Abstract
Evidence suggests that the peak skeletal muscle mitochondrial ATP synthesis rate (Vmax) in patients with peripheral artery disease (PAD) may be attenuated due to disease-related impairments in O2 supply. However, in vitro assessments suggest intrinsic deficits in mitochondrial respiration despite ample O2 availability. To address this conundrum, Doppler ultrasound, near-infrared spectroscopy, phosphorus magnetic resonance spectroscopy, and high-resolution respirometry were combined to assess convective O2 delivery, tissue oxygenation, Vmax, and skeletal muscle mitochondrial capacity (complex I + II, state 3 respiration), respectively, in the gastrocnemius muscle of 10 patients with early stage PAD and 11 physical activity-matched healthy control (HC) subjects. All participants were studied in free-flow control conditions (FF) and with reactive hyperemia (RH) induced by a period of brief ischemia during the last 30 s of submaximal plantar flexion exercise. Patients with PAD repeated the FF and RH trials under hyperoxic conditions (FF + 100% O2 and RH + 100% O2). Compared with HC subjects, patients with PAD exhibited attenuated O2 delivery at the same absolute work rate and attenuated tissue reoxygenation and Vmax after relative intensity-matched exercise. Compared with the FF condition, only RH + 100% O2 significantly increased convective O2 delivery (~44%), tissue reoxygenation (~54%), and Vmax (~60%) in patients with PAD (P < 0.05), such that Vmax was now not different from HC subjects. Furthermore, there was no evidence of an intrinsic mitochondrial deficit in PAD, as assessed in vitro with adequate O2. Thus, in combination, this comprehensive in vivo and in vitro investigation implicates O2 supply as the predominant factor limiting mitochondrial oxidative capacity in early stage PAD.
NEW & NOTEWORTHY Currently, there is little accord as to the role of O2 availability and mitochondrial function in the skeletal muscle dysfunction associated with peripheral artery disease. This is the first study to comprehensively use both in vivo and in vitro approaches to document that the skeletal muscle dysfunction associated with early stage peripheral artery disease is predominantly a consequence of limited O2 supply and not the impact of an intrinsic mitochondrial defect in this pathology.
Keywords: blood flow, exercise, magnetic resonance spectroscopy, metabolism, mitochondria, peripheral vascular disease, ultrasound
INTRODUCTION
Peripheral artery disease (PAD) is characterized by insufficient tissue O2 supply secondary to reduced blood flow both at rest and during even submaximal exercise (49, 64, 67). This form of atherosclerosis currently affects ~8–12 million Americans (49, 50) and translates to $4 billion in Medicare costs annually (26). The clinical diagnosis of PAD is confirmed by an ankle brachial index (ABI) of ≤0.90, with a progressively lower ABI indicating greater severity of the disease (13). Somewhat surprisingly, only ~10% of patients with PAD are symptomatic, experiencing calf muscle pain while walking, termed claudication (25). However, as highlighted by this observation, the impact of this disease is not isolated to a clearly affected limb. In fact, individuals diagnosed with PAD are faced with a 5-yr mortality rate as high as 30% (49), further demonstrating the need to better understand the pathophysiology of this far-reaching disease.
The limited function of the locomotor muscles in patients with PAD was originally attributed solely to altered hemodynamics as a consequence of lower limb ischemia (54, 55). Indeed, data collected in vivo with 31P magnetic resonance spectroscopy (MRS) revealed that the peak skeletal muscle mitochondrial ATP synthesis rate (Vmax) was attenuated in the skeletal muscle of patients with PAD (28, 29, 31, 32), and this was attributed to a disease-related impairment in O2 supply (28, 29, 31, 32). However, several in vitro assessments of skeletal muscle mitochondrial respiration, during which there is ample O2 availability, have revealed functional deficits in patients with PAD, alluding to mitochondrial dysfunction as a component of this disease (10, 56). However, it should be noted that this may be a consequence of studying the later stages of this disease associated with critical limb ischemia. In contrast, our group recently documented preserved mitochondrial respiratory function despite increased mitochondria-derived reactive oxygen species in patients with relatively early stage PAD (19). Therefore, perhaps fueled, at least in part, by end-stage metabolic changes in patients with PAD, currently there is little accord as to the role of O2 availability and mitochondrial function in PAD.
With the aim to assess mitochondrial oxidative capacity in both health and disease, previous studies have increased the fraction of inspired O2 () to augment the Po2 in blood and subsequently increase muscle O2 availability. This approach has revealed a metabolic reserve in individuals with an O2 delivery limitation (21, 36, 61). More recently, the influence of O2 delivery on mitochondrial oxidative capacity, defined here as the intrinsic mitochondrial capacity for ATP synthesis, was investigated by superimposing reactive hyperemia (RH), induced by a period of brief ischemia toward the end of exercise, on the recovery from plantar flexion exercise (36, 37). The consequence of this intervention was assessed with an integrative approach combining 31P MRS, near-infrared spectroscopy (NIRS), and Doppler ultrasound. RH significantly increased convective O2 delivery and Vmax in both young and old healthy subjects (36, 37). Interestingly, neither the impact of hyperoxia nor RH on the oxidative capacity of the skeletal muscle of patients with PAD, a population recognized to have reduced muscle O2 saturation and perfusion (12, 14), has been evaluated and could provide new insights into the role of O2 availability and skeletal muscle mitochondrial oxidative capacity in patients with PAD.
Therefore, this study sought to determine the role that O2 availability and utilization play in the skeletal muscle dysfunction associated with documented but relatively early stage PAD by combining both in vivo and in vitro assessments of skeletal muscle oxidative capacity and the manipulation of O2 availability. We hypothesized that increased postexercise O2 availability would restore tissue reoxygenation and Vmax in patients with early stage PAD to that of well-matched healthy control subjects. Furthermore, based on this expected capacity for O2 availability to influence skeletal muscle oxidative capacity, we hypothesized that mitochondrial respiration per milligram of tissue assessed in vitro would not be different between patients with early stage PAD and healthy control (HC) subjects, further implicating O2 availability and not intrinsic mitochondrial dysfunction in the fundamental pathology of PAD.
METHODS
Subjects.
A total of 21 subjects (10 patients with clear but relatively early stage PAD and 11 physical activity-matched HC subjects) were recruited to participate in this study. Inclusion criteria for patients with PAD included a clinical diagnosis with evidence of femoropopliteal occlusive disease (ABI ≤ 0.90), intermittent claudication, and no prior PAD-related surgical interventions. Patients presented with clinical symptoms characteristic of early stage PAD corresponding to Fontaine stages IIa and IIb, indicating mild to moderate intermittent claudication (18). All HC subjects were normotensive (<140/90 mmHg), not taking any medication recognized to alter blood flow or metabolism, and showed no evidence of overt cardiovascular disease. Participants were recruited based on being moderately physically active (assessed by both interview and 7 days of accelerometry measurements) and capable of performing the required plantar flexion exercise. All women were postmenopausal and not taking any form of estrogen replacement therapy, minimizing the influence of female hormones. All participants reported to the laboratory for testing in a fasted state (>8 h postprandial) and refrained from caffeine or strenuous exercise before any assessments (>24 h). The protocol was approved by the Human Research Protection Program at the University of Utah and the Salt Lake City Veterans Affairs Medical Center, and written informed consent was attained from all subjects before participation. The comprehensive assessment of skeletal muscle mitochondrial respiratory function of these subjects in vitro has been previously published (19).
Exercise protocols.
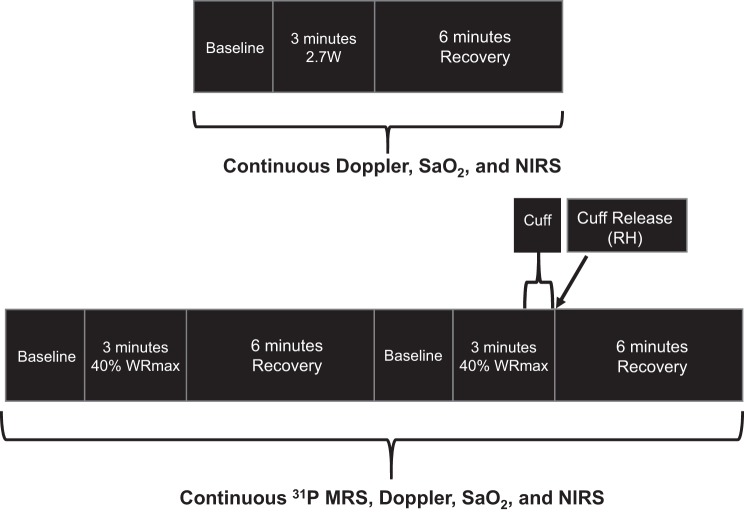
After familiarization, the individual maximum plantar flexion work rate (WRmax) was determined by performing incremental dynamic plantar flexion exercise with 1-min stages with 0.5- to 1-W increments and a cadence of 1 Hz until exhaustion. A schematic of the experimental protocols is shown in Fig. 1. On separate days, subjects performed constant-load submaximal plantar flexion at an absolute work rate of 2.7 W followed by a moderate-intensity work rate of ~40% of WRmax (frequency of 1 Hz) either under conditions of free flow (FF) or RH induced by a cuff occlusion during the last 30 s of exercise. These assessments were performed in the whole body magnetic resonance imaging system (TimTrio 3T Siemens Medical Systems, Erlangen, Germany) operating at 49.9 MHz for 31P to facilitate the MRS. Specifically, after 2 min of rest, subjects exercised for 3 min followed by 5 min of recovery. Patients with PAD performed two additional protocols in the magnet under FF + 100% O2 and RH + 100% O2 conditions. During these hyperoxic protocols, patients with PAD breathed through a low-resistance two-way breathing valve (model 2700, Hans-Rudolph, Kansas City, MO) connected to a Douglas bag containing the hyperoxic gas (: 1.0). All protocols were repeated on separate days to measure limb blood flow as well as tissue oxygenation using Doppler ultrasound and NIRS, respectively. Heart rate and arterial O2 saturation were monitored continuously throughout the experiments with a finger probe oximeter (OxiMax N-600x, Nellcor, Pleasanton, CA). Before initiation of these protocols, a blood sample was collected to perform a complete blood cell count and a 10-m walk time test was performed to assess functional disability.
Fig. 1.
Schematics for the plantar flexion exercise protocols performed at both absolute (top) and relative (bottom) exercise intensities. SaO2, O2 saturation; NIRS, near-infrared spectroscopy; RH, reactive hyperemia; WRmax, maximum work rate; MRS, magnetic resonance spectroscopy.
31P MRS.
31P MRS data were acquired with a dual-tuned 31P-1H surface coil with linear polarization (Rapid biomedical, Rimpar, Germany) positioned around the calf at its maximum diameter as previously described (20). Briefly, before each experiment, three fully relaxed spectra were acquired at rest with three accumulations per spectrum and a repetition time of 30 s. MRS data were acquired throughout the rest-exercise-recovery protocol. Each spectrum was time averaged over 6 s (3 scans/spectrum). Saturation factors were quantified by the comparison between relaxed (repetition time: 30 s) and partially relaxed spectra (repetition time: 2 s). As previously described (34), relative concentrations of phosphocreatine (PCr), inorganic phosphate, and ATP were obtained by a time-domain fitting routine using the advanced method for the accurate, robust, and efficient spectral fitting algorithm (71) incorporated into CSIAPO software (38).
Popliteal blood flow, O2 delivery, and tissue oxygenation.
Measurements of popliteal artery blood velocity and vessel diameter were performed in the popliteal fossa of the exercising leg, proximal to the branching of the medial inferior genicular artery, with a Logic 7 Doppler ultrasound system (General Electric Medical Systems, Milwaukee, WI), as previously described (20). Mean arterial pressure (MAP) was determined with a Finometer (Finapress Medical Systems, Amsterdam, The Netherlands). Arterial O2 content was calculated as the sum of hemoglobin (Hb)-bound O2 (1.34 × Hb × O2 saturation) and dissolved O2 (0.003/Po2) using Hb content, with Hb saturation measured by a pulse oximeter (OxiMax, Nellcor) and Po2 calculated from Hb saturation based on a normal Hb association curve (65). Convective O2 delivery (bulk delivery of O2) was then calculated as the product of O2 contents and popliteal artery blood flow. Tissue oxygenation, providing some insight into diffusive O2 delivery (movement of O2 from blood to tissue), was assessed with a near-infrared frequency resolved spectroscopy oximeter (Oxiplex TS, ISS) using the NIRS technique, as previously described (20). The use of NIRS to assess changes in O2 saturation in the calf muscles during exercise has been previously documented (17, 42, 43).
Muscle biopsy.
A core biopsy from the anteromedial aspect of the gastrocnemius muscle belly, ~10 cm distal to the tibial tuberosity, was obtained by a percutaneous needle biopsy at a depth of ~1.0 cm under sterile conditions. Immediately after removal of the muscle sample (~150 mg) from the leg, ~20% of the sample was immersed in biopsy preservation fluid (BIOPS) at 4°C for respiratory analysis, as previously described (52), and the remaining muscle sample was snap frozen and stored at −80°C for biochemical analyses.
Mitochondrial respiration in vitro.
Muscle samples were prepared and permeabilized as previously described by Pesta et al. (52). Briefly, BIOPS-immersed fibers were carefully separated with fine-tip forceps and subsequently bathed in a BIOPS-based saponin solution (50 μg saponin/ml BIOPS) for 30 min. After saponin treatment, muscle fibers were rinsed twice in ice-cold mitochondrial respiration fluid (MIR05) for 10 min each rinse. The wet weight of the muscle samples (~4 mg wet wt) was then assessed on a calibrated scale. Muscle fibers were then placed in two temperature-controlled respiration chambers (Oxytherm, Hansatech Instruments, Norfolk, UK) in 2 ml of MIR05 solution and warmed to 37°C, allowing duplicate measurements. Specifically, state 3 complex I + II respiration was assessed with malate (2 mM) + glutamate (10 mM) + ADP (5 mM) + succinate (10 mM). Cytochrome c (10 µM) was added to the bath to verify membrane integrity, as recommended by Pesta et al. (52). Step changes in respiration lasted as long as required to produce a steady-state respiration rate, typically ~3 min in duration. Mitochondrial respiration was corrected for background respiration inherent to the experimental setup. Respiration data for each of the two separate muscle fiber samples were then averaged. The peak mitochondrial respiration rate was mathematically adjusted to account for an increase in muscle temperature during exercise from 37 to 38°C and expressed as milliliters per kilogram per minute, as previously described (15).
Citrate synthase activity.
After the assessment of respiration, muscle samples (~4 mg wet wt) were homogenized with homogenization buffer [containing (in mM) 250 sucrose, 40 KCl, 2 EGTA, and 20 Tris·HCl, Qiagen, Hilden, Germany]. The citrate synthase (CS) activity assay was performed as previously described (53) and read with a spectrophotometer (Synergy 4, Biotek Instruments, Winooski, VT).
Data analysis.
PCr and tissue oxygenation index (TOI) recovery kinetics were determined by fitting the time-dependent changes during the recovery period to a single-exponential curve, as previously described (20). Muscle Vmax (in mM/min) was calculated using the initial rate of PCr synthesis (ViPCr) during the postexercise recovery period and the ADP concentration obtained at the end of exercise, as previously described (69):
in which Km is ∼30 µM in skeletal muscle (30).
Statistical analysis.
Statistical analyses were performed using commercially available software (SigmaStat 11.0, Systat Software, San Jose, CA). Repeated-measures ANOVA was used to identify significant changes in measured variables within and between FF, RH, FF + 100% O2, and RH + 100% O2 conditions as well as between HC subjects and patients with PAD. For popliteal blood flow and convective O2 delivery, the cumulative area under the curve (AUC) was calculated as the summed second-by-second response during the first 90 s of recovery and used to identify how differences over time were affected by the various conditions. Peak postexercise blood flow and convective O2 delivery were determined by the greatest 6-s average recorded during recovery. When a significant main effect was identified, the Holm-Sidak method was used for α adjustment and post hoc analysis. Student’s t-tests were used to identify significant differences in subject characteristics between HC subjects and patients with PAD. Significance was established at P < 0.05. All group data are expressed as means ± SE.
RESULTS
Subject characteristics.
Subject characteristics and physical activity/functional assessments are shown in Table 1; Table 2 shows subject blood, comorbidity, and medication data. Participants were well matched for sex, age, height, weight, body mass index, MAP, and lower leg volume and muscle mass. By experimental design, patients with PAD had a significantly lower ABI (P < 0.05) than HC subjects. Plantar flexion WRmax, determined by an incremental test, was ~50% lower in patients with PAD compared with HC subjects (Fig. 2). Also of note, while participants were well matched for physical activity, HC subjects exhibited a significantly faster 10-m walk time compared with patients with PAD (P < 0.05; Table 1), indicating functional disability. Blood characteristics were well matched with the exception of red blood cells and low- and high-density lipoproteins, which were significantly lower in patients with PAD compared with HC subjects (P < 0.05). Furthermore, compared with HC subjects, patients with PAD had a greater number of comorbidities and were taking more medications.
Table 1.
Subject characteristics and physical activity/functional assessments
| Healthy Control Subjects | Patients With Peripheral Artery Disease | |
|---|---|---|
| Total number of participants/group (women/men) | 11 (2/9) | 10 (2/8) |
| Age, yr | 61 ± 6 | 65 ± 9 |
| Height, cm | 173 ± 8 | 168 ± 8 |
| Weight, kg | 82 ± 19 | 79 ± 13 |
| Body mass index, kg/m2 | 27 ± 5 | 28 ± 4 |
| Mean arterial pressure, mmHg | 106 ± 3 | 107 ± 6 |
| Ankle brachial index | 1.19 ± 0.11 | 0.67 ± 0.10* |
| Smoker (former/present) | (2/0) | (4/5) |
| Lower leg volume, liters | 2.4 ± 0.2 | 2.4 ± 0.5 |
| Lower leg muscle mass, kg | 1.1 ± 0.2 | 1.1 ± 0.2 |
| Plantar flexor maximal work rate, W | 10.5 ± 1.1 | 5.4 ± 0.6* |
| Physical activity | ||
| Steps, number/day | 6,491 ± 904 | 5,208 ± 1,203 |
| Sedentary activity, min/day | 1,239 ± 28 | 1,383 ± 80 |
| Light activity, min/day | 97 ± 16 | 100 ± 14 |
| Moderate activity, min/day | 83 ± 12 | 65 ± 12 |
| Vigorous activity, min/day | 36 ± 7 | 22 ± 7 |
| 10-meter walk time, s | 6.8 ± 1.1 | 9.2 ± 2.1* |
Values are expressed as means ± SE.
Significant difference between groups (P < 0.05).
Table 2.
Subject blood, comorbidity, and medication data
| Healthy Control Subjects | Patients With Peripheral Artery Disease | |
|---|---|---|
| Glucose, mg/dl | 88 ± 6 | 110 ± 9 |
| Cholesterol, mg/dl | 199 ± 33 | 165 ± 14 |
| Triglycerides, mg/dl | 101 ± 15 | 147 ± 28 |
| High-density lipoprotein, mg/dl | 58 ± 6 | 42 ± 2* |
| Low-density lipoprotein, mg/dl | 130 ± 6 | 95 ± 14* |
| White blood cells, k/μl | 6.3 ± 0.7 | 7.9 ± 0.6 |
| Red blood cells, k/μl | 4.9 ± 0.1 | 4.5 ± 0.1* |
| Hemoglobin, g/dl | 14.2 ± 1.0 | 14.5 ± 0.5 |
| Hematocrit, % | 45.5 ± 0.8 | 43.2 ± 1.2 |
| O2 saturation, % | 95.2 ± 1.3 | 94.3 ± 1.7 |
| Coronary artery disease, n | 0 | 40 |
| Myocardial infraction, n | 0 | 20 |
| Hypertension, n | 0 | 30 |
| Diabetes mellitus, n | 0 | 10 |
| Renal insufficiency, n | 0 | 10 |
| Medications, % | ||
| Statins medication | 0 | 40 |
| Aspirin/clopidogrel | 20 | 100 |
Values are expressed as means ± SE; n, number of patients/group.
Significant difference between groups (P < 0.05).
Fig. 2.
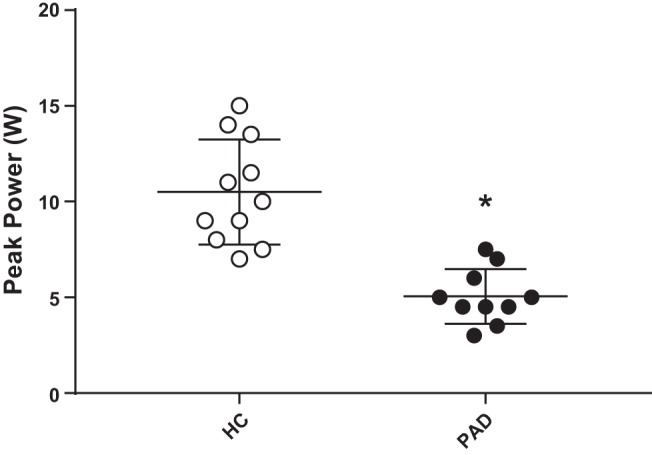
Peak power output during maximal plantar flexion exercise in healthy control (HC) subjects and patients with peripheral artery disease (PAD). Data are presented as means ± SE. *Significant difference between groups (P < 0.05).
Blood flow and tissue oxygenation at rest, 2.7 W, and during recovery.
At rest, popliteal blood flow (HC subjects: 93 ± 19 ml/min and patients with PAD: 80 ± 18 ml/min) and arterial O2 contents (HC subjects: 19.5 ± 0.6 ml O2/dl and patients with PAD: 19 ± 0.8 ml O2/dl) were not significantly different between HC subjects and patients with PAD. To specifically assess the adequacy of the hemodynamic response to exercise in PAD, we compared the blood flow response in HC subjects and patients with PAD at the same absolute work rate. During plantar flexion exercise at 2.7 W, convective O2 delivery and tissue oxygenation were significantly lower in patients with PAD compared with HC subjects (P < 0.05; Fig. 3). Likewise, during recovery from this 2.7 W work rate, both peak postexercise blood flow (HC subjects: 794 ± 50 ml/min and patients with PAD: 482 ± 50 ml/min) and convective O2 delivery (HC subjects: 146 ± 9 ml/min and patients with PAD: 93 ± 14 ml/min) were significantly lower in patients with PAD compared with HC subjects (P < 0.05). Furthermore, TOI during exercise and throughout the first minute postexercise (Fig. 3) was significantly lower in patients with PAD compared with HC subjects (P < 0.05). There was no difference in MAP between the groups during exercise or recovery.
Fig. 3.
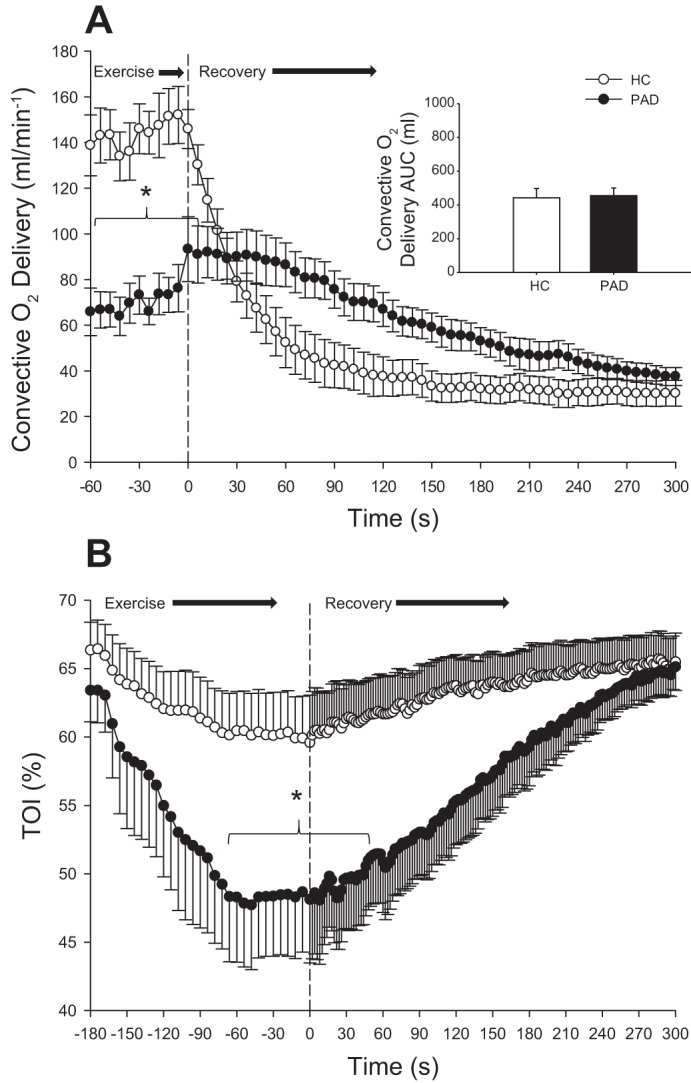
Convective O2 delivery during the last minute of exercise at 2.7 W and throughout recovery in healthy control (HC) subjects and patients with peripheral artery disease (PAD; A), with the inset displaying convective O2 delivery area under the curve (AUC) calculated from the first 90 s post exercise. The tissue oxygenation index (TOI) throughout exercise and recovery was assessed with near-infrared spectroscopy (B). Data are presented as means ± SE. *Significant difference between groups (P < 0.05).
Blood flow and convective O2 delivery at 40% WRmax and during recovery.
As the main goal of this study was to assess the interplay between peripheral O2 availability and O2 utilization, a second protocol was used where the participants exercised at the same relative intensity to account for metabolic disturbances attributed to different peak work rates. Average blood flow and convective O2 delivery during the last minute of plantar flexion exercise at 40% WRmax and postexercise recovery kinetics in the FF and RH conditions are shown in Figs. 4 and 5, respectively. Blood flow and convective O2 delivery (steady state, peak, and AUC) both during plantar flexion exercise (steady state) and postexercise were significantly greater in HC subjects compared with patients with PAD during FF and RH conditions (P < 0.05). This was likely attributable to differences in the absolute work rate corresponding to 40% WRmax in the two groups (HC subjects: 4.2 ± 0.3 W and patients with PAD: 2.1 ± 0.2 W). It is also worth recognizing that the 40% WRmax in patients with PAD was similar to the absolute workload of 2.7 W chosen to assess hemodynamic responses in both groups, at which work rate, attenuated blood flow, and convective O2 delivery were established in patients with PAD compared with HC subjects. In HC subjects, RH superimposed upon recovery resulted in a significant increase in both peak postexercise blood flow (FF: 950 ± 62 ml/min and RH: 1405 ± 62 ml/min; Fig. 4B) and convective O2 delivery (FF: 176 ± 15 ml/min and RH: 254 ± 15 ml/min; Fig. 5B) compared with the FF condition (P < 0.05). In contrast, there was no significant change in blood flow or convective O2 delivery during recovery in patients with PAD with RH or FF + 100% O2 compared with the FF condition. However, RH + 100% O2 resulted in a significant increase in both peak postexercise blood flow (FF: 459 ± 40 ml/min and RH + 100% O2: 656 ± 91 ml/min; Fig. 4B) and convective O2 delivery (FF: 87 ± 10 ml/min and RH + 100% O2: 125 ± 16 ml/min; Fig. 5B) compared with the FF condition (P < 0.05). O2 saturation, a component of convective O2 transport, was not different at rest (Table 2) between patients with PAD and HC subjects and did not change in the FF and RH conditions. However, when patients with PAD breathed 100% O2 (FF + 100% O2 and RH + 100% O2), O2 saturation rose significantly to ~100%. MAP was not different between groups in either the FF or RH condition or in patients with PAD across all conditions (FF, RH, FF + 100% O2, and RH + 100% O2).
Fig. 4.
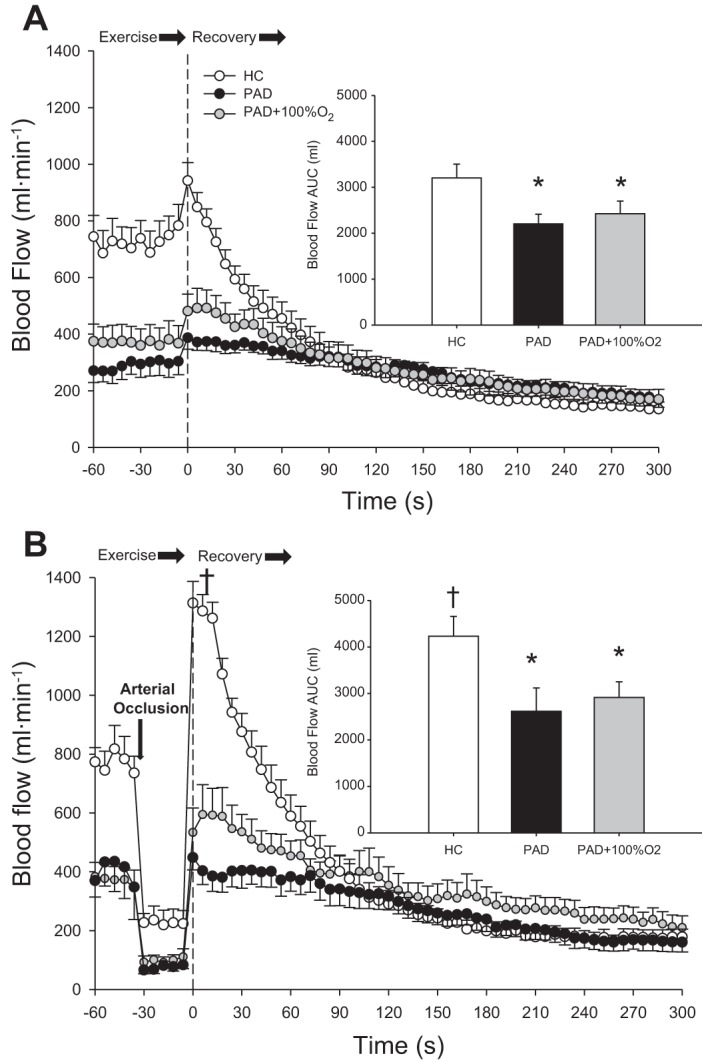
Popliteal blood flow during the last minute of exercise and throughout recovery assessed with Doppler ultrasound during free flow (FF; A) and reactive hyperemia (RH; B) in healthy control (HC) subjects and patients with peripheral artery disease (PAD) during normoxia and with patients with PAD breathing 100% O2 (PAD + 100% O2). Insets display the blood flow area under the curve (AUC) calculated from the first 90 s postexercise. Data are presented as means ± SE. *Significant difference between groups; †significant difference compared with the FF condition within group (P < 0.05).
Fig. 5.
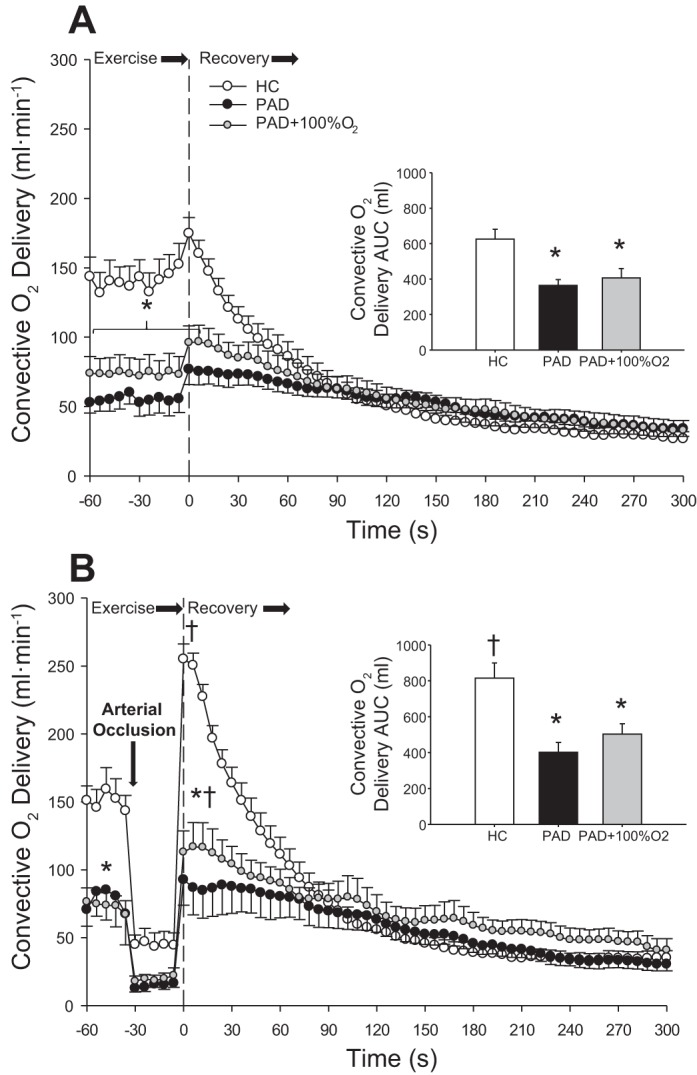
Convective O2 delivery during the last minute of exercise and throughout recovery during free flow (FF; A) and reactive hyperemia (RH; B) in healthy control (HC) subjects and patients with peripheral artery disease (PAD) during normoxia and patients with PAD while breathing 100% O2 (PAD + 100% O2). Insets display the convective O2 delivery area under the curve (AUC) calculated from the first 90 s postexercise. Data are presented as means ± SE. *Significant difference between groups; †significant difference compared with the FF condition within group (P < 0.05).
Tissue oxygenation at 40% WRmax and during recovery.
Tissue oxygenation throughout plantar flexion exercise at 40% WRmax and recovery is shown in Fig. 6. During exercise in the FF condition, average TOI was significantly lower in patients with PAD compared with HC subjects (HC subjects: 58 ± 3% and patients with PAD: 44 ± 3%). The greater postexercise blood flow and convective O2 delivery in HC subjects during both FF and RH was accompanied by faster tissue reoxygenation compared with patients with PAD, as indicated by the significantly faster TOI recovery kinetics (τ) and shorter mean response time (P < 0.05; Table 3 and Fig. 6, A and B). Compared with the FF condition, RH resulted in no difference in τ in patients with PAD (Table 3). However, FF + 100% O2 resulted in a significant increase in TOI (54 ± 3%) compared with the FF condition (P < 0.05) in patients with PAD, such that there was no longer a significant difference in TOI recorded during exercise in HC subjects and patients with PAD (P = 0.22; Table 3 and Fig. 6, A and B).
Fig. 6.
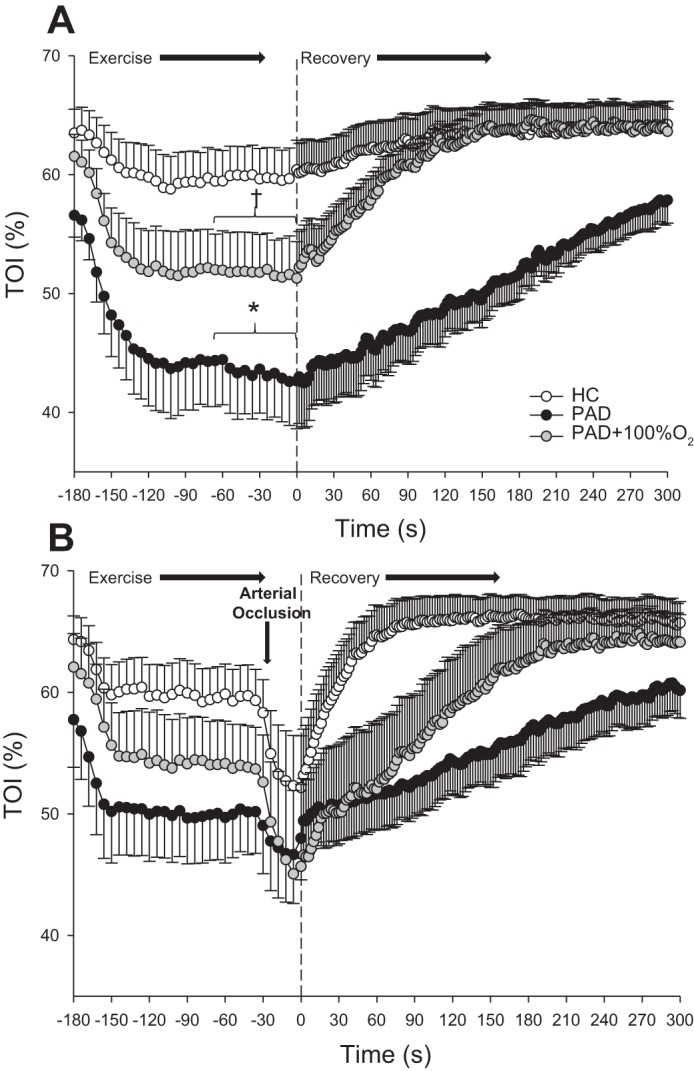
The tissue oxygenation index (TOI) throughout exercise and recovery assessed with near-infrared spectroscopy during free flow (FF; A) and reactive hyperemia (RH; B) in healthy control (HC) subjects and patients with peripheral artery disease (PAD) during normoxia and in patients with PAD while breathing 100% O2 (PAD + 100% O2). Data are presented as means ± SE. *Significant difference between groups; †significant difference compared with the FF condition within group (P < 0.05).
Table 3.
Deoxyhemoglobin/myoglobin kinetics assessed by near-infrared spectroscopy after submaximal plantar flexion exercise
| HC-FF | PAD-FF | PAD-FF + 100% O2 | HC-RH | PAD-RH | PAD-RH + 100% O2 | |
|---|---|---|---|---|---|---|
| Resting tissue oxygenation index | 64 ± 2 | 58 ± 2* | 62 ± 2 | 65 ± 2 | 63 ± 2 | 64 ± 3 |
| Time delay, s | 6 ± 9 | 24 ± 9* | 15 ± 7 | 3 ± 4 | 24 ± 12* | 17 ± 9 |
| Amplitude | 5 ± 3 | 25 ± 5* | 18 ± 2* | 16 ± 10 | 23 ± 7 | 21 ± 4 |
| τ, s | 49 ± 39 | 140 ± 32* | 84 ± 33 | 14 ± 16 | 173 ± 33* | 98 ± 39 |
| 95% Confidence interval | 14 ± 19 | 9 ± 3 | 8 ± 4 | 0.9 ± 0.5 | 12 ± 3 | 7 ± 4 |
| Mean response time, s | 54 ± 43 | 164 ± 33 | 99 ± 39* | 17 ± 16 | 197 ± 31* | 115 ± 45* |
Values are expressed as means ± SE. HC, healthy control; FF, free flow conditions; PAD, peripheral artery disease; RH, reactive hyperemia; τ, tissue oxygenation index recovery time constant.
Significant difference between groups (P < 0.05).
High-energy phosphate compounds, intracellular pH, and PCr recovery kinetics.
At rest, there was no difference in PCr, inorganic phosphate, pH, and ADP between HC subjects and patients with PAD (Table 4). In the FF condition, after exercise at 40% WRmax, patients with PAD exhibited a significantly slower PCr recovery τ than HC subjects (P < 0.05; Table 4), corresponding to a significantly lower Vmax in patients with PAD compared with HC subjects (P < 0.05; Fig. 7). In HC subjects, there was a significant increase in Vmax (~36%) after RH compared with FF (P < 0.05; Fig. 7). In contrast, in patients with PAD, Vmax was unaltered during FF + 100% O2 and RH conditions and consequentially remained significantly lower compared with HC subjects (Table 4). However, RH + 100% O2 resulted in a significant ~50% increase in Vmax compared with FF (P < 0.05), such that the difference in Vmax between HC subjects and patients with PAD was no longer evident (Fig. 7).
Table 4.
High-energy phosphate compounds, intracellular pH, and PCr recovery kinetics
| HC-FF | PAD-FF | PAD-FF + 100% O2 | HC-RH | PAD-RH | PAD-RH + 100% O2 | |
|---|---|---|---|---|---|---|
| Rest | ||||||
| PCr, mM | 31 ± 1 (26–41) | 34 ± 2 (26–43) | 31 ± 2 (26–46) | |||
| Pi, mM | 1.5 ± 0.4 (1.1–2.2) | 1.5 ± 0.1 (0.8–2.1) | 1.8 ± 0.2 (0.6–2.4) | |||
| ADP, µM | 8.0 ± 0.2 (7.2–8.8) | 8.4 ± 0.2 (7.2–9.6) | 8.3 ± 0.3 (7.2–9.5) | |||
| pH | 6.96 ± 0.01 (6.92–7.00) | 6.98 ± 0.01 (6.92–7.04) | 6.98 ± 0.02 (6.92–7.04) | |||
| End-exercise and recovery parameters | ||||||
| PCr, mM | 14 ± 1 (8.1–16.9) | 13 ± 1 (6.5–19.3) | 15 ± 2 (7.3–18.8) | 11 ± 1 (5–15)† | 14 ± 1 (7–18)* | 14 ± 1 (7–19) |
| ADP, µM | 67 ± 1 (45–97) | 68 ± 4 (40–88) | 57 ± 5 (27–111) | 92 ± 11 (46–182)† | 78 ± 8 (46–134)* | 63 ± 12 (27–112) |
| pH min | 6.91 ± 0.01 (6.86–6.97) | 6.83 ± 0.06 (6.37–7.00)* | 6.75 ± 0.07 (6.42–7.00)*,† | 6.81 ± 0.04 (6.45–6.97)† | 6.81 ± 0.03 (6.69–6.99) | 6.91 ± 0.04 (6.67–7.00)* |
| pH end | 6.98 ± 0.01 (6.91–7.03) | 6.92 ± 0.03 (6.72–7.02)* | 6.90 ± 0.04 (6.71–7.05)* | 6.97 ± 0.02 (6.86–7.06) | 6.94 ± 0.02 (6.89–7.05) | 7.01 ± 0.02 (6.92–7.09)† |
| τ, s−1 | 48 ± 5 (31–96) | 90 ± 11 (33–164)* | 94 ± 18 (29–189)* | 45 ± 4 (27–78) | 86 ± 10 (58–147)* | 68 ± 12 (31–118)* |
| 95% Confidence interval | 8 ± 1 (5–15) | 13 ± 1 (6–18)* | 16 ± 2 (7–24)* | 6 ± 1 (2–10) | 12 ± 2 (8–22) | 10 ± 2 (5–21) |
Values are expressed as means ± SE with the range of minimum and maximum values in parentheses. HC, healthy control; FF, free flow conditions; PAD, peripheral artery disease; RH, reactive hyperemia; PCr, phosphocreatine; τ, PCr recovery time constant.
Significant difference between groups;
significant difference compared with the FF condition within group (P < 0.05).
Fig. 7.
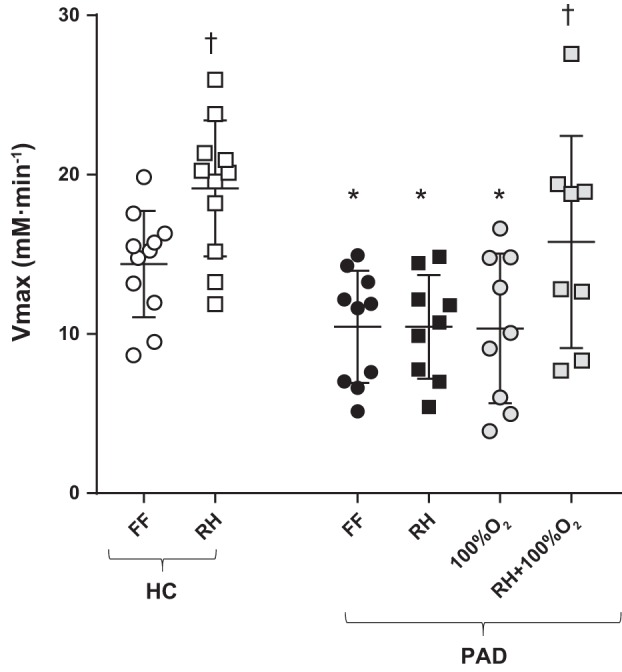
Peak skeletal muscle mitochondrial ATP synthesis rate (Vmax) determined from postexercise phosphocreatine recovery assessed with 31P magnetic resonance spectroscopy in healthy control (HC) subjects and patients with peripheral artery disease (PAD) under conditions of free flow (FF), reactive hyperemia (RH), FF with 100% inspired O2 (100% O2), and RH with 100% inspired O2 (RH + 100% O2) in patients with PAD. Vertical bars represent group means ± SE. *Significant difference between groups; †significant difference compared with the FF condition within group (P < 0.05).
Mitochondrial respiration rate in vitro.
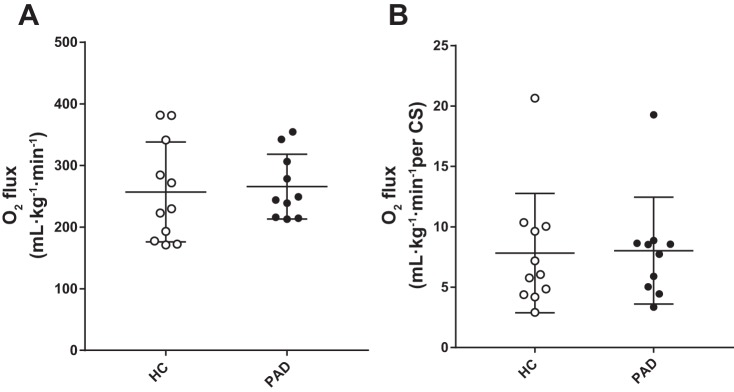
The ADP-stimulated complex I + II peak mitochondrial respiration rate per muscle tissue wet weight assessed in vitro was not different between HC subjects and patients with PAD (Fig. 8). When the ADP-stimulated complex I + II peak mitochondrial respiration rate was expressed as milliliters per kilogram per minute, these values were within the range of previously documented mitochondrial state 3 respiration rates in the human vastus lateralis (15). CS activity, a measure of mitochondrial volume density (33, 59), was also not different between the two groups (HC subjects: 38 ± 6 nmol·min−1·mg tissue−1 and patients with PAD: 40 ± 7 nmol·min−1·mg tissue−1). Furthermore, the ADP-stimulated complex I + II peak mitochondrial respiration rate normalized to CS activity was not different between HC subjects and patients with PAD (Fig. 8).
Fig. 8.
Comparison of state 3 and complex I and II mitochondrial respiration rate assessed in vitro in healthy control (HC) subjects and patients with peripheral artery disease (PAD). Individual state 3 and complex I and II mitochondrial respiration rate (O2 flux) data are expressed as both per muscle tissue wet weight (m·kg−1·min−1; A) and normalized to citrate synthase activity (B). Values are expressed as means ± SE.
DISCUSSION
This study sought to determine the role that O2 supply and utilization play in the skeletal muscle dysfunction associated with early stage PAD by combining both in vivo and in vitro assessments of skeletal muscle oxidative capacity and the manipulation of O2 availability. Patients with PAD exhibited attenuated O2 delivery at the same absolute work rate and attenuated tissue reoxygenation as well as Vmax after submaximal plantar flexion exercise that was matched for relative work rate. Compared with the FF condition, RH + 100% O2 significantly increased convective O2 delivery and concomitantly improved tissue reoxygenation in patients with PAD, resulting in a considerable increase in Vmax, such that differences in skeletal muscle oxidative capacity, assessed in vivo, no longer persisted between HC subjects and patients with PAD. Furthermore, peak mitochondrial respiration rates assessed in vitro with ample O2 availability were not different between HC subjects and patients with PAD. Taken together, these findings reveal that the skeletal muscle dysfunction associated with early stage PAD, when assessed in vivo, is predominantly a consequence of limited O2 supply and not the impact of an intrinsic mitochondrial defect influencing oxidative capacity in this pathology.
RH and mitochondrial oxidative capacity in patients with PAD and HC subjects.
Patients with PAD exhibited an attenuated convective O2 delivery and tissue oxygenation compared with HC subjects during plantar flexion exercise at 2.7 W (Fig. 3), confirming the expected O2 delivery limitation in patients with PAD that is likely a consequence of the disease-related arterial occlusion. The observed differences between groups in tissue oxygenation during exercise and recovery are in agreement with previously documented evidence of an altered time course for tissue oxygenation with exercise in patients with PAD (40, 42, 46). After the assessment at a matched absolute work rate, it was necessary to use a relative work rate for the in vivo assessment of mitochondrial oxidative capacity to elicit a measurable PCr depletion and recovery in both groups. Therefore, the remaining experiments were performed using a work rate of 40% WRmax. With this approach, in the FF condition, patients with PAD exhibited a significantly slower PCr recovery τ (Table 4) and lower Vmax (~36%) than HC subjects (Fig. 7), suggestive of either differences in oxidative capacity between the groups or a metabolic limitation because of limited O2 availability in patients with PAD. With the intent to increase postexercise blood flow and subsequently convective O2 delivery, in HC subjects, RH did indeed result in a significant increase in both peak blood flow (~53%) and blood flow AUC (~32%) compared with the FF condition (Fig. 4). This increase in blood flow significantly augmented both peak convective O2 delivery (~49%) and convective O2 delivery AUC (~30%; Fig. 5). Furthermore, this significant improvement in O2 availability after RH in HC subjects resulted in an ~33% increase in Vmax (Fig. 7). These findings are in agreement with previous data from our group (36, 37) that documented an ~77% and ~35% increase in convective O2 delivery and a resultant ~43% and ~33% increase in Vmax after RH compared with FF conditions in a group of young and old healthy adults, respectively. Interestingly, these results support the concept that in health there is an O2 supply-dependent metabolic reserve and document that this is the case even in older individuals.
In contrast to HC subjects, RH in patients with PAD failed to augment blood flow compared with the FF condition (Fig. 4), and, as a consequence, neither convective O2 delivery nor Vmax were altered (Figs. 5 and 7). There are several potential explanations for the attenuated blood flow response after the RH intervention in patients with PAD compared with HC subjects. Specifically, in patients with PAD, there was likely a RH response, due to some degree of stenosis in the conduit arteries, which not only impedes blood flow but also contributes to a loss of kinetic energy because of turbulent flow (24, 77). In addition, prior evidence in patients with PAD has revealed an impaired vasodilatory response in the conduit arteries, distal to the stenosis that limits the potential to offset the fixed resistance caused by the arterial lesions (9). In addition to these structural and functional abnormalities at the level of the conduit arteries in patients with PAD, a multitude of vasoactive substances that influence postexercise hyperemia at the microvascular level, including locally released ions and metabolites (6), have been correlated with the severity of this disease (3, 24, 72). Specifically, nitric oxide (NO) bioavailability within endothelial cells has been associated with exercise intolerance in patients with PAD (3). Indeed, recent evidence suggests that the attenuated NO bioavailability in patients with PAD may be secondary to increased levels of reactive oxygen species produced by elevated levels of NADPH oxidase in the skeletal muscle of this population, which promotes uncoupling of endothelial NO synthase and results in superoxide rather than NO formation (72).
Recognizing that the role of NO during exercise and throughout recovery remains controversial in both health and disease (1, 11, 16, 66), it is important to note that a growing body of evidence suggests that a number of endothelium-derived compounds act in a redundant and potentially synergistic manner to induce exercise hyperemia (23, 47). Indeed, in the face of the limited NO bioavailability exhibited by patients with PAD, muscle prostacyclin synthase appears to play a greater vasoregulatory role in this population. Additionally, impaired vasodilation during exercise and recovery in patients with PAD could also be attributed to an exercise-induced release of the potent vasoconstrictor endothelin-1 (41), likely further limiting blood flow during recovery in those suffering from this condition. Therefore, in combination, the consequences of PAD pathology, in terms of vascular structure and function, likely contribute to the blunted RH response, limiting the efficacy of this intervention as a means by which to increase convective O2 delivery in this population.
Hyperoxia and mitochondrial oxidative capacity in patients with PAD.
Despite a significant increase in TOI in patients with PAD (Fig. 6), the addition of 100% inspired O2 under FF conditions did not result in a significant improvement in postexercise convective O2 delivery, and, subsequently, Vmax was unaltered (Fig. 7). In a previous study, Kemp and colleagues (32) concluded that impaired PCr recovery in patients with PAD is a consequence of reduced O2 supply. However, while the causal relationship between limited O2 supply and attenuated skeletal muscle oxidative capacity may appear intuitive in patients with PAD, there was no attempt to manipulate O2 availability in support of this conclusion. Of note, it has previously been demonstrated that hyperoxia enhances both the convective (dissolved O2 contents can rise from 0.3 to 2 ml/dl) and diffusive (arterial Po2 can rise from 100 to 650 mmHg) components of O2 transport from blood to muscle, resulting in an increase in intracellular Po2 that facilitates greater oxidative metabolism in O2 supply-limited, healthy trained subjects (22). Interestingly, in the present study, FF + 100% O2 significantly increased mean TOI during exercise in patients with PAD by ~23% compared with the normoxic FF condition (Fig. 6). However, this improvement in tissue oxygenation did not translate into an increase in Vmax in patients with PAD. This absence of an improvement in Vmax after FF + 100% O2 in patients with PAD could be explained by a previous study reporting a decreased muscle capillary density in this patient population (62), resulting in a decreased skeletal muscle perfusion and limited O2 transport within the muscle capillary bed. Furthermore, additional factors influencing perfusion and O2 transport related to capillary function, such as red blood cell flux, velocity, and hematocrit (60), may be altered by PAD, although the importance of these factors in this pathology remain unknown. Based on the present findings, it appears that the increase in O2 availability and subsequently the muscle oxygenation afforded by FF + 100% O2 was insufficient to overcome the likely extensive convective restrictions to O2 delivery imposed by the occlusive disease in patients with PAD. As a consequence, hyperoxia alone was insufficient to increase O2 availability and augment skeletal muscle mitochondrial oxidative capacity in patients with PAD.
A potential explanation for the absence of a marked improvement in skeletal muscle oxidative capacity in the FF + 100% O2 condition could be the development of hyperoxic vasoconstriction as a consequence of the high levels of O2 in the vasculature. Indeed, a decline in muscle blood flow during exercise under hyperoxic compared with normoxic conditions, accompanied by a trend toward lower maximal O2 consumption, has been previously documented in young healthy adults (51, 75). However, it is unlikely that hyperoxic vasoconstriction explains the inability of hyperoxia to impact skeletal muscle oxidative capacity in the present study, as blood flow was not different between the FF and FF + 100% O2 conditions during the final minute of exercise in patients with PAD (Fig. 4). Further work is needed to determine whether the recently documented autonomic dysfunction associated with oxidative stress in patients with PAD (40, 46, 48) alters hemodynamic responses to different .
RH combined with hyperoxia and mitochondrial oxidative capacity in patients with PAD.
To our knowledge, this is the first study to alter O2 availability during exercise in patients with PAD in an attempt to augment mitochondrial oxidative capacity. To this end, combined RH + 100% O2 elicited a significant improvement in both O2 availability and oxidative capacity in patients with PAD compared with the FF condition, as evidenced by an ~44% increase in postexercise convective O2 delivery (Fig. 5) and an ~50% increase in Vmax (Fig. 7), such that there was no longer a difference in Vmax between patients with PAD and HC subjects. The additional assessment of mitochondrial oxidative capacity in vitro, under conditions of ample O2 availability, supports these in vivo observations by confirming that peak mitochondrial respiration rate in the patients with PAD and HC subjects was not different. The exact mechanism responsible for the efficacy of the RH + 100% O2 treatment is not entirely clear but may be related to changes in both microcirculatory blood flow and the subsequently more widespread availability of the additional O2 across the muscle bed. Interestingly, Spires and colleagues (68) demonstrated that the diffusive component of O2 transport has a greater impact when convective O2 delivery is limited, in their case by limited blood flow during skeletal muscle contractions. This could potentially play a role in the observed efficacy of the combined RH + 100% O2 treatment in patients with PAD, a population with impaired blood flow. Nevertheless, in combination, these findings suggest that both convective and diffusive O2 transport limit mitochondrial oxidative capacity in patients with PAD and document unaltered intrinsic mitochondrial respiratory capacity in this patient population.
The observation in the present study that skeletal muscle mitochondrial oxidative capacity is not lower in patients with PAD is a departure from the conclusions of several previous studies (4, 7, 8, 56–58). However, Anderson and colleagues (4) failed to observe a correlation between calf muscle perfusion and the PCr recovery time constant, an in vivo index of mitochondrial oxidative capacity, in a similar cohort of patients with PAD. Unlike the PCr recovery time constant, Vmax, as used in the present study, has been repeatedly documented as a robust index of skeletal muscle mitochondrial oxidative capacity independent from end-exercise alterations in pH (39, 73). Consequently, the broad range of end-exercise pH (6.74–7.26) reported by Anderson and colleagues (4) may have influenced the observed PCr recovery time constants and subsequently the apparent uncoupling between calf muscle perfusion and metabolism. More recently, AlGhatrif and colleagues (2) examined the relationship between borderline attenuated ABI (0.91−1.10) and mitochondrial energy production in a large group of men and women without a diagnosis of PAD. The PCr recovery time constant, measured in the left thigh after ballistic knee extension exercise, was decreased in participants with borderline attenuated ABI compared with participants with ABI > 1.1. Furthermore, a weak but statistically significant correlation (r = 0.12282) was documented between the PCr recovery rate constant and ABI (2), suggesting some degree of O2 delivery dependency for skeletal muscle mitochondrial oxidative capacity. However, unlike the present study (Fig. 7), none of the prior investigations attempted to alter O2 availability to strengthen their conclusions of an impaired intrinsic mitochondrial oxidative capacity independent of O2 delivery in patients with PAD.
Evidence of a mitochondrial reserve capacity in patients with PAD and HC subjects.
Both HC subjects and patients with PAD revealed a mitochondrial reserve capacity after interventions to increase O2 delivery. Specifically, RH increased Vmax in HC subjects, and this was achieved in patients with PAD using RH + 100% O2. A previous study has suggested that a mitochondrial reserve capacity in young exercise-trained subjects serves to improve substrate selection and therefore efficiency during endurance performance and improves the mitochondrial buffering capacity during periods of very high metabolic demand (15). However, for patients with PAD, a more likely explanation for the increased mitochondrial reserve capacity is a compensatory response to the increased level of oxidative stress and mitochondrial toxins (e.g., superoxide) associated with this disease (19, 56, 63). Such a response likely provides a greater resistance to further metabolic insults and cell death. Not surprisingly, exercise training has proven to be one of the most effective treatments to improve both muscle function and blood flow, in addition to improving mechanisms responsible for minimizing oxidative stress in patients with PAD (24). Therefore, it is noteworthy that in the present study, subjects were carefully matched for physical activity. Based on the number of steps per day for patients with PAD (~5,208 steps/day), these subjects are considered to have a low level of physical activity compared with typical healthy, age-matched adults (70). However, the patients in this study were relatively active compared with previous studies that evaluated mitochondrial oxidative capacity in patients with greater PAD severity (56–58). Therefore, the presence of a mitochondrial reserve capacity in the patients who participated in this study is, at least in part, likely attributable to the relatively high level of physical activity and functional status, which contributed to their preserved mitochondrial oxidative capacity compared with other less physically active patients with PAD. Of note, given the broad range of physical functional capacity in patients with PAD (44), the present findings were restricted to patients with intermittent claudication (Fontaine stage II) and should not be generalized to patients with critical limb ischemia and greater functional impairment, which is more commonly observed in advanced PAD etiology.
Experimental considerations.
The estimation of the peak oxidative ATP synthesis rate, Vmax, from the 31P MRS measurements relies on several key assumptions that should be acknowledged. First, the kinetic parameters are based on a sigmoidal relationship between mitochondrial respiration and ADP concentration. However, there is clear evidence that, when assessed in vivo in skeletal muscle, this is actually, at the very least, a second-order kinetic function (27, 35, 76). From the literature, we assumed a constant value for the Hill coefficient (nH = 2.2) and Km (30 μM). Slightly different values have been directly estimated in the skeletal muscle of healthy humans (nH = 2.6 and Km = 25 μM), and these values have been documented to vary in some pathological conditions (35, 45). However, from simulations (PCr resynthesis rate = 18 mM/min), it was estimated that the variability in Vmax associated with these parameters would be at most 7%, which is far less than the experimental effects observed in the present study. We are therefore confident that the assumptions related those parameters do not confound the interpretation of the current data.
This study provides the first evidence of preserved intrinsic mitochondrial capacity for ATP synthesis in patients with PAD after a combined intervention that increased both convective and diffusive O2 delivery in patients with PAD. However, the methods used in this study cannot entirely rule out the potential existence of underlying deficits affecting mitochondrial quality related to increased uncoupling, excess free radical production, and impaired autophagic flux, all of which have been proposed to contribute to the progressive decline in skeletal muscle function with advanced PAD (56, 57, 74). Currently, the time course for progressive impairments in mitochondrial function and stages of PAD severity is not known.
Conclusions.
The unique combination of both in vivo and in vitro assessments of skeletal muscle mitochondrial oxidative capacity used in this study emphasizes the important role that O2 availability plays in skeletal muscle function in patients with PAD. Specifically, there was no evidence of an intrinsic mitochondrial deficit in patients with PAD assessed in vitro in the absence of any restrictions on O2 availability. Furthermore, in vivo observations revealed that increased postexercise convective O2 delivery was able to augment skeletal muscle oxidative capacity, strongly implicating O2 supply as the primary factor limiting mitochondrial oxidative capacity in this population of patients with a less severe form of PAD. These findings support the use of both surgical and pharmacological therapies before the onset of debilitating physical impairment and critical limb ischemia to improve O2 availability and subsequently preserve mitochondrial oxidative capacity in patients with PAD.
GRANTS
This work was funded in part by National Heart, Lung, and Blood Institute Grants K99-HL-125756 and PO1-HL-1091830, the Flight Attendant Medical Research Institute, Veterans Administration Rehabilitation Research and Development Service Grants E6910-R, E1697-R, E1433-P, and E9275-L, and Small Projects in Rehabilitation Research Grant E1572P.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R.H., G.L., J.D.T., and R.S.R. conceived and designed research; C.R.H., G.L., J.D.T., and H.L.C. performed experiments; C.R.H., G.L., J.D.T., Y.L.F., and J.R.G. analyzed data; C.R.H., G.L., J.D.T., and R.S.R. interpreted results of experiments; C.R.H. prepared figures; C.R.H. drafted manuscript; C.R.H., G.L., J.D.T., Y.L.F., J.R.G., H.L.C., and R.S.R. edited and revised manuscript; R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank all subjects who partook in this study for the dedicated participation in this research.
REFERENCES
- 1.Health Quality Ontario Stenting for peripheral artery disease of the lower extremities: an evidence-based analysis. Ont Health Technol Assess Ser 10: 1–88, 2010. [PMC free article] [PubMed] [Google Scholar]
- 2.AlGhatrif M, Zane A, Oberdier M, Canepa M, Studenski S, Simonsick E, Spencer RG, Fishbein K, Reiter D, Lakatta EG, McDermott MM, Ferrucci L. Lower mitochondrial energy production of the thigh muscles in patients with low-normal ankle-brachial index. J Am Heart Assoc 6: e006604, 2017. doi: 10.1161/JAHA.117.006604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, Dobrosielski DA, Annex BH. Plasma nitrite flux predicts exercise performance in peripheral arterial disease after 3months of exercise training. Free Radic Biol Med 49: 1138–1144, 2010. doi: 10.1016/j.freeradbiomed.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JD, Epstein FH, Meyer CH, Hagspiel KD, Wang H, Berr SS, Harthun NL, Weltman A, Dimaria JM, West AM, Kramer CM. Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol 54: 628–635, 2009. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bangsbo J, Hellsten Y. Muscle blood flow and oxygen uptake in recovery from exercise. Acta Physiol Scand 162: 305–312, 1998. doi: 10.1046/j.1365-201X.1998.0331e.x. [DOI] [PubMed] [Google Scholar]
- 7.Bauer TA, Regensteiner JG, Brass EP, Hiatt WR. Oxygen uptake kinetics during exercise are slowed in patients with peripheral arterial disease. J Appl Physiol 87: 809–816, 1999. doi: 10.1152/jappl.1999.87.2.809. [DOI] [PubMed] [Google Scholar]
- 8.Baum O, Torchetti E, Malik C, Hoier B, Walker M, Walker PJ, Odriozola A, Graber F, Tschanz SA, Bangsbo J, Hoppeler H, Askew CD, Hellsten Y. Capillary ultrastructure and mitochondrial volume density in skeletal muscle in relation to reduced exercise capacity of patients with intermittent claudication. Am J Physiol Regul Integr Comp Physiol 310: R943–R951, 2016. doi: 10.1152/ajpregu.00480.2015. [DOI] [PubMed] [Google Scholar]
- 9.Bragadeesh T, Sari I, Pascotto M, Micari A, Kaul S, Lindner JR. Detection of peripheral vascular stenosis by assessing skeletal muscle flow reserve. J Am Coll Cardiol 45: 780–785, 2005. doi: 10.1016/j.jacc.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 10.Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med 5: 55–59, 2000. doi: 10.1177/1358836X0000500109. [DOI] [PubMed] [Google Scholar]
- 11.Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 589: 1477–1488, 2011. doi: 10.1113/jphysiol.2010.203539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comerota AJ, Throm RC, Kelly P, Jaff M. Tissue (muscle) oxygen saturation (StO2): a new measure of symptomatic lower-extremity arterial disease. J Vasc Surg 38: 724–729, 2003. doi: 10.1016/S0741-5214(03)01032-2. [DOI] [PubMed] [Google Scholar]
- 13.Gardner AW, Afaq A. Management of lower extremity peripheral arterial disease. J Cardiopulm Rehabil Prev 28: 349–357, 2008. doi: 10.1097/HCR.0b013e31818c3b96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner AW, Parker DE, Webb N, Montgomery PS, Scott KJ, Blevins SM. Calf muscle hemoglobin oxygen saturation characteristics and exercise performance in patients with intermittent claudication. J Vasc Surg 48: 644–649, 2008. doi: 10.1016/j.jvs.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gifford JR, Garten RS, Nelson AD, Trinity JD, Layec G, Witman MA, Weavil JC, Mangum T, Hart C, Etheredge C, Jessop J, Bledsoe A, Morgan DE, Wray DW, Rossman MJ, Richardson RS. Symmorphosis and skeletal muscle V̇o2max: in vivo and in vitro measures reveal differing constraints in the exercise-trained and untrained human. J Physiol 594: 1741–1751, 2016. doi: 10.1113/JP271229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon MB, Jain R, Beckman JA, Creager MA. The contribution of nitric oxide to exercise hyperemia in the human forearm. Vasc Med 7: 163–168, 2002. doi: 10.1191/1358863x02vm439oa. [DOI] [PubMed] [Google Scholar]
- 17.Grassi B, Quaresima V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Opt 21: 091313, 2016. doi: 10.1117/1.JBO.21.9.091313. [DOI] [PubMed] [Google Scholar]
- 18.Hardman RL, Jazaeri O, Yi J, Smith M, Gupta R. Overview of classification systems in peripheral artery disease. Semin Intervent Radiol 31: 378–388, 2014. doi: 10.1055/s-0034-1393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart CR, Layec G, Trinity JD, Kwon OS, Zhao J, Reese VR, Gifford JR, Richardson RS. Increased skeletal muscle mitochondrial free radical production in peripheral arterial disease despite preserved mitochondrial respiratory capacity. Exp Physiol 103: 838–850, 2018. doi: 10.1113/EP086905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart CR, Layec G, Trinity JD, Liu X, Kim SE, Groot HJ, Le Fur Y, Sorensen JR, Jeong EK, Richardson RS. Evidence of preserved oxidative capacity and oxygen delivery in the plantar flexor muscles with age. J Gerontol A Biol Sci Med Sci 70: 1067–1076, 2015. doi: 10.1093/gerona/glu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol 86: 2013–2018, 1999. doi: 10.1152/jappl.1999.86.6.2013. [DOI] [PubMed] [Google Scholar]
- 22.Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J Appl Physiol 97: 1077–1081, 2004. doi: 10.1152/japplphysiol.01321.2003. [DOI] [PubMed] [Google Scholar]
- 23.Hearon CM Jr, Dinenno FA. Regulation of skeletal muscle blood flow during exercise in ageing humans. J Physiol 594: 2261–2273, 2016. doi: 10.1113/JP270593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiatt WR, Armstrong EJ, Larson CJ, Brass EP. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res 116: 1527–1539, 2015. doi: 10.1161/CIRCRESAHA.116.303566. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 286: 1317–1324, 2001. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med 13: 209–215, 2008. doi: 10.1177/1358863X08089277. [DOI] [PubMed] [Google Scholar]
- 27.Jeneson JA, Wiseman RW, Westerhoff HV, Kushmerick MJ. The signal transduction function for oxidative phosphorylation is at least second order in ADP. J Biol Chem 271: 27995–27998, 1996. doi: 10.1074/jbc.271.45.27995. [DOI] [PubMed] [Google Scholar]
- 28.Kemp GJ. Mitochondrial dysfunction in chronic ischemia and peripheral vascular disease. Mitochondrion 4: 629–640, 2004. doi: 10.1016/j.mito.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Kemp GJ, Hands LJ, Ramaswami G, Taylor DJ, Nicolaides A, Amato A, Radda GK. Calf muscle mitochondrial and glycogenolytic ATP synthesis in patients with claudication due to peripheral vascular disease analysed using 31P magnetic resonance spectroscopy. Clin Sci (Lond) 89: 581–590, 1995. doi: 10.1042/cs0890581. [DOI] [PubMed] [Google Scholar]
- 30.Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q 10: 43–63, 1994. [PubMed] [Google Scholar]
- 31.Kemp GJ, Roberts N, Bimson WE, Bakran A, Frostick SP. Muscle oxygenation and ATP turnover when blood flow is impaired by vascular disease. Mol Biol Rep 29: 187–191, 2002. doi: 10.1023/A:1020325812680. [DOI] [PubMed] [Google Scholar]
- 32.Kemp GJ, Roberts N, Bimson WE, Bakran A, Harris PL, Gilling-Smith GL, Brennan J, Rankin A, Frostick SP. Mitochondrial function and oxygen supply in normal and in chronically ischemic muscle: a combined 31P magnetic resonance spectroscopy and near infrared spectroscopy study in vivo. J Vasc Surg 34: 1103–1110, 2001. doi: 10.1067/mva.2001.117152. [DOI] [PubMed] [Google Scholar]
- 33.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590: 3349–3360, 2012. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Layec G, Bringard A, Vilmen C, Micallef JP, Fur YL, Perrey S, Cozzone PJ, Bendahan D. Accurate work-rate measurements during in vivo MRS studies of exercising human quadriceps. MAGMA 21: 227–235, 2008. doi: 10.1007/s10334-008-0117-3. [DOI] [PubMed] [Google Scholar]
- 35.Layec G, Gifford JR, Trinity JD, Hart CR, Garten RS, Park SY, Le Fur Y, Jeong EK, Richardson RS. Accuracy and precision of quantitative 31P-MRS measurements of human skeletal muscle mitochondrial function. Am J Physiol Endocrinol Metab 311: E358–E366, 2016. doi: 10.1152/ajpendo.00028.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Layec G, Haseler LJ, Trinity JD, Hart CR, Liu X, Le Fur Y, Jeong EK, Richardson RS. Mitochondrial function and increased convective O2 transport: implications for the assessment of mitochondrial respiration in vivo. J Appl Physiol 115: 803–811, 2013. doi: 10.1152/japplphysiol.00257.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Layec G, Trinity JD, Hart CR, Le Fur Y, Sorensen JR, Jeong EK, Richardson RS. Evidence of a metabolic reserve in the skeletal muscle of elderly people. Aging (Albany NY) 9: 52–67, 2016. doi: 10.18632/aging.101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le Fur Y, Nicoli F, Guye M, Confort-Gouny S, Cozzone PJ, Kober F. Grid-free interactive and automated data processing for MR chemical shift imaging data. MAGMA 23: 23–30, 2010. [Erratum in MAGMA 23: 123, 2010.] doi: 10.1007/s10334-009-0186-y. [DOI] [PubMed] [Google Scholar]
- 39.Lodi R, Kemp GJ, Iotti S, Radda GK, Barbiroli B. Influence of cytosolic pH on in vivo assessment of human muscle mitochondrial respiration by phosphorus magnetic resonance spectroscopy. MAGMA 5: 165–171, 1997. doi: 10.1007/BF02592248. [DOI] [PubMed] [Google Scholar]
- 40.Luck JC, Miller AJ, Aziz F, Radtka JF III, Proctor DN, Leuenberger UA, Sinoway LI, Muller MD. Blood pressure and calf muscle oxygen extraction during plantar flexion exercise in peripheral artery disease. J Appl Physiol 123: 2–10, 2017. doi: 10.1152/japplphysiol.01110.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangiafico RA, Malatino LS, Spada RS, Santonocito M, Messina R, Dell’Arte S, Attinà T. Treadmill exercise-induced release of endothelin-1 in patients with peripheral arterial occlusive disease at Fontaine stage IIb. Int Angiol 19: 14–17, 2000. [PubMed] [Google Scholar]
- 42.McCully KK, Halber C, Posner JD. Exercise-induced changes in oxygen saturation in the calf muscles of elderly subjects with peripheral vascular disease. J Gerontol 49: B128–B134, 1994. doi: 10.1093/geronj/49.3.B128. [DOI] [PubMed] [Google Scholar]
- 43.McCully KK, Landsberg L, Suarez M, Hofmann M, Posner JD. Identification of peripheral vascular disease in elderly subjects using optical spectroscopy. J Gerontol A Biol Sci Med Sci 52: B159–B165, 1997. doi: 10.1093/gerona/52A.3.B159. [DOI] [PubMed] [Google Scholar]
- 44.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA 286: 1599–1606, 2001. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 45.Mettauer B, Zoll J, Sanchez H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J Am Coll Cardiol 38: 947–954, 2001. doi: 10.1016/S0735-1097(01)01460-7. [DOI] [PubMed] [Google Scholar]
- 46.Miller AJ, Luck JC, Kim DJ, Leuenberger UA, Proctor DN, Sinoway LI, Muller MD. Blood pressure and leg deoxygenation are exaggerated during treadmill walking in patients with peripheral artery disease. J Appl Physiol 123: 1160–1165, 2017. doi: 10.1152/japplphysiol.00431.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009. doi: 10.1161/HYPERTENSIONAHA.109.130880. [DOI] [PubMed] [Google Scholar]
- 48.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012. doi: 10.1113/jphysiol.2012.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E III, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K; TASC II Working Group . Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 33, Suppl 1: S1–S75, 2007. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Pasternak RC, Criqui MH, Benjamin EJ, Fowkes FG, Isselbacher EM, McCullough PA, Wolf PA, Zheng ZJ; American Heart Association . Atherosclerotic Vascular Disease Conference: writing group I: epidemiology. Circulation 109: 2605–2612, 2004. doi: 10.1161/01.CIR.0000128518.26834.93. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen PK, Kiens B, Saltin B. Hyperoxia does not increase peak muscle oxygen uptake in small muscle group exercise. Acta Physiol Scand 166: 309–318, 1999. doi: 10.1046/j.1365-201x.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 52.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810: 25–58, 2012. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- 53.Picard M, Csukly K, Robillard ME, Godin R, Ascah A, Bourcier-Lucas C, Burelle Y. Resistance to Ca2+-induced opening of the permeability transition pore differs in mitochondria from glycolytic and oxidative muscles. Am J Physiol Regul Integr Comp Physiol 295: R659–R668, 2008. doi: 10.1152/ajpregu.90357.2008. [DOI] [PubMed] [Google Scholar]
- 54.Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg 41: 481–489, 2008. doi: 10.1177/1538574407311106. [DOI] [PubMed] [Google Scholar]
- 55.Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovascular Surg 42: 101–112, 2008. doi: 10.1177/1538574408315995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pipinos II, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, Baxter BT, Lynch TG, Dodd SL. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med 41: 262–269, 2006. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Pipinos II, Sharov VG, Shepard AD, Anagnostopoulos PV, Katsamouris A, Todor A, Filis KA, Sabbah HN. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Surg 38: 827–832, 2003. doi: 10.1016/S0741-5214(03)00602-5. [DOI] [PubMed] [Google Scholar]
- 58.Pipinos II, Shepard AD, Anagnostopoulos PV, Katsamouris A, Boska MD. Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. J Vasc Surg 31: 944–952, 2000. doi: 10.1067/mva.2000.106421. [DOI] [PubMed] [Google Scholar]
- 59.Pipinos II, Swanson SA, Zhu Z, Nella AA, Weiss DJ, Gutti TL, McComb RD, Baxter BT, Lynch TG, Casale GP. Chronically ischemic mouse skeletal muscle exhibits myopathy in association with mitochondrial dysfunction and oxidative damage. Am J Physiol Regul Integr Comp Physiol 295: R290–R296, 2008. doi: 10.1152/ajpregu.90374.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poole DC, Copp SW, Ferguson SK, Musch TI. Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol 98: 1645–1658, 2013. doi: 10.1113/expphysiol.2013.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richardson RS, Sheldon J, Poole DC, Hopkins SR, Ries AL, Wagner PD. Evidence of skeletal muscle metabolic reserve during whole body exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159: 881–885, 1999. doi: 10.1164/ajrccm.159.3.9803049. [DOI] [PubMed] [Google Scholar]
- 62.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR, Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol 111: 81–86, 2011. doi: 10.1152/japplphysiol.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sansbury BE, Jones SP, Riggs DW, Darley-Usmar VM, Hill BG. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem Biol Interact 191: 288–295, 2011. doi: 10.1016/j.cbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation 110: 738–743, 2004. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 65.Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol 46: 599–602, 1979 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- 66.Shabeeh H, Seddon M, Brett S, Melikian N, Casadei B, Shah AM, Chowienczyk P. Sympathetic activation increases NO release from eNOS but neither eNOS nor nNOS play an essential role in exercise hyperemia in the human forearm. Am J Physiol Heart Circ Physiol 304: H1225–H1230, 2013. doi: 10.1152/ajpheart.00783.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health Risk Manag 3: 229–234, 2007. doi: 10.2147/vhrm.2007.3.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spires J, Gladden LB, Grassi B, Goodwin ML, Saidel GM, Lai N. Distinguishing the effects of convective and diffusive O2 delivery on V̇o2 on-kinetics in skeletal muscle contracting at moderate intensity. Am J Physiol Regul Integr Comp Physiol 305: R512–R521, 2013. doi: 10.1152/ajpregu.00136.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trenell MI, Sue CM, Kemp GJ, Sachinwalla T, Thompson CH. Aerobic exercise and muscle metabolism in patients with mitochondrial myopathy. Muscle Nerve 33: 524–531, 2006. doi: 10.1002/mus.20484. [DOI] [PubMed] [Google Scholar]
- 70.Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, Ewald B, Gardner AW, Hatano Y, Lutes LD, Matsudo SM, Ramirez-Marrero FA, Rogers LQ, Rowe DA, Schmidt MD, Tully MA, Blair SN. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act 8: 80, 2011. doi: 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129: 35–43, 1997. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 72.Walker MA, Hoier B, Walker PJ, Schulze K, Bangsbo J, Hellsten Y, Askew CD. Vasoactive enzymes and blood flow responses to passive and active exercise in peripheral arterial disease. Atherosclerosis 246: 98–105, 2016. doi: 10.1016/j.atherosclerosis.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 73.Walter G, Vandenborne K, McCully KK, Leigh JS. Noninvasive measurement of phosphocreatine recovery kinetics in single human muscles. Am J Physiol Cell Physiol 272: C525–C534, 1997. doi: 10.1152/ajpcell.1997.272.2.C525. [DOI] [PubMed] [Google Scholar]
- 74.Weiss DJ, Casale GP, Koutakis P, Nella AA, Swanson SA, Zhu Z, Miserlis D, Johanning JM, Pipinos II. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. J Transl Med 11: 230, 2013. doi: 10.1186/1479-5876-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol 42: 385–390, 1977. doi: 10.1152/jappl.1977.42.3.385. [DOI] [PubMed] [Google Scholar]
- 76.Wüst RC, Grassi B, Hogan MC, Howlett RA, Gladden LB, Rossiter HB. Kinetic control of oxygen consumption during contractions in self-perfused skeletal muscle. J Physiol 589: 3995–4009, 2011. doi: 10.1113/jphysiol.2010.203422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young DF, Cholvin NR, Kirkeeide RL, Roth AC. Hemodynamics of arterial stenoses at elevated flow rates. Circ Res 41: 99–107, 1977. doi: 10.1161/01.RES.41.1.99. [DOI] [PubMed] [Google Scholar]