Abstract
Heart failure with preserved ejection fraction (HFpEF) is caused, or exacerbated by, a wide range of extracardiac conditions. Diabetes, obesity, and metabolic dysfunction are associated with a unique HFpEF phenotype, characterized by inflammation, cardiac fibrosis, and microvascular dysfunction. Development of new therapies for HFpEF is hampered by the absence of reliable animal models. The leptin-resistant db/db mouse has been extensively studied as a model of diabetes-associated cardiomyopathy; however, data on the functional and morphological alterations in db/db hearts are conflicting. In the present study, we report a systematic characterization of the cardiac phenotype in db/db mice, focusing on the time course of functional and histopathological alterations and on the identification of sex-specific cellular events. Although both male and female db/db mice developed severe obesity, increased adiposity, and hyperglycemia, female mice had more impressive weight gain and exhibited a modest but significant increase in blood pressure. db/db mice had hypertrophic ventricular remodeling and diastolic dysfunction with preserved ejection fraction; the increase in left ventricular mass was accentuated in female mice. Histological analysis showed that both male and female db/db mice had cardiomyocyte hypertrophy and interstitial fibrosis, associated with marked thickening of the perimysial collagen, and expansion of the periarteriolar collagen network, in the absence of replacement fibrosis. In vivo and in vitro experiments showed that fibrotic changes in db/db hearts were associated with increased collagen synthesis by cardiac fibroblasts, in the absence of periostin, α-smooth muscle actin, or fibroblast activation protein overexpression. Male db/db mice exhibited microvascular rarefaction. In conclusion, the db/db mouse model recapitulates functional and histological features of human HFpEF associated with metabolic dysfunction. Development of fibrosis in db/db hearts, in the absence of myofibroblast conversion, suggests that metabolic dysfunction may activate an alternative profibrotic pathway associated with accentuated extracellular matrix protein synthesis.
NEW & NOTEWORTHY We provide a systematic analysis of the sex-specific functional and structural myocardial alterations in db/db mice. Obese diabetic C57BL6J db/db mice exhibit diastolic dysfunction with preserved ejection fraction, associated with cardiomyocyte hypertrophy, interstitial/perivascular fibrosis, and microvascular rarefaction, thus recapitulating aspects of human obesity-related heart failure with preserved ejection fraction. Myocardial fibrosis in db/db mice is associated with a matrix-producing fibroblast phenotype, in the absence of myofibroblast conversion, suggesting an alternative mechanism of activation.
Keywords: diabetes, diastolic dysfunction, fibroblast, fibrosis, hypertrophy
INTRODUCTION
Approximately 50% of patients with heart failure (HF) in the community have preserved ejection fraction (HFpEF). Because of the absence of effective treatment strategies, HFpEF is associated with a high mortality, a low quality of life, and a high incidence of hospitalizations (13). Development of new therapies for patients with HFpEF is hampered by the remarkable pathophysiological heterogeneity of the syndrome, which is caused or exacerbated by a wide range of cardiac, or extracardiac, comorbidities (16) and by the absence of reliable animal models that recapitulate the human condition (51). Obesity, diabetes, and metabolic dysfunction markedly increase the risk of HFpEF, independent of the occurrence of coronary artery disease (27, 38). A growing body of evidence supports the existence of a distinct obesity-associated phenotype of human HFpEF (37). Patients with “obese HFpEF” exhibit increased plasma volume, more concentric left ventricular (LV) hypertrophy, and greater right ventricular dilatation, despite lower plasma levels of natriuretic peptides (5, 37). However, the cell biological basis and molecular mechanisms responsible for the unique HFpEF phenotype associated with obesity remain unknown.
To date, treatment strategies for HFpEF are largely restricted to symptomatic relief (1). Development of new therapeutics is impeded by the lack of understanding of the cellular and molecular basis of HFpEF; our limited knowledge is often attributed to the absence of translationally relevant animal models of the disease. Considering the heterogeneity of human HFpEF and its relation with a range of comorbid conditions, designing an animal model that mimics the broad spectrum of pathophysiologies underlying the human condition is practically impossible. Instead, our focus should be to systematically characterize and understand models that recapitulate specific pathophysiological aspects of human HFpEF (42, 51). Rodent models of cardiomyopathy associated with diabetes, obesity, and metabolic dysfunction have been extensively used to investigate the mechanisms of HF associated with metabolic disease. Interpretation of the findings is often challenging because of differences in the observed phenotypes and is further hampered by the unclear relations between the functional changes observed in the animal model and the human pathological condition.
The db/db mouse has a point mutation in the gene encoding the leptin receptor, resulting in a protein with a truncated cytoplasmic domain that is functionally inactive (10). As a result, db/db mice are resistant to the central effects of leptin and develop a voracious appetite at an early age, leading to severe obesity and the development of overt diabetes. Although obesity, diabetes, and metabolic dysregulation in db/db mice have been associated with significant alterations in myocardial structure and function (11, 29, 46, 56), the findings are conflicting. Although some studies have reported that db/db mice exhibit a significant reduction in ejection fraction (53, 56), other investigations have suggested that db/db mice may have an early improvement in systolic function (2). In contrast, numerous other studies have shown evidence of diastolic dysfunction associated with preserved systolic function (21, 22), whereas some investigations reported no significant functional and structural differences between db/db mice and lean age-matched control mice (28). The age and strain of the experimental animals, sex-specific effects, and different methods used for functional analysis may account for the contrasting observations.
In the present investigation, we provide a systematic analysis of the functional and structural myocardial alterations in db/db mice. Our findings suggest that db/db mice in the C57BL6J background exhibit diastolic dysfunction with preserved ejection fraction, associated with cardiomyocyte hypertrophy, interstitial/perivascular fibrosis, and microvascular rarefaction, thus recapitulating aspects of human obesity-related HFpEF. Sex-specific analysis suggested that female db/db mice exhibit modest arterial hypertension, associated with accentuated hypertrophic remodeling, whereas male mice have exaggerated microvascular loss. Myocardial fibrosis in db/db mice was associated with activation of a matrix-producing fibroblast phenotype, in the absence of myofibroblast conversion, suggesting that activation of diabetic fibroblasts may involve an alternative mechanism, distinct from the cellular events leading to fibrosis in infarcted and pressure-overloaded hearts.
MATERIALS AND METHODS
Animals.
Animal experiments were approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine and the Animal Protocol Review Committee of Baylor College of Medicine. We used Leprdm/+ on a C57BL6J background (db/+) and wild-type (WT) C57BL6J animals from our own colonies (originally purchased from Jackson Laboratories, Bar Harbor, ME) (4, 18). Genotyping was performed using standard protocols. Mice were fed a regular chow diet (LabDiet 5001).
Assessment of body fat content.
Body composition (body fat mass) of WT and db/db mice was quantitatively assessed at 2 and 6 mo of age using nuclear magnetic resonance (EchoMRI Whole Body Composition Analyzer, Echo Medical System, Houston, TX) at the Albert Einstein College of Medicine Diabetes Research Center, Animal Physiology Core. In a separate group of WT and db/db animals, fat tissue mass was measured at 4 mo of age by dual-energy X-ray absorptiometry using the PIXImus Densitometer (Lunar, Madison, WI). Each mouse was anesthetized in a chamber for the entire procedure (5% isoflurane-95% oxygen). Once anesthetized, each mouse was placed in the densitometer in the prone position, with the limbs and tail extended. Data were analyzed with PIXImus software (2.0, GE/Lunar).
Assessment of plasma glucose.
Animals used for the assessment of plasma glucose were euthanized at 2 or 6 mo of age, and blood was collected by aortic puncture in EDTA anticoagulant-coated tubes. Plasma was extracted by centrifugation at 850 g for 15 min at 4°C and stored at −80°C. Mice were fasted for 12 h before blood collection. Glucose levels were measured using a glucometer (One Touch Ultra, LifeScan, Milpitas, CA).
Echocardiography.
For echocardiographic analysis, WT and db/db mice were imaged at 2 and 6 mo of age (29–81 mice/group). The procedures used complied with guidelines for measuring cardiac physiology in mice (34). Mice were initially anesthetized in a chamber (2% isoflurane-95% oxygen at 0.8 l/min) and afterward were supinely placed on a heating pad at 37°C on maintenance anesthesia (1.5% isoflurane). Echocardiographic assessment was performed using a Vevo770 ultrasound (Visualsonics, Toronto, ON, Canada) with a real-time microvisualization transducer (RMVB710B, 12–38 MHz at a frame rate of 110–120 frames/s) applied parasternally to the shaved chest wall. Images were taken in the parasternal long-axis, parasternal short-axis, and short-axis M-mode (SAMM). SAMM images were acquired by vertically placing the M-mode cursor at the parasternal short-axis view when both papillary muscles were visualized. Views and data were exported for offline calculation using dedicated Vevo 770 quantification software (Vevo 770 version 3.0.0). Images from SAMM were used to measure LV end-diastolic volume (LVEDV), LV mass, and ejection fraction. To compare the remodeling response between groups, we measured the global “remodeling index” (remodeling index = EDV1/3/t, where t is time), an indicator of maladaptive hypertrophic remodeling that has been used for risk stratification in patients with hypertension (17). The echocardiographic offline analysis was performed by a sonographer blinded to the study groups. Echocardiographic parameters were not normalized to body weight.
Doppler echocardiography and tissue Doppler imaging.
WT and db/db mice underwent Doppler echocardiography and tissue Doppler imaging (TDI) at 2 and 6 mo of age (14–35 mice/group). Images from the apical four-chamber view were acquired to assess LV filling and diastolic function. Transmitral LV inflow velocities were measured by pulsed-wave Doppler. Peak early E wave (E) and late A wave (A) filling velocities and the E-to-A ratio (E/A) were measured. TDI was obtained by placing a 1.0-mm sample volume at the medial annulus of the mitral valve. Analysis was performed for the early (e′) and late (a′) diastolic velocity. The mitral inflow E velocity-to-tissue Doppler e′ wave velocity ratio (E/e′) and tissue Doppler early e′ velocity-to-tissue Doppler late a′ velocity ratio were calculated to assess diastolic function. All Doppler spectra were recorded for three to five cardiac cycles at a sweep speed of 100 mm/s. The color Doppler preset was at a Nyquist limit of 0.44 m/s. The echocardiographic offline analysis was performed by a sonographer blinded to the study groups.
Immunohistochemistry and histology.
For histopathological analysis, mice were euthanized at 6 or 12 mo of age, and hearts were fixed in Z-fix (Anatech, Battle Creek, MI) and embedded in paraffin (8 mice/group, 4 male and 4 female mice). Sequential 5-µm sections were cut by microtomy. Collagen fibers were identified by picrosirius red staining using protocols established in our laboratory (6). Endomysial collagen was semiquantitatively assessed in 10 different ×200 fields from each mouse using the following scale: 0, normal endomysial collagen pattern; 1, increased endomysial collagen deposition involving <50% of the field; and 2, increased endomysial collagen deposition involving >50% of the field. The mean score was calculated for each mouse. Perimysial collagen thickness was assessed by measuring the average thickness of each perimysial strand by averaging measurements at three different points (which included the thickest and thinnest dimensions and a third random point). To assess the perimysial collagen thickness for each mouse, measurements for at least 10 perimysial strands were averaged. To assess periarteriolar collagen deposition, at least 50 arterioles from each group were identified in picrosirius red-stained sections and selected for analysis only if the ratio of the major to minor axes was <3:1 (to exclude vessels sectioned longitudinally). The medial area and adventitial collagenous area were quantitatively assessed for each vessel using Axiovision software.
To identify cardiac arterioles and to label myofibroblasts, immunofluorescence for α-smooth muscle actin (α-SMA) was performed using FITC-conjugated anti-α-SMA antibody (F3777, mouse monoclonal antibody, 1:100, Sigma). To further investigate myofibroblast activation in the db/db myocardium, immunohistochemistry for periostin (no. 92460, rabbit polyclonal antibody, 1:200, Abcam) and fibroblast activation protein (FAP) was performed (no. 53066, rabbit polyclonal antibody, 1:200, Abcam). Sections from infarcted mouse hearts after 7 days of permanent coronary occlusion (49) were used as a positive control. Substitution of primary antibodies with corresponding species IgG in blocking buffer was used as a negative control, and no background staining was noted. Staining was performed using a peroxidase-based technique with the Vectastain ELITE kit (Vector Laboratories). After antigen retrieval with citrate buffer, sections were treated with 3% hydrogen peroxide to inhibit endogenous peroxidase activity and blocked with 10% serum to block nonspecific protein binding. Peroxidase activity was detected using diaminobenzidine with nickel.
To perform collagen immunofluorescence, freshly harvested hearts of WT and db/db mice (8 animals/group, 4 male and 4 female mice) were embedded in OCT compound (no. 4585, Fisher HealthCare) and frozen in 2-methylbutane (Millipore) kept in dry ice. Cryosections (7 μm) were cut, acetone fixed, and then probed for collagen I (no. 21286, rabbit polyclonal antibody, 1:500, Abcam).
Griffonia Simplicifolia-I lectin histochemistry and assessment of microvascular density.
To identify microvessels in WT and db/db hearts, we used histochemical staining for Griffonia Simplicifolia (GSL)-I lectin (DL1207, Vector) as previously described (44). Assessment of microvascular density was performed for each heart by counting the number of GSL-I lectin-positive profiles in at least 10 fields for each mouse at ×200 magnification.
Wheat germ agglutinin lectin histochemistry and quantification of cardiomyocyte size.
Cardiomyocyte size was assessed using staining with Alexa Fluor 488-conjugated wheat germ agglutinin (WGA) lectin (W11261, Invitrogen) to label cardiomyocyte membranes (12, 20). For each mouse, the area of 50 cardiomyocytes from subendocardial or subepicardial regions cut in cross section was measured. Cardiomyocytes were identified from five different random subendocardial or subepicardial fields, scanned from two different sections for each mouse. The mean of these cardiomyocyte cross-sectional areas was calculated for each mouse.
Blood pressure measurements.
Invasive blood pressure measurements were performed with a commercial pressure-volume analysis system (Scisense) as previously described (4). Mice undergoing blood pressure measurement were anesthetized with isoflurane and allowed to rest for 10 min to stabilize blood pressure. The right carotid artery was exposed by blunt dissection and tied in three spots 3 mm apart with 6-0 silk suture. Distal suture was tied permanently with two knots, whereas mid and proximal sutures were tied with one knot. With the use of fine scissors, a small incision was made between the distal and mid suture. A 1.2-Fr pressure-volume catheter (Scisense) was inserted and advanced until the tip of the catheter passed midsuture, which was tightened to secure the catheter. Subsequently, the proximal suture was removed, and the catheter was advanced to the aortic arch and secured with a suture. The catheter was left in place for 10 min to allow blood pressure equilibration. Pressure tracings were recorded for 5 min and analyzed to derive average systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Invasive assessment of LV end-diastolic pressure.
LV pressure was recorded in WT and db/db mice as previously described (12). The right carotid artery was cannulated with a modified RADI PressureWire catheter (RADI Medical Systems, Upsala, Sweden). Subsequently, the catheter was advanced in the ascending aorta and then in the LV (41). LV end-diastolic pressure (LVEDP) was calculated from the LV pressure signal.
Fibroblast isolation.
Mouse fibroblasts from WT and db/db hearts were isolated as previously described (49) by enzymatic digestion and cultured in DMEM-F-12 (GIBCO Invitrogen, Carlsbad, CA) with 10% FCS. At passage 3, cardiac fibroblasts were serum starved for 24 h, harvested, and suspended in 1× serum-free MEM (GIBCO Invitrogen). Cells were stimulated with transforming growth factor (TGF)-β1 (10 ng/ml) for 4 h. At the end of the experiment, cell lysates were used for RNA extraction using standard protocols.
mRNA extraction and quantitative PCR.
Isolated total RNA from cardiac fibroblasts was reverse transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad) following the manufacturer’s guidelines. Quantitative PCR was performed using the SYBR green (Bio-Rad) method on an iQ5 Real-Time PCR Detection System (Bio-Rad). Collagen type I and type III mRNA levels were assessed using the following primers: collagen type I, forward 5′-GTATGCTTGATCTGTATCTG-3′ and reverse: 5′-CGACTCCTACATCTTCTG-3′; and collagen type III, forward 5′-CCTTGGTCAGTCCTATGAG-3′ and reverse 5′-CAGGAGCAGGTGTAGAAG-3′.
Statistical analysis.
For comparisons of two groups, an unpaired two-tailed Student’s t-test using (when appropriate) Welch’s correction for unequal variances was performed. The Mann-Whitney test was used for comparisons between two groups that did not show Gaussian distribution. For comparisons of multiple groups, one-way ANOVA was performed followed by Sidak’s multiple-comparison test. The Kruskal-Wallis test, followed by Dunn’s multiple-comparison posttest, was used when one or more groups did not show Gaussian distribution. Data are expressed as means ± SE. Statistical significance was set at 0.05.
RESULTS
db/db mice exhibit severe obesity, high adiposity, and hyperglycemia.
db/db mice in a C57BL6J background exhibited rapid weight gain when fed a regular chow diet. Body weight was significantly higher in both male and female db/db mice compared with age- and sex-matched control C57BL6J mice (Fig. 1, A–C). Compared with age-matched WT mice, female db/db mice had a more impressive increase in weight than male mice (fold increase in mean body weight vs. WT mice, female mice: 2.38-fold at 6 mo of age and 2.37-fold at 12 mo and male mice: 1.88-fold at 6 mo and 1.74-fold at 12 mo). Both magnetic resonance imaging (Fig. 1, D–I) and dual-energy X-ray absorptiometry (Fig. 1, J and K) showed a marked increase in adiposity and in abdominal fat content in both male and female db/db animals compared with age-matched WT control animals (Fig. 1, D–F, J, and K). In contrast, lean weight was comparable between groups (Fig. 1, G–I). Plasma glucose levels were markedly increased in both male and female db/db mice at 2 and 6 mo of age compared with age-matched WT control mice (Fig. 1, L–N).
Fig. 1.
Both male and female db/db (db) mice in a C57BL6J background exhibit severe obesity, increased adiposity, and overt diabetes. A–C: body weight (BW) was markedly higher in db/db mice compared with age-matched wild-type (WT) control mice. Compared with age-matched WT mice, female db/db mice had a more impressive increase in body weight than male mice (female db/db mice: 2.38-fold higher body weight than age-matched WT mice at 6 mo of age and 2.37-fold higher at 12 mo vs. male db/db mice: 1.88-fold at 6 mo and 1.74-fold at 12 mo of age). D–F: magnetic resonance imaging showed a marked increase in body fat weight in both male and female db/db mice at 2 and 6 mo of age. G–I: in contrast, lean mass was comparable between age- and sex-matched WT and db/db mice. J and K: dual-energy X-ray absorptiometry performed at 4 mo of age also showed that db/db mice had a marked increase in total (G) and abdominal (H) fat content. L–N: although there was significant variability, both male (M) and female (N) db/db animals exhibited a marked increase in fasting plasma glucose levels at 2 and 6 mo of age. **P < 0.01, ***P < 0.001, ****P < 0.0001, and ^P < 0.05 vs. corresponding 2-mo-old mice; ^^^P < 0.001 vs. corresponding 2-mo-old mice. Body weight sample size: WT mice: 1 mo n = 37, 2 mo n = 63, 4 mo n = 58, 6 mo n = 91, 12 mo n = 22; db/db mice: 1 mo n = 11, 2 mo n = 35, 4 mo n = 45, 6 mo n = 68, 12 mo n = 24. Male mice: WT 1 mo n = 16, 2 mo n = 23, 4 mo n = 32, 6 mo n = 53, 12 mo n = 11; db/db: 1 mo n = 7, 2 mo n = 22, 4 mo n = 29, 6 mo n = 38, 12 mo n = 10. Female mice: WT 1 mo n = 21, 2 mo n = 40, 4 mo n = 31, 6 mo n = 38, 12 mo n = 11; db/db: 1 mo n = 4, 2 mo n = 13, 4 mo n = 16, 6 mo n = 30, 12 mo n = 14. Magnetic resonance imaging fat content: n = 12–16 mice/group, n = 5–9 male mice/group, n = 4–8 female mice/group. Dual-energy X-ray absorptiometry: n = 7–16/group. Fasting plasma glucose: n = 18–49 mice/group, n = 9–27 male mice/group, and n = 9–22 female mice/group.
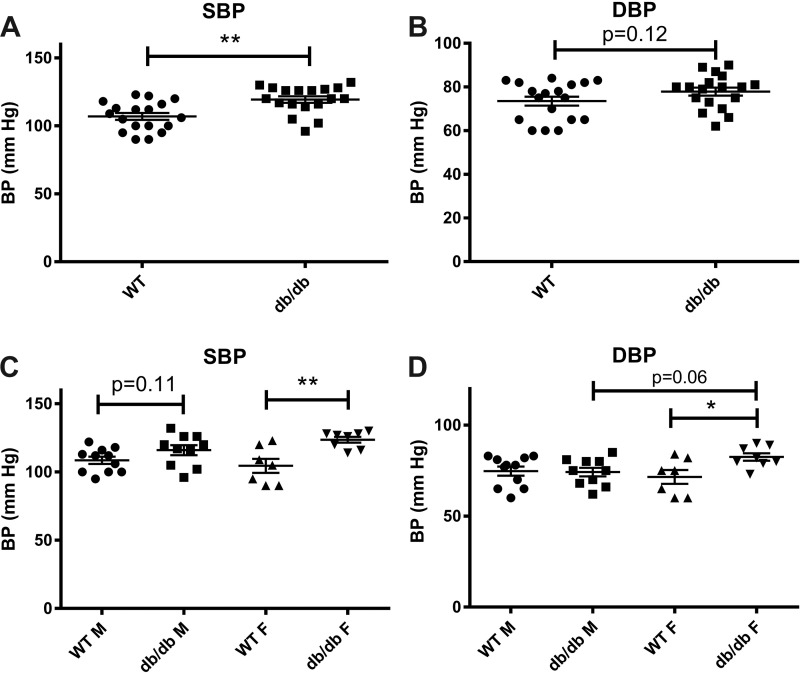
Female but not male db/db mice exhibit mild hypertension.
Compared with age-matched WT animals, 4-mo-old db/db mice exhibited a modest but significant elevation of SBP and a trend toward increased DBP (Fig. 2, A and B). Both SBP and DBP were significantly increased in female db/db mice. In contrast, male db/db animals had a trend toward increased SBP and comparable DBP with WT control animals (Fig. 2, C and D).
Fig. 2.
Female db/db mice have a modest but significant elevation in systolic blood pressure (SBP) and diastolic blood pressure (DBP). A and B: at 4 mo of age, obese diabetic db/db mice had a significant elevation in SBP and a trend toward higher DBP. C and D: sex-specific analysis showed that male db/db mice had a trend toward increased SBP (C) and no significant difference in DBP (D) compared with lean WT control mice. Female db/db mice had significantly increased SBP (C) and DBP (D) compared with age-matched WT control mice (n = 10–11 male mice/group, n = 7–8 female mice/group, n = 18 male + female mice/group). *P < 0.05 and **P < 0.01. BP, blood pressure.
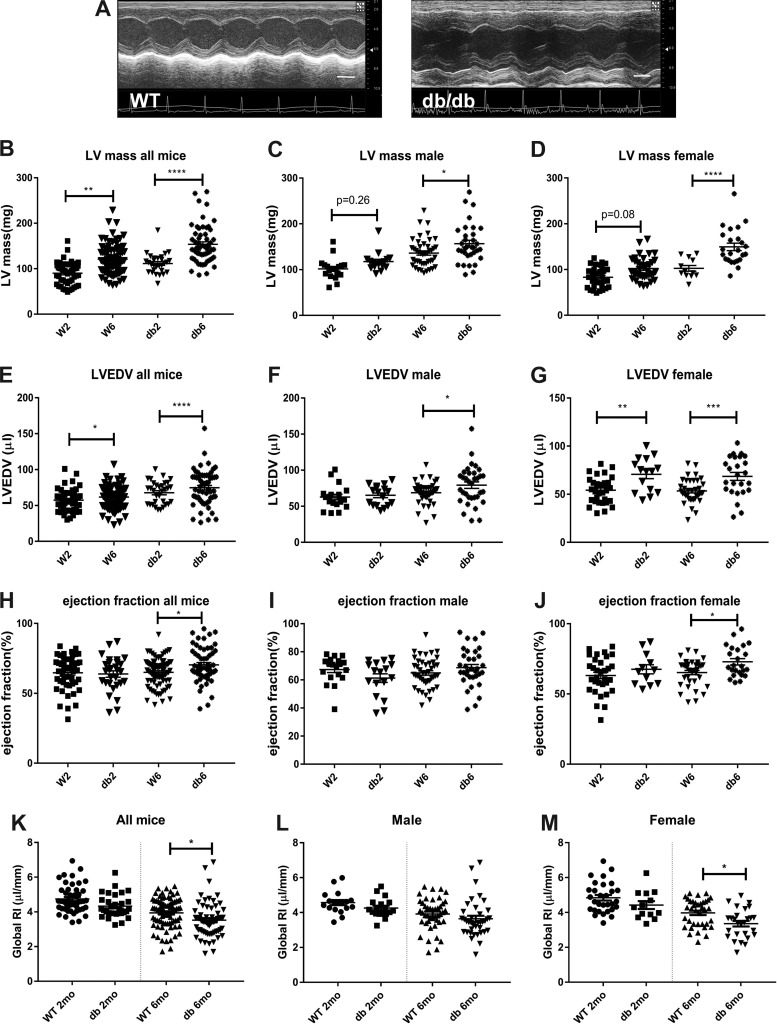
db/db mice exhibit cardiac remodeling in the absence of systolic dysfunction.
Compared with age-matched WT mice, db/db mice exhibited significantly higher LV mass (Fig. 3, A and B) at 2 and 6 mo of age accompanied by increased LVEDV (Fig. 3E). At 6 mo of age, ejection fraction was slightly but significantly higher in db/db mice (Fig. 3H). To compare the remodeling response between groups, we measured the remodeling index, an indicator of maladaptive hypertrophic remodeling that has been used for risk stratification in patients with hypertension. At 6 mo of age, db/db mice had a significantly lower remodeling index compared with lean WT mice, indicating accentuated hypertrophic remodeling (Fig. 3J).
Fig. 3.
Obese diabetic db/db mice exhibit cardiac remodeling with preserved ejection fraction. A: echocardiographic imaging was performed in wild-type (WT) and db/db mice. Representative images show evidence of cardiac remodeling in db/db mice at 6 mo of age compared with lean WT control mice (scale bar = 2 mm). B: db/db mice had significantly higher left ventricular (LV) mass at 2 and 6 mo of age. C and D: both male and female db/db mice had increased LV mass at 6 mo of age. However, the increase in LV mass was exaggerated in female db/db mice. At 6 mo of age, male db/db mice had a 15.1% higher LV mass than age-matched WT mice, whereas female db/db animals at the same age exhibited a 46.4% increase. E: LV end-diastolic volume (LVEDV) was increased in db/db mice. F and G: female mice had earlier ventricular dilation than male mice. H–J: obese diabetic db/db mice exhibited slightly but significantly higher ejection fraction at 6 mo of age than age-matched WT control mice. K–M: the global remodeling index was assessed to compare maladaptive hypertrophic remodeling between groups. K: at 6 mo of age, db/db mice had a lower remodeling index than corresponding lean WT control mice. L and M: female but not male db/db mice had a significant reduction in the global remodeling index, indicating accentuated hypertrophic remodeling (n = 19–44 male mice/group, n = 12–37 female mice/group, n = 29–81 male + female mice/group). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Cardiac remodeling is accentuated in female db/db mice.
Sex-specific analysis demonstrated that cardiac remodeling was exaggerated in female db/db mice. Six-month-old male db/db mice had a 15.1% increase in LV mass compared with age-matched WT mice, whereas female db/db mice at the same age exhibited a 46.4% increase (Fig. 3, C and D). The obesity-associated increase in LVEDV was also more impressive in female mice (Fig. 3, F and G). At 6 mo of age, female db/db mice had slightly higher ejection fraction than WT mice. In contrast, no significant difference in ejection fraction was noted in male animals (Fig. 3, I and J). The remodeling index was significantly reduced in female but not male mice at 6 mo of age (Fig. 3, L and M).
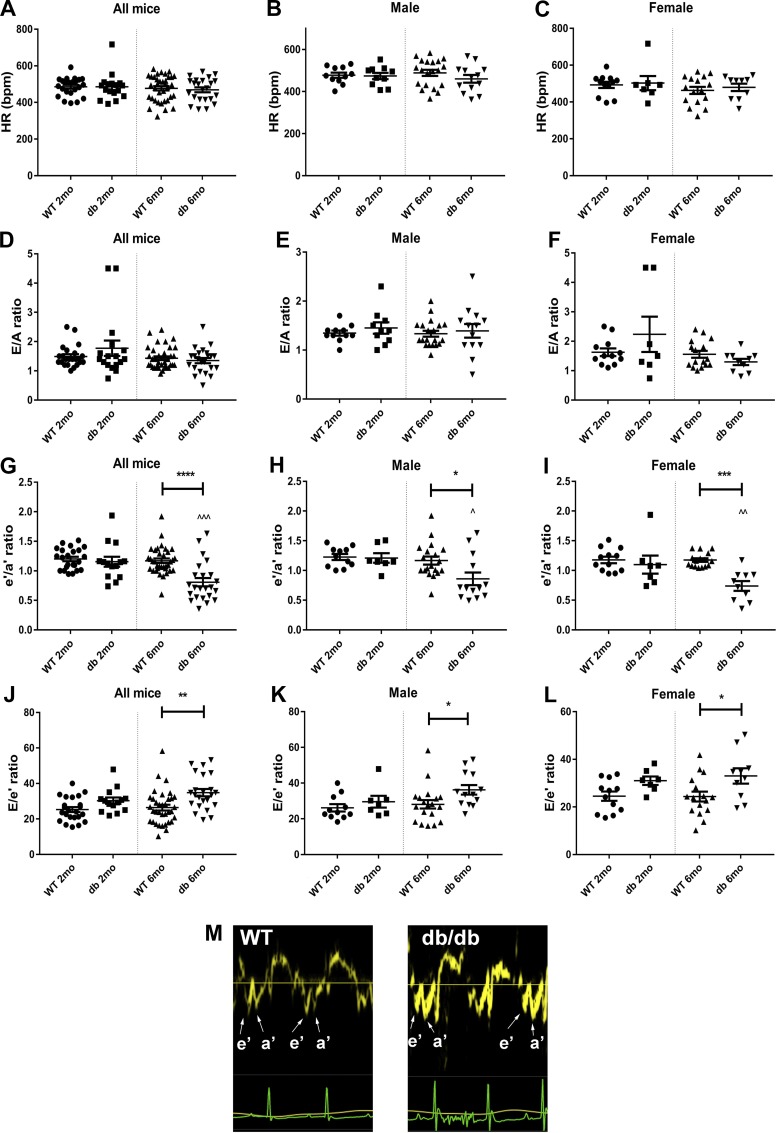
TDI suggests that both male and female db/db mice have evidence of diastolic dysfunction.
Mitral inflow Doppler echocardiography and TDI were used to assess diastolic function. At 2 and 6 mo of age, male and female db/db mice had comparable heart rates with age- and sex-matched WT control mice (Fig. 4, A–C). Mitral inflow Doppler experiments showed no significant differences in the E/A ratio between WT and db/db mice (Fig. 4, D–F). TDI showed that at 6 mo of age, db/db mice had a significant reduction in the e′/a′ ratio compared with WT control mice (Fig. 4G). The e′/a′ ratio was significantly reduced in both male (by 26.3%) and female (by 37.1%) animals (Fig. 4, H and I). The E/e′ ratio was significantly increased in db/db mice at 6 mo of age (Fig. 4, J–L). Male mice exhibited a statistically significant increase of the ratio by 28.8% (P < 0.05), whereas female mice had a 35.6% increase (P < 0.05). The representative images in Fig. 4M show the changes in the e′/a′ ratio in db/db mice at 6 mo of age.
Fig. 4.
Tissue Doppler imaging suggests that db/db mice develop diastolic dysfunction. A–C: both male and female db/db mice and age-matched wild-type (WT) control mice had comparable heart rates. D–F: mitral inflow Doppler showed no significant differences in the E-to-A ratio between groups. G–I: tissue Doppler imaging showed that the e′-to-a′ ratio was significantly reduced in both male and female db/db mice at 6 mo of age. J–L: the E-to-e′ ratio was significantly increased in both male and female db/db mice at 6 mo of age. M: representative images of tissue Doppler tracings showing the changes in the e′-to-a′ ratio in 6-mo-old db/db mice compared with age-matched lean control mice. These findings suggest that db/db mice develop diastolic dysfunction. Tissue Doppler imaging may be more sensitive than mitral inflow Doppler in detecting changes in diastolic function in mice (n = 7–19 male mice/group, n = 7–16 female mice/group, n = 14–35 male + female mice/group). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 vs. corresponding lean mice; ^P < 0.05, ^^P < 0.01, and ^^^P < 0.0001 vs. corresponding 2-mo-old mice.
Female db/db mice have elevated LVEDP.
At 6 mo of age, db/db mice had higher LVEDP than age-matched WT mice (Fig. 5A). Compared with sex-matched WT mice, male db/db mice exhibited a trend toward increased LVEDP (Fig. 5B). Female db/db mice had significantly higher LVEDP than corresponding WT mice (P < 0.05; Fig. 5C).
Fig. 5.
Obese diabetic db/db mice had significantly higher left ventricular end-diastolic pressure (LVEDP) than lean wild-type (WT) mice at 6 mo of age. A: db/db mice had significantly higher LVEDP than age-matched lean WT mice. B and C: sex-specific analysis showed that male db/db mice had a trend toward increased LVEDP (B), whereas female mice had significantly higher LVEDP (C; n = 6–7 male mice/group, n = 3–7 female mice/group, n = 10–13 male + female mice/group). *P < 0.05.
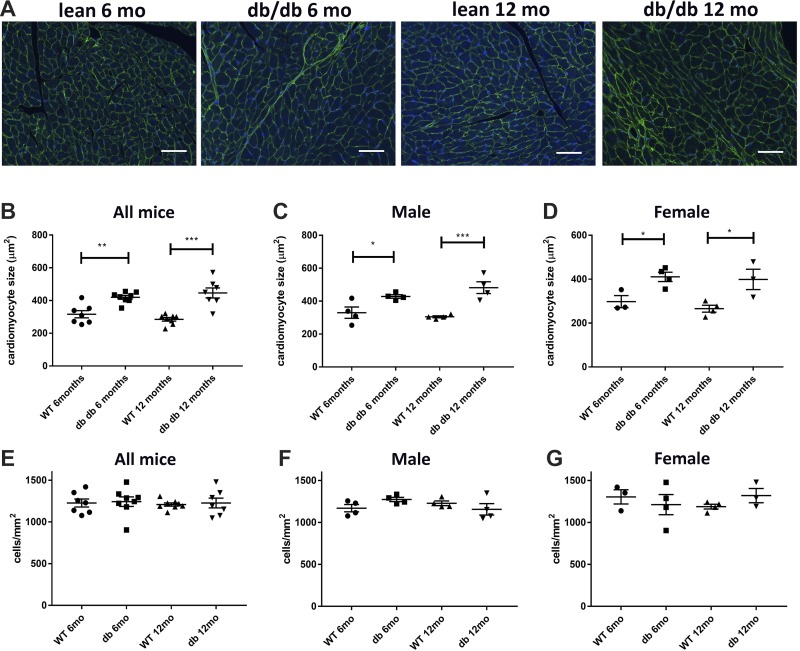
Both male and female db/db mice exhibit cardiomyocyte hypertrophy.
WGA histochemistry was used to assess cardiomyocyte size in WT and db/db mice (Fig. 6A). At 6 and 12 mo of age, both male and female db/db mice had markedly larger cardiomyocytes than age-matched WT mice (Fig. 6, B–D). To examine the effects of obesity on the noncardiomyocyte compartment, we assessed the density of interstitial cells in WT and db/db mice. No significant differences in interstitial cellularity were noted between the groups at 6 and 12 mo of age (Fig. 6, E–G).
Fig. 6.
Obese diabetic db/db mice exhibit a marked increase in cardiomyocyte size without a significant increase in the density of interstitial cells. A: wheat germ agglutinin (WGA) lectin histochemistry was used to quantitatively assess cardiomyocyte size. DAPI staining was used for the quantification of interstitial cell density. B–D: both male and female db/db mice had a marked increase in cardiomyocyte size at 2 and 6 mo of age. E–G: interstitial cell density was comparable between groups (n = 4 male mice/group, n = 4 female mice/group, n = 8 male + female mice/group). Scale bar = 50 μm. *P < 0.05, **P < 0.01, and ***P < 0.001.
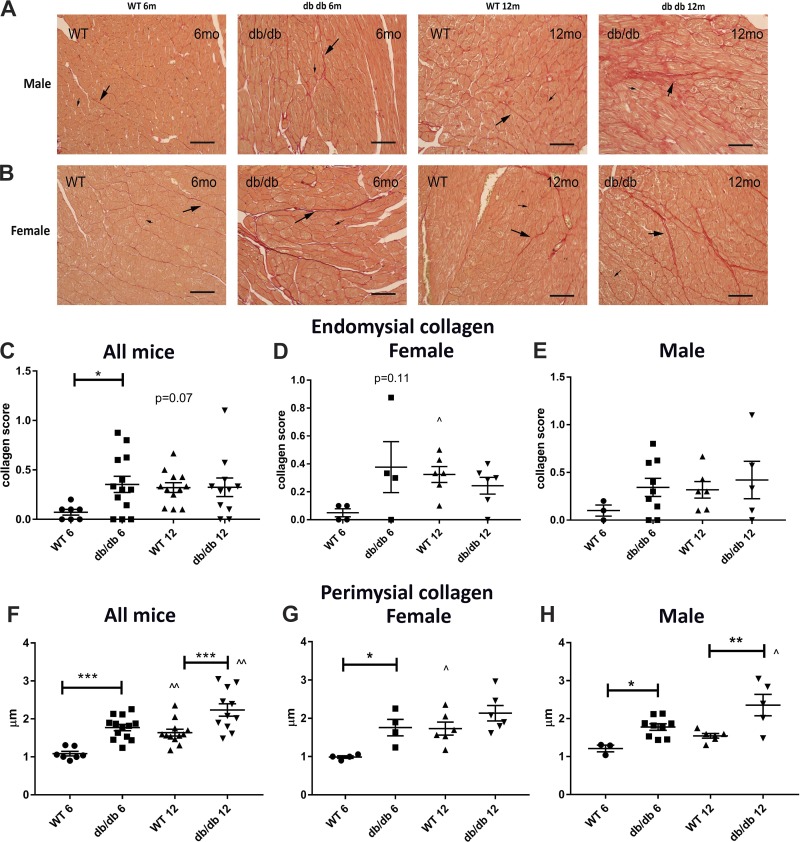
db/db mice have increased perimysial collagen thickness and a higher endomysial collagen content.
Endomysial collagen content and thickness of perimysial collagen were assessed in picrosirius red-stained sections (Fig. 7, A and B). Compared with age-matched WT mice, db/db mice had higher endomysial collagen content at 6 mo of age but not at 12 mo of age (Fig. 7C). Sex-specific analysis showed no significant differences in endomysial collagen content between sex-matched db/db and WT animals (Fig. 7, D and E). Compared with WT mice, db/db mice had markedly higher perimysial collagen thickness at 6 and 12 mo of age (Fig. 7F). Whereas male db/db mice had significantly higher perimyisial collagen thickness at both time points studied, the difference in female mice reached statistical significance only for the 6-mo time point (Fig. 7, G and H).
Fig. 7.
Obese diabetic db/db mice do not have replacement fibrosis but exhibit thickening of the perimysial collagen network and increased endomysial collagen. A and B: picrosirius red staining was used to identify perimysial collagen fibers that form the sheath that groups cardiomyocytes into bundles (long arrows) and endomysial collagen fibers surrounding each individual cardiomyocyte (short arrows). There was no evidence of replacement fibrosis in any of the db/db hearts. C–E: semiquantitative analysis showed that db/db mice have accentuated endomysial collagen at 6 mo of age. The increased endomysial collagen score in male or female mice did not reach statistical significance. F–H: perimysial collagen thickness was markedly increased in db/db mice at 6 and 12 mo of age. Both female and male mice had increased perimysial collagen thickness at 6 mo of age (n = 4 male mice/group, n = 4 female mice/group, n = 8 male + female mice/group). Scale bar = 50 μm. *P < 0.05, **P < 0.01, ***P < 0.001, ^P < 0.05, and ^^P < 0.01 vs. the corresponding 6-mo group.
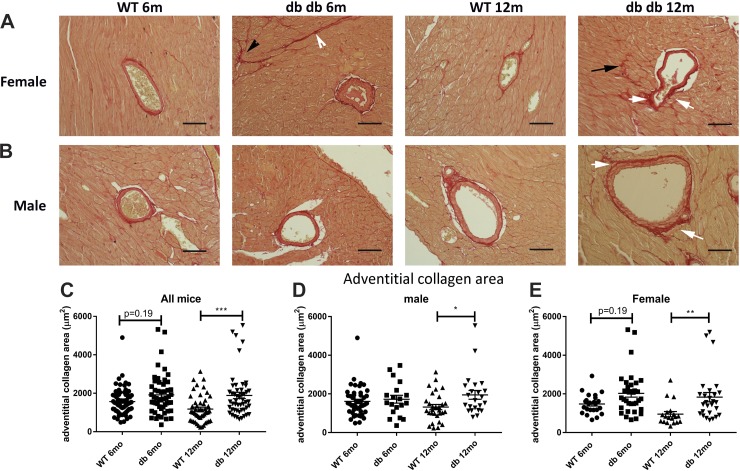
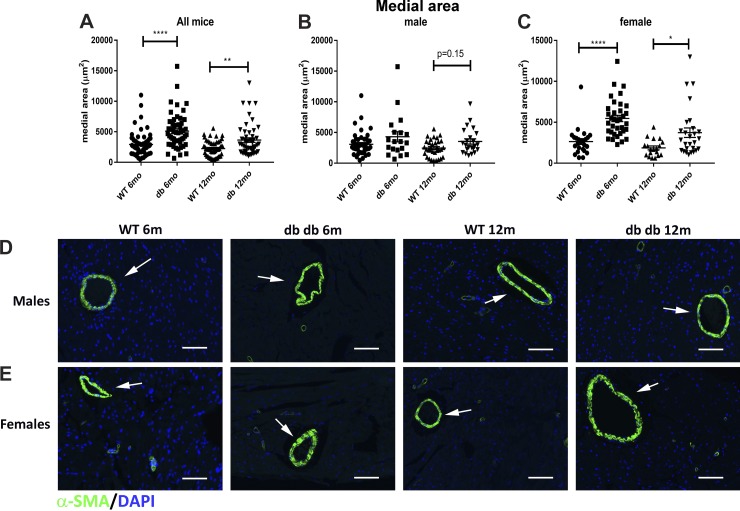
Coronary arterioles in db/db mice exhibit expansion of the media, associated with an increase in the area of perivascular collagen.
Cardiac fibrosis is typically associated with expansion of the perivascular adventitial collagen. We used picrosirius red-stained sections to quantitatively assess the adventitial collagen network in WT and db/db mice (Fig. 8, A and B). Quantitative analysis showed that the area of adventitial collagen was significantly increased in db/db mice at 12 mo of age (Fig. 8C). Although both male and female mice had increased adventitial collagen content, adventitial expansion was more marked in female mice (Fig. 8, D and E). Arterioles in db/db mice had a higher mean medial area than in age-matched WT mice (Fig. 9A). Arteriolar medial area was significantly increased in female db/db mice; in contrast, male mice showed a trend toward medial expansion (Fig. 9, B and C). Immunofluorescence for α-SMA showed the hypertrophy of arteriolar media in db/db hearts (Fig. 9, D and E).
Fig. 8.
db/db mice exhibit expansion of the periadventitial collagen network in coronary arterioles. A and B: periarteriolar collagen was identified using picrosirius red staining in male and female wild-type (WT) and db/db mouse hearts (white arrows). Please note the increased perimysial thickness (arrowheads) and the accentuated deposition of endomysial collagen (black arrow) in db/db mouse hearts (quantified in Fig. 7). C: quantitative analysis showed that at 12 mo of age, the periadventitial collagen area was higher in arterioles of db/db mice than in the corresponding vessels of WT mice. D and E: both male (D) and female (E) animals exhibited expansion of the periadventitial collagen area (n = 19–45 vessels/group for male mice, n = 19–35 vessels/group for female mice, n = 50–70 vessels/group for male + female mice). Scale bar = 50 μm. *P < 0.05, **P < 0.01, and ***P < 0.001.
Fig. 9.
Female db/db mice show significant hypertrophy of the coronary arteriolar media. A: quantitative analysis of the picrosirius red-stained sections (shown in Fig. 8) suggested that db/db mice had a significantly higher mean arteriolar area compared with wild-type (WT) mice at 6 and 12 mo of age. B: male mice had a trend toward increased arteriolar area at 12 mo of age. C: female mice had significantly higher arteriolar area at both 6- and 12-mo time points. D and E: α-smooth muscle actin (α-SMA) immunofluorescence showed hypertrophy of the arteriolar media (arrows) in female db/db mice (n = 19–45 vessels/group for male mice, n = 19–35 vessels/group for female mice, n = 50–70 vessels/group for male + female mice). Scale bar = 50 μm. *P < 0.05, **P < 0.01, and ****P < 0.0001.
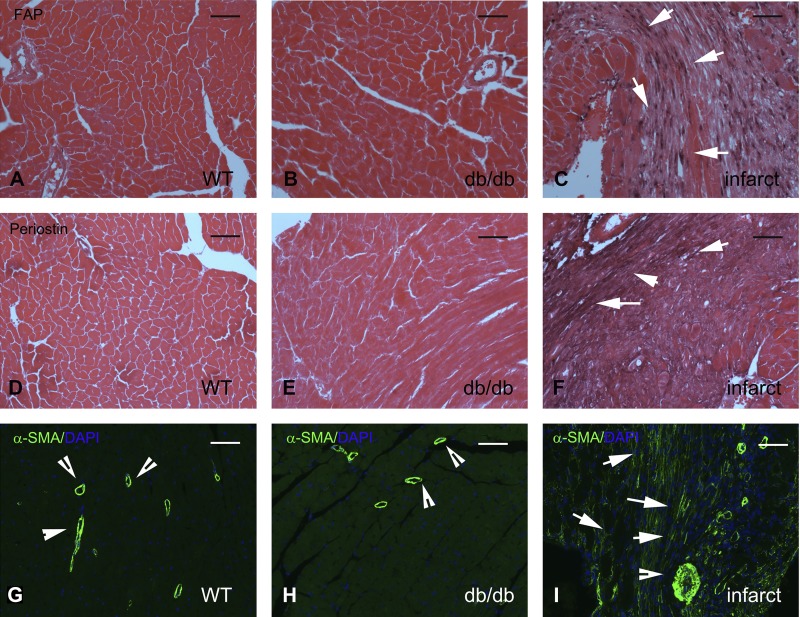
Fibroblasts in db/db mice do not undergo myofibroblast conversion.
In the fibrotic myocardium, deposition of collagen is typically associated with myofibroblast conversion, since activated fibroblasts incorporate contractile proteins, such as α-SMA, in the cytoskeleton and express matricellular proteins, such as periostin (14, 30). Moreover, in infarcted and remodeling hearts, fibroblasts express activation markers, such as FAP (50). In contrast to the robust expression of FAP in fibroblast-like cells infiltrating the healing infarct, WT and db/db mouse hearts showed negligible FAP immunoreactivity (Fig. 10, A–C). Moreover, fibroblasts in db/db hearts had no expression of the myofibroblast conversion markers periostin (Fig. 10, D–F) and α-SMA (Fig. 10, G–I).
Fig. 10.
Increased collagen deposition in db/db hearts is not associated with myofibroblast conversion. A–C: immunohistochemical staining for fibroblast activation protein (FAP), a marker for activated fibroblasts, in lean wild-type (WT; A), uninjured diabetic (B), and infarcted WT C57BL6J (C) mouse hearts. No FAP+ cells were noted in WT or diabetic myocardium. In contrast, abundant FAP+ fibroblasts infiltrated the infarcted myocardium 7 days after coronary occlusion (arrows in C). Images were counterstained with eosin. D–F: periostin staining in lean WT (D), uninjured diabetic (E), and infarcted WT C57BL6J (F) mouse hearts. In injury sites and in fibrotic tissues, activated myofibroblasts typically exhibited periostin expression. E: please note the complete absence of periostin immunoreactivity in db/db hearts. In contrast, infarcted hearts (F) exhibited periostin expression in activated myofibroblasts and in the surrounding extracellular matrix (arrows). G–I: α-smooth muscle actin (α-SMA) immunofluorescence was used to identify activated myofibroblasts as spindle-shaped immunoreactive cells located outside the vascular media. In uninjured WT (G) and db/db (H) hearts, α-SMA was exclusively localized in the arteriolar media (arrowheads). I: please note the abundant α-SMA-expressing myofibroblasts in the infarcted myocardium (arrows). Images show sections from 6-mo-old-mice representative of at least 4 different animals/group. Scale bar = 50 μm.
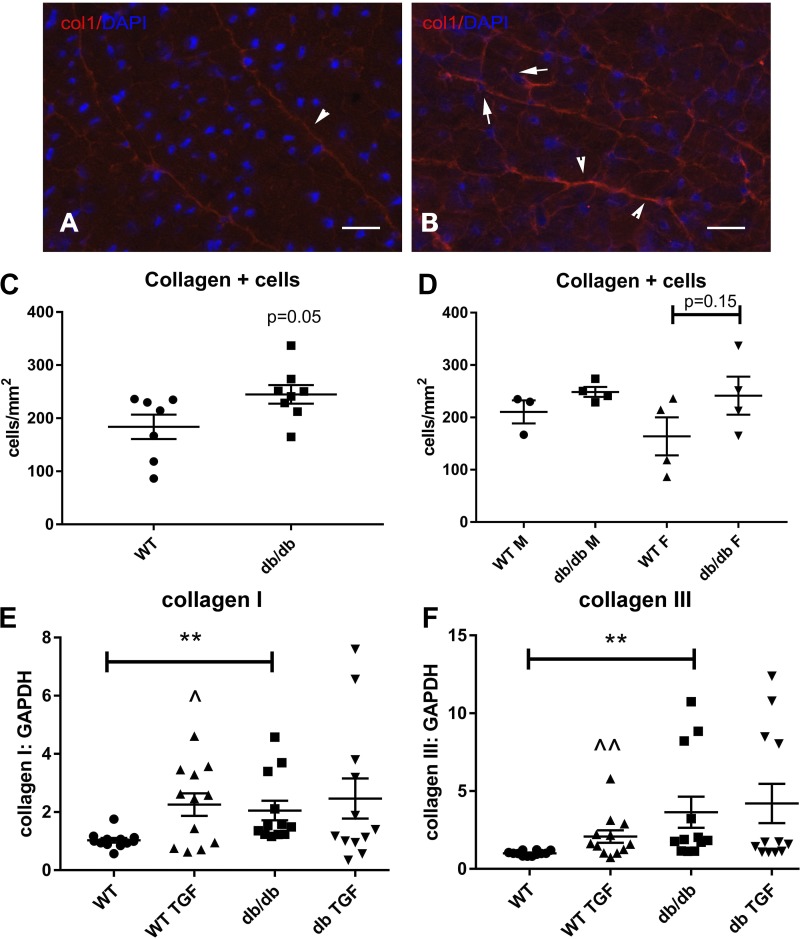
db/db mice exhibit expansion of the population of collagen-producing cells.
Increased synthesis of structural extracellular matrix proteins, such as collagens, is an indicator of fibroblast activation. We performed immunohistochemical staining for collagen type I using cryosections to compare the density of collagen-producing fibroblasts in WT and db/db hearts (Fig. 11, A and B). Fibroblasts were identified as collagen-immunoreactive cells. db/db hearts exhibited a trend toward higher number of collagen-positive fibroblasts (P = 0.05; Fig. 11C). Sex-specific analysis did not reveal significant differences in the density of collagen-positive cells between male and female animals (Fig. 11D).
Fig. 11.
Fibroblasts in db/db hearts show increased collagen synthesis. A and B: cryosections from 6-mo-old lean wild-type (WT) and db/db mouse hearts were stained with an anticollagen type I antibody. DAPI counterstaining was used to identify collagen type I-expressing interstitial cells. C: db/db hearts had a higher density of collagen type I-expressing interstitial cells that did not reach statistical significance (n = 8 hearts/group, P = 0.05). D: sex-specific analysis showed a trend toward higher density of collagen type I-expressing cells in female db/db hearts (n = 4 hearts/group). E and F: in vitro, cardiac fibroblasts harvested from 4-mo-old db/db mice had a 2.0- to 3.0-fold increase in baseline collagen type I (E) and type III (F) mRNA expression compared with fibroblasts from WT hearts. Activated fibroblasts from db/db hearts were less responsive to transforming growth factor (TGF)-β1 stimulation. TGF-β1 (10 ng/ml) stimulation for 4 h stimulated collagen type I and type III mRNA synthesis in WT cells but did not significantly increase expression of collagens in db/db fibroblasts (n = 7–8/group). Scale bar = 25 μm. **P < 0.01, ^P < 0.05, and ^^P < 0.01 vs. corresponding unstimulated cells. F, female; M, male.
Isolated cardiac fibroblasts from db/db mice have increased baseline expression of collagen but exhibit blunted responses to TGF-β1 stimulation.
In vitro, cardiac fibroblasts harvested from db/db mice had significantly higher collagen type I and type III mRNA expression compared with fibroblasts from WT mice (Fig. 11, E and F). TGF-β1 stimulation for 4 h induced collagen mRNA synthesis in WT but not in db/db fibroblasts (Fig. 11, E and F).
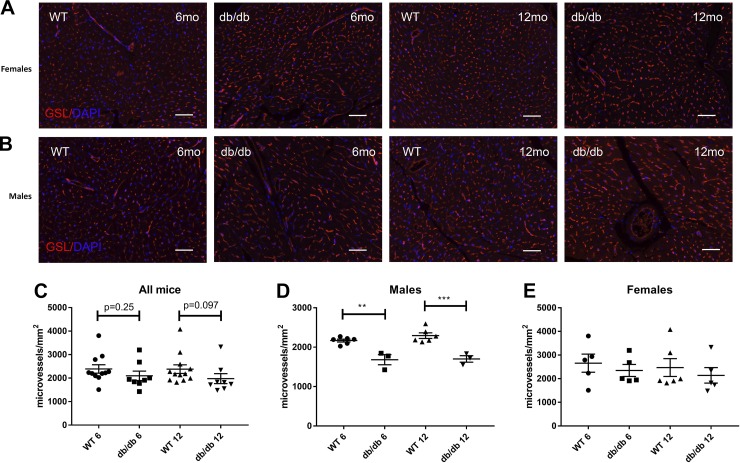
Male db/db mice exhibit significant microvascular rarefaction.
Microvascular density was quantitatively assessed in WT and db/db hearts using histochemical staining for GSL-I (Fig. 12, A and B). db/db mice had trends toward reduced microvascular density at 6 and 12 mo of age (Fig. 12C). Sex-specific analysis showed a significant reduction in microvascular density in male (Fig. 12E) but not female db/db mice (Fig. 12D) compared with age-matched WT control mice.
Fig. 12.
Male db/db mice had microvascular rarefaction. A and B: microvascular density was assessed in db/db and lean wild-type (WT) mouse hearts using Griffonia simplicifolia (GSL)-I lectin staining. C: db/db mice had a trend toward reduced microvascular density at 6 and 12 mo of age. D and E: although female db/db mice had comparable microvascular density with age-matched WT control mice, male mice exhibited a markedly lower microvascular density (n = 4 male mice/group, n = 4 female mice/group, n = 8 male + female mice/group). Scale bar = 50 μm. **P < 0.01 and ***P < 0.001.
DISCUSSION
Our study characterized the sex-specific functional, structural, and histopathological alterations in the db/db mouse model of type 2 diabetes, obesity, and metabolic dysfunction. Here, we report that 1) hyperglycemia and markedly increased adiposity in C57BL6J db/db mice are associated with increases in LV mass and with diastolic dysfunction, in the absence of a significant reduction in ejection fraction; 2) the functional changes in db/db mice are associated with cardiomyocyte hypertrophy, perimysial collagen thickening, mild endomysial and perivascular fibrosis, and vascular rarefaction; 3) fibrosis in db/db mice is not associated with generation of myofibroblast-like cells but involves acquisition of a matrix-synthetic phenotype by interstitial fibroblasts in vivo and in vitro; and 4) sex is a major determinant of the functional and structural alterations in db/db mice. Female db/db animals exhibit accentuated weight gain, hypertension, and exaggerated increases in LV mass, accompanied by worse diastolic dysfunction and early expansion of the perivascular collagen network, whereas male mice have accelerated microvascular rarefaction. Our findings highlight the sex-specific aspects of the cardiomyopathy associated with metabolic disease and show that the db/db mouse recapitulates important features of human HFpEF found in patients with diabetes, obesity, and metabolic dysfunction.
The contribution of metabolic dysregulation in the pathogenesis of a distinct clinical phenotype of human HFpEF.
In human patients, HFpEF involves a wide range of pathophysiological mechanisms that result in a spectrum of clinical phenotypes (32, 47). It has been suggested that, in contrast to HF with reduced ejection fraction, which is predominantly driven by cardiomyocyte loss, HFpEF reflects a myocardial response to extracardiac comorbidities (such as hypertension, diabetes, obesity, dyslipidemia, and renal dysfunction) that may stimulate myocardial microvascular inflammation, fibrosis, and cardiomyocyte hypertrophy, ultimately reducing compliance of the cardiac muscle (39, 47). Studies that have examined endomyocardial biopsy samples showed that patients with HFpEF have significant interstitial fibrosis, accompanied by infiltration with inflammatory leukocytes (54). Diabetes and obesity may accentuate inflammatory and fibrogenic signaling, leading to exaggerated interstitial remodeling in patients with HFpEF with metabolic dysfunction (8, 45). This notion is supported by the following clinical evidence: circulating levels of biomarkers reflecting inflammation and fibrosis, such as galectin-3 and COOH-terminal telopeptide of collagen type I, were higher in patients with diabetes versus nondiabetic patients with HFpEF (33). Whether the inflammatory activation and fibrogenic stimulation in diabetic hearts play a direct role in the pathogenesis of diastolic dysfunction remains unknown. Dissection of the cell biological events and molecular signals requires systematic characterization of experimental models of diabetic cardiomyopathy that recapitulate features of human HFpEF.
The db/db mouse as a model of metabolic disease-associated HFpEF.
Although the db/db mouse has been extensively studied to investigate the cellular basis and molecular mechanisms of diabetic cardiomyopathy, reported findings on the cardiac functional phenotype and structural characteristics of these animals are conflicting. Some studies have reported that, compared with lean controls, db/db mice have a significantly reduced ejection fraction, associated with cardiomyocyte apoptosis (3, 40, 48). In contrast, numerous other studies have suggested that db/db mice have preserved ejection fraction but develop premature diastolic dysfunction as they age (4, 18, 21, 22, 25, 43). Some investigations found no functional or structural differences between db/db mice and age-matched control mice (28). Several factors may explain the conflicting observations. First, genetic background is an important determinant of phenotype in db/db mice. C57BL/KsJ mice are more susceptible to the development of diabetes than C57BL6J mice in both genetic and streptozotocin-induced models (31) and may have accelerated cardiac involvement. Comparison of the cardiac phenotype in different strains of db/db mice has not been performed. Second, the female sex is associated with a greater predisposition for HFpEF in human patients (19) and with accentuated diastolic dysfunction in mouse models of diabetes and obesity (9). Many published investigations that have studied db/db mice were limited to the study of male animals; moreover, in the studies that have included both male and female animals, no sex-specific analysis was performed. Third, diet plays an important role in the severity of metabolic dysfunction in mouse models of obesity and may be a major factor driving development of complications. Differences in the composition of the diet used in various studies may explain, at least in part, conflicting observations. Fourth, the time points examined are different in various studies. Conflicting conclusions may reflect observations performed at different time points, capturing changes at different stages of the cardiomyopathic process in db/db mice. Finally, methodological differences in functional assessment of the ventricle between studies may affect the sensitivity and specificity of documentation of myocardial dysfunction.
Our study analyzed data from large populations of male and female WT and db/db mice in a C57BL6J background from our own colony. Our findings suggest that db/db mice in the C57BL6J background exhibit both functional and histopathological features of human HFpEF associated with metabolic dysfunction. db/db mice have evidence of diastolic dysfunction based on both TDI and hemodynamic assessment, in the absence of a significant reduction in ejection fraction (Figs. 3–5). The increased LV filling pressures observed in our present study (Fig. 5) may explain the left atrial remodeling previously reported in db/db mice (23). Assessment of structural parameters demonstrated that db/db animals had predominant hypertrophic LV remodeling, associated with a reduction in the global remodeling index (Fig. 3). Increases in LV mass and in LVEDP were accentuated in female db/db animals, highlighting the importance of gender in the cardiac phenotype in models of metabolic dysfunction. The increased susceptibility of female db/db mice may be related, at least in part, to the more impressive weight gain observed in female mice (Fig. 1) and to an exaggerated pressure load resulting from a modest but significant sex-specific increase in systemic blood pressure (Fig. 2).
Histopathological myocardial changes in db/db mice: cardiomyocyte hypertrophy, interstitial fibrosis, and vascular rarefaction.
Functional changes in db/db mice were associated with histopathological abnormalities. Cardiomyocyte size was markedly and consistently increased in both male and female db/db animals (Fig. 6). Although db/db hearts had no evidence of replacement fibrosis, they exhibited marked thickening of the perimysial collagen network, mild endomysial fibrosis associated with an increased density of interstitial collagen-expressing cells, and expansion of the collagenous adventitia of myocardial arterioles (Figs. 7 and 8).
Microvascular rarefaction has been previously documented in both human diabetic hearts and in animal models of diabetic cardiomyopathy (24, 59). Our study demonstrated a significant reduction in microvascular density in db/db mice, showing that capillary loss is more pronounced in male mice (Fig. 12). The cellular basis for microvascular loss in diabetic hearts is likely multifactorial and may involve pericyte loss, endothelial cell apoptosis, and diabetes-induced alterations in the composition of the extracellular matrix, leading to the induction and deposition of angiostatic matricellular proteins (15, 18).
Fibrosis in db/db mice does not involve myofibroblast conversion but is associated with increased fibroblast-derived collagen synthesis.
We demonstrate that, in contrast to the marked expansion of α-SMA-positive/periostin-positive interstitial cells observed in the infarcted myocardium (49, 55), db/db hearts do not contain significant numbers of α-SMA-positive myofibroblasts and have negligible expression of the matricellular protein periostin and the fibroblast activation marker FAP (Fig. 10). However, despite the absence of indicators of myofibroblast transdifferentiation in the db/db myocardium, cardiac fibroblasts harvested from db/db hearts exhibited a 2.0- to 3.0-fold increase in baseline collagen type I and type III mRNA synthesis (Fig. 11). This finding suggests that, in the diabetic heart, fibrotic remodeling does not require conversion of fibroblasts into myofibroblasts but may involve an alternative activation pathway that stimulates synthesis of structural extracellular matrix proteins, without inducing expression of contractile proteins, such as α-SMA.
The relative contribution of collagen type I and type III in the cardiac matrix network has been implicated in the regulation of myocardial compliance. Collagen type I forms thicker and stiffer fibers, whereas the finer reticular collagen type III fibers are more compliant. In patients with dilated cardiomyopathy, expansion of collagen type I fibers has been associated with reduced ventricular compliance (36). Whether diastolic dysfunction in diabetes, obesity, and metabolic dysfunction is due at least in part to perturbations of the collagen type I-to-type III ratio has not been investigated.
Cardiac fibrosis in mouse models of obesity and type 2 diabetes.
Fibrotic changes have been also described in other models of obesity and type 2 diabetes. Studies in leptin-deficient ob/ob mice have produced conflicting results. Although some studies have reported pericoronary fibrosis in ob/ob mice (57), other investigations found no significant fibrotic changes in the ob/ob myocardium (52). Documentation of cardiac fibrosis in mouse models of diet-induced metabolic dysfunction is dependent on the type and duration of the diet used, the sex and strain of the mice, and the sensitivity of the methodology used to assess fibrotic changes. Administration of a high-fat diet for 8–16 mo caused a significant increase in myocardial collagen type I and type III levels in male C57BL6J mice (7). High-fat diets with a high content of simple carbohydrates may accelerate the development of fibrosis. Male C57BL/6 mice fed a high-fat diet that was also rich in simple carbohydrates for 6 mo exhibited increased deposition of cross-linked collagen, associated with diastolic dysfunction (58). Male C57BL6 mice fed a high-fat diet containing high-fructose corn syrup exhibited myocardial fibrosis after 16 wk of feeding (26). Female mice may be more susceptible to fibrosis and diastolic dysfunction when fed a high-fat/high-fructose diet, exhibiting accentuated collagen type I deposition and increased myocardial stiffness after 8 wk of feeding (35).
Conclusions.
Our study shows that the db/db mouse in the C57BL6J background can serve as a pathophysiologically relevant model of human HFpEF associated with metabolic dysfunction. Much like in human patients with diabetes and obesity-associated HFpEF, db/db mice exhibit evidence of diastolic dysfunction with preserved ejection fraction, associated with cardiomyocyte hypertrophy, interstitial fibrosis, and microvascular rarefaction. Interpretation of studies using db/db mice requires careful consideration of sex-specific effects. The absence of myofibroblast conversion in db/db hearts highlights the distinct mechanisms of fibroblast activation in the repair of the infarcted heart and in metabolic dysfunction.
GRANTS
N. Frangogiannis’ laboratory is supported by National Heart, Lung, and Blood Institute Grants R01-HL-76246 and R01-HL-85440 and by Department of Defense Grants PR151134 and PR151029.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.A. and N.G.F. conceived and designed research; L.A., I.R., and V.H. performed experiments; L.A., I.R., V.H., and N.G.F. analyzed data; L.A., I.R., V.H., and N.G.F. interpreted results of experiments; L.A., I.R., and N.G.F. prepared figures; L.A. and N.G.F. drafted manuscript; L.A., I.R., and N.G.F. edited and revised manuscript; L.A., I.R., V.H., and N.G.F. approved final version of manuscript.
REFERENCES
- 1.Abbate A, Arena R, Abouzaki N, Van Tassell BW, Canada J, Shah K, Biondi-Zoccai G, Voelkel NF. Heart failure with preserved ejection fraction: refocusing on diastole. Int J Cardiol 179: 430–440, 2015. doi: 10.1016/j.ijcard.2014.11.106. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez JA, Reyes M, Escobedo D, Freeman GL, Steinhelper ME, Feldman MD. Enhanced left ventricular systolic function early in type 2 diabetic mice: clinical implications. Diab Vasc Dis Res 1: 89–94, 2004. doi: 10.3132/dvdr.2004.013. [DOI] [PubMed] [Google Scholar]
- 3.Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, Kittleson MM, Minhas KM, Berkowitz DE, Wei C, Hare JM. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res 98: 119–124, 2005. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 4.Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, Gonzalez-Quesada C, Rai V, Dobaczewski M, Lee DW, Wang XF, Frangogiannis NG. Smad3 signaling promotes fibrosis while preserving cardiac and aortic geometry in obese diabetic mice. Circ Heart Fail 8: 788–798, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley LF, Canada JM, Del Buono MG, Carbone S, Trankle CR, Billingsley H, Kadariya D, Arena R, Van Tassell BW, Abbate A. Low NT-proBNP levels in overweight and obese patients do not rule out a diagnosis of heart failure with preserved ejection fraction. ESC Heart Fail 5: 372–378, 2018. doi: 10.1002/ehf2.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation 116: 2127–2138, 2007. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 7.Calligaris SD, Lecanda M, Solis F, Ezquer M, Gutiérrez J, Brandan E, Leiva A, Sobrevia L, Conget P. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS One 8: e60931, 2013. doi: 10.1371/journal.pone.0060931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalera M, Wang J, Frangogiannis NG. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl Res 164: 323–335, 2014. doi: 10.1016/j.trsl.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandramouli C, Reichelt ME, Curl CL, Varma U, Bienvenu LA, Koutsifeli P, Raaijmakers AJA, De Blasio MJ, Qin CX, Jenkins AJ, Ritchie RH, Mellor KM, Delbridge LMD. Diastolic dysfunction is more apparent in STZ-induced diabetic female mice, despite less pronounced hyperglycemia. Sci Rep 8: 2346, 2018. doi: 10.1038/s41598-018-20703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495, 1996. doi: 10.1016/S0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen JX, Zeng H, Reese J, Aschner JL, Meyrick B. Overexpression of angiopoietin-2 impairs myocardial angiogenesis and exacerbates cardiac fibrosis in the diabetic db/db mouse model. Am J Physiol Heart Circ Physiol 302: H1003–H1012, 2012. doi: 10.1152/ajpheart.00866.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, Frangogiannis NG. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem 61: 555–570, 2013. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 14: 591–602, 2017. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 14.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res 48: 89–100, 2000. doi: 10.1016/S0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 15.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 127: 1600–1612, 2017. doi: 10.1172/JCI87491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgiopoulou VV, Velayati A, Burkman G, Li S, Farooq K, Samman-Tahhan A, Papadimitriou L, Butler J, Kalogeropoulos AP. Comorbidities, sociodemographic factors, and hospitalizations in outpatients with heart failure and preserved ejection fraction. Am J Cardiol 121: 1207–1213, 2018. doi: 10.1016/j.amjcard.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Goh VJ, Le TT, Bryant J, Wong JI, Su B, Lee CH, Pua CJ, Sim CPY, Ang B, Aw TC, Cook SA, Chin CWL. Novel index of maladaptive myocardial remodeling in hypertension. Circ Cardiovasc Imaging 10: e006840, 2017. doi: 10.1161/CIRCIMAGING.117.006840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Quesada C, Cavalera M, Biernacka A, Kong P, Lee DW, Saxena A, Frunza O, Dobaczewski M, Shinde A, Frangogiannis NG. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ Res 113: 1331–1344, 2013. doi: 10.1161/CIRCRESAHA.113.302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gori M, Lam CS, Gupta DK, Santos AB, Cheng S, Shah AM, Claggett B, Zile MR, Kraigher-Krainer E, Pieske B, Voors AA, Packer M, Bransford T, Lefkowitz M, McMurray JJ, Solomon SD, Investigators P; PARAMOUNT Investigators . Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail 16: 535–542, 2014. doi: 10.1002/ejhf.67. [DOI] [PubMed] [Google Scholar]
- 20.Gros D, Bruce B, Challice CE, Schrevel J. Ultrastructural localization of concanavalin A and wheat germ agglutinin binding sites in adult and embryonic mouse myocardium. J Histochem Cytochem 30: 193–200, 1982. doi: 10.1177/30.3.7061821. [DOI] [PubMed] [Google Scholar]
- 21.Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, Barron B, Mayoux E, Rector RS, Whaley-Connell A, DeMarco VG. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol 16: 9, 2017. doi: 10.1186/s12933-016-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall ME, Maready MW, Hall JE, Stec DE. Rescue of cardiac leptin receptors in db/db mice prevents myocardial triglyceride accumulation. Am J Physiol Endocrinol Metab 307: E316–E325, 2014. doi: 10.1152/ajpendo.00005.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanif W, Alex L, Su Y, Shinde AV, Russo I, Li N, Frangogiannis NG. Left atrial remodeling, hypertrophy, and fibrosis in mouse models of heart failure. Cardiovasc Pathol 30: 27–37, 2017. doi: 10.1016/j.carpath.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinkel R, Howe A, Renner S, Ng J, Lee S, Klett K, Kaczmarek V, Moretti A, Laugwitz KL, Skroblin P, Mayr M, Milting H, Dendorfer A, Reichart B, Wolf E, Kupatt C. Diabetes mellitus-induced microvascular destabilization in the myocardium. J Am Coll Cardiol 69: 131–143, 2017. doi: 10.1016/j.jacc.2016.10.058. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson KR, Lord CK, West TA, Stewart JA Jr. Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS One 8: e72080, 2013. doi: 10.1371/journal.pone.0072080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia G, Habibi J, Bostick BP, Ma L, DeMarco VG, Aroor AR, Hayden MR, Whaley-Connell AT, Sowers JR. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension 65: 531–539, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med 347: 305–313, 2002. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 28.Ko KY, Wu YW, Liu CW, Cheng MF, Yen RF, Yang WS. Longitudinal evaluation of myocardial glucose metabolism and contractile function in obese type 2 diabetic db/db mice using small-animal dynamic 18F-FDG PET and echocardiography. Oncotarget 8: 87795–87808, 2017. doi: 10.18632/oncotarget.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koka S, Aluri HS, Xi L, Lesnefsky EJ, Kukreja RC. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1α signaling. Am J Physiol Heart Circ Physiol 306: H1558–H1568, 2014. doi: 10.1152/ajpheart.00865.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol 305: H1363–H1372, 2013. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leiter EH, Le PH, Coleman DL. Susceptibility to db gene and streptozotocin-induced diabetes in C57BL mice: control by gender-associated, MHC-unlinked traits. Immunogenetics 26: 6–13, 1987. doi: 10.1007/BF00345448. [DOI] [PubMed] [Google Scholar]
- 32.Lewis GA, Schelbert EB, Williams SG, Cunnington C, Ahmed F, McDonagh TA, Miller CA. Biological phenotypes of heart failure with preserved ejection fraction. J Am Coll Cardiol 70: 2186–2200, 2017. doi: 10.1016/j.jacc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Lindman BR, Dávila-Román VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, de las Fuentes L, Joseph SM, Vader J, Hernandez AF, Redfield MM. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 64: 541–549, 2014. doi: 10.1016/j.jacc.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol 314: H733–H752, 2018. doi: 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manrique C, DeMarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, Hayden MR, Sowers JR. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology 154: 3632–3642, 2013. doi: 10.1210/en.2013-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marijianowski MM, Teeling P, Mann J, Becker AE. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol 25: 1263–1272, 1995. doi: 10.1016/0735-1097(94)00557-7. [DOI] [PubMed] [Google Scholar]
- 37.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 136: 6–19, 2017. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Packer M, Kitzman DW. Obesity-related heart failure with a preserved ejection fraction: the mechanistic rationale for combining inhibitors of aldosterone, neprilysin, and sodium-glucose cotransporter-2. JACC Heart Fail 6: 633–639, 2018. doi: 10.1016/j.jchf.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Paulus WJ, Dal Canto E. Distinct myocardial targets for diabetes therapy in heart failure with preserved or reduced ejection fraction. JACC Heart Fail 6: 1–7, 2018. doi: 10.1016/j.jchf.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Plante E, Menaouar A, Danalache BA, Yip D, Broderick TL, Chiasson JL, Jankowski M, Gutkowska J. Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology 156: 1416–1428, 2015. doi: 10.1210/en.2014-1718. [DOI] [PubMed] [Google Scholar]
- 41.Reddy AK, Taffet GE, Li YH, Lim SW, Pham TT, Pocius JS, Entman ML, Michael LH, Hartley CJ. Pulsed Doppler signal processing for use in mice: applications. IEEE Trans Biomed Eng 52: 1771–1783, 2005. doi: 10.1109/TBME.2005.855709. [DOI] [PubMed] [Google Scholar]
- 42.Regan JA, Mauro AG, Carbone S, Marchetti C, Gill R, Mezzaroma E, Valle Raleigh J, Salloum FN, Van Tassell BW, Abbate A, Toldo S. A mouse model of heart failure with preserved ejection fraction due to chronic infusion of a low subpressor dose of angiotensin II. Am J Physiol Heart Circ Physiol 309: H771–H778, 2015. doi: 10.1152/ajpheart.00282.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reil JC, Hohl M, Reil GH, Granzier HL, Kratz MT, Kazakov A, Fries P, Müller A, Lenski M, Custodis F, Gräber S, Fröhlig G, Steendijk P, Neuberger HR, Böhm M. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J 34: 2839–2849, 2013. doi: 10.1093/eurheartj/ehs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem 50: 71–79, 2002. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 45.Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol 90: 84–93, 2016. doi: 10.1016/j.yjmcc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db -hGLUT4 mice. Am J Physiol Heart Circ Physiol 283: H976–H982, 2002. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- 47.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 134: 73–90, 2016. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen E, Li Y, Li Y, Shan L, Zhu H, Feng Q, Arnold JM, Peng T. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes 58: 2386–2395, 2009. doi: 10.2337/db08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shinde AV, Humeres C, Frangogiannis NG. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta 1863: 298–309, 2017. doi: 10.1016/j.bbadis.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, Galuppo P, Bauersachs J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol 87: 194–203, 2015. doi: 10.1016/j.yjmcc.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 51.Valero-Muñoz M, Backman W, Sam F. Murine models of heart failure with preserved ejection fraction: a “fishing expedition”. JACC Basic Transl Sci 2: 770–789, 2017. doi: 10.1016/j.jacbts.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van den Bergh A, Vanderper A, Vangheluwe P, Desjardins F, Nevelsteen I, Verreth W, Wuytack F, Holvoet P, Flameng W, Balligand JL, Herijgers P. Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness. Cardiovasc Res 77: 371–379, 2008. doi: 10.1093/cvr/cvm001. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Wang B, Wang Y, Tong Q, Liu Q, Sun J, Zheng Y, Cai L. Zinc prevents the development of diabetic cardiomyopathy in db/db mice. Int J Mol Sci 18: 18, 2017. doi: 10.3390/ijms18030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschöpe C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4: 44–52, 2011. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 55.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol 145: 868–875, 1994. [PMC free article] [PubMed] [Google Scholar]
- 56.Yue P, Arai T, Terashima M, Sheikh AY, Cao F, Charo D, Hoyt G, Robbins RC, Ashley EA, Wu J, Yang PC, Tsao PS. Magnetic resonance imaging of progressive cardiomyopathic changes in the db/db mouse. Am J Physiol Heart Circ Physiol 292: H2106–H2118, 2007. doi: 10.1152/ajpheart.00856.2006. [DOI] [PubMed] [Google Scholar]
- 57.Zaman AK, Fujii S, Goto D, Furumoto T, Mishima T, Nakai Y, Dong J, Imagawa S, Sobel BE, Kitabatake A. Salutary effects of attenuation of angiotensin II on coronary perivascular fibrosis associated with insulin resistance and obesity. J Mol Cell Cardiol 37: 525–535, 2004. doi: 10.1016/j.yjmcc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Zibadi S, Vazquez R, Moore D, Larson DF, Watson RR. Myocardial lysyl oxidase regulation of cardiac remodeling in a murine model of diet-induced metabolic syndrome. Am J Physiol Heart Circ Physiol 297: H976–H982, 2009. doi: 10.1152/ajpheart.00398.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zoja C, Cattaneo S, Fiordaliso F, Lionetti V, Zambelli V, Salio M, Corna D, Pagani C, Rottoli D, Bisighini C, Remuzzi G, Benigni A. Distinct cardiac and renal effects of ETA receptor antagonist and ACE inhibitor in experimental type 2 diabetes. Am J Physiol Renal Physiol 301: F1114–F1123, 2011. doi: 10.1152/ajprenal.00122.2011. [DOI] [PubMed] [Google Scholar]