Abstract
Weight loss from exercise is often less than expected. Putative compensatory mechanisms may limit exercise-induced reductions in body fat and might be proportional to exercise energy expenditure (ExEE). This study was conducted to determine compensation for (the difference between accumulated exercise energy expenditure and changes in body tissue energy stores) and compensatory responses to 1,500 or 3,000 kcal/wk of ExEE. Overweight-to-obese (n = 36) sedentary men and women were randomized to groups expending 300 or 600 kcal/exercise session, 5 days/wk, for 12 wk. Fourteen participants in the 300-kcal group and 15 in the 600-kcal group completed the study. The primary outcome was energy compensation assessed through changes in body tissue energy stores. Secondary outcomes were putative compensatory responses of resting metabolic rate, food reinforcement, dietary intake, and serum acylated ghrelin and glucagon-like peptide-1. All measures were determined pre- and posttraining. The 3,000 kcal/wk group decreased (P < 0.01) percentage and kilograms of body fat, while the 1,500 kcal/wk group did not. The 1,500 and 3,000 kcal/wk groups compensated for 943 (−164 to 2,050) and 1,007 (32 to 1,982) kcal/wk (mean, 95% CI, P ≥ 0.93), or 62.9% and 33.6% of ExEE, respectively. Resting metabolic rate and energy intake did not change. Food reinforcement and glucagon-like peptide-1 decreased (P < 0.02), whereas acylated ghrelin increased (P ≤ 0.02). Compensation is not proportional to ExEE. Similar energy compensation occurred in response to1,500 and 3,000 kcal/wk of ExEE. ExEE of 3,000 kcal/wk is sufficient to exceed compensatory responses and reduce fat mass.
Keywords: energy compensation, food reinforcement, ghrelin, glucagon-like peptide-1, obesity, weight loss
INTRODUCTION
To achieve weight loss, specifically, reductions in fat mass (FM), energy expenditure must exceed energy intake. Exercise can attenuate weight gain and produce fat loss (12), prompting the American College of Sports Medicine to issue separate recommendations to maintain health (23) or promote weight loss (12) through exercise. Many studies have demonstrated successful reductions in weight and FM with aerobic exercise training that accumulates 2,000–4,900 kcal/wk of energy expenditure (14, 34).
However, the amount of weight loss from exercise training is often disappointingly less than expected (45). It is generally expected that greater amounts of exercise energy expenditure (ExEE) would promote greater decreases in body weight and body fat. However, we previously (9) observed no differences in weight loss after exercise at 50%, 100%, or 150% of public health recommendations. Kraus et al. reported minimal (0.2–1.5 kg) weight loss and no group differences when adults exercised at 14 or 23 kcal·kg−1·wk−1 (28). Others found no changes in weight compared with a sedentary control group after 4 mo of exercise (10). It is apparent that greater amounts of exercise do not always lead to greater amounts of weight loss, which is likely explained by compensatory mechanisms that resist maintenance of an exercise-induced energy deficit. It is possible that engaging in sufficient amounts of exercise may overcome the compensatory responses that occur during an exercise program (43); however, greater amounts of exercise may also evoke a proportionally greater compensatory response that resists accumulation of an energy deficit. Rosenkilde et al. (33) demonstrated that 1,800 and 3,600 kcal/wk of ExEE produced nearly identical energy deficits, likely due to a proportionally twofold greater compensatory response from 600 than 300 kcal/day of ExEE.
Compensatory responses that may resist exercise-induced weight loss include physiological and behavioral factors such as reduced metabolic rate and nonexercise activity thermogenesis, increased metabolic efficiency, and increased energy intake (39, 45). Alterations in serum concentrations of hormonal mediators of appetite (41), in particular. increases in ghrelin with exercise training, may contribute to increased energy intake (29). Alterations in satiety-inducing signals [e.g., peptide YY (PYY), glucagon-like peptide-1 (GLP-1), pancreatic polypeptide, and leptin] may also promote greater energy intake during exercise training (21).
Another factor that influences energy intake is food reinforcement. Food reinforcement is a strong predictor of greater energy intake and weight status (16), and the reinforcing value of a food is a more robust predictor of food intake than the hedonic value (liking) of the food (17). Food deprivation that results in energy deficits increases the reinforcing value of food (17); thus exercise-induced energy deficits may also increase food reinforcement. However, it is less clear how exercise impacts energy intake, as some studies show changes in ab libitum daily energy intake, whereas others do not (13). This could be due to assessment methodology, specifically, the limitations to self-reported dietary intake.
To improve the effectiveness of exercise as a weight control strategy, it is important to understand whether ExEE can be great enough to overcome putative compensatory responses. This knowledge of aerobic exercise-induced weight/FM loss is also important to avoid giving false hope to individuals who will ultimately feel discouraged due to a lack of successful weight loss with exercise. The primary aim of the present study was to determine whether energy compensation in response to 1,500 kcal/wk of ExEE differed from that in response to 3,000 kcal/wk of ExEE and whether changes in putative compensatory mechanisms differed between groups and were associated with weight loss and/or change in body composition. The main study hypothesis was that accumulated negative energy balance and increases in food reinforcement, energy intake, and ghrelin would be greater in response to 3,000 than 1,500 kcal/wk of ExEE.
MATERIALS AND METHODS
Participants
A total of 36 participants (26 female) between the ages of 18 and 49 yr volunteered for the study and were randomized into study groups. Twenty-nine participants completed the study (21 female), six (5 female) voluntarily withdrew for personal reasons, and one was dismissed for noncompliance with exercise training. All participants were sedentary (i.e., exercised less than twice per week) with body mass index (BMI) ranging from 25 to 35 kg/m2. Recruitment occurred between April and October 2016 in the greater Grand Forks, ND, metropolitan area. Participants were a sample of the individuals who responded to recruitment media, including printed brochures and fliers and online advertisements placed on the Grand Forks Human Nutrition Research Center website. All participants were nonsmokers, did not take medication that influenced hunger or metabolism, and were healthy enough to participate in an exercise program assessed by the physical activity readiness questionnaire (PAR-Q). The study was approved by the University of North Dakota Institutional Review Board, and all participants provided written informed consent.
Procedures
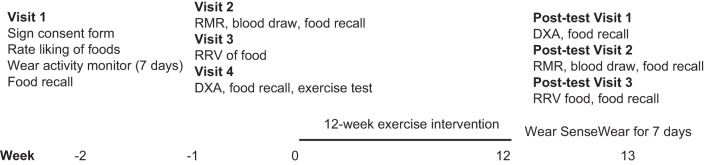
During the initial visit, participants were measured for anthropometrics and provided with a SenseWear mini armband (27) to assess usual energy expenditure for 7 days before baseline assessments. Participant’s liking of the test foods used in the food reinforcement task was assessed on a 1–10 scale of how much they liked each of the following foods after tasting a sample: Doritos, Snickers, Chips Ahoy cookies, powdered donuts, and Oreo cookies. The highest-scoring test food was used in the food reinforcement task. All participants had to rate at least a moderate liking (6 of 10) for at least one test food to be eligible for participation. Subsequent visits included assessments of resting metabolic rate (RMR), food reinforcement, dietary intake, body composition, aerobic fitness, and a fasted blood draw (see below) (Fig. 1).
Fig. 1.
Study time line. RMR, resting metabolic rate; RRV, reinforcing value of food; DXA, dual-energy absorptiometry.
Study Design
The study was a randomized trial that included a 12-wk exercise intervention of 300 or 600 kcal per exercise session, 5 sessions (days) per week, or intervention groups of 1,500 or 3,000 kcal/wk. The random allocation sequence was generated using the Plan procedure in SAS, with a block size of 4 and both treatments randomly occurring twice within each block. The study statistician generated and maintained the allocation sequence and concealed the sequence until the participants were enrolled and the interventions were assigned. There was no blinding of assignment to interventions. The trial is registered with ClinicalTrials.gov identifier NCT02152501. Participant recruitment began in April 2016, and all testing was completed in January 2017. Participants were assessed at baseline and immediately after the intervention. Food reinforcement and body composition were assessed 24–48 h after completion of the final exercise session of the 12-wk intervention. RMR measurements and a fasted blood sample were obtained 48–72 h after completion of the final exercise session of the intervention, and total energy expenditure was assessed for 7 days after completion of the intervention.
Exercise Intervention
Each participant received personalized heart rate (HR)-based exercise prescriptions so that they expended the assigned energy per exercise session (300 or 600 kcal). A graded exercise treadmill test was used to determine each participant’s rate of energy expenditure in four different HR zones. Resting and exercise HRs were measured using a Vivofit activity tracker (Garmin, Kansas City, KS). O2 consumed (V̇o2) and CO2 expired (V̇co2) were analyzed by indirect calorimetry (Oxycon Mobile, CareFusion, San Diego, CA). Upon completion of a 5-min warm-up walk at 0% grade, 3.0 mph, the treadmill grade increased to 2.5% for 3 min. The treadmill grade was then increased every 3 min to produce an ~10 beat/min increase in HR from the previous stage with the speed fixed at 3.0 mph. The test continued until 85% of HR reserve (HRR) was attained or the participant felt unable to continue. Energy expenditure (kcal/min) was determined from V̇o2 and V̇co2 using the Weir equation (46). Rate of energy expenditure was regressed against HR measured during the last minute of each stage of the treadmill test to calculate the rate of energy expenditure in different HR zones. HR zones were calculated using the HRR formula as follows: (220 − age) − resting HR × %HRR + resting HR (42). HR zone 1 ranged from 45 to 54% HRR, zone 2 from 55 to 64% HRR, zone 3 from 65 to 74% HRR, and zone 4 from 75 to 85% HRR. Energy expenditure (in kcal/min) was then averaged across each HR zone for determination of energy expenditure per minute per zone. Exercise sessions were carefully formulated for each participant so that they met the group-assigned energy expenditure prescription. Two lower-intensity exercise sessions per week (%HRR zones 1 and 2) and three interval-based sessions per week that included exercise time in zones 3 and 4 were included to provide variability and keep participants engaged. These individual sessions were tailored to each participant’s aerobic fitness; for example, some participants could not maintain HR in zone 4, whereas others could tolerate several minutes in zone 4. These differences were reflected in their prescribed workouts. The Vivofit activity tracker was used to monitor compliance with the exercise prescriptions over the 12-wk intervention and returned each week for downloading of workout HRs. Compliance was monitored and defined as expending 85–115% of the prescribed kilocalories per session. If participants were found to be noncompliant, they were required to make up missed exercise time in the following week if possible. The average energy expenditure of each HR zone was recalculated after the fitness test was repeated at 6 wk, and the subsequent exercise sessions were prescribed to reflect any changes in the HR-V̇o2 (energy expenditure) relationship. Upon beginning the exercise intervention, the participants were provided with a 12-wk pass to a local fitness center to ensure access to exercise facilities.
Assessments
Resting metabolic rate.
RMR was measured using indirect calorimetry (TrueOne 2400, Parvo Medics, Sandy, UT) and a ventilated canopy. After refraining from eating or drinking anything besides water for ≥10 h and without exercising for the previous 48 h, the participants traveled by automobile to the Grand Forks Human Nutrition Research Center. Participants completed a compliance questionnaire to ensure that these testing criteria were met before the RMR test. The TrueOne 2400 metabolic cart is a mixing chamber system that uses a paramagnetic O2 analyzer (range 0–25%) and an infrared, single-beam, single-wavelength CO2 analyzer (range 0–10%). Before each test, calibrations were performed on the flowmeter using a 3.0-liter syringe and on the gas analyzers using verified gases of known concentrations. After 30 min of quiet rest in the supine position in a dimly lit, temperature-controlled (22–24°C) room, RMR was measured for 30 min. The test was monitored to ensure that participants remained awake and fractional expired CO2 was between 0.8 and 1.2%. The criterion for a valid RMR was ≥15 min of steady state, determined as <10% fluctuation in V̇o2 and <5% fluctuation in respiratory quotient (RQ). The Weir equation (46) was used to determine RMR from the measured V̇o2 and V̇co2. The TrueOne 2400 metabolic cart is reliable, with across-day Pearson correlation coefficients of 0.994 and 0.991 for V̇o2 and V̇co2, respectively, and coefficients of variation of 4.7% and 5.7%, for V̇o2 and V̇co2, respectively (11).
Energy expenditure.
For 7 days before beginning any baseline testing and upon completion of the intervention, when the participants had completed their prescribed exercise, a SenseWear mini device (27) was worn on the arm to provide estimates of total energy expenditure (TEE). The SenseWear device uses heat flux, galvanic skin response, skin temperature, and activity, in addition to physical characteristics and demographics (e.g., sex, age, height, and weight), to estimate TEE. The SenseWear device estimates TEE within 22 kcal/day, with an absolute error rate of 8.3 ± 6.5% (27). Participants wore the SenseWear device throughout their waking hours; they removed it only for showers or other activities that would cause the device to be submersed in water.
Food reinforcement.
Participants’ reinforcing value of food was assessed using their favorite test food relative to their most-liked sedentary, noneating behavior (watching TV, reading magazines, or doing crossword puzzles or Sudoku). Reinforcing value was measured by evaluating the amount of operant responding (number of “mouse clicks”) by the participant to gain access to each alternative (food vs. sedentary activity) (5, 8, 16). The testing environment included two workstations with computers in the same room. On one computer, a game was set up for participants to earn points toward their most-liked snack food; on the other computer, the same game was set up for the participants to earn points toward the most-liked noneating sedentary behavior. Participants could switch between stations as much as they wished. The computer programs presented a game that mimicked a slot machine; 1 point was earned each time three shapes matched after the shapes were clicked. For every 5 points, a schedule was completed, and the participant received a 100-calorie portion of the snack food or 5 min of sedentary activity time, depending on what was earned. Initially, points were delivered after every four mouse clicks; thereafter, the schedule of reinforcement doubled (4, 8, 16, 32, [. . .] 1,024) each time 5 points were earned. For instance, initially, four mouse clicks were required to earn each point for schedule 1. After the first 5 points were earned, schedule 1 was complete, and the participant earned either a 100-calorie portion of food or 5 min of sedentary time. Eight clicks were required to earn each of the next 5 points for schedule 2 before another portion of the reinforcer was earned. Schedule 3 required 16 clicks to earn 1 point, schedule 4 required 32 clicks to earn 1 point, and so on (5). Participants received the food earned and/or were awarded the time they earned for sedentary activities after completing the game, which ended when the participant no longer wished to earn points for either behavior. Participants were not allowed to take food out of the laboratory but were not required to consume all the food they earned in the event a participant earned more food than they could eat in one sitting. Each test was conducted 2–4 h postprandially between usual lunch or dinner times, when snack foods are likely to be consumed at the same time for each participant. Before each task, participants rated how hungry, how full, or how satisfied they were, as well as how much they thought they could eat and how strong their desire to eat was on visual analog scales (VAS) (18) to be used as potential covariates for food reinforcement. The reinforcing value of food was conceptualized as the break point, or Pmax (5), which was the last schedule of reinforcement completed for the food choice.
Dietary intake.
For 3 days (2 week days and 1 weekend day) at baseline and during week 12 of the exercise intervention, the Automated Self-Administered 24-Hour Dietary Recall (ASA24) was used to estimate dietary intake (total energy and grams of carbohydrates, fat, protein, and alcohol) (30). The ASA24 is web-based software that allows for automated and self-administered 24-h recalls to be completed by research study participants. The ASA24 system is based on the multipass method of collecting dietary recall data using prompts and list of foods and beverages from the US Department of Agriculture Food and Nutrient Database for Dietary Studies. The system guides the participant through the multiple passes of dietary intake. Multiple images are shown to help respondents estimate portion size (40).
Body composition.
Body composition was measured using a GE Lunar iDXA machine before the exercise test. The iDXA allows the noninvasive assessment of soft tissue composition by region with a precision of 1–3% (35). A total body scan was conducted with participants lying supine on the table and arms positioned to the side. Most scans were completed using the thick mode suggested by the software, as participants were overweight-to-obese. All scans were analyzed using GE Lunar enCORE software (13.60.033). Automatic edge detection was used for scan analyses. The GE Lunar calibration phantom was used to calibrate the machine before each scanning session.
Compensation.
To calculate compensation for the energy expended during the exercise program (ExEE), the accumulated energy balance (AEB) was calculated from changes in FM and fat-free mass (FFM) upon completion of the study, as body composition changes reflect long-term alterations in energy balance (33). Gains of 1 kg of FM or 1 kg of FFM were assumed to reflect 12,000 and 1,780 kcal, respectively (15). Losses of 1 kg of FM or 1 kg of FFM were assumed to equal 9,417 and 884 kcal, respectively (19). ExEE was calculated from the training-induced energy expenditure (TrEE) of 300 or 600 kcal/session with the addition of 15% excess postexercise energy expenditure (1). The resting energy expenditure (REE) that would have occurred during the exercise sessions (REE × 1.2) was subtracted. Thus, ExEE = (TrEE × 0.15) + [TrEE – training duration × (REE × 1.2)] (33). Compensation in response to the increase in ExEE was assessed as described by Rosenkilde et al. (33), with the compensation index calculated as (ExEE + AEB)/ExEE × 100%. When the compensation index equals zero, AEB equals −ExEE, or changes in the energy equivalent of FM and FFM equal ExEE. Positive compensation suggests that changes in body composition indicate a less-negative-than-expected energy balance based on ExEE, whereas negative compensation indicates a greater-than-expected negative energy balance. ExEE, AEB, and compensation index (CI) could be calculated only for those participants who completed the study, as both pre- and posttreatment data points were needed to calculate these variables.
Fasting blood sample.
A fasting blood sample was collected in EDTA-coated and serum tubes. Serum PYY-(3–36), GLP-1, acylated ghrelin, irisin, and myostatin concentrations were measured using ELISA (Millipore, Phoenix Pharmaceuticals, Alpco). Serum insulin concentrations were measured with a chemiluminescent immunometric assay (Siemens, Erlangen, Germany), and serum glucose was measured via the hexokinase glucose-6-phosphate dehydrogenase reaction (Roche Diagnostics, Indianapolis, IN). Samples were frozen and batch-analyzed to reduce interassay variability.
Analytic Plan
Group differences in baseline characteristics and TrEE, AEB, and CI were tested using t-tests. Differences in the mean Δ (Δ = final – initial) values for secondary treatment outcomes (weight, FM, FFM, and %body fat) and putative compensatory mechanisms (RMR, TEE, food reinforcement, dietary intake, satiety hormones, and peptides) for the two treatment groups were tested for statistical significance using analysis of covariance, as was whether the mean Δ value for each treatment group was significantly different from 0. Baseline values were included as a covariate in all models. VAS scores for hunger and desire to eat were measured before the food reinforcement test to determine other possible covariates to be controlled for in this test. Neither sex nor age was a significant covariate for any of the outcomes; therefore, sex and age are not included in the models. The models were fit with the Glimmix procedure in SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Demographics and Physical Characteristics
As shown in Table 1, there were no baseline group differences in age, weight, height, BMI, RMR, TEE, food reinforcement, dietary intake, body composition, or aerobic fitness among the enrolled participants.
Table 1.
Baseline measures of study participants enrolled in an exercise intervention expending 1,500 or 3,000 kcal/wk
| 1,500 kcal (n = 18) | 3,000 kcal (n = 18) | |
|---|---|---|
| %Female | 72.2 | 66.7 |
| Age, yr | 26.6 ± 5.5 | 29.4 ± 5.4 |
| Weight, kg | 87.3 ± 16.2 | 85.5 ± 15.3 |
| Height, cm | 168.2 ± 9.2 | 169.3 ± 10.6 |
| BMI, kg/m2 | 30.7 ± 4.3 | 29.6 ± 3.0 |
| RMR, kcal/24 h | 1,984 ± 481 | 1,876 ± 321 |
| TEE, kcal/day | 2,574 ± 511 | 2,615 ± 596 |
| Food reinforcement | 33.6 ± 33.4 | 29.6 ± 30.8 |
| PWC, ml O2·kg–1·min–1 | ||
| 150 beats/min | 21.9 ± 6.1 | 21.6 ± 6.3 |
| 170 beats/min | 26.0 ± 7.1 | 25.8 ± 7.4 |
Values are means ± SD for all subjects who were randomized to a study group. An exercise intervention consisted of 5 sessions/wk for 12 wk. BMI, body mass index; RMR, 24-h resting metabolic rate energy expenditure; TEE, estimated total 24-h energy expenditure; food reinforcement, Pmax (the last schedule of reinforcement completed for the most-liked food); PWC, performance work capacity (rate of O2 consumption) at heart rate of 150 and 170 beats/min.
Treatment Fidelity, Body Composition, Energy Balance, and CI
All participants were ≥85% compliant to the exercise energy prescribed per session. As shown in Fig. 2, participants in the 3,000 kcal/wk group had an objectively measured ExEE of 35,780 ± 214 (SE) kcal across the 12 wk of the study, which was, by design, greater (P < 0.001) than the 17,640 ± 116 kcal of the 1,500 kcal/wk group. There was a group difference (P = 0.04) in AEB. Participants in the 1,500 kcal/wk group had an accumulated negative energy balance of 541 ± 496 kcal/wk or 6,492 kcal for the 12-wk intervention, while those in the 3,000 kcal/wk group had an accumulated negative energy balance of 1,993 ± 453 kcal/wk, or 23,919 kcal across the entire intervention. As shown in Table 2, this negative AEB was driven by a loss (P < 0.01) of FM in the 3,000 kcal/wk, but not (P ≥ 0.16) in the 1,500 kcal/wk, group. Loss of FM was greater (P < 0.05) in the 3,000 than the 1,500 kcal/wk group. Likewise, percent body fat was reduced in the 3,000 kcal/wk, but not the 1,500 kcal/wk, group (P < 0.01), and the changes in percent body fat differed (P < 0.03) between groups. Twelve of 15 participants in the 3,000 kcal/wk group lost FM, while 7 of 14 participants in the 1,500 kcal/wk group lost FM. This was also reflected in the magnitude of decreases in FM, as 7 of the 9 study participants who lost >10% of their initial FM and 5 of the 7 who lost 5–10% of their initial FM were in the 3,000 kcal/wk group. Initial body weight and initial FM were not significant covariates in changes in body weight or FM (P > 0.2). FFM did not change in either group. When expressed as a percentage of ExEE calories, the 1,500 kcal/wk group compensated for 63 ± 34% (mean ± SE) and the 3,000 kcal/wk group compensated for 33 ± 15% of the kilocalories expended during exercise. These group differences in CI, however, were not significant (P ≥ 0.43).
Fig. 2.
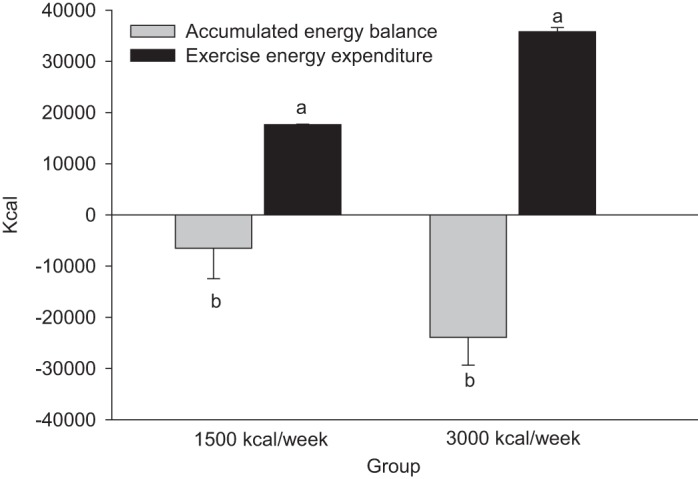
Accumulated energy balance and exercise energy expenditure of participants after exercise to expend 1,500 or 3,000 kcal/wk for 12 wk. Like letters indicate differences (P < 0.05) between treatment groups. Values are means ± SE.
Table 2.
Outcome variables at baseline and 12 wk for participants exercising to expend 1,500 or 3,000 kcal/wk for 12 wk
| 1,500 kcal/wk |
3,000 kcal/wk |
|||||
|---|---|---|---|---|---|---|
| Baseline | 12 wk | Adjusted group change (95% CI) | Baseline | 12 wk | Adjusted group change (95% CI) | |
| Weight, kg | 83.6 ± 3.6 | 82.4 ± 3.4 | –1.3 (–2.6, 0.1) | 85.0 ± 3.8 | 82.3 ± 3.8 | –2.6 (–3.9, –1.4)* |
| FFM, kg | 50.1 ± 2.8 | 49.8 ± 2.7 | –0.4 (–1.0, 0.3) | 49.8 ± 2.8 | 49.7 ± 2.7 | –0.1 (–0.8, 0.6) |
| FM,† kg | 30.4 ± 2.4 | 29.6 ± 2.3 | –0.9 (–2.1, 0.4) | 32.0 ± 2.1 | 29.3 ± 2.2 | –2.6 (–3.8, –1.4)* |
| %Body fat† | 36.3 ± 2.3 | 35.9 ± 2.2 | –0.4 (–1.6, 0.7) | 37.6 ± 1.9 | 35.5 ± 2.0 | –2.2 (–3.2, –1.1)* |
| RMR, kcal/day | 1,909 ± 110 | 1,874 ± 105 | –32 (–115, 51) | 1,866 ± 86 | 1,808 ± 76 | –61 (–141, 19) |
| TEE, kcal·kg FFM−1·day−1 | 51.0 ± 1.8 | 52.2 ± 2.1 | 0.9 (–2.8, 4.7) | 52.5 ± 2.3 | 51.1 ± 2.5 | –1.3 (–4.9, 2.3) |
| Food reinforcement | 24.9 ± 6.2 | 10.9 ± 3.3 | –17.6 (–26.4, –8.9)* | 32.0 ± 8.5 | 14.7 ± 4.7 | –13.9 (–22.4, –5.5)* |
| Energy intake, kcal/day | 2,188 ± 148 | 2,035 ± 144 | –278 (–567, 11) | 2,476 ± 228 | 2,034 ± 131 | –324 (–603, –45)* |
| %kcal | ||||||
| Protein | 14.4 ± 0.7 | 14.7 ± 0.8 | 0.17 (–2.2, 2.5) | 15.4 ± 0.6 | 17.5 ± 1.3 | 2.4 (0.2, 4.7)* |
| Fat† | 36.3 ± 1.8 | 40.0 ± 1.3 | 3.0 (0.3, 5.6)* | 38.3 ± 0.8 | 35.4 ± 1.3 | –2.2 (–4.7, 0.4) |
| Carbohydrate | 49.6 ± 2.5 | 44.6 ± 2.1 | –4.1 (–8.1, –0.2)* | 46.1 ± 1.1 | 47.6 ± 2.1 | 0.73 (–3.1, 4.5) |
Values are means ± SE. Only results from participants who completed the intervention and all assessments (n = 29) are included. Food reinforcement, Pmax, the last schedule of reinforcement completed for access to the most-liked test food; energy intake, average intake across 3 days from ASA24 recall. CI, confidence interval; FFM, fat-free mass; FM, fat mass; RMR, resting metabolic rate; TEE, total energy expenditure. Change score (adjusted group change) data are adjusted for baseline values.
Different from zero (P < 0.05).
Change scores differ between groups (P < 0.05).
Energy Expenditure
Neither RMR nor TEE per kilogram of lean mass (both P > 0.40; Table 2) changed in either group during treatment. Substrate oxidation (RQ = V̇co2/V̇o2) shifted toward a greater preference for fatty acid oxidation during the incremental exercise test (0.99 ± 0.02 and 0.95 ± 0.02 at baseline and 6 wk, respectively, P < 0.05). There were no changes in resting RQ (0.72 ± 0.01 and 0.71 ± 0.02 at baseline and 12 wk, respectively, P = 0.25). Neither initial RQ nor changes in RQ predicted changes in FM (P > 0.77 and P > 0.56, respectively).
Liking and Food Reinforcement
Liking of the test foods was measured at baseline and did not differ (P > 0.6) between the 1,500 and 3,000 kcal/wk groups [9.0 ± 1.3 and 8.8 ± 1.2 (means ± SD), respectively]. The reinforcing value of snack foods as measured by Pmax (final schedule completed) decreased (P < 0.01; Table 2) in both groups; there were no differences in the change scores between groups. VAS responses for “how strong is your desire to eat” correlated with food reinforcement (r = 0.53, P < 0.01) at the 12-wk visit. When “how strong is your desire to eat” was included as a covariate, the food reinforcement results did not change.
Dietary Intake
Dietary intakes are presented in Table 2. Energy intake decreased (P = 0.03) and percentage of kilocalories from protein increased (P = 0.04) in the 3,000 kcal/wk group. Percentage of kilocalories from fat increased (P = 0.03) and percentage of kilocalories from carbohydrate (P = 0.04) decreased in the 1,500 kcal/wk group. The change scores for percentage of kilocalories from fat differed (P = 0.01) between groups.
Satiety Signals
Fasting glucose, insulin, irisin, myostatin, PYY, acylated ghrelin, and GLP-1 concentrations were not changed in either group. GLP-1 and ghrelin concentrations trended toward significance in both groups. As shown in Table 3, when change scores were analyzed by pooling all participants, GLP-1 increased (P = 0.02) and ghrelin decreased (P < 0.01). Neither the changes in acylated ghrelin (r = −0.10, P > 0.60) nor the changes in GLP-1 (r = 0.13, P > 0.50) were associated with CI. The change in food reinforcement was correlated (r = −0.45, P = 0.01) with the change in serum GLP-1 concentration, but not (r = 0.11, P > 0.56) with the change in serum acylated ghrelin concentrations.
Table 3.
Changes in fasting acylated ghrelin and GLP-1 concentrations of participants exercising to expend 1,500 or 3,000 kcal/wk for 12 wk
| Baseline | Week 12 | Adjusted Difference (95% CI) | |
|---|---|---|---|
| GLP-1, pM | 59.2 ± 4.1 | 52.6 ± 3.5 | −6.7 (−11.6, −1.8)* |
| Acylated ghrelin, pg/ml | 842.6 ± 65.0 | 909.1 ± 64.3 | 66.5 (8.6, 124.4)* |
| Peptide-YY, pg/ml | 202.8 ± 14.0 | 189.9 ± 14.1 | −13.1 (−35.8, 9.7)* |
| Myostatin, mg/l | 41.0 ± 1.4 | 42.3 ± 1.4 | 1.0 (−0.8, 2.7) |
| Irisin, µg/ml | 9.0 ± 0.5 | 8.8 ± 0.6 | −0.3 (−1.1, 0.43) |
| Insulin, mIU/l | 9.0 ± 1.0 | 8.2 ± 0.9 | −0.9 (−2.4, 0.69) |
| Glucose, mg/dl | 94.5 ± 1.4 | 93.9 ± 1.4 | −0.6 (−3.3, 2.0) |
Values are means ± SE. GLP-1, glucagon-like peptide 1; CI, confidence interval.
Different from zero (P < 0.01).
DISCUSSION
The primary finding of the present study is that compensation for ExEE occurs but that compensation is not commensurate with the amount of exercise performed. As such, expending 600 kcal/session, 5 days/wk (3,000 kcal/wk), resulted in a decrease in body weight, which was driven by the decrease in FM and percent body fat, while expending 300 kcal per session did not. While compensation for ExEE occurred, it was nearly equal for the 1,500 and 3,000 kcal/wk groups (compensation of 943 and 1,007 kcal/wk, respectively). Thus, for loss of weight (specifically, fat) across 12 wk of exercise training, ExEE should exceed 1,500 kcal/wk and likely be closer to 3,000 kcal/wk to overcome the compensation that occurs place when previously sedentary individuals begin an exercise program. The current results are in contrast to the work of Rosenkilde et al. (33), who found no difference in the amounts of body weight and FM lost between adults who engaged in 300 kcal/session and adults who engaged in 600 kcal/session (6 sessions/wk) for 13 wk. While the present study found no difference in CI between the 1,500 and 3,000 kcal/wk groups, Rosenkilde et al. concluded that CI increases proportionally with ExEE, although a ceiling effect of ExEE on CI was not tested. The disparate results between the current study and the study of Rosenkilde et al. are surprising, given the similar exercise interventions, age, and BMI of the participants.
The results of the present study are more aligned with those of the STRRIDE study (37), where FM loss was greater in overweight men and women expending 23 kcal·kg body wt−1·wk−1 than in those expending 14 kcal·kg body wt−1·wk−1. One possibility for the discrepancies between the studies may be that Rosenkilde et al. (33) relied on a calorie-tracking HR monitor, instead of indirect calorimetry, to prescribe exercise sessions. This methodology may have contributed to their finding that CI for the 300 kcal/session (1,800 kcal/wk) group was −83%, indicating an AEB in excess of what could be expected from the energy expended by the participants during exercise. Thus the 300 kcal/session (1,800 kcal/wk) group either expended more energy through exercise than estimated, or they concurrently restricted their energy intake, which was not observed in either group. Sex differences may be another explanation for the contrasting results, as Rosenkilde et al. entered only men into their study, while the present study’s sample mostly consisted of women. A recent review concluded that acute exercise did not affect men and women differently in terms of appetite and energy compensation (44); however, less is known about whether this holds true for long-term exercise interventions. It is also known that women have a greater reliance on fat as an energy substrate during exercise than men; thus carbohydrate and amino acids are spared (7, 25). This is also observed in the postexercise recovery period, during which women demonstrate greater homeostatic control over lipolytic rate, postprandial triglyceride concentration, blood glucose concentration, and fuel selection (24). Future studies may benefit from focusing on this potential effect of sex and its potential impact on weight loss and energy compensation with exercise. Additionally, lifestyle and environmental factors, such as access to healthy food and physical activity, and attitudes regarding eating may have played a role in the different compensatory responses to exercise between the two studies.
An additional aim of the present investigation was to identify mechanisms for compensation for ExEE. Putative behavioral compensatory factors include an increase in energy intake and decreased physical activity outside of exercise (45). Similar to the results of Rosenkilde et al. (33), energy intake remained unchanged during the exercise intervention in both groups. This was surprising based on the amount of energy compensated for in both groups, and this result should be interpreted with caution, considering the well-known underreporting bias in self-reported dietary intake (31). The reality is that 24-h recalls assessing food intake for 3 days at two time points are not sufficient to capture the observed compensatory response. This measure may have been more robust if these recalls were completed for 7–10 consecutive days. Underestimation of portion size may have been another issue with the 24-h recall; thus future studies may look into other methods of assessing dietary intake, such as detailed food records. Another unexpected finding was the decrease in the reinforcing value of food in both groups. This may be due to the foods available in the reinforcement task, as all foods might be considered less-healthy (candy, donuts, and chips) food choices. Participants may have been less motivated to consume less-healthy snack foods upon completion of the exercise intervention. Indeed, engagement in an exercise program can exert a “spillover” effect, resulting in other health behavior changes, including dietary modification, where the intake of sweets and desserts is reduced spontaneously upon initiation of an exercise program (22). It is therefore possible that overall food reinforcement and reinforcement for foods perceived as being “healthy” could have increased after the 12 wk of exercise training. It would have been interesting to test changes in the reinforcing values of both healthy and less-healthy snack foods to determine if a particular type of food became more reinforcing with exercise training. It is also interesting to note that the 3,000 kcal/wk group did not change their dietary composition with exercise while the 1,500 kcal/wk group increased their percent energy from fat and decreased their percent energy from carbohydrate. Perhaps the 1,500 kcal/wk group decreased their intake of sweets, as previously noted (22), shifting their macronutrient distribution upon exercise initiation. Careful study of changes in macronutrient distribution with exercise training could be an interesting topic for future studies. In addition to dietary intake, reducing physical activity outside of an exercise program is another putative behavioral compensatory mechanism. However, the present study did not detect changes in nonexercise energy expenditure after the exercise program.
Putative biological mechanisms may have also influenced compensatory responses and the CI. When assessed independently, neither group’s change in acylated ghrelin or GLP-1 reached statistical significance. Analysis of change scores for the entire sample showed that the increase in serum acylated ghrelin concentration and the reduction in serum GLP-1 concentration were significant. It would be expected that concurrent elevations in the orexigenic hormone ghrelin and reductions in the anorexigenic GLP-1 would promote a greater food intake and compensatory response. However, neither the change in serum acylated ghrelin nor the change in GLP-1 was associated with CI, which may be a result of most participants having a positive CI. Only seven participants did not have a positive CI, and one participant, who had a very negative CI, had paradoxically decreased ghrelin and increased GLP-1 concentrations. A larger sample and, possibly, a control group for comparison are needed to confirm or deny the link between these hunger hormones and CI. However, the change in serum GLP-1 concentration was inversely correlated with the change in snack food reinforcement. GLP-1 reduces hunger-driven feeding and the motivation to consume food (36), and a decrease in GLP-1 would be expected to increase hunger-driven feeding and eating motivation. Thus the present results raise additional questions regarding how exercise, total energy intake, and energy balance may moderate the relationship between GLP-1 and food reinforcement, which could explain the less-than-expected weight loss with exercise.
The present study found no change in RMR or resting RQ in response to exercise training. Previous studies of changes in RMR with exercise and/or weight loss offer conflicting results (3, 20, 38). The effects of exercise on RMR likely depend on factors such as the daily and total duration of training, the mode and intensity of exercise training, the amount of weight loss, and the changes in body composition that occur during the training period. For instance, the lack of change in RMR in the present study may be explained by the lack of changes in FFM or the self-selected nature of the intensity of the aerobic exercise sessions, which were not designed to build muscle (2, 26). Exercise training decreased RQ during the incremental exercise test. This shift in substrate oxidation toward a greater preference for fatty acid oxidation during exercise is an expected consequence when previously sedentary, obese individuals adopt an aerobic exercise program (6, 32). However there were no changes in resting RQ after 12 wk of exercise, and, in contrast to previous work (4), changes in RQ did not predict changes in fat loss.
This study is not without limitations. Self-reported dietary intake is often biased toward underreporting, potentially preventing detection of the expected increase in energy intake (31). Similarly, the use of only energy-dense snack foods in the reinforcing value of food test may have limited our ability to detect changes in food reinforcement, as might be expected with decreases in GLP-1 and a positive CI. Additionally, of the 29 participants who completed the current study, 26 were Caucasian (1 was American Indian, 1 was multiracial, and 1 was African American), thus limiting the generalizability to other race/ethnic groups. The duration of the intervention (12 wk) is similar to that of most exercise interventions in this area of research, although a longer period may have been required to detect changes in the 300 kcal/wk group. This study also was not designed to detect sex differences and included an unbalanced sample of women; thus sex effects cannot be concluded. The lack of a control group raises the possibility that the changes in body composition and fasting hunger hormones were not related to the exercise intervention.
Perspectives and Significance
Current recommendations state that exercise programs should exceed 225 min/wk to induce clinically significant weight loss (12), which is greater than the amount of exercise recommended by the Centers for Disease Control for general health. The current study prescribed the amount of exercise training by calories per session, as opposed to minutes; however, participants in the 1,500 kcal/wk (300 kcal/session) group exercised an average of 167 min/wk. The 3,000 kcal/wk group exercised an average of 335 min/wk. Results of the current study suggest that the recommendation should be closer to 300 min/wk to achieve appreciable fat loss. As there was no difference in the amount of compensation between exercise interventions with a twofold (1,500 kcal/wk) difference in energy expenditure, it seems that, with sufficient amounts of exercise, the inevitable compensatory factors that resist an exercise-induced negative energy balance can be overcome.
There are still many other factors in an exercise program that may influence an individual’s amount of compensation, and it appears that there are large variations between individuals in their energy compensation. In the current sample, initial body weight, initial FM, minutes spent exercising, and both initial RMR and RQ and changes in RMR and RQ were not correlated to CI or changes in FM. Other variables, such as mode of exercise (resistance training or circuit training), exercise intensity (high-intensity interval training), exercise frequency (sessions per week or multiple sessions in 1 day), and time of exercise (early morning or evening), may be targets of future research to better understand the compensatory response to exercise and why exercise training results in less-than-expected weight loss.
GRANTS
This study was funded by the US Department of Agriculture, Agricultural Research Service, and Project 3062-51000-051-00D.
DISCLAIMERS
Mention of trade names, commercial products, or organizations does not imply endorsement from the US government.
The US Department of Agriculture is an equal opportunity provider and employer. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.D.F., J.S.F., and J.N.R. conceived and designed research; K.D.F. and K.U. performed experiments; K.D.F., L.J., and J.N.R. analyzed data; K.D.F., K.U., L.J., J.S.F., and J.N.R. interpreted results of experiments; K.D.F. and J.N.R. prepared figures; K.D.F. drafted manuscript; K.D.F., K.U., L.J., J.S.F., and J.N.R. edited and revised manuscript; K.D.F., K.U., L.J., J.S.F., and J.N.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Doreen Rolshoven, Jackie Nelson, and student interns for assistance with implementation of the protocol, data collection, and data entry.
REFERENCES
- 1.Bahr R, Ingnes I, Vaage O, Sejersted OM, Newsholme EA. Effect of duration of exercise on excess postexercise O2 consumption. J Appl Physiol (1985) 62: 485–490, 1987. doi: 10.1152/jappl.1987.62.2.485. [DOI] [PubMed] [Google Scholar]
- 2.Ballor DL, Keesey RE. A meta-analysis of the factors affecting exercise-induced changes in body mass, fat mass and fat-free mass in males and females. Int J Obes 15: 717–726, 1991. [PubMed] [Google Scholar]
- 3.Ballor DL, Poehlman ET. A meta-analysis of the effects of exercise and/or dietary restriction on resting metabolic rate. Eur J Appl Physiol Occup Physiol 71: 535–542, 1995. doi: 10.1007/BF00238557. [DOI] [PubMed] [Google Scholar]
- 4.Barwell ND, Malkova D, Leggate M, Gill JM. Individual responsiveness to exercise-induced fat loss is associated with change in resting substrate utilization. Metabolism 58: 1320–1328, 2009. doi: 10.1016/j.metabol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology (Berl) 153: 44–56, 2000. doi: 10.1007/s00213,0000589. [DOI] [PubMed] [Google Scholar]
- 6.Blaak EE, Saris WH. Substrate oxidation, obesity and exercise training. Best Pract Res Clin Endocrinol Metab 16: 667–678, 2002. doi: 10.1053/beem.2002.0226. [DOI] [PubMed] [Google Scholar]
- 7.Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab 280: E898–E907, 2001. doi: 10.1152/ajpendo.2001.280.6.E898. [DOI] [PubMed] [Google Scholar]
- 8.Casperson SL, Johnson L, Roemmich JN. The relative reinforcing value of sweet versus savory snack foods after consumption of sugar- or non-nutritive sweetened beverages. Appetite 112: 143–149, 2017. doi: 10.1016/j.appet.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA 297: 2081–2091, 2007. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 10.Church TS, Earnest CP, Thompson AM, Priest EL, Rodarte RQ, Saunders T, Ross R, Blair SN. Exercise without weight loss does not reduce C-reactive protein: the INFLAME Study. Med Sci Sports Exerc 42: 708–716, 2010. doi: 10.1249/MSS.0b013e3181c03a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crouter SE, Antczak A, Hudak JR, DellaValle DM, Haas JD. Accuracy and reliability of the ParvoMedics TrueOne 2400 and MedGraphics VO2000 metabolic systems. Eur J Appl Physiol 98: 139–151, 2006. doi: 10.1007/s00421-006-0255-0. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine . American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 41: 459–471, 2009. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly JE, Herrmann SD, Lambourne K, Szabo AN, Honas JJ, Washburn RA. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PLoS One 9: e83498, 2014. doi: 10.1371/journal.pone.0083498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly JE, Honas JJ, Smith BK, Mayo MS, Gibson CA, Sullivan DK, Lee J, Herrmann SD, Lambourne K, Washburn RA. Aerobic exercise alone results in clinically significant weight loss for men and women: Midwest Exercise Trial 2. Obesity (Silver Spring) 21: E219–E228, 2013. doi: 10.1002/oby.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elia M, Stratton R, Stubbs J. Techniques for the study of energy balance in man. Proc Nutr Soc 62: 529–537, 2003. doi: 10.1079/PNS2003255. [DOI] [PubMed] [Google Scholar]
- 16.Epstein LH, Carr KA, Lin H, Fletcher KD. Food reinforcement, energy intake, and macronutrient choice. Am J Clin Nutr 94: 12–18, 2011. doi: 10.3945/ajcn.110.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA. Effects of deprivation on hedonics and reinforcing value of food. Physiol Behav 78: 221–227, 2003. doi: 10.1016/S0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- 18.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 24: 38–48, 2000. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 19.Forbes GB. Do obese individuals gain weight more easily than nonobese individuals? Am J Clin Nutr 52: 224–227, 1990. doi: 10.1093/ajcn/52.2.224. [DOI] [PubMed] [Google Scholar]
- 20.Gim MN, Choi JH. The effects of weekly exercise time on V̇o2max and resting metabolic rate in normal adults. J Phys Ther Sci 28: 1359–1363, 2016. doi: 10.1589/jpts.28.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagobian TA, Sharoff CG, Stephens BR, Wade GN, Silva JE, Chipkin SR, Braun B. Effects of exercise on energy-regulating hormones and appetite in men and women. Am J Physiol Regul Integr Comp Physiol 296: R233–R242, 2009. doi: 10.1152/ajpregu.90671.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halliday TM, Davy BM, Clark AG, Baugh ME, Hedrick VE, Marinik EL, Flack KD, Savla J, Winett S, Winett RA. Dietary intake modification in response to a participation in a resistance training program for sedentary older adults with prediabetes: findings from the Resist Diabetes Study. Eat Behav 15: 379–382, 2014. doi: 10.1016/j.eatbeh.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39: 1423–1434, 2007. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 24.Henderson GC. Sexual dimorphism in the effects of exercise on metabolism of lipids to support resting metabolism. Front Endocrinol (Lausanne) 5: 162, 2014. doi: 10.3389/fendo.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Mau TL, Luke-Zeitoun M, Brooks GA. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol 584: 963–981, 2007. doi: 10.1113/jphysiol.2007.137331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev 15: 310–321, 2014. doi: 10.1111/obr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med Sci Sports Exerc 42: 2134–2140, 2010. doi: 10.1249/MSS.0b013e3181e0b3ff. [DOI] [PubMed] [Google Scholar]
- 28.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 347: 1483–1492, 2002. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 29.Mason C, Xiao L, Imayama I, Duggan CR, Campbell KL, Kong A, Wang CY, Alfano CM, Blackburn GL, Foster-Schubert KE, McTiernan A. The effects of separate and combined dietary weight loss and exercise on fasting ghrelin concentrations in overweight and obese women: a randomized controlled trial. Clin Endocrinol (Oxf) 82: 369–376, 2015. doi: 10.1111/cen.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 76: 1261–1271, 2002. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 31.Mullaney L, O’Higgins AC, Cawley S, Doolan A, McCartney D, Turner MJ. An estimation of periconceptional under-reporting of dietary energy intake. J Public Health (Oxf) 37: 728–736, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Romijn JA, Klein S, Coyle EF, Sidossis LS, Wolfe RR. Strenuous endurance training increases lipolysis and triglyceride-fatty acid cycling at rest. J Appl Physiol (1985) 75: 108–113, 1993. doi: 10.1152/jappl.1993.75.1.108. [DOI] [PubMed] [Google Scholar]
- 33.Rosenkilde M, Auerbach P, Reichkendler MH, Ploug T, Stallknecht BM, Sjödin A. Body fat loss and compensatory mechanisms in response to different doses of aerobic exercise—a randomized controlled trial in overweight sedentary males. Am J Physiol Regul Integr Comp Physiol 303: R571–R579, 2012. doi: 10.1152/ajpregu.00141.2012. [DOI] [PubMed] [Google Scholar]
- 34.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 12: 789–798, 2004. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 35.Rothney MP, Martin FP, Xia Y, Beaumont M, Davis C, Ergun D, Fay L, Ginty F, Kochhar S, Wacker W, Rezzi S. Precision of GE Lunar iDXA for the measurement of total and regional body composition in nonobese adults. J Clin Densitom 15: 399–404, 2012. doi: 10.1016/j.jocd.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci 7: 181, 2013. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, Houmard JA, Bales CW, Kraus WE. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med 164: 31–39, 2004. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 38.Speakman JR, Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc 62: 621–634, 2003. doi: 10.1079/PNS2003282. [DOI] [PubMed] [Google Scholar]
- 39.Stubbs RJ, Hughes DA, Johnstone AM, Whybrow S, Horgan GW, King N, Blundell J. Rate and extent of compensatory changes in energy intake and expenditure in response to altered exercise and diet composition in humans. Am J Physiol Regul Integr Comp Physiol 286: R350–R358, 2004. doi: 10.1152/ajpregu.00196.2003. [DOI] [PubMed] [Google Scholar]
- 40.Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, Willis G, Islam NG, Baranowski T, McNutt S, Potischman N. The Automated Self-Administered 24-hour Dietary Recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet 112: 1134–1137, 2012. doi: 10.1016/j.jand.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 365: 1597–1604, 2011. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 42.Swain DP, Leutholtz BC, King ME, Haas LA, Branch JD. Relationship between % heart rate reserve and % V̇o2 reserve in treadmill exercise. Med Sci Sports Exerc 30: 318–321, 1998. doi: 10.1097/00005768-199802000-00022. [DOI] [PubMed] [Google Scholar]
- 43.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis 56: 441–447, 2014. doi: 10.1016/j.pcad.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thackray AE, Deighton K, King JA, Stensel DJ. Exercise, appetite and weight control: are there differences between men and women? Nutrients 8: 583, 2016. doi: 10.3390/nu8090583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas DM, Kyle TK, Stanford FC. The gap between expectations and reality of exercise-induced weight loss is associated with discouragement. Prev Med 81: 357–360, 2015. doi: 10.1016/j.ypmed.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weir JBD. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]