Abstract
Cremaster muscle arteriolar smooth muscle cells (SMCs) display inositol 1,4,5-trisphosphate receptor-dependent Ca2+ waves that contribute to global myoplasmic Ca2+ concentration and myogenic tone. However, the contribution made by voltage-gated Ca2+ channels (VGCCs) to arteriolar SMC Ca2+ waves is unknown. We tested the hypothesis that VGCC activity modulates SMC Ca2+ waves in pressurized (80 cmH2O/59 mmHg, 34°C) hamster cremaster muscle arterioles loaded with Fluo-4 and imaged by confocal microscopy. Removal of extracellular Ca2+ dilated arterioles (32 ± 3 to 45 ± 3 μm, n = 15, P < 0.05) and inhibited the occurrence, amplitude, and frequency of Ca2+ waves (n = 15, P < 0.05), indicating dependence of Ca2+ waves on Ca2+ influx. Blockade of VGCCs with nifedipine (1 μM) or diltiazem (10 μM) or deactivation of VGCCs by hyperpolarization of smooth muscle with the K+ channel agonist cromakalim (10 μM) produced similar inhibition of Ca2+ waves (P < 0.05). Conversely, depolarization of SMCs with the K+ channel blocker tetraethylammonium (1 mM) constricted arterioles from 26 ± 3 to 14 ± 2 μm (n = 11, P < 0.05) and increased wave occurrence (9 ± 3 to 16 ± 3 waves/SMC), amplitude (1.6 ± 0.07 to 1.9 ± 0.1), and frequency (0.5 ± 0.1 to 0.9 ± 0.2 Hz, n = 10, P < 0.05), effects that were blocked by nifedipine (1 μM, P < 0.05). Similarly, the VGCC agonist Bay K8644 (5 nM) constricted arterioles from 14 ± 1 to 8 ± 1 μm and increased wave occurrence (3 ± 1 to 10 ± 1 waves/SMC) and frequency (0.2 ± 0.1 to 0.6 ± 0.1 Hz, n = 6, P < 0.05), effects that were unaltered by ryanodine (50 μM, n = 6, P > 0.05). These data support the hypothesis that Ca2+ waves in arteriolar SMCs depend, in part, on the activity of VGCCs.
NEW & NOTEWORTHY Arterioles that control blood flow to and within skeletal muscle depend on Ca2+ influx through voltage-gated Ca2+ channels and release of Ca2+ from internal stores through inositol 1,4,5-trisphosphate receptors in the form of Ca2+ waves to maintain pressure-induced smooth muscle tone.
Keywords: arterioles, calcium, calcium waves, voltage-gated Ca2+ channels
INTRODUCTION
Arterioles in the skeletal muscle microcirculation contribute to the distribution of blood flow in muscle, total peripheral resistance, and blood pressure regulation (29). Vascular smooth muscle cells (SMCs) are the effectors of these functions. They determine vessel internal diameter, which, in turn, is controlled by the contractile activity of SMCs (i.e., “tone”) in these resistance microvessels. We have previously shown that cremaster arteriolar SMCs display spontaneous, asynchronous inositol 1,4,5-trisphosphate (IP3) receptor-dependent Ca2+ waves that contribute to global myoplasmic Ca2+ concentration ([Ca2+]in) and myogenic tone in mouse and hamster cremaster arterioles (14, 34, 35). In addition, myogenic tone in these microvessels is strongly dependent on Ca2+ influx through voltage-gated Ca2+ channels (VGCCs) (2, 14). Taken together, these data suggest that Ca2+ influx through VGCCs may interact with Ca2+ release through IP3 receptors and contribute to Ca2+ signaling and the regulation of myogenic tone in cremaster arterioles. However, examination of the literature reveals no clear picture regarding VGCC activity and Ca2+ waves in pressure-induced myogenic tone. Jaggar (16) found that depolarization of SMCs in rat cerebral arteries by increased pressure (10−60 mmHg) or elevated extracellular K+ concentration (6−30 mM K+) increased the frequency of Ca2+ waves, effects that were inhibited by the VGCC blocker diltiazem. These data suggest that Ca2+ waves are stimulated by Ca2+ entry through VGCCs. This idea is also supported by studies of agonist-induced Ca2+ waves in rat basilar arteries (30) and human mesenteric arteries (24) studied by wire myography. Conversely, also in rat cerebral arteries, Mufti et al. (23) showed that although the occurrence and frequency of Ca2+ waves increased with increased pressure, blockade of VGCCs with diltiazem or nifedipine had no effect on Ca2+ wave parameters. These investigators also found that membrane depolarization or hyperpolarization, which will activate and deactivate VGCCs, respectively, did not influence the occurrence and frequency of Ca2+ waves. These findings suggest that pressure-induced Ca2+ waves are voltage independent and not influenced by Ca2+ entry through VGCCs, in contrast to the findings of Jaggar (16). Voltage independence and lack of effects of VGCC blockade on Ca2+ oscillations in unpressurized rat iridial arterioles has also been reported (11). In addition, Misfeldt et al. (22) found that nifedipine increased the occurrence of agonist-induced Ca2+ oscillations in rat retinal resistance arteries studied by wire myography. Therefore, there remains no consensus on how the activity of VGCCs influences Ca2+ waves in vascular smooth muscle, and there are few studies of VGCC activity and Ca2+ waves in arterioles.
The purpose of the present study was to test the hypothesis that spontaneous IP3 receptor-dependent Ca2+ waves are modulated by the activity of VGCCs in SMCs of hamster cremaster arterioles. In support of this hypothesis, we found that the occurrence and frequency of Ca2+ waves were inhibited upon removal of extracellular Ca2+, by exposure to the VGCC blockers nifedipine or diltiazem or by hyperpolarization of SMCs to deactivate VGCCs. Conversely, Ca2+ wave occurrence and frequency were augmented by the VGCC agonist Bay K8644 or depolarization produced by blockade of large-conductance Ca2+-activated K+ (BKCa) channels. Our results support the hypothesis that VGCC activity strongly modulates Ca2+ waves in cremaster arteriolar SMCs. Our data conflict with reports in rat cerebral arteries (23) and iridial arterioles (11), supporting the hypothesis that there are regional differences in the regulation of Ca2+ waves in vascular SMCs.
MATERIALS AND METHODS
Animals.
All experiments were approved by the Institutional Animal Care and Use Committee of Michigan State University and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (3). Male Golden Syrian hamsters (2–4 mo old, 100–150 g, Harlan) were maintained on a 12:12-h light-dark cycle and were provided food and water ad libitum. Animals were euthanized by CO2 asphyxiation followed by cervical dislocation during the day (normal light period of the light-dark cycle), and cremaster muscles were removed and placed in cold (4°C) Ca2+-free physiological salt solution (PSS) containing (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.4, 295 mosmol/kgH2O) to which 10 μM sodium nitroprusside, 10 μM diltiazem, and 1% BSA (USB, Affymetrix, Cleveland, OH) had been added as previously described (2, 15, 34, 35).
Arteriole isolation and study.
Second-order arterioles were isolated, cannulated onto glass micropipettes, and pressurized to 20 cmH2O (15 mmHg) as previously described (2, 15, 34, 35). Vessels were then loaded with Fluo-4-AM (5 μM) in Ca2+-free PSS (Invitrogen, Carlsbad, CA) for 2 h at room temperature as previously described (35). After washout of Fluo-4-AM and a 30-min equilibration period to allow deesterification of the Fluo-4 and gradual warming to 34°C in PSS containing 1.8 mM Ca2+, pressure was increased to 80 cmH2O (59 mmHg) with no luminal flow, and vessels were allowed to develop myogenic tone for 15–30 min. Smooth muscle layer Fluo-4 fluorescence at 526 nm (proportional to intracellular Ca2+) was recorded in each vessel using a high-speed (500 frames at 30 frames/s, 16.7-s duration) spinning-disk confocal microscope consisting of a Leica DMLFSA microscope (Leica Microsystems, Buffalo Grove, IL) equipped with a Yokagawa CSU-10 confocal head (Solamere, Salt Lake City, UT) and a Leica ×40 long-working-distance, water-immersion objective (numerical aperture: 0.8, working distance: 3 mm, Leica Microsystems). Illumination was provided by a Coherent 488-nm diode laser (Coherent, Santa Clara, CA). Images were captured by an XR Mega-10 intensified charge-coupled device camera (Stanford Photonics, Palo Alto, CA) coupled to the confocal head and controlled using Piper software (Stanford Photonics).
Fluo-4 fluorescence data were analyzed using SparkAn (obtained from A. Bonev and M. T. Nelson, University of Vermont) and ImageJ (27) as previously described (14, 34, 35). SparkAn allows placement of regions of interest (10 pixels/10 pixels) at the peak of a fluorescent event. Baseline fluorescence (Fo) was estimated by the software from at least 10 images that did not contain an event [defined as an increase in fluorescence intensity (F) that was at least 15% above Fo], and data are expressed as F/Fo as previously reported (14, 34, 35).
Arteriolar internal diameters were measured asynchronously from transmitted light images of vessels at the midplane, in the steady state, acquired immediately after the acquisition of fluorescence images using ImageJ calibrated with a stage micrometer as previously described (14, 34, 35).
Experiment protocol.
Experiments were started after arterioles were loaded with Fluo-4, pressurized, and allowed to develop a steady level of myogenic tone as previously described (14, 34, 35). Vessels were superfused with PSS or PSS containing a drug for 5–10 min [time determined by monitoring vessel diameter with dim red (586 nm) transillumination until a steady state was achieved]. An image sequence was then acquired for Fluo-4 fluorescence, as outlined above, followed by acquisition of a sequence of images using transmitted light for diameter measurements. A new site on the vessel then was selected, the superfusate was switched to the next solution to be studied, and another Fluo-4 image sequence and diameter image sequence were acquired after a steady diameter was achieved (5–10 min). We changed imaging positions to limit phototoxicity and Fluo-4 bleaching. The sequence of PSS/drug application was randomized in all experiments. In prior studies (14, 34, 35), we found that up to six different sites could be studied in a given arteriole using this approach with comparable Ca2+ events detected among the sites.
Drugs and chemicals.
All compounds were purchased from Sigma-Aldrich (St. Louis, MO) unless noted otherwise. Except for nifedipine and (±)Bay K8644 (catalog no. B-112), drugs were prepared daily as 10 mM stock solutions in distilled H2O and diluted in PSS as indicated. Nifedipine and Bay K8644 were dissolved in ethanol to yield a 10 mM stock and then diluted in PSS to yield the working concentration. Fluo-4-AM was dissolved in DMSO and then diluted in Ca2+-free PSS containing 1% albumin to yield a working concentration of 5 μM.
Data analysis and statistics.
Summary data are expressed as means ± SE. Sample sizes (n values) stated in the text refer to the number of vessels studied from at least three animals with no more than two arterioles studied from any hamster. Statistical significance was determined using Student’s paired and unpaired t-tests and appropriate ANOVA designs followed by Sidak’s multiple-comparison tests to assess differences among means using Prism Software 8.0 (Graphpad Software, San Diego, CA), with P < 0.05 considered statistically significant.
RESULTS
Spontaneous Ca2+ waves in pressurized arterioles depend on extracellular Ca2+.
Hamster cremaster arterioles pressurized to 80 cmH2O and superfused with PSS containing 1.8 mM Ca2+ developed significant myogenic tone, constricting 30 ± 2% from their maximum diameter (45 ± 3 μm, n = 15). As previously reported (14, 35), SMCs in these microvessels displayed spontaneous Ca2+ waves (Fig. 1). Consistent with the hypothesis that Ca2+ entry through VGCCs stimulates Ca2+ waves, we found that 5−10 min of exposure of arterioles to 0 Ca2+ PSS decreased global [Ca2+]in, dilated the arterioles, and inhibited Ca2+ waves (Fig. 1).
Fig. 1.
Removal of extracellular Ca2+ inhibits Ca2+ waves. A–C: typical records of Ca2+ waves recorded in arteriolar smooth muscle cells (SMCs). A: image of a Fluo-4-loaded arteriole with regions of interest (ROIs) used for measuring Ca2+ waves shown on 20 SMCs. The ROIs have been colored and enlarged for display purposes. B: Ca2+ waves recorded in an arteriole in physiological salt solution (PSS) containing 1.8 mM Ca2+ from the 20 ROIs shown in A. C: recordings from 20 ROIs from the same arteriole as in A and B after exposure to nominally Ca2+-free PSS (0 mM Ca2+ PSS). D and E: pseudocolored images of a Fluo-4-loaded arteriole (average projection of a stack of 500 images cropped to show images of the vessels only) in PSS and in 0 mM Ca2+ PSS. F–H: means ± SE (n = 9). F: global Fluo-4 intensity relative to the mean value in PSS (a measure of global myoplasmic Ca2+ concentration). G: diameter. H: occurrence (number of waves/cell), amplitude (F/Fo), and frequency (Hz) of Ca2+ waves in the presence of 1.8 mM Ca2+ (PSS) and after exposure to nominally Ca2+-free PSS (0 mM Ca2+ PSS). *P < 0.05 compared with PSS via paired t-tests. Scale bars in D and E are 20 μm. F, fluorescence intensity; Fo, baseline fluorescence.
Inhibition of VGCC activity attenuates Ca2+ waves.
Consistent with the effects of removal of extracellular Ca2+, myogenic tone and Ca2+ wave occurrence, frequency, and amplitude were also inhibited by 5−10 min of exposure to the VGCC blocker nifedipine (1 μM), as shown in Fig. 2. Because nifedipine has been proposed to affect refilling of Ca2+ stores in retinal arterioles through a VGCC-independent mechanism (5), similar experiments were performed using the benzothiazepine VGCC blocker diltiazem. We found that diltiazem (10 μM) produced comparable results. This nondihydropyridine VGCC blocker dilated arterioles from 28 ± 1.8 to 33 ± 1.8 μm (n = 5, P < 0.05) and reduced the occurrence (1 ± 0.3 to 0.3 ± 0.07 waves/cell), amplitude (1.7 ± 0.1 to 1.4 ± 0.03 F/Fo), and frequency (0.2 ± 0.06 to 0.08 ± 0.02 Hz) of Ca2+ waves (n = 5, P < 0.05 for each comparison).
Fig. 2.
Voltage-gated Ca2+ channel (VGCC) blocker inhibits Ca2+ waves. Data are means ± SE (n = 4) before [physiological salt solution (PSS)] and in the presence of the VGCC blocker nifedipine (1 μM) for global Fluo-4 intensity relative to the mean value in PSS (a measure of global myoplasmic Ca2+ concentration; A), internal diameter (B), and the occurrence (number of waves/cell), amplitude (F/Fo), and frequency (Hz) of Ca2+ waves as indicated (C). *P < 0.05 compared with PSS via paired t-tests. F, fluorescence intensity; Fo, baseline fluorescence.
As another means to reduce Ca2+ influx through VGCCs, we exposed arterioles to the ATP-sensitive K+ channel agonist cromakalim (10 μM) (26), which we have previously shown to hyperpolarize SMCs in pressurized hamster cremaster arterioles from a resting potential of −30 mV to approximately −55 mV (2). Similar to the results obtained with the removal of extracellular Ca2+ (Fig. 1) and exposure to VGCC blockers (Fig. 2), 5–10 min of exposure to cromakalim decreased global [Ca2+]in, dilated the arterioles, and reduced the occurrence, amplitude, and frequency of Ca2+ waves (Fig. 3).
Fig. 3.
Ca2+ waves are inhibited by the ATP-sensitive K+ channel agonist cromakalim. Data are means ± SE (n = 4) before [physiological salt solution (PSS)] and in the presence of cromakalim (10 μM) for global Fluo-4 intensity relative to the mean value in PSS (a measure of global myoplasmic Ca2+ concentration; A), internal diameter (B), and occurrence (number of waves/cell], amplitude (F/Fo), and frequency (Hz) of Ca2+ waves as indicated (C). *P < 0.05 compared with PSS via paired t-tests. F, fluorescence intensity; Fo, baseline fluorescence.
Activation of VGCCs augments Ca2+ waves.
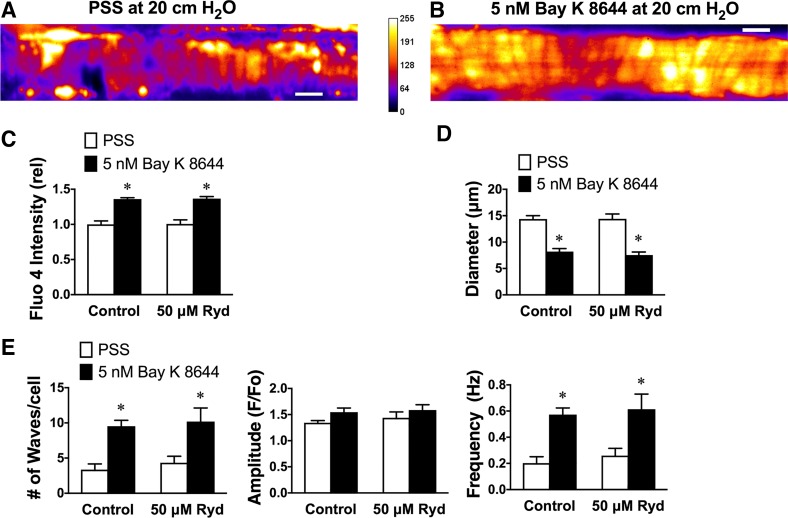
If the hypothesis that Ca2+ entry through VGCCs stimulates Ca2+ waves is correct, then augmentation of the activity of these channels should stimulate Ca2+ wave activity. We accomplished this by exposing arterioles to the VGCC agonist Bay K8644 (28). To reduce the pressure-dependent activity of VGCCs, we performed these experiments at 20 cmH2O (15 mmHg), where these arterioles display little myogenic tone and resting Ca2+ wave activity is reduced (35). Lowering pressure from 80 to 20 cmH2O (59 to 15 mmHg) resulted in a fall in global Ca2+ (79 ± 9% of value at 80 cmH2O, n = 6, P < 0.05 vs. 100%), a reduction in Ca2+ wave occurrence (from 6.4 ± 0.8 to 3.5 ± 0.6 waves/cell, P < 0.05), and the frequency of Ca2+ waves (from 0.4 ± 0.05 to 0.2 ± 0.04 Hz; P < 0.05) with no significant change in Ca2+ wave amplitude (from 1.5 ± 0.08 to 1.4 ± 0.04, P > 0.05). Consistent with our hypothesis and producing results opposite to the data shown in Figs. 1–3, we found that 5–10 min of exposure to Bay K8644 (5 nM) caused an increase in global [Ca2+]in, vasoconstriction and an increase in the occurrence, amplitude and frequency of Ca2+ waves in hamster cremaster arteriolar SMCs (Fig. 4).
Fig. 4.
Ca2+ waves are stimulated by the voltage-gated Ca2+ channel agonist Bay K8644 independent from ryanodine (Ryd) receptors. A and B: pseudocolored images (average projection of a stack of 500 images cropped to show only the segment of arteriole that was imaged) of a Fluo-4-loaded arteriole pressurized to 20 cmH2O in physiological salt solution (PSS; A) and in the presence of 5 nM Bay K8644 (B). C−E: global Fluo-4 intensity relative to the mean value in PSS (a measure of global myoplasmic Ca2+ concentration; C), internal diameter (D), and occurrence (number of waves/cell], amplitude (F/Fo), and frequency (Hz) of Ca2+ waves in the presence of 1.8 mM Ca2+ (PSS) and after exposure to nominally Ca2+-free PSS (0 mM Ca2+; E). Values are means ± SE (n = 6). Two-way ANOVA indicated significant effects of Bay K8644 (P < 0.05) but no significant effect of ryanodine (P > 0.05) and no significant interaction between Bay K8644 and ryanodine (P > 0.05) for each variable except wave amplitude. *P < 0.05 compared with PSS via two-way ANOVA and a subsequent Sidak test for multiple comparison of means. Scale bar = 20 μm. F, fluorescence intensity; Fo, baseline fluorescence.
Ca2+ influx through VGCCs may trigger Ca2+ release through ryanodine receptors in some vascular SMCs (1, 4, 17). To evaluate the role of these intracellular channels in the stimulation of cremaster arteriolar SMC Ca2+ waves by Bay K8644, the effects of ryanodine (50 μM) on Ca2+ waves in the absence and presence of Bay K8644 also were examined. We have previously shown that this concentration of ryanodine effectively blocks ryanodine receptors in hamster cremaster arterioles but has no effect on Ca2+ waves (35). In the present study, we found that 5–10 min of exposure to ryanodine (50 μM) had no significant effect on global [Ca2+]in, resting diameter, or Ca2+ waves (Fig. 4), consistent with the previous study (35). Importantly, ryanodine (50 μM) had no significant effect on Bay K8644 (5 nM)-induced increases in [Ca2+]in, constriction, or augmentation of Ca2+ waves (Fig. 4). These data exclude a role for ryanodine receptors in linking VGCC activity to Ca2+ wave activity.
As an additional test of the hypothesis that Ca2+ influx through VGCCs stimulates Ca2+ waves, we examined the effects of blockade of BKCa channels on Ca2+ waves in hamster cremaster arterioles and the role played by VGCCs in this process. In hamster cremaster arterioles (35), as in other vessels (12, 32), BKCa channels participate in the negative feedback regulation of vascular SMC membrane potential and myogenic tone. Thus, blockade of BKCa channels should lead to membrane depolarization, opening of VGCCs, and increased Ca2+ influx through VGCCs. We have previously shown that inhibition of BKCa channels with drugs like tetraethylammonium (TEA; 1 mM) (13, 18) constricts cremaster arterioles and augments smooth muscle Ca2+ waves (35), consistent with this idea. We obtained similar results in the present study. In arterioles pressurized to 80 cmH2O, 5–10 min of exposure to TEA (1 mM) led to an increase in global [Ca2+]in (Fig. 5A), vasoconstriction (Fig. 5B), and augmented occurrence and frequency of Ca2+ waves (Fig. 5C). The stimulatory effect of TEA could be prevented by blockade of VGCCs with nifedipine (see Fig. 2 for the effects of nifedipine alone) or diltiazem (see text above for the effects of diltiazem alone; Fig. 6). In the presence of nifedipine or diltiazem, TEA was without effect on diameter (Fig. 6, A and B) or on Ca2+ wave parameters (Fig. 6, C and D). Thus, consistent with our hypothesis, blockade of BKCa channels with TEA (1 mM) led to activation of VGCCs and augmentation of Ca2+ waves.
Fig. 5.
Ca2+ waves are stimulated by the large-conductance Ca2+-activated K+ channel antagonist tetraethylammonium (TEA). Data are means ± SE (n = 10–11) before [physiological salt solution (PSS)] and in the presence of TEA (1 mM) for global Fluo-4 intensity relative to the mean value in PSS (a measure of global myoplasmic Ca2+ concentration; A), internal diameter (B), and occurrence (number of waves/cell), amplitude (F/Fo), and frequency (Hz) of Ca2+ waves as indicated (C). *P < 0.05 compared with PSS via paired t-tests. F, fluorescence intensity; Fo, baseline fluorescence.
Fig. 6.
Effects of tetraethylammonium (TEA) are prevented by voltage-gated Ca2+ channel (VGCC) blockers. Data are means ± SE (n = 5) during superfusion with physiological salt solution (PSS), nifedipine, nifedipine + TEA, diltiazem, or diltiazem + TEA as shown. *P < 0.05 vs. PSS; ⊗P > 0.05 vs. PSS and diltiazem alone. TEA had no significant effect on diameter or the number, frequency, or amplitude of Ca2+ waves in the presence of VGCCs (P > 0.05 vs. same VGCC blocker alone). All comparisons were by repeated-measures ANOVA and a subsequent Sidak test for multiple comparisons. F, fluorescence intensity; Fo, baseline fluorescence.
IP3 receptors mediate Ca2+ waves in hamster cremaster arterioles.
We have previously shown that Ca2+ waves in hamster cremaster arterioles are mediated by IP3 receptors (35). Consistent with that report, we found that the IP3 receptor antagonist 2-aminoethyl diphenylborinate (2-APB; 100 μM) nearly abolished the occurrence and frequency of Ca2+ waves in the present study (Fig. 7C). Importantly, and consistent with our hypothesis, this IP3 receptor antagonist also prevented augmentation of the Ca2+ waves by TEA (1 mM; Fig. 7C), supporting the idea that the Ca2+ waves observed during TEA exposure (Fig. 5C) are mediated by IP3 receptors. In contrast, although 2-APB reduced global [Ca2+]in, TEA still produced a robust increase in global [Ca2+]in in the presence of 2-APB (Fig. 7A) that resulted in constriction of the arterioles (Fig. 7B). These data suggest that the lack of effect of TEA on Ca2+ waves in the presence of 2-APB is not due to lack of an increase in VGCC activity.
Fig. 7.
Inositol 1,4,5-trisphosphate (IP3) receptor blocker prevents tetraethylammonium (TEA) effects on Ca2+ waves. Data are means ± SE (n = 6) in the absence [physiological salt solution (PSS)] and the presence of TEA (1 mM) to block large-conductance Ca2+-activated K+ channels before (control) and during exposure to 2-aminoethyl diphenylborinate (2-APB; 100 μM) to block IP3 receptors. For global Fluo-4 intensity (a measure of global myoplasmic Ca2+ concentration), two-way ANOVA indicated significant effects of both TEA (P < 0.05) and 2-APB (P < 0.05) with no significant interaction (P > 0.05) indicating a lack of effect of 2-APB on the global myoplasmic Ca2+ concentration response to TEA (A). For internal diameter, two-way ANOVA indicated significant effects of TEA (P < 0.05) and 2-APB (P < 0.05) and a significant interaction between TEA and 2-APB (P < 0.05) on diameter. Although TEA still produced a significant decrease in diameter in the presence of 2-APB, the magnitude of the response was blunted in the presence of 2-APB (P < 0.05; B). For occurrence (number of waves/cell), amplitude (F/Fo), and frequency (Hz) of Ca2+ waves, two-way ANOVA indicated significant effects of TEA (P < 0.05) and 2-APB (P < 0.05) and a significant interaction between TEA and 2-APB (P < 0.05) on the number of waves and wave frequency. In the presence of 2-APB, TEA failed to have any significant effect of wave parameters (P > 0.05) (C). *Significantly different from control PSS (P < 0.05). #Significantly different from adjacent value (P < 0.05) but not significantly different from control PSS (P > 0.05). **Significantly different from control PSS but not different from adjacent value (P > 0.05). Multiple comparisons were by Sidak test. F, fluorescence intensity; Fo, baseline fluorescence.
DISCUSSION
The role of VGCCs in the development of SMC Ca2+ waves remains unclear and appears to vary based on both vascular bed and experimental conditions. This is the first study to describe VGCC-dependent spontaneous SMC Ca2+ waves in arterioles (diameter < 50 μm, single layer of SMCs) with significant pressure-induced myogenic tone.
We have previously shown that the frequency and occurrence of spontaneous Ca2+ waves in hamster cremaster arteriole SMCs are abolished by inhibition of phospholipase C with U73122, blockade of IP3 receptors with 2-APB or xestospongin-D, and depletion of intracellular Ca2+ stores with thapsigargin, whereas block of ryanodine receptors with ryanodine or tetracaine had no effect on Ca2+ waves (35). These data indicate that Ca2+ waves in hamster cremaster arteriole SMCs are mediated by IP3 receptors. We confirmed these findings in the present study by showing that 2-APB inhibited the frequency and occurrence of Ca2+ waves, whereas ryanodine was without effect. We used 2-APB to block IP3 receptor signaling in the present study because its effects on Ca2+ signaling and diameter are identical to those produced by xestospongin-D and U73122 in hamster cremaster arterioles (35), indicating selectivity for IP3 receptor signaling in this model. In addition, 2-APB effects are rapid in onset (5–10 min) and completely reversible with rapid washout of effects. These characteristics allow randomization of the sequence of treatments. This drug is also inexpensive, reducing experiment costs. Finally, we have also shown that 100 μM 2-APB is without effect on myogenic tone in hamster cheek pouch arterioles (where SMCs do not display Ca2+ waves), suggesting that off-target effects, such as the blockade of transient receptor potential channels, are minimal in hamster arterioles (14). We used 2-APB in the present study to confirm that Ca2+ waves were IP3 receptor dependent and to demonstrate that the augmented Ca2+ waves during TEA exposure did not originate from some other source, such as oscillations in VGCC activity.
In the present investigation, we found that removal of extracellular Ca2+, blockade of VGCCs with nifedipine or diltiazem, or SMC hyperpolarization by activation of ATP-sensitive K+ channels to deactivate VGCCs all reduced the frequency and occurrence of spontaneous IP3 receptor-dependent Ca2+ waves. Conversely, SMC depolarization-induced by blockade of BKCa channels or pharmacological activation of VGCC with Bay K8644 increased the occurrence and frequency of Ca2+ waves. Together, these observations support our hypothesis that Ca2+ waves in hamster cremaster arteriolar SMCs depend, in part, on the activity of VGCCs and are the first to report this phenomenon in cremaster or any arteriolar SMCs. Our results are consistent with a previous study of pressure-induced Ca2+ waves in rat middle and posterior cerebral arteries (16) and for agonist-induced Ca2+ waves in rat basilar arteries (30) and human mesenteric arteries (24) studied by wire myography. However, our data do not agree with observations in rat middle and posterior cerebral arteries (23) where activation of VGCCs by membrane depolarization, deactivation of VGCCs by membrane hyperpolarization, or blockade of VGCCs with diltiazem had no effect on the occurrence or frequency of pressure-induced Ca2+ waves (23). Global Ca2+ oscillations in unpressurized rat iridial arteriolar SMCs also appear to be voltage and VGCC independent (11). Our results also differ from the observations of Misfeldt et al. (22) in rat retinal resistance arteries, who found that nifedipine increased the proportion of SMCs displaying Ca2+ oscillations without affecting the amplitude or frequency of these events. We have no explanation that can reconcile these divergent results other than to invoke methodological differences, particularly for Mufti et al.’s findings (23), or regional differences in mechanisms to explain the findings in rat retinal resistance arteries (22). In the present study, and in Jaggar’s experiments (16), vessels were studied with an intact endothelium, whereas in the study performed by Mufti et al. (23), vessels were endothelium denuded. Thus, it is possible that myoendothelial signaling somehow contributes to coupling VGCC activity and Ca2+ wave mechanisms. However, in contrast to this idea, VGCC-independent Ca2+ oscillations were observed in rat iridial arterioles with a presumably intact endothelium (11). Additional research will be required to test the hypothesis that the endothelium promotes voltage-dependent modulation of Ca2+ waves in vascular SMCs.
Vascular SMC IP3 receptor-dependent Ca2+ waves significantly contribute to global Ca2+ levels and myogenic tone in both hamster and mouse cremaster arterioles (present study and Refs. 34 and 35). Ca2+ waves also contribute to global Ca2+ levels and myogenic tone in rat cerebral arteries; however, in these vessels, ryanodine receptors also contribute to Ca2+ waves (16, 23). These results differ from those obtained in rat (21) and mouse (36) mesenteric arteries where Ca2+ waves were infrequent in vessels that developed myogenic tone. It is not clear if methodological differences or regional differences in Ca2+ signaling mechanisms contribute to these discordant results.
Our present and previous (34, 35) findings implicating IP3 receptor-dependent Ca2+ waves in the Ca2+ signals underlying myogenic tone in vitro also do not agree with observations in mouse cremaster muscle arterioles, studied in vivo, where basal tone appeared not to be accompanied by Ca2+ waves (20). Despite the lack of resolution of Ca2+ waves in cremaster arterioles in vivo, inhibition of phospholipase C with U73122, blockade of IP3 receptors with 2-APB, or blockade of VGCCs with nifedipine resulted in significant arteriolar dilation, although intracellular Ca2+ measurements were not performed during these experiments (20). These data support roles for IP3, IP3 receptors, and VGCCs in the basal tone of mouse cremaster arterioles in the living microcirculation, consistent with our findings. Why Ca2+ waves were not discernable in mouse cremaster arterioles, in vivo, is not clear and will require additional research. However, the use of anesthetics, in vivo, and the complex in vivo milieu with circulating hormones, etc., might contribute. Alternatively, it has been proposed that myogenic tone does not depend on IP3 and IP3 receptors but on diacylglycerol produced by phosphatidyl choline-specific phospholipase C and protein kinase C, although these findings were obtained in mouse mesenteric resistance arteries such that regional differences in mechanisms cannot be excluded (19). Protein kinase C does not contribute to the mechanisms underlying myogenic tone of hamster cremaster arterioles studied by pressure myography (14), supporting the regional heterogeneity hypothesis.
Our data indicate that in contrast to SMCs in cerebral arteries (23) and iridial arterioles (11), skeletal muscle arteriolar SMC Ca2+ waves depend in part on Ca2+ entry through VGCCs. Our findings also suggest that membrane potential, through activation of VGCCs, strongly impacts arteriolar Ca2+ signals involving IP3 receptors but not ryanodine receptors [which appear to be silent in the SMCs of these arterioles (34, 35)]. Agonist-induced Ca2+ waves may also be independent of ryanodine receptor activity in porcine retinal resistance arteries, where application of ryanodine over a range of concentrations failed to alter the presence or kinetics of Ca2+ oscillations (22). In contrast, observations in cerebral arteries (16, 23), skeletal muscle feed arteries (34, 35), and retinal arterioles (6, 31) indicate that ryanodine receptors contribute to Ca2+ waves. These observation support the hypothesis that there are significant regional differences in Ca2+ signaling mechanisms, as we have previously posited (34, 35).
The mechanism coupling VGCC activity to IP3 receptor activity and Ca2+ waves in cremaster arterioles remains to be established. Ca2+ influx through VGCCs has been shown to trigger Ca2+-induced Ca2+ release through IP3 receptors in renal arteriolar SMCs (25). It is also possible that by contributing to global Ca2+ levels, Ca2+ influx through VGCCs contributes to the state of fullness of intracellular Ca2+ stores and IP3 receptor activity (32), as has been proposed for coupling between VGCCs and ryanodine receptors (9, 31). Voltage-dependent, metabotropic release of Ca2+ through IP3 receptors in vascular SMCs, independent from Ca2+ influx through VGCCs, has been proposed (7, 8, 10, 33). A study (10) in aortic SMCs from mice lacking functional Cav1.2 channels has underscored the importance of VGCCs as the sensor in this depolarization-induced Ca2+ release, as cells lacking Cav1.2 VGCCs do not undergo depolarization-induced Ca2+ release from the sarcoplasmic reticulum in response to membrane depolarization. These studies also support IP3 receptors as the relevant effectors because Ca2+ release through ryanodine receptors was unaffected in Cav1.2 knockout mice. Our data do not allow us to distinguish among these mechanisms, and additional experiments will be required.
Our data support the hypothesis that VGCC activity strongly modulates the activity of IP3 receptors and the Ca2+ waves that they produce. We think that IP3 receptors amplify Ca2+ signals that originate from VGCCs and importantly contribute to the regulation of myogenic tone in skeletal muscle arterioles. Our findings also support the idea that there are significant regional differences in Ca2+ signaling mechanisms in vascular SMCs.
A better understanding of regional heterogeneity in Ca2+ signaling mechanisms in vascular SMCs may provide new therapeutic targets to treat cardiovascular disease in an organ-specific manner. In particular, further defining unique signaling pathways in the microcirculation versus larger, more commonly studied resistance vessels remains an important goal in the treatment of many cardiovascular diseases. An improved ability to modulate arteriolar SMC Ca2+ signals and myogenic tone in an organ-specific manner might lead to new therapeutics with reduced off-target effects, such as orthostatic hypotension, that accompany drugs that more generally target vascular smooth muscle contractile activity.
GRANTS
This research was supported by National Heart, Lung and Blood Institute grants HL-32469 and PO1-HL-070687 (to W. Jackson) and American Heart Association Fellowship 0815778G (to E. Boerman).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.F.J. and E.M.B. conceived and designed research; E.M.B. performed experiments; W.F.J. and E.M.B. analyzed data; W.F.J. and E.M.B. interpreted results of experiments; W.F.J. prepared figures; W.F.J. and E.M.B. drafted manuscript; W.F.J. and E.M.B. edited and revised manuscript; W.F.J. and E.M.B. approved final version of manuscript.
REFERENCES
- 1.Bolton TB, Gordienko DV. Confocal imaging of calcium release events in single smooth muscle cells. Acta Physiol Scand 164: 567–575, 1998. doi: 10.1046/j.1365-201X.1998.00464.x. [DOI] [PubMed] [Google Scholar]
- 2.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation 11: 279–293, 2004. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee for the Update of the Guide for the Care and Use of Laboratory Animals NRC Guide for the Care and Use of Laboratory Animals (8th ed.). Washington, DC: National Academies, 2011. [Google Scholar]
- 4.Coussin F, Macrez N, Morel JL, Mironneau J. Requirement of ryanodine receptor subtypes 1 and 2 for Ca2+-induced Ca2+ release in vascular myocytes. J Biol Chem 275: 9596–9603, 2000. doi: 10.1074/jbc.275.13.9596. [DOI] [PubMed] [Google Scholar]
- 5.Curtis TM, Scholfield CN. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J Physiol 532: 609–623, 2001. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis TM, Tumelty J, Dawicki J, Scholfield CN, McGeown JG. Identification and spatiotemporal characterization of spontaneous Ca2+ sparks and global Ca2+ oscillations in retinal arteriolar smooth muscle cells. Invest Ophthalmol Vis Sci 45: 4409–4414, 2004. doi: 10.1167/iovs.04-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.del Valle-Rodríguez A, Calderón E, Ruiz M, Ordoñez A, López-Barneo J, Ureña J. Metabotropic Ca2+ channel-induced Ca2+ release and ATP-dependent facilitation of arterial myocyte contraction. Proc Natl Acad Sci USA 103: 4316–4321, 2006. doi: 10.1073/pnas.0508781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.del Valle-Rodríguez A, López-Barneo J, Ureña J. Ca2+ channel-sarcoplasmic reticulum coupling: a mechanism of arterial myocyte contraction without Ca2+ influx. EMBO J 22: 4337–4345, 2003. doi: 10.1093/emboj/cdg432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Essin K, Welling A, Hofmann F, Luft FC, Gollasch M, Moosmang S. Indirect coupling between Cav1.2 channels and ryanodine receptors to generate Ca2+ sparks in murine arterial smooth muscle cells. J Physiol 584: 205–219, 2007. doi: 10.1113/jphysiol.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Tenorio M, González-Rodríguez P, Porras C, Castellano A, Moosmang S, Hofmann F, Ureña J, López-Barneo J. Short communication: genetic ablation of L-type Ca2+ channels abolishes depolarization-induced Ca2+ release in arterial smooth muscle. Circ Res 106: 1285–1289, 2010. doi: 10.1161/CIRCRESAHA.109.213967. [DOI] [PubMed] [Google Scholar]
- 11.Haddock RE, Hirst GD, Hill CE. Voltage independence of vasomotion in isolated irideal arterioles of the rat. J Physiol 540: 219–229, 2002. doi: 10.1113/jphysiol.2001.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson WF. Ion channels and vascular tone. Hypertension 35: 173–178, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson WF, Blair KL. Characterization and function of Ca2+-activated K+ channels in arteriolar muscle cells. Am J Physiol Heart Circ Physiol 274: H27–H34, 1998. doi: 10.1152/ajpheart.1998.274.1.H27. [DOI] [PubMed] [Google Scholar]
- 14.Jackson WF, Boerman EM. Regional heterogeneity in the mechanisms of myogenic tone in hamster arterioles. Am J Physiol Heart Circ Physiol 313: H667–H675, 2017. doi: 10.1152/ajpheart.00183.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle alpha1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol 155: 514–524, 2008. doi: 10.1038/bjp.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 281: C439–C448, 2001. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 17.Kamishima T, McCarron JG. Depolarization-evoked increases in cytosolic calcium concentration in isolated smooth muscle cells of rat portal vein. J Physiol 492: 61–74, 1996. doi: 10.1113/jphysiol.1996.sp021289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langton PD, Nelson MT, Huang Y, Standen NB. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol Heart Circ Physiol 260: H927–H934, 1991. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- 19.Mauban JR, Zacharia J, Fairfax S, Wier WG. PC-PLC/sphingomyelin synthase activity plays a central role in the development of myogenic tone in murine resistance arteries. Am J Physiol Heart Circ Physiol 308: H1517–H1524, 2015. doi: 10.1152/ajpheart.00594.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauban JR, Zacharia J, Zhang J, Wier WG. Vascular tone and Ca2+ signaling in murine cremaster muscle arterioles in vivo. Microcirculation 20: 269–277, 2013. doi: 10.1111/micc.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miriel VA, Mauban JR, Blaustein MP, Wier WG. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J Physiol 518: 815–824, 1999. doi: 10.1111/j.1469-7793.1999.0815p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misfeldt MW, Aalkjaer C, Simonsen U, Bek T. Voltage-gated calcium channels are involved in the regulation of calcium oscillations in vascular smooth muscle cells from isolated porcine retinal arterioles. Exp Eye Res 91: 69–75, 2010. doi: 10.1016/j.exer.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Mufti RE, Brett SE, Tran CH, Abd El-Rahman R, Anfinogenova Y, El-Yazbi A, Cole WC, Jones PP, Chen SR, Welsh DG. Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves. J Physiol 588: 3983–4005, 2010. doi: 10.1113/jphysiol.2010.193300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro-Dorado J, Garcia-Alonso M, van Breemen C, Tejerina T, Fameli N. Calcium oscillations in human mesenteric vascular smooth muscle. Biochem Biophys Res Commun 445: 84–88, 2014. doi: 10.1016/j.bbrc.2014.01.150. [DOI] [PubMed] [Google Scholar]
- 25.Povstyan OV, Harhun MI, Gordienko DV. Ca2+ entry following P2X receptor activation induces IP3 receptor-mediated Ca2+ release in myocytes from small renal arteries. Br J Pharmacol 162: 1618–1638, 2011. doi: 10.1111/j.1476-5381.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 77: 1165–1232, 1997. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schramm M, Thomas G, Towart R, Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature 303: 535–537, 1983. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- 29.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation 12: 33–45, 2005. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 30.Syyong HT, Yang HH, Trinh G, Cheung C, Kuo KH, van Breemen C. Mechanism of asynchronous Ca2+ waves underlying agonist-induced contraction in the rat basilar artery. Br J Pharmacol 156: 587–600, 2009. doi: 10.1111/j.1476-5381.2008.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tumelty J, Scholfield N, Stewart M, Curtis T, McGeown G. Ca2+-sparks constitute elementary building blocks for global Ca2+-signals in myocytes of retinal arterioles. Cell Calcium 41: 451–466, 2007. doi: 10.1016/j.ceca.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tykocki NR, Boerman EM, Jackson WF. Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Compr Physiol 7: 485–581, 2017. doi: 10.1002/cphy.c160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ureña J, del Valle-Rodríguez A, López-Barneo J. Metabotropic Ca2+ channel-induced calcium release in vascular smooth muscle. Cell Calcium 42: 513–520, 2007. doi: 10.1016/j.ceca.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Westcott EB, Goodwin EL, Segal SS, Jackson WF. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol 590: 1849–1869, 2012. doi: 10.1113/jphysiol.2011.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol 300: H1616–H1630, 2011. doi: 10.1152/ajpheart.00728.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zacharia J, Zhang J, Wier WG. Ca2+ signaling in mouse mesenteric small arteries: myogenic tone and adrenergic vasoconstriction. Am J Physiol Heart Circ Physiol 292: H1523–H1532, 2007. doi: 10.1152/ajpheart.00670.2006. [DOI] [PubMed] [Google Scholar]