Abstract
Although improvements in timing and approach for early reperfusion with acute coronary syndromes have occurred, myocardial injury culminating in a myocardial infarction (MI) remains a common event. Although a multifactorial process, an imbalance between the induction of proteolytic pathways, such as matrix metalloproteinases (MMPs) and endogenous tissue inhibitors of metalloproteinase (TIMPs), has been shown to contribute to this process. In the present study, a full-length TIMP-3 recombinant protein (rTIMP-3) was encapsulated in a specifically formulated hyaluronic acid (HA)-based hydrogel that contained MMP-cleavable peptide cross-links, which influenced the rate of rTIMP-3 release from the HA gel. The effects of localized delivery of this MMP-sensitive HA gel (HAMMPS) alone and containing rTIMP-3 (HAMMPS/rTIMP-3) were examined in terms of the natural history of post-MI remodeling. Pigs were randomized to one of the following three different groups: MI and saline injection (MI/saline group, 100-μl injection at nine injection sites, n = 7), MI and HAMMPS injection (MI/HAMMPS group; 100-μl injection at nine injection sites, n = 7), and MI and HAMMPS/rTIMP-3 injection (MI/HAMMPS/rTIMP-3 group; 20-μg/100-μl injection at nine injection sites, n = 7). Left ventricular (LV) echocardiography was serially performed up to 28 days post-MI. LV dilation, as measured by end-diastolic volume, and the degree of MI wall thinning were reduced by ~50% in the HAMMPS/rTIMP-3 group (P < 0.05). Furthermore, indexes of heart failure progression post-MI, such as LV filling pressures and left atrial size, were also attenuated to the greatest degree in the HAMMPS/rTIMP-3 group. At 28 days post-MI, HAMMPS/rTIMP-3 caused a relative reduction in the transcriptional profile for myofibroblasts as well as profibrotic pathways, which was confirmed by subsequent histochemistry. In conclusion, these findings suggest that localized delivery of a MMP-sensitive biomaterial that releases a recombinant TIMP holds promise as a means to interrupt adverse post-MI remodeling.
NEW & NOTEWORTHY The present study targeted a myocardial matrix proteolytic system, matrix metalloproteinases (MMPs), through the use of a recombinant tissue inhibitor of MMPs incorporated into a MMP-sensitive hydrogel, which was regionally injected using a large animal model of myocardial infarction. Left ventricular geometry and function and indexes of myocardial remodeling were improved with this approach and support the advancement of localized therapeutic strategies that specifically target the myocardial matrix.
Keywords: extracellular matrix, heart failure, matrix metalloproteinase, myocardial remodeling, tissue inhibitors of metalloproteinase
INTRODUCTION
Although advancements in the timing and approach for early reperfusion with acute coronary syndromes have resulted in improved survival, a degree of myocardial injury culminating in a myocardial infarction (MI) remains a common event. Within the MI region, a cascade of structural/biological events occurs, which include inflammation and the induction of a number of proteolytic pathways, such as matrix metalloproteinases (MMPs) (3, 9, 11, 17, 18, 26, 28, 33, 39). Although the substrates for these MMPs were canonically thought to be extracellular matrix proteins, such as collagens, it is now recognized that the substrate portfolio is diverse and includes processing a number of biological signaling molecules (1, 8, 24, 31). In clinical observational studies, plasma levels of MMPs increase significantly and have been related to the degree of adverse left ventricular (LV) remodeling in the early MI period (19, 22, 37). In animal studies, a cause-and-effect relationship between MMP induction and LV injury and remodeling in the context of ischemia has been established (2, 5, 10, 20, 21, 23, 29, 42). However, a past clinical study using an oral MMP pharmacological inhibitor in the early post-MI period yielded equivocal results (16). The reasons for the failure of this initial post-MI clinical trial were likely multifactorial and included problematic issues, from dosing and reaching therapeutic levels to that of small cohorts of patients enrolled from national and international sites with surprisingly modest degrees of LV remodeling post-MI. In a more recent study using doxycycline that putatively influenced MMP activity, LV functional outcomes were improved in patients with a significant MI (6, 7). Moreover, this clinical trial identified shifts in plasma profiles of the endogenous tissue inhibitors of metalloproteinase (TIMPs), which were associated with indexes of LV remodeling post-MI (38). Thus, targeting the MMP system in the post-MI context continues to hold therapeutic relevance, whereby more targeted approaches would be a logical research direction.
MMP activity is tightly controlled at several levels, including both transcriptional and posttranslational regulation (1, 9, 24, 31). With respect to posttranscriptional regulation, TIMPs have been shown to play an important role in the context of LV remodeling (1, 24, 31). For example, transgenic deletion of a specific TIMP, TIMP-3, was shown to exacerbate LV remodeling and dysfunction after an ischemic stimulus (5, 21, 23). In clinical observational studies, relative plasma TIMP-3 levels are reduced in the early post-MI period compared with the magnitude of MMP release (13). In a murine model of MI in which injection of an adenoviral construct expressing TIMP-3 was performed, indexes of adverse LV remodeling were reduced, and favorable effects on myocardial structure, such as a stabilized matrix, were reported (34). Thus, although it cannot be discounted that other TIMP types hold biological and pathophysiological relevance in the context of post-MI remodeling, these past murine TIMP-3 studies provided the rationale for advancing to translational studies in a large animal model. In initial large animal feasibility studies, myocardial supplementation of recombinant TIMP-3 (rTIMP-3) has been shown to attenuate adverse LV remodeling in the early postischemic period (2, 10). However, these past studies did not evaluate the effects of rTIMP-3 supplementation over a longer post-MI period or whether and to what degree a more controlled release of rTIMP-3 could be developed. Our laboratory has previously reported the concept of MMP-sensitive biomaterials, such as hyaluronic acid (HA)-based hydrogels, which may provide a means to provide localized release of biomolecules (29). In a short-term feasibility study, it was established that incorporation of rTIMP-3 into these designed HA hydrogels could be achieved and identified the potential for reducing adverse post-MI remodeling (29). However, whether this localized approach would influence LV function and geometry over a relevant post-MI time course (~1 mo) remained to be established. Accordingly, the first objective was to induce MI in a large animal model and perform myocardial injections of the designed MMP-sensitive HA gel for mediating rTIMP-3 release and to serially assess LV function and geometry.
Although a major focus on adverse LV remodeling post-MI has been on cardiomyocyte cell salvage and viability, it is recognized that another critical cell type emerges and proliferates with critical consequences on extracellular matrix remodeling: the fibroblast (9, 11, 14, 28, 33, 35). Specifically, an emergence of a myofibroblast phenotype occurs in the post-MI myocardium and may significantly influence extracellular matrix stability and remodeling. Past studies have suggested that alterations in TIMP levels can influence fibroblast viability and potentially differentiation into the myofibroblast phenotype (5, 10, 21, 40). Accordingly, the second objective of this study was to perform LV regional transcriptional profiling for myofibroblasts post-MI and to identify whether and to what degree localized HA gel-mediated release of rTIMP-3 would influence this myofibroblast signature. The third objective of this study was to determine if these shifts in transcriptional profiles would be translated into definable changes in myofibroblast density and extracellular structure.
METHODS
In this study, a full-length rTIMP-3 protein, rTIMP-3, was encapsulated in a specifically formulated HA-based hydrogel that contained a MMP-cleavable peptide, which influenced the rate of rTIMP-3 release from the HA gel. The specific methodological and biochemical details for rTIMP-3 and the MMP-sensitive HA gel have been previously reported (10, 29) and therefore are only briefly outlined below. The major focus and important new direction for the present study was to examine the effects of localized delivery of this MMP-sensitive HA gel (heretofore designated as HAMMPS) alone and containing rTIMP-3 (HAMMPS/rTIMP-3) on the natural history of post-MI remodeling.
For this study, a previously described pig model of surgically induced MI was used (10) whereby serial measurements of LV function and geometry were performed. MI induction in this species results in uniformity of MI size and predictable temporal changes in MI expansion and LV geometry (10, 12). All animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed., Washington, DC: The National Academies Press, 2011), and all protocols were approved by the University of South Carolina’s Institutional Animal Care and Use Committee. Serial experiments were carried out until 28 days post-MI because this time period encompasses a rapid change in LV geometry and function in both animals and patients (3, 6, 7, 33). After the final set of LV function measurements, LV regions were subjected to mRNA analysis for myofibroblast phenotype expression (14, 32, 35, 41).
rTIMP-3 protein synthesis and MMP-sensitive HA gel.
Human full-length rTIMP-3 was expressed in a Chinese hamster ovary cell line using a vector with a cytomegalovirus promoter, whereby conditioned media was concentrated and purified by size exclusion chromatography (Ni-NTA resin, Qiagen, Valencia, CA) (10). Using a validated global MMP fluorescent peptide assay, inhibition of MMP activity occurred with increasing concentrations of either rTIMP-3 with an approximate 50% inhibitory concentration of 2–6 μg/ml (0.4–5 nM) in a manner consistent with native TIMP-3.
The objectives for the HAMMPS gel formulation for rTIMP-3 delivery were threefold: 1) to generate a HAMMPS gel amenable to myocardial injection through a conventional syringe, 2) the HAMMPS gel would only degrade in the presence of active MMPs, and 3) to minimize passive release of encapsulated rTIMP-3. The specific chemistry, in vitro validation experiments, and short-term HAMMPS gel injection experiments that accomplished these objectives have been previously described in detail (29). Briefly, we developed a three-component polymer system consisting of the following: 1) HA modified with aldehydes, 2) dextran sulfate modified with aldehydes, and 3) HA modified with a peptide consisting of a hydrazide end group. When mixed, these polymers cross-link through the reaction of aldehydes and hydrazides into a hydrazone bond. Dextran sulfate was incorporated into the hydrogels to act as a heparin mimetic to retain TIMP-3 in the hydrogel through electrostatic interactions until gel degradation. The peptide GGRMSMPV was included to introduce specific susceptibility to MMP-mediated proteolysis into the gel (29). This approach resulted in an HA gel that would release rTIMP-3 with increasing concentrations of an active MMP (29).
MI induction and myocardial injection of hydrogels.
For this protocol, pigs (n = 21, 20 kg, male) were randomized to one of the following three different groups: MI and saline injection (MI/saline group; 100-μl injection at nine injection sites, n = 7), MI and HAMMPS injection (MI/HAMMPS group; 100-μl injection at nine injection sites, n = 7), and MI and HAMMPS/rTIMP-3 injection (MI/HAMMPS/rTIMP-3 group; 20-μg/100-μl injection at nine injection sites, n = 7). Before MI induction, pigs were administered amiodarone (200 mg po) and aspirin (81 mg po) for 3 days preoperatively and a broad-spectrum antibiotic [Draxxin (2.5 mg/kg im)] at least once preoperatively. On the evening before surgery, pigs were randomized using a random number table, and the treatment assignments were coded until the completion of the protocol. On the day of surgery, pigs were sedated [ketamine (22 mg/kg im), acepromazine (1.1 mg/kg im), and atropine (0.04 mg/kg im)], intubated, and then maintained on 2% isoflurane delivered in an oxygen-nitrous mixture (3:1 l/min). Through a left thoracotomy, the LV free wall was exposed, and the first two obtuse marginal arteries of the circumflex artery were ligated. This provides for a uniform and consistent magnitude of myocardial injury, as previously described (10, 12), and thereby removed this potential confounding factor from the experimental design. After coronary ligation, myocardial injections were performed as described in further detail below, and the incision was then closed. Buprenorphine (0.05 mg/kg im) was administered as presurgery analgesia. A cohort (n = 5) of age/weight-matched pigs was treated in identical fashion (sham procedures) and served as referent controls for myocardial biochemistry and histochemistry.
The HAMMPS precursor solutions (aldehyde and hydrazine solutions) were mixed in a sterile fashion, drawn into separate 1-ml syringes, and loaded into a dual-barrel syringe injection system (FibriJet blending connector, SA-3670, 27-gauge needle, Nordson Micromedics). This allowed for the aldehyde and hydrazine polymers to blend in equal ratios immediately at the point of injection, providing for in situ cross-linking. These injections were placed in the central portion of the MI through nine equivalently spaced 100-μl injections. This was achieved by temporarily suturing a 2 × 2 cm, 9-point grid onto the MI region and, using markings placed on the syringe, ensuring the depth of each injection was 0.4–0.5 cm. The same injection system was used for the saline delivery. Therefore, the total bulk volume of HAMMPS injected into the MI region was 900 μl. For the HAMMPS/rTIMP-3 injections, the formulation contained 20 μg rTIMP-3/100 μl within the aldehyde precursor. Thus, a total of 1.8 mg rTIMP-3 contained within the HAMMPS gel was injected into the targeted MI region. Because the HAMMPS gel releases rTIMP-3 in a responsive fashion dependent on local MMP activity, only estimates for local steady-state concentrations of rTIMP-3 for this system were possible. Using several assumptions, such as that the total injected myocardial region was 4 ml (2 × 2 × 1 cm, specific gravity of 1 cm3/ml) and that the HAMMPS gel would be degraded within 14 days (based on in vitro erosion experiments), and steady-state release kinetics, ~3 μg/ml rTIMP-3 would be released per day, which was within the computed in vitro EC50 for rTIMP-3 (10, 29). However, these computations for rTIMP-3 kinetics are based on steady-state release and do not reflect the responsive release associated with local MMP activity. Moreover, these computations do not take into account other confounding influences that would affect rTIMP-3 release, such as biophysical myocardial properties like compression of the HAMMPS gel.
Serial measurements of LV geometry and function, referent controls, and sampling.
The day before randomization and MI induction, animals were sedated [diazepam (200 mg po), Barr Laboratories, Pomona, NY] and echocardiography was performed (GE VIVID 7 Dimension Ultrasound System, M4S 1.5- to 4.3-MHz active matrix array sector transducer probe) to measure LV volumes, left atrial (LA) area, posterior LV free wall thickness, and ejection fraction, as previously described (2, 10). In addition, mitral valve inflow velocities and tissue Doppler were used to compute an estimate of pulmonary capillary wedge pressure. These indexes of LV function and geometry are shown in Table 1. Pigs were returned to the laboratory under identical sedation/study conditions at 3, 7, 14, 21, and 28 days post-MI. At the completion of the study interval, pigs were again anesthetized, the LV was harvested, and a 0.5-cm ring was cut from the central region of the MI, which was subsequently sectioned into equivalent quadrants containing the MI region, two border regions (anterior lateral and posterior lateral), and a remote region (posterior LV served by posterior descending artery). These sections were then processed for RNA extraction as well as formalin fixed for histology.
Table 1.
MMP-sensitive gel release of recombinant TIMP-3: effects on LV function and geometry
| EF, % | LVEDV, ml | LVESV, ml | LVPWThd, cm | LV Mass, g | LA Area, cm2 | E/A | PCWP, mmHg | |
|---|---|---|---|---|---|---|---|---|
| Baseline (n = 21) | ||||||||
| Day 0 | 60.1 ± 0.7 | 37.3 ± 0.9 | 11.8 ± 0.7 | 0.79 ± 0.01 | 66.3 ± 1.14 | 6.60 ± 0.16 | 1.15 ± 0.02 | 4.01 ± 0.07 |
| MI/saline (n = 7) | ||||||||
| Day 3 | 45.8 ± 1.9* | 68.9 ± 4.0* | 38.8 ± 4.0* | 0.64 ± 0.01* | 80.2 ± 3.06* | 8.10 ± 0.45* | 1.33 ± 0.05* | 6.34 ± 0.71* |
| Day 7 | 42.9 ± 1.8* | 77.4 ± 4.8* | 45.4 ± 5.6* | 0.56 ± 0.03* | 79.9 ± 4.0* | 9.25 ± 0.36* | 1.45 ± 0.04* | 8.45 ± 1.17* |
| Day 14 | 39.2 ± 1.7* | 84.4 ± 5.5* | 50.2 ± 5.7* | 0.52 ± 0.02* | 85.3 ± 3.9* | 10.09 ± 0.43* | 1.56 ± 0.05* | 10.89 ± 1.09* |
| Day 21 | 37.4 ± 1.9* | 98.4 ± 9.4* | 57.2 ± 7.9* | 0.49 ± 0.03* | 90.3 ± 5.4* | 10.95 ± 0.43* | 1.70 ± 0.07* | 10.93 ± 0.90* |
| Day 28 | 34.4 ± 2.0* | 109.1 ± 10.5* | 66.4 ± 9.9* | 0.47 ± 0.03* | 94.3 ± 6.1* | 11.88 ± 0.29* | 1.80 ± 0.04* | 12.75 ± 1.00* |
| MI/HAMMPS (n = 7) | ||||||||
| Day 3 | 47.9 ± 1.5* | 62.4 ± 5.8* | 32.2 ± 4.0* | 0.69 ± 0.01*† | 82.2 ± 5.4* | 9.46 ± 0.40*† | 1.38 ± 0.05* | 5.94 ± 0.72* |
| Day 7 | 45.1 ± 1.8* | 69.9 ± 6.0* | 36.4 ± 4.1* | 0.60 ± 0.02* | 78.9 ± 4.5* | 9.74 ± 0.44* | 1.42 ± 0.05* | 6.68 ± 0.68* |
| Day 14 | 41.1 ± 2.0* | 78.4 ± 9.8* | 43.5 ± 7.7* | 0.57 ± 0.03* | 86.6 ± 7.3* | 10.83 ± 0.38* | 1.52 ± 0.05* | 8.26 ± 0.78* |
| Day 21 | 39.2 ± 2.1* | 91.0 ± 12.4* | 52.1 ± 10.8* | 0.53 ± 0.02* | 92.8 ± 8.7* | 11.55 ± 0.53* | 1.64 ± 0.07* | 9.56 ± 0.72* |
| Day 28 | 37.1 ± 2.0* | 102.4 ± 13.5* | 59.0 ± 9.8* | 0.49 ± 0.02* | 96.8 ± 8.7* | 12.59 ± 0.55* | 1.71 ± 0.07* | 10.44 ± 0.58* |
| MI/HAMMPS/rTIMP-3 (n = 7) | ||||||||
| Day 3 | 54.6 ± 0.9*†‡ | 50.0 ± 1.8*† | 20.5 ± 1.6*†‡ | 0.73 ± 0.02*† | 77.2 ± 2.0* | 7.87 ± 0.20*‡ | 1.26 ± 0.02* | 4.19 ± 0.11†‡ |
| Day 7 | 51.5 ± 1.6*†‡ | 55.4 ± 1.7*†‡ | 25.8 ± 2.5*† | 0.67 ± 0.02*† | 75.6 ± 2.4* | 8.56 ± 0.27*‡ | 1.30 ± 0.02*†‡ | 5.42 ± 0.49*† |
| Day 14 | 49.4 ± 1.8*†‡ | 60.1 ± 2.5*† | 31.7 ± 2.2*† | 0.63 ± 0.03*† | 74.1 ± 2.6*† | 9.28 ± 0.19*‡ | 1.37 ± 0.02*†‡ | 6.43 ± 0.36*† |
| Day 21 | 47.7 ± 1.5*†‡ | 66.8 ± 2.8*† | 34.2 ± 2.5* | 0.61 ± 0.03*†‡ | 82.4 ± 3.0* | 9.86 ± 0.20*†‡ | 1.46 ± 0.02*†‡ | 7.13 ± 0.43*†‡ |
| Day 28 | 45.5 ± 1.3*†‡ | 72.2 ± 2.5*†‡ | 38.1 ± 1.8*† | 0.60 ± 0.02*†‡ | 85.7 ± 2.4* | 10.27 ± 0.24*†‡ | 1.57 ± 0.03*† | 7.21 ± 0.42*†‡ |
Values are means ± SE; n, number of samples. EF, LV ejection fraction; LVEDV, left ventricular (LV) end-diastolic volume; LVESV, LV end-systolic volume; LVPWThd, diastolic LV posterior wall thickness; LA area, left atrial area; E/A, early-to-late transmitral filling velocity rates; PCWP, pulmonary capillary wedge pressure; MI, myocardial infarction; HAMMPS, matrix metalloproteinase (MMP)-sensitive hyaluronic acid (HA) gel; rTIMP-3, recombinant tissue inhibitor of metalloproteinase-3.
P < 0.05 vs. baseline;
P < 0.05 vs. the respective MI group only;
P < 0.05 vs. the respective MI/HAMMPS group.
Myocardial mRNA and profiles.
RNA was extracted from the LV samples (Experion Automated Electrophoresis System, Bio-Rad Laboratories, Hercules, CA) and reverse transcribed (iScript cDNA Synthesis Kit, Bio-Rad), and cDNA was amplified with the gene/pig-specific primer/probe sets (RT2 Profiler PCR Custom Array, Qiagen) shown in Table 2. Although there has been no definitive consensus on transcriptional profiles that define myofibroblasts, this array was designed to contain primers for representative markers of fibroblast transdifferentiation to myofibroblasts (10, 14, 32, 35, 41, 42). In addition, MMP and TIMP transcriptional profiles were also quantified within the designated MI region and are shown in Table 3. The reaction was performed (RT2 SYBR Green@qPCR Mastermix, Qiagen) and quantified by real-time PCR (CFX96 real-time PCR detection system, Bio-Rad). The real-time PCR fluorescence signal was converted to threshold cycle (Ct) and then normalized to GAPDH (ΔCt) and then to referent control samples (ΔΔCt). All PCR assays were performed in duplicate.
Table 2.
Porcine-specific PCR array: myofibroblast transcriptional profiling
| UniGene | GenBank No. | |
|---|---|---|
| ACTA | Ssc.53896 | NM_001164650 |
| CCN | Ssc.70001 | NM_213833 |
| COL1A1 | Ssc.55931 | XM_003483014 |
| COL6A3 | Ssc.12068 | XM_001928087 |
| FN1(EDA) | Ssc.16743 | XM_003133641 |
| ITGA3 | Ssc.78753 | XM_003131594 |
| LOX | Ssc.10386 | NM_001206403 |
| MYH14 | Ssc.93295 | XM_003127360 |
| P311 | Ssc.55114 | XM_003123844 |
| SPP1 | Ssc.23321 | NM_214023 |
| TGFB | Ssc.76 | NM_214015 |
| VIM | Ssc.42613 | XM_003130721 |
ACTA, α-smooth muscle actin; CCN, connective tissue growth factor; COL1A1, collagen type Iα1; COL6A, collagen type VIα; FN1, fibronectin (extra domain); ITGA3, α3-integrin (antigen CD49C α3-subunit of the VLA-3 receptor); LOX, lysyl oxidase; MYH14, myosin heavy chain 14; P311, P311 protein; SPP1, secreted phosphoprotein; TGFB, transforming growth factor-β; VIM, vimentin-like.
Table 3.
Porcine-specific PCR array: MMP and TIMP
| UniGene | GenBank No. | |
|---|---|---|
| COL3A1 | Ssc.24309 | NM_001243297 |
| LTBP-1 | Ssc.41162 | XM_003354867 |
| MMP-2 | Ssc.5713 | NM_214192 |
| MMP-7 | Ssc.548 | NM_214207 |
| MMP-13 | Ssc.16053 | XM_003129808 |
| MMP-14 | Ssc.734 | NM_214239 |
| TIMP-1 | Ssc.11784 | NM_213857 |
| TIMP-2 | Ssc.57257 | NM_001145985 |
| TIMP-3 | Ssc.16028 | XM_003126073 |
| TIMP-4 | Ssc.16027 | XM_003358524 |
COL3A1, collagen type IIIα1; LTBP-1, latent transforming growth factor-β-binding protein 1; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
Myocardial histochemistry.
LV formalin-fixed samples were embedded, sectioned (7 μm), and stained with picrosirius red for fibrillar collagen, and percent areas of collagen within the designated regions were computed using computer-assisted morphometry (10, 40). For this analysis, a minimum of five fields were digitized within each region. LV sections from the MI region were also subjected to immunostaining to localize cells that stained positive for α-smooth muscle actin (α-SMA; 1:100, A5228, Sigma). α-SMA-positive images were subjected to densitometry to quantitate α-SMA-positive cells, which, although imprecise, provides an index of myofibroblast density (12, 13, 25, 35).
Computation and data analysis.
Statistical analyses were performed (STATA, College Station, TX) whereby LV geometry and function were initially examined by two-way ANOVA in which time and treatment were considered main effects. Post hoc separation after ANOVA was performed using pairwise comparisons with a least-significant analysis (LSD module, STATA). For the histological measurements, because these were performed at one point in time, one-way ANOVA with post hoc comparisons was performed. If the assumption regarding equal variances between groups or normality in the data distribution were not met, then a nonparametric pairwise comparison between groups at matched time points was performed using a Wilcoxon test (nonparametric module, STATA). This was encountered for the mRNA measurements. Results are presented as means ± SE. Values of P < 0.05 were considered to be statistically significant.
RESULTS
All of the pigs randomized to the different MI treatment groups successfully completed the protocol. Baseline (pre-MI) values reflecting LV function and geometry are shown in Table 1 along with specific time points up to 28 days post-MI. As expected and consistent with a past report (40), a time-dependent fall in LV ejection fraction occurred post-MI irrespective of the treatment group. This was associated with a concordant increase in LV end-diastolic and systolic volume in the post-MI period. Using targeted M-mode ultrasound imaging, LV end-diastolic posterior wall thickness at the site of the MI decreased significantly from baseline values, indicative of myocyte loss and remodeling of this region. LV mass increased from baseline values, reflective of LV hypertrophy of the residual, viable myocardium. LA area increased, as did an index of LV filling rates, the Doppler-derived E-to-A ratio. A key physiological index of impaired LV performance and reflective of progression to LV failure (32), pulmonary capillary wedge pressure increased in the post-MI period. Although these indexes of LV function and geometry changed in all groups post-MI, the magnitude of these changes was attenuated in the MI/HAMMPS/rTIMP-3 group. Specifically, by 28 days post-MI, LV ejection fraction was higher, LV end-diastolic and systolic volumes were lower, and indexes of LV filling pressures (LA area and pulmonary capillary wedge pressure) were reduced in the MI/HAMMPS/rTIMP-3 group.
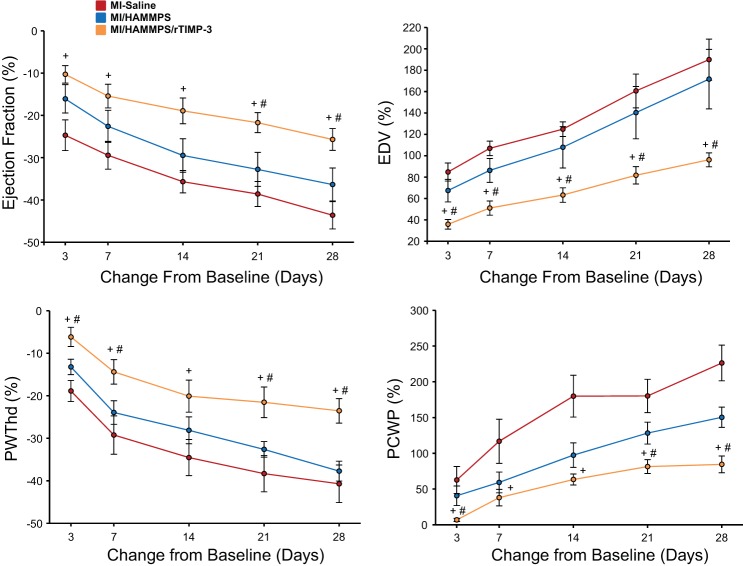
To more carefully examine the time-dependent changes in LV function and geometry post-MI, key response variables were transformed to a change from baseline (pre-MI) values and are shown in Fig. 1. Although LV ejection fraction decreased and LV end-diastolic volume increased in a time-dependent manner in all groups (ANOVA for time, P < 0.05), a significant treatment effect was also observed (ANOVA for treatment, P < 0.05). Individual pairwise analysis revealed that the changes in these response variables were significantly attenuated in the MI/HAMMPS/rTIMP-3 group compared with the MI-saline or MI/HAMMPS groups, particularly at later post-MI time points. Similarly, the degree of LV posterior wall thinning and increases in pulmonary capillary wedge pressure occurred in a time-dependent manner post-MI (ANOVA for time for both variables, P < 0.05). However, LV posterior wall thickness that incorporated the MI region was preserved to the greatest degree in the MI/HAMMPS/rTIMP-3 group, and the trajectory for pulmonary capillary wedge pressure was blunted.
Fig. 1.
To examine more carefully time-dependent changes in key response variables of left ventricular (LV) function and geometry, percent changes from baseline [before myocardial infarction (MI) induction] were computed. The absolute values for these variables are shown in Table 1. LV ejection fraction fell in a time-dependent manner post-MI in all groups, but the relative magnitude of this functional decline was blunted in the MI/matrix metalloproteainase (MMP)-sensitive hyaluronic acid (HA) gel (HAMMPS)/recombinant tissue inhibitor of metalloproteinase (rTIMP)-3 group. LV end-diastolic volume (EDV), a measure of LV dilation, increased significantly in all groups. For example, in the MI/saline group, LV end-diastolic volume increased by over 150% from baseline. However, at all post-MI time points, the degree of LV dilation was reduced in the MI/HAMMPS/rTIMP-3 group. LV end-diastolic posterior wall thickness (PWThd) fell significantly from baseline values, but the degree of LV thinning within this targeted MI region was reduced in the MI/HAMMPS/rTIMP-3 group. Pulmonary capillary wedge pressure (PCWP), an index of LV filling pressures, increased in a time-dependent manner post-MI, but in relation to MI/saline and MI/HAMMPS values was reduced in the MI/HAMMPS/rTIMP-3 group. In all of the data points shown, the change in values was significantly different from baseline (P < 0.05) and thus for clarity have not been marked. Sample sizes for each of the MI groups are shown in Table 1. +P < 0.05 vs. MI/saline; #P < 0.05 vs. MI/HAMMPS.
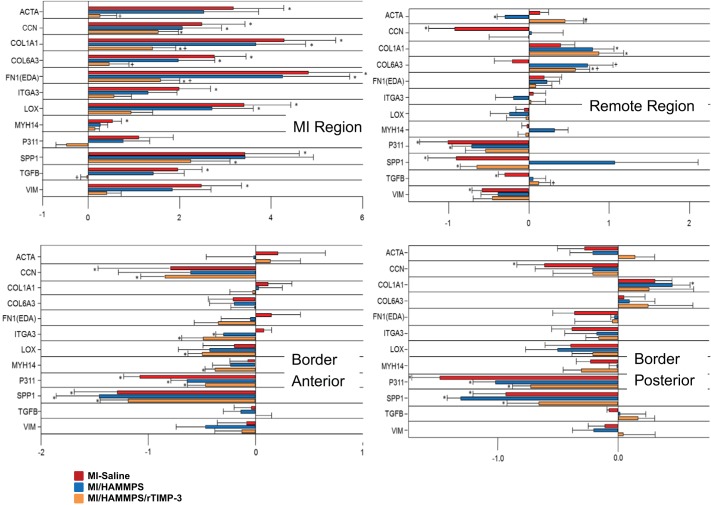
Transcriptional profiling for markers of myofibroblasts was performed within the MI, border, and remote regions and compared with referent control values. LV function and geometry in these referent controls were equivalent to those of the baseline values shown in Table 1. Relative mRNA levels normalized to these referent control values are shown in Fig. 2. Steady-state mRNA levels for a number of factors recognized to emerge in myofibroblasts and myofibroblast transdifferentiation were determined at 28 days post-MI within specific LV regions. Significantly different mRNA profiles were observed within the MI region, the border regions, and the remote regions. Within the MI region, a transcriptional profile consistent with myofibroblasts was increased in the MI/saline group, which included α-SMA, vimentin, myosin heavy chain 14, secreted phosphoprotein-1, fibronectin-1 EDA, and collagen type 6A3 (13, 21, 24, 32, 35, 39). In addition, factors associated with a profibrotic response and interaction with myofibroblasts, such as connective tissue growth factor, collagen type 1A, lysyl oxidase, and transforming growth factor-β, were increased in the MI/saline group. In the MI/HAMMPS group, a similar pattern of mRNA levels emerged within the MI region. However, this transcriptional profile for both myofibroblasts and profibrotic pathways was reduced in the MI/HAMMPS/rTIMP-3 group. A more heterogeneous transcription profile was observed in LV samples taken from the border regions, where a number of myofibroblast and transcriptional factors fell from referent normal values. Overall, the transcriptional profile was modestly affected by HAMMPS alone or HAMMPS/rTIMP-3 injection.
Fig. 2.
Steady-state mRNA levels for a number of factors recognized to emerge in myofibroblasts and myofibroblast transdifferentiation were determined at 28 days postmyocardial infarction (post-MI) within specific left ventricular (LV) regions. A LV midwall circumferential section was taken encompassing the midregion of the MI, the viable anterior region (anterior border), the viable posterior region (posterior border), and the region comprising the posterior descending artery (remote region). mRNA values are expressed as ΔΔCt values (where Ct is threshold cycle) (normalized to a standardized housekeeping gene and to referent control values) and constitute the x-axis. The full nomenclature and gene identification numbers are shown in Table 2. Significantly different mRNA profiles were observed within the MI region, the border regions, and the remote regions. Within the MI region, a transcriptional profile consistent with myofibroblasts was increased in the MI/saline group, which included smooth muscle actin (ACTA), vimentin (VIM), myosin heavy chain 14 (MYH14), secreted phosphoprotein-1 (SPP1), fibronectin-1 EDA [FN1(EDA)], and collagen type 6A3 (COL6A3). In addition, factors associated with a profibrotic response and interaction with myofibroblasts, such as connective tissue growth factor (CCN), collagen type 1A (COL1A1), lysyl oxidase (LOX), and transforming growth factor-β (TGFB), were increased in the MI/saline group. In the MI/HAMMPS group, the overall trend in mRNA levels was similar to that of the MI/saline group. However, this transcriptional profile for myofibroblasts and profibrotic pathways was reduced in the MI/matrix metalloproteainase (MMP)-sensitive hyaluronic acid (HA) gel (HAMMPS)/recombinant tissue inhibitor of metalloproteinase (rTIMP)-3 group. A more heterogeneous transcription profile was observed in LV samples taken from the border regions where a number of myofibroblast and transcriptional factors fell from referent normal values. A similar transcriptional pattern was observed in the LV remote region. Sample sizes for the different treatment groups are shown in Table 1. *P < 0.05 vs. ΔΔCt of 0 (i.e., different from referent control); +P < 0.05 vs. MI/saline; #P < 0.05 vs. HAMMPS.
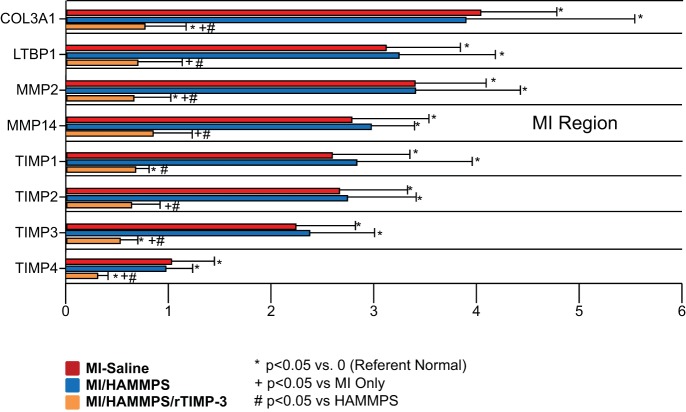
To examine more carefully whether and to what degree responsive release of rTIMP-3 would alter steady-state mRNA levels for endogenous MMPs and TIMPs, transcriptional profiling was performed (Table 3), and those values with significant changes from referent control values are shown in Fig. 3. MMP-2 and MMP-14 mRNA levels were increased in the MI/saline and MI/HAMMPS groups but reduced in the MI/HAMMPS/rTIMP-3 group. A similar shift in TIMP-1, TIMP-2, TIMP-3, and TIMP-4 levels was observed whereby TIMP levels within the MI region were increased by over twofold in the MI/saline and MI/HAMMPS groups, with a significant reduction in the MI/HAMMPS/rTIMP-3 group. These shifts in MMP/TIMP transcriptional profiles were also associated with shifts in the indexes of a profibrotic response, such as fibrillar collagen type 3a1 and latent TGF-β-binding protein (LTBP-1). The steady-state mRNA levels for collagen type 3a1 and LTBP-1 were increased by over threefold in the MI/saline and MI/HAMMPS groups and attenuated in the MI/HAMMPS/rTIMP-3 group.
Fig. 3.
Steady-state mRNA levels for a number of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinase (TIMPs) as well as for fibrillar collagen type 3a1 (COL3A1) and latent transforming growth factor-β-binding protein (LTBP-1) were assessed within the myocardial infarct (MI) region at 28 days post-MI. The full mRNA array, nomenclature, and gene identification numbers are shown in Table 3. mRNA values are expressed as ΔΔCt values (where Ct is threshold cycle) (normalized to a standardized housekeeping gene and to referent control values) and constitute the x-axis. The steady-state mRNA levels that were identified to have significant fold changes at the 28-day post-MI time point are shown. COL3A1 and LTBP-1 increased by over threefold in the MI/saline and MI/MMP-sensitive hyaluronic acid (HA) gel (HAMMPS) groups and were reduced in the MI/HAMMPS/recombinant (r)TIMP-3 group. MMP-2 and MMP-14 as well as endogenous TIMP-1, TIMP-2, TIMP-3, and TIMP-4 mRNA levels increased in the MI/saline and MI/HAMMPS groups with a significant reduction in the MI/HAMMPS/rTIMP-3 group. *P < 0.05 vs. ΔΔCt of 0 (i.e., different from referent control); +P < 0.05 vs. MI/saline; #P < 0.05 vs. HAMMPS.
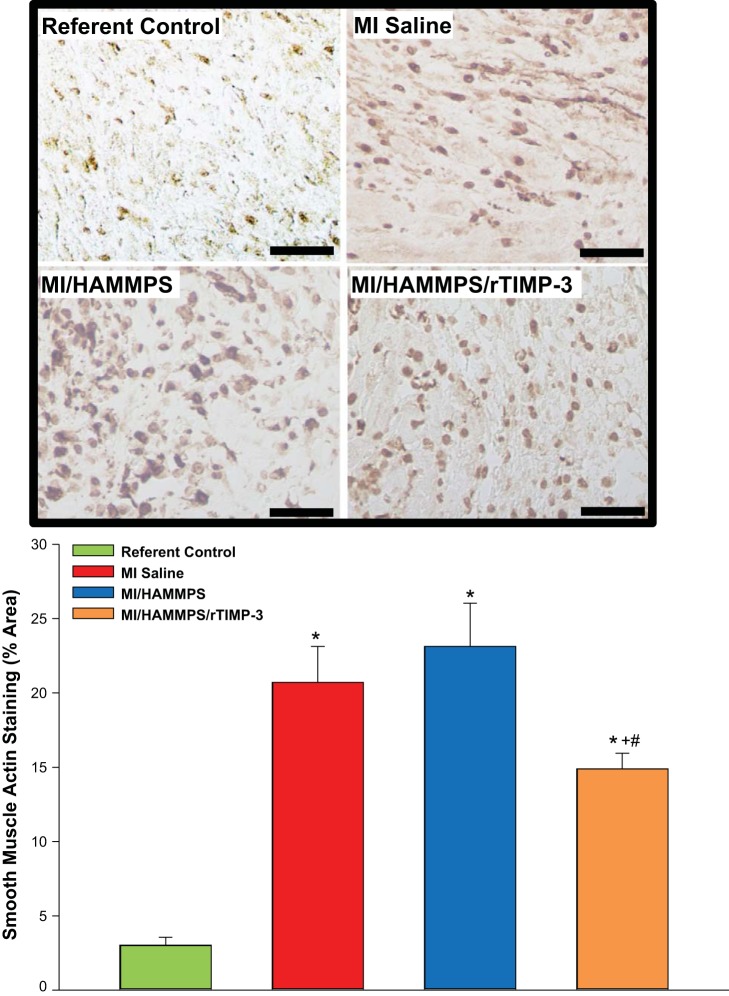
To determine if the observed changes in transcriptional profiles post-MI and in HAMMPS-mediated r-TIMP-3 delivery were translated into measurable posttranslational effects, histochemical staining and morphometric measurements were performed. Because the predominant changes in α-SMA mRNA levels were within the MI region, α-SMA staining of LV sections taken from this region was performed, and the results are shown in Fig. 4. Within referent control LV sections, positive α-SMA staining was primarily localized to the vasculature, but within the MI region it was most strongly associated with α-SMA-positive cells, putatively myofibroblasts. Robust α-SMA staining was observed within the MI region for the MI/saline and MI/HAMMPS groups but was reduced within the MI/HAMMPS/rTIMP-3 group. Thus, the directional changes observed with respect to α-SMA mRNA levels post-MI and with rTIMP-3 delivery were paralleled by changes in relative α-SMA content.
Fig. 4.
Top: representative α-smooth muscle actin (α-SMA) histochemical staining of left ventricular (LV) sections from the myocardial infarction (MI) region for all treatment groups and the corresponding LV region for referent controls. Within referent control LV sections, positive α-SMA staining was primarily localized to the vasculature, but within the MI region, it was most strongly associated with αSMA-positive cells, putatively myofibroblasts. Robust α-SMA staining was observed within the MI region for the MI/saline and MI/matrix metalloproteinase (MMP)-sensitive hyaluronic acid (HA) gel (HAMMPS) groups but appeared reduced within the MI/HAMMPS/recombinant tissue inhibitor of metalloproteinase (rTIMP)-3 group. Bottom: morphometric analysis for α-SMA staining density was performed, and a significant increase occurred in all MI groups compared with referent control values but was reduced in the MI/HAMMPS/rTIMP-3 group. Bar = 40 μm. *P < 0.05 vs. referent control; +P < 0.05 vs. MI/saline; #P < 0.05 vs. HAMMPS.
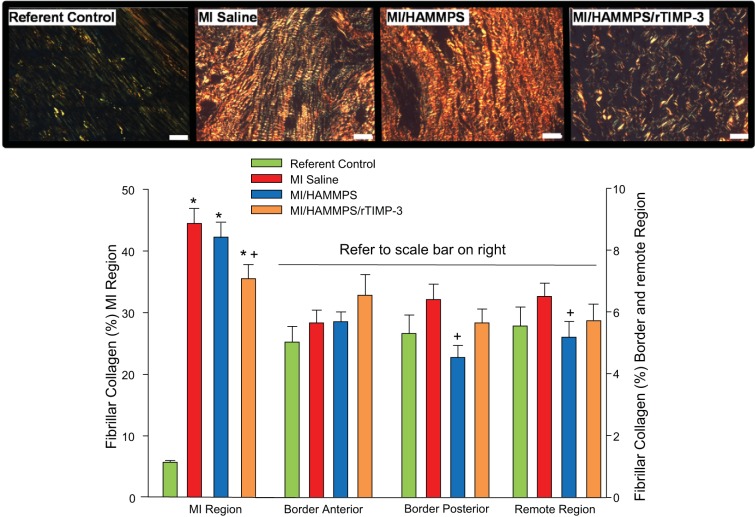
Relative LV myocardial content of fibrillar collagen was assessed by histomorphometric measurements of picrosirius red-positive staining, and these results are shown in Fig. 5. As expected, fibrillar collagen content increased substantially within the MI region in all groups at 28 days post-MI. However, fibrillar collagen content was reduced within the MI region in the MI/HAMMPS/rTIMP-3 group. Within the border and remote regions, a trend toward higher fibrillar collagen content was observed in the MI/saline and MI/HAMMPS/rTIMP-3 groups but did not reach statistical significance (P > 0.20). However, within the border-posterior and remote regions, relative fibrillar collagen content was reduced in the MI/HAMMPS group compared with MI/saline values.
Fig. 5.
Top: representative picrosirius red (PSR)-stained sections from the myocardial infarct (MI) region and corresponding referent control left ventricular (LV) region imaged using polarized light microscopy. As expected, fibrillar collagen content increased substantially within the MI region in all groups at 28 days post-MI, but the relative content and distribution appeared reduced within the MI/HAMMPS/rTIMP-3 group. Bottom: morphometric measurements of PSR-stained LV sections was performed and revealed a robust increase in fibrillar collagen content within the MI region, which was reduced in the MI/HAMMPS/rTIMP-3 group. Within the border and remote regions, a trend toward higher fibrillar collagen content was observed in the MI/saline and MI/matrix metalloproteinase (MMP)-sensitive hyaluronic acid (HA) gel (HAMMPS)/recombinant tissue inhibitor of metalloproteinase (rTIMP)-3 groups but did not reach statistical significance (P > 0.20). However, within the border-posterior and remote regions, relative fibrillar collagen content was reduced in the MI/HAMMPS group compared with MI/saline values. Note the scale difference for MI vs. other LV regions. Bar = 40 μm. *P < 0.05 vs. referent control; +P < 0.05 vs. MI/saline; #P < 0.05 vs. HAMMPS.
DISCUSSION
MI remains one of the leading causes of morbidity and mortality, whereby progressive changes in LV structure and function (post-MI remodeling) contribute to the development and progression of heart failure. Specifically, the rate and magnitude of LV dilation and the progressive rise in filling pressures, such as pulmonary capillary wedge pressure, are harbingers of poor outcomes and risk for heart failure. In this context, the predominant pharmacotherapies are those directed toward attenuation of the signs and symptoms of heart failure. Although a number of signaling and proteolytic pathways contribute to the adverse post-MI remodeling and progression to heart failure, the early and sustained induction of MMPs has been uniformly identified. Thus, targeting this pathway may provide a means to interrupt adverse post-MI remodeling and the progression to heart failure. In the present study, we built upon our previous feasibility report (29) whereby a HA hydrogel containing a MMP-sensitive element (HAMMPS) was loaded with rTIMP-3 (HAMMPS/rTIMP-3) and injected into the myocardium within the infarct after MI induction in pigs. There were several new and unique findings from the present study that significantly extended and expanded from our initial observations. First, targeted HAMMPS/rTIMP-3 injections significantly attenuated key indexes of LV remodeling, such as LV dilation and thinning of the MI region for up to 28 days post-MI compared with saline or HAMMPS injections alone. Second, indexes of HF progression, such as LA size and pulmonary capillary wedge pressure, were specifically reduced with HAMMPS/rTIMP-3 injections. Third, at 28 days post-MI, HAMMPS/rTIMP-3 injections altered the transcription profile for myofibroblasts within the MI region, which was translated into reduced α-SMA-positive cells within this region. Although additional studies are necessary, these findings suggest that localized delivery of a MMP-sensitive biomaterial that releases a rTIMP alters the natural history of post-MI remodeling and holds promise as a means to interrupt this inexorable process.
The present study builds on past reports using rTIMP-3 injections in the post-MI context in a number of important ways. Specifically, a past study from this laboratory incorporated rTIMP-3 in a hydrogel, which allowed for “passive” release into the MI region where a hydrogel sustained the release of rTIMP-3 through hydrolytic degradation (10). This study identified a beneficial effect of rTIMP-3 release in the early (14 days) post-MI period. The present study identified that using a MMP-sensitive hydrogel, which we previously developed to release rTIMP-3 in response to local proteolytic activity (29), further improves over the passive release approach with respect to LV dilation and dysfunction. Moreover, the present study identified that the effects of local rTIMP-3 release were sustained over a much longer (28 day) post-MI period. In a recent study (2), intracoronary delivery of rTIMP-3 at the time of MI induction in a pig model has been described. However, although this approach showed that rTIMP-3 was successfully delivered to the MI region, this recombinant molecule was cleared by ~5 days post-MI (2). In addition, the spatial control for rTIMP-3 delivery via the intracoronary approach is much less than that of specific targeted injections, such as those of the present study. Nevertheless, the study confirmed that a localized bolus of rTIMP-3 did reduce LV dilation in the early post-MI period. Taken together, these previous studies and the present report clearly suggest that localized delivery of a rTIMP, such as TIMP-3, can significantly modify the natural history of post-MI remodeling and the progression to heart failure.
One of the important findings of the present study was that not only did HAMMPS/rTIMP-3 delivery attenuate LV dilation and improve LV pump performance but also reduced key indexes of heart failure progression. Specifically, HAMMPS/r-TIMP3 injections reduced LA enlargement and pulmonary capillary wedge pressure, which both reflect a relative reduction in LV filling pressures. Concordant with these measurements, HAMMPS/r-TIMP3 also improved early LV filling times (lowered Doppler E/A), which also suggests reduced LV pressures at the onset of diastole. The favorable effects of improved passive LV filling with HAMMPS/r-TIMP3 would, in turn, be reflective of reduced pulmonary artery diastolic pressure (i.e., pulmonary capillary wedge pressure) and, in turn, reduced risk for pulmonary edema, the primary presenting clinical symptom of heart failure (32). However, it must be recognized that although the present study was performed in a large animal model, whether and to what degree these findings could be translated to a clinical context remains unknown. Specifically, the present study examined the effects of MMP-sensitive delivery of rTIMP-3 in the absence of other established pharmacotherapies (i.e., angiotensin-converting enzyme and β-receptor inhibition). Thus, to what degree HAMMPS/rTIMP-3 myocardial injections would attenuate adverse LV remodeling with conventional background therapeutics remains to be established.
The present study used a modified HA hydrogel construct that incorporated an MMP-sensitive domain to facilitate the release of a bioactive molecule (29). There are several considerations in this approach that should be taken into account. The approach uses an “on-demand” concept, in which therapeutic release is tuned to the local changes in proteolytic activity. Here, this releases an inhibitor that can block this activity; however, the approach could be used with numerous other therapeutics themselves or in combination with the protease inhibitors (30). The material design builds from HA, which is used in many clinical products (e.g., dermal fillers and viscosupplements) (4, 15); although we have not observed any significant inflammation, further investigation of inflammatory and immune responses may be needed to evaluate the overall biocompatibility of the specific material. Additionally, although the work illustrates proof of concept of on-demand release, further work could tailor the susceptibility of the peptides to specific protease levels or the cross-link density of the hydrogel to alter release profiles (27, 36). In a past study (10), denatured rTIMP-3 was incorporated into a HA gel formulation and demonstrated to eliminate any beneficial effects on post-MI remodeling, and, hence, the observations from the present study are likely due to the local release of a bioactive TIMP-3 protein. Although the present study incorporated a saline MI injection as well as a hydrogel formulation injection as comparator groups, the design did not include injection of rTIMP-3 independent of the hydrogel formulation, and this warrants further study.
Local myocardial injections of HAMMPS/rTIMP-3 resulted in a shift in the transcriptional myofibroblast transcriptional profile. Although a unifying definition and phenotype for myofibroblasts has not been established, the present study identified that recognized factors associated with fibroblast transdifferentiation were increased within the MI region at 28 days post-MI (12, 13, 21, 24, 32, 35, 39). A number of these factors, such as α-SMA and vimentin, are not necessarily specific for myofibroblasts. However, sampling from the central portion of the MI region in a permanent coronary occlusion model is likely to be predominated by fibroblasts and transdifferentiated myofibroblasts. Several other factors that also would be expected to be increased with a greater myofibroblast population include fibronectin-1 EDA, myosin heavy chain 14, and secreted phosphoprotein (12, 13, 35). Thus, at 28 days post-MI, the emergence of this transcriptional profile specifically within the MI region would likely represent the myofibroblast phenotype. In the present study, this transcriptional profile for myofibroblasts was selectively reduced in the HAMMPS/rTIMP-3 group. This observation would suggest that the biological underpinning for this shift in transcriptional profiles was due to the release of rTIMP-3 rather than a nonspecific effect of the MMP-sensitive HA gel alone. Moreover, this effect appeared to be restricted to the MI region, as the myofibroblast transcriptional profile within the border and remote zones was similar across all post-MI groups. It must be recognized that steady-state mRNA levels do not provide insights into myofibroblast density or distribution within the MI region, and therefore histochemical staining for α-SMA-positive cells was performed. The findings from this analysis paralleled those of the mRNA profiles for α-SMA and suggest that localized delivery of rTIMP-3 altered the density of myofibroblasts within the MI region. However, whether this is due to an inhibition of the fibroblast transdifferentiation process, an alteration in myofibroblast viability, or a combination of both remains to be determined.
In terms of a “fibrotic” transcriptional profile, the mRNA array used in the present study identified steady-state mRNA levels increased within the MI region for connective tissue growth factor and TGF-β in both the saline and HAMMPS groups but reduced with HAMMPS/rTIMP-3 injections. In turn, relative expression of key determinants of collagen synthesis and posttranslational control, such as collagen types IA and 3A1 as well as lysyl oxidase, were reduced with HAMMPS/rTIMP-3 injections. In addition, LTBP-1 and MMP-14 were both significantly reduced with regional release of rTIMP-3, which would have specific implications in terms of the profibrotic response. Specifically, LTBP-1 sequesters TGF-β and prevents binding to the cognate receptors, whereas MMP-14 cleaves LTBP-1 and causes release of TGF-β (31). Thus, the relative reduction in MMP-14 mRNA levels within the MI region with HAMMPS/r-TIMP-3 injections may have reduced LTBP-1 proteolysis and release of TGF-β. Although the relative reduction in a “profibrotic” mRNA signature occurred with rTIMP-3 supplementation, this does not imply that overall fibrillar collagen accumulation may have resulted. Accordingly, histochemical analysis was performed and revealed that, although not as robust as the relative reductions in mRNA collagen profiles, collagen content was reduced within the MI region with rTIMP-3 supplementation. Moreover, because these measurements were performed at 28 days post-MI when the biomaterial would have been fully degraded and rTIMP-3 release completed, it is unclear whether the shift in transcriptional profiles observed in the present study were due to the direct biological effects of rTIMP-3, biophysical effects of reduced adverse LV remodeling, or a combination thereof. For example, HAMMPS/rTIMP-3 injections reduced LV dilation and filling pressures and maintained a greater MI thickness throughout the 28-day post-MI period. These effects on LV geometry and pressure would be translated into a reduction in LV circumferential wall stress, particularly within the MI region, and thereby would reduce a biophysical stimulus for myofibroblast transdifferentiation and proliferation (13, 35). Nevertheless, the present study clearly identified that targeted injections of HAMMPS/rTIMP-3 at the time of MI induction caused a shift in transcriptional profiles relevant to extracellular matrix remodeling at 1 mo post-MI.
In past transgenic studies, it has been shown that TIMP-3 deletion exacerbated adverse LV remodeling in the setting of myocardial ischemia/infarction as well as in pressure overload (5, 20, 21). However, these past studies as well as others also identified that the biological effects of TIMP-3 are likely to extend beyond inhibition of active MMPs (1, 8, 24). For example, TIMP-3 can inhibit cytokine processing as well as influence cell growth and viability, including fibroblasts (1). Therefore, one mechanism that may have contributed to the relative reduction in α-SMA-positive fibroblasts within the MI region with rTIMP-3 delivery may have been an attenuation in the magnitude and duration of the proinflammatory cascade. Although it is likely that the effects of rTIMP-3 delivery altered MMP activity (at both transcriptional and posttranslational levels), it must be recognized that the effects of this TIMP extend beyond this single functionality. Moreover, it remains unclear whether other TIMPs released from this MMP-sensitive biomaterial would impart similar effects on post-MI remodeling. For example, in a transgenic mouse model, overexpression of TIMP-4 reduced the magnitude of post-MI remodeling (42). Future studies that determine whether and to what degree augmentation of TIMP levels within the MI produce a generalized effect on LV remodeling or are specific to the TIMP type are warranted. In addition, the present study performed myocardial injections at the time of MI induction rather than at a later post-MI time point. Thus, whether delayed myocardial injections of the MMP-sensitive biomaterial containing rTIMP-3 would alter the course of post-MI remodeling and slow the progression to heart failure remains to be established.
Elevated MMP plasma levels occur in patients and animals after MI and appear to serve as a predictor for the subsequent development of heart failure (9, 11, 17, 19, 22, 24, 28, 31, 33, 37–39). Although initial clinical trials using systemic inhibitors of MMPs in the post-MI setting were disappointing, results from recent clinical trials suggest that targeting the MMP system remains an important therapeutic target (6, 7, 16). The results from the present study provide a critical proof-of-concept study that localized delivery of a bioresponsive hydrogel into the MI region, which releases a recombinant TIMP in response to local MMP activity, can significantly attenuate adverse LV remodeling and key hemodynamic indexes of heart failure progression. Although future studies that are focused on issues of timing as well as tuning the response of the MMP-sensitive biomaterial are required, this approach holds promise as a specific therapeutic to halt the inexorable progression of post-MI remodeling and heart failure.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-130972 and HL-063954 and a Veterans Affairs Health Administration Merit Award.
DISCLOSURES
A. Y. Khakoo and T. Lee are employees of Amgen Incorporated, which manufactures drugs for a wide range of diseases, including cardiovascular diseases. The rTIMP-3 formulation was furnished as a material transfer agreement from Amgen to F. G. Spinale.
AUTHOR CONTRIBUTIONS
A.Y.K., T.L., J.A.B., and F.G.S. conceived and designed research; B.P.P., S.C.B., P.E.P., L.F., H.D., J.J., A.H., K.N.Z., J.A.B., and F.G.S. performed experiments; J.A.B. and F.G.S. analyzed data; J.A.B. and F.G.S. interpreted results of experiments; J.A.B. and F.G.S. prepared figures; J.A.B. and F.G.S. drafted manuscript; J.A.B. and F.G.S. edited and revised manuscript; J.A.B. and F.G.S. approved final version of manuscript.
REFERENCES
- 1.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 44-46: 247–254, 2015. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Barlow SC, Doviak H, Jacobs J, Freeburg LA, Perreault PE, Zellars KN, Moreau K, Villacreses CF, Smith S, Khakoo AY, Lee T, Spinale FG. Intracoronary delivery of recombinant TIMP-3 after myocardial infarction: effects on myocardial remodeling and function. Am J Physiol Heart Circ Physiol 313: H690–H699, 2017. doi: 10.1152/ajpheart.00114.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks GC, Lee BK, Rao R, Lin F, Morin DP, Zweibel SL, Buxton AE, Pletcher MJ, Vittinghoff E, Olgin JE; PREDICTS Investigators . Predicting persistent left ventricular dysfunction following myocardial infarction: the PREDICTS study. J Am Coll Cardiol 67: 1186–1196, 2016. doi: 10.1016/j.jacc.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 23: H41–H56, 2011. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carnevale D, Cifelli G, Mascio G, Madonna M, Sbroggiò M, Perrino C, Persico MG, Frati G, Lembo G. Placental growth factor regulates cardiac inflammation through the tissue inhibitor of metalloproteinases-3/tumor necrosis factor-α-converting enzyme axis: crucial role for adaptive cardiac remodeling during cardiac pressure overload. Circulation 124: 1337–1350, 2011. doi: 10.1161/CIRCULATIONAHA.111.050500. [DOI] [PubMed] [Google Scholar]
- 6.Cerisano G, Buonamici P, Parodi G, Santini A, Moschi G, Valenti R, Migliorini A, Colonna P, Bellandi B, Gori AM, Antoniucci D. Early changes of left ventricular filling pattern after reperfused ST-elevation myocardial infarction and doxycycline therapy: Insights from the TIPTOP trial. Int J Cardiol 240: 43–48, 2017. doi: 10.1016/j.ijcard.2017.03.125. [DOI] [PubMed] [Google Scholar]
- 7.Cerisano G, Buonamici P, Valenti R, Sciagrà R, Raspanti S, Santini A, Carrabba N, Dovellini EV, Romito R, Pupi A, Colonna P, Antoniucci D. Early short-term doxycycline therapy in patients with acute myocardial infarction and left ventricular dysfunction to prevent the ominous progression to adverse remodelling: the TIPTOP trial. Eur Heart J 35: 184–191, 2014. doi: 10.1093/eurheartj/eht420. [DOI] [PubMed] [Google Scholar]
- 8.Cieplak P, Strongin AY. Matrix metalloproteinases - from the cleavage data to the prediction tools and beyond. Biochim Biophys Acta 1864: 1952–1963, 2017. doi: 10.1016/j.bbamcr.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLeon-Pennell KY, Meschiari CA, Jung M, Lindsey ML. Matrix metalloproteinases in myocardial infarction and heart failure. Prog Mol Biol Transl Sci 147: 75–100, 2017. doi: 10.1016/bs.pmbts.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckhouse SR, Purcell BP, McGarvey JR, Lobb D, Logdon CB, Doviak H, O’Neill JW, Shuman JA, Novack CP, Zellars KN, Pettaway S, Black RA, Khakoo A, Lee T, Mukherjee R, Gorman JH, Gorman RC, Burdick JA, Spinale FG. Local hydrogel release of recombinant TIMP-3 attenuates adverse left ventricular remodeling after experimental myocardial infarction. Sci Transl Med 6: 223ra21, 2014. doi: 10.1126/scitranslmed.3007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 127: 1600–1612, 2017. doi: 10.1172/JCI87491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldsmith EC, Bradshaw AD, Zile MR, Spinale FG. Myocardial fibroblast-matrix interactions and potential therapeutic targets. J Mol Cell Cardiol 70: 92–99, 2014. doi: 10.1016/j.yjmcc.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gourdie RG, Dimmeler S, Kohl P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov 15: 620–638, 2016. doi: 10.1038/nrd.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammoud L, Lu X, Lei M, Feng Q. Deficiency in TIMP-3 increases cardiac rupture and mortality post-myocardial infarction via EGFR signaling: beneficial effects of cetuximab. Basic Res Cardiol 106: 459–471, 2011. doi: 10.1007/s00395-010-0147-7. [DOI] [PubMed] [Google Scholar]
- 15.Highley CB, Prestwich GD, Burdick JA. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol 40: 35–40, 2016. doi: 10.1016/j.copbio.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaber WA, Weaver WD. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol 48: 15–20, 2006. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 17.Iyer RP, de Castro Brás LE, Patterson NL, Bhowmick M, Flynn ER, Asher M, Cannon PL, Deleon-Pennell KY, Fields GB, Lindsey ML. Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution. J Mol Cell Cardiol 100: 109–117, 2016. doi: 10.1016/j.yjmcc.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer RP, Jung M, Lindsey ML. MMP-9 signaling in the left ventricle following myocardial infarction. Am J Physiol Heart Circ Physiol 311: H190–H198, 2016. doi: 10.1152/ajpheart.00243.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kampourides N, Tziakas D, Chalikias G, Papazoglou D, Maltezos E, Symeonides D, Konstantinides S. Usefulness of matrix metalloproteinase-9 plasma levels to identify patients with preserved left ventricular systolic function after acute myocardial infarction who could benefit from eplerenone. Am J Cardiol 110: 1085–1091, 2012. doi: 10.1016/j.amjcard.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 20.Kandalam V, Basu R, Abraham T, Wang X, Awad A, Wang W, Lopaschuk GD, Maeda N, Oudit GY, Kassiri Z. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am J Physiol Heart Circ Physiol 299: H1012–H1023, 2010. doi: 10.1152/ajpheart.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassiri Z, Defamie V, Hariri M, Oudit GY, Anthwal S, Dawood F, Liu P, Khokha R. Simultaneous transforming growth factor beta-tumor necrosis factor activation and cross-talk cause aberrant remodeling response and myocardial fibrosis in Timp3-deficient heart. J Biol Chem 284: 29893–29904, 2009. doi: 10.1074/jbc.M109.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly D, Khan SQ, Thompson M, Cockerill G, Ng LL, Samani N, Squire IB. Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J 29: 2116–2124, 2008. doi: 10.1093/eurheartj/ehn315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limana F, Esposito G, D’Arcangelo D, Di Carlo A, Romani S, Melillo G, Mangoni A, Bertolami C, Pompilio G, Germani A, Capogrossi MC. HMGB1 attenuates cardiac remodelling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PLoS One 6: e19845, 2011. doi: 10.1371/journal.pone.0019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey ML, Iyer RP, Jung M, DeLeon-Pennell KY, Ma Y. Matrix metalloproteinases as input and output signals for post-myocardial infarction remodeling. J Mol Cell Cardiol 91: 134–140, 2016. doi: 10.1016/j.yjmcc.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML. Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharmacol Sci 38: 448–458, 2017. doi: 10.1016/j.tips.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangion K, Carrick D, Hennigan BW, Payne AR, McClure J, Mason M, Das R, Wilson R, Edwards RJ, Petrie MC, McEntegart M, Eteiba H, Oldroyd KG, Berry C. Infarct size and left ventricular remodelling after preventive percutaneous coronary intervention. Heart 102: 1980–1987, 2016. doi: 10.1136/heartjnl-2015-308660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 31: 7836–7845, 2010. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 28.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119: 91–112, 2016. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN, Doviak H, Pettaway S, Logdon CB, Shuman JA, Freels PD, Gorman JH III, Gorman RC, Spinale FG, Burdick JA. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat Mater 13: 653–661, 2014. doi: 10.1038/nmat3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rufaihah AJ, Seliktar D. Hydrogels for therapeutic cardiovascular angiogenesis. Adv Drug Deliv Rev 96: 31–39, 2016. doi: 10.1016/j.addr.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Spinale FG, Janicki JS, Zile MR. Membrane-associated matrix proteolysis and heart failure. Circ Res 112: 195–208, 2013. doi: 10.1161/CIRCRESAHA.112.266882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spinale FG. Assessment of cardiac function - basic principles and approaches. Compr Physiol 5: 1911–1946, 2015. doi: 10.1002/cphy.c140054. [DOI] [PubMed] [Google Scholar]
- 33.Suthahar N, Meijers WC, Silljé HHW, de Boer RA. From inflammation to fibrosis-molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr Heart Fail Rep 14: 235–250, 2017. doi: 10.1007/s11897-017-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takawale A, Zhang P, Azad A, Wang W, Wang X, Murray AG, Kassiri Z. Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects by promoting angiogenesis and suppressing early proteolysis. Am J Physiol Heart Circ Physiol 313: H224–H236, 2017. doi: 10.1152/ajpheart.00108.2017. [DOI] [PubMed] [Google Scholar]
- 35.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res 118: 1021–1040, 2016. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wade RJ, Bassin EJ, Rodell CB, Burdick JA. Protease-degradable electrospun fibrous hydrogels. Nat Commun 6: 6639, 2015. doi: 10.1038/ncomms7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang KF, Huang PH, Chiang CH, Hsu CY, Leu HB, Chen JW, Lin SJ. Usefulness of plasma matrix metalloproteinase-9 level in predicting future coronary revascularization in patients after acute myocardial infarction. Coron Artery Dis 24: 23–28, 2013. doi: 10.1097/MCA.0b013e32835aab4a. [DOI] [PubMed] [Google Scholar]
- 38.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation 114: 1020–1027, 2006. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 39.Yan AT, Yan RT, Spinale FG, Afzal R, Gunasinghe HR, Stroud RE, McKelvie RS, Liu PP. Relationships between plasma levels of matrix metalloproteinases and neurohormonal profile in patients with heart failure. Eur J Heart Fail 10: 125–128, 2008. doi: 10.1016/j.ejheart.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarbrough WM, Mukherjee R, Escobar GP, Mingoia JT, Sample JA, Hendrick JW, Dowdy KB, McLean JE, Lowry AS, O’Neill TP, Spinale FG. Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarction remodeling. Circulation 108: 1753–1759, 2003. doi: 10.1161/01.CIR.0000091087.78630.79. [DOI] [PubMed] [Google Scholar]
- 41.Zamilpa R, Ibarra J, de Castro Brás LE, Ramirez TA, Nguyen N, Halade GV, Zhang J, Dai Q, Dayah T, Chiao YA, Lowell W, Ahuja SS, D’Armiento J, Jin YF, Lindsey ML. Transgenic overexpression of matrix metalloproteinase-9 in macrophages attenuates the inflammatory response and improves left ventricular function post-myocardial infarction. J Mol Cell Cardiol 53: 599–608, 2012. doi: 10.1016/j.yjmcc.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zavadzkas JA, Stroud RE, Bouges S, Mukherjee R, Jones JR, Patel RK, McDermott PJ, Spinale FG. Targeted overexpression of tissue inhibitor of matrix metalloproteinase-4 modifies post-myocardial infarction remodeling in mice. Circ Res 114: 1435–1445, 2014. doi: 10.1161/CIRCRESAHA.114.303634. [DOI] [PMC free article] [PubMed] [Google Scholar]