Abstract
High dietary sodium intake has been linked to alterations in neurally mediated cardiovascular function, but the effects of high sodium on cardiovagal baroreflex sensitivity (cBRS) in healthy adults are unknown. The purpose of this study was to determine whether high dietary sodium alters cBRS and heart rate variability (HRV) and whether acute intravenous sodium loading similarly alters cBRS and HRV. High dietary sodium (300 mmol/day, 7 days) was compared with low dietary sodium (20 mmol/day, 7 days; randomized) in 14 participants (38 ± 4 yr old, 23 ± 1 kg/m2 body mass index, 7 women). Acute sodium loading was achieved via a 23-min intravenous hypertonic saline infusion (HSI) in 14 participants (22 ± 1 yr old, 23 ± 1 kg/m2 body mass index, 7 women). During both protocols, participants were supine for 5 min during measurement of beat-to-beat blood pressure (photoplethysmography) and R-R interval (ECG). cBRS was evaluated using the sequence method. Root mean square of successive differences in R-R interval (RMSSD) was used as an index of HRV. Serum sodium (137.4 ± 0.7 vs. 139.9 ± 0.5 meq/l, P < 0.05), plasma osmolality (285 ± 1 vs. 289 ± 1 mosmol/kgH2O, P < 0.05), cBRS (18 ± 2 vs. 26 ± 3 ms/mmHg, P < 0.05), and RMSSD (62 ± 6 vs. 79 ± 10 ms, P < 0.05) were increased following high-sodium diet intake compared with low-sodium diet intake. HSI increased serum sodium (138.1 ± 0.4 vs. 141.1 ± 0.5 meq/l, P < 0.05) and plasma osmolality (286 ± 1 vs. 290 ± 1 mosmol/kgH2O, P < 0.05) but did not change cBRS (26 ± 5 vs. 25 ± 3 ms/mmHg, P = 0.73) and RMSSD (63 ± 9 vs. 63 ± 8 ms, P = 0.99). These data suggest that alterations in dietary sodium intake alter cBRS and HRV but that acute intravenous sodium loading does not alter these indexes of autonomic cardiovascular regulation.
Keywords: baroreflex sensitivity, cardiovagal, heart rate variability, high-salt diet, hypertonic saline, plasma osmolality, sequence method, serum sodium
INTRODUCTION
There is evidence that increased dietary sodium intake alters neurally mediated control of the cardiovascular system. Data from rats indicate that elevated dietary sodium intake alters cardiovascular control, as documented by increases in blood pressure (BP) variability (43). This has potential clinical implications, because increased BP variability is associated with development of end-organ damage and may be a predictor of future cardiovascular events (39). Furthermore, a number of studies indicate that reflexes evoked from the rostral ventrolateral medulla (1–3, 29) and via the baroreceptors (24) are altered following excess dietary sodium intake. Rats fed high-sodium (HS) diets demonstrate exaggerated sympathetic nerve activity (SNA) and BP responses to stimulation of the aortic depressor nerve or vagal afferents (43), volume expansion (43), sciatic nerve stimulation (43), and exercise (46) compared with rats fed low-sodium (LS) diets. Additionally, increased dietary sodium intake enhances the sensitivity of baroreflex-mediated changes in SNA in animals (21, 23, 38) and humans (24). Reflex control of heart rate (HR) via the baroreflex, however, occurs via the parasympathetic branch of the autonomic nervous system and, therefore, may differ from control of SNA (19). It is not known if high dietary sodium intake affects cardiovagal baroreflex sensitivity (cBRS) in normotensive adults.
One way in which high dietary sodium may influence autonomic cardiovascular regulation is through increases in serum sodium and plasma osmolality acting on the central nervous system. Intracerebroventricular hypertonic saline infusion (HSI) in rats has recently been shown to increase discharge from the organum vasculosum of the lamina terminalis, leading to increases in sympathetic activity and arterial BP (31). In humans, acute increases in serum sodium and plasma osmolality have been shown to increase baroreflex control of sympathetic activity (13, 45), but not cBRS (13).
The primary purpose of this study was to determine the effects of 7 days of high dietary sodium intake on cBRS. Additionally, we evaluated HR variability (HRV) as a secondary index of cardiac autonomic function. We hypothesized that high dietary sodium intake would increase cBRS and HRV. Because of the potential for HS diets to increase serum sodium and plasma osmolality (18, 27), we also used a short, intravenous infusion of 3% NaCl (HSI) to assess cBRS and HRV before and after an acute increase in serum sodium and plasma osmolality. Comparison of the responses to the acute infusions with the responses to the 7-day diets was intended to provide additional information on the time course of the responses. We hypothesized that, unlike 7 days of HS diet intake, a brief increase in serum sodium and plasma osmolality, induced via intravenous HSI, would not be sufficient to increase cBRS or HRV.
METHODS
Subjects
The Institutional Review Board at the University of Delaware approved the protocols for these studies, which were performed in accordance with the Declaration of Helsinki. A total of 28 participants completed the studies: 14 in study 1 and 14 in study 2. No participants volunteered for both studies. All participants provided written informed consent before enrollment in the study. During an initial screening visit for each study, participants completed a medical history questionnaire, a 12-lead electrocardiogram (ECG) was performed, and resting brachial BP was measured (Dash 2000, GE Medical Systems, Milwaukee, WI). Height, weight, and a fasted blood sample were obtained. All 28 participants were healthy adults (20–59 yr old), with resting systolic BP (SBP) <140 mmHg and diastolic BP (DBP) <90 mmHg. Study participants were not pregnant and were free of any known cardiovascular, metabolic, neurological, renal, or pulmonary disease. Participants were nonobese [<30 kg/m2 body mass index (BMI)] and did not use nicotine products.
Experimental Measures
Beat-to-beat BP was measured using a photoplethysmography device (Finometer, Finapres Medical Systems, Enschede, The Netherlands) calibrated to brachial BPs according to the manufacturer’s recommended calibration procedures. Brachial BPs were measured using an automated oscillometric sphygmomanometer (Dash 2000, GE Medical Systems) to verify beat-to-beat BP. Simultaneously, HR was recorded continuously using a single-lead ECG (Dash 2000, GE Medical Systems). Respiratory excursions were recorded via a strain gauge pneumograph (Pneumotrace, UFI) placed around the abdomen.
After instrumentation, participants rested quietly in a dimly lit, temperature-controlled room for 5 min, during which beat-to-beat BP, HR, and respiration data were collected continuously at 1,000 Hz using data-acquisition software (LabChart 7, ADInstruments, Colorado Springs, CO) and stored on a personal computer for offline analysis.
Study 1: High Dietary Sodium Intake
Study 1 participants are a subset of the participants from a large controlled feeding study described previously (9). Beat-to-beat BP was recorded, and the participants were found to have salt-resistant BP, defined as a change in ambulatory MAP of <5 mmHg from LS to HS intake (18, 25, 34), and adequate baroreflex sequences (see below) for inclusion in the present study. Participants completed a 21-day controlled feeding study. Participants first completed a standardized 7-day run-in (100 mmol sodium/day) diet. Immediately following day 7, participants completed a 7-day LS (20 mmol sodium/day) and a 7-day HS (300 mmol sodium/day) diet, in random order. Dietary potassium was not different among the three diets (70 mmol potassium/day). A registered dietitian prepared all food. All diets were designed to be eucaloric; therefore, energy content was appropriately adjusted using the Mifflin-St. Jeor equation to ensure that the participants maintained a constant body weight for the duration of the study (22). The macronutrient content comprised ~50% carbohydrates, ~30% fats, and ~20% protein. Participants were instructed to consume all provided food. Because of the large differences in dietary sodium content, it was not possible to blind the participants to the condition. All analyses were performed by an investigator who was unaware of the dietary condition.
During the final 24 h of each diet preceding the study visit (see below), participants collected their urine and underwent ambulatory BP monitoring. Urine volume, collection time, electrolytes (EasyElectrolyte Analyzer, Medica, Bedford, MA), and osmolality (3D3 osmometer, Advanced Instruments, Norwood, MA) were recorded. Sodium, chloride, and potassium excretion was determined. Ambulatory BP (model 90207, Spacelabs Medical, Issaquah, WA) was measured every 20 min during waking hours and every 30 min during sleep.
On the final day of each diet, participants reported to the laboratory for testing. Upon arrival, participants rested in the supine position, and a catheter was placed in the antecubital space for collection of venous blood samples before BP and HR recording. Additionally, a 24-h urine sample was collected and assessed on the final day of each diet.
Participants were instructed to abstain from caffeine, alcohol, and exercise for 24 h and to fast for ≥4 h before their study visit. They reported to the laboratory at the same time of day (±1 h) for each study visit and were outfitted with the required equipment for collection of beat-to-beat BP, brachial BP, HR, and respiratory excursions, as described above. For determination of cBRS and HRV, participants rested quietly in a supine position in a dimly lit, temperature-controlled room for 5 min, during which beat-to-beat BP, HR, and respiration data were collected continuously.
Study 2. Acute Sodium Loading
Participants from study 2 are a subset of 18 participants in a recently published study (10); cBRS and HRV analyses in these participants have not been previously published. Of the 18 participants previously described (10), 14 had adequate baroreflex sequences (detailed below) for inclusion in the present study. Consistent with previous studies examining acute increases in serum sodium, participants in study 2 received a 23-min HSI (3% NaCl) (26). For 3 days before testing, participants were asked to consume a run-in diet (100 mmol sodium/day), maintain ad libitum hydration, and keep a food log. After completion of the run-in diet, participants reported to the Cardiovascular Physiology Laboratory for acute sodium manipulation and testing. Participants were instructed to abstain from caffeine, alcohol, and exercise for 24 h and to fast for ≥6 h before their study visit.
Upon arrival, participants rested in the supine position, and one catheter was placed in the antecubital space of the dominant arm for collection of venous blood samples. A second catheter was placed in the antecubital space of the nondominant arm for saline infusion. Participants were outfitted with the required equipment for collection of beat-to-beat BP, brachial BP, HR, and respiratory excursion data.
After instrumentation and ≥30 min of supine rest, venous blood samples were collected. Participants then rested quietly in a supine position in a dimly lit, temperature-controlled room for 5 min, during which beat-to-beat BP, HR, and respiration data were collected continuously at 1,000 Hz using data-acquisition software (LabChart 7, ADInstruments). After the baseline period, HSI was administered at 0.15 ml·kg−1·min−1 for 23 min. Immediately after HSI, participants rested again for 5 min under conditions similar to those described above, and beat-to-beat BP, HR and respiration data were collected continuously.
Blood Analysis
Venous blood samples were analyzed for electrolyte concentration (EasyElectrolyte Analyzer, Medica) and osmolality (3D3 osmometer, Advanced Instruments, Norwood, MA) during each sodium manipulation. Hemoglobin (Hb 201+, Hemocue, Lake Forest, CA) and hematocrit (precalibrated Clay Adams Readacrit centrifuge, Becton Dickinson, Sparks, MD) were analyzed from whole blood samples. Plasma volume expansion (expressed as a percentage) was calculated using the following equation (17): {[100 × (Hb1/Hb2)] × [1 − (Hct2/100)]/[1 − (Hct1/100)]} − 100, where Hb1 represents preinfusion or LS hemoglobin, Hb2 represents postinfusion or HS hemoglobin, Hct1 represents preinfusion or LS hematocrit, and Hct2 represents postinfusion or HS hematocrit.
Data Analysis
Spontaneous cBRS.
Beat-to-beat time series of SBP and R-R interval were analyzed using the sequence method for estimating spontaneous cBRS (HemoLab version 8.9, Harald Stauss Scientific, Iowa City, IA). A detailed description of this method has been published previously (8). Briefly, sequences of four or more consecutive cardiac cycles in which SBP and R-R interval change in the same direction were identified as baroreflex sequences. Sequences were detected only when the variation in R-R interval was >0.5 ms and SBP changes were >1 mmHg. A linear regression was applied to each individual sequence, and only those sequences in which R2 was >0.80 were accepted. Values of cBRS were accepted when the number of sequences was ≥3 for both up and down sequences. The slopes of those individual linear regressions were then calculated and averaged for a measure of spontaneous cBRS. cBRS was determined for all sequences combined and also separately for up (increase in both SBP and R-R interval) and down (decrease in both SBP and R-R interval) sequences.
HR variability.
R-R intervals from the ECG recordings were analyzed using Kubios HRV software (version 2.2, Biosignal Analysis and Medical Imaging Group, Kuopio, Finland). Time domain HRV was determined by the SD of the normal-to-normal R-R intervals (SDNN) and the root mean square of successive differences in R-R interval (RMSSD). SDNN is considered to be an estimate of overall HRV, and RMSSD is an estimate of short-term components of HRV (and, therefore, an index of parasympathetic modulation of HR) (44). Power spectral analysis using fast Fourier transformation was performed in the high-frequency (HF) range (0.15–0.4 Hz). Similar to RMSSD, HF power predominantly represents parasympathetic activity.
Statistical Analysis
All data are presented as means ± SE. Data were analyzed using Prism 5 (GraphPad Software, La Jolla, CA), and significance was set a priori at P < 0.05. Baseline screening anthropometrics and biochemical parameters were compared between the acute and chronic study participants by unpaired, two-tailed t-tests. Normality of distribution for continuous variables was checked qualitatively via histograms as well as quantitatively using the Shapiro-Wilk test. Normally distributed biochemical, cBRS, and HRV data were compared using paired, two-tailed t-tests for the acute and chronic arms of the study. Because HF power was not normally distributed, differences were compared using Wilcoxon’s matched-pairs signed-rank test.
RESULTS
Baseline Characteristics
Baseline demographic, anthropometric, and biochemical parameters for studies 1 and 2 are displayed in Table 1. Participants in both studies had normal BP and normal BMI. In both groups, screening serum electrolytes were normal.
Table 1.
Participant screening characteristics
| Baseline Demographic Data | Study 1 | Study 2 |
|---|---|---|
| n (women) | 14 (7) | 14 (7) |
| Age, yr | 38 ± 4 | 22 ± 1 |
| Height, cm | 170.7 ± 2.6 | 171.6 ± 2.4 |
| Mass, kg | 68.3 ± 2.6 | 69.1 ± 3.1 |
| BMI, kg/m2 | 23.3 ± 0.6 | 23.3 ± 0.7 |
| SBP, mmHg | 116 ± 2 | 113 ± 4 |
| DBP, mmHg | 71 ± 2 | 68 ± 2 |
| MAP, mmHg | 86 ± 2 | 83 ± 3 |
| Serum sodium, mmol/l | 139.9 ± 0.3 | 139.7 ± 0.3 |
| Serum potassium, mmol/l | 4.49 ± 0.12 | 4.28 ± 0.06 |
| Serum chloride, mmol/l | 104.6 ± 0.6 | 104.4 ± 0.4 |
Values are means ± SE. BMI, body mass index; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
Study 1
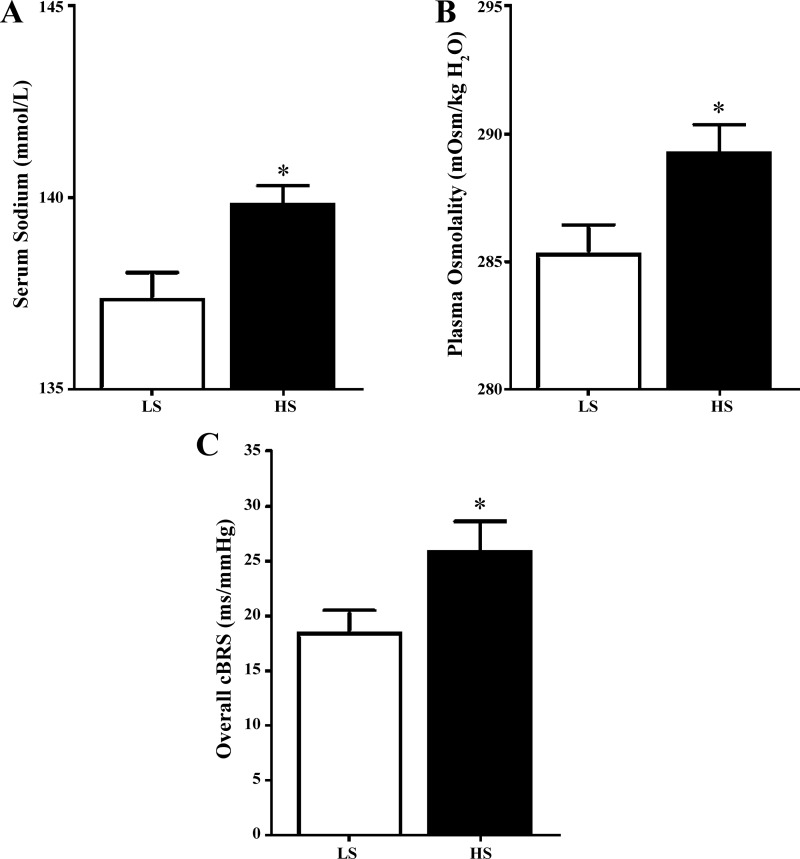
After the run-in diet, 24-h sodium excretion was 61.5 ± 6.3 mmol/24 h, serum sodium was 138.2 ± 0.6 mmol/l, and plasma osmolality was 286.5 ± 1.0 mosmol/kgH2O. Twenty-four-hour sodium excretion was higher during the HS than LS diet (197.6 ± 10.8 vs. 21.0 ± 3.7 mmol/24 h, P < 0.05). Similarly, serum sodium (Fig. 1A) and plasma osmolality (Fig. 1B) were higher after the HS than LS diet. Serum chloride was also increased (Table 2; P < 0.05) following the HS diet, but serum potassium was similar between conditions. Hemoglobin and hematocrit were lower following the HS than LS diet (Table 2; P < 0.05), indicating a plasma volume expansion of 6.4 ± 2.0%. SBP, DBP, and MAP were not different between diets. HR was significantly lower after the HS than LS diet (P < 0.05). Overall cBRS was analyzed in 12 of 14 participants following the run-in diet and was 20.6 ± 2.7 ms/mmHg (all participants completed the run-in diet, but adequate baroreflex sequences were only available for 12 of the 14 participants). Overall cBRS was higher on day 7 of the HS diet than day 7 of the LS diet (Fig. 1C; P < 0.05). Figure 2 demonstrates the effect of HS and LS diets relative to the recommended sodium run-in diet. cBRS of down sequences was increased on the HS compared with the LS diet (P = 0.03), while up-sequence cBRS tended to be increased on the HS diet (P = 0.12). RMSSD was increased following the HS diet (Table 3; P < 0.05), while SDNN and HF power were not different at the end of the HS diet compared with the LS diet (P = 0.23 and P = 0.24, respectively).
Fig. 1.
Effects of dietary sodium manipulation on serum sodium, plasma osmolality, and cardiovagal baroreflex sensitivity (cBRS). A: serum sodium was significantly increased from day 7 of the low-sodium (LS) compared with the high-sodium (HS) diet. B: plasma osmolality was significantly increased from day 7 of the LS compared with the HS diet. C: overall cBRS (all sequences) was significantly increased at the end of 7 days of the HS compared with the LS diet. Values are means ± SE (n = 14). *P < 0.05.
Table 2.
Effects of altered dietary sodium intake
| Run-In | Low Sodium | High Sodium | |
|---|---|---|---|
| Biological variables | |||
| Serum potassium, mmol/l | 4.00 ± 0.14 | 3.94 ± 0.12 | 4.17 ± 0.15 |
| Serum chloride, mmol/l | 105.3 ± 0.6 | 102.3 ± 0.6 | 106.6 ± 0.8* |
| Hemoglobin, g/dl | 13.3 ± 0.3 | 13.8 ± 0.3 | 12.8 ± 0.5* |
| Hematocrit, % | 40.9 ± 0.7 | 42.3 ± 0.7 | 39.8 ± 1.4* |
| SBP, mmHg | 113 ± 1 | 113 ± 2 | 113 ± 2 |
| DBP, mmHg | 70 ± 1 | 70 ± 2 | 67 ± 1 |
| MAP, mmHg | 84 ± 1 | 84 ± 2 | 83 ± 2 |
| HR, beats/min | 59.7 ± 2.3 | 62.2 ± 1.7 | 58.0 ± 2.6* |
| Respiration rate, min−1 | 15.6 ± 0.9 | 15.5 ± 1.0 | 15.9 ± 0.8 |
| cBRS | |||
| Up sequences, ms/mmHg | 20.0 ± 4.0 | 17.6 ± 2.4 | 24.6 ± 3.7 |
| Down sequences, ms/mmHg | 21.0 ± 2.3 | 18.3 ± 2.1 | 25.5 ± 3.8* |
| HRV | |||
| SDNN, ms | 74.6 ± 8.3 | 69.1 ± 5.4 | 75.3 ± 7.7 |
| RMSSD, ms | 68.7 ± 8.6 | 61.9 ± 5.7 | 79.0 ± 10.1* |
| HF power, ms2 | 1,301 ± 318 | 1,244 ± 370 | 1,645 ± 467 |
Values are means ± SE. cBRS, cardiovagal baroreflex sensitivity; DBP, diastolic blood pressure; HF, high frequency; HR, heart rate; HRV, HR variability; MAP, mean arterial pressure; RMSSD, root mean square of successive differences in R-R interval; SBP, systolic blood pressure; SDNN, SD of normal-to-normal R-R intervals.
Significantly different from low sodium (P < 0.05).
Fig. 2.

Effects of low-sodium (LS) and high-sodium (HS) diets relative to a recommended sodium run-in diet. Overall cardiovagal baroreflex sensitivity (cBRS, all sequences) tends to demonstrate differential responses with alterations in dietary sodium intake (P = 0.10). Values are means ± SE (n = 12 participants).
Table 3.
Effects of acute sodium loading
| Preinfusion | Postinfusion | |
|---|---|---|
| Biological variables | ||
| Serum potassium, mmol/l | 3.95 ± 0.11 | 4.01 ± 0.15 |
| Serum chloride, mmol/l | 104.2 ± 1.0 | 108.0 ± 0.8* |
| Hemoglobin, g/dl | 12.8 ± 0.5 | 12.6 ± 0.5 |
| Hematocrit, % | 39.6 ± 1.5 | 38.1 ± 1.5* |
| SBP, mmHg | 112 ± 3 | 116 ± 3* |
| DBP, mmHg | 65 ± 2 | 64 ± 2 |
| MAP, mmHg | 79 ± 3 | 81 ± 2 |
| HR, beats/min | 57 ± 2 | 59 ± 2* |
| Respiration rate, min−1 | 14.5 ± 1.0 | 14.9 ± 0.7 |
| cBRS | ||
| Up sequences, ms/mmHg | 23.7 ± 4.2 | 22.0 ± 62.7 |
| Down sequences, ms/mmHg | 27.0 ± 4.7 | 28.6 ± 4.2 |
| HRV | ||
| SDNN, ms | 66.6 ± 6.3 | 73.6 ± 6.4 |
| RMSSD, ms | 63.2 ± 8.9 | 63.2 ± 8.0 |
| HF power, ms2 | 1,614 ± 472 | 1,569 ± 425 |
Values are means ± SE. cBRS, cardiovagal baroreflex sensitivity; DBP, diastolic blood pressure; HF, high frequency; HR, heart rate; HRV, HR variability; MAP, mean arterial pressure; RMSSD, root mean square of successive differences in R-R interval; SBP, systolic blood pressure; SDNN, SD of normal-to-normal R-R intervals.
P < 0.05.
Study 2
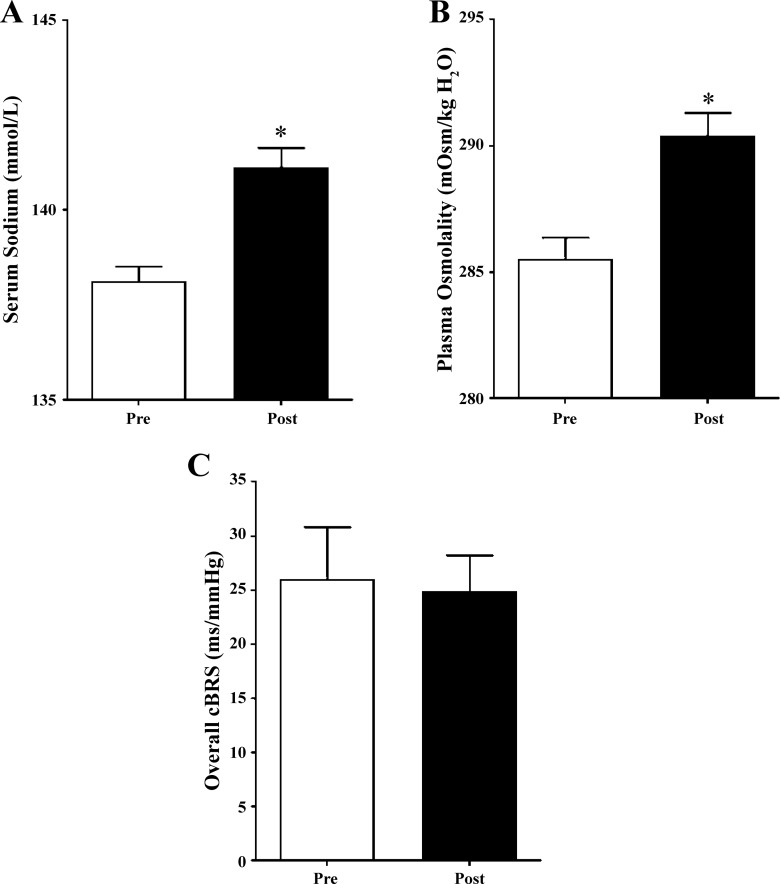
Participants received 268 ± 12 ml of hypertonic saline during the 23-min HSI, which increased serum sodium (Fig. 3A) and plasma osmolality (Fig. 3B). Serum chloride was also increased following HSI (Table 3; P < 0.05), but serum potassium remained unchanged. Hemoglobin was similar before and after the infusion; however, hematocrit was reduced (Table 3; P < 0.05), indicating a plasma volume expansion of 6.2 ± 3.1%. SBP was modestly increased (P < 0.05), but DBP and MAP were not different, after HSI. HR was higher following the infusion (P = 0.01). Overall cBRS was not different following HSI (Fig. 3C; P = 0.73). Table 2 displays measures of cBRS and HRV. There were no differences in cBRS of up (P = 0.57) or down (P = 0.94) sequences. No measure of HRV was different following acute HSI.
Fig. 3.
Effects of acute sodium manipulation on serum sodium, plasma osmolality, and cardiovagal baroreflex sensitivity (cBRS). A: serum sodium was significantly increased from preinfusion (Pre) to postinfusion (Post) of hypertonic saline. B: plasma osmolality was significantly increased from pre- to postinfusion of hypertonic saline. C: overall cBRS (all sequences) was not different between pre- and postinfusion (P = 0.73). Values are means ± SE (n = 14 participants). *P < 0.05.
DISCUSSION
The novel finding of the present study is that alterations in dietary sodium intake affect cBRS. Few studies have examined the influence of excess dietary sodium intake on cBRS in healthy young adults with salt-resistant BP. Our data also suggest that dietary sodium intake alters RMSSD, which is similar to previous findings in normotensive subjects with salt-sensitive BP (35). These data build on animal studies suggesting that chronic HS intake affects neurally mediated cardiovascular control. We also found that acute changes in plasma osmolality and serum sodium achieved via HSI are not sufficient to alter cBRS or HRV. Consistent with this finding, Charkoudian et al. (13) found no effect of acute increases in plasma osmolality on cBRS. Collectively, these studies suggest that dietary sodium intake, but not acute intravenous sodium loading, increases cBRS and RMSSD, a measure of parasympathetic activity.
The present study assessed alterations in cBRS and HRV following modest increases in plasma osmolality (~3–5 mosmol/kgH2O) and serum sodium (~2.5 mmol/l). The magnitudes of the diet- and HSI-induced increases in plasma osmolality were similar to those observed in previous human studies (13, 14). Elevations of this magnitude represent physiologically relevant changes that may occur with a HS diet (as demonstrated here). For example, He et al. report plasma sodium concentrations ~3.0 mmol/l higher when individuals consume a ~350 mmol sodium/day diet compared with a ~10–20 mmol sodium/day diet (27, 28).
Changes in plasma osmolality greater than those observed in the present study have been used in animal models to investigate the relation between acute changes in plasma osmolality and cBRS. Very large increases in plasma osmolality (~15–20 mosmol/kgH2O) have been achieved via a HSI-induced decrease in cBRS (5–7) in rats. Changes in plasma osmolality of this magnitude may be achievable in humans under conditions of severe dehydration; however, the effects on cBRS remain unknown.
Our focus was on normotensive adults. However, several reports have demonstrated that excess dietary sodium increases both sympathetic baroreflex sensitivity [i.e., the relation between muscle SNA and DBP (sBRS)] (24) and cBRS (16, 24) in adults with hypertension or salt-sensitive BP (12). We extend these findings by demonstrating that excess dietary sodium intake increases cBRS in healthy young normotensive adults with salt-resistant BP. Further study is required to evaluate the effect of excess dietary sodium intake on sBRS in healthy young adults.
Similar to previously published observations (4, 35), our findings regarding HRV suggest that parasympathetic activity is affected following extended periods (i.e., 7 days) of altered dietary sodium intake. Following the HS diet RMSSD was significantly increased compared with the LS diet. RMSSD is generally considered to be associated with short-term changes in HRV and is believed to reflect parasympathetic outflow (44). These findings indicate that parasympathetic activity is also affected by alterations in dietary sodium intake.
Acute sodium loading via intravenous HSI raised plasma osmolality and serum sodium but did not elicit changes in cBRS or HRV. However, several studies in humans have demonstrated that acute sodium loading affects sBRS (13, 45). It has been observed that sBRS is increased following HSI when assessed by both the modified Oxford technique (sequential bolus of nitroprusside and phenylephrine) (13) and spontaneous fluctuations of SNA and DBP (45). Although these parallel reflex pathways share a similar input with cBRS (distension of the arterial baroreceptors), their sensitivities are not always well correlated (19), which is consistent with the present findings. That is, acute increases in plasma osmolality appear to increase sBRS (13, 45) but have no effect on cBRS, as demonstrated here and by others (13).
As expected, alterations in dietary sodium intake resulted in plasma volume expansion (~6.4%, as approximated from changes in hematocrit and hemoglobin). This was similar to the plasma volume expansion incurred via HSI (~6.2%). Evidence indicates that plasma volume and sBRS function are inversely related (13–15, 30, 41). Additionally, data from patients with unexplained syncope indicate that sodium supplementation resulting in a similar plasma volume expansion (~6.0–6.5%) reduces cBRS, as measured via application of external neck pressure (20, 37). While the present data suggest that cBRS may not follow the same pattern after plasma volume expansion in healthy young adults, methodological differences make direct comparisons of these studies difficult. For example, these previous studies used neck suction in a patient population, while the present study uses spontaneous fluctuations in SBP and R-R interval. Furthermore, because the previous studies do not report serum sodium or plasma osmolality, it is unclear how much (if at all) these variables may have been changed in these patients. In the present study, plasma volume expansion occurred concomitantly with increases in serum sodium and plasma osmolality, which affect both sBRS and cBRS; therefore, we are unable to separate the effects of plasma volume expansion from the effects of increased serum sodium and plasma osmolality. It may be that the increases in serum sodium and plasma osmolality simply offset the reductions in cBRS that may have otherwise been observed during the HSI, resulting in our observation that cBRS is unchanged. Further study is needed to fully characterize the effects of isosmotic plasma volume expansion on cBRS.
It remains unclear why increased dietary sodium intake increases cBRS sensitivity. The present findings may be somewhat contrary to expectations, as high dietary sodium intake is associated with increased cardiovascular disease risk, as is reduced, not increased, cBRS. However, these findings are consistent with recent animal evidence (1, 2, 43), which suggests that HS diets increase the gain of central autonomic circuits. This sensitization would lead to increased cBRS (i.e., a greater change in HR for a given change in BP), similar to that in study 1. Increases in serum sodium and plasma osmolality following the HS diet may be similar to changes incurred by dehydration, which concentrates serum electrolytes and raises osmolality. Findings in humans following dehydrating exercise indicate that cBRS is increased when serum sodium and plasma osmolality are increased to an extent similar to that in study 1 (14). In the case of dehydration, a greater increase in HR following a decline in BP may be beneficial for increasing cardiac output and, therefore, preventing hypotension. Further research into the effects of dehydration on cBRS may provide further insight into this relationship.
The present study indicates that plasma osmolality and/or serum sodium must be elevated for days (i.e., 7 days), not minutes, to change cBRS and HRV. Data from animal models suggest that high dietary sodium intake for >7 days is required to sensitize cardiovascular control neurons and exaggerate reflexes (43). Because cBRS and HRV were changed following high dietary sodium intake, but not acute HSI, sensitization of the neurons in the cardiovascular control center (nucleus tractus solitarius and others) may underlie these changes, as proposed in previous research (2, 43). It is also possible that hormonal changes contributed to the changes in cBRS. We previously demonstrated that the level of sodium in the diet used in the current study (300–350 mmol/day) suppresses circulating levels of angiotensin II compared with a LS (20 mmol/day) diet (18). There is some evidence that circulating levels of angiotensin II affect cBRS (11, 36) and, therefore, may contribute to the differences in the present study.
Limitations
Participants in study 1 were older than those in study 2. Because our findings were limited to within-comparisons, this difference does not affect our ability to draw the described conclusions. Nevertheless, participants were well matched otherwise (resting SBP, DBP, MAP, BMI, and serum electrolytes). However, because of the potential confounding effects of age on cBRS (32, 33) and HRV (40), caution is warranted in a comparison of study 1 with study 2.
Previous reports indicate that cBRS is affected by sodium to a lesser extent in individuals with salt-sensitive BP (12). Only participants with salt-resistant BP were included in study 1, as reported above. We are unable to determine the salt sensitivity of participants in study 2, as they were not subjected to manipulations in their dietary sodium intake. However, participants in study 2 were young, normotensive adults, a population in which salt-sensitive BP is uncommon. Furthermore, after the HSI, no participant in study 2 had a change in BP large enough to indicate that they may have salt-sensitive BP. Therefore, it is unlikely that the different responses were caused by differences in salt sensitivity.
Finally, use of the sequence method for determination of baroreflex sensitivity only allows for determination of gain around the operating point, rather than full characterization of the baroreflex curve that is generated via pharmacologically induced pressure changes. While the modified Oxford technique is the gold standard for evaluating baroreflex sensitivity, estimations using the sequence method provide clinically relevant indications of cardiovascular disease risk (42). Furthermore, previous research regarding acute changes in plasma osmolality and cBRS utilizing the modified Oxford technique yielded results (13) similar to the findings in study 2.
Perspectives and Significance
The findings of the present study indicate that changes in dietary sodium intake impact autonomic neural control of the cardiovascular system. cBRS and RMSSD were increased following 7 days of high dietary sodium intake, but not following an acute HSI. This may indicate that neurons in the cardiovascular control centers of the brain become sensitized and, therefore, are more responsive to input in the presence of chronic high serum sodium and plasma osmolality.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants R01-HL-104106 and R01-HL-128388.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.B., M.S.B., J.C.W., D.G.E., M.M.W., and W.B.F. performed experiments; M.C.B. and J.C.W. analyzed data; M.C.B. and W.B.F. interpreted results of experiments; M.C.B. prepared figures; M.C.B. and W.B.F. drafted manuscript; M.C.B., M.S.B., J.C.W., D.G.E., S.D.S., M.M.W., and W.B.F. edited and revised manuscript; M.C.B., M.S.B., J.C.W., D.G.E., S.D.S., M.M.W., and W.B.F. approved final version of manuscript; M.S.B., D.G.E., S.D.S., M.M.W., and W.B.F. conceived and designed research.
ACKNOWLEDGMENTS
We thank Carolyn Haines and the University of Delaware Nurse Managed Primary Care Center for assistance and the participants for their time and cooperation.
REFERENCES
- 1.Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension 54: 308–314, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension 50: 354–359, 2007. doi: 10.1161/HYPERTENSIONAHA.107.091843. [DOI] [PubMed] [Google Scholar]
- 3.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension 52: 932–937, 2008. doi: 10.1161/HYPERTENSIONAHA.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen AR, Gullixson LR, Wolhart SC, Kost SL, Schroeder DR, Eisenach JH. Dietary sodium influences the effect of mental stress on heart rate variability: a randomized trial in healthy adults. J Hypertens 32: 374–382, 2014. doi: 10.1097/HJH.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 5.Bealer SL. Central control of cardiac baroreflex responses during peripheral hyperosmolality. Am J Physiol Regul Integr Comp Physiol 278: R1157–R1163, 2000. doi: 10.1152/ajpregu.2000.278.5.R1157. [DOI] [PubMed] [Google Scholar]
- 6.Bealer SL. Peripheral hyperosmolality reduces cardiac baroreflex sensitivity. Auton Neurosci 104: 25–31, 2003. doi: 10.1016/S1566-0702(02)00265-5. [DOI] [PubMed] [Google Scholar]
- 7.Bealer SL. Increased dietary sodium inhibits baroreflex-induced bradycardia during acute sodium loading. Am J Physiol Regul Integr Comp Physiol 288: R1211–R1219, 2005. doi: 10.1152/ajpregu.00244.2004. [DOI] [PubMed] [Google Scholar]
- 8.Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–S81, 1985. [PubMed] [Google Scholar]
- 9.Brian MS, Dalpiaz A, Matthews EL, Lennon-Edwards S, Edwards DG, Farquhar WB. Dietary sodium and nocturnal blood pressure dipping in normotensive men and women. J Hum Hypertens 31: 145–150, 2017. doi: 10.1038/jhh.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brian MS, Matthews EL, Watso JC, Babcock MC, Wenner MM, Rose WC, Stocker SD, Farquhar WB. The influence of acute elevations in plasma osmolality and serum sodium on sympathetic outflow and blood pressure responses to exercise. J Neurophysiol 119: 1257–1265, 2018. doi: 10.1152/jn.00559.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campagnole-Santos MJ, Diz DI, Ferrario CM. Baroreceptor reflex modulation by angiotensin II at the nucleus tractus solitarii. Hypertension 11: I167–I171, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Castiglioni P, Parati G, Lazzeroni D, Bini M, Faini A, Brambilla L, Brambilla V, Coruzzi P. Hemodynamic and autonomic response to different salt intakes in normotensive individuals. J Am Heart Assoc 5: e003736, 2016. doi: 10.1161/JAHA.116.003736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charkoudian N, Eisenach JH, Joyner MJ, Roberts SK, Wick DE. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. Am J Physiol Heart Circ Physiol 289: H2456–H2460, 2005. doi: 10.1152/ajpheart.00601.2005. [DOI] [PubMed] [Google Scholar]
- 14.Charkoudian N, Halliwill JR, Morgan BJ, Eisenach JH, Joyner MJ. Influences of hydration on post-exercise cardiovascular control in humans. J Physiol 552: 635–644, 2003. doi: 10.1113/jphysiol.2003.048629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charkoudian N, Martin EA, Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Influence of increased central venous pressure on baroreflex control of sympathetic activity in humans. Am J Physiol Heart Circ Physiol 287: H1658–H1662, 2004. doi: 10.1152/ajpheart.00265.2004. [DOI] [PubMed] [Google Scholar]
- 16.Coruzzi P, Parati G, Brambilla L, Brambilla V, Gualerzi M, Novarini A, Castiglioni P, Di Rienzo M. Effects of salt sensitivity on neural cardiovascular regulation in essential hypertension. Hypertension 46: 1321–1326, 2005. doi: 10.1161/01.HYP.0000189183.50301.5c. [DOI] [PubMed] [Google Scholar]
- 17.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 18.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 31: 530–536, 2013. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutoit AP, Hart EC, Charkoudian N, Wallin BG, Curry TB, Joyner MJ. Cardiac baroreflex sensitivity is not correlated to sympathetic baroreflex sensitivity within healthy, young humans. Hypertension 56: 1118–1123, 2010. doi: 10.1161/HYPERTENSIONAHA.110.158329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Sayed H, Hainsworth R. Salt supplement increases plasma volume and orthostatic tolerance in patients with unexplained syncope. Heart 75: 134–140, 1996. doi: 10.1136/hrt.75.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari A, Gordon FJ, Mark AL. Impairment of cardiopulmonary baroreflexes in Dahl salt-sensitive rats fed low salt. Am J Physiol Heart Circ Physiol 247: H119–H123, 1984. doi: 10.1152/ajpheart.1984.247.1.H119. [DOI] [PubMed] [Google Scholar]
- 22.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc 105: 775–789, 2005. doi: 10.1016/j.jada.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Gordon FJ, Mark AL. Impaired baroreflex control of vascular resistance in prehypertensive Dahl S rats. Am J Physiol Heart Circ Physiol 245: H210–H217, 1983. doi: 10.1152/ajpheart.1983.245.2.H210. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Bolla G, Mancia G. Baroreflex impairment by low sodium diet in mild or moderate essential hypertension. Hypertension 29: 802–807, 1997. doi: 10.1161/hyp.29.3.802. [DOI] [PubMed] [Google Scholar]
- 25.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol 590: 5519–5528, 2012. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greaney JL, Ray CA, Prettyman AV, Edwards DG, Farquhar WB. Influence of increased plasma osmolality on sympathetic outflow during apnea. Am J Physiol Regul Integr Comp Physiol 299: R1091–R1096, 2010. doi: 10.1152/ajpregu.00341.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He FJ, Markandu ND, MacGregor GA. Importance of the renin system for determining blood pressure fall with acute salt restriction in hypertensive and normotensive whites. Hypertension 38: 321–325, 2001. doi: 10.1161/hyp.38.3.321. [DOI] [PubMed] [Google Scholar]
- 28.He FJ, Markandu ND, Sagnella GA, MacGregor GA. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension 32: 820–824, 1998. doi: 10.1161/hyp.32.5.820. [DOI] [PubMed] [Google Scholar]
- 29.Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 276: R1600–R1607, 1999. 10.1152/ajpregu.1999.276.6.R1600. [DOI] [PubMed] [Google Scholar]
- 30.Kamiya A, Iwase S, Kitazawa H, Mano T, Vinogradova OL, Kharchenko IB. Baroreflex control of muscle sympathetic nerve activity after 120 days of 6° head-down bed rest. Am J Physiol Regul Integr Comp Physiol 278: R445–R452, 2000. doi: 10.1152/ajpregu.2000.278.2.R445. [DOI] [PubMed] [Google Scholar]
- 31.Kinsman BJ, Browning KN, Stocker SD. NaCl and osmolarity produce different responses in organum vasculosum of the lamina terminalis neurons, sympathetic nerve activity and blood pressure. J Physiol 595: 6187–6201, 2017. doi: 10.1113/JP274537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laitinen T, Hartikainen J, Vanninen E, Niskanen L, Geelen G, Länsimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol 84: 576–583, 1998. doi: 10.1152/jappl.1998.84.2.576. [DOI] [PubMed] [Google Scholar]
- 33.Matsukawa T, Sugiyama Y, Mano T. Age-related changes in baroreflex control of heart rate and sympathetic nerve activity in healthy humans. J Auton Nerv Syst 60: 209–212, 1996. doi: 10.1016/0165-1838(96)00057-4. [DOI] [PubMed] [Google Scholar]
- 34.Matthews EL, Brian MS, Ramick MG, Lennon-Edwards S, Edwards DG, Farquhar WB. High dietary sodium reduces brachial artery flow-mediated dilation in humans with salt-sensitive and salt-resistant blood pressure. J Appl Physiol (1985) 118: 1510–1515, 2015. doi: 10.1152/japplphysiol.00023.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNeely JD, Windham BG, Anderson DE. Dietary sodium effects on heart rate variability in salt sensitivity of blood pressure. Psychophysiology 45: 405–411, 2008. doi: 10.1111/j.1469-8986.2007.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michelini LC, Bonagamba LG. Angiotensin II as a modulator of baroreceptor reflexes in the brainstem of conscious rats. Hypertension 15: I45–I50, 1990. doi: 10.1161/01.HYP.15.2_Suppl.I45. [DOI] [PubMed] [Google Scholar]
- 37.Mtinangi BL, Hainsworth R. Early effects of oral salt on plasma volume, orthostatic tolerance, and baroreceptor sensitivity in patients with syncope. Clin Auton Res 8: 231–235, 1998. doi: 10.1007/BF02267786. [DOI] [PubMed] [Google Scholar]
- 38.Panzenbeck MJ, Harrison PC, Madwed JB, McFarland ML, Winquist RJ, Frei P, Weldon S, Desai SN. Sodium depletion in conscious cynomolgus monkeys attenuates baroreflex sensitivity independently of prostaglandins. Am J Physiol Heart Circ Physiol 266: H2430–H2435, 1994. doi: 10.1152/ajpheart.1994.266.6.H2430. [DOI] [PubMed] [Google Scholar]
- 39.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol 10: 143–155, 2013. [Erratum in Nat Rev Cardiol 11: 314, 2014]. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 40.Pikkujämsä SM, Mäkikallio TH, Sourander LB, Räihä IJ, Puukka P, Skyttä J, Peng CK, Goldberger AL, Huikuri HV. Cardiac interbeat interval dynamics from childhood to senescence: comparison of conventional and new measures based on fractals and chaos theory. Circulation 100: 393–399, 1999. doi: 10.1161/01.CIR.100.4.393. [DOI] [PubMed] [Google Scholar]
- 41.Fu Q, Shook RP, Okazaki K, Hastings JL, Shibata S, Conner CL, Palmer MD, Levine BD. Vasomotor sympathetic neural control is maintained during sustained upright posture in humans. J Physiol 577: 679–687, 2006. doi: 10.1113/jphysiol.2006.118158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol 13: 191–207, 2008. doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmonds SS, Lay J, Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension 64: 583–589, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93: 1043–1065, 1996. doi: 10.1161/circ.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 45.Wenner MM, Rose WC, Delaney EP, Stillabower ME, Farquhar WB. Influence of plasma osmolality on baroreflex control of sympathetic activity. Am J Physiol Heart Circ Physiol 293: H2313–H2319, 2007. doi: 10.1152/ajpheart.01383.2006. [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi K, Tsuchimochi H, Stone AJ, Stocker SD, Kaufman MP. Increased dietary salt intake enhances the exercise pressor reflex. Am J Physiol Heart Circ Physiol 306: H450–H454, 2014. doi: 10.1152/ajpheart.00813.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]