Abstract
During mammalian pregnancy, the uterine circulation must undergo substantial vasodilation and growth to maintain sufficient uteroplacental perfusion. Although we and others have shown that nitric oxide (NO) is a key mediator of these processes, the mechanisms that augment uterine artery NO signaling during gestation have not been identified. We hypothesized that Piezo1, a recently discovered cation channel, may be involved in the process of shear stress mechanotransduction, as other studies have shown that it is both mechanosensitive and linked to NO production. Surprisingly, there are no studies on Piezo1 in the uterine circulation. Our aims in the present study were to determine whether this novel channel is 1) present in uterine arteries, 2) regulated by gestation, 3) functionally relevant (able to elicit rises in intracellular Ca2+ concentration and vasodilation), and 4) linked to NO. Immunohistochemistry confirmed that Piezo1 is present in uterine arteries, primarily but not exclusively in endothelial cells. Western blot analysis showed that its protein expression was elevated during gestation. In pressurized main uterine arteries, pharmacological activation of Piezo1 by Yoda1 produced near maximal vasodilation and was associated with significant increases in intracellular Ca2+ concentration in endothelial cell sheets. Shear stress induced by intraluminal flow produced reversible vasodilations that were inhibited >50% by GsMTx-4, a Piezo1 inhibitor, and by Nω-nitro-l-arginine methyl ester/Nω-nitro-l-arginine, inhibitors of NO synthase. These findings are the first to implicate a functional role for Piezo1 in the uterine circulation as a mechanosensor of endothelial shear stress. Moreover, our data demonstrate that Piezo1 activation leads to vasodilation via NO and indicate that its molecular expression is upregulated during pregnancy.
NEW & NOTEWORTHY This is the first study to highlight Piezo1 in the uterine circulation. As a potentially important endothelial mechanosensor of shear stress, Piezo1 may be linked to mechanisms that support increased uteroplacental perfusion during pregnancy.
Listen to this article’s corresponding podcast at https://ajpheart.podbean.com/e/piezo1-mechanotransduction-in-the-uterine-circulation/.
Keywords: endothelial cell calcium, Piezo1, uterine artery, vasodilation
INTRODUCTION
The maternal uterine circulation is a unique vascular bed in that it undergoes vasodilation and growth during pregnancy, with significant increases in both arterial and venous diameter (34, 37, 39, 40). These processes are essential for normal pregnancy outcome because they facilitate adequate placental perfusion. At term, ~20% of total body blood volume is directed to the uterine circulation and delivered at a rate approaching 1 l/m (41). Failure to provide adequate blood supply increases the risk for maternal and fetal diseases such as preeclampsia and intrauterine growth restriction, underscoring the importance of maternal uterine vascular adaptation (7, 25, 31, 40).
Although mammalian pregnancy is characterized by high levels of circulating sex steroids, growth factors, and other endocrine signals, local rather than systemic factors play the principal role in maternal uterine vascular remodeling (40). A variety of mechanisms mediate arterial dilation and enlargement. In humans and rodents, ablation of the uterine microcirculation by spiral artery trophoblast invasion during hemochorial placentation decreases distal resistance and increases upstream blood flow velocity (although the velocity of blood entering the placenta may decrease) (8, 40). This in turn increases endothelial shear stress, which is known to stimulate both vasodilation and growth (39). Although the molecular identity of the mechanosensor(s) has not been established, we and others have shown that nitric oxide (NO) is a key mediator of both processes (4, 36, 45, 50, 51). Notably, several studies have established a relationship between tone and remodeling, showing that these processes are not exclusive (3, 6, 11, 33, 35, 47).
In view of their location on the luminal surface of the endothelium and reported mechanosensitivity, ion channels are attractive candidates for sensing and transducing shear stress. Cation channels, whose activation causes an increase in endothelial intracellular Ca2+ concentration ([Ca2+]i), are particularly appealing in this regard because Ca2+ elevation represents a common mechanism underlying endothelial vasodilator (e.g., NO, EDHF, and prostacyclin) production and release (24, 55, 58, 59).
Here, we hypothesized that the Piezo1 channel may be an important mechanosensor in the uterine circulation. First identified in 2010, Piezo proteins make up a unique class of large (1.2 Da) mechanically activated cation channels (13). Their structure resembles that of a propeller: a trimeric complex with a central pore topped by a cap, and whose subunits contain numerous transmembrane domains (57). A role for Piezo1 as a shear stress sensor upstream from NO synthase (NOS) activation is supported by several lines of evidence. Transfection of Piezo1 into human embryonic kidney-293 cells, which lack endogenous Piezo1 channels and normally do not respond to shear stress, resulted in shear stress-evoked elevations in human embryonic kidney cell [Ca2+]i (29). In human umbilical vein endothelial cells (ECs), Piezo1 was linked to endothelial NO synthase (eNOS) protein production and demonstrated involvement in mediating VEGF phosphorylation of eNOS (29). In Piezo1 haplo-insufficient animals, EC organization and alignment appeared abnormal and directionless compared with wild-type controls, revealing a distinct role for Piezo1 in shear stress-induced cell polarization (29). The linkage between Piezo1 activation by shear stress and NO release may involve other factors such as ATP, as shown in one study in which loss of this signaling pathway resulted in the development of hypertension (53). From a developmental standpoint, knockout experiments in mice have highlighted the importance of Piezo1 in embryonic vascular development. Embryos that lacked Piezo1 displayed inadequate vascular growth and maturation, which eventually proved to be lethal by days 9.5–11.5 of gestation (29, 42). Together, these studies indicate that Piezo1 channels are 1) sensitive to shear stress and 2) sufficient to evoke shear stress-induced Ca2+ entry, which is 3) linked to eNOS activation and 4) flow-induced dilation, and 5) play a role in vascular development. Surprisingly, neither their presence nor functionality have been examined in the uterine circulation, and it is not known whether Piezo1 expression is altered by gestation.
In this study, we used a combination of techniques to examine Piezo1 localization and protein expression in rat uterine arteries from pregnant and age-matched nonpregnant (NP) rats and to evaluate how Piezo1 channel activation and inhibition affect endothelial cytosolic Ca2+ and arterial vasodilatory responses. We also determined whether Piezo1 can be directly activated by flow-induced shear stress and, if so, whether their activation is linked to increases in EC Ca2+, NO production, and vasodilation. The results indicate that Piezo1 may be a physiologically important, functional, and formerly unrecognized molecular sensor for shear stress in the maternal uterine circulation and that its expression is upregulated during gestation.
METHODS
Animals
Twelve- to fourteen-week-old NP and late pregnant (LP; day 20/22 of gestation) female Sprague-Dawley rats (n = 32) were purchased from Charles River Laboratories (Saint-Constant, QC, Canada) and housed at the animal care facility at the University of Vermont. Food and water were provided ad libitum. After acclimation for at least 4 days, animals were anesthetized using 3% isoflurane and euthanized with a small animal guillotine. The uterus and its vasculature were removed, placed in a silicone-coated Petri dish, and immersed in HEPES-physiological salt solution (PSS) buffer (see Solutions). Segments of the main uterine artery (MUA) were carefully dissected from perivascular connective and adipose tissue and used in subsequent experiments. All protocols were approved by the University of Vermont Institutional Animal Care and Use Committee.
Immunohistochemistry
Freshly dissected segments of the MUA were fixed in 10% formalin overnight and stored in 70% ethanol. Tissues were embedded and sectioned by the Surgical Pathology Department at the University of Vermont Medical Center. After being processed, slides were deparaffinized using xylene and ethanol and rinsed with deionized H2O. Heat-induced epitope retrieval was carried out by immersing slides in DAKO target retrieval solution (50% glycerol + H2O, Agilent, Carpinteria, CA) and incubating at 95°C for 20 min. Slides were permitted to cool to room temperature in DAKO target retrieval solution and rinsed with PBS. Immunohistochemistry was performed by blocking sections with 10% normal goat serum in PBS-1% BSA-0.1% Triton X-100 for 1 h. Slides were then rinsed in PBS-1% BSA and incubated at room temperature overnight with rabbit polyclonal Piezo1 antibody (1:50, catalog no. APC-087, Alomone Laboratories, Jerusalem, Israel) (12). A negative control was performed by combining the Piezo1 antibody and Piezo1 peptide for 30 min before overnight incubation. After overnight incubation, slides were washed in PBS-1% BSA and incubated for 1 h with secondary antibody (Alexa Fluor GAR-488, 1:500, ThermoFisher Scientific, Waltham, MA). Slides were rinsed in PBS-1% BSA and stained with DAPI (1:500, Invitrogen, ThermoFisher Scientific) in PBS-1% BSA (10 μg/ml final concentration) for 15 min. Finally, slides were washed in PBS-1% BSA and affixed with coverslips using Aqua Polymount (Polysciences, Warrington, PA). Images were captured at the University of Vermont Microscopy Imaging Center using a Zeiss 510 META scanning confocal microscope.
Western Blot Quantification of Piezo1 Protein
Protein expression of Piezo1 was quantified by Western blot analysis. MUAs were freshly dissected from NP and LP rats. MUAs were lysed with Pierce RIPA buffer (ThermoFisher Scientific) supplemented with Halt protease inhibitor cocktail (ThermoFisher Scientific) and homogenized with Lysis Matrix D tubes (MP Biomedicals, Solon, OH) using two 30-s pulses on a FastPrep-24 instrument (MP Biomedicals). Protein quantities were determined by a BCA protein assay kit (ThermoFisher Scientific), and samples (15 μg of soluble protein each) were analyzed using 4–15% Mini-PROTEAN TGX gels. Rabbit polyclonal Piezo1 antibody (1:800, Alomone Laboratories), rabbit polyclonal β-tubulin antibody (1:3,000, Cell Signaling Technology, Danvers, MA), and horseradish peroxidase-conjugated secondary antibody (Abcam, Cambridge, UK) were used to detect specific proteins on the blot. Protein bands were detected by SuperSignal West Pico chemiluminescent substrate (ThermoFisher Scientific) and analyzed by densitometry using ImageJ software (National Institutes of Health).
Enzymatic Isolation of EC Sheets From MUAs and Fura 2-Based Measurements of Endothelial Intracellular [Ca2+]i
Isolated sheets of ECs (EC sheets) were obtained as previously described (20, 23). After dissection and careful cleaning, MUAs were cut open and placed in dissociation media (see composition below) for 10 min at 4–6°C before being placed in a solution containing neutral protease (dispase, 0.5 mg/ml) and elastase (0.5 mg/ml) for 60 min at 37°C; 0.5 mg/ml of collagenase type 1 was included for the final minute. Gentle trituration with a glass pipette induced dissociation of EC sheets from the arterial wall. To study [Ca2+]i responses, a suspension of EC sheets was placed into an experimental chamber. After 15 min to allow for equilibration and attachment of EC sheets to the glass bottom, the chamber was superfused with HEPES-PSS at 37°C for 20 min. Loading of ECs with fura-2 was performed during the next 10 min at room temperature under no-flow conditions followed by washout with HEPES-PSS for the next 15 min to allow intracellular deesterification of fura-2 AM. For each experiment, fura-2 fluorescence was recorded from one EC sheet containing 60–100 cells. Ca2+ measurements were initially made under basal conditions and after exposure to Yoda1 (10, 20, and 30 µM), a Piezo1 activator (10, 49).
Isolated Vessel Preparation
Segments of the MUA from NP and LP animals were cannulated in an arteriograph filled with HEPES-PSS and pressurized to 10 mmHg using a pressure servo pump (Living Systems Instrumentation, St. Albans, VT). Pressure was then gradually raised to 90 mmHg, and vessels were equilibrated at 37°C for 60 min. After equilibration, vessels were preconstricted by adding a thromboxane agonist U-46619 (5–10 nM) to the superfusate to produce a 40–60% reduction in lumen diameter. For all vessel experiments, changes in intraluminal diameter were measured using a video dimension analyzer (Living Systems Instrumentation) and WinDaq software (DATAQ Instruments, Akron, OH).
Role of NO in Vasodilation Induced by Pharmacological Activation of Piezo1
Segments of the MUA from NP and LP animals were cannulated and pressurized as indicated above. Before constriction by U-46619, vessels were pretreated for 20 min with a combination of Nω-nitro-l-arginine methyl ester (l-NAME; 100 μM) and Nω-nitro-l-arginine (l-NNA; 100 μM) to inhibit NO production. Once diameter had stabilized, the effect of Piezo1 activation was tested by the addition of an intermediate concentration of Yoda1 (20 µM) to the superfusate while lumen diameter was recorded (30 min).
Flow-Induced Vasodilation and Pharmacological Inhibition of Piezo1
Intraluminal flow was induced in cannulated MUA segments by creating a symmetrical pressure differential of 5–7 mmHg around a mean pressure of 90 mmHg using two independent pressure servo pumps connected to the inflow and outflow tubing. A constant flow rate of 60 μl/min was maintained for all experiments by measuring the volume of solution displaced by each turn of the pump and keeping a constant rate of rotation thereafter.
After preconstriction with U-46619, each vessel was exposed to three cycles of flow (10 min) versus no flow (25–35 min). Vessels that repeatedly dilated to flow and constricted when flow was shut off were deemed viable; others were discarded. The Piezo1 blocker Grammostola spatulata mechanotoxin 4 (GsMTx-4) (2, 19) was delivered intralumenally and allowed to equilibrate for 45–60 min before the induction of flow for a fourth time. Superfusion with acetylcholine (10−5 M, to confirm endothelial functionality) was followed by relaxing solution containing diltiazem and papaverine to achieve maximal dilation.
Role of NO in Flow-Induced Vasodilation
Vessels were cannulated, preconstricted, and repeatedly exposed to flow as stated above. The NOS inhibitors l-NAME (100 μM) and l-NNA (100 μM) were introduced into the vessel lumen and the superfusate for 30 min before the induction of flow for a fourth time. Both NOS inhibitors were used based on previous studies that showed that l-NAME and l-NNA in combination are more effective in blocking NOS than either alone (54, 56). As stated above, superfusion with acetylcholine was followed by relaxing solution containing diltiazem and papaverine.
Drugs and Solutions
HEPES-PSS contained the following (in mM): 141.8 sodium chloride, 4.7 potassium chloride, 1.7 magnesium sulfate, 2.8 calcium chloride, 1.2 potassium phosphate, 10.0 HEPES, 0.5 EDTA, and 5.0 dextrose. HEPES-PSS was prepared with deionized water and titrated with sodium hydroxide to a physiological pH of 7.4 at 37°C. All chemicals for HEPES-PSS were purchased from Fisher Scientific (Fair Lawn, NJ). l-NAME, l-NNA, papaverine, diltiazem, and acetylcholine were purchased from Sigma-Aldrich (St. Louis, MO). GsMTx-4 was purchased from Alomone Laboratories, Yoda1 was from AOBIOUS (Gloucester, MA), and U-46619 was from Cayman Chemical (Ann Arbor, MI). Dissociation medium for enzymatic isolation of ECs contained 55 mM NaCl, 80 mM Na glutamate, 5.9 mM KCl, 2 mM MgCl2, 0.1 mM CaCl2, 5 mM glucose, and 10 mM HEPES (pH was adjusted to 7.3). For the fura-2 calibration procedure, we used a solution of the following composition: 140 mM KCl, 20 mM NaCl, 5 mM HEPES, 5 mM EGTA, 1 mM MgCl2, 5 µM nigericin, and 10 µM ionomycin, pH 7.1. Fura-2 AM and pluronic acid were purchased from Invitrogen (Carlsbad, CA). Protease, elastase, and collagenase were purchased from Worthington Biochemical (Lakewood, NJ). Fura-2 AM was dissolved in dehydrated DMSO as a 1 mM stock solution, refrigerated in small aliquots, and used within 1 wk of preparation.
Calculations and Statistical Analysis
Western blot analysis.
Densitometric data were quantified using ImageJ software and analyzed using an unpaired t-test. Significant differences in Piezo1 protein expression between NP and LP groups were identified according to P < 0.05. Data are expressed as means ± SE.
EC [Ca2+]i.
EC [Ca2+]i was calculated using the following equation: [Ca2+]i = Kdβ(R − Rmin)/(Rmax − R), where R is an experimentally measured ratio (340/380 nm) of fluorescence intensities, Rmin is the ratio in the absence of [Ca2+]i, Rmax is the ratio under Ca2+-saturated fura-2 conditions, and β is the ratio of the fluorescence intensities at 380-nm excitation wavelength at Rmin and Rmax (22). Rmin, Rmax, and β were determined by an in situ calibration procedure from EC sheets loaded with fura-2 and treated with ionomycin (10 µM) and nigericin (5 µM). These values were then pooled and used to convert the ratio values into [Ca2+]i (in nM). Kd was 282 nM, as determined by in situ titration of Ca2+ in fura-2-loaded small arteries (26).
Ratio values were recorded using an IonOptix data acquisition program and imported into SigmaPlot verion 12.5 for graphical representation, calculations, and statistical analysis. Data are expressed as means ± SE, where n is the number of EC sheets studied. One EC sheet isolated from MUAs of NP or LP groups was used for a particular protocol. Two-way repeated-measures ANOVA was used to determine the significance of differences between sets of data, with P < 0.05 considered significant.
Vasodilation
Vasodilations to Yoda1 or flow were calculated as the percent change in lumen diameter relative to complete vasodilation obtained in response to acetylcholine or papaverine and diltiazem. Occasional flow-induced constrictions were observed and are reported as zero percent vasodilation. All data were analyzed using GraphPad Prism version 7.01. Two-way ANOVA was used to compare effects of NOS inhibition on Piezo1 activation by Yoda1 within and between NP and LP groups, with P < 0.05 considered significant. To compare the effect of Piezo1 and NOS inhibition within vessels from NP and LP animals, paired t-tests were used, with P < 0.05 considered significant. Data are expressed as means ± SE.
RESULTS
Piezo1 Is Localized to ECs and Smooth Muscle Cells in MUAs From NP and LP Rats
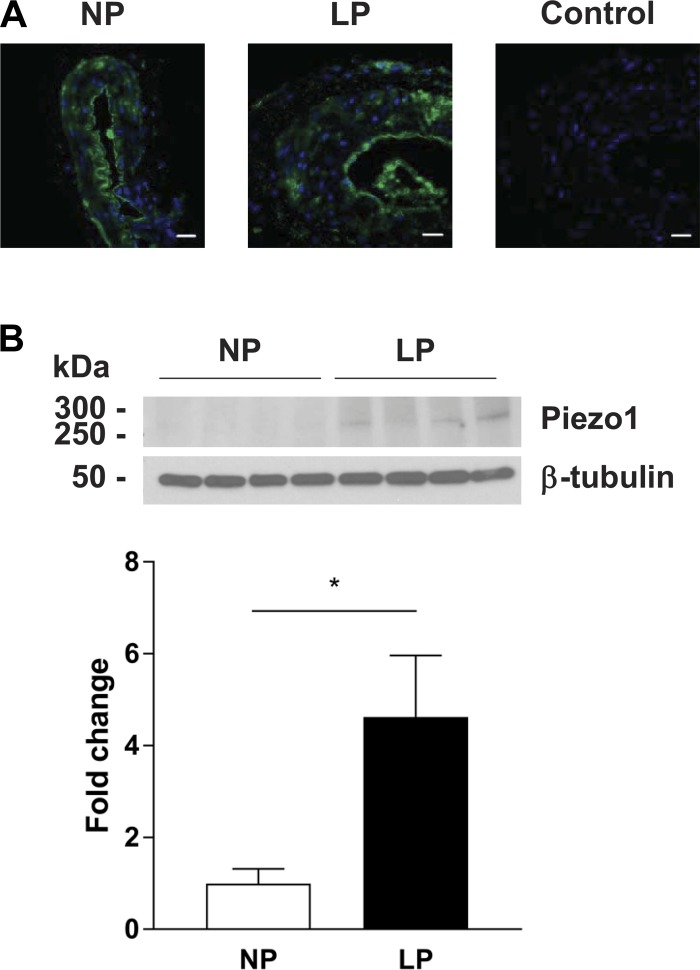
Stained cross-sections of MUAs showed a strong signal for Piezo1 in the EC layer in both vessels from NP and LP rats, with much weaker punctate staining detected in vascular smooth muscle (Fig. 1A).
Fig. 1.
Piezo1 channel localization and expression in segments of the main uterine artery (MUA). A: representative cross-sections of MUAs showing positive staining (green) in the endothelial layer; some punctate staining was also evident in vascular smooth muscle. Photographs show a vessel from an nonpregnant (NP) rat (left) and a corresponding section from a late pregnant (LP) rat (middle). The photo on the right is of a negative LP control showing absence of staining after exposure to primary antibody preincubated with the Piezo1 peptide (scale bar = 20 µm). Immunohistochemistry was representative of several independent experiments (NP: n = 3 and LP: n = 3). B: Western blot gel showing Piezo1 and β-tubulin protein expression in MUAs from NP and LP rats (n = 4 rats/group); densitometric analysis normalized to β-tubulin. Protein expression was significantly (P = 0.03) increased in vessels in LP vs. NP animals. Data are expressed as mean ± SE.
Piezo1 Protein Expression Is Upregulated During Pregnancy
As shown in Fig. 1B, although Piezo1 was detectable in MUAs from NP animals, protein expression was significantly increased by late pregnancy.
Chemical Activation of Piezo 1 With Yoda1 Results in Elevation of EC [Ca2+]i
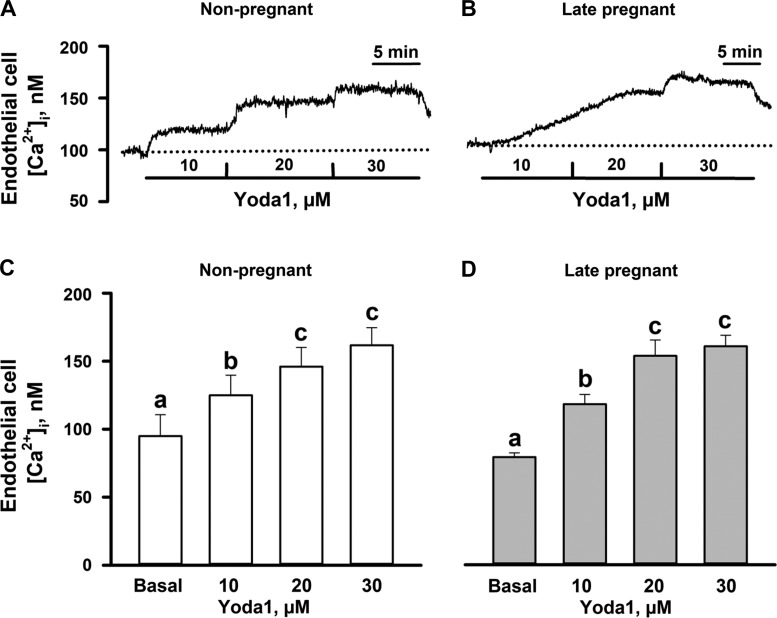
Basal levels of EC [Ca2+]i were similar in MUA ECs from NP (95 ± 17 nM, n = 6) and LP (79 ± 3 nM, n = 6) rats. Application of Yoda1 (10, 20, and 30 μM) resulted in comparable concentration-dependent elevations in [Ca2+]i in EC sheets isolated from MUAs of NP (125 ± 15, 146 ± 14, and 162 ± 13 nM) and LP (118 ± 7, 154 ± 12, and 161 ± 8 nM) rats (Fig. 2, A–D).
Fig. 2.
Activation of Piezo1 channels with Yoda1 results in concentration-dependent elevation of cytosolic Ca2+ concentration ([Ca2+]i) in main uterine artery (MUA) endothelial cell (EC) sheets. A and B: original tracings of [Ca2+]i elevations in response to increasing concentrations of Yoda1 in MUA EC sheets from a nonpregnant (NP) rat and a late pregnant (LP) rat. Dotted lines indicate basal (unstimulated) levels of [Ca2+]i. C and D: Yoda1 exposure led to significant EC [Ca2+]i elevations in EC sheets from NP and LP animals. Data are expressed as means ± SE. Significant differences at P < 0.05 (n = 6 per group, one-way repeated measures ANOVA). There were no differences in vessels from NP vs. LP animals (two-way repeated-measures ANOVA, P > 0.05).
Piezo1 Activation With Yoda1 Produces Vasodilation That Is Sensitive to NOS Inhibition
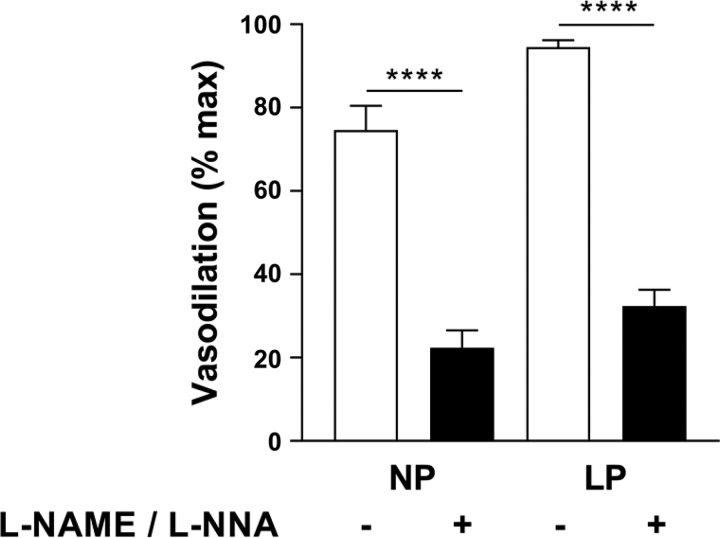
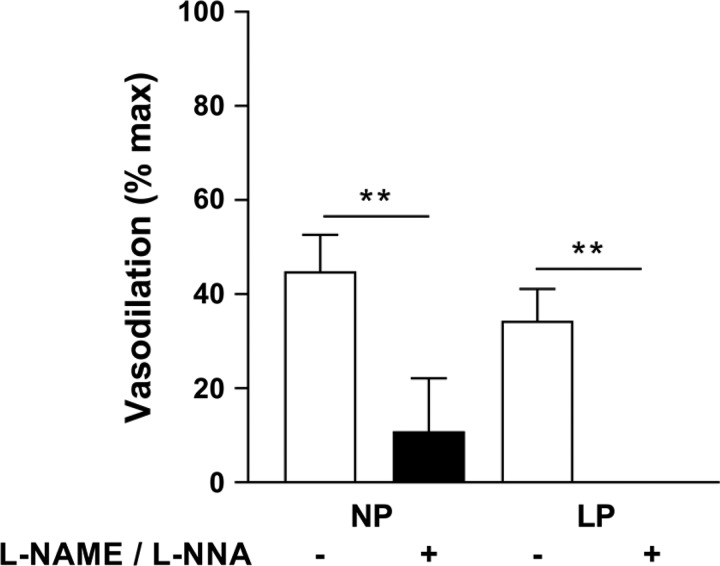
Application of Yoda1 (20 µM) to MUAs preconstricted with U-46619 induced significant vasodilation, with a larger effect in vessels from LP animals (P < 0.05). Preincubation with l-NAME + l-NNA significantly diminished vasodilation in MUAs from NP and LP rats and to a similar extent (60–70%; Fig. 3).
Fig. 3.
Nitric oxide synthase (NOS) inhibition significantly reduces main uterine artery (MUA) vasodilation to Piezo1 activation by Yoda1. Shown is vasodilation to Yoda1 (20 µM) in MUAs from nonpregnant (NP) and late pregnant (LP) rats with (+) and without (−) NOS inhibition [Nω-nitro-l-arginine methyl ester (l-NAME) and Nω-nitro-l-arginine (l-NNA), 100 µM each]. Data are expressed as mean percentages of maximal vasodilation using diltiazem and papaverine ± SE. P < 0.0001 for both groups (n = 4 per group; two-way ANOVA).
Intraluminal Flow Stimulates MUA Vasodilation Via Piezo1 Activation
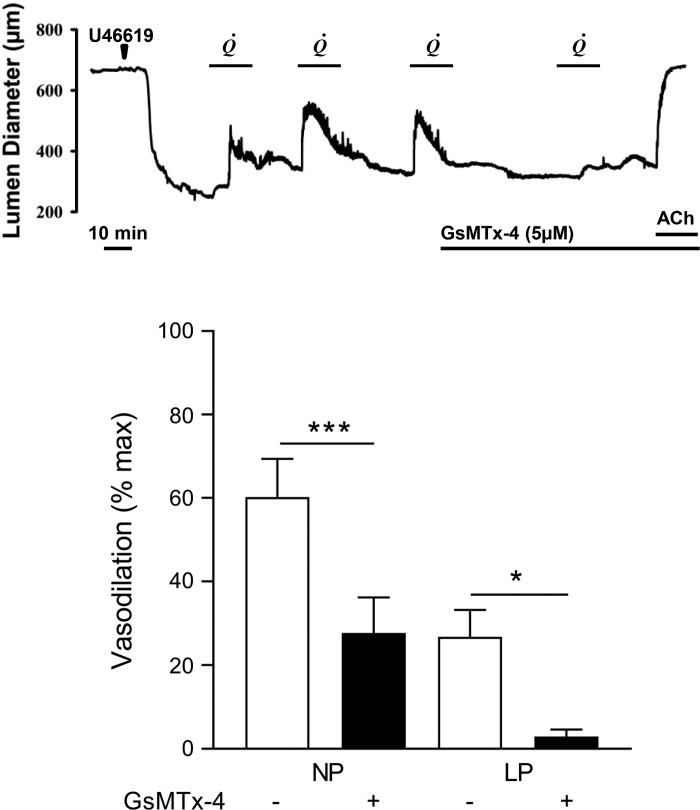
The induction of lumenal flow (60 µl/min) produced reversible vasodilation of MUAs preconstricted with U-46619 (representative tracing shown in Fig. 4A). Because of gestational expansive remodeling (39), vessels from LP rats were significantly larger than those from NP rats under both relaxed and preconstricted conditions (data not shown). Flow-induced vasodilation of vessels from LP animals was significantly smaller than that of NP animals. Preincubation with GsMTx-4, a Piezo1 inhibitor, significantly inhibited vasodilation in vessels from both NP and LP rats (n = 7 per group, P < 0.05; Fig. 4B).
Fig. 4.
Piezo1 inhibition by Grammostola spatulata mechanotoxin 4 (GsMTx-4) diminishes flow-induced main uterine artery (MUA) vasodilation. A: representative tracing from a flow experiment using a segment of the MUA from a late pregnant (LP) rat. After equilibration, the vessel was constricted with U-46619 (7 nM, arrowhead) and then exposed to three cycles of intraluminal flow (60 µl/min), which induced vasodilation. Introduction of the Piezo1 inhibitor GsMTx-4 at the end of the third cycle reduced vasodilation to a subsequent flow stimulus. Application of acetylcholine (ACh; 10 µM) induced complete vasodilation. B: summary showing the effects of GsMTx-4 on flow-induced vasodilation in MUAs from nonpregnant (NP) and LP rats (n = 7 per group). Data are expressed as mean ± SE. Significant differences at P < 0.05 within NP and LP groups (paired t-test).
NOS Inhibition Significantly Diminishes Flow-Induced MUA Dilations
Preincubation in l-NAME and l-NNA significantly decreased the maximal vasodilatory response to flow in MUAs from NP and LP animals (n = 4 per group). Moreover, in vessels from LP animals, pretreatment with NOS inhibitors resulted in small vasoconstriction instead of vasodilation in response to lumenal flow (Fig. 5).
Fig. 5.
Nitric oxide synthase (NOS) inhibition diminishes flow-induced dilations in main uterine arteries (MUAs) from nonpregnant (NP) and late pregnant (LP) rats. Flow-induced dilations with (+) and without (−) NOS inhibition [Nω-nitro-l-arginine methyl ester (l-NAME) and Nω-nitro-l-arginine (l-NNA), 100 µM each] in vessels from NP and LP rats (n = 4 per group). In vessels from LP animals, vasodilation was completely abolished and often resulted in vasoconstriction. Data are expressed as mean ± SE. Significant differences at P < 0.01 within NP and LP groups (paired t-test).
DISCUSSION
Involvement of Piezo1 Channels in Mechanotransduction of Endothelial Shear Stress
The working hypothesis in this study was that Piezo1 channels are important endothelial mechanosensors for shear stress in the uterine circulation and that their activation will stimulate arterial vasodilation (and, presumably, outward remodeling).
Localization and Expression of Piezo1 Channels in MUAs From NP and LP Animals
Consistent with a role for Piezo1 sensing shear stress in the uterine circulation, Piezo1 expression was localized primarily in the EC layer in MUAs from NP and LP animals. Piezo1 was also detectable in the medial layer, although its distribution in vascular smooth muscle was more punctate and less dense than in the endothelium. Other studies have shown Piezo1 to be present in smooth muscle (44), nerves, and tissues exposed to stretch and pressure (e.g., renal epithelial and bladder urothelial cells) (57) in addition to the endothelium (e.g., aortic, pulmonary, and cerebral) (29).
As shown by the Western blot results, Piezo1 protein levels were significantly higher in arteries from LP versus NP animals. This indicates that pregnancy affects Piezo1 expression, although neither the time course nor the factors responsible for this effect (e.g., the endocrine milieu of pregnancy or altered shear stress secondary to placentation) are known.
Effects of Piezo1 Activation on Endothelial Ca2+
Consistent with results from a study by Li et al. (28) pharmacological activation of Piezo1 by Yoda1 in freshly isolated sheets of uterine artery ECs induced concentration-dependent rises in EC [Ca2+]i, although there were no significant differences between EC sheets from NP versus LP animals in the extent of Ca2+ elevation as a function of Yoda1 concentration. This was surprising in view of the differences in Piezo1 protein expression and may have to do with the absence of mechanical load (normally present in the intact artery because of intravascular pressure) in isolated EC sheets.
Functional Effects of Piezo1 Activation
Having demonstrated a linkage between Piezo1 activation and elevations in EC [Ca2+]i, we shifted our focus to pressurized segments of MUAs to examine a role for Piezo1 in vasodilation and to determine if there is a link between Piezo1 and the production of NO. Consistent with Wang et al. (53), who used the Piezo1-specific activator Yoda1 to induce concentration-dependent dilations in mesenteric arteries, we observed significant dilations to Yoda1 in both NP and LP vessels, with a larger effect in the latter. This is consistent with the Western blot data and may also be a result of the increased NO signaling often reported in pregnancy (36).
Vasodilations that occurred in the presence of NOS inhibition, although diminished significantly, were not completely abolished in either case, suggesting that the effect of Piezo1 activation was not limited to the release of NO. Rather, this cation channel leads to increases in EC Ca2+, an event that has been associated with the Ca2+-induced release of other endothelial vasodilators, e.g., EDHF and prostacyclin (9, 17, 21, 38, 52), which may account for the residual vasodilation (Fig. 3).
Piezo1 Involvement in Flow-Induced Vasodilatory Responses
To evaluate the functional effect of Piezo1 in the uterine circulation, an in vitro system for inducing shear stress by luminal flow while measuring changes in the lumen diameter of preconstricted uterine arteries was used. Although repeated and reversible vasodilations could be induced, their magnitude was smaller in vessels from LP animals. This may be because of their larger diameter, which would reduce shear stress at the same flow rate. Consistent with previous data on remodeled vessels, our pressurized diameter measurements reflected expansive changes that typically occur during pregnancy (40).
Consistent with experiments on Piezo1 knockout mice, whose mesenteric arteries almost completely lost the ability to respond to flow (53), reversible dilations to intraluminal flow were significantly reduced after incubation with GsMTx-4, a Piezo1 antagonist. As with any pharmacological probe, we cannot exclude nonspecific effects of GsMTx-4 on Na+ voltage-gated channels and certain K+ channels as well as transient receptor potential (TRP)C1 and TRPC6 channels (1, 43, 48).
Shear stress-induced activation of Piezo1 appears to be primarily linked to vasodilation via an NO mechanism, as preincubation with l-NNA and l-NAME significantly (>70%) reduced flow-induced responses. Furthermore, we observed flow-induced constrictions in LP vessels treated with NOS inhibitors, suggesting that in the absence of NOS activity, Piezo1 activation may lead to smooth muscle depolarization leading to constriction (44).
Study Limitations
We were unable to directly evaluate the effect of Piezo1 on uterine arterial remodeling, as this would require longer-term in vivo intervention. Instead, we examined basic functional properties of the channel that support well-documented physiological processes (e.g., channel stimulation leading to Ca2+ influx and vasodilation). These two processes may be related, as others have shown that constriction leads to inward remodeling (3, 16, 32) and that inhibition of arterial smooth muscle activation leads to outward eutrophic remodeling (6, 11, 33, 35, 47).
In addition, our flow/perfusion system did not replicate physiological conditions in which flow and shear stress are oscillatory. Finally, we acknowledge that other channels (notably those of the TRP family) may also be involved in uterine artery endothelial mechanotransduction (15, 24, 27, 28, 30, 58) and that they may interact with Piezo1 channels in ways that are complementary or synergistic (5, 14, 18, 46).
Conclusions
This is the first report showing that Piezo1 channels are present in the uterine circulation and regulated by pregnancy. Their activation by shear stress leads to arterial vasodilation through a mechanism linked to NO. Piezo1 channels may therefore be a previously unrecognized, physiologically important endothelial mechanosensor involved in the regulation of uteroplacental blood flow.
GRANTS
Confocal microscopy was performed on a Zeiss 510 META laser scanning confocal microscope supported by National Institutes of Health (NIH) Grant 1-S10-RR-019246. This work was supported by NIH Grants R21-HD-080156 and RO1-HL-134371 (to G. Osol).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.J., N.L.K., A.G., and N.G. performed experiments; L.J., N.L.K., A.G., N.G., and G.O. analyzed data; L.J., A.G., N.G., and G.O. interpreted results of experiments; L.J., N.L.K., and N.G. prepared figures; L.J. drafted manuscript; L.J., N.G., M.M., and G.O. edited and revised manuscript; L.J., N.L.K., A.G., N.G., M.M., and G.O. approved final version of manuscript; M.M. and G.O. conceived and designed research.
ACKNOWLEDGMENTS
We acknowledge the Microscopy Imaging Center at the University of Vermont.
REFERENCES
- 1.Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci 29: 6217–6228, 2009. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 50: 6295–6300, 2011. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker EN, van der Meulen ET, van den Berg BM, Everts V, Spaan JA, VanBavel E. Inward remodeling follows chronic vasoconstriction in isolated resistance arteries. J Vasc Res 39: 12–20, 2002. doi: 10.1159/000048989. [DOI] [PubMed] [Google Scholar]
- 4.Barron C, Mandala M, Osol G. Effects of pregnancy, hypertension and nitric oxide inhibition on rat uterine artery myogenic reactivity. J Vasc Res 47: 463–471, 2010. doi: 10.1159/000313874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci Signal 8: ra15, 2015. doi: 10.1126/scisignal.2005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briet M, Schiffrin EL. Treatment of arterial remodeling in essential hypertension. Curr Hypertens Rep 15: 3–9, 2013. doi: 10.1007/s11906-012-0325-0. [DOI] [PubMed] [Google Scholar]
- 7.Brosens I. A study of the spiral arteries of the decidua basalis in normotensive and hypertensive pregnancies. J Obstet Gynaecol Br Commonw 71: 222–230, 1964. doi: 10.1111/j.1471-0528.1964.tb04270.x. [DOI] [PubMed] [Google Scholar]
- 8.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30: 473–482, 2009. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–380, 2002. doi: 10.1016/S0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 10.Cahalan SM, Lukacs V, Ranade SS, Chien S, Bandell M, Patapoutian A. Piezo1 links mechanical forces to red blood cell volume (Abstract). eLife 4: e07370, 2015. doi: 10.7554/eLife.07370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen KL, Mulvany MJ. Vasodilatation, not hypotension, improves resistance vessel design during treatment of essential hypertension: a literature survey. J Hypertens 19: 1001–1006, 2001. doi: 10.1097/00004872-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in rats with ligated femoral arteries. Am J Physiol Heart Circ Physiol 310: H1233–H1241, 2016. doi: 10.1152/ajpheart.00974.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coste B, Murthy SE, Mathur J, Schmidt M, Mechioukhi Y, Delmas P, Patapoutian A. Piezo1 ion channel pore properties are dictated by C-terminal region. Nat Commun 6: 7223, 2015. doi: 10.1038/ncomms8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dragovich MA, Chester D, Fu BM, Wu C, Xu Y, Goligorsky MS, Zhang XF. Mechanotransduction of the endothelial glycocalyx mediates nitric oxide production through activation of TRP channels. Am J Physiol Cell Physiol 311: C846–C853, 2016. doi: 10.1152/ajpcell.00288.2015. [DOI] [PubMed] [Google Scholar]
- 16.Dunn WR, Wallis SJ, Gardiner SM. Remodelling and enhanced myogenic tone in cerebral resistance arteries isolated from genetically hypertensive Brattleboro rats. J Vasc Res 35: 18–26, 1998. doi: 10.1159/000025561. [DOI] [PubMed] [Google Scholar]
- 17.Félétou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 117: 139–155, 2009. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- 18.Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Li R, Gao N, Xiao B, Yang M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 527: 64–69, 2015. doi: 10.1038/nature15247. [DOI] [PubMed] [Google Scholar]
- 19.Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS, Sukharev SI, Suchyna TM. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophys J 112: 31–45, 2017. doi: 10.1016/j.bpj.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokina NI, Bonev AD, Gokin AP, Goloman G. Role of impaired endothelial cell Ca2+ signaling in uteroplacental vascular dysfunction during diabetic rat pregnancy. Am J Physiol Heart Circ Physiol 304: H935–H945, 2013. doi: 10.1152/ajpheart.00513.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gokina NI, Kuzina OY, Vance AM. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am J Physiol Heart Circ Physiol 299: H1642–H1652, 2010. doi: 10.1152/ajpheart.00227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 23.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31: 1175–1186, 2011. doi: 10.1038/jcbfm.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: performing under pressure and going with the flow. Physiology (Bethesda) 29: 343–360, 2014. doi: 10.1152/physiol.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev 71, Suppl 1: S18–S25, 2013. doi: 10.1111/nure.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohler R, Hoyer J. Role of TRPV4 in the mechanotransduction of shear stress in endothelial cells. In: TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, edited by Liedtke WB, Heller S. Boca Raton, FL: CRC, 2007. [PubMed] [Google Scholar]
- 28.Li J, Hou B, Beech DJ. Endothelial Piezo1: life depends on it. Channels (Austin) 9: 1–2, 2015. doi: 10.4161/19336950.2014.986623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad RK, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature 515: 279–282, 2014. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, Wong CO, Huang Y, Yao X. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol 30: 851–858, 2010. doi: 10.1161/ATVBAHA.109.196584. [DOI] [PubMed] [Google Scholar]
- 31.Mandala M, Osol G. Physiological remodelling of the maternal uterine circulation during pregnancy. Basic Clin Pharmacol Toxicol 110: 12–18, 2012. doi: 10.1111/j.1742-7843.2011.00793.x. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Lemus LA, Hill MA, Bolz SS, Pohl U, Meininger GA. Acute mechanoadaptation of vascular smooth muscle cells in response to continuous arteriolar vasoconstriction: implications for functional remodeling. FASEB J 18: 708–710, 2004. doi: 10.1096/fj.03-0634fje. [DOI] [PubMed] [Google Scholar]
- 33.Mathiassen ON, Buus NH, Larsen ML, Mulvany MJ, Christensen KL. Small artery structure adapts to vasodilatation rather than to blood pressure during antihypertensive treatment. J Hypertens 25: 1027–1034, 2007. doi: 10.1097/HJH.0b013e3280acac75. [DOI] [PubMed] [Google Scholar]
- 34.Moll W, Espach A, Wrobel KH. Growth of mesometrial arteries in guinea pigs during pregnancy. Placenta 4: 111–123, 1983. doi: 10.1016/S0143-4004(83)80024-1. [DOI] [PubMed] [Google Scholar]
- 35.Mulvany MJ. Abnormalities of the resistance vasculature in hypertension: correction by vasodilator therapy. Pharmacol Rep Suppl 57: 144–150, 2005. [PubMed] [Google Scholar]
- 36.Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circ Res 87: 406–411, 2000. doi: 10.1161/01.RES.87.5.406. [DOI] [PubMed] [Google Scholar]
- 37.Osol G, Cipolla M. Pregnancy-induced changes in the three-dimensional mechanical properties of pressurized rat uteroplacental (radial) arteries. Am J Obstet Gynecol 168: 268–274, 1993. doi: 10.1016/S0002-9378(12)90924-2. [DOI] [PubMed] [Google Scholar]
- 38.Osol G, Ko NL, Mandalà M. Altered endothelial nitric oxide signaling as a paradigm for maternal vascular maladaptation in preeclampsia. Curr Hypertens Rep 19: 82, 2017. doi: 10.1007/s11906-017-0774-6. [DOI] [PubMed] [Google Scholar]
- 39.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osol G, Moore LG. Maternal uterine vascular remodeling during pregnancy. Microcirculation 21: 38–47, 2014. doi: 10.1111/micc.12080. [DOI] [PubMed] [Google Scholar]
- 41.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol 80: 1000–1006, 1992. [PubMed] [Google Scholar]
- 42.Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li YS, Chien S, Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA 111: 10347–10352, 2014. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redaelli E, Cassulini RR, Silva DF, Clement H, Schiavon E, Zamudio FZ, Odell G, Arcangeli A, Clare JJ, Alagón A, de la Vega RC, Possani LD, Wanke E. Target promiscuity and heterogeneous effects of tarantula venom peptides affecting Na+ and K+ ion channels. J Biol Chem 285: 4130–4142, 2010. doi: 10.1074/jbc.M109.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ, Bailey MA, Yuldasheva NY, Ludlow MJ, Cubbon RM, Li J, Futers TS, Morley L, Gaunt HJ, Marszalek K, Viswambharan H, Cuthbertson K, Baxter PD, Foster R, Sukumar P, Weightman A, Calaghan SC, Wheatcroft SB, Kearney MT, Beech DJ. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun 8: 350, 2017. doi: 10.1038/s41467-017-00429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 101: 731–736, 1998. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Servin-Vences MR, Moroni M, Lewin GR, Poole K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife 6: 6, 2017. doi: 10.7554/eLife.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorop O, Bakker EN, Pistea A, Spaan JA, VanBavel E. Calcium channel blockade prevents pressure-dependent inward remodeling in isolated subendocardial resistance vessels. Am J Physiol Heart Circ Physiol 291: H1236–H1245, 2006. doi: 10.1152/ajpheart.00838.2005. [DOI] [PubMed] [Google Scholar]
- 48.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586–16591, 2006. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, Petrassi HM, Schumacher AM, Montal M, Bandell M, Patapoutian A. Chemical activation of the mechanotransduction channel Piezo1 (Abstract). eLife 4: e07369, 2015. doi: 10.7554/eLife.07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol 281: H1380–H1389, 2001. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- 51.van der Heijden OW, Essers YP, Fazzi G, Peeters LL, De Mey JG, van Eys GJ. Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol Reprod 72: 1161–1168, 2005. doi: 10.1095/biolreprod.104.033985. [DOI] [PubMed] [Google Scholar]
- 52.Vanhoutte PM, Zhao Y, Xu A, Leung SW. Thirty years of saying NO: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res 119: 375–396, 2016. doi: 10.1161/CIRCRESAHA.116.306531. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest 126: 4527–4536, 2016. doi: 10.1172/JCI87343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wedel Jones C, Mandala M, Barron C, Bernstein I, Osol G. Mechanisms underlying maternal venous adaptation in pregnancy. Reprod Sci 16: 596–604, 2009. doi: 10.1177/1933719109332820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. TRPV4: molecular conductor of a diverse orchestra. Physiol Rev 96: 911–973, 2016. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- 56.Williams JM, Hull AD, Pearce WJ. Maturational modulation of endothelium-dependent vasodilatation in ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol 288: R149–R157, 2005. doi: 10.1152/ajpregu.00427.2004. [DOI] [PubMed] [Google Scholar]
- 57.Wu J, Lewis AH, Grandl J. Touch, tension, and transduction - the function and regulation of piezo ion channels. Trends Biochem Sci 42: 57–71, 2017. doi: 10.1016/j.tibs.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang DX, Gutterman DD. Transient receptor potential channel activation and endothelium-dependent dilation in the systemic circulation. J Cardiovasc Pharmacol 57: 133–139, 2011. doi: 10.1097/FJC.0b013e3181fd35d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y, Vanhoutte PM, Leung SW. Vascular nitric oxide: beyond eNOS. J Pharmacol Sci 129: 83–94, 2015. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]