Biological mechanisms meant to heal the body, when left unchecked in a disease setting, often can lead to problems more insidious and potentially more detrimental than the disease itself. Fibrosis, marked by the activation, proliferation, and, in some organs, invasion of fibroblasts into tissues, has obvious advantages in the context of wound healing, where proper scar formation can limit serious injury like hemorrhage or myocardial infarction. However, progressive and uncontrolled fibrosis and deposition of excessive extracellular martix (ECM) has been shown to cause adverse pathological effects in virtually every major organ system in the body (1, 2). Evidence gathered in the last 20–30 yr within the field of cardiology has highlighted the previously underappreciated role of cardiac fibrosis in heart failure pathologies, prompting the characterization of specific disease phenotypes such as heart failure with preserved ejection fraction, which may be responsible for a significant percentage of the patient population with heart disease (3, 7). Despite these advances in diagnosis and classification of disease, there is currently no approved method for treating cardiac fibrosis, thus indicating an unmet clinical need for therapeutic strategies to prevent or potentially reverse cardiac fibrosis to enhance cardiac function.
Research has been dedicated to investigating the molecular mechanisms that trigger fibroblast activation, leading to the discovery of key pathways that induce significant phenotypic changes corresponding to the progressive disease state. The process of fibroblast activation is known to be directly regulated by prolonged stimulation of fibroblasts by secreted proteins, cytokines and growth factors, most notably matrix metalloproteases, interleukins (e.g., interleukin-6), and transforming growth factor (TGF)-β (5, 6). These signals synergistically cause transdifferentiation of fibroblasts into myofibroblasts (1), a subset of hyperactivated fibroblast cells identified by their altered gene expression patterns, morphological structure, and significantly increased production of ECM proteins like collagen. Excessive deposition of ECM proteins in heart tissue by myofibroblasts is therefore thought to be causative for inducing the fibrotic phenotype, thus identifying a target for therapeutic intervention (2, 4). However, studies showing specific modulation of these regulatory factors have proven to be inconsequential due to prevalence of side effects upon drug treatment or genetic manipulation. Therefore, alternative strategies are necessary to find other ways to affect the pathways that ultimately lead to downstream activation of fibrotic ECM remodeling.
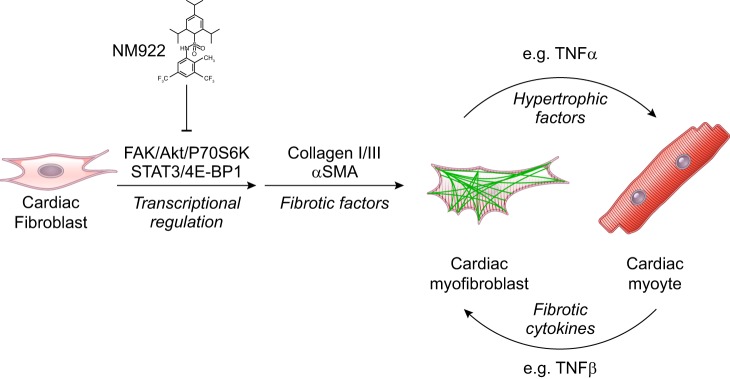
Along these lines, in a recent article published in the American Journal of Physiology-Heart and Circulatory Physiology, Bradley et al. (1a) described the utility of a novel compound, NM922, as an antifibrotic drug to prevent fibroblast to myofibroblast transdifferentiation (Fig. 1). Rather than targeting conventional fibrotic targets, the drug was shown to inhibit the activation of both the focal adhestion kinase/Akt/p70 S6 kinase and STAT3/4E-bindging protein 1 pathways, as measured by decreased phosphorylation of the signaling components upon coadministration of TGF-β and NM922 in vitro. This finding signifies a potential strategy for targeting the downstream effector molecules that transduce the signal for fibroblast activation and myofibroblast differentiation at the level of transcriptional regulation. The in vitro characterization of the compound also suggested a role for regulation of prostaglandin synthesis (via upregulation of cyclooxygenase 2) and the cell cycle (via downregulation of cyclin D3). The authors also showed that treatment with NM922 significantly reduced aberrant cardiac remodeling in an in vivo mouse model of cardiac hypertrophy and heart failure induced by transaortic constriction. The clinically relevant in vivo approach (treatment with the drug after pathology was already in place) resulted in decreased cardiac hypertrophy, decreased left ventricular dilation, increased ejection fraction, and ultimately increased survival. Characterization of tissues from the in vivo model showed a TGF-β-independent decrease in fibrosis and myofibroblast cell infiltration as measured by decreased expression of fibrotic markers such as collagen types I/III, α-smooth muscle actin, TNF-α, and lysyl oxidase.
Fig. 1.
NM922 inhibits fibroblast-to-myofibroblast transdifferentiation. Shown is a cartoon diagram depicting the proposed mechanism for NM922 to prevent downstream cardiac fibrosis and hypertrophic remodeling via upstream signal regulation [i.e., focal adhesion kinase (FAK)/Akt/p70 S6 kinase (P70S6K) and STAT3/4E-binding protein (4E-BP1) pathways].
Taken together, the study introduces an interesting paradigm for how blocking fibroblast activation likely plays a critical role upstream of cardiac remodeling in heart failure as embodied by intercellular communication between fibroblasts and myocytes in the myocardium. The strong corroboration of in vitro and in vivo data from this preclinical study demonstrates the potential for eventual use of this compound in a clinical setting to treat cardiac fibrosis. Most strikingly, the positive effects of the compound were observed in an interventional model of the drug delivery (i.e., 6 wk after the induction of transaortic constriction), implying that the compound may be used similarly in a clinical setting after the onset of disease. Given further pharmacological characterization of the drug, identifying the specific cellular target(s) of the compound will greatly enhance future drug design research in the field, providing hope for a potential therapy for those suffering from cardiac fibrosis or other forms of fibrotic disease.
GRANTS
Funding for this work was partly provided by National Institutes of Health Grants HL-091071 and AG-052722 and the Veterans Administration Grant BX001963 (to H. H. Patel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.J.H. and H.H.P. drafted manuscript; K.J.H. and H.H.P. approved final version of manuscript; H.H.P. edited and revised manuscript.
REFERENCES
- 1.Bagalad BS, Mohan Kumar KP, Puneeth HK. Myofibroblasts: master of disguise. J Oral Maxillofac Pathol 21: 462–463, 2017. doi: 10.4103/jomfp.JOMFP_146_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Bradley JM, Spaletra P, Li Z, Sharp TE 3rd, Goodchild TT, Corral LG, Fung L, Chan KW, Sullivan RW, Swindlehurst CA, DJ Lefer. A novel fibroblast activation inhibitor attenuates left ventricular remodeling and preserves cardiac function in heart failure. Am J Physiol Heart Circ Physiol 315: H563–H570, 2018. doi: 10.1152/ajpheart.00603.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iozzo RV, Gubbiotti MA. Extracellular matrix: the driving force of mammalian diseases. Matrix Biol. In press. doi: 10.1016/j.matbio.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis GA, Schelbert EB, Williams SG, Cunnington C, Ahmed F, McDonagh TA, Miller CA. Biological phenotypes of heart failure with preserved ejection fraction. J Am Coll Cardiol 70: 2186–2200, 2017. doi: 10.1016/j.jacc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Zhao Q, Kong W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol 68-69: 490–506, 2018. doi: 10.1016/j.matbio.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML. Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharmacol Sci 38: 448–458, 2017. doi: 10.1016/j.tips.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack M. Inflammation and fibrosis. Matrix Biol 68-69: 106-121, 2018. doi: 10.1016/j.matbio.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Zile MR, Baicu CF. Biomarkers of diastolic dysfunction and myocardial fibrosis: application to heart failure with a preserved ejection fraction. J Cardiovasc Transl Res 6: 501–515, 2013. doi: 10.1007/s12265-013-9472-1. [DOI] [PubMed] [Google Scholar]