Abstract
Age represents a major risk factor for multiple organ failure, including cardiac dysfunction, in patients with sepsis. AMP-activated protein kinase (AMPK) is a crucial regulator of energy homeostasis that controls mitochondrial biogenesis by activation of peroxisome proliferator-activated receptor-γ coactivator-1α and disposal of defective organelles by autophagy. We investigated whether AMPK dysregulation contributes to age-dependent cardiac injury in young (2–3 mo) and mature adult (11–13 mo) male mice subjected to sepsis by cecal ligation and puncture and whether AMPK activation by 5-amino-4-imidazole carboxamide riboside affords cardioprotective effects. Plasma proinflammatory cytokines and myokine follistatin were similarly elevated in vehicle-treated young and mature adult mice at 18 h after sepsis. However, despite equivalent troponin I and T levels compared with similarly treated young mice, vehicle-treated mature adult mice exhibited more severe cardiac damage by light and electron microscopy analyses with more marked intercellular edema, inflammatory cell infiltration, and mitochondrial derangement. Echocardiography revealed that vehicle-treated young mice exhibited left ventricular dysfunction after sepsis, whereas mature adult mice exhibited a reduction in stroke volume without apparent changes in load-dependent indexes of cardiac function. At molecular analysis, phosphorylation of the catalytic subunits AMPK-α1/α2 was associated with nuclear translocation of peroxisome proliferator-activated receptor-γ coactivator-1α in vehicle-treated young but not mature adult mice. Treatment with 5-amino-4-imidazole carboxamide riboside ameliorated cardiac architecture derangement in mice of both ages. These cardioprotective effects were associated with attenuation of the systemic inflammatory response and amelioration of cardiac dysfunction in young mice only, not in mature adult animals.
NEW & NOTEWORTHY Our data suggest that sepsis-induced cardiac dysfunction manifests with age-dependent characteristics, which are associated with a distinct regulation of AMP-activated protein kinase-dependent metabolic pathways. Consistent with this age-related deterioration, pharmacological activation of AMP-activated protein kinase may afford cardioprotective effects allowing a partial recovery of cardiac function in young but not mature age.
Keywords: 5-amino-4-imidazole carboxamide riboside, cardiac dysfunction, cecal ligation and puncture, coactivator-1α, mitochondria, peroxisome proliferator-activated receptor-γ
INTRODUCTION
Sepsis is a life-threatening disorder caused by a dysregulated host response to infection and is characterized by overlapping circulatory, cellular, and metabolic abnormalities (53). Standard therapeutic approaches include antibiotics in combination with hemodynamic and respiratory support to maintain proper oxygen delivery. Despite significant advances in our understanding of the underlying pathophysiology and improved therapeutic strategies, mortality due to sepsis and the resultant organ failure remains high (44, 46). The incidence of sepsis is disproportionately increased in the elderly, and age is an independent predictor of mortality (34).
Cardiac dysfunction is a well-recognized manifestation of sepsis, and patients with myocardial depression have a higher mortality rate than those without (14, 31, 48). Mitochondrial dysfunction has been proposed as an important cause of sepsis-related organ failure in sepsis, and, indeed, mitochondrial ultrastructural damage and diminished ATP production have been demonstrated in cardiomyocytes during sepsis (5, 9, 15, 56).
AMP-activated protein kinase (AMPK) is a crucial regulator of energy homeostasis by directing biogenesis of new organelles via activation of peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α and controlling the disposal of damaged mitochondria via autophagy (38). Autophagy acts as a survival mechanism under conditions of stress, maintaining cellular integrity by regenerating metabolic precursors and clearing subcellular debris (39). Several studies have demonstrated that the activation of AMPK signaling declines with aging, which impairs the maintenance of efficient cellular homeostasis (45, 58). The precise role that AMPK plays in cardiac injury during sepsis remains undefined.
In a previous experimental study (20), we demonstrated that AMPK-dependent metabolic mechanisms become dysfunctional with aging and correlate with liver injury in mature adult male mice compared with young mice after sepsis. Similarly, we demonstrated that age-dependent AMPK dysregulation may play a role in myocardial and lung injury in mature adult male mice after hemorrhagic shock (25, 36, 37). To further understand the underlying age-dependent molecular mechanisms of cardiovascular function during sepsis, the purpose of the present study was to investigate whether changes of the AMPK signaling pathway would correlate with the hemodynamic compromise and systemic inflammatory response in a clinically relevant model of polymicrobial sepsis in young (2–3 mo old) and middle-aged mature adult (11–13 mo old) mice. Additionally, we wanted to determine whether pharmacological treatment with the AMP analog 5-amino-4-imidazole carboxamide riboside (AICAR) (13) would affect AMPK signaling pathway and, consequently, ameliorate cardiac injury.
MATERIALS AND METHODS
Murine model of polymicrobial sepsis.
The investigation conformed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and commenced with the approval of the Institutional Animal Care and Use Committee of the Cincinnati Children’s Hospital Medical Center. C57BL/6 male young (2–3 mo) and male mature adult (11–13 mo) mice were obtained from Charles River Laboratories (Wilmington, MA) and were acclimatized for at least 48 h. Male mice only were chosen for the experimentation to avoid interference from female hormonal fluctuations in sepsis responses during the estrous cycle in the young groups and during the decline of fertility in the mature groups (2). All mice were allowed free access to water and a maintenance diet on a 12:12-h light-dark cycle with room temperature at 21 ± 2°C. Polymicrobial sepsis was induced by cecal ligation and puncture (CLP), a model that closely mimics the pathophysiology of human sepsis, with positive bacterial cultures already detectable at 6 h after the procedure and early lethality starting at 18–24 h after the procedure (20, 47). Briefly, mice were anesthetized intraperitoneally with pentobarbital sodium (40 mg/kg ip). The body weight of each mouse was recorded before the CLP procedure. After the abdomen had been opened, the cecum was exteriorized, ligated at its base without obstructing intestinal continuity, punctured twice with a 22-gauge needle, and squeezed to allow excretion of a small amount of fecal material into the peritoneum. The cecum was then returned into the peritoneal cavity, and the abdominal incision was closed. After the procedure, mice of both age groups were randomly assigned to two treatment regimens: the vehicle-treated group received distilled water (200 µl/mouse ip) and the AICAR-treated group received the AMPK activator AICAR (500 mg/kg ip) at 1 and 6 h after CLP. All groups of mice also received fluid resuscitation (35 ml/kg normal saline with 5% dextrose subcutaneously) immediately after and at 6 h after the CLP procedure. In a separate study, the effects of AICAR or vehicle were examined in control mice, which were treated with the same dosage and at the same time interval as mice in the CLP groups. Mice were euthanized 18 h after surgery or sham procedure. Blood and cardiac tissue were collected for analysis.
Echocardiographic assessment of left ventricular structure and function.
Cardiac function was assessed by echocardiography as previously described, using a VisualSonics 2100 system equipped with a 30 MHz transducer (1). Echocardiographic measurements were obtained 6 h before and 18 h after CLP. Mice were lightly anesthetized with inhaled 1% isoflurane. Heart rate (HR), ejection fraction (EF), fractional shortening (FS), left ventricular (LV) internal dimensions, including end-diastolic and end-systolic dimensions (LVIDd and LVIDs, respectively), interventricular septal thickness at diastole and systole (IVSd and IVSs, respectively), and LV posterior wall thickness at diastole and systole (LVPWd and LVPWs, respectively) were measured by M-mode. Ventricular volumes were calculated using M-mode measurement, stroke volume (SV) was calculated by subtracting end-systolic LV volume from end-diastolic volume, and cardiac output (CO) was calculated as the product of SV × HR.
Histological evaluation of the myocardium.
For histological evaluation, heart tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and then stained with hematoxylin and eosin. Samples were evaluated by four independent observers. Myocardial injury was determined by a semiquantitative score based on the following histological features: presence of edema, infiltration of red blood and inflammatory cells, and myofibril derangement. A score of 0 represented normal findings, and scores of 1, 2, 3, and 4 represented minimal (<25% tissue involvement), mild (25–50% tissue involvement), significant (50–75% tissue involvement), and severe (>75% tissue involvement) injury, respectively. The three variables were summed to represent the cardiac injury score (total score, 0–12).
Transmission electron microscopy.
Heart samples were fixed in 3% glutaraldehyde, postfixed in 1% osmium tetroxide in sodium phosphate buffer, and cut with an ultramicrotome. Samples were stained with 2% uranyl acetate and lead citrate. Sections were viewed and photographed on a Hitachi H-7650 transmission electron microscope (TEM) at 120 kV. The total number of mitochondria and autophagosomes and the presence of elongated or abnormal mitochondria with loose matrix and fragmented cristae and membranes were determined in blinded fashion in nine adjacent cells in three different sections for each animal by National Institutes of Health ImageJ analysis (50).
Plasma levels of cytokines and cardiovascular injury markers.
Using a multiplex array system (Milliplex, Millipore, Billerica, MA), plasma levels of IL-1β, IL-6, IL-10, IL-17F, IL-27, and TNF-α were measured as indexes of the systemic inflammatory response, whereas plasma levels of troponin I, troponin T, and follistatin were measured as indexes of cardiac injury.
Subcellular fractionation and nuclear protein extraction.
Heart samples were homogenized using a Polytron homogenizer in a buffer containing 0.32 M sucrose, 10 mM Tris·HCl (pH 7.4), 1 mM EGTA, 2 mM EDTA, 5 mM NaN3, 10 mM β-mercaptoethanol, 20 µM leupeptin, 0.15 µM pepstatin A, 0.2 mM phenylmethanesulfonyl fluoride, 50 mM NaF, 1 mM sodium orthovanadate, and 0.4 nM microcystin. Homogenates were centrifuged at 1,000 g for 10 min at 4°C, and the supernatants were collected as cytosol extracts. The pellets were then solubilized in Triton buffer (1% Triton X-100, 250 mM NaCl, 50 mM Tris·HCl at pH 7.5, 3 mM EGTA, 3 mM EDTA, 0.1 mM phenylmethanesulfonyl fluoride, 0.1 mM sodium orthovanadate, 10% glycerol, 2 mM p-nitrophenyl phosphate, 0.5% Nonidet P-40, and 46 µM aprotinin). The lysates were centrifuged at 15,000 g for 30 min at 4°C, and the supernatant was collected as nuclear extracts.
Western blot analysis.
Immunoblot analyses were used to quantify cytosol and nuclear content of AMPK-α1/α2 as well as the phosphorylated active forms p-AMPKα1/α2 and PGC-1α. Cytosol and nuclear extracts were boiled in equal volumes of NuPAGE LDS Sample Buffer (4×), and 25–40 μg of protein were loaded per lane on a 16% Tris-glycine gradient gel. Proteins were separated electrophoretically and transferred to nitrocellulose membranes. For immunoblot analysis of PGC-1α, membranes were blocked with 5% nonfat dried milk in Tris-buffered saline (TBS) and probed with primary antibodies. Membranes were washed in TBS with 0.1% Tween 20 and incubated with secondary peroxidase-conjugated antibody. Membranes were also reprobed with primary antibody against β-actin to ensure equal loading of samples. Immunoreaction was visualized by chemiluminescence. Densitometric analysis was performed using Quantity One (Bio-Rad Laboratories, Des Plaines, IL), and fold changes of relative intensity of PGC-1α were calculated versus the value of young control mice, which was set to 1 upon data normalization with β-actin. For immunoblot analysis of AMPK-α1/α2 and p-AMPK-α1/α2, membranes were blocked with Odyssey blocking buffer and incubated with specific primary antibodies; β-actin was concomitantly probed as a loading control. Membranes were washed in PBS with 0.1% Tween 20 and incubated with LI-COR secondary antibodies. The immunoreaction was detected by near-infrared fluorescence. The Odyssey LI-COR scanner and software (LI-COR Biotechnology, Lincoln, NE) were used for detection and densitometric analysis of blots. Fold changes of relative intensity of both detected bands of AMPK-α1/α2 and p-AMPK-α1/α2 were calculated versus the value of young control mice, with was set to 1 upon data normalization with β-actin.
Materials.
AICAR was obtained from LC Laboratories (Woburn, MA) and was reconstituted in sterile distilled water. The primary antibodies for AMPK-α1/α2 and p-AMPK-α1/α2 were obtained from Cell Signaling Technology (Danvers, MA). The primary antibody for PGC-1α was obtained from Abcam (Cambridge, MA). The primary antibody for β-actin and secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The Odyssey blocking and loading buffers and LI-COR secondary antibodies were obtained from LI-COR Biotechnology. All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Statistical analysis.
Statistical analysis was performed using SigmaPlot 13.0 (Systat Software, San Jose, CA). All values in the figures and text are expressed as medians with 25th and 75th percentiles or means ± SE; n = 4–10 animals/group. For multiple group analysis at a single time point, one-way ANOVA with the Student-Newman-Keuls correction was used. If data failed to follow a normal distribution, a Mann-Whitney rank-sum test or an ANOVA on ranks test was performed. P values of <0.05 were considered significant.
RESULTS
Age-dependent effect of AICAR on cardiac dysfunction during sepsis.
To mimic the acute onset of organ damage of clinical sepsis, we used a severe model of CLP (20, 47). With this procedure, the onset of clinical symptoms, such as piloerection and inactivity, were observed early by 6–12 h in both young and mature mice. To characterize the cardiac damage produced by the infection, we performed echocardiographic analysis (Table 1). Vehicle-treated young mice exhibited a significant decrease in systolic function, as evidenced by lower FS and EF at 18 h after CLP, compared with baseline values before the CLP procedure. This dysfunction was also associated with a significant bradycardia and decrease in CO. In addition to systolic dysfunction, vehicle-treated young mice also experienced a significant LVIDd and SV decrease. Treatment with AICAR restored systolic and diastolic parameters in young mice after sepsis to values like baseline functions but did not affect HR or CO. Interestingly, mature mice exhibited a significant lower systolic function already at baseline before sepsis compared with baseline values of young mice. In contrast to the systolic dysfunction observed in vehicle-treated young mice, vehicle-treated mature mice did not experience changes in FS or EF after sepsis. However, vehicle-treated mature mice had a significant LVIDd and SV decline after sepsis. Treatment with AICAR did not modify these echocardiographic parameters in mature mice but caused a reduction of HR or CO compared with vehicle treatment.
Table 1.
Echocardiographic measurements of LV function and volumes
| Vehicle |
5-Amino-4-Imidazole Carboxamide Riboside |
|||
|---|---|---|---|---|
| Echocardiographic Parameters and Units | Baseline | After sepsis | Baseline | After sepsis |
| Young group | ||||
| Ejection fraction, % | 60.9 ± 2.6 | 38.5 ± 10.0* | 57.9 ± 3.0 | 54.9 ± 5.2 |
| Fractional shortening, % | 32.3 ± 1.8 | 19.3 ± 6.0* | 30.5 ± 2.0 | 28.2 ± 3.4 |
| IVSd, mm | 0.94 ± 0.07 | 1.20 ± 0.13* | 0.90 ± 0.07 | 1.01 ± 0.05 |
| IVSs, mm | 1.45 ± 0.03 | 1.39 ± 0.12 | 1.34 ± 0.07 | 1.49 ± 0.06 |
| LVIDd, mm | 4.05 ± 0.17 | 3.15 ± 0.24* | 4.33 ± 0.08 | 3.67 ± 0.25* |
| LVIDs, mm | 2.75 ± 0.16 | 2.54 ± 0.29 | 2.95 ± 0.13 | 2.62 ± 0.17 |
| LVPWd, mm | 0.94 ± 0.09 | 1.03 ± 0.15 | 0.85 ± 0.03 | 0.98 ± 0.05 |
| LVPWs, mm | 1.31 ± 0.07 | 1.32 ± 0.15 | 1.20 ± 0.05 | 1.34 ± 0.08 |
| LV Vol d, µl | 73.4 ± 7.1 | 41.2 ± 6.7* | 84.7 ± 3.9 | 58.6 ± 10.3* |
| LV Vol s, µl | 29.3 ± 3.9 | 25.9 ± 6.6 | 35.9 ± 3.6 | 25.7 ± 3.7 |
| SV, µl | 44.1 ± 3.8 | 15.3 ± 4.6* | 48.8 ± 2.6 | 32.8 ± 6.7*† |
| HR, beats/min | 436 ± 18 | 255 ± 38* | 494 ± 23 | 266 ± 45* |
| CO, ml/min | 19 ± 2 | 5 ± 2* | 24 ± 2 | 10 ± 4* |
| Body weight, g | 29.3 ± 1.5 | ND | 29.8 ± 1.1 | ND |
| Mature group | ||||
| Ejection fraction, % | 48.8 ± 2.6‡ | 49.9 ± 6.8 | 49.5 ± 2.0‡ | 38.6 ± 6.2‡ |
| Fractional shortening, % | 24.6 ± 1.5‡ | 25.9 ± 4.3 | 24.5 ± 1.1‡ | 18.9 ± 3.5‡ |
| IVSd, mm | 0.93 ± 0.05 | 1.03 ± 0.06 | 0.94 ± 0.04 | 1.13 ± 0.09* |
| IVSs, mm | 1.41 ± 0.06 | 1.32 ± 0.10 | 1.40 ± 0.04 | 1.51 ± 0.08 |
| LVIDd, mm | 4.31 ± 0.11 | 3.70 ± 0.30* | 4.34 ± 0.14 | 3.61 ± 0.31* |
| LVIDs, mm | 3.29 ± 0.13‡ | 2.87 ± 0.32 | 3.26 ± 0.12 | 2.94 ± 0.31 |
| LVPWd, mm | 0.89 ± 0.04 | 0.99 ± 0.07 | 0.90 ± 0.01 | 1.06 ± 0.07 |
| LVPWs, mm | 1.22 ± 0.07 | 1.24 ± 0.07 | 1.26 ± 0.04 | 1.29 ± 0.07 |
| LV Vol d, µl | 87.0 ± 5.8 | 62.1 ± 11.5* | 85.9 ± 6.6 | 58.4 ± 12.1* |
| LV Vol s, µl | 45.8 ± 4.9 | 33.1 ± 8.9 | 43.5 ± 4.1 | 36.9 ± 9.2 |
| SV, µl | 41.2 ± 2.4 | 29.0 ± 6.1* | 42.4 ± 3.2 | 21.5 ± 4.8* |
| HR, beats/min | 472 ± 17 | 402 ± 43 | 486 ± 29 | 275 ± 43*† |
| CO, ml/min | 20 ± 2 | 14 ± 3 | 20 ± 2 | 6 ± 4*† |
| Body weight, g | 35.2 ± 1.0 | ND | 35.1 ± 1.3 | ND |
Data are means ± SE of 8 mice/group. IVSd and IVSs, intraventricular septum dimension at diastole and systole; LVIDd and LVIDs, left ventricular (LV) internal dimensions at diastole and systole; LVPWd, LV posterior wall dimension at diastole and systole; LV Vol d and LV Vol s, LV volume at diastole and systole; SV, stroke volume; HR, heart rate; CO, cardiac output; ND, not determined. The body weight of each mouse was recorded before cardiac examination and before the cecal ligation and puncture procedure.
P < 0.05 vs. baseline of the same age group;
P < 0.05 vs. sepsis with vehicle treatment of the same age group;
P < 0.05 vs. young mice as determined by ANOVA with Student-Newman-Keuls correction.
Age-dependent cardiac pathological changes.
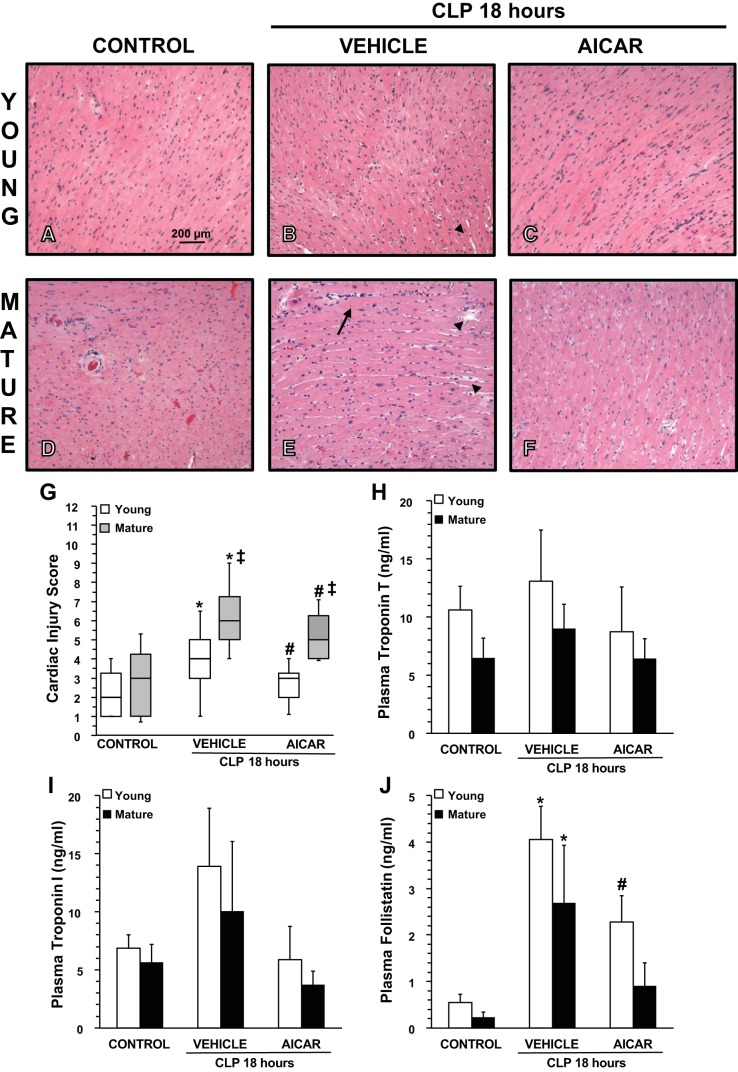
At 18 h after CLP, histological analysis revealed that vehicle-treated mature mice had more severe myocardial injury, which was characterized by perivascular and intercellular edema and a remarkable vascular engulfment of neutrophils compared with young mice (Fig. 1, A–G). To confirm the degree of cardiac injury, cardiovascular injury markers were measured. Plasma levels of troponin I and troponin T were slightly, but not significantly, increased after sepsis in mice in both vehicle-treated age groups compared with age-matched control mice (Fig. 1, H–I). Plasma levels of the myokine follistatin were significantly increased in both vehicle-treated young and mature adult mice compared with age-matched control mice (Fig. 1J). Treatment with AICAR significantly ameliorated myocardial damage as evidenced by histological analysis in both young and mature mice, which also exhibited troponin I and troponin T levels similar to baseline values. Furthermore, after treatment with AICAR, plasma levels of follistatin were significantly reduced in young mice, whereas they returned to baseline levels in mature mice compared with vehicle treatment (Fig. 1).
Fig. 1.
A–F: representative histology photomicrographs of heart sections. CLP, cecal ligation and puncture. A and D: normal myocardium architecture in control young (A) and mature (D) mice. Myocardial damage in vehicle-treated young male mice was characterized by mild endocardial edema (arrowhead) at 18 h after sepsis (B). Myocardial damage in vehicle-treated mature mice was characterized by severe endocardial edema (arrowheads) and engulfment of inflammatory cells (arrows) at 18 h after sepsis (E). C and F: significant amelioration of the heart architecture in 5-amino-4-imidazole carboxamide riboside (AICAR)-treated young (C) and mature (F) mice, which, however, still exhibited some mild edema. Magnification: ×100. G: histopathological scores of heart sections (n = 5 mice/group). Myocardial injury was scored from 0 (no damage) to 12 (maximum damage). Box plots represent 25th percentile, median, and 75th percentile; error bars define 10th and 90th percentiles. *P < 0.05 vs. age-matched control mice; #P < 0.05 vs. age-matched mice treated with vehicle; ‡P < 0.05 vs. young mice as determined by a Mann-Whitney rank-sum test. H–J: plasma levels of troponin T (H), troponin I (I), and follistatin (J). Data represent means ± SE of 5 control mice and 6 vehicle- or AICAR-treated mice for each age group. *P < 0.05 vs. age-matched control mice; #P < 0.05 vs. age-matched mice treated with vehicle; ‡P < 0.05 vs. young mice as determined by ANOVA with Student-Newman-Keuls correction.
Age-dependent changes of the myocardium mitochondrial ultrastructure in sepsis.
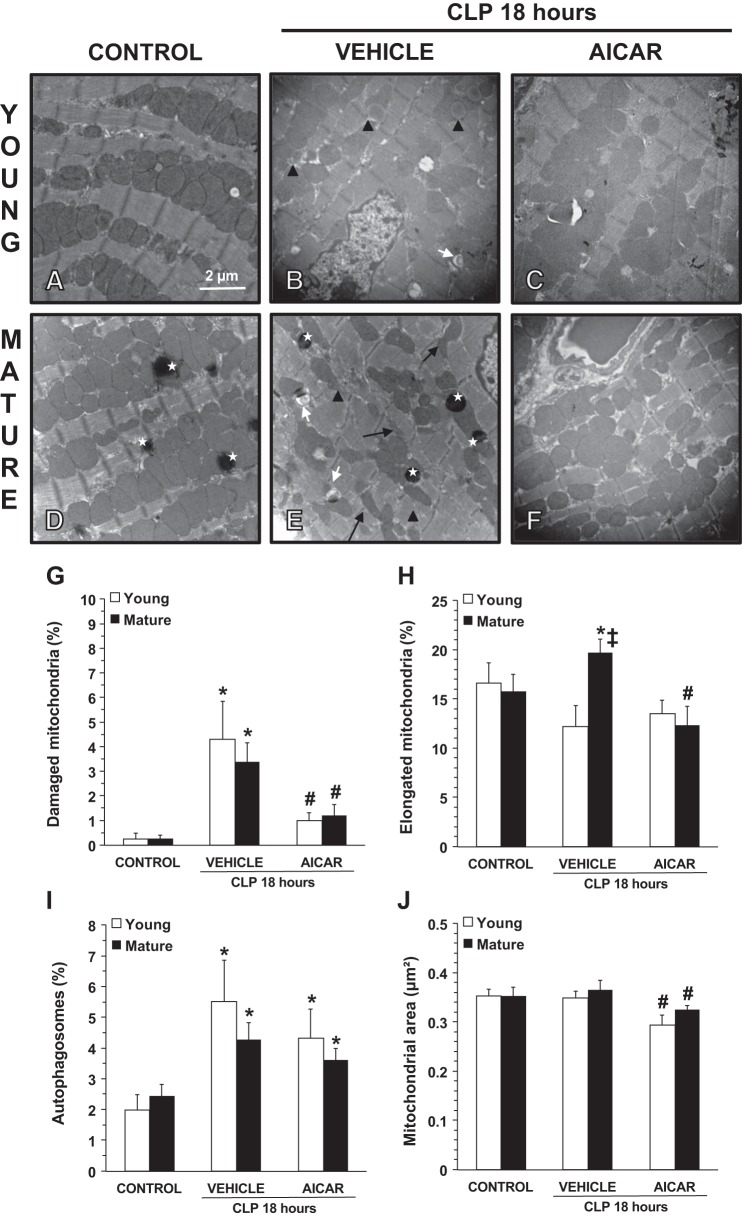
To further assess the effect of sepsis on the myocardium, TEM was performed to evaluate structural changes (Fig. 2). At 18 h after CLP, examination of cardiomyocytes of vehicle-treated mice of both age groups demonstrated loss of sarcomere structural integrity and alterations of mitochondria, characterized by formation of internal vesicles, disruption of cristae, and clearer matrix. In vehicle-treated mature adult mice, TEM analysis also revealed a higher number of thin and elongated mitochondria compared with younger mice. Cardiomyocytes of control young and mature mice displayed a similar number of autophagosomes. At 18 h after CLP, vehicle-treated mice of both age groups exhibited a significant increase in autophagosome formation compared with age-matched control mice. In AICAR-treated mice, integrity of sarcomeres and mitochondria was well maintained in both young and middle-aged mature mice. Furthermore, in AICAR-treated mice of both age groups mitochondria with fusion/fission-associated morphology and of smaller size were observed, suggesting active processes of mitochondrial regeneration. Treatment with AICAR did not affect autophagosome formation in either age group (Fig. 2).
Fig. 2.
A–F: transmission electronic microscopy of cardiac cells. CLP, cecal ligation and puncture. A and D: normal cellular structure in control young (A) and mature (D) mice. B and E: structural changes in vehicle-treated young (B) and mature (E) mice at 18 h after sepsis. C and F: amelioration of mitochondrial damage in 5-amino-4-imidazole carboxamide riboside (AICAR)-treated young (C) and mature (F) mice with normal electron dense mitochondria. *Lysosomes. Black arrows show thin and elongated mitochondria. Black arrowheads show damaged mitochondria presenting translucent matrix, disrupted membrane, and cristae. White arrows show autophagic vesicles. G–J: quantification of damaged mitochondria (G), elongated mitochondria (H), autophagosomes (I), and average mitochondrial area (J) in cardiac cells as determined using NIH ImageJ software. Damaged and elongated mitochondria and autophagosomes were determined as percent total numbers of mitochondria. Data are expressed as means ± SE; n = 4 mice/group. *P < 0.05 vs. age-matched control mice; #P < 0.05 vs. age-matched mice treated with vehicle; ‡P < 0.05 vs. young mice as determined by ANOVA with Student-Newman-Keuls correction.
Age-dependent effect of AICAR on plasma levels of inflammatory cytokines.
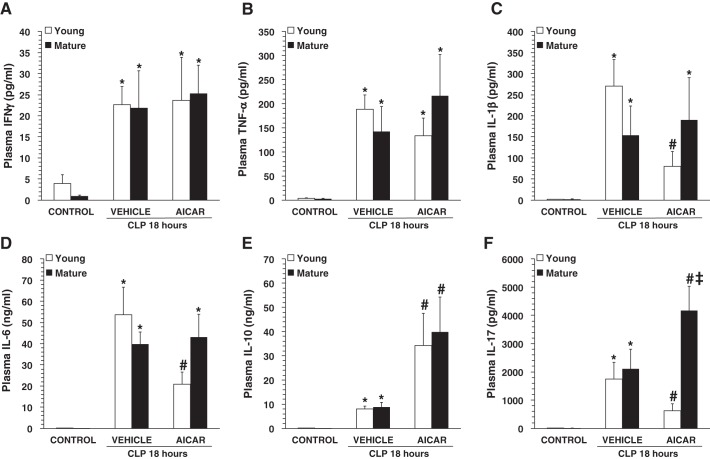
To assess the extent of the systemic inflammatory response, a panel of T helper (Th)1/Th2/Th17 cytokines was measured (Fig. 3). At 18 h after CLP, plasma levels of interferon-γ, TNF-α, IL-1β, IL-6, IL-10, and IL-17 were significantly increased in both vehicle-treated young and mature adult mice compared with age-matched control mice. AICAR treatment did not affect plasma levels of interferon-γ or TNF-α in either age group (Fig. 3, A and B). Interestingly, AICAR treatment significantly decreased levels of IL-1β and IL-6 in young but not mature mice (Fig. 3, C and D). In contrast, AICAR significantly increased plasma levels of IL-10 in both age groups compared with vehicle treatment (Fig. 3E). AICAR treatment showed age-dependent effects on plasma levels of IL-17, with a significant decrease in young mice and a significant increase in mature mice compared with vehicle treatment (Fig. 3).
Fig. 3.
Plasma levels of interferon (IFN)-γ (A), TNF-α (B), IL-1β (C), IL-6 (D), IL-10 (D), and IL-17 (F). CLP, cecal ligation and puncture. Data represent means ± SE of 9 control mice and 10 vehicle- or 5-amino-4-imidazole carboxamide riboside (AICAR)-treated mice for each age group. *P < 0.05 vs. baseline values of age-matched control mice; #P < 0.05 vs. age-matched mice treated with vehicle; ‡P < 0.05 vs. young mice as determined by ANOVA with Student-Newman-Keuls correction.
Age-dependent effect of AICAR on AMPK cellular localization and activation.
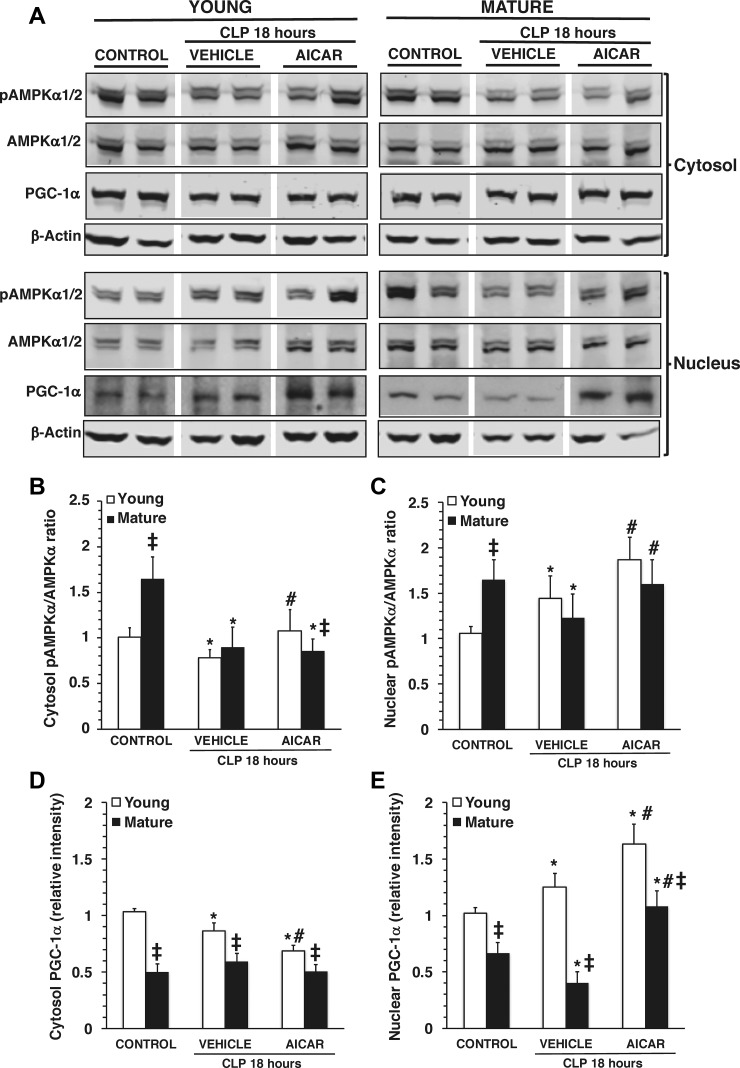
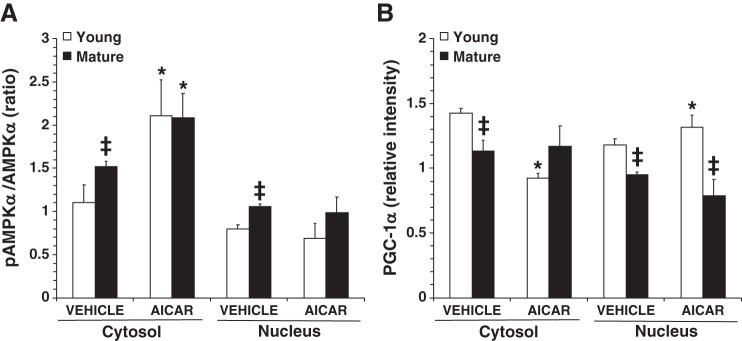
Because of the central role of AMPK in mitochondrial quality control, we measured the activation of the catalytic α1- and α2-subunits, both of which are present in the heart (Fig. 4) (24). In vehicle-treated young mice, p-AMPK-α1/α2 expression was significantly reduced in the cytosol, whereas it was increased in the nuclear compartment at 18 h after CLP compared with age-matched control mice, thus suggesting the occurrence of nuclear translocation. Interestingly, there was marked p-AMPK-α1/α2 expression in control mature mice at basal conditions compared with young control mice. Despite this high basal level of p-AMPK-α1/α2, vehicle-treated mature mice experienced a marked downregulation of AMPK activation in both cytosol and the nucleus at 18 h after CLP compared with age-matched control mice, thus suggesting impairment to promote metabolic recovery after stress (Fig. 4). AICAR treatment had age-dependent effects on AMPK cellular localization and activation that were significantly increased in both cytosol and nucleus in young mice but were increased only in the nucleus in mature mice compared with vehicle treatment (Fig. 4). In a separate study, the effect of this therapeutic dose was also examined in control young and mature mice under normal conditions only. Treatment with AICAR was able to increase the activation of AMPK in the cytosol only, not in the nucleus, in both age groups (Fig. 5).
Fig. 4.
A: representative Western blot analysis of phosphorylated (p)AMP-activated protein kinase-α (AMPK-α), AMPK-α, peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α, and β-actin (used as loading control protein) in heart cytosol and nuclear extracts. CLP, cecal ligation and puncture. Images were converted and adjusted in grayscale after detection by near-infrared fluorescence or chemiluminescence. B and C: image analyses of the pAMPK-α-to-AMPK-α ratio as determined by densitometry in the cytosol (B) and in the nucleus (C). D and E: image analyses of PGC-1α expression as determined by densitometry in the cytosol (D) and in the nucleus (E). Data are means ± SE of 6 control mice and 8 vehicle- or 5-amino-4-imidazole carboxamide riboside (AICAR)-treated mice for each age group and are expressed as relative intensity units. *P < 0.05 vs. baseline values of age-matched control mice; #P < 0.05 vs. age-matched mice treated with vehicle; ‡P < 0.05 vs. young mice as determined by ANOVA on ranks test.
Fig. 5.
Image analyses of the phosphorylated (p)AMP-activated protein kinase-α (AMPK-α)-to-AMPK-α ratio (A) and peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α expression (B) as determined by densitometry in the cytosol and in the nucleus of hearts of control mice under basal conditions. AICAR, 5-amino-4-imidazole carboxamide riboside. Data represent means ± SE of 4 mice/group and are expressed as relative intensity units. *P < 0.05 vs. baseline values of age-matched control mice; #P < 0.05 vs. age-matched mice treated with vehicle; ‡P < 0.05 vs. young mice as determined by ANOVA on ranks test.
Age-dependent effect of AICAR on PGC-1α nuclear expression.
Since AMPK regulates mitochondrial biogenesis through activation of PGC-1α (38), we assessed the intracellular expression of this transcription cofactor. In young mice, cytosolic levels of PGC-1α decreased at 18 h after CLP and further decreased with AICAR administration. These events paralleled the increase of nuclear levels of PGC-1α after CLP and further significant increased with AICAR administration, thus suggesting nuclear translocation of this cofactor. Interestingly, in mature mice, cytosol levels of PGC-1α were unaffected by sepsis regardless of whether vehicle or AICAR was administered. However, in mature mice, nuclear levels of PGC-1α decreased with sepsis, a change that was reversed by AICAR treatment (Fig. 4). It is important to note that under all conditions cytosol and nuclear levels of PGC-1α were significantly lower in mature mice than in young mice (Fig. 4). Furthermore, when AICAR was administered to normal control mice, it induced nuclear translocation of PGC-1α in young mice only and had no influence on the levels of PGC-1α protein in mature mice (Fig. 5).
DISCUSSION
Age is known to be a significant risk factor for the development of multiple organ failure and mortality in adult septic patients. While the exact mechanism of organ failure is yet to be understood, loss of metabolic capacity is likely a major pathophysiological mechanism (5, 15, 48).
We (20) have previously reported in a murine model of sepsis that the degree of severity and the pathophysiological characteristics of liver injury are both age dependent and associated with heightened mitochondrial dysfunction. We also observed that this liver metabolic derangement occurred during the early phase of sepsis, i.e., at 6 h, and further increased at 18 h after the CLP procedure in mature mice compared with young mice. In this study, we extended our investigation to cardiac injury and demonstrated that age also influences the hemodynamic response in sepsis. Specifically, despite the presence of a similar systemic inflammatory response, pathological characteristics of sepsis-induced cardiac damage and function were different between young and mature adult mice. Compared with young mice, mature adult mice exhibited an exuberant neutrophil infiltration and interstitial edema associated with greater mitochondrial structural derangement in cardiomyocytes. We also demonstrated that treatment with the AMPK activator AICAR improved cardiomyocyte ultrastructure in both young and mature adult mice but systolic function was improved only in young AICAR-treated mice.
Cardiac dysfunction is a well-recognized cardiovascular complication in sepsis, characterized by a reversible decrease in systolic and/or diastolic LV function (41, 59). Various mechanisms, including mitochondrial dysfunction, have been proposed to explain septic myocardial depression (48). It has been speculated that during severe sepsis cellular oxygen consumption is lower due to mitochondrial dysfunction and that adequate early maintenance or subsequent restoration of mitochondrial function plays an important role in recovery from severe sepsis (7, 16, 26). Myocardial mitochondrial damage and dysfunction during endotoxemia or CLP-induced sepsis have been demonstrated in a number of animal studies (22, 28, 32, 55). In humans, examination of postmortem hearts from septic patients identified abnormalities of mitochondrial structures in cardiomyocytes, but cardiomyocyte cell death was rarely observed despite the magnitude of organ dysfunction (56). In our study, both age groups had similar negligible degrees of myocyte degeneration after sepsis, as evidenced by the slight, but not significant, plasma increase in the biomarkers of cardiomyocyte damage, cardiac troponin I and T. Furthermore, all experimental groups experienced a similar cardiac and systemic inflammation, as confirmed by similar changes in plasma levels of cytokines of the Th1/Th2/Th17 immune response and levels of follistatin, a cardiokine induced in response to ischemic insults (52). Despite this, at the tissue level, septic vehicle-treated mature mice exhibited greater edema and neutrophil infiltration. Importantly, treatment with AICAR significantly reduced the histologically evident cardiac damage in both age groups of mice but was associated with improved systolic function in young mice only.
At the ultrastructural level, whereas there was no age-related difference in the number of swollen mitochondria with poorly arranged cristae, vehicle-treated mature adult mice exhibited a higher number of elongated organelles compared with young mice, suggesting an impairment of mitochondrial dynamics. Using cellular models, other studies have suggested that impairment of fusion/fission dynamics results in mitochondrial heterogeneity and decreased rates of respiration (8, 27). For example, decreased fission can generate long and highly interconnected mitochondria (51). Such elongation has been suggested to account for the occurrence of hyperfused dysfunctional mitochondria in aging muscles (35) and has been associated with increased cellular senescence (29).
At functional analysis, the cardiac damage in vehicle-treated young mice correlated with LV dysfunction, as evidenced by a decrease in load-dependent indexes (EF and FS) at 18 h after CLP compared with baseline conditions. Surprisingly, EF and FS, while exhibiting an age-associated decline of the cardiovascular capacity at baseline conditions, were unchanged in vehicle-treated mature mice during sepsis. This result was a contradiction between an apparently normal LV function and the several pieces of evidence of myocardial damage and mitochondrial derangement in aged mice. However, such conflicting results have also been reported in clinical studies. LV systolic dysfunction is common in septic patients and potentially reversible in survivors. Paradoxically, some clinical studies have reported more cardiac depression in sepsis survivors than in nonsurvivors (21, 41). Other studies have reported no significant difference in mortality rates between septic patients with reduced EF compared with patients with normal EF (49). A likely explanation of this phenomenon is that the presence of profound myocardial depression defined by a low EF may represent preload optimization and adaptation, whereas a normal EF could be caused by persistent preload deficiency and/or ongoing harmful adrenergic stimulation (23). In our study, we also noted that, after CLP, vehicle-treated young but not mature mice exhibited bradycardia and low CO, which persisted even with AICAR treatment. Interestingly, AICAR treatment of mature mice induced a reduction in HR and CO. Although we did not determine the molecular mechanisms contributing to the bradycardia, a recent study has demonstrated that AMPK activation, through the γ2-subunit, is a crucial regulator of intrinsic HR during high energy demand, and this event may represent an adaptive event to stress or exercise to maintain cardiac energy homeostasis (60). Therefore, it is plausible that during sepsis young mice have the capability to mitigate cardiac energy expenditure through activity of AMPK. This important energy-sparing event may be less efficient with age. Nevertheless, pharmacological activation with AICAR in mature mice was effective in inducing a bradycardic response. Our findings are also consistent with previous studies demonstrating that Gram-positive or Gram-negative bacteria injection in young mice can induce bradycardia (17). It is important to note that clinical studies in a large cohort of patients with septic shock reported that relative bradycardia was associated with significantly higher survival, even after control for multiple covariates (4).
However, as a limitation of our study, we relied on echocardiographic parameters of load-dependent indexes, which may not reflect the intrinsic myocardial contractility function during sepsis. Recently, Hestenes et al. (19) reported that LV longitudinal strain was significantly declined due to reduced afterload while LV EF remained unaltered in Escherichia coli-induced sepsis in a pig model. Similarly, circumferential and radial strain declined after endotoxin-induced shock in mice and was consistent with myocardial damage, as indicated by serum and histological analyses, contrary to time-dependent and variable changes of EF as evaluated by conventional echocardiography (11). Of clinical importance, treatment with AICAR provided significant improvement of the LV function in young mice. However, despite providing amelioration of tissue damage and better preservation of mitochondria structure and morphology, we did not observe changes in conventional echocardiographic parameters in AICAR-treated mature adult mice. Our current findings are in agreement with our previous reports demonstrating that AICAR treatment did not result in improvement of cardiovascular parameters during experimental hemorrhagic shock in mature adult mice compared with young animals, even in the presence of beneficial effects in lung and cardiac tissue at histology (25, 36).
We investigated the molecular mechanisms of mitochondrial organization changes and the potential mechanisms of action of AICAR by analyzing the contribution of the AMPK signaling pathway. AMPK is a trimeric molecule consisting of one catalytic α-subunit and two regulatory β- and γ-subunits. Phosphorylation of the catalytic α-subunit by upstream kinases is essential for AMPK activation. Although the kinase is ubiquitously expressed, different catalytic isoforms (α1 and α2) display tissue-specific distribution. In the heart, both the catalytic α1- and α2-isoforms are expressed, with high prevalence of the α2-isoform (3, 24). Here, we demonstrated, for the first time, that phosphorylation of the AMPK α-isoform has a distinct age-dependent cellular localization during sepsis. In young mice, activation of AMPK-α decreased in the cytosol while increasing in the nuclear compartment. This event well paralleled the nuclear translocation of PGC-1α, thus raising the possibility that AMPK might regulate gene expression in response to cellular stresses. Interestingly, compared with young mice, baseline levels of phosphorylated AMPK were higher in mature hearts and were not responsive to sepsis-induced stress. This is consistent with previous findings of multiorgan (e.g., heart, liver, or brain) baseline elevation of p-AMPK, which is not further elevated in response to hemorrhagic shock, hypoxia, or stroke (33, 36, 40). This baseline increase in AMPK activity with aging may reflect an adaptive mechanism to chronic age-related oxidative damage but consequently resulting in an inability to respond to acute stressors. In our study, the impairment of AMPK-α activation was associated with downregulation of nuclear PGC-1α expression in vehicle-treated mature adult animals compared with young animals. This might explain why vehicle-treated mature adult animals also exhibited cardiomyocyte mitochondrial heterogeneity compared with young animals. In line with this age-dependent dysregulation of AMPK cellular distribution and activation, treatment with AICAR activated the p-AMPK-α/PGC-1α axis in mature adult mice to a lesser degree than mice in the young group and was unable to improve cardiac function. It is also important to note that in our study the therapeutic dose of AICAR had different molecular effects in healthy conditions than in disease conditions, as AICAR treatment was able to increase activation of AMPK in the cytosol only, not in the nucleus, in both age groups under normal conditions. Furthermore, AICAR treatment in normal mice induced nuclear translocation of PGC-1α in young mice only, failing to increase nuclear levels of PGC-1α protein in mature mice. Overall, these results further confirmed that altered basal AMPK expression in aging renders tissues less sensitive to changes in cellular redox balance and consequently impairs the ability to counteract metabolic disturbances. We cannot exclude, however, that the lack of a full effect of AICAR in aging mice may also be due, in part, to inefficient dosage of the drug. Nevertheless, it should be noted that the dosage chosen for our study (500 mg/kg) has been reported to be efficacious in improving skeletal muscle function during exercise (43) and in improving systolic and diastolic function after myocardial infarction (12) in aging mouse models. On the contrary, we refrained from using a higher dosage of the drug to avoid toxic effects. In this regard, it has been shown that AICAR at 1000 mg/kg in mice is accompanied by hypoglycemia and elevated blood lactate levels (54), which could pose a risk of aggravating dysmetabolism in our model of sepsis.
Another plausible mechanism of AICAR in attenuating cardiac dysfunction in young mice may be secondary to an anti-inflammatory effect. Among proinflammatory cytokines, IL-1β and IL-6 have been implicated in the pathogenesis of myocardial dysfunction in sepsis (6, 42, 57). In vitro experiments have demonstrated that AICAR diminishes nuclear translocation of NF-κB and reduces release of proinflammatory cytokines (18, 61). In our study, we demonstrated that AICAR treatment significantly decreased plasma levels of IL-1 β, IL-6, and IL-17 in young mice only. Thus, our data suggest that AMPK may also be involved in the regulation of age-dependent immune responses.
Another downstream pathway of AMPK activation is autophagy via inhibition of mammalian target of rapamycin signaling. Autophagy is a cellular process that degrades damaged mitochondria and other organelles (10). In our TEM analysis, we observed an increase of the number of the autophagic vacuoles after sepsis in both young and mature animals, thus suggesting the capability to mount an authophagic event in response to cellular stress. AICAR treatment did not affect the number of authophagosomes in either age group. Although autophagy has been shown to decline in the heart with age (30), our data suggest that in mature middle-aged animals autophagy is maintained and may be also regulated by AMPK-independent mechanisms, given the lack of response to AICAR. This important aspect of metabolic recovery and autophagic function needs further investigation.
In conclusion, we have demonstrated that AMPK-dependent metabolic recovery pathways become active during sepsis in young mice and that the capacity for metabolic recovery is lost in mature mice. Age-dependent impairment of AMPK activation is associated with distinct hemodynamics and cardiac ultrastructural changes in mature animals. Pharmacological activation of AMPK by AICAR lessens tissue cardiac injury and protects mitochondrial structure in cardiomyocytes. However, the beneficial effects of AICAR appear to be age dependent, with different molecular consequences in mature adult mice compared with younger animals. Whether AMPK activation may serve as a novel therapeutic approach of myocardial depression in human sepsis warrants further investigation.
GRANTS
This work was supported by the National Institute of General Medical Sciences Grants R01 GM-067202 and GM-115973 (to B. Zingarelli).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.I. and B.Z. conceived and designed research; Y.I., G.P., P.W.H., M.O., P.L., V.W., C.S., and V.M. performed experiments; Y.I., G.P., P.L., J.M.J., and B.Z. analyzed data; Y.I., G.P., J.M.J., and B.Z. interpreted results of experiments; Y.I., G.P., and B.Z. prepared figures; Y.I., G.P., and B.Z. drafted manuscript; Y.I., G.P., J.M.J., and B.Z. edited and revised manuscript; Y.I., G.P., P.W.H., M.O., P.L., V.W., C.S., V.M., J.M.J., and B.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of Dr. Y. Inata: Dept. of Intensive Care Medicine, Osaka Women's and Children's Hospital, Osaka 565-0871, Japan.
REFERENCES
- 1.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, Khuchua Z. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem 286: 899–908, 2011. doi: 10.1074/jbc.M110.171439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence 5: 12–19, 2014. doi: 10.4161/viru.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res 100: 474–488, 2007. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 4.Beesley SJ, Wilson EL, Lanspa MJ, Grissom CK, Shahul S, Talmor D, Brown SM. Relative bradycardia in patients with septic shock requiring vasopressor therapy. Crit Care Med 45: 225–233, 2017. doi: 10.1097/CCM.0000000000002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 6.Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, Harken AH. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit Care Med 27: 1309–1318, 1999. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Carré JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, Stotz M, Singer M. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med 182: 745–751, 2010. doi: 10.1164/rccm.201003-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280: 26185–26192, 2005. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 9.Chen HW, Hsu C, Lu TS, Wang SJ, Yang RC. Heat shock pretreatment prevents cardiac mitochondrial dysfunction during sepsis. Shock 20: 274–279, 2003. doi: 10.1097/00024382-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 368: 651–662, 2013. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 11.Chu M, Gao Y, Zhang Y, Zhou B, Wu B, Yao J, Xu D. The role of speckle tracking echocardiography in assessment of lipopolysaccharide-induced myocardial dysfunction in mice. J Thorac Dis 7: 2253–2261, 2015. doi: 10.3978/j.issn.2072-1439.2015.12.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cieslik KA, Taffet GE, Crawford JR, Trial J, Mejia Osuna P, Entman ML. AICAR-dependent AMPK activation improves scar formation in the aged heart in a murine model of reperfused myocardial infarction. J Mol Cell Cardiol 63: 26–36, 2013. doi: 10.1016/j.yjmcc.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 14.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit Care 6: 500–508, 2002. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 4: 729–741, 2004. doi: 10.1016/j.mito.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Exline MC, Crouser ED. Mitochondrial mechanisms of sepsis-induced organ failure. Front Biosci 13: 5030–5041, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairchild KD, Srinivasan V, Moorman JR, Gaykema RP, Goehler LE. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am J Physiol Regul Integr Comp Physiol 300: R330–R339, 2011. doi: 10.1152/ajpregu.00487.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci 24: 479–487, 2004. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hestenes SM, Halvorsen PS, Skulstad H, Remme EW, Espinoza A, Hyler S, Bugge JF, Fosse E, Nielsen EW, Edvardsen T. Advantages of strain echocardiography in assessment of myocardial function in severe sepsis: an experimental study. Crit Care Med 42: e432–e440, 2014. doi: 10.1097/CCM.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 20.Inata Y, Kikuchi S, Samraj RS, Hake PW, O’Connor M, Ledford JR, O’Connor J, Lahni P, Wolfe V, Piraino G, Zingarelli B. Autophagy and mitochondrial biogenesis impairment contributes to age-dependent liver injury in experimental sepsis: dysregulation of AMP-activated protein kinase pathway. FASEB J 32: 728–741, 2018. doi: 10.1096/fj.201700576R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jardin F, Fourme T, Page B, Loubières Y, Vieillard-Baron A, Beauchet A, Bourdarias JP. Persistent preload defect in severe sepsis despite fluid loading: a longitudinal echocardiographic study in patients with septic shock. Chest 116: 1354–1359, 1999. doi: 10.1378/chest.116.5.1354. [DOI] [PubMed] [Google Scholar]
- 22.Joshi MS, Julian MW, Huff JE, Bauer JA, Xia Y, Crouser ED. Calcineurin regulates myocardial function during acute endotoxemia. Am J Respir Crit Care Med 173: 999–1007, 2006. doi: 10.1164/rccm.200411-1507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakihana Y, Ito T, Nakahara M, Yamaguchi K, Yasuda T. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care 4: 22, 2016. doi: 10.1186/s40560-016-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim M, Tian R. Targeting AMPK for cardiac protection: opportunities and challenges. J Mol Cell Cardiol 51: 548–553, 2011. doi: 10.1016/j.yjmcc.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klingbeil LR, Kim P, Piraino G, O’Connor M, Hake PW, Wolfe V, Zingarelli B. Age-dependent changes in AMPK metabolic pathways in the lung in a mouse model of hemorrhagic shock. Am J Respir Cell Mol Biol 56: 585–596, 2017. doi: 10.1165/rcmb.2016-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozlov AV, Bahrami S, Calzia E, Dungel P, Gille L, Kuznetsov AV, Troppmair J. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann Intensive Care 1: 41, 2011. doi: 10.1186/2110-5820-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuznetsov AV, Hermann M, Troppmair J, Margreiter R, Hengster P. Complex patterns of mitochondrial dynamics in human pancreatic cells revealed by fluorescent confocal imaging. J Cell Mol Med 14: 417–425, 2010. doi: 10.1111/j.1582-4934.2009.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larche J, Lancel S, Hassoun SM, Favory R, Decoster B, Marchetti P, Chopin C, Neviere R. Inhibition of mitochondrial permeability transition prevents sepsis-induced myocardial dysfunction and mortality. J Am Coll Cardiol 48: 377–385, 2006. doi: 10.1016/j.jacc.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Jeong S-Y, Lim W-C, Kim S, Park Y-Y, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem 282: 22977–22983, 2007. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- 30.Leon LJ, Gustafsson AB. Staying young at heart: autophagy and adaptation to cardiac aging. J Mol Cell Cardiol 95: 78–85, 2016. doi: 10.1016/j.yjmcc.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy RJ, Deutschman CS. Evaluating myocardial depression in sepsis. Shock 22: 1–10, 2004. doi: 10.1097/01.shk.0000129198.53836.15. [DOI] [PubMed] [Google Scholar]
- 32.Levy RJ, Vijayasarathy C, Raj NR, Avadhani NG, Deutschman CS. Competitive and noncompetitive inhibition of myocardial cytochrome C oxidase in sepsis. Shock 21: 110–114, 2004. doi: 10.1097/01.shk.0000108400.56565.ab. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Benashski SE, Persky R, Xu Y, Li J, McCullough LD. Age-related changes in AMP-activated protein kinase after stroke. Age (Dordr) 34: 157–168, 2012. doi: 10.1007/s11357-011-9214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 34: 15–21, 2006. doi: 10.1097/01.CCM.0000194535.82812.BA. [DOI] [PubMed] [Google Scholar]
- 35.Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol 45: 2288–2301, 2013. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsiukevich D, Piraino G, Klingbeil LR, Hake PW, Wolfe V, O’Connor M, Zingarelli B. The AMPK activator Aicar ameliorates age-dependent myocardial injury in murine hemorrhagic shock. Shock 47: 70–78, 2017. doi: 10.1097/SHK.0000000000000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsiukevich D, Piraino G, Lahni P, Hake PW, Wolfe V, O’Connor M, James J, Zingarelli B. Metformin ameliorates gender-and age-dependent hemodynamic instability and myocardial injury in murine hemorrhagic shock. Biochim Biophys Acta 1863, 10 Pt B: 2680–2691, 2017. doi: 10.1016/j.bbadis.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13: 1016–1023, 2011. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulligan JD, Gonzalez AA, Kumar R, Davis AJ, Saupe KW. Aging elevates basal adenosine monophosphate-activated protein kinase (AMPK) activity and eliminates hypoxic activation of AMPK in mouse liver. J Gerontol A Biol Sci Med Sci 60: 21–27, 2005. doi: 10.1093/gerona/60.1.21. [DOI] [PubMed] [Google Scholar]
- 41.Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 100: 483–490, 1984. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 42.Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, McCabe C, Welch SB, Whitney A, O’Gara P, Nadel S, Relman DA, Harding SE, Levin M. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet 363: 203–209, 2004. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 43.Pauly M, Chabi B, Favier FB, Vanterpool F, Matecki S, Fouret G, Bonafos B, Vernus B, Feillet-Coudray C, Coudray C, Bonnieu A, Ramonatxo C. Combined strategies for maintaining skeletal muscle mass and function in aging: myostatin inactivation and AICAR-associated oxidative metabolism induction. J Gerontol A Biol Sci Med Sci 70: 1077–1087, 2015. doi: 10.1093/gerona/glu147. [DOI] [PubMed] [Google Scholar]
- 44.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC; ProCESS Investigators . A randomized trial of protocol-based care for early septic shock. N Engl J Med 370: 1683–1693, 2014. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5: 151–156, 2007. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med 45: 486–552, 2017. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 47.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4: 31–36, 2009. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35: 1599–1608, 2007. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 49.Sanfilippo F, Corredor C, Fletcher N, Landesberg G, Benedetto U, Foex P, Cecconi M. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med 41: 1004–1013, 2015. [Erratum in Intensive Care Med 41: 1178–1179, 2015]. doi: 10.1007/s00134-015-3748-7. [DOI] [PubMed] [Google Scholar]
- 50.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol 147: 699–706, 1999. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimano M, Ouchi N, Nakamura K, van Wijk B, Ohashi K, Asaumi Y, Higuchi A, Pimentel DR, Sam F, Murohara T, van den Hoff MJ, Walsh K. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci USA 108: E899–E906, 2011. doi: 10.1073/pnas.1108559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810, 2016. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoppani J, Hildebrandt AL, Sakamoto K, Cameron-Smith D, Goodyear LJ, Neufer PD. AMP-activated protein kinase activates transcription of the UCP3 and HKII genes in rat skeletal muscle. Am J Physiol Endocrinol Metab 283: E1239–E1248, 2002. doi: 10.1152/ajpendo.00278.2002. [DOI] [PubMed] [Google Scholar]
- 55.Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res 64: 279–288, 2004. doi: 10.1016/j.cardiores.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187: 509–517, 2013. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tateishi Y, Oda S, Nakamura M, Watanabe K, Kuwaki T, Moriguchi T, Hirasawa H. Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock 28: 549–553, 2007. doi: 10.1097/shk.0b013e3180638d1. [DOI] [PubMed] [Google Scholar]
- 58.Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell 9: 592–606, 2010. doi: 10.1111/j.1474-9726.2010.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vieillard-Baron A. Septic cardiomyopathy. Ann Intensive Care 1: 6, 2011. doi: 10.1186/2110-5820-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yavari A, Bellahcene M, Bucchi A, Sirenko S, Pinter K, Herring N, Jung JJ, Tarasov KV, Sharpe EJ, Wolfien M, Czibik G, Steeples V, Ghaffari S, Nguyen C, Stockenhuber A, Clair JRS, Rimmbach C, Okamoto Y, Yang D, Wang M, Ziman BD, Moen JM, Riordon DR, Ramirez C, Paina M, Lee J, Zhang J, Ahmet I, Matt MG, Tarasova YS, Baban D, Sahgal N, Lockstone H, Puliyadi R, de Bono J, Siggs OM, Gomes J, Muskett H, Maguire ML, Beglov Y, Kelly M, Dos Santos PPN, Bright NJ, Woods A, Gehmlich K, Isackson H, Douglas G, Ferguson DJP, Schneider JE, Tinker A, Wolkenhauer O, Channon KM, Cornall RJ, Sternick EB, Paterson DJ, Redwood CS, Carling D, Proenza C, David R, Baruscotti M, DiFrancesco D, Lakatta EG, Watkins H, Ashrafian H. Mammalian γ2 AMPK regulates intrinsic heart rate. Nat Commun 8: 1258, 2017. doi: 10.1038/s41467-017-01342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L497–L504, 2008. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]