Abstract
Many cardiovascular diseases are associated with pathological remodeling of the extracellular matrix (ECM) in the myocardium. ECM remodeling is a complex, multifactorial process that often contributes to declines in myocardial function and progression toward heart failure. However, the direct effects of the many forms of ECM remodeling on myocardial cell and tissue function remain elusive, in part because conventional model systems used to investigate these relationships lack robust experimental control over the ECM. To address these shortcomings, microphysiological systems are now being developed and implemented to establish direct relationships between distinct features in the ECM and myocardial function with unprecedented control and resolution in vitro. In this review, we will first highlight the most prominent characteristics of ECM remodeling in cardiovascular disease and describe how these features can be mimicked with synthetic and natural biomaterials that offer independent control over multiple ECM-related parameters, such as rigidity and composition. We will then detail innovative microfabrication techniques that enable precise regulation of cellular architecture in two and three dimensions. We will also describe new approaches for quantifying multiple aspects of myocardial function in vitro, such as contractility, action potential propagation, and metabolism. Together, these collective technologies implemented as cardiac microphysiological systems will continue to uncover important relationships between pathological ECM remodeling and myocardial cell and tissue function, leading to new fundamental insights into cardiovascular disease, improved human disease models, and novel therapeutic approaches.
Keywords: biomaterials, contractility, electrophysiology, metabolism, microfabrication
INTRODUCTION
The healthy adult myocardium consists of layers of rhythmically contracting, synchronized cardiac myocytes that are elongated and aligned into fiber-like structures to maximize force generation parallel to the long axis of the tissue (Fig. 1A) (186). Cardiac myocytes are embedded in a compliant extracellular matrix (ECM) network that provides alignment cues, biochemical signals, and mechanical support and resistance to myocytes. Myocardial ECM contains mostly collagen fibers aligned in parallel to cardiac myocytes to provide structural stability and tensile strength. Cardiac myocytes anchor to collagen fibers through a basement membrane composed largely of the glycoproteins fibronectin and laminin (151, 186). The ECM network is synthesized and remodeled by cardiac fibroblasts, which are interspersed among cardiac myocytes and are mostly quiescent in the healthy myocardium (29).
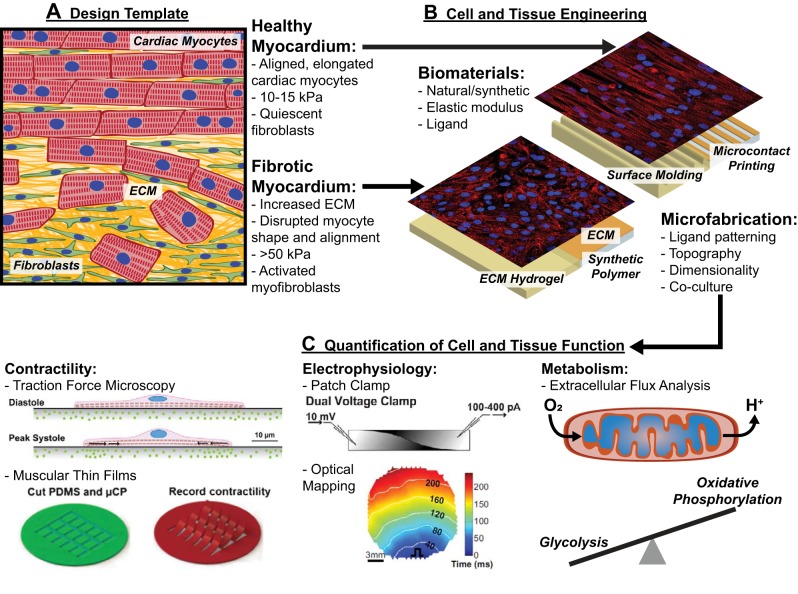
Fig. 1.
Engineering cardiac microphysiological systems to define the functional impacts of pathological extracellular matrix remodeling. A: distinct features of native healthy and diseased/fibrotic myocardium, such as myocyte shape, tissue alignment, extracellular matrix (ECM) rigidity, and cell demographics, are used as design templates for engineering cardiac microphysiological systems. B: features of native healthy and diseased/fibrotic myocardium are replicated by combining appropriate biomaterials and microfabrication techniques. C: engineered cardiac cells and tissues are interrogated with functional assays to quantify contractility, electrophysiology, and metabolism as a function of their microenvironment. Collectively, these approaches implemented as cardiac microphysiological systems can identify the functional impact of ECM remodeling to streamline mechanistic studies and therapeutic development. Images in C were adapted from Ref. 119 (top left), Ref. 2 with permission from The Royal Society of Chemistry (bottom left), Ref. 117 (top middle), and Ref. 199 (bottom middle) with permission from Elsevier.
Many cardiac diseases are associated with extensive remodeling of both the cellular and extracellular components of the myocardium (50). For example, after a myocardial infarction, immune cells first remove necrotic cardiac myocytes. Subsequently, fibroblasts and their activated derivatives, myofibroblasts, migrate to the affected area and deposit scar tissue to stabilize the ventricle (181). The infarcted myocardium has more collagen and less fibronectin and laminin than the healthy myocardium (176) and is considerably stiffer (20, 48, 176), changing the biochemical environment and increasing the mechanical load on surviving myocytes. Additionally, the transitional tissue from infarcted to healthy myocardium, known as the infarct border zone, experiences a loss of myocyte alignment and remodeling of myocyte shape due to infiltration of scar tissue and fibroblasts/myofibroblasts (Fig. 1A) (115). These remodeling processes compromise the function of the ventricle and contribute to the progression toward heart failure. Similar patterns of ECM remodeling coupled with tissue dysfunction have been observed in many other pathological conditions, such as tachyarrythmia (171, 198), pathological hypertrophy (43, 73), and aortic valve disease (183), as well as aging (9). Thus, understanding the direct impact of distinct forms of ECM remodeling on the myocardium could lead to more effective treatment options for many forms of cardiac disease and dysfunction.
The effects of ECM remodeling on myocardial function remain elusive, which is in part due to the limitations of conventional model systems. For example, independently dictating the many diverse features of the ECM is extremely challenging, if not impossible, with animal models. Furthermore, complex microenvironmental features, such as tissue alignment and ECM elasticity, are not controllable with conventional cell culture substrates. To bridge the gap between in vivo and conventional in vitro models, new in vitro platforms, known as cardiac microphysiological systems, are now being developed and implemented. These are microfabricated platforms engineered to mimic many of the diverse microenvironmental cues observed in myocardial tissue in vivo while the level of user control and experimental accessibility provided by in vitro models is maintained (Fig. 1B). Additionally, these platforms are developed with integrated functional assays to enable well-designed biological studies, provide deeper mechanistic insights, and facilitate medium-throughput screening (Fig. 1C). In this review, we will describe present approaches for engineering and using cardiac microphysiological systems to interrogate the functional impact of pathological ECM remodeling on the myocardium. These platforms have unique potential for establishing relationships between the ECM and cardiac function with more granularity than previously possible, which will complement existing model systems, greatly enhance our understanding of cardiac disease, and pave the way for new treatment options.
TUNABLE BIOMATERIALS FOR ENGINEERING CARDIAC MICROPHYSIOLOGICAL SYSTEMS
Biomaterials can be used to replicate multiple features of the native ECM in vitro. Biomaterials are usually polymers and can be naturally derived or manmade. In this section, we will detail the characteristics, uses, advantages, and disadvantages of some of the most common natural and synthetic biomaterials used in cardiac microphysiological systems, which are also shown in Table 1.
Table 1.
Biomaterials implemented in cardiac microphysiological systems
| Advantages | Disadvantages | Examples with References | |
|---|---|---|---|
| Natural | |||
| Mammalian ECM-derived hydrogels | • Tunable elastic modulus | • Difficult to decouple elastic modulus and ECM ligand | • Matrigel (52, 146, 158) |
| • Naturally adhesive to cells and amenable to cellular remodeling | • Certain isolated proteins (fibronectin, etc.) are expensive | • Gelatin (116, 125, 156) | |
| • Compatible with 2-D and 3-D tissue engineering | • Limited range of elastic moduli | • Fibrin (24, 28, 196) | |
| • Physiological | |||
| Nonmammalian ECM-derived hydrogels | • Tunable elastic modulus | • Not physiological | • Chitosan (41) |
| • Easier to decouple mechanical properties and ECM ligand | • Limited range of elastic moduli | • Alginate (1)• Silk (140, 175) | |
| • Some are naturally adhesive to cells and amenable to cellular remodeling | |||
| • Relatively compatible with 2-D and 3-D tissue engineering | |||
| • Relatively inexpensive | |||
| ECM from decellularized tissues | • Closely mimic the chemical and mechanical properties of native ECM | • Not tunable• Difficult to acquire• Highly heterogeneous and variable | • Decellularized rat and pig heart slices (25)• Solubilized decellularized ECM (59, 176) |
| Synthetic | |||
| Elastomers | • Easy to decouple elastic modulus and ECM ligand | • Not naturally adhesive to cells or amenable to cellular remodeling | • Polydimethylsiloxane (31, 54, 111, 134, 147) |
| • Compatible with 2-D tissue engineering• Relatively inexpensive | • Not compatible with 3-D tissue engineering | ||
| • Extensive range of elastic moduli | |||
| Hydrogels | • Easy to decouple elastic modulus and ECM ligand | • Not naturally adhesive to cells or amenable to cellular remodeling | • Polyacrylamide (11, 59, 69, 119, 176) |
| • Compatible with 2-D tissue engineering• Relatively inexpensive | • Not compatible with 3D tissue engineering | • Polyethylene glycol (86, 87) | |
2-D, two-dimensional; 3-D, three-dimensional; ECM, extracellular matrix.
Mammalian ECM-Derived Hydrogels
For decades, cardiac myocytes have been cultured on glass or plastic surfaces coated with ECM proteins present in native myocardium, such as collagen, fibronectin, and laminin. These proteins are usually isolated from other ECM-rich tissues that are relatively easy to acquire, such as porcine skin, rat tails, blood plasma, and the placenta (37). Coating culture surfaces with isolated ECM molecules has been used to delineate the effects of distinct ECM ligands on cell phenotype and define the integrin types expressed and activated in cardiac myocytes in developmental, physiological, and pathological settings. For example, both neonatal and adult rat cardiac myocytes adhere to surfaces coated with basement membrane proteins such as laminin and collagen type IV. However, only neonatal rat cardiac myocytes adhere to interstitial ECM proteins, such as other types of collagen and fibronectin (27, 109), which is suggestive of developmentally regulated integrin expression patterns. Additionally, studies have shown that phenylephrine-induced hypertrophy occurs when cardiac myocytes are cultured on fibronectin or laminin, but not bovine serum albumin, implicating ECM composition and the subsequent activation of specific integrin receptors in hypertrophic responses (152).
Although coating ECM proteins onto glass or plastic surfaces can be used to interrogate the phenotypic effects of ECM composition on cardiac myocytes, this approach offers minimal control over other properties of the ECM, including rigidity. One approach for tuning the rigidity of many ECM molecules is to synthesize them as hydrogels. Hydrogels are hydrophilic, cross-linked polymer networks with significant water content and elastic moduli in the range of soft biological tissues (101). Because of their high water content, porosity, and biocompatibility, hydrogels synthesized from ECM molecules are relatively versatile. For example, they can be used as two-dimensional (2-D) culture substrates (116) or for encapsulating cells to engineer three-dimensional (3-D) tissues (180). Many ECM molecules are already commercialized and widely used as hydrogels for cell culture, such as Matrigel. Matrigel is a cell-derived, laminin-rich mixture of ECM proteins and growth factors that self-polymerize into a hydrogel at 37°C (94). Matrigel is commonly used for culturing stem cell-derived cardiac myocytes as either a monolayer coating on a surface or as a thicker hydrogel (52, 146, 158). However, because Matrigel is a heterogeneous mixture of ECM molecules, it can vary between batches and offers imprecise control of the ECM.
Collagen and its partially denatured derivative, gelatin, can also be cross-linked into hydrogels. Collagen type I is soluble at acidic pH and spontaneously cross-links into a thermostable hydrogel at neutral pH due to intermolecular bond formation between lysine and hydroxylysine residues (126). Gelatin requires cross-linking by chemical or enzymatic means, such as glutaraldehyde (14, 128) or microbial transglutaminase (130, 197), respectively, to form thermostable hydrogels. Likely because of the biomimetic composition and mechanical properties of gelatin hydrogels, neonatal rat cardiac myocytes naturally adhere to and maintain longer-term viability and contractility on these substrates compared with ECM-coated synthetic polymers (116). Gelatin and other ECM proteins, such as tropoelastin (8), can also be modified with methacrylate groups, which are cross-linked by light (125). Because methacrylated gelatin is photo-cross-linkable, users have both spatial and temporal control over hydrogel cross-linking while still preserving biocompatibility and cell adhesion (125, 156). Another protein widely used to fabricate hydrogels is fibrin, which is involved in blood clotting and is synthesized by combining fibrinogen and thrombin to form fibrin fibers (89). Although fibrin is non-native to the myocardium, fibrin hydrogels are still highly biocompatible and can be remodeled by cardiac cells, which have been shown to replace fibrin with their own collagen matrix (24). Fibrin can also be combined with collagen to fabricate hybrid hydrogels with tunable stiffness, which also increases the longevity of 3-D tissues because fibrin fibers are more robust than collagen (28).
Many properties of ECM-derived hydrogels, including stability, rigidity, optical clarity, and porosity, can be tuned by altering the concentration(s) of polymer and/or cross-linker. This feature can be used to controllably mimic both the chemical and mechanical features of healthy and diseased ECM environments in vitro. However, a major disadvantage of these hydrogels is that many of their physical properties are coupled (37). For example, gelatin hydrogels can be stiffened by increasing the concentration of gelatin, but this also increases ligand density and decreases porosity (99). These hydrogels also have a relatively limited range of elastic moduli and thus cannot always recapitulate the range of mechanical environments observed in vivo throughout development and disease.
Decellularized ECM Scaffolds
A more recent approach for defining the physiological and pathological effects of native myocardial ECM is decellularization. Decellularization entails using detergents to remove all cells from a tissue, leaving behind the intact ECM that can be used as a scaffold for tissue engineering. Entire intact hearts have been decellularized and repopulated with cardiac myocytes, with the long-term goal of organ regeneration (131, 188). This approach has also been adapted for in vitro studies by attaching thin slices of decellularized rat and pig hearts to coverslips, seeding the slices with neonatal rat cardiac myocytes, and measuring contractility and electrophysiology (25). The main advantage of decellularized tissue scaffolds is that they maintain the composition, architecture, and mechanical properties of native cardiac ECM, and therefore, they have high physiological relevance. Decellularized ECM from healthy and postinfarct rat ventricular myocardium has also been solubilized and incorporated into mechanically tunable polyacrylamide hydrogels to determine the effects of completely native ECM on mesenchymal stem cell differentiation toward cardiac lineages (59, 176). Despite its advantages related to physiological relevance, the major limitations of using ECM acquired from decellularized hearts are the limited availability of human samples and the relatively high amount of processing required.
Nonmammalian Natural Biomaterials
Macromolecules from nonmammalian organisms have also been isolated and used as biomaterials for in vitro cardiac models. For example, alginate and chitosan are polysaccharides isolated from algae and crustacean shells, respectively, that are widely used tissue engineering scaffolds due to their availability, low cost, and biocompatibility. Alginate and chitosan hydrogels are cross-linked with agents such as calcium chloride and glutaraldehyde, respectively (37, 101). The degree of cross-linking dictates their elastic modulus. However, because these materials are not found in native myocardium, they must be combined or functionalized with proteins or peptides that promote cardiac myocyte adhesion. Although this adds an additional fabrication step, this can be advantageous for gaining independent control over the chemical and mechanical properties of the scaffold. One approach for functionalization is to add ECM proteins directly to the hydrogel prepolymer solution. For example, chitosan has been functionalized with azidobenzoic acid and mixed with collagen to create photocrosslinkable collagen-chitosan hydrogels compatible with cardiac myocyte culture (41). Alternatively, alginate hydrogels have been functionalized by doping the hydrogel with streptavidin, which enables surface binding of biotinylated fibronectin and subsequent attachment of cardiac myocytes (1).
Silk protein has also been used as a cardiac tissue scaffold. Silk fibroin isolated from cocoons of Bombyx mori, one species of silkworm, has been mixed with collagen or ECM isolated from decellularized pig hearts to create fibrous scaffolds adherent for cardiac myocytes (175). Silk fibroin isolated from the silk glands of Antheraea myllita, another species of silkworm, contains more Arg-Gly-Asp groups than silk fibroin from B. mori, resembling the structure of fibronectin. Thus, cardiac myocytes adhere directly to coverslips coated with solutions of A. myllita silk (140), reducing the need for additional functionalization. In summary, macromolecules derived from nonmammalian organisms are a unique source of nontoxic biomaterials that can also be used to engineer tunable substrates for physiological and pathological in vitro models of the myocardium.
Synthetic Hydrogels
Synthetic biomaterials generally provide greater flexibility in terms of composition, fabrication, and processing compared with natural biomaterials. Similar to natural biomaterials, the mechanical properties of synthetic biomaterials are dictated by their molecular structure, including the degree of polymer cross-linking. In most cases, synthetic biomaterials are nonadhesive to cells and must be functionalized with peptides or proteins before cell culture. Hence, the elasticity and protein composition of synthetic biomaterial scaffolds can be tuned independently, which is advantageous for decoupling mechanical and chemical features.
Polyacrylamide hydrogels are a common synthetic hydrogel for culturing cardiac myocytes. These hydrogels are typically prepared by inducing free-radical polymerization of acrylamide and bis-acrylamide with ammonium persulfate and a catalyst. The elastic modulus of the resulting hydrogel is controlled by adjusting the ratio of acrylamide to bis-acrylamide (10, 48, 69, 81, 82, 118). To induce myocyte adhesion, ECM proteins can be directly mixed into the hydrogel prepolymer solution before cross-linking (59, 176) or attached to the surface of the hydrogel using sulfo-SANPAH and ultraviolet light followed by ECM protein coating (10, 47, 69). Polyacrylamide hydrogels can also be doped with streptavidin-acrylamide to enable surface attachment of biotinylated ECM proteins such as fibronectin (119) or laminin (11). Polyethylene glycol (PEG) hydrogels have also been used as mechanically tunable synthetic substrates for in vitro cardiac models. For example, PEG-diacrylate is an ultraviolet-curable modified version of PEG that can be coated with ECM molecules. These hydrogels have been successfully used to culture neonatal rat cardiac myocytes (87) and cardiosphere-derived cells (86). In summary, synthetic hydrogels offer independent control over ECM elasticity and protein composition, which is a useful feature for probing multiple extracellular variables in healthy and diseased myocardium.
Synthetic Elastomers
Another widely used synthetic biomaterial is polydimethylsiloxane (PDMS), an organic, silicon-based, elastomeric polymer that is optically clear, moldable, biocompatible, and easy to fabricate. The most common PDMS, Sylgard 184, is prepared by mixing a base and curing agent at a defined ratio, coating or molding this prepolymer solution onto different surfaces (such as spin coating onto coverslips) and temperature curing the substrate. The ratio of base to curing agent can be adjusted to define the elastic modulus of the final elastomer (189). Another approach for adjusting the elastic modulus of Sylgard 184 is to mix with different ratios of Sylgard 527 silicone dielectric gel, which is much softer than Sylgard 184 (111, 134). To promote cell adhesion, PDMS surfaces are oxidized in an ultraviolet-ozone cleaner or plasma treater to induce covalent binding to ECM proteins such as fibronectin (31, 54, 63), laminin (147), or Matrigel (40). Hence, PDMS offers independent control over ECM rigidity and protein composition. Because of its versatility and biocompatibility, PDMS is also widely used to fabricate cell culture chambers (76) and microfluidic devices (114) for cardiac microphysiological systems.
In summary, as shown in Table 1, mammalian ECM-derived biomaterials are advantageous for in vitro cardiac models because they closely match the ECM of native myocardium and are naturally adhesive to cells. However, decoupling their chemical, physical, and/or mechanical properties can be challenging. In contrast, nonmammalian natural biomaterials and synthetic biomaterials are a “blank slate” that offer greater independent control over chemical and mechanical properties at the expense of additional modifications required for myocyte adhesion, a characteristic similar to synthetic biomaterials. Furthermore, most synthetic biomaterials cannot be used to encapsulate cells for engineering 3-D tissues because they are not amenable to cellular remodeling and/or are toxic as a 3-D substrate. Therefore, researchers must weigh these tradeoffs and select biomaterials that best fit their research question when designing in vitro models to probe the effects of ECM remodeling.
ENGINEERING AND INTERROGATING 2-D CARDIAC MICROPHYSIOLOGICAL SYSTEMS
In the healthy myocardium, cardiac myocytes are uniaxially aligned and embedded in a compliant ECM network. Both of these microenvironmental features can be affected by ECM remodeling, but neither can be easily controlled with conventional in vitro approaches. Thus, to determine the direct effects of ECM remodeling on cardiac structure and function, microfabrication techniques have been developed to engineer customizable microenvironments for cardiac myocyte cells and tissues in vitro. In this section, we will detail approaches for engineering cardiac tissues in 2-D and interrogating their key functions, including contractility, as a function of their microenvironment.
Microcontact Printing
Patterned growth of cardiac tissue was originally achieved by culturing cardiac myocytes on photoresist-coated coverslips selectively exposed to ultraviolet light through a custom photomask (150). A similar photolithography approach is still used today to fabricate wafer templates for microcontact printing. Microcontact printing is a technique similar to rubber ink stamping that is used to define the 2-D architecture of cells and tissues in vitro (144), including cardiac myocytes (57, 136). The first step in microcontact printing is to fabricate a silicon wafer with a relief of the desired pattern. PDMS is then cured on the wafer and carefully removed to create “stamps” containing the desired pattern as raised features on the surface. These PDMS stamps are then coated with ECM proteins, such as fibronectin, and inverted onto substrates, such as coverslips spin coated with a layer of PDMS, to transfer the desired pattern from the stamp to the substrate. Cardiac myocytes seeded on these substrates will attach only to the protein-printed areas and, therefore, conform to the pattern.
Many cardiac diseases are associated with distinct changes in myocyte shape and/or tissue architecture, which can be recapitulated in vitro with microcontact printing. For example, the aspect ratio of healthy cardiac myocytes is 7:1, but this decreases in concentric hypertrophy and increases in eccentric hypertrophy (58, 68). To mimic these changes, neonatal rat cardiac myocytes have been cultured on 2,500-µm2 rectangles of fibronectin microcontact printed on PDMS with length-to-width aspect ratios ranging from 1:1 to 7:1, which demonstrated that cell geometry directly impacts sarcomere alignment (31) and nuclear morphology (30).
To engineer confluent cardiac tissues with controlled alignment, cardiac myocytes have been cultured on PDMS-coated coverslips microcontact printed with parallel lines of fibronectin. Early studies engineered aligned neonatal rat ventricular tissues by patterning 10- or 20-µm-wide lines of high-concentration fibronectin, separated by 10- or 20-µm-wide regions of low-concentration fibronectin. In these studies, aligned tissues generated greater contractile stress, propagated action potentials more rapidly, and had distinct Ca2+ handling compared with unaligned tissues (53, 63, 143). More recently, 15-µm-wide lines of high-concentration fibronectin separated by 2-µm-wide spacing have been used to engineer confluent neonatal rat (2) and human-induced pluripotent stem cell (hiPSC)-derived cardiac tissues (187) for a variety of studies. More complex cardiac tissues with adjacent regions of distinct alignment have also been engineered to determine how sharp changes in tissue architecture affect conduction (35) and contractility (93). These tissues replicate some of the drastic and heterogeneous changes in tissue alignment that can occur in many pathological settings, such as the infarct border zone. To more closely mimic native cardiac microstructure, micropatterns that match the fiber orientation of mouse hearts have also been developed, acquired by high-resolution diffusion tensor magnetic resonance imaging (15). This approach could also be adopted to determine the functional impact of disrupted fiber alignment in disease. Thus, microcontact printing is a powerful technique for mimicking changes in cell and tissue architecture that often accompany pathological remodeling of the ECM in the myocardium.
To probe the joint effects of cell/tissue architecture and ECM elasticity, cardiac myocytes can be cultured on microcontact-printed polyacrylamide hydrogels with tunable rigidity. Polyacrylamide hydrogels can be microcontact printed by inverting stamps coated with biotinylated ECM proteins onto partially dehydrated, streptavidin-doped polyacrylamide hydrogels (119). Alternatively, polyacrylamide hydrogels can be polymerized on chemically treated glass coverslips microcontact printed with ECM proteins such as Matrigel (Fig. 2A) (121, 146). This transfers the patterned protein but avoids stamping directly on the hydrogel, which can introduce defects due to its low rigidity. Microcontact-printed polyacrylamide hydrogels have been used to demonstrate that sarcomere alignment and density are coregulated by both myocyte shape and ECM elasticity in single neonatal rat cardiac myocytes (119). A similar technique was used to engineer pairs of cardiac myocytes and show that electrical (117) and mechanical (118) cell-cell coupling is impacted by cell shape and/or ECM rigidity. Square aligned cardiac microtissues on custom ECM have also been engineered by microcontact printing 200 × 200-µm squares of arrayed lines of fibronectin or laminin on soft or stiff polyacrylamide gels. These substrates were seeded with primary neonatal rat ventricular myocytes, which also contain a small population of cardiac fibroblasts. Thus, these “micromyocardium” tissues consist of both cardiac myocytes and fibroblasts with ratios that are relatively tunable, adding another level of microenvironmental control to this platform (11). Tunable PDMS substrates can also be microcontact printed and used to control both tissue alignment and ECM elasticity for engineered cardiac tissues (111), although PDMS generally offers less fine control over elastic modulus in lower ranges compared with hydrogels. In summary, microcontact printing combined with tunable synthetic biomaterials can be used to independently define features such as cell/tissue architecture, ECM elasticity, and ECM composition to establish the direct intra- and intercellular effects of healthy and pathological microenvironments.
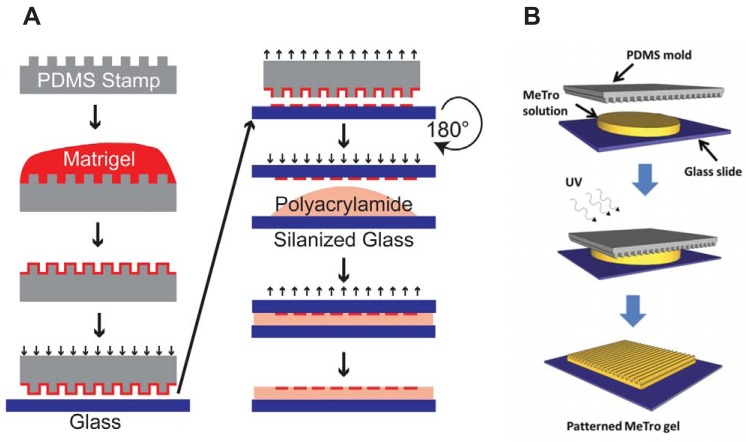
Fig. 2.
Dictating two-dimensional tissue architecture with substrate micropatterning. Customizable polydimethylsiloxane (PDMS) stamps (gray) are fabricated with photolithography and soft lithography. A: in one example of microcontact printing, stamps are coated with proteins (red) such as Matrigel and inverted onto glass coverslips (blue), transferring the pattern to the coverslip. These patterned coverslips are inverted onto polyacrylamide hydrogel prepolymer solution to transfer the extracellular matrix protein pattern to the hydrogel as it polymerizes. B: micromolding is performed by inverting PDMS stamps onto a hydrogel prepolymer solution (yellow) such as methacrylated tropoelastin (MeTro). The stamp remains in position during hydrogel polymerization, which is initiated by ultraviolet (UV) light in this example, to mold the pattern from the PDMS stamp into the hydrogel. Images were adapted from Ref. 146 with permission from the National Academy of Sciences (A) and from Ref. 8 with permission from John Wiley & Sons (B).
Surface Molding
Micromolding and nanomolding entail casting desired patterns, typically parallel lines with controlled height, ridge width, and groove width, onto the surface of a substrate. These topographical features also induce cell alignment and are similar to the fiber-like topography of native myocardial ECM (87). Microgrooved PDMS substrates have been molded on templates prepared by photolithography and soft lithography (145) or acoustic micromachining (193) and subsequently coated with fibronectin. Neonatal rat (193) and hiPSC-derived (145) cardiac myocytes cultured on microgrooved PDMS surfaces demonstrate substrate-mediated changes in calcium dynamics. Microgrooved PDMS stamps can also be inverted onto hydrogel prepolymer solutions to mold the surface of the hydrogel as it polymerizes (Fig. 2B). This approach has been used for gelatin (116), tropoelastin (8), and chitosan-collagen hydrogels (41), which are already adhesive to cardiac myocytes, and alginate, which requires additional functionalization with ECM proteins (1). Similar techniques can also be used to fabricate isolated microchannels of gelatin hydrogel embedded with cardiac myocytes (13).
Templates for nanomolding have been fabricated by using electron beam lithography to etch nanometer scale features into photoresist-coated silicon wafers. These wafers are then used to mold ultraviolet-curable poly(urethane acrylate) templates, which are subsequently inverted onto the surface of ultraviolet-curable PEG hydrogel prepolymer solution (88, 90). These PEG hydrogels can then be coated with fibronectin and seeded with cardiac myocytes. Nanoscale features have been shown to impact cell geometry, action potential conduction velocity, and connexin43 expression in cardiac myocytes, demonstrating the functional effects of topographical ECM remodeling (38, 87). In summary, micromolding and nanomolding can also be used to engineer substrates for probing the combined effects of tissue alignment and ECM mechanics on cardiac tissue function, with additional versatility for investigating the impact of 3-D topographical features.
Quantifying Contractility
Quantifying cardiac cell and tissue contractility is essential for understanding the direct functional effects of pathological ECM remodeling and evaluating the efficacy of possible therapeutic interventions. Contractility can be indirectly quantified by measuring sarcomere shortening (67), spontaneous beating frequency (165), or contraction velocity (76, 77). Atomic force microscopy (155) and manipulation of magnetic beads attached to the cell surface (194) have also been used to measure contractile forces generated by single cardiac myocytes, although these techniques are relatively invasive and low-throughput. Contractile forces can also be quantified by culturing cells on a bed of PDMS micropillars and tracking the displacement of each micropillar (Fig. 3A) (62, 133, 201). Micropillar length and width can be adjusted to tune the stiffness of the substrate and model conditions such as fibrosis (55, 179). This method has been used to measure forces generated by single cells (56, 62) and cell pairs (107). However, micropillar platforms are relatively difficult to fabricate, limiting their scalability.
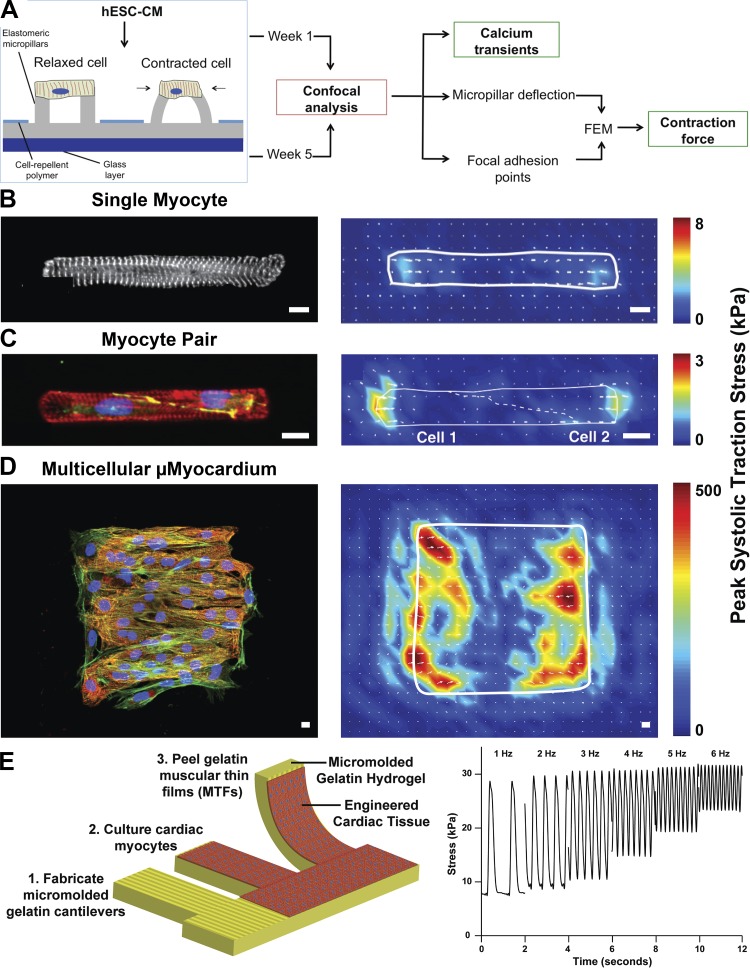
Fig. 3.
Quantifying contractility in engineered two-dimensional cardiac myocytes and tissues. A: micropillar substrates can be used to quantify contractile forces generated by cardiac myocytes. In this example, Ca2+ transients were also measured in engineered tissues on micropillar substrates. hESC-CM, human embryonic stem cell-derived cardiomyocytes. FEM, finite-element modeling. B–D: traction force microscopy and microcontact-printed polyacrylamide hydrogels have been implemented to quantify the combined effects of extracellular matrix elasticity and cell/tissue architecture on peak systolic traction stress generated by single cardiac myocytes (B), cardiac myocyte pairs (C), and multicellular cardiac tissues (D). Scale bars = 10 μm. In B, white denotes α-actinin. In C, red denotes actin, green denotes β-catenin, and blue denotes nuclei. In D, red denotes α-actinin, green denotes actin, and blue denotes nuclei. E: muscular thin film assay entails culturing cardiac myocytes on precut polymer cantilevers (shown here, micromolded gelatin hydrogels), which are released from the substrate at the time of analysis. Stress is calculated based on cantilever deflection. Images were adapted from Ref. 62 with permission from the American Chemical Society (A), Ref. 119 (B), Ref. 118 with permission from the National Academy of Sciences (C), Ref. 11 with permission from the Royal Society of Chemistry (D), and Ref. 116 with permission from Elsevier (E).
Another approach for quantifying contractility in vitro is traction force microscopy (TFM). In this assay, strains, stresses, and forces generated by cells are determined based on the displacement of their substrate. Typically, this assay is performed by culturing cells on polyacrylamide hydrogels doped with fluorescent beads and coated or microcontact printed with ECM proteins (10, 148). TFM has also been performed on alternative substrate such as PDMS (160) or gelatin hydrogels (100). As the cardiac myocytes contract, they displace the hydrogel and embedded beads. By capturing and analyzing videos of bead motion, the displacement field generated by the contracting cells can be quantified noninvasively. Based on the displacement field and the elastic modulus of the hydrogel, stresses and forces can also be calculated (36), providing a quantitative readout of contractility. Importantly, TFM substrates are relatively easy to fabricate and thus this assay has moderate scalability.
TFM studies have shown that ECM elasticity regulates contractility in unpatterned embryonic quail (48), neonatal rat (22), and hiPSC-derived (69) cardiac myocytes. In general, these studies agree that displacement decreases and stress increases as the elastic modulus of the ECM increases. TFM has also been combined with microcontact printing to assess the contractility of cardiac myocytes as a function of their architecture and the elasticity of the ECM (146). For example, neonatal rat cardiac myocytes have been cultured on polyacrylamide gels tuned to match the elasticity of healthy and fibrotic myocardium and microcontact printed with rectangles of fibronectin of distinct aspect ratios (Fig. 3B). Interestingly, myocytes with a healthy aspect ratio (7:1) generated the most amount of contractile work on substrates with physiological elasticity (98), whereas myocytes with aspect ratios closer to those in concentric hypertrophy (2:1) generated the most amount of work on substrates with fibrotic rigidity (119). This suggests that changes in myocyte shape observed in concentric hypertrophy may be an adaptation to a stiffer fibrotic ECM and/or other forms of increased afterload observed in this disease. A similar technique was used to engineer “pairs” of neonatal rat cardiac myocytes and quantify stresses transmitted both extracellularly and intercellularly (Fig. 3C). With this system, a weakened relationship between cytoskeletal organization and force generation and defects in mechanical cell-cell coupling was identified on stiffer hydrogels compared with softer hydrogels (118), suggesting that remodeling of the ECM can directly affect cell-cell junction formation. To probe the combined effects of ECM rigidity, ECM ligand, and fibroblasts on cardiac tissue contractility, aligned MicroMyocardium with distinct myocyte-to-fibroblast ratios was engineered on polyacrylamide hydrogels microcontact printed with fibronectin or laminin (11) (Fig. 3D). On stiffer polyacrylamide hydrogels, tissues generated similar amounts of force but less work, as also demonstrated by other studies (139). Interestingly, contractile force was not significantly affected by ECM protein composition or cardiac myocyte to fibroblast ratio (11), suggesting that ECM rigidity dominates over changes in ECM ligand or fibroblast density. In summary, TFM combined with microcontact printing is powerful for delineating the effects of multiple forms of cellular and extracellular remodeling on cardiac myocyte contractility. However, scalability can be limited due to the amount of time required for substrate fabrication, reliance on high-speed, high-resolution imaging, and relatively intensive data processing.
Cardiac tissue contractility has also been quantified by measuring the deflection of cells grown on deformable membranes. For example, cardiac myocytes have been cultured on circular silicone membranes that deflect with each contraction. Tensile stress can be calculated from membrane deflection (60). A similar approach is to culture and monitor cardiac myocytes on PDMS microcantilevers (135). However, in both of these examples, the cardiac myocytes are not aligned, confounding force measurements. To overcome this, muscular thin films (MTFs) were established to provide a more accurate quantification of force by combining microcontact printing with deformable cantilevers (Fig. 3E). MTFs are fabricated by laser-engraving releasable, millimeter-scale cantilevers into microcontact-printed PDMS (2, 63, 187) or micromolded gelatin (116) or alginate (1) hydrogels selectively attached to glass coverslips at their base. Cantilevers are cut parallel to the micropatterning such that seeded cells form tissues aligned parallel to the length of the cantilever. After tissue formation, the user peels each cantilever and electrically paces the tissues, causing tissue contraction and cantilever deflection. Film movement is recorded and analyzed to calculate stresses generated by the tissue based on cantilever curvature (3, 53, 63). This platform has been successfully used to quantify contractile stresses in response to tissue architecture (53), genetic mutations (187), and drug exposure (2, 116), demonstrating its utility for a wide array of disease modeling studies. However, although MTFs can be fabricated from hydrogels, the range of elastic moduli compatible with this assay is relatively narrow compared with TFM. Thus, MTFs are well suited for determining how remodeling of select disease-relevant parameters, such as tissue architecture or genetic mutations, compromise contractility but have limitations related to probing ECM mechanics.
Quantifying Electrophysiology
Myofibril contraction is dependent on the release of Ca2+ from the sarcoplasmic reticulum, which is initiated by an action potential (21). Action potentials are rapidly propagated across myocyte cell-cell junctions by low-resistance gap junction channels to ensure that myocardial tissue contracts in synchrony (91). Thus, the morphology, dynamics, and propagation velocity of action potentials and Ca2+ transients are critical functional metrics because each can directly impact cell and tissue contractility. Historically, patch clamp has been one of the most common techniques to record changes in membrane voltage or current from single cardiac myocytes (19). However, although this technique is highly sensitive, it is low throughput and requires extensive technical expertise and specialized equipment. Another approach that is more scalable is to use voltage- and Ca2+-sensitive fluorescent probes to optically record the morphology and/or propagation of action potentials and Ca2+ transients in cardiac cells and tissues (83). Recently, these dyes have been implemented with optogenetic stimulation of cardiac myocytes in multiwell plates as an all-optical, contact-free approach to electrophysiology screening (92), further improving throughput and automation.
Several studies have investigated relationships between the ECM and electrophysiology. At the single-cell level, the elastic modulus of the ECM has been shown to alter the morphology of Ca2+ transients (82) and action potentials (26) in unpatterned neonatal rat cardiac myocytes, as measured by intracellular Ca2+ dyes and patch clamp, respectively. At the two-cell level, micropatterned neonatal rat cardiac myocytes and dual voltage clamp have demonstrated that myocyte shape and cell-cell junction morphology impacts connexin43 density and intercellular conductance (118). At the tissue level, high-speed imaging of transmembane voltage and/or intracellular Ca2+ dyes has shown that tissue alignment induced by microcontact printing (35, 53, 63), microtopography (42), or nanotopography (87) increases action potential duration and the anisotropy ratio of conduction. Furthermore, the elastic modulus and topography of the ECM have been shown to impact the anisotropy ratio of Ca2+ wave propagation velocity (142). Another technique for recording conduction velocity is to culture cardiac myocytes on microelectrode arrays, which are glass substrates with a grid of electrodes on the surface. The microelectrode array surface is compatible with select 2-D tissue engineering techniques such as microcontact printing (124, 172). Micromolded gelatin hydrogels have also been engineered on microelectrode arrays (97), which minimally interfere with electrical signals due to their high water content. Collectively, these studies indicate that properties of the ECM impact electrophysiological parameters in cardiac myocytes at the cell and tissue levels.
Metabolic Profiling
Cardiac myocytes have relatively high metabolic demands, as the human heart consumes ∼30 kg/day ATP (46). In healthy adult cardiac myocytes, ATP is produced primarily by the oxidative phosphorylation of products generated by the β-oxidation of fatty acids in the mitochondria. Consequently, cardiac myocytes are densely packed with mitochondria that align into parallel lanes packed between myofibrils to efficiently deliver the ATP that fuels sarcomere shortening (7, 78, 127). However, in many pathological settings, cardiac myocytes revert to a neonatal metabolic phenotype (61, 173, 177) that is characterized by increased reliance on glycolysis and glucose oxidative phosphorylation (39, 108, 185) and changes in the turnover of the mitochondrial network (5, 182). However, the mechanisms driving these metabolic changes, including any effects secondary to ECM remodeling, are poorly understood. In other cell types, mitochondria have been shown to be mechanosensitive (16, 32), suggesting that ECM remodeling could impact mitochondria structure and/or function in cardiac myocytes. Additionally, because of their proximity to myofibrils, mitochondria are mechanically compressed with each contraction (127) and are known to interact with other cytoskeletal proteins, such as microtubules (6, 65). Thus, the cytoskeleton could transduce signals from the ECM directly to the mitochondria to modulate metabolism based on the microenvironment. However, few studies have investigated the impact of ECM remodeling on mitochondria in cardiac myocytes specifically, as described below.
Several techniques exist for metabolically profiling cardiac myocytes in vitro. Intracellular ATP production can be quantified by lysing cells and using luciferin/luciferase-based assays (132, 141). However, it is not possible to differentiate between ATP generated by glycolytic or mitochondrial pathways with this approach. ATP can also be quantified using liquid chromatography-mass spectroscopy (33, 159), which can be combined with radioactive carbon-13 tagging to identify the specific metabolic pathways preferred by the cells (44, 137). Mitochondrial membrane potential can be assessed by imaging cells loaded with mitochondrial dyes, such as TMRM/TMRE (Fig. 4A) (45, 169, 170, 191) and rhodamine 123 (103, 113), although these dyes have relatively low sensitivity. Many imaging and plate reader-based assays also exist for quantifying oxidative stress and reactive oxygen species, a byproduct of oxidative phosphorylation (Fig. 4A) (64, 187, 191). Mitochondrial function can be more directly characterized in vitro by measuring oxygen consumption rates (OCRs). Historically, OCRs have been measured with a Clark electrode (45, 141), which measures ambient oxygen concentration in a liquid using a catalytic platinum surface. Another approach is to evaluate OCRs in mitochondria isolated from tissue samples or cells in suspension using an oxygraph, a closed chamber that contains an oxygen sensor (74, 75). However, both of these techniques are relatively low throughput and incompatible with cells adhered to culture surfaces, which is essential for establishing direct relationships between the microenvironment and metabolism in cardiac myocytes.
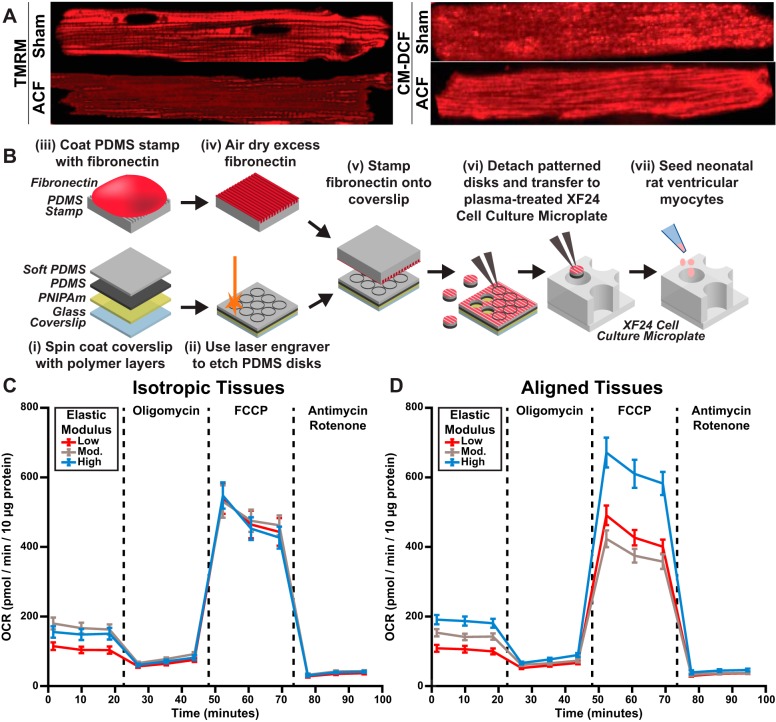
Fig. 4.
Metabolic profiling of cardiac myocytes and engineered two-dimensional cardiac tissues. A: cardiac myocytes isolated from adult rats subjected to sham surgery or aortocaval fistula (ACF) and tagged for mitochondrial membrane potential (TMRM) or reactive oxygen species (CM-DCF). B: microcontact-printed polydimethylsiloxane (PDMS) disks of distinct elasticities were fabricated in a multistep process (i–vi), transferred to the wells of modified microplates (vi), and seeded with neonatal rat ventricular myocytes (vii). These plates were inserted into an extracellular flux analyzer to quantify the effects of extracellular matrix elasticity and tissue architecture on oxygen consumption rates (OCRs). C and D: representative OCR profiles for isotropic (C) and aligned (D) tissues demonstrate distinct profiles due to extracellular matrix rigidity and tissue alignment. Images were adapted from Ref. 191 (A) and Ref. 111 (B–D).
A more recent technique that is compatible with conventional cell culture is extracellular flux analysis. This approach simultaneously measures changes in both oxygen concentration and pH to quantify OCRs and extracellular acidification rate for cultured cells or isolated mitochondria (4, 167). These experiments require cells or tissues to be cultured within a modified microplate, which can be easily coated with gelatin hydrogels or tunable PDMS to determine the effects of these biomaterials on cardiac myocyte metabolism (116). To establish how both ECM elasticity and tissue architecture regulate mitochondrial function, PDMS disks with distinct elastic moduli have been microcontact printed with fibronectin lines, transferred to the bottom of microplate wells, and seeded with neonatal rat ventricular myocytes (Fig. 4B). In this study, baseline and ATP production OCR values increased with ECM rigidity for both isotropic and aligned tissues, suggesting that mitochondria consume more oxygen in stiffer microenvironments (Fig. 4C). However, maximum respiration and spare respiratory OCR values were independent of ECM rigidity for isotropic tissues but were significantly higher in aligned tissues on the most rigid substrate (Fig. 4D) (111). These data suggest that ECM rigidity regulates baseline metabolic function, but both ECM rigidity and tissue architecture influence metabolic stress responses. In summary, cardiac microphysiological systems integrated with functional assays have provided many new insights into the effects of multiple forms of ECM remodeling on contractility, electrophysiology, and metabolism.
ENGINEERING AND INTERROGATING 3-D CARDIAC MICROPHYSIOLOGICAL SYSTEMS
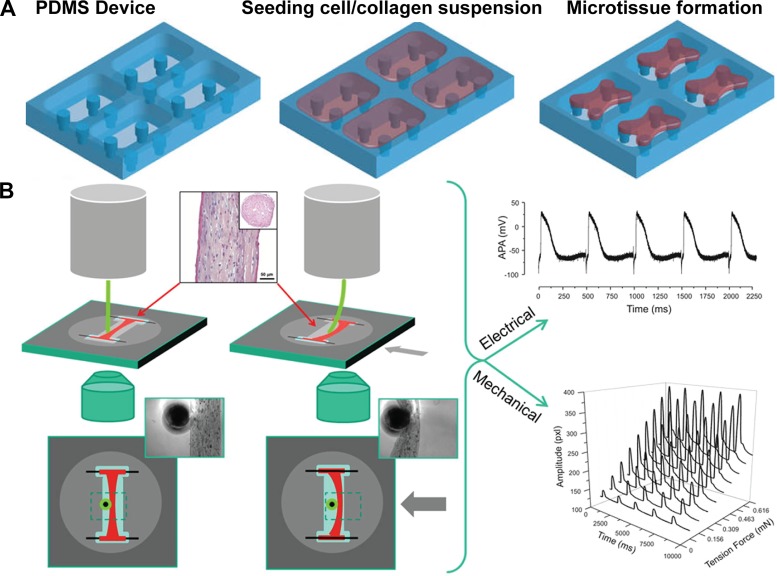
In general, tissues engineered in 3-D replicate the architecture of native myocardium more closely than 2-D engineered tissues, and, therefore, they are potentially more accurate representations of healthy and diseased myocardium. The simplest approach to engineering 3-D tissues is to create spherical aggregates of cells grown in suspension (17, 18, 84) or adhered to a substrate (168). Previous work has shown that chick cardiac cells in these 3-D spheroids exhibit differences in morphology, contractile ability, and myofibril organization compared with cells in 2-D (168). Another approach is to fabricate cell sheets by culturing cells on dishes coated with a temperature-sensitive polymer and ECM hydrogel. After tissue maturation, the temperature is reduced to release cell sheets from the dish, which can be stacked to form 3-D tissues (66). Although spheroids and stacked cell sheets replicate the 3-D interactions of cells, they lack user control over key architectural features, such as ECM and myocyte alignment. 3-D cardiac tissues with aligned, elongated cardiac myocytes have been engineered by mixing cells into a fiber-forming ECM-derived hydrogel prepolymer solution and injecting this mixture into a mold with supportive structures, such as pillars. As the hydrogel polymerizes around the support structures, cells are encapsulated inside and gradually remodel the ECM, ultimately creating tension and forming a 3-D tissue with aligned fibers and cardiac myocytes (Fig. 5A). Thus, these 3-D tissues replicate both the alignment and dense cell-cell interactions of native myocardium. 3-D tissues in the shape of an elongated bundle have been fabricated on PDMS posts manufactured using soft lithography (28), laser-etched molds (123), silk sutures (190), or titanium wires (166). Larger square tissue constructs have been engineered by molding cell-matrix mixtures on arrays of PDMS pillars supported by nylon frames (23, 80, 199). Recently, this approach was implemented to engineer a 5 mm-wide “giga-patch” of iPSC-derived cardiac myocytes (163), an impressive demonstration of the scalability of this method.
Fig. 5.
Engineering and functionally assessing engineered three-dimensional cardiac tissues. A: aligned three-dimensional cardiac tissues are fabricated by filling a custom polydimethylsiloxane (PDMS) mold with a mixture of cells and an extracellular matrix hydrogel prepolymer solution, which gradually solidifies and compacts into a tissue supported by the PDMS pillars. B: the electrical and mechanical functionality of “iWire” engineered three-dimensional cardiac tissue constructs are quantified using patch clamp and optical recording of a flexible probe, respectively. Images were adapted from Refs. 157 (A) and Ref. 166 with permission from Elsevier (B).
A variety of ECM mixtures, including combinations of collagen, Matrigel, and fibrin (49, 196), have been used as scaffolds for 3-D tissue constructs. Thus, the composition and elasticity of the ECM is somewhat tunable in engineered 3-D tissues. However, ECM composition does have some constraints because it must be robust enough to maintain the structural integrity of the tissue. For example, whereas collagen hydrogels have high physiological relevance, collagen fibers are relatively rigid, and, therefore, bundles fabricated solely with collagen are susceptible to breakage. Fibrin is more extensible than collagen (106), and therefore, bundles with fibrin or a mixture of fibrin and collagen are typically more robust than pure collagen bundles. Additionally, supporting cell populations, such as cardiac fibroblasts or other stromal cell populations, can be easily mixed into hydrogel mixtures before polymerization, demonstrating another tunable aspect of engineered 3-D tissues (149, 154, 156). Because cardiac fibroblasts are a critical part of the fibrotic response, integrating these cells into 3-D tissues can be leveraged to mimic aspects of pathological ECM remodeling. Thus, engineering 3-D cardiac tissues on microfabricated molds enables control over some microenvironmental features, such as macroscale tissue alignment, but ECM composition and elasticity are somewhat limited to combinations that can maintain stable tissues. Furthermore, controlling microscale features in 3-D is not feasible with this approach.
The contractility of 3-D engineered cardiac tissue bundles is quantified by imaging movements at tissue attachment sites (28), attaching tissues to force sensors (23, 153), or measuring the deflection of a flexible probe (Fig. 5B) (161). Similar to 2-D tissues, electrophysiological function can be characterized using patch clamp (166) or optical mapping of cell membrane potentials or Ca2+ transients (28, 104). However, although approaches for engineering 3-D cardiac tissues and quantifying their function have been developed, these platforms have not been extensively used for modeling pathological changes in the ECM, in part because of the challenges associated with robustly controlling ECM composition and tissue architecture in 3-D compared with 2-D.
OUTLOOK
Largely because of parallel advances in microfabrication, materials science, imaging, and cellular reprogramming, cardiac microphysiological systems have become increasingly sophisticated and have already contributed many important new insights into cardiac disease processes. Here, we will highlight ongoing challenges and areas of growth that will continue to advance these platforms.
Renewable Sources of Human Cardiac Myocytes
Because adult cardiac myocytes are postmitotic and human heart biopsies are extremely rare, human stem cell-derived cardiac myocytes are the only practical source of human cardiac myocytes (122). hiPSC-derived cardiac myocytes are especially attractive because they are reprogrammed from adult somatic cells (typically skin fibroblasts) (34, 102), and, therefore, they can be used for patient-specific disease modeling and drug screening, especially when integrated into microphysiological systems (70, 76, 187). However, existing protocols for differentiating cardiac myocytes from stem cell populations generate cardiac myocytes at an embryonic or fetal state (192), with a weaker contractile phenotype (164). The relevance of these immature cardiac myocytes to adult cardiac myocytes is questionable. Thus, protocols for promoting the maturation of human stem cell-derived cardiac myocytes (110), which will likely include modulating the microenvironment (12, 195), are critically needed to enhance the relevance of cardiac microphysiological systems to model healthy and diseased human adult myocardium.
Fabrication Technologies
New fabrication technologies are presently under development to further increase experimental control, reduce cost, and improve throughput for cardiac microphysiological systems. As described above, 2-D engineered tissues poorly mimic the multilayered architecture of the myocardium, whereas existing approaches to engineer 3-D tissues offer limited spatial control over cell and ECM microarchitecture. To address these limitations, 3-D bioprinting systems are presently being explored for engineering cardiac tissues with enhanced control over cell and ECM placement in all three dimensions, including incorporation of vascular networks (95, 200). However, to date, most 3-D bioprinting systems are extrusion based, for which the “ink” of biomaterials, with or without cells, is extruded through a nozzle. For this reason, the inks have been limited primarily to purely scaffolding materials (178), cardiac spheroids (129), or relatively robust cell populations, such as fibroblasts (95), endothelial cells (79, 200), or myoblasts (71). It has yet to be shown whether suspensions of cardiac myocytes can be viably printed in extrusion-based bioprinting systems. Alternative approaches that avoid extrusion, such as digital light processing-based 3-D bioprinting (202), may be more supportive of cell viability. This technique projects patterns of light to selectively cross-link a solution of light-sensitive hydrogel prepolymer mixed with cells. Despite its present limitations, 3-D bioprinting has vast potential for engineering cardiac microphysiological systems with enhanced spatial control over multiple cell populations and the ECM, which will greatly improve our ability to model diverse forms of pathological remodeling in health and disease.
Sensing Technologies
Today, many of the functional assays integrated into microphysiological systems, especially for contractility, rely on imaging-based techniques, which inherently have limited scalability due to the requirement for microscopy. Thus, integrating sensors that provide direct functional readouts with high spatial and temporal resolution will increase throughput and accuracy. For example, force sensors were recently integrated into MTFs to provide electrical readouts of contractility without the use of a microscope (105). In terms of electrophysiology, microelectrode arrays can provide real-time measurements of extracellular field potentials in 2-D tissues, but with limited spatial resolution (174). Electrophysiological measurements from 3-D tissues still rely largely on voltage- or Ca2+-sensitive dyes. Recently, flexible sensor arrays have been developed to readout electrical parameters from whole hearts (51). A similar system could potentially be adapted for assessing electrophysiology in engineered heart tissues as well. Ultimately, integrating sensors that provide real-time, constant measurements of multiple functional outputs will greatly enhance disease modeling studies within cardiac microphysiological systems.
Disease Modeling
Microphysiological systems can be leveraged to model many diverse forms of acquired and inherited cardiac diseases. As described above, pathological hypertrophy, increases in afterload, and fibrosis can be modeled in 2-D by engineering tissues on stiffer biomaterials, which has been shown to impact contractility (11, 48, 119), cell-cell coupling (118), and metabolism (111). These same pathologies can be modeled with 3-D tissue systems using stiffer support posts, which induces myocyte enlargement, decreases force generation, and activates fetal gene programs (72). Sophisticated microfluidic systems can also be used to simulate the hypoxic microenvironment following a myocardial infarction (85).
Inherited cardiomyopathies can be modeled with microphysiological systems by integrating iPSC-derived cardiac myocytes acquired directly from afflicted patients. Importantly, microphysiological systems are critical for robustly extracting quantitative functional metrics from these cells, as demonstrated in a 2-D engineered tissue model of Barth Syndrome (187) and a 3-D engineered tissue model of titinopathies (70). In both of these examples, microphysiological systems were used to engineer patient iPSC-derived cardiac myocytes into aligned tissues and quantify contractile stresses in response to specific interventions. Because many inherited cardiomyopathies are also associated with ECM remodeling, such as fibrosis, utilizing microphysiological systems will lead to unique insight into how cell phenotype and ECM remodeling jointly impact cardiac tissue function in a diverse range of human diseases.
Integration of Supporting Cell Populations
Most cardiac microphysiological systems today consist primarily of cardiac myocytes. However, supporting cells in the myocardium, such as fibroblasts and endothelial cells, also play a role in disease progression. Thus, biological relevance of existing cardiac microphysiological systems can be improved by integrating these supporting cell populations. Existing studies have shown that mixing fibroblasts (138, 156) or endothelial cells (162) with cardiac myocytes improves tissue structure and function. However, randomly coculturing these cells does not mimic their relative positioning in native myocardium. To overcome this, microfluidic devices have been used to culture a layer of endothelial cells on a porous PDMS membrane suspended above a layer of cardiac myocytes. The endothelial cell layer was used to regulate drug transport to the underlying cardiac myocytes, which more closely recapitulates the relationship between these cells in vivo (112). Channels for vasculature have also been fabricated in hydrogels by using removable spacers (184) or 3-D bioprinting a dissolvable filament (96, 120), which can then be removed. The evacuated channels can then be seeded with endothelial cells, which form a lumen. More recently, tubes of endothelial cells have been directly bioprinted and subsequently coated with cardiac myocytes to generate endothelialized myocardium (200). Ongoing refinement of these sophisticated techniques for controllably integrating supporting cell populations will continue to improve the physiological relevance of cardiac microphysiological systems.
In summary, cardiac microphysiological systems are powerful new in vitro platforms for quantifying the functional impact of physiological and pathological ECM remodeling and many other disease-relevant variables. Although these platforms still have many fundamentally artificial characteristics, their key advantage is user control over many of the diverse extracellular cues observed in healthy and diseased myocardium. Additionally, these platforms provide real-time access to cell and tissue structure and physiology with relatively high spatial and temporal resolution. Thus, these platforms can provide new insights into biological processes that complement but do not replace existing in vivo and ex vivo models. Ongoing efforts to improve the maturation of human stem cell-derived cardiac myocytes, develop new fabrication and sensing technologies, and integrate supporting cell populations will continue to evolve these platforms for human-relevant disease modeling, leading to new therapeutic targets and treatment strategies for many diverse forms of cardiac dysfunction.
GRANTS
This project was supported by the USC Viterbi School of Engineering, USC Women in Science and Engineering, American Heart Association Scientist Development Grant 16SDG29950005 (to M. L. McCain), a Saban Research Institute Team Science Grant (to M. L. McCain), and the USC Graduate School (Provost Fellowship to N. R. Ariyasinghe and an Annenberg Fellowship to D. M. Lyra-Leite).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.R.A., D.M.L.-L., and M.L.M. prepared figures; N.R.A., D.M.L.-L., and M.L.M. drafted manuscript; N.R.A., D.M.L.-L., and M.L.M. edited and revised manuscript; N.R.A., D.M.L.-L., and M.L.M. approved final version of manuscript.
REFERENCES
- 1.Agarwal A, Farouz Y, Nesmith AP, Deravi LF, McCain ML, Parker KK. Micropatterning alginate substrates for in vitro cardiovascular muscle on a chip. Adv Funct Mater 23: 3738–3746, 2013. doi: 10.1002/adfm.201203319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 13: 3599–3608, 2013. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alford PW, Feinberg AW, Sheehy SP, Parker KK. Biohybrid thin films for measuring contractility in engineered cardiovascular muscle. Biomaterials 31: 3613–3621, 2010. doi: 10.1016/j.biomaterials.2010.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andres AM, Hernandez G, Lee P, Huang C, Ratliff EP, Sin J, Thornton CA, Damasco MV, Gottlieb RA. Mitophagy is required for acute cardioprotection by simvastatin. Antioxid Redox Signal 21: 1960–1973, 2014. doi: 10.1089/ars.2013.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andres AM, Stotland A, Queliconi BB, Gottlieb RA. A time to reap, a time to sow: mitophagy and biogenesis in cardiac pathophysiology. J Mol Cell Cardiol 78: 62–72, 2015. doi: 10.1016/j.yjmcc.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anesti V, Scorrano L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta 1757: 692–699, 2006. doi: 10.1016/j.bbabio.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Anmann T, Varikmaa M, Timohhina N, Tepp K, Shevchuk I, Chekulayev V, Saks V, Kaambre T. Formation of highly organized intracellular structure and energy metabolism in cardiac muscle cells during postnatal development of rat heart. Biochim Biophys Acta 1837: 1350–1361, 2014. doi: 10.1016/j.bbabio.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, Weiss AS. Highly elastic micropatterned hydrogel for engineering functional cardiac tissue. Adv Funct Mater 23: 23, 2013. doi: 10.1002/adfm.201300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annoni G, Luvarà G, Arosio B, Gagliano N, Fiordaliso F, Santambrogio D, Jeremic G, Mircoli L, Latini R, Vergani C, Masson S. Age-dependent expression of fibrosis-related genes and collagen deposition in the rat myocardium. Mech Ageing Dev 101: 57–72, 1998. doi: 10.1016/S0047-6374(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 10.Aratyn-Schaus Y, Oakes PW, Stricker J, Winter SP, Gardel ML. Preparation of complaint matrices for quantifying cellular contraction. J Vis Exp: 2173, 2010. doi: 10.3791/2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariyasinghe NR, Reck CH, Viscio AA, Petersen AP, Lyra-Leite DM, Cho N, McCain ML. Engineering micromyocardium to delineate cellular and extracellular regulation of myocardial tissue contractility. Integr Biol 9: 730–741, 2017. doi: 10.1039/C7IB00081B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arshi A, Nakashima Y, Nakano H, Eaimkhong S, Evseenko D, Reed J, Stieg AZ, Gimzewski JK, Nakano A. Rigid microenvironments promote cardiac differentiation of mouse and human embryonic stem cells. Sci Technol Adv Mater 14: 025003, 2013. doi: 10.1088/1468-6996/14/2/025003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aubin H, Nichol JW, Hutson CB, Bae H, Sieminski AL, Cropek DM, Akhyari P, Khademhosseini A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 31: 6941–6951, 2010. doi: 10.1016/j.biomaterials.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azami M, Rabiee M, Moztarzadeh F. Glutaraldehyde crosslinked gelatin/hydroxyapatite nanocomposite scaffold, engineered via compound techniques. Polym Compos 31: 2112–2120, 2010. doi: 10.1002/pc.21008. [DOI] [Google Scholar]
- 15.Badie N, Bursac N. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys J 96: 3873–3885, 2009. doi: 10.1016/j.bpj.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartolák-Suki E, Imsirovic J, Parameswaran H, Wellman TJ, Martinez N, Allen PG, Frey U, Suki B. Fluctuation-driven mechanotransduction regulates mitochondrial-network structure and function. Nat Mater 14: 1049–1057, 2015. doi: 10.1038/nmat4358. [DOI] [PubMed] [Google Scholar]
- 17.Beachley VZ, Wolf MT, Sadtler K, Manda SS, Jacobs H, Blatchley MR, Bader JS, Pandey A, Pardoll D, Elisseeff JH. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat Methods 12: 1197–1204, 2015. doi: 10.1038/nmeth.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beauchamp P, Moritz W, Kelm JM, Ullrich ND, Agarkova I, Anson BD, Suter TM, Zuppinger C. Development and characterization of a scaffold-free 3D spheroid model of induced pluripotent stem cell-derived human cardiomyocytes. Tissue Eng Part C Methods 21: 852–861, 2015. doi: 10.1089/ten.tec.2014.0376. [DOI] [PubMed] [Google Scholar]
- 19.Bébarová M. Advances in patch clamp technique: towards higher quality and quantity. Gen Physiol Biophys 31: 131–140, 2012. doi: 10.4149/gpb_2012_016. [DOI] [PubMed] [Google Scholar]
- 20.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol 290: H2196–H2203, 2006. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 21.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 22.Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng 105: 1148–1160, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Bian W, Jackman CP, Bursac N. Controlling the structural and functional anisotropy of engineered cardiac tissues. Biofabrication 6: 024109–24109, 2014. doi: 10.1088/1758-5082/6/2/024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black LD III, Meyers JD, Weinbaum JS, Shvelidze YA, Tranquillo RT. Cell-induced alignment augments twitch force in fibrin gel-based engineered myocardium via gap junction modification. Tissue Eng Part A 15: 3099–3108, 2009. doi: 10.1089/ten.tea.2008.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blazeski A, Kostecki GM, Tung L. Engineered heart slices for electrophysiological and contractile studies. Biomaterials 55: 119–128, 2015. doi: 10.1016/j.biomaterials.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boothe SD, Myers JD, Pok S, Sun J, Xi Y, Nieto RM, Cheng J, Jacot JG. The effect of substrate stiffness on cardiomyocyte action potentials. Cell Biochem Biophys 74: 527–535, 2016. doi: 10.1007/s12013-016-0758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borg TK, Rubin K, Lundgren E, Borg K, Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol 104: 86–96, 1984. doi: 10.1016/0012-1606(84)90038-1. [DOI] [PubMed] [Google Scholar]
- 28.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A 18: 910–919, 2012. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol 48: 474–482, 2010. doi: 10.1016/j.yjmcc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bray MA, Adams WJ, Geisse NA, Feinberg AW, Sheehy SP, Parker KK. Nuclear morphology and deformation in engineered cardiac myocytes and tissues. Biomaterials 31: 5143–5150, 2010. doi: 10.1016/j.biomaterials.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton 65: 641–651, 2008. doi: 10.1002/cm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bretón-Romero R, Acín-Perez R, Rodríguez-Pascual F, Martínez-Molledo M, Brandes RP, Rial E, Enríquez JA, Lamas S. Laminar shear stress regulates mitochondrial dynamics, bioenergetics responses and PRX3 activation in endothelial cells. Biochim Biophys Acta 1843: 2403–2413, 2014. doi: 10.1016/j.bbamcr.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Bround MJ, Wambolt R, Luciani DS, Kulpa JE, Rodrigues B, Brownsey RW, Allard MF, Johnson JD. Cardiomyocyte ATP production, metabolic flexibility, and survival require calcium flux through cardiac ryanodine receptors in vivo. J Biol Chem 288: 18975–18986, 2013. doi: 10.1074/jbc.M112.427062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat Methods 11: 855–860, 2014. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res 91: e45–e54, 2002. doi: 10.1161/01.RES.0000047530.88338.EB. [DOI] [PubMed] [Google Scholar]
- 36.Butler JP, Tolić-Nørrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 37.Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture. Nat Methods 13: 405–414, 2016. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carson D, Hnilova M, Yang X, Nemeth CL, Tsui JH, Smith AS, Jiao A, Regnier M, Murry CE, Tamerler C, Kim DH. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. ACS Appl Mater Interfaces 8: 21923–21932, 2016. doi: 10.1021/acsami.5b11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatham JC, Young ME. Metabolic remodeling in the hypertrophic heart: fuel for thought. Circ Res 111: 666–668, 2012. doi: 10.1161/CIRCRESAHA.112.277392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaw KC, Manimaran M, Tay FE, Swaminathan S. Matrigel coated polydimethylsiloxane based microfluidic devices for studying metastatic and non-metastatic cancer cell invasion and migration. Biomed Microdevices 9: 597–602, 2007. doi: 10.1007/s10544-007-9071-5. [DOI] [PubMed] [Google Scholar]
- 41.Chiu LL, Janic K, Radisic M. Engineering of oriented myocardium on three-dimensional micropatterned collagen-chitosan hydrogel. Int J Artif Organs 35: 237–250, 2012. doi: 10.5301/ijao.5000084. [DOI] [PubMed] [Google Scholar]
- 42.Chung CY, Bien H, Entcheva E. The role of cardiac tissue alignment in modulating electrical function. J Cardiovasc Electrophysiol 18: 1323–1329, 2007. doi: 10.1111/j.1540-8167.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- 43.Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 91: 161–170, 1995. doi: 10.1161/01.CIR.91.1.161. [DOI] [PubMed] [Google Scholar]
- 44.Correia C, Koshkin A, Duarte P, Hu D, Teixeira A, Domian I, Serra M, Alves PM. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci Rep 7: 8590, 2017. doi: 10.1038/s41598-017-08713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dedkova EN, Blatter LA. Measuring mitochondrial function in intact cardiac myocytes. J Mol Cell Cardiol 52: 48–61, 2012. doi: 10.1016/j.yjmcc.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorn GW., II Mitochondrial dynamics in heart disease. Biochim Biophys Acta 1833: 233–241, 2013. doi: 10.1016/j.bbamcr.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J 86: 617–628, 2004. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci 121: 3794–3802, 2008. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schäfer H, Bishopric N, Wakatsuki T, Elson EL. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J 11: 683–694, 1997. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 50.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 5: 15, 2012. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang H, Yu KJ, Gloschat C, Yang Z, Song E, Chiang CH, Zhao J, Won SM, Xu S, Trumpis M, Zhong Y, Han SW, Xue Y, Xu D, Cauwenberghs G, Kay M, Huang Y, Viventi J, Efimov IR, Rogers JA. Capacitively coupled arrays of multiplexed flexible silicon transistors for long-term cardiac electrophysiology. Nat Biomed Eng 1: 0038, 2017. doi: 10.1038/s41551-017-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feaster TK, Cadar AG, Wang L, Williams CH, Chun YW, Hempel JE, Bloodworth N, Merryman WD, Lim CC, Wu JC, Knollmann BC, Hong CC. Matrigel mattress: a method for the generation of single contracting human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res 117: 995–1000, 2015. doi: 10.1161/CIRCRESAHA.115.307580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feinberg AW, Alford PW, Jin H, Ripplinger CM, Werdich AA, Sheehy SP, Grosberg A, Parker KK. Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials 33: 5732–5741, 2012. doi: 10.1016/j.biomaterials.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feinberg AW, Feigel A, Shevkoplyas SS, Sheehy S, Whitesides GM, Parker KK. Muscular thin films for building actuators and powering devices. Science 317: 1366–1370, 2007. doi: 10.1126/science.1146885. [DOI] [PubMed] [Google Scholar]
- 55.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods 7: 733–736, 2010.[Erratum in Nat Methods 8: 184, 2011. doi: 10.1038/nmeth0211-184a.] doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganz A, Lambert M, Saez A, Silberzan P, Buguin A, Mège RM, Ladoux B. Traction forces exerted through N-cadherin contacts. Biol Cell 98: 721–730, 2006. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- 57.Geisse NA, Sheehy SP, Parker KK. Control of myocyte remodeling in vitro with engineered substrates. In Vitro Cell Dev Biol Anim 45: 343–350, 2009. doi: 10.1007/s11626-009-9182-9. [DOI] [PubMed] [Google Scholar]
- 58.Gerdes AM, Kellerman SE, Moore JA, Muffly KE, Clark LC, Reaves PY, Malec KB, McKeown PP, Schocken DD. Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation 86: 426–430, 1992. doi: 10.1161/01.CIR.86.2.426. [DOI] [PubMed] [Google Scholar]
- 59.Gershlak JR, Resnikoff JI, Sullivan KE, Williams C, Wang RM, Black LD III. Mesenchymal stem cells ability to generate traction stress in response to substrate stiffness is modulated by the changing extracellular matrix composition of the heart during development. Biochem Biophys Res Commun 439: 161–166, 2013. doi: 10.1016/j.bbrc.2013.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goßmann M, Frotscher R, Linder P, Neumann S, Bayer R, Epple M, Staat M, Artmann AT, Artmann GM. Mechano-pharmacological characterization of cardiomyocytes derived from human induced pluripotent stem cells. Cell Physiol Biochem 38: 1182–1198, 2016. doi: 10.1159/000443124. [DOI] [PubMed] [Google Scholar]
- 61.Gottlieb RA, Bernstein D. Mitochondrial remodeling: rearranging, recycling, and reprogramming. Cell Calcium 60: 88–101, 2016. doi: 10.1016/j.ceca.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grespan E, Martewicz S, Serena E, Le Houerou V, Rühe J, Elvassore N. Analysis of calcium transients and uniaxial contraction force in single human embryonic stem cell-derived cardiomyocytes on microstructured elastic substrate with spatially controlled surface chemistries. Langmuir 32: 12190–12201, 2016. doi: 10.1021/acs.langmuir.6b03138. [DOI] [PubMed] [Google Scholar]
- 63.Grosberg A, Alford PW, McCain ML, Parker KK. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip 11: 4165–4173, 2011. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guan J, Mishra S, Qiu Y, Shi J, Trudeau K, Las G, Liesa M, Shirihai OS, Connors LH, Seldin DC, Falk RH, MacRae CA, Liao R. Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of amyloidogenic light chain-mediated cardiotoxicity. EMBO Mol Med 6: 1493–1507, 2014. doi: 10.15252/emmm.201404190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guzun R, Gonzalez-Granillo M, Karu-Varikmaa M, Grichine A, Usson Y, Kaambre T, Guerrero-Roesch K, Kuznetsov A, Schlattner U, Saks V. Regulation of respiration in muscle cells in vivo by VDAC through interaction with the cytoskeleton and MtCK within Mitochondrial Interactosome. Biochim Biophys Acta 1818: 1545–1554, 2012. doi: 10.1016/j.bbamem.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 66.Haraguchi Y, Shimizu T, Sasagawa T, Sekine H, Sakaguchi K, Kikuchi T, Sekine W, Sekiya S, Yamato M, Umezu M, Okano T. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc 7: 850–858, 2012. doi: 10.1038/nprot.2012.027. [DOI] [PubMed] [Google Scholar]
- 67.Harmer AR, Abi-Gerges N, Morton MJ, Pullen GF, Valentin JP, Pollard CE. Validation of an in vitro contractility assay using canine ventricular myocytes. Toxicol Appl Pharmacol 260: 162–172, 2012. doi: 10.1016/j.taap.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 68.Harvey PA, Leinwand LA. The cell biology of disease: cellular mechanisms of cardiomyopathy. J Cell Biol 194: 355–365, 2011. doi: 10.1083/jcb.201101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, Delgado SM, Wakatsuki T, Crone WC, Pruitt BL, Palecek SP. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol 2012: 508294, 2012. doi: 10.1155/2012/508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, Haghighi A, Homsy J, Hubner N, Church G, Cook SA, Linke WA, Chen CS, Seidman JG, Seidman CE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349: 982–986, 2015. doi: 10.1126/science.aaa5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv 1: e1500758, 2015. doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirt MN, Sörensen NA, Bartholdt LM, Boeddinghaus J, Schaaf S, Eder A, Vollert I, Stöhr A, Schulze T, Witten A, Stoll M, Hansen A, Eschenhagen T. Increased afterload induces pathological cardiac hypertrophy: a new in vitro model. Basic Res Cardiol 107: 307, 2012. doi: 10.1007/s00395-012-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG, Díez J, Seidman CE. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med 363: 552–563, 2010. doi: 10.1056/NEJMoa1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holzem KM, Vinnakota KC, Ravikumar VK, Madden EJ, Ewald GA, Dikranian K, Beard DA, Efimov IR. Mitochondrial structure and function are not different between nonfailing donor and end-stage failing human hearts. FASEB J 30: 2698–2707, 2016. doi: 10.1096/fj.201500118R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horan MP, Pichaud N, Ballard JW. Review: quantifying mitochondrial dysfunction in complex diseases of aging. J Gerontol A Biol Sci Med Sci 67: 1022–1035, 2012. doi: 10.1093/gerona/glr263. [DOI] [PubMed] [Google Scholar]
- 76.Huebsch N, Loskill P, Deveshwar N, Spencer CI, Judge LM, Mandegar MA, Fox CB, Mohamed TM, Ma Z, Mathur A, Sheehan AM, Truong A, Saxton M, Yoo J, Srivastava D, Desai TA, So PL, Healy KE, Conklin BR. Miniaturized iPS-cell-derived cardiac muscles for physiologically relevant drug response analyses. Sci Rep 6: 24726, 2016. doi: 10.1038/srep24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huebsch N, Loskill P, Mandegar MA, Marks NC, Sheehan AS, Ma Z, Mathur A, Nguyen TN, Yoo JC, Judge LM, Spencer CI, Chukka AC, Russell CR, So PL, Conklin BR, Healy KE. Automated video-based analysis of contractility and calcium flux in human-induced pluripotent stem cell-derived cardiomyocytes cultured over different spatial scales. Tissue Eng Part C Methods 21: 467–479, 2015. doi: 10.1089/ten.tec.2014.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ishihara T, Ban-Ishihara R, Maeda M, Matsunaga Y, Ichimura A, Kyogoku S, Aoki H, Katada S, Nakada K, Nomura M, Mizushima N, Mihara K, Ishihara N. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol Cell Biol 35: 211–223, 2015. doi: 10.1128/MCB.01054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Izadifar M, Chapman D, Babyn P, Chen X, Kelly ME. UV-assisted 3D bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng Part C Methods 24: 74–88, 2018. doi: 10.1089/ten.TEC.2017.0346. [DOI] [PubMed] [Google Scholar]
- 80.Jackman CP, Ganapathi AM, Asfour H, Qian Y, Allen BW, Li Y, Bursac N. Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials 159: 48–58, 2018. doi: 10.1016/j.biomaterials.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacot JG, Kita-Matsuo H, Wei KA, Chen HS, Omens JH, Mercola M, McCulloch AD. Cardiac myocyte force development during differentiation and maturation. Ann N Y Acad Sci 1188: 121–127, 2010. doi: 10.1111/j.1749-6632.2009.05091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95: 3479–3487, 2008. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaimes R III, Walton RD, Pasdois P, Bernus O, Efimov IR, Kay MW. A technical review of optical mapping of intracellular calcium within myocardial tissue. Am J Physiol Heart Circ Physiol 310: H1388–H1401, 2016. doi: 10.1152/ajpheart.00665.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelm JM, Ehler E, Nielsen LK, Schlatter S, Perriard JC, Fussenegger M. Design of artificial myocardial microtissues. Tissue Eng 10: 201–214, 2004. doi: 10.1089/107632704322791853. [DOI] [PubMed] [Google Scholar]
- 85.Khanal G, Chung K, Solis-Wever X, Johnson B, Pappas D. Ischemia/reperfusion injury of primary porcine cardiomyocytes in a low-shear microfluidic culture and analysis device. Analyst (Lond) 136: 3519–3526, 2011. doi: 10.1039/c0an00845a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim DH, Kshitiz, Smith RR, Kim P, Ahn EH, Kim HN, Marbán E, Suh KY, Levchenko A. Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integr Biol 4: 1019–1033, 2012. doi: 10.1039/c2ib20067h. [DOI] [PubMed] [Google Scholar]
- 87.Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci USA 107: 565–570, 2010. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim DH, Seo CH, Han K, Kwon KW, Levchenko A, Suh KY. Guided Cell Migration on Microtextured Substrates with Variable Local Density and Anisotropy. Adv Funct Mater 19: 1579–1586, 2009. doi: 10.1002/adfm.200801174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J, Rubin N, Huang Y, Tuan TL, Lien CL. In vitro culture of epicardial cells from adult zebrafish heart on a fibrin matrix. Nat Protoc 7: 247–255, 2012. doi: 10.1038/nprot.2011.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim P, Kim DH, Kim B, Choi SK, Lee SH, Khademhosseini A, Langer R, Suh KY. Fabrication of nanostructures of polyethylene glycol for applications to protein adsorption and cell adhesion. Nanotechnology 16: 2420–2426, 2005. doi: 10.1088/0957-4484/16/10/072. [DOI] [PubMed] [Google Scholar]
- 91.Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84: 431–488, 2004. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 92.Klimas A, Ambrosi CM, Yu J, Williams JC, Bien H, Entcheva E. OptoDyCE as an automated system for high-throughput all-optical dynamic cardiac electrophysiology. Nat Commun 7: 11542, 2016. doi: 10.1038/ncomms11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knight MB, Drew NK, McCarthy LA, Grosberg A. Emergent global contractile force in cardiac tissues. Biophys J 110: 1615–1624, 2016. doi: 10.1016/j.bpj.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kohen NT, Little LE, Healy KE. Characterization of Matrigel interfaces during defined human embryonic stem cell culture. Biointerphases 4: 69–79, 2009. doi: 10.1116/1.3274061. [DOI] [PubMed] [Google Scholar]
- 95.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci USA 113: 3179–3184, 2016. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 26: 3124–3130, 2014. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 97.Kujala VJ, Pasqualini FS, Goss JA, Nawroth JC, Parker KK. Laminar ventricular myocardium on a microelectrode array-based chip. J Mater Chem B Mater Biol Med 4: 3534–3543, 2016. doi: 10.1039/C6TB00324A. [DOI] [PubMed] [Google Scholar]
- 98.Kuo PL, Lee H, Bray MA, Geisse NA, Huang YT, Adams WJ, Sheehy SP, Parker KK. Myocyte shape regulates lateral registry of sarcomeres and contractility. Am J Pathol 181: 2030–2037, 2012. doi: 10.1016/j.ajpath.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lai JY, Ma DH, Lai MH, Li YT, Chang RJ, Chen LM. Characterization of cross-linked porous gelatin carriers and their interaction with corneal endothelium: biopolymer concentration effect. PLoS One 8: e54058, 2013. doi: 10.1371/journal.pone.0054058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee J. The use of gelatin substrates for traction force microscopy in rapidly moving cells. Methods Cell Biol 83: 297–312, 2007. doi: 10.1016/S0091-679X(07)83012-3. [DOI] [PubMed] [Google Scholar]
- 101.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev 101: 1869–1880, 2001. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 102.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc 8: 162–175, 2013. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liao XD, Wang XH, Jin HJ, Chen LY, Chen Q. Mechanical stretch induces mitochondria-dependent apoptosis in neonatal rat cardiomyocytes and G2/M accumulation in cardiac fibroblasts. Cell Res 14: 16–26, 2004. doi: 10.1038/sj.cr.7290198. [DOI] [PubMed] [Google Scholar]
- 104.Liau B, Christoforou N, Leong KW, Bursac N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials 32: 9180–9187, 2011. doi: 10.1016/j.biomaterials.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park SJ, Kotikian A, Nesmith AP, Campbell PH, Vlassak JJ, Lewis JA, Parker KK. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat Mater 16: 303–308, 2017. doi: 10.1038/nmat4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu W, Jawerth LM, Sparks EA, Falvo MR, Hantgan RR, Superfine R, Lord ST, Guthold M. Fibrin fibers have extraordinary extensibility and elasticity. Science 313: 634, 2006. doi: 10.1126/science.1127317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci USA 107: 9944–9949, 2010. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol 56: 130–140, 2010. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 109.Lundgren E, Terracio L, Mårdh S, Borg TK. Extracellular matrix components influence the survival of adult cardiac myocytes in vitro. Exp Cell Res 158: 371–381, 1985. doi: 10.1016/0014-4827(85)90462-8. [DOI] [PubMed] [Google Scholar]
- 110.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev 22: 1991–2002, 2013. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lyra-Leite DM, Andres AM, Petersen AP, Ariyasinghe NR, Cho N, Lee JA, Gottlieb RA, McCain ML. Mitochondrial function in engineered cardiac tissues is regulated by extracellular matrix elasticity and tissue alignment. Am J Physiol Heart Circ Physiol 313: H757–H767, 2017. doi: 10.1152/ajpheart.00290.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maoz BM, Herland A, Henry OYF, Leineweber WD, Yadid M, Doyle J, Mannix R, Kujala VJ, FitzGerald EA, Parker KK, Ingber DE. Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 17: 2294–2302, 2017. doi: 10.1039/C7LC00412E. [DOI] [PubMed] [Google Scholar]
- 113.Mathur A, Hong Y, Kemp BK, Barrientos AA, Erusalimsky JD. Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc Res 46: 126–138, 2000. doi: 10.1016/S0008-6363(00)00002-X. [DOI] [PubMed] [Google Scholar]
- 114.Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Healy KE. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep 5: 8883, 2015. doi: 10.1038/srep08883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matsushita T, Oyamada M, Fujimoto K, Yasuda Y, Masuda S, Wada Y, Oka T, Takamatsu T. Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ Res 85: 1046–1055, 1999. doi: 10.1161/01.RES.85.11.1046. [DOI] [PubMed] [Google Scholar]
- 116.McCain ML, Agarwal A, Nesmith HW, Nesmith AP, Parker KK. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials 35: 5462–5471, 2014. doi: 10.1016/j.biomaterials.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McCain ML, Desplantez T, Geisse NA, Rothen-Rutishauser B, Oberer H, Parker KK, Kleber AG. Cell-to-cell coupling in engineered pairs of rat ventricular cardiomyocytes: relation between Cx43 immunofluorescence and intercellular electrical conductance. Am J Physiol Heart Circ Physiol 302: H443–H450, 2012. doi: 10.1152/ajpheart.01218.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McCain ML, Lee H, Aratyn-Schaus Y, Kléber AG, Parker KK. Cooperative coupling of cell-matrix and cell-cell adhesions in cardiac muscle. Proc Natl Acad Sci USA 109: 9881–9886, 2012. doi: 10.1073/pnas.1203007109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McCain ML, Yuan H, Pasqualini FS, Campbell PH, Parker KK. Matrix elasticity regulates the optimal cardiac myocyte shape for contractility. Am J Physiol Heart Circ Physiol 306: H1525–H1539, 2014. doi: 10.1152/ajpheart.00799.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11: 768–774, 2012. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moeller J, Denisin AK, Sim JY, Wilson RE, Ribeiro AJS, Pruitt BL. Controlling cell shape on hydrogels using lift-off protein patterning. PLoS One 13: e0189901, 2018. doi: 10.1371/journal.pone.0189901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 111: 344–358, 2012. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Munarin F, Kaiser NJ, Kim TY, Choi BR, Coulombe KLK. Laser-Etched Designs for Molding Hydrogel-Based Engineered Tissues. Tissue Eng Part C Methods 23: 311–321, 2017. doi: 10.1089/ten.tec.2017.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Natarajan A, Stancescu M, Dhir V, Armstrong C, Sommerhage F, Hickman JJ, Molnar P. Patterned cardiomyocytes on microelectrode arrays as a functional, high information content drug screening platform. Biomaterials 32: 4267–4274, 2011. doi: 10.1016/j.biomaterials.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31: 5536–5544, 2010. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nimni ME. Mechanism of inhibition of collagen crosslinking by penicillamine. Proc R Soc Med 70, Suppl 3: 65–72, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nozaki T, Kagaya Y, Ishide N, Kitada S, Miura M, Nawata J, Ohno I, Watanabe J, Shirato K. Interaction between sarcomere and mitochondrial length in normoxic and hypoxic rat ventricular papillary muscles. Cardiovasc Pathol 10: 125–132, 2001. doi: 10.1016/S1054-8807(01)00071-0. [DOI] [PubMed] [Google Scholar]
- 128.Olde Damink LHH, Dijkstra PJ, Van Luyn MJ, Van Wachem PB, Nieuwenhuis P, Feijen J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J Mater Sci Mater Med 6: 460–472, 1995. doi: 10.1007/BF00123371. [DOI] [Google Scholar]
- 129.Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, DiSilvestre D, Vricella L, Conte J, Tung L, Tomaselli GF, Hibino N. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci Rep 7: 4566, 2017. doi: 10.1038/s41598-017-05018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA. Crosslinking of collagen gels by transglutaminase. J Biomed Mater Res A 68A: 756–762, 2004. doi: 10.1002/jbm.a.20110. [DOI] [PubMed] [Google Scholar]
- 131.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 14: 213–221, 2008. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 132.Oyarzún AP, Westermeier F, Pennanen C, López-Crisosto C, Parra V, Sotomayor-Flores C, Sánchez G, Pedrozo Z, Troncoso R, Lavandero S. FK866 compromises mitochondrial metabolism and adaptive stress responses in cultured cardiomyocytes. Biochem Pharmacol 98: 92–101, 2015. doi: 10.1016/j.bcp.2015.08.097. [DOI] [PubMed] [Google Scholar]
- 133.Oyunbaatar NE, Lee DH, Patil SJ, Kim ES, Lee DW. Biomechanical characterization of cardiomyocyte using PDMS pillar with microgrooves. Sensors (Basel) 16: 1258, 2016. doi: 10.3390/s16081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Palchesko RN, Zhang L, Sun Y, Feinberg AW. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS One 7: e51499, 2012. doi: 10.1371/journal.pone.0051499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park J, Ryu J, Choi SK, Seo E, Cha JM, Ryu S, Kim J, Kim B, Lee SH. Real-time measurement of the contractile forces of self-organized cardiomyocytes on hybrid biopolymer microcantilevers. Anal Chem 77: 6571–6580, 2005. doi: 10.1021/ac0507800. [DOI] [PubMed] [Google Scholar]
- 136.Parker KK, Tan J, Chen CS, Tung L. Myofibrillar architecture in engineered cardiac myocytes. Circ Res 103: 340–342, 2008. doi: 10.1161/CIRCRESAHA.108.182469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parker SJ, Svensson RU, Divakaruni AS, Lefebvre AE, Murphy AN, Shaw RJ, Metallo CM. LKB1 promotes metabolic flexibility in response to energy stress. Metab Eng, 43: 208–217, 2017. doi: 10.1016/j.ymben.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Parrag IC, Zandstra PW, Woodhouse KA. Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnol Bioeng 109: 813–822, 2012. doi: 10.1002/bit.23353. [DOI] [PubMed] [Google Scholar]
- 139.Pasqualini FS, Agarwal A, O’Connor BB, Liu Q, Sheehy SP, Parker KK. Traction force microscopy of engineered cardiac tissues. PLoS One 13: e0194706, 2018. doi: 10.1371/journal.pone.0194706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Patra C, Talukdar S, Novoyatleva T, Velagala SR, Mühlfeld C, Kundu B, Kundu SC, Engel FB. Silk protein fibroin from Antheraea mylitta for cardiac tissue engineering. Biomaterials 33: 2673–2680, 2012. doi: 10.1016/j.biomaterials.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 141.Pennanen C, Parra V, López-Crisosto C, Morales PE, Del Campo A, Gutierrez T, Rivera-Mejías P, Kuzmicic J, Chiong M, Zorzano A, Rothermel BA, Lavandero S. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. J Cell Sci 127: 2659–2671, 2014. doi: 10.1242/jcs.139394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Petersen AP, Lyra-Leite DM, Ariyasinghe NR, Cho N, Goodwin CM, Kim JY, McCain ML. Microenvironmental modulation of calcium wave propagation velocity in engineered cardiac tissues. Cell Mol Bioeng 1–15 2018. doi: 10.1007/s12195-018-0522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pong T, Adams WJ, Bray MA, Feinberg AW, Sheehy SP, Werdich AA, Parker KK. Hierarchical architecture influences calcium dynamics in engineered cardiac muscle. Exp Biol Med (Maywood) 236: 366–373, 2011. doi: 10.1258/ebm.2010.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qin D, Xia Y, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nat Protoc 5: 491–502, 2010. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 145.Rao C, Prodromakis T, Kolker L, Chaudhry UA, Trantidou T, Sridhar A, Weekes C, Camelliti P, Harding SE, Darzi A, Yacoub MH, Athanasiou T, Terracciano CM. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials 34: 2399–2411, 2013. doi: 10.1016/j.biomaterials.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci USA 112: 12705–12710, 2015. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ribeiro AJ, Zaleta-Rivera K, Ashley EA, Pruitt BL. Stable, covalent attachment of laminin to microposts improves the contractility of mouse neonatal cardiomyocytes. ACS Appl Mater Interfaces 6: 15516–15526, 2014. doi: 10.1021/am5042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ribeiro AJS, Schwab O, Mandegar MA, Ang YS, Conklin BR, Srivastava D, Pruitt BL. Multi-imaging method to assay the contractile mechanical output of micropatterned human iPSC-derived cardiac myocytes. Circ Res 120: 1572–1583, 2017. doi: 10.1161/CIRCRESAHA.116.310363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roberts MA, Tran D, Coulombe KL, Razumova M, Regnier M, Murry CE, Zheng Y. Stromal cells in dense collagen promote cardiomyocyte and microvascular patterning in engineered human heart tissue. Tissue Eng Part A 22: 633–644, 2016. doi: 10.1089/ten.tea.2015.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rohr S, Schölly DM, Kléber AG. Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circ Res 68: 114–130, 1991. doi: 10.1161/01.RES.68.1.114. [DOI] [PubMed] [Google Scholar]
- 151.Ross RS, Borg TK. Integrins and the myocardium. Circ Res 88: 1112–1119, 2001. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 152.Ross RS, Pham C, Shai SY, Goldhaber JI, Fenczik C, Glembotski CC, Ginsberg MH, Loftus JC. Beta1 integrins participate in the hypertrophic response of rat ventricular myocytes. Circ Res 82: 1160–1172, 1998. doi: 10.1161/01.RES.82.11.1160. [DOI] [PubMed] [Google Scholar]
- 153.Rupert CE, Coulombe KLK. IGF1 and NRG1 enhance proliferation, metabolic maturity, and the force-frequency response in hESC-derived engineered cardiac tissues. Stem Cells Int 2017: 7648409, 2017. doi: 10.1155/2017/7648409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sadeghi AH, Shin SR, Deddens JC, Fratta G, Mandla S, Yazdi IK, Prakash G, Antona S, Demarchi D, Buijsrogge MP, Sluijter JPG, Hjortnaes J, Khademhosseini A. Engineered 3D cardiac fibrotic tissue to study fibrotic remodeling. Adv Healthc Mater 6: 6, 2017. doi: 10.1002/adhm.201601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saenz Cogollo JF, Tedesco M, Martinoia S, Raiteri R. A new integrated system combining atomic force microscopy and micro-electrode array for measuring the mechanical properties of living cardiac myocytes. Biomed Microdevices 13: 613–621, 2011. doi: 10.1007/s10544-011-9531-9. [DOI] [PubMed] [Google Scholar]
- 156.Saini H, Navaei A, Van Putten A, Nikkhah M. 3D cardiac microtissues encapsulated with the co-culture of cardiomyocytes and cardiac fibroblasts. Adv Healthc Mater 4: 1961–1971, 2015. doi: 10.1002/adhm.201500331. [DOI] [PubMed] [Google Scholar]
- 157.Sakar MS, Eyckmans J, Pieters R, Eberli D, Nelson BJ, Chen CS. Cellular forces and matrix assembly coordinate fibrous tissue repair. Nat Commun 7: 11036, 2016. doi: 10.1038/ncomms11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Salick MR, Napiwocki BN, Sha J, Knight GT, Chindhy SA, Kamp TJ, Ashton RS, Crone WC. Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials 35: 4454–4464, 2014. doi: 10.1016/j.biomaterials.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sato Y, Kobayashi H, Higuchi T, Shimada Y, Ida H, Ohashi T. Metabolomic profiling of pompe disease-induced pluripotent stem cell-derived cardiomyocytes reveals that oxidative stress is associated with cardiac and skeletal muscle pathology. Stem Cells Transl Med 6: 31–39, 2017. doi: 10.5966/sctm.2015-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schaefer JA, Tranquillo RT. Tissue Contraction Force Microscopy for Optimization of Engineered Cardiac Tissue. Tissue Eng Part C Methods 22: 76–83, 2016. doi: 10.1089/ten.tec.2015.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schroer AK, Shotwell MS, Sidorov VY, Wikswo JP, Merryman WD. I-Wire Heart-on-a-Chip II: Biomechanical analysis of contractile, three-dimensional cardiomyocyte tissue constructs. Acta Biomater 48: 79–87, 2017. doi: 10.1016/j.actbio.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, Kurosawa H, Kobayashi E, Okano T. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation 118, Suppl: S145–S152, 2008. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 163.Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, Bursac N. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun 8: 1825, 2017. doi: 10.1038/s41467-017-01946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sheehy SP, Pasqualini F, Grosberg A, Park SJ, Aratyn-Schaus Y, Parker KK. Quality metrics for stem cell-derived cardiac myocytes. Stem Cell Reports 2: 282–294, 2014. doi: 10.1016/j.stemcr.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shin SR, Zihlmann C, Akbari M, Assawes P, Cheung L, Zhang K, Manoharan V, Zhang YS, Yüksekkaya M, Wan KT, Nikkhah M, Dokmeci MR, Tang XS, Khademhosseini A. Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small 12: 3677–3689, 2016. doi: 10.1002/smll.201600178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sidorov VY, Samson PC, Sidorova TN, Davidson JM, Lim CC, Wikswo JP. I-Wire Heart-on-a-Chip I: three-dimensional cardiac tissue constructs for physiology and pharmacology. Acta Biomater 48: 68–78, 2017. doi: 10.1016/j.actbio.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sin J, Andres AM, Taylor DJ, Weston T, Hiraumi Y, Stotland A, Kim BJ, Huang C, Doran KS, Gottlieb RA. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 12: 369–380, 2016. doi: 10.1080/15548627.2015.1115172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pontes Soares C, Midlej V, de Oliveira ME, Benchimol M, Costa ML, Mermelstein C. 2D and 3D-organized cardiac cells shows differences in cellular morphology, adhesion junctions, presence of myofibrils and protein expression. PLoS One 7: e38147, 2012. doi: 10.1371/journal.pone.0038147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Song M, Franco A, Fleischer JA, Zhang L, Dorn GW II. Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab 26: 872–883.e5, 2017. doi: 10.1016/j.cmet.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW II. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab 21: 273–286, 2015. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Spinale FG, Tomita M, Zellner JL, Cook JC, Crawford FA, Zile MR. Collagen remodeling and changes in LV function during development and recovery from supraventricular tachycardia. Am J Physiol 261: H308–H318, 1991. doi: 10.1152/ajpheart.1991.261.2.H308. [DOI] [PubMed] [Google Scholar]
- 172.Stancescu M, Molnar P, McAleer CW, McLamb W, Long CJ, Oleaga C, Prot JM, Hickman JJ. A phenotypic in vitro model for the main determinants of human whole heart function. Biomaterials 60: 20–30, 2015. doi: 10.1016/j.biomaterials.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stanley WC, Chandler MP. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Fail Rev 7: 115–130, 2002. doi: 10.1023/A:1015320423577. [DOI] [PubMed] [Google Scholar]
- 174.Stett A, Egert U, Guenther E, Hofmann F, Meyer T, Nisch W, Haemmerle H. Biological application of microelectrode arrays in drug discovery and basic research. Anal Bioanal Chem 377: 486–495, 2003. doi: 10.1007/s00216-003-2149-x. [DOI] [PubMed] [Google Scholar]
- 175.Stoppel WL, Hu D, Domian IJ, Kaplan DL, Black LD III. Anisotropic silk biomaterials containing cardiac extracellular matrix for cardiac tissue engineering. Biomed Mater 10: 034105, 2015. doi: 10.1088/1748-6041/10/3/034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sullivan KE, Quinn KP, Tang KM, Georgakoudi I, Black LD III. Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res Ther 5: 14, 2014. doi: 10.1186/scrt403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, Gropler RJ, Holzhuetter H-G, Kizer JR, Lewandowski ED, Malloy CR, Neubauer S, Peterson LR, Portman MA, Recchia FA, Van Eyk JE, Wang TJ; American Heart Association Council on Basic Cardiovascular Sciences . Assessing cardiac metabolism: a scientific statement from the american heart association. Circ Res 118: 1659–1701, 2016. doi: 10.1161/RES.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tijore A, Irvine SA, Sarig U, Mhaisalkar P, Baisane V, Venkatraman SS. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication 10: 025003, 2018. doi: 10.1088/1758-5090/aaa15d. [DOI] [PubMed] [Google Scholar]
- 179.Trichet L, Le Digabel J, Hawkins RJ, Vedula SR, Gupta M, Ribrault C, Hersen P, Voituriez R, Ladoux B. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc Natl Acad Sci USA 109: 6933–6938, 2012. doi: 10.1073/pnas.1117810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.van Spreeuwel AC, Bax NA, Bastiaens AJ, Foolen J, Loerakker S, Borochin M, van der Schaft DW, Chen CS, Baaijens FP, Bouten CV. The influence of matrix (an)isotropy on cardiomyocyte contraction in engineered cardiac microtissues. Integr Biol 6: 422–429, 2014. doi: 10.1039/C3IB40219C. [DOI] [PubMed] [Google Scholar]
- 181.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res 69: 604–613, 2006. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 182.Vásquez-Trincado C, García-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, Lavandero S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol 594: 509–525, 2016. doi: 10.1113/JP271301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Villari B, Campbell SE, Hess OM, Mall G, Vassalli G, Weber KT, Krayenbuehl HP. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol 22: 1477–1484, 1993. doi: 10.1016/0735-1097(93)90560-N. [DOI] [PubMed] [Google Scholar]
- 184.Vollert I, Seiffert M, Bachmair J, Sander M, Eder A, Conradi L, Vogelsang A, Schulze T, Uebeler J, Holnthoner W, Redl H, Reichenspurner H, Hansen A, Eschenhagen T. In vitro perfusion of engineered heart tissue through endothelialized channels. Tissue Eng Part A 20: 854–863, 2014. doi: 10.1089/ten.TEA.2013.0214. [DOI] [PubMed] [Google Scholar]
- 185.Wai T, García-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, Rupérez FJ, Barbas C, Ibañez B, Langer T. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350: aad0116, 2015. doi: 10.1126/science.aad0116. [DOI] [PubMed] [Google Scholar]
- 186.Walker CA, Spinale FG. The structure and function of the cardiac myocyte: a review of fundamental concepts. J Thorac Cardiovasc Surg 118: 375–382, 1999. doi: 10.1016/S0022-5223(99)70233-3. [DOI] [PubMed] [Google Scholar]
- 187.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 20: 616–623, 2014. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang RM, Christman KL. Decellularized myocardial matrix hydrogels: In basic research and preclinical studies. Adv Drug Deliv Rev 96: 77–82, 2016. doi: 10.1016/j.addr.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang Z, Volinsky AA, Gallant ND. Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom‐built compression instrument. J Appl Polym Sci 131 131, 2014. [Google Scholar]
- 190.Xiao Y, Zhang B, Liu H, Miklas JW, Gagliardi M, Pahnke A, Thavandiran N, Sun Y, Simmons C, Keller G, Radisic M. Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle. Lab Chip 14: 869–882, 2014. doi: 10.1039/C3LC51123E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yancey DM, Guichard JL, Ahmed MI, Zhou L, Murphy MP, Johnson MS, Benavides GA, Collawn J, Darley-Usmar V, Dell’Italia LJ. Cardiomyocyte mitochondrial oxidative stress and cytoskeletal breakdown in the heart with a primary volume overload. Am J Physiol Heart Circ Physiol 308: H651–H663, 2015. doi: 10.1152/ajpheart.00638.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res 114: 511–523, 2014. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yin L, Bien H, Entcheva E. Scaffold topography alters intracellular calcium dynamics in cultured cardiomyocyte networks. Am J Physiol Heart Circ Physiol 287: H1276–H1285, 2004. doi: 10.1152/ajpheart.01120.2003. [DOI] [PubMed] [Google Scholar]
- 194.Yin S, Zhang X, Zhan C, Wu J, Xu J, Cheung J. Measuring single cardiac myocyte contractile force via moving a magnetic bead. Biophys J 88: 1489–1495, 2005. doi: 10.1529/biophysj.104.048157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 32: 1002–1009, 2011. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yuan Ye K, Sullivan KE, Black LD. Encapsulation of cardiomyocytes in a fibrin hydrogel for cardiac tissue engineering. J Vis Exp: 3251, 2011. doi: 10.3791/3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yung CW, Wu LQ, Tullman JA, Payne GF, Bentley WE, Barbari TA. Transglutaminase crosslinked gelatin as a tissue engineering scaffold. J Biomed Mater Res A 83A: 1039–1046, 2007. doi: 10.1002/jbm.a.31431. [DOI] [PubMed] [Google Scholar]
- 198.Zellner JL, Spinale FG, Eble DM, Hewett KW, Crawford FA Jr. Alterations in myocyte shape and basement membrane attachment with tachycardia-induced heart failure. Circ Res 69: 590–600, 1991. doi: 10.1161/01.RES.69.3.590. [DOI] [PubMed] [Google Scholar]
- 199.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34: 5813–5820, 2013. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 110: 45–59, 2016. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao Y, Lim CC, Sawyer DB, Liao R, Zhang X. Simultaneous orientation and cellular force measurements in adult cardiac myocytes using three-dimensional polymeric microstructures. Cell Motil Cytoskeleton 64: 718–725, 2007. doi: 10.1002/cm.20218. [DOI] [PubMed] [Google Scholar]
- 202.Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J, Wang P, Lai CS, Gou M, Xu Y, Zhang K, Chen S. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 124: 106–115, 2017. doi: 10.1016/j.biomaterials.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]