenged with a 5-day HFD in agreement with previous findings conducted over a longer time course.
Perspectives and Significance
Herein, we have provided a detailed analysis of the acute metabolic and behavioral responses of mice housed at standard vivarium (22°C) and thermoneutral (30°C) temperatures, while consuming an HFD (45% calories from fat) or CON (10% calories from fat diet). We found that 1 day of HFD was sufficient to impair glucose tolerance despite no evidence of a consistent parallel increase in immune response. Although housing temperature did not influence HFD induced glucose tolerance, the tissue-specific responses resulting in reduced glucose disposal were altered by housing temperature. Furthermore, short-term (8–12 days) thermoneutral housing decreases energy expenditure, BAT mass, fasting blood glucose and scWAT/BAT “browning” genes and scWAT anti-inflammatory genes. Considering the aforementioned variables have been implicated in mechanisms that initiate insulin resistance in obesity, it is important that housing conditions that alter the physiology of the model system be considered in interpretation of metabolic data.
GRANTS
Research reported in this publication was supported by National Institute of Diabetes and Digestive and Kidney Diseases under Award Number R01-DK-095926 (to C. E. McCurdy). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the Eugene Evonuk Memorial Graduate Fellowship in Environmental and Stress Physiology (to Z. S. Clayton). Parts of this study were presented in abstract form at Experimental Biology 2017, Chicago, Illinois, 22–26 April 2017.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.S.C. conceived and designed research; Z.S.C. performed experiments; Z.S.C. and C.E.M. analyzed data; Z.S.C. and C.E.M. interpreted results of experiments; Z.S.C. and C.E.M. prepared figures; Z.S.C. and C.E.M. drafted manuscript; Z.S.C. and C.E.M. edited and revised manuscript; Z.S.C. and C.E.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We kindly acknowledge Dr. Byron Hetrick and William Campodonico-Burnett for technical assistance. We also acknowledge University of Oregon Terrestrial Animal Care services for mouse husbandry.
REFERENCES
- 1.Acosta-Martínez M, Luo J, Elias C, Wolfe A, Levine JE. Male-biased effects of gonadotropin-releasing hormone neuron-specific deletion of the phosphoinositide 3-kinase regulatory subunit p85alpha on the reproductive axis. Endocrinology 150: 4203–4212, 2009. doi: 10.1210/en.2008-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298: E1244–E1253, 2010. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 3.Bonet ML, Mercader J, Palou A. A nutritional perspective on UCP1-dependent thermogenesis. Biochimie 134: 99–117, 2017. doi: 10.1016/j.biochi.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 5.Collins S, Daniel KW, Petro AE, Surwit RS. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology 138: 405–413, 1997. doi: 10.1210/endo.138.1.4829. [DOI] [PubMed] [Google Scholar]
- 6.Cornier MA, Bergman BC, Bessesen DH. The effects of short-term overfeeding on insulin action in lean and reduced-obese individuals. Metabolism 55: 1207–1214, 2006. doi: 10.1016/j.metabol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Cousin B, Casteilla L, Dani C, Muzzin P, Revelli JP, Penicaud L. Adipose tissues from various anatomical sites are characterized by different patterns of gene expression and regulation. Biochem J 292: 873–876, 1993. doi: 10.1042/bj2920873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 103: 931–942, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Cui X, Nguyen NL, Zarebidaki E, Cao Q, Li F, Zha L, Bartness T, Shi H, Xue B. Thermoneutrality decreases thermogenic program and promotes adiposity in high-fat diet-fed mice. Physiol Rep 4: e12799, 2016. doi: 10.14814/phy2.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David JM, Chatziioannou AF, Taschereau R, Wang H, Stout DB. The hidden cost of housing practices: using noninvasive imaging to quantify the metabolic demands of chronic cold stress of laboratory mice. Comp Med 63: 386–391, 2013. [PMC free article] [PubMed] [Google Scholar]
- 11.DeRuisseau LR, Parsons AD, Overton JM. Adaptive thermogenesis is intact in B6 and A/J mice studied at thermoneutrality. Metabolism 53: 1417–1423, 2004. doi: 10.1016/j.metabol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Even PC, Blais A. Increased cost of motor activity and heat transfer between non-shivering thermogenesis, motor activity, and thermic effect of feeding in mice housed at room temperature - implications in pre-clinical studies. Front Nutr 3: 43, 2016. doi: 10.3389/fnut.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209, 2009. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Graspeuntner S, Cappelletti M, Wu D, Mukherjee R, Chan CC, Lawson MJ, Klarquist J, Sünderhauf A, Softic S, Kahn CR, Stemmer K, Iwakura Y, Aronow BJ, Karns R, Steinbrecher KA, Karp CL, Sheridan R, Shanmukhappa SK, Reynaud D, Haslam DB, Sina C, Rupp J, Hogan SP, Divanovic S. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med 23: 829–838, 2017. [Erratum in Nat Med 23: 1241, 2017.] 10.1038/nm.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon CJ. The mouse thermoregulatory system: its impact on translating biomedical data to humans. Physiol Behav 179: 55–66, 2017. doi: 10.1016/j.physbeh.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 102: 412–420, 1998. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann H, Beckh K, Jungermann K. Direct control of glycogen metabolism in the perfused rat liver by the sympathetic innervation. Eur J Biochem 123: 521–526, 1982. doi: 10.1111/j.1432-1033.1982.tb06562.x. [DOI] [PubMed] [Google Scholar]
- 18.Hocking SL, Chisholm DJ, James DE. Studies of regional adipose transplantation reveal a unique and beneficial interaction between subcutaneous adipose tissue and the intra-abdominal compartment. Diabetologia 51: 900–902, 2008. doi: 10.1007/s00125-008-0969-0. [DOI] [PubMed] [Google Scholar]
- 19.Jankovic A, Golic I, Markelic M, Stancic A, Otasevic V, Buzadzic B, Korac A, Korac B. Two key temporally distinguishable molecular and cellular components of white adipose tissue browning during cold acclimation. J Physiol 593: 3267–3280, 2015. doi: 10.1113/JP270805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J-Y, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O’Brien WR, Littman DR, Shulman GI. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114: 823–827, 2004. doi: 10.1172/JCI200422230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108: 437–446, 2001. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 350: 2549–2557, 2004. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 24.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60: 2474–2483, 2011. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S, Cantley LC. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol 25: 9491–9502, 2005. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 87: 2784–2791, 2002. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 27.National Institutes of Health The Guide for the Care and Use of Laboratory Animals. Washington, DC: National Research Council, 2016. [Google Scholar]
- 28.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab 20: 396–407, 2014. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 18: 363–374, 2012. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 30.Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 13: 572–587, 2017. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 32: 1068–1075, 2009. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 127: 1–4, 2017. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, De Rekeneire N, Kanaya AM, Newman AB, Tylavsky FA, Seidell JC; Health ABC Study . Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 48: 301–308, 2005. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 34.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, Tseng YH, Goodyear LJ. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64: 2002–2014, 2015. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stemmer K, Kotzbeck P, Zani F, Bauer M, Neff C, Müller TD, Pfluger PT, Seeley RJ, Divanovic S. Thermoneutral housing is a critical factor for immune function and diet-induced obesity in C57BL/6 nude mice. Int J Obes 39: 791–797, 2015. doi: 10.1038/ijo.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tankó LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation 107: 1626–1631, 2003. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 38.Tao C, Holland WL, Wang QA, Shao M, Jia L, Sun K, Lin X, Kuo YC, Johnson JA, Gordillo R, Elmquist JK, Scherer PE. Short-term versus long-term effects of adipocyte toll-like receptor 4 activation on insulin resistance in male mice. Endocrinology 158: 1260–1270, 2017. doi: 10.1210/en.2017-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, Tangirala RK, Tontonoz P, Chawla A. Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab 23: 165–178, 2016. doi: 10.1016/j.cmet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7: 410–420, 2008. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 42.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes 50: 2786–2791, 2001. doi: 10.2337/diabetes.50.12.2786. [DOI] [PubMed] [Google Scholar]
- 44.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO; Washington University School of Medicine CALERIE Group . Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 84: 1033–1042, 2006. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winn NC, Vieira-Potter VJ, Gastecki ML, Welly RJ, Scroggins RJ, Zidon TM, Gaines TL, Woodford ML, Karasseva NG, Kanaley JA, Sacks HS, Padilla J. Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. Am J Physiol Regul Integr Comp Physiol 312: R74–R84, 2017. doi: 10.1152/ajpregu.00425.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]