Abstract
Sudden cardiac death from ventricular arrhythmias is more common in adult patients with with heart failure compared with pediatric patients with heart failure. We identified age-specific differences in arrhythmogenesis using a guinea pig model of acute β-adrenergic stimulation. Young and adult guinea pigs were exposed to the β-adrenergic agonist isoproterenol (ISO; 0.7 mg/kg) for 30 min in the absence or presence of flecainide (20 mg/kg), an antiarrhythmic that blocks Na+ and ryanodine channels. Implanted cardiac monitors (Reveal LINQ, Medtronic) were used to monitor heart rhythm. Alterations in phosphorylation and oxidation of ryanodine receptor 2 (RyR2) were measured in left ventricular tissue. There were age-specific differences in arrhythmogenesis and sudden death associated with acute β-adrenergic stimulation in guinea pigs. Young and adult guinea pigs developed arrhythmias in response to ISO; however, adult animals developed significantly more premature ventricular contractions and experienced higher arrhythmia-related mortality than young guinea pigs treated with ISO. Although there were no significant differences in the phosphorylation of left ventricular RyR2 between young and adult guinea pigs, adult guinea pigs exposed to acute ISO had significantly more oxidation of RyR2. Flecainide treatment significantly improved survival and decreased the number of premature ventricular contractions in young and adult animals in association with lower RyR2 oxidation. Adult guinea pigs had a greater propensity to develop arrhythmias and suffer sudden death than young guinea pigs when acutely exposed to ISO. This was associated with higher oxidation of RyR2. The incidence of sudden death can be rescued with flecainide treatment, which decreases RyR2 oxidation.
NEW & NOTEWORTHY Clinically, adult patients with heart failure are more likely to develop arrhythmias and sudden death than pediatric patients with heart failure. In the present study, older guinea pigs also showed a greater propensity to arrhythmias and sudden death than young guinea pigs when acutely exposed to isoproterenol. Although there are well-described age-related cardiac structural changes that predispose patients to arrhythmogenesis, the present data suggest contributions from dynamic changes in cellular signaling also play an important role in arrhythmogenesis.
Keywords: arrhythmia, guinea pig, premature ventricular contraction, ryanodine oxidation
INTRODUCTION
Although pediatric heart failure (HF) is less prevalent than adult HF, it is associated with high mortality and high costs of care. The most common cause of noncongenital HF in children is idiopathic dilated cardiomyopathy (IDC) (65). In adults, IDC is one of the most common nonischemic causes of HF (36). Importantly, therapies that improve outcomes in adult patients with IDC fail to provide such benefits in pediatric patients with IDC (54, 55, 65). We have previously shown fundamental differences in the myocellular mechanisms involved in cardiac pathology between children and adults with IDC, which we propose lead to differences in response to therapies (7, 38, 42, 63, 71). Further investigation of these differences will improve understanding of both patient populations and ultimately lead to identification of age-specific targeted treatments that could improve clinical outcomes.
One clinically apparent age difference in patients with HF is the incidence of ventricular arrhythmias and sudden death. For example, primary prevention implantable defibrillators are the standard of care in adults with systolic HF for the prevention of sudden cardiac death, whereas pediatric patients with IDC show a much lower incidence of arrhythmias and sudden death and rarely require implantable defibrillators (13, 49, 53). In addition, adult patients with IDC demonstrate an increased incidence of arrhythmias and sudden death when treated with phosphodiesterase 3 inhibitors, whereas phosphodiesterase 3 inhibitors are well tolerated by pediatric patients with HF, and this therapy is often used as a bridge to transplantation (12, 15, 16, 20, 44, 47, 48). It has been proposed that the adult heart is more prone to arrhythmias because of the development of fibrotic regions, whereas fibrosis in the pediatric failing heart is less prominent (57, 71). We hypothesize that age-dependent differences in adaptations to HF, including alterations in proteins involved in Ca2+ handling, contribute to differences in propensities to develop arrhythmias between the two populations.
Posttranslational modifications of Ca2+-handling proteins occur in response to changes in circulating catecholamines. Catecholamine excess is present in HF regardless of age, resulting in stimulation of the β-adrenergic receptor (β-AR), leading to phosphorylation of downstream targets through PKA or Ca2+/calmodulin-dependent protein kinase II (CaMKII) (18, 35, 41, 52, 62, 74). Furthermore, β-AR stimulation leads to increased production of ROS, which can also lead to posttranslational modifications of Ca2+-handling proteins through oxidation (5). In particular, phosphorylation and oxidation of the sarcoplasmic reticulum Ca2+ release channel, ryanodine receptor 2 (RyR2), can cause Ca2+ leak, leading to delayed afterdepolarizations and Ca2+ waves that propagate between cardiomyocytes. These alterations have the potential to trigger aberrant contractions (4, 9, 14, 31, 39, 45, 66, 69) or premature ventricular contractions (PVCs). PVCs that occur during a vulnerable repolarization period in the heart can lead to ventricular tachyarrhythmias, inadequate perfusion, and death (34, 43).
In the present study, we used guinea pigs to investigate age-related differences in catecholamine-induced ventricular arrhythmias. We hypothesized that acute exposure to the β-AR agonist isoproterenol (ISO) would result in arrhythmogenesis and RyR2 changes in an age-dependent fashion. We found that ISO treatment resulted in the occurrence of ventricular arrhythmias and oxidation of RyR2 that was improved with flecainide treatment. Importantly, the arrhythmia burden and oxidation of RyR2 in response to ISO was higher in adult animals compared with young animals.
MATERIALS AND METHODS
Animal Protocols
Young (24 days old) male and female Dunkin-Hartley guinea pigs (average weight: 260 g, n = 71) and adult (over 6 mo of age) male and female Dunkin-Hartley guinea pigs (average weight: 981.2 g, n = 35, Charles River Laboratories, Kingston, NY) were housed at the University of Colorado Anschutz Medical campus. Briefly, animals were anesthetized with isoflurane and received an intraperitoneal injection of saline (0.9% NaCl), flecainide (20 mg/kg, Sigma-Aldrich, St. Louis, MO), or quinidine (37.5 mg/kg, Sigma-Aldrich) before incision. Flecainide is a Vaughan Williams class Ic agent with secondary RyR2 inhibitory effects used clinically to treat catecholaminergic polymorphic ventricular tachycardia (CPVT), whereas quinidine is a class Ia agent acting on Na+ channels without RyR2 blockade. These inhibitors were used to differentiate the effects of functional RyR2 inhibition from Na+ channel effects in the guinea pig model (21, 67, 68). A subcutaneous incision was made between the scapulae, 1 in. behind the head. Blunt dissection through the incision created a subcutaneous pocket on each side of the animal. REVEAL LINQ Implantable Cardiac Monitors (Medtronic, Minneapolis, MN) were placed into the pocket on the animal’s upper left side. Osmotic pumps (2ML1, 2ML2, and 2ML4, Alzet, Cupertino, CA) containing either saline (control) or ISO (16 or 32 mg·kg−1·day−1, Sigma-Aldrich) were implanted on the upper right side as previously described (58, 60). The incisions were closed with staples. Animals were either monitored for a full hour after surgery or euthanized with Fatal Plus [phenobarbital (100 mg/kg), MWI Veterinary Supply, Boise, ID] 30 min after the surgery was completed. For survival analyses, animals who received either 16 or 32 mg·kg−1·day−1 ISO were grouped, as there were no differences in survival or collapse between these two dosages. All other assessments and experiments were conducted on animals that received 32 mg·kg−1·day−1 ISO. Heart rhythm was recorded from 4−10 and 24–30 min after surgery. Chest compressions (estimated 144 compressions/min) were performed on any animal that collapsed until visible evidence of return of spontaneous circulation or for 10 min of total resuscitation time. Rhythm was collected on the LINQ device specifically during this time to capture initiation of the rhythm disturbance in addition to the prespecified recordings outlined above. Hearts were excised immediately after euthanasia and perfused with saline, and the right ventricle and left ventricle were then separated and flash frozen at −80°C.
All animal experiments were approved by University of Colorado Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines.
Analysis of LINQ Tracings
ECG recordings from REVEAL LINQ Implantable Cardiac Monitors (Medtronic) were obtained 4–10 min and 24–30 min after pump implantations. Recordings were converted to .csv files using a proprietary script created by Medtronic. ECG parameters, including P-R interval, duration of QRS, Q-T interval, interval from the peak of the T wave to the end of the T wave (Tp-e), R-R interval, corrected QT interval (QTc), and Q-Tapex, were manually calculated using an average of 5–10 beats derived from the .csv files. Tp-e is a measurement of transmural dispersion of repolarization. Further analysis was done to calculate heart rate (HR) based on R-R interval, and the Tp-e-to-QTc ratio (a marker of arrhythmogenesis) was calculated (19, 29, 50). Numbers of PVCs were counted in tracings recorded 4–10 min and 24–30 min after surgery.
Protein Isolation
RyR2 protein was isolated as previously described with modifications (6). Briefly, 100 mg of frozen left ventricular tissue were homogenized using a bead homogenizer in 20 mM HEPES, 250 mM sucrose, and protease and phosphatase inhibitors. The homogenates were centrifuged at 4°C at 1,500 × g for 15 min, and the supernatant was transferred to ultracentrifuge tubes. The pellet was resuspended in buffer and centrifuged again at 4°C for 15 min at 1,500 × g, and this supernatant was added to the first supernatant. The combined supernatants were centrifuged at 110,000 × g for 60 min at 4°C, and the resulting pellets were resuspended using 20 mM HEPES, 250 mM sucrose buffer with inhibitors, and 2% N-dodecyl-β-d-maltoside (DDM; Sigma-Aldrich). Protein concentration was determined using the DC assay (Bio-Rad, Hercules, CA).
Protein was isolated from 10–20 mg of frozen left ventricular tissue in isoelectric focusing buffer (8 M urea, 2.5 M thiourea, 4% CHAPS, 2 mM EDTA, 1 mM DTT, and 1% TBP) with protease and phosphatase inhibitors and homogenized at 4°C as previously described (60). Protein concentration was determined using the Bradford assay (Bio-Rad).
Western Blot Analysis
Western blots were performed as previously described with the following modifications (61).
RyR2.
Sarcoplasmic reticulum-enriched proteins were isolated as described above and separated using a 4–20% Criterion gel (Bio-Rad) for 4 h at 130 V. Proteins were transferred to 0.45 μM PVDF membranes in transfer buffer (National Diagnostics, Atlanta, GA) + 0.01% SDS at 30 V for 16 h and 80 V for 1 h on ice. Blots were blocked with 5% BSA in Tris-buffered saline plus 0.1% Tween (Bio-Rad). Dephosphorylated Ser2030 (1:1,000, a gift from Dr. Valdivia), Ser2808 (1:8,000, Badrilla, Leeds, UK), Ser2814 (1:3,000, Badrilla), and total RyR2 (1:2,000, a gift from Dr. Marks) were incubated on separate blots at 4°C overnight, and the appropriate secondary antibodies were applied for 1 h at room temperature (goat anti-rabbit, 1:25,000, Jackson Immunology Research Laboratories, West Grove, PA; and sheep anti-mouse, 1:25,000, GE Healthcare Life Sciences, Pittsburg, PA).
Phospholamban.
Proteins isolated by isoelectric focusing buffer were separated using 15% Criterion gels (Bio-Rad) at 130 V for 2 h and transferred onto 0.2 μM PVDF membranes (EMD Millipore, Billerica, MA) at 80 V for 80 min. Blots were blocked with 10% milk in Tris-buffered saline plus 0.1% Tween. Ser16 (Badrilla), Thr17 (Badrilla), and total phospholamban (PLN; EMD Millipore) were incubated on separate membranes at 4°C overnight, and the appropriate secondary antibodies were applied for 1 h (goat anti-rabbit, 1:50,000, Jackson Immunology Research Laboratories; and sheep anti-mouse, 1:25,000, GE Healthcare Life Sciences).
Oxyblot.
Carbonyl groups on proteins from the sarcoplasmic reticulum-enriched protein fraction were derivatized to 2,4-dinitrophenylhydrazone (DNP) by treatment with 2,4-dinitrophenylhydrazine as instructed in the OxyBlot Protein Oxidation Detection kit (EMD Millipore). As a negative control, protein samples from the same animal were exposed to a control buffer lacking the enzyme. Proteins were then separated on a 4–20% Criterion gel (Bio-Rad) and treated as described above for the RyR2 Western blots. Blots were incubated with anti-DNP (1:150, EMD Millipore) for 1 h at room temperature followed by anti-rabbit secondary (1:300, EMD Millipore), and membranes were exposed as described above. RyR2 protein was identified based on molecular weight and confirmed by incubation with total RyR2 antibody. Oxidized RyR2 was normalized to total RyR2.
All blots were incubated with ECL reagent (West Pico or West Femto, ThermoFisher Scientific, Pittsburg, PA) and exposed to autoradiography film. Proteins were normalized to GAPDH, and ratios of the phosphorylated protein to total protein were calculated (except for oxyblot samples, total oxidized RyR2 was normalized to total RyR2, Santa Cruz Biotechnology, Dallas, TX) and quantified using ImageJ (version 1.49v).
Data Analysis and Statistics
Statistical analyses were performed using Prism Version 6 (GraphPad Software, San Diego, CA). Consistent with American Physiological Society recommendations on studies that include higher-level mammals and the goal of identifying putative mechanisms that could lead to further studies, statistical significance was set a priori at P < 0.1, and all data are presented as means ± SD (8). Data were tested for normality, and data that were non-normally distributed were log transformed before statistical testing. Mortality data were assessed using the Mantel-Cox log-rank test. Comparison of three or more groups was conducted using one-way ANOVA, and Bonferroni’s post hoc tests were performed. Since transformation of the PVC data was not possible with the zero counts, PVC data were analyzed using a Kruskal-Wallis test with Dunn’s multiple-comparisons test.
RESULTS
Adult Guinea Pigs Are More Susceptible to Sudden Death With acute ISO Exposure Than Young Guinea Pigs
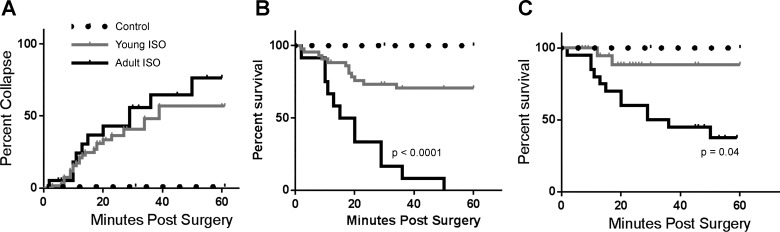
Acute exposure to either concentration of ISO (16 or 32 mg·kg−1·day−1) resulted in guinea pigs collapsing. Overall, 50–60% of guinea pigs (11 of 18 adult guinea pigs and 37 of 71 young guinea pigs) collapsed within 1 h of completion of the surgery (Fig. 1A). Although chest compressions rescued 68% of young animals (25 of 37 guinea pigs), none of the adult animals (0 of 11 guinea pigs) survived (Fig. 1B). As a result, mortality was significantly higher in adult guinea pigs within the first hour of acute ISO exposure when compared with young guinea pigs (Fig. 1C).
Fig. 1.
Age-specific responses to acute isoproterenol (ISO) exposure. A: percentage of guinea pigs that collapsed in the first hour of ISO exposure; 37 of 71 young guinea pigs collapsed (52%) and 11 of 18 adult guinea pigs collapsed (61%). B: percent survival in guinea pigs that collapsed when exposed to ISO. Sixty-eight percent of young guinea pigs (25 of 37 guinea pigs) recovered after collapse, whereas none of the adult guinea pigs (0 of 11 guinea pigs) recovered after collapse. Significantly more adult ISO-treated guinea pigs died after collapse than did young ISO-treated guinea pigs by Mantel-Cox log-rank test (P < 0.0001). C: percent survival in guinea pigs in the first hour of ISO exposure; 59 of 71 young guinea pigs (83%) survived, whereas 7 of 18 adult guinea pigs (39%) survived. Mortality in adult guinea pigs was statistically significant by Mantel-Cox log-rank test (P = 0.04). Young control group: n = 36, young ISO-treated group: n = 71, adult control group: n = 13, and adult ISO-treated group: n = 18.
ISO Treatment Increased PVCs and Repolarization Abnormalities to a Greater Extent in Adult Guinea Pigs
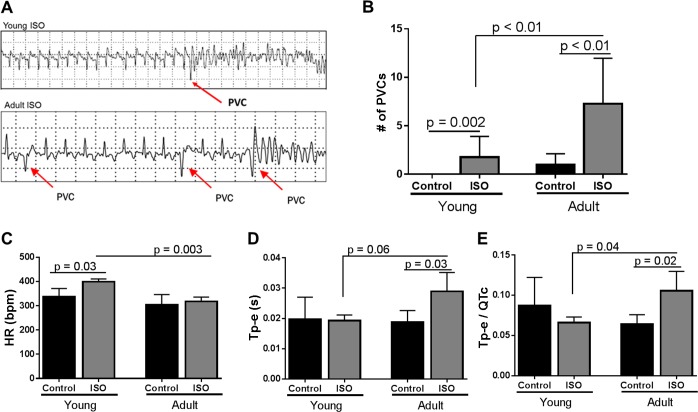
Heart rhythm data from LINQ recorders demonstrated increased PVCs in response to acute exposure to ISO (32 mg·kg−1·day−1). When these PVCs occurred during ventricular repolarization, animals developed ventricular fibrillation and collapsed (Fig. 2A). Importantly, all episodes of collapse captured on LINQ recorders were associated with ventricular tachycardia/ventricular fibrillation, and no ventricular tachycardia/ventricular fibrillation was documented in animals that did not collapse. PVC number was significantly higher in ISO-treated animals compared with control animals regardless of age (Fig. 2B). However, adult guinea pigs treated with ISO developed significantly more PVCs than young guinea pigs treated with ISO. Young animals exposed to ISO had significantly higher HRs than young control animals and adult animals exposed to ISO (Fig. 2C). There were no differences in QRS duration, QTc, or P-R interval between ISO-treated young and adult animals (data not shown). Adult guinea pigs exposed to ISO had prolonged Tp-e compared with age-matched control guinea pigs (Fig. 2D) and young ISO-treated guinea pigs. The Tp-e-to-QTc ratio was also significantly higher in adult guinea pigs treated with ISO compared with age-matched control guinea pigs (Fig. 2E) and young ISO-treated guinea pigs. There was no difference in Tp-e or the Tp-e-to-QTc ratio between control and ISO-treated young animals.
Fig. 2.
Arrhythmogenic changes in guinea pigs upon acute isoproterenol (ISO) exposure. A: representative images of cardiac rhythms from young and adult guinea pigs exposed to ISO demonstrating premature ventricular contractions (PVCs) leading to polymorphic ventricular tachycardia/ventricular fibrillation. These episodes were associated with collapse of the animal and sudden death. B: quantification of PVCs in young and adult guinea pigs for 14 min of recording within 30 min of pump implantation. Both young and adult guinea pigs had significantly more PVCs than age-matched control guinea pigs when exposed to ISO (young: P = 0.002 and adult: P < 0.01). Adult guinea pigs treated with ISO had more PVCs than young guinea pigs treated with ISO (P < 0.01). Statistical analysis of data was by Kruskal-Wallis test with Dunn’s multiple comparisons. Young control group: n = 15, young ISO-treated group: n = 29, adult control group: n = 9, and adult ISO-treated group: n = 7. C: heart rate (HR) averaged over first 10 min after pump implantation. Within the first 10 min of ISO exposure, young guinea pigs had a significantly higher HR (P = 0.03). There was a significant difference between young and adult guinea pig HRs with exposure to ISO (P = 0.003). Statistical analysis of transformed data was done using one-way ANOVA with Bonferroni’s multiple-comparisons analysis. Young control group: n = 6, young ISO-treated group: n = 5, adult control group: n = 5, and adult ISO-treated group: n = 6. D: ISO exposure for 10 min significantly increased the time from the peak to the end of the T wave (Tp-e) in adult guinea pigs (P = 0.03). Adult guinea pigs exposed to ISO had longer duration of Tp-e than young guinea pigs treated with ISO (P = 0.06). Statistical analysis of transformed data was done using one-way ANOVA with Bonferroni’s multiple-comparisons analysis. Young control group: n = 6, young ISO-treated group: n = 5, adult control group: n = 5, and adult ISO-treated group: n = 6. E: time from Tp-e over corrected QT interval (Tp-e/QTc) increased in adult guinea pigs treated with ISO (P = 0.02). Adult guinea pigs treated with ISO had a significantly higher Tp-e/QTc than young guinea pigs treated with ISO (P = 0.04). Statistical analysis of transformed data was done using one-way ANOVA with Bonferroni’s multiple-comparisons analysis. Young control group: n = 6, young ISO-treated group: n = 5, adult control group: n = 5, and adult ISO-treated group: n = 6.
Flecainide But Not Quinidine Decreases Sudden Death Due to Acute ISO exposure by Decreasing PVCs
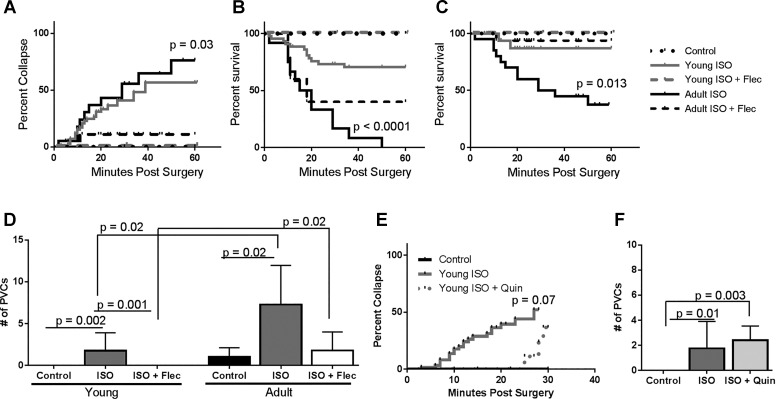
Treatment with flecainide before acute ISO exposure lowered the incidence of arrhythmias and sudden death in both young and adult guinea pigs (Fig. 3). Although 52% of young guinea pigs (37 of 71 guinea pigs) treated with ISO collapsed in the first hour, none of the young guinea pigs (0 of 27 guinea pigs) treated with ISO + flecainide collapsed or died (Fig. 3, A–C). In contrast, although 61% of adult guinea pigs (11 of 18 guinea pigs) collapsed when exposed to ISO, 26% of the adult guinea pigs (5 of 19 guinea pigs) that received ISO + flecainide collapsed (Fig. 3A). Importantly, although none of the adult guinea pigs exposed to ISO survived if they developed ventricular fibrillation, even with chest compressions (0 of 11 guinea pigs), 40% of guinea pigs (2 of 5 guinea pigs) survived when treated with ISO + flecainide and chest compressions (Fig. 3B). Young guinea pigs treated with ISO + flecainide developed significantly fewer PVCs than young guinea pigs treated with ISO (Fig. 3D). Although adult guinea pigs that received ISO + flecainide had fewer PVCs compared with adult guinea pigs treated with ISO, these guinea pigs still experienced significantly more PVCs than young guinea pigs that received ISO + flecainide. Interestingly, young guinea pigs treated with ISO + quinidine still collapsed (2 of 6 guinea pigs), and there was no difference in the number of PVCs compared with young control and ISO-treated guinea pigs (Fig. 3, E and F).
Fig. 3.
Flecainide (Flec) decreases sudden death due to acute isoproterenol (ISO) exposure, but quinidine (Quin) does not. A: percentage of guinea pigs that collapsed in the first hour of ISO exposure with and without Flec; 52% of young guinea pigs (37 of 71 guinea pigs) collapsed with ISO exposure, whereas none of the young guinea pigs (0 of 27 guinea pigs) treated with ISO + Flec collapsed, and 61% of adult guinea pigs (11 of 18 guinea pigs) exposed to ISO collapsed, whereas 26% of adult GPs (5 of 19 guinea pigs) treated with ISO + Flec collapsed. The percentage of adult GPs that collapsed was statistically significant by a Mantel-Cox log-rank test (P = 0.03). Young control group: n = 8, young ISO-treated group: n = 29, young ISO + Flec-treated group n = 27, adult control group: n = 9, adult ISO-treated group: n = 7, and adult ISO + Flec-treated group: n = 19. B: percent survival of guinea pigs that collapsed when treated with ISO or ISO + Flec. When exposed to ISO, 68% of young guinea pigs (25 of 37 guinea pigs) survived after collapse, whereas 100% of young guinea pigs treated with Flec survived. When treated with ISO, none of the adult guinea pigs (0 of 11 guinea pigs) that collapsed survived, but when treated with Flec at the time of pump implantation, 40% of the guinea pigs (2 of 5 guinea pigs) that collapsed survived (Mantel-Cox log-rank test, P < 0.0001). Young control group: n = 8, young ISO-treated group: n = 29, young ISO + Flec-treated group: n = 27, adult control group: n = 9, adult ISO-treated group: n = 7, and adult ISO + Flec-treated group: n = 19. C: percent survival in guinea pigs in the first hour of ISO exposure; 83% of young guinea pigs (59 of 71 guinea pigs) survived acute ISO exposure, 100% of young guinea pigs (27 of 27 guinea pigs) treated with Flec survived ISO exposure, 39% of adult guinea pigs (7 of 18 guinea pigs) survived acute ISO exposure, and 84% of adult guinea pigs (16 of 19 guinea pigs) treated with Flec survived ISO exposure. Mortality in adult guinea pigs was statistically significant by Mantel-Cox log-rank test (P = 0.013). Young control group: n = 8, young ISO-treated group: n = 29, young ISO + Flec-treated group: n = 27, adult control group: n = 9, adult ISO-treated group: n = 7, and adult ISO + Flec-treated group: n = 19. D: quantification of premature ventricular contractions (PVCs) from 14 min of recordings within 30 min of pump implantation. Flec significantly decreased the number of PVCs in young animals compared with young animals treated with ISO alone (P = 0.001). Adult animals treated with ISO + Flec had significantly more PVCs than young animals treated with ISO + Flec (P = 0.02). Statistical analysis of data was performed by Kruskal-Wallis test with Dunn’s multiple comparisons. Young control group: n = 15, young ISO-treated group: n = 29, young ISO + Flec-treated group: n = 16, adult control group: n = 9, adult ISO-treated group: n = 7, and adult ISO + Flec-treated group: n = 9. E: percentage of young guinea pigs that collapsed in 30 min of ISO exposure with and without Quin; 52% of young guinea pigs (37 of 71 guinea pigs) collapsed with ISO exposure, whereas 33% of young guinea pigs (2 of 6 guinea pigs) collapsed when treated with ISO + Quin (P = 0.07). Young control group: n = 8, young ISO-treated group: n = 29, and young ISO + Quin-treated group: n = 5. F: young guinea pigs treated with ISO + Quin had significantly more PVCs than young guinea pigs treated with saline (P = 0.003). There were no differences in the number of PVCs in young animals treated with ISO + Quin compared with young animals treated with ISO. Statistical analysis of data was performed by Kruskal-Wallis test with Dunn’s multiple comparisons. Young control group: n = 8, young ISO-treated group: n = 29, and young ISO + Quin-treated group: n = 5.
There Are No Significant Differences Between Age or Treatment Groups in Phosphorylation of Important Ca2+-Handling Proteins
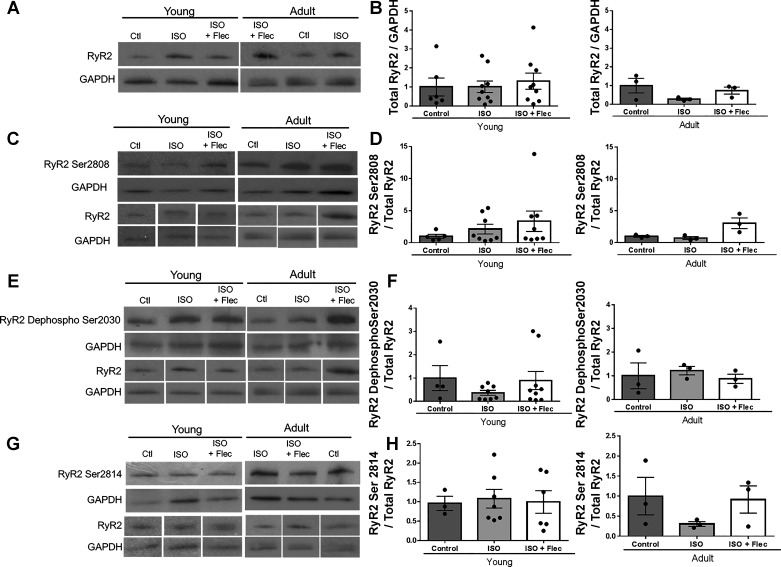
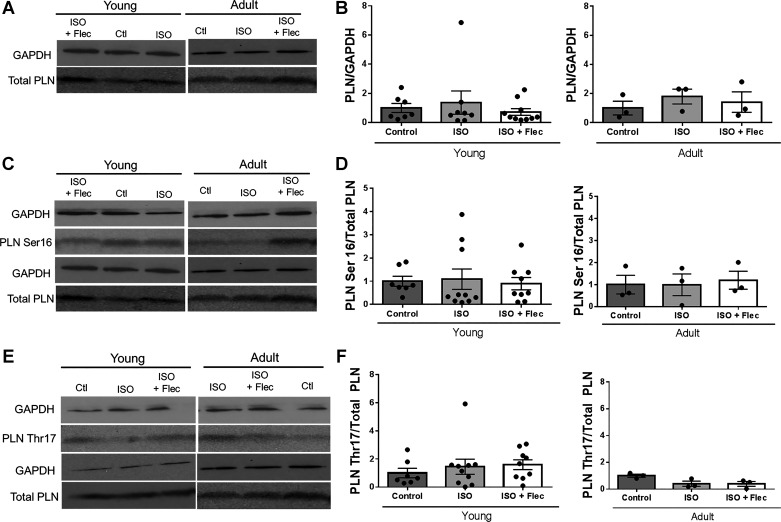
There were no differences in total RyR2 protein between young and adult guinea pigs under any treatment condition (Fig. 4, A and B). There are two sites on RyR2 phosphorylated by PKA: Ser2808 and Ser2030 (4), and no differences in phosphorylation at either site in young or adult GPs across treatment groups were observed (Fig. 4, C–F). In addition, RyR2 is phosphorylated at Ser2814 by CaMKII. Phosphorylation at this site has also been shown to increase Ca2+ leak (4, 70); however, acute exposure to ISO did not alter phosphorylation of RyR2 at Ser2814, and there were no differences in phosphorylation at Ser2814 between young and adult guinea pigs (Fig. 4, G and H). To determine phosphorylation of other PKA targets, we investigated phosphorylation of PLN at Ser16. There were no differences in total PLN between age or treatment groups (Fig. 5A). Ser16 phosphorylation is significantly lower in adult guinea pigs than in young guinea pigs (control young guinea pigss: 1.0 ± 0.33 and control adult guinea pigs: 0.44 ± 0.06, P < 0.04), but there were no differences in phosphorylation with ISO or ISO + flecainide compared with age-matched control guinea pigs. There were also no differences between age or treatment groups in the phosphorylation of PLN at Thr17 (CaMKII site; Fig. 5, E and F).
Fig. 4.
Isoproterenol (ISO) and flecainide (Flec) do not significantly change phosphorylation of ryanodine receptor 2 (RyR2). A: representative Western blot of total RyR2 in guinea pig left ventricles (LVs). B: quantification of total RyR2/GAPDH normalized to control groups. There were no significant differences in transformed data of total RyR2 expression in young or adult hearts by one-way ANOVA with Bonferroni’s multiple comparisons. Young control group: n = 6, young ISO-treated group: n = 8, young ISO + Flec-treated group: n = 6, adult control group: n = 3, adult ISO-treated group: n = 3, and adult ISO + Flec-treated group: n = 3. C: representative Western blot of phosphorylation of RyR2 at Ser2808 in guinea pig LVs. D: quantification of phosphorylation of Ser2808/total RyR2 normalized to control groups. There were no significant differences in transformed data by one-way ANOVA with Bonferroni’s multiple comparisons. Young control group: n = 5, young ISO-treated group: n = 8, young ISO + Flec-treated group: n = 8, adult control group: n = 3, adult ISO-treated group: n = 3, and adult ISO + Flec-treated group: n = 3. E: representative Western blot of phosphorylation of Ser2030 RyR2. F: quantification of RyR2 phosphorylation at Ser2030/total RyR2 normalized to control groups. There were no significant differences in transformed data by one-way ANOVA with Bonferroni’s multiple comparisons. Young control group: n = 4, young ISO-treated group: n = 8, young ISO + Flec-treated group: n = 9, adult control group: n = 3, adult ISO-treated group: n = 3, and adult ISO + Flec-treated group: n = 3. G: representative Western blot of RyR2 phosphorylation at Ser2814 in guinea pig LVs. H: quantification of RyR2 phosphorylation at Ser2814/total RyR2 normalized to control groups. There were no significant differences in transformed data by one-way ANOVA with Bonferroni’s multiple comparisons. Young control group: n = 3, young ISO-treated group: n = 7, young ISO + Flec-treated group: n = 6, adult control group: n = 3, adult ISO-treated group: n = 3, and adult ISO + Flec-treated group: n = 3. The blots for RYR2 and GAPDH are the appropriate and same controls for the adult samples in C and E.
Fig. 5.
Isoproterenol (ISO) and flecainide (Flec) do not significantly change phosphorylation of phospholamban (PLN). A: representative Western blot of total PLN in guinea pig left ventricles (LVs). B: quantification of total PLN/GAPDH normalized to control groups. There were no significant differences in transformed data by one-way ANOVA with Bonferroni’s multiple comparisons. Young control group: n = 7, young ISO-treated group: n = 7, young ISO + Flec-treated group: n = 10, adult control group: n = 3, adult ISO-treated group: n = 3, and adult ISO + Flec-treated group: n = 3. C: representative Western blot of phosphorylation of PLN at Ser16 in GP LVs. D: quantification of phosphorylation of Ser16/total PLN normalized to control groups. There were no significant differences in phosphorylation of Ser16 between treatment groups in adult and young guinea pigs. Young control group: n = 7, young ISO-treated group: n = 10, young ISO + Flec-treated group: n = 9, adult control group: n = 3, adult ISO-treated group: n = 3, and adult ISO + Flec-treated group: n = 3. E: representative Western blot of phosphorylation of PLN at Thr17. F: quantification of phosphorylation of PLN at Thr17/total PLN normalized to control groups. There were no significant differences in transformed data by one-way ANOVA with Bonferroni’s multiple comparisons. Young control group: n = 7, young ISO-treatet group: n = 10, young ISO + Flec-treated group: n = 9, adult control group: n = 3, adult ISO-treated group: n = 3, and adult ISO + Flec-treated group: n = 3. Blots for GAPDH and total PLN are the appropriate and same controls for samples presented in A and C (young) and A, C, and E (adult).
Acute Exposure to ISO in Adult Guinea Pigs Increases Oxidation of RyR2
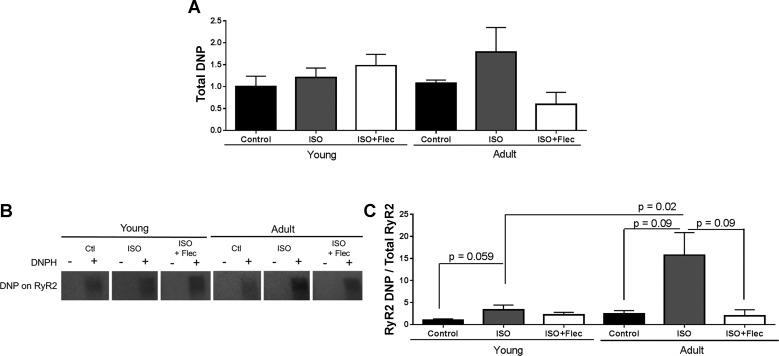
ISO treatment can result in increased protein oxidation (5, 40, 76, 77). To determine if oxidation increases in the left ventricle of guinea pigs in response to 30-min exposure to ISO, expression of carbonyl groups in proteins was assessed. When carbonyl groups were derivatized to DNP by 2,4-dinitrophenylhydrazine (a marker used to indicate oxidative state) (10), there was a trend toward higher overall expression of carbonyl groups in proteins from adult guinea pigs treated with ISO (Fig. 6A). Importantly, RyR2 had significantly more oxidative modifications in adult guinea pigs treated with ISO than young guinea pigs treated with ISO (Fig. 6C). Both young and adult guinea pigs treated with ISO had significantly more oxidation in RyR2 than control guinea pigs. Furthermore, RyR2 oxidation was not different between young animals treated with ISO + flecainide and young control animals, whereas in adult animals treated with ISO + flecainide, there was a significant decrease in the oxidative modifications of RyR2 (Fig. 6C).
Fig. 6.
Oxidation of ryanodine receptor 2 (RyR2) is higher in adult guinea pigs treated with isoproterenol (ISO). A: quantification of total 2,4-dinitrophenylhydrazone (DNP) in protein lysates from guinea pig left ventricles (LVs). There was a trend toward increased DNP in adult ISO-treated animals compared with young ISO-treated animals. Young control group: n = 6, young ISO-treated group: n = 8, young ISO + Flec-treated group: n = 5, adult control group: n = 3, adult ISO-treated group: n = 3, and adult ISO + Flec-treated group: n = 3. B: representative image of DNP at RyR2 in guinea pig LVs with and without 2,4-dinitrophenylhydrazine (DNPH) treatment. C: quantification of DNP at RyR2/total RyR2. There was significantly more oxidation of RyR2 in young guinea pigs treated with ISO compared with control guinea pigs (P = 0.1). RyR2 was more oxidized in adult guinea pigs treated with ISO than control or ISO + Flec-treated guinea pigs (P = 0.04 and P = 0.004). RyR2 was significantly more oxidized in adult guinea pigs treated with ISO compared with young guinea pigs treated with ISO (P = 0.01). Statistical analysis of transformed data was by one-way ANOVA with Bonferroni’s multiple comparisons. Young control group: n = 6, young ISO-treated group: n = 8, young ISO + Flec-treated group: n = 5, adult control group: n = 3, adult ISO-treated group: n = 3, and adult ISO + Flec-treated group: n = 3. Flec, flecainide.
DISCUSSION
The present study is the first, to our knowledge, to examine age differences in arrhythmogenic sudden cardiac death in guinea pigs. Implantable loop recorders were used to document changes in the underlying rhythm, electrocardiographic intervals, and timing of arrhythmic events in real time. Similar to what is observed clinically where adult patients with HF are more likely to develop arrhythmias and sudden death than pediatric patients with HF, older guinea pigs also showed a greater propensity to arrhythmias and sudden death than young guinea pigs when acutely exposed to ISO. After acute treatment with ISO, older guinea pigs had significant changes in ECG parameters and more PVCs than young guinea pigs, which were associated with an increased incidence of lethal ventricular arrhythmias. Older guinea pigs also had higher oxidation of RyR2 than did young guinea pigs with ISO-mediated β-AR stimulation. Treatment with the anti-arrhythmic RyR2 channel blocker flecainide blunted arrhythmogenic events and resulted in less RyR2 oxidation in older guinea pigs. Although there are well-described age-related cardiac structural changes, in particular increasing wall thickness (59) and fibrotic changes (23), that predispose patients to arrhythmogenesis (11, 37), the present data suggest contributions from dynamic changes in cellular signaling.
The initial mortality in guinea pigs in response to acute exposure to 32 mg/kg ISO initiated the present study. We chose the dose based on dosing studies in mice and guinea pigs that produced pathological hypertrophy under chronic stimulation (58, 60). Several groups have previously demonstrated early mortality in guinea pigs after ISO treatment at varying doses [Liu et al. (33): 1 mg·kg−1·day−1, Overholser et al. (46): 9.6 µg·kg−1·day−1 and 0.312, 0.48, and 4.8 mg·kg−1·day−1, and Soltysinska et al. (58): 19.2 mg·kg−1·day−1]. In line with the occurrence of sudden death associated with a wide range of ISO doses, we found that high (32 mg·kg−1·day−1) and low (16 mg·kg−1·day−1) doses of ISO led to similar mortality in both age groups. Indeed, Soltysinska et al. (58) found that 20% of guinea pigs that received chronic ISO died suddenly, and the authors attributed this death to arrhythmias but the acute mechanism was not described. The present study extends these findings by not only demonstrating an age difference in sudden death but also documenting the underlying electrocardiographic mechanism as well as a putative molecular mechanism through changes in RyR2 oxidation.
We hypothesized that these age-specific differences in sudden death may be due to differences in rhythm characteristics between young and adult guinea pigs. Greer-Short et al. (17) reported increased ectopic activity in Langendorff-perfused adult guinea pig hearts stimulated acutely with ISO when compared with young guinea pig hearts. In our in vivo model, we found that ISO increased the number of PVCs (ectopic activity) in both adult and young animals (Fig. 2B) but that older animals had a greater PVC burden than the young.
Soltysinska et al. (58) also demonstrated that chronic ISO administration (daily injections with increasing dosage over 3 mo) lengthened ventricular repolarization in guinea pigs. In the present study, although there were no age differences at baseline, ISO induced an age-dependent change in two repolarization parameters associated with increased arrhythmogenesis and sudden death. Tp-e (a marker for transmural dispersion of repolarization) and the Tp-e-to-QTc ratio (an index of arrhythmogenesis) (19, 29, 50) were significantly higher in adult guinea pigs treated with ISO compared with age-matched control guinea pigs (Fig. 2, D and E) and compared with young guinea pigs treated with ISO. The acute nature of the ISO treatment in the present study suggests that there are significant age-related differences in cellular signaling in response to β-AR agonism that contribute to rhythm disturbances.
CPVT is a condition in humans in which acute adrenergic stimulation results in episodes of ventricular tachycardia (25, 27). The most commonly identified mutations in patients with CPVT occur in RyR2 channels and are hypothesized to lead to increased Ca2+ leak (25, 27). CPVT is often treated with flecainide, a class I antiarrhythmic that blocks Na+ and RyR2 channels (21, 67, 68). In the present study, administration of flecainide lowered the number of PVCs induced by exposure to ISO in both adult and young guinea pigs (Fig. 3) (21, 67, 73). In contrast, quinidine (a class I antiarrhythmic that blocks Na+ channels but not RyR2 channels) did not lower the number of PVCs in ISO-treated guinea pigs. Since there were fewer PVCs in the animals treated with flecainide, we hypothesize that the RyR2 channel is central to the development of PVCs with acute β-AR stimulation.
β-AR stimulation increases phosphorylation of the RyR2 channel, but there is a lack of consensus about how phosphorylation at three well-documented sites (Ser2030, Ser2808, and Ser2814) affects the function of the RyR2 channel (22, 23, 43, 53–63). It has been suggested that phosphorylation of RyR2 at Ser2808 and Ser2814 occurs during HF, and this phosphorylation increases Ca2+ leak from the sarcoplasmic reticulum (4, 24, 26, 42a, 51, 56, 69). This increased Ca2+ can cause delayed afterdepolarizations and aberrant contractions as well as depletion of sarcoplasmic reticulum Ca2+ stores, thereby leading to decreased contractility (3, 25, 26). In guinea pigs acutely exposed to ISO, there were no significant differences in the phosphorylation of the three known sites on RyR2, which suggests that phosphorylation changes in RyR2 channels are not responsible for the PVCs in guinea pigs acutely exposed to β-AR stimulation (Fig. 4).
It is possible that changes in sarcoplasmic Ca2+ loading could contribute to changes in cytoplasmic Ca2+ and lead to arrhythmogenesis. PLN is phosphorylated at Ser16 by PKA, resulting in increased sarco(endo)plasmic reticulum Ca2+-ATPase 2 activity and increased Ca2+ reuptake into the sarcoplasmic reticulum (3). Changes in PLN structure or phosphorylation can alter this Ca2+ handling and could potentially increase arrhythmogenic propensity (32). However, there were no significant differences in PLN phosphorylation in adult or young guinea pigs exposed to ISO.
β-AR stimulation also adversely affects cell function through increasing production of ROS. In fact, acute β-AR stimulation has been shown to increase ROS within 15 min of stimulation (28). Increased ROS can lead to increased oxidation of cellular proteins (5, 40, 76, 77). Although several studies have demonstrated that phosphorylation of RyR2 at Ser2808 is not sufficient to cause Ca2+ leak (5, 75), it has been suggested that oxidation of the RyR2 channel increases Ca2+ leak (22, 64, 72). Furthermore, ROS have been shown to oxidize RyR2, altering the activity of the channel (5, 30, 42a, 64, 77). Becerra et al. (2) demonstrated that blockade of oxidative modifications of RyR2 in an ischemia-reperfusion model leads to decreased arrhythmias. Since, in the guinea pigs model, every episode of ventricular arrhythmia was initiated by a PVC, we hypothesized that differences in ROS production between adult and young guinea pigs mediated oxidation of RyR2, which, in turn, influenced PVC generation. Importantly, RyR2 was significantly more oxidized in adult animals treated with ISO than in young animals treated with ISO. Additionally, treatment with flecainide prevented RyR2 oxidation in adult and young animals, and this was associated with fewer PVCs and improved survival. Therefore, we believe acute ISO exposure in guinea pigs leads to ROS production, which oxidizes RyR2 channels, leading to PVC production. A higher PVC burden in combination with the repolarization abnormalities could lead to an increased chance that a PVC will occur during ventricular repolarization (R-on-T phenomena), which leads to ventricular arrhythmias and death.
Limitations
There are several limitations of this study. First, the amount of ISO eluted from the pumps immediately upon implantation is not known. All pumps were primed for similar durations before implant to mitigate any variability. It is also interesting to note that this is an acute phenomenon, and after the first hour postsurgery, no animals experienced ventricular tachycardia. We originally hypothesized that β-adrenergic signaling would activate CaMKII and PKA pathways; however, phosphorylation at known PKA and CaMKII sites for both RyR2 and PLN did not show significant changes. We believe the timing of this experiment might play a role, and it is possible that earlier or later assessment might demonstrate activation of these pathways. Second, there may be differences in the blockade of Na+ channels between quinidine and flecainide that were incompletely characterized. It is also possible that there is an age-dependent difference in the response to quinidine that was not captured in the present experiments. However, the remarkable response to flecainide and the known RyR effects in CPVT along with the changes in RyR oxidation in guinea pigs provide significant support for the critical role of RyR in this model. Although we suspect ROS production is related to PVC generation, this is an associative finding, and these experiments do not conclusively define the cause of the PVCs. Finally, there are many factors that contribute to arrhythmogenesis, and RyR2 oxidation does not explain the differences in repolarization demonstrated with acute ISO exposure in adult guinea pigs. It is clear RyR2 is only one contributor, and the differences between young and adult guinea pigs in response to acute ISO stimulation is multifactorial.
Conclusions
In this study, the underlying rhythm, electrocardiographic intervals, and timing of arrhythmic changes in response to acute β-AR stimulation were characterized in guinea pigs. Importantly, we evaluated the relationship between rhythm disturbances responsible for age-related differences in sudden death in guinea pigs. Flecainide, but not quinidine, lowered the number of PVCs, suggesting that RyR2 channels contribute to rhythm changes in response to acute β-AR stimulation in guinea pigs. Importantly, age-specific differences in RyR2 oxidation resulted from acute ISO treatment, and RyR2 oxidation was lower with the use of flecainide. The increased number of PVCs, repolarization abnormalities, and oxidation of RyR2 in adult guinea pigs in response to ISO led to a higher incidence of fatal ventricular arrhythmias compared with their younger counterparts.
GRANTS
This work was supported by funding from National Heart, Lung, and Blood Institute Grants R01-HL-07715 (to B. L. Stauffer), R01-HL-16928 (to S. D. Miyamoto), T32-HL-007171 (to K. C. Woulfe), and R25-HL-103286 (to S. Nau) and American Heart Association Grant 16POST29970010 (to K. C. Woulfe). Medtronic provided research support to investigate the use of LINQ cardiac monitors in animal research.
DISCLAIMERS
The contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health views.
DISCLOSURES
C. C. Sucharov, B. L. Stauffer, and S. D. Miyamoto are founders and scientific advisors for CoramiR Biomedical, LLC.
AUTHOR CONTRIBUTIONS
K.C.W. and B.L.S. conceived and designed research; K.C.W., C.E.W., S.N., S.C., and E.K.P. performed experiments; K.C.W., C.E.W., S.N., S.Z., and C.T. analyzed data; K.C.W., S.Z., C.T., C.C.S., S.D.M., and B.L.S. interpreted results of experiments; K.C.W. prepared figures; K.C.W. drafted manuscript; K.C.W., C.C.S., S.D.M., and B.L.S. edited and revised manuscript; K.C.W., C.T., C.C.S., S.D.M., and B.L.S. approved final version of manuscript.
REFERENCES
- 2.Becerra R, Román B, Di Carlo MN, Mariangelo JI, Salas M, Sanchez G, Donoso P, Schinella GR, Vittone L, Wehrens XH, Mundiña-Weilenmann C, Said M. Reversible redox modifications of ryanodine receptor ameliorate ventricular arrhythmias in the ischemic-reperfused heart. Am J Physiol Heart Circ Physiol 311: H713–H724, 2016. doi: 10.1152/ajpheart.00142.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM. Cardiac ryanodine receptor phosphorylation: target sites and functional consequences. Biochem J 396: e1–e3, 2006. doi: 10.1042/BJ20060377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bovo E, Lipsius SL, Zima AV. Reactive oxygen species contribute to the development of arrhythmogenic Ca2+ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes. J Physiol 590: 3291–3304, 2012. doi: 10.1113/jphysiol.2012.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck ED, Lachnit WG, Pessah IN. Mechanisms of delta-hexachlorocyclohexane toxicity: I. Relationship between altered ventricular myocyte contractility and ryanodine receptor function. J Pharmacol Exp Ther 289: 477–485, 1999. [PubMed] [Google Scholar]
- 7.Chatfield KC, Sparagna GC, Sucharov CC, Miyamoto SD, Grudis JE, Sobus RD, Hijmans J, Stauffer BL. Dysregulation of cardiolipin biosynthesis in pediatric heart failure. J Mol Cell Cardiol 74: 251–259, 2014. doi: 10.1016/j.yjmcc.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Regul Integr Comp Physiol 287: R247–R249, 2004. doi: 10.1152/ajpregu.00346.2004. [DOI] [PubMed] [Google Scholar]
- 9.Curran J, Hinton MJ, Ríos E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res 100: 391–398, 2007. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 10.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329: 23–38, 2003. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 11.de Jong S, van Veen TA, van Rijen HV, de Bakker JM. Fibrosis and cardiac arrhythmias. J Cardiovasc Pharmacol 57: 630–638, 2011. doi: 10.1097/FJC.0b013e318207a35f. [DOI] [PubMed] [Google Scholar]
- 12.DiBianco R, Shabetai R, Kostuk W, Moran J, Schlant RC, Wright R. A comparison of oral milrinone, digoxin, and their combination in the treatment of patients with chronic heart failure. N Engl J Med 320: 677–683, 1989. doi: 10.1056/NEJM198903163201101. [DOI] [PubMed] [Google Scholar]
- 13.Dimas VV, Denfield SW, Friedman RA, Cannon BC, Kim JJ, Smith EO, Clunie SK, Price JF, Towbin JA, Dreyer WJ, Kertesz NJ. Frequency of cardiac death in children with idiopathic dilated cardiomyopathy. Am J Cardiol 104: 1574–1577, 2009. doi: 10.1016/j.amjcard.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Ellison GM, Torella D, Karakikes I, Purushothaman S, Curcio A, Gasparri C, Indolfi C, Cable NT, Goldspink DF, Nadal-Ginard B. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem 282: 11397–11409, 2007. doi: 10.1074/jbc.M607391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewy GA. Inotropic infusions for chronic congestive heart failure: medical miracles or misguided medicinals? J Am Coll Cardiol 33: 572–575, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Felker GM, O’Connor CM. Inotropic therapy for heart failure: an evidence-based approach. Am Heart J 142: 393–401, 2001. doi: 10.1067/mhj.2001.117606. [DOI] [PubMed] [Google Scholar]
- 17.Greer-Short A, Poelzing S. Temporal response of ectopic activity in guinea pig ventricular myocardium in response to isoproterenol and acetylcholine. Front Physiol 6: 278, 2015. doi: 10.3389/fphys.2015.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm M, Brown JH. Beta-adrenergic receptor signaling in the heart: role of CaMKII. J Mol Cell Cardiol 48: 322–330, 2010. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, Yan GX. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol 41: 567–574, 2008. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Hauptman PJ, Mikolajczak P, George A, Mohr CJ, Hoover R, Swindle J, Schnitzler MA. Chronic inotropic therapy in end-stage heart failure. Am Heart J 152: 1096.e1–1096.e8, 2006. doi: 10.1016/j.ahj.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, Piston DW, Huke S, Knollmann BC. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol 48: 293–301, 2010. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho HT, Stevens SC, Terentyeva R, Carnes CA, Terentyev D, Györke S. Arrhythmogenic adverse effects of cardiac glycosides are mediated by redox modification of ryanodine receptors. J Physiol 589: 4697–4708, 2011. doi: 10.1113/jphysiol.2011.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horn MA, Trafford AW. Aging and the cardiac collagen matrix: novel mediators of fibrotic remodelling. J Mol Cell Cardiol 93: 175–185, 2016. doi: 10.1016/j.yjmcc.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houser SR. Role of RyR2 phosphorylation in heart failure and arrhythmias: protein kinase A-mediated hyperphosphorylation of the ryanodine receptor at serine 2808 does not alter cardiac contractility or cause heart failure and arrhythmias. Circ Res 114: 1320–1327, 2014. doi: 10.1161/CIRCRESAHA.114.300569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res 97: 1173–1181, 2005. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 26.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res 91: 1015–1022, 2002. doi: 10.1161/01.RES.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 27.Kawata H, Ohno S, Aiba T, Sakaguchi H, Miyazaki A, Sumitomo N, Kamakura T, Nakajima I, Inoue YY, Miyamoto K, Okamura H, Noda T, Kusano K, Kamakura S, Miyamoto Y, Shiraishi I, Horie M, Shimizu W. Catecholaminergic polymorphic ventricular tachycardia (CPVT) associated with ryanodine receptor (RyR2) gene mutations−long-term prognosis after initiation of medical treatment. Circ J 80: 1907–1915, 2016. doi: 10.1253/circj.CJ-16-0250. [DOI] [PubMed] [Google Scholar]
- 28.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Böhm M, O’Rourke B, Maack C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation 121: 1606–1613, 2010. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp-Te interval and its diagnostic value. J Electrocardiol 41: 575–580, 2008. doi: 10.1016/j.jelectrocard.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol Cell Physiol 275: C1–C24, 1998. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- 31.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 118: 2230–2245, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu GS, Morales A, Vafiadaki E, Lam CK, Cai WF, Haghighi K, Adly G, Hershberger RE, Kranias EG. A novel human R25C-phospholamban mutation is associated with super-inhibition of calcium cycling and ventricular arrhythmia. Cardiovasc Res 107: 164–174, 2015. doi: 10.1093/cvr/cvv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu T, Takimoto E, Dimaano VL, DeMazumder D, Kettlewell S, Smith G, Sidor A, Abraham TP, O’Rourke B. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ Res 115: 44–54, 2014. doi: 10.1161/CIRCRESAHA.115.303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luebbert J, Auberson D, Marchlinski F. Premature ventricular complexes in apparently normal hearts. Card Electrophysiol Clin 8: 503–514, 2016. doi: 10.1016/j.ccep.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Mangmool S, Shukla AK, Rockman HA. β-Arrestin-dependent activation of Ca2+/calmodulin kinase II after β1-adrenergic receptor stimulation. J Cell Biol 189: 573–587, 2010. doi: 10.1083/jcb.200911047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention . Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113: 1807–1816, 2006. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 37.Mirza M, Strunets A, Shen WK, Jahangir A. Mechanisms of arrhythmias and conduction disorders in older adults. Clin Geriatr Med 28: 555–573, 2012. doi: 10.1016/j.cger.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto SD, Stauffer BL, Nakano S, Sobus R, Nunley K, Nelson P, Sucharov CC. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J 35: 33–41, 2014. doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morimoto S, O-Uchi J, Kawai M, Hoshina T, Kusakari Y, Komukai K, Sasaki H, Hongo K, Kurihara S. Protein kinase A-dependent phosphorylation of ryanodine receptors increases Ca2+ leak in mouse heart. Biochem Biophys Res Commun 390: 87–92, 2009. doi: 10.1016/j.bbrc.2009.09.071. [DOI] [PubMed] [Google Scholar]
- 40.Murakami W, Kobayashi S, Susa T, Nanno T, Ishiguchi H, Myoren T, Nishimura S, Kato T, Hino A, Oda T, Okuda S, Yamamoto T, Yano M. Recombinant atrial natriuretic peptide prevents aberrant Ca2+ leakage through the ryanodine receptor by suppressing mitochondrial reactive oxygen species production induced by isoproterenol in failing cardiomyocytes. PLoS One 11: e0163250, 2016. doi: 10.1371/journal.pone.0163250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Najafi A, Sequeira V, Kuster DW, van der Velden J. β-Adrenergic receptor signalling and its functional consequences in the diseased heart. Eur J Clin Invest 46: 362–374, 2016. doi: 10.1111/eci.12598. [DOI] [PubMed] [Google Scholar]
- 42.Nakano SJ, Miyamoto SD, Movsesian M, Nelson P, Stauffer BL, Sucharov CC. Age-related differences in phosphodiesterase activity and effects of chronic phosphodiesterase inhibition in idiopathic dilated cardiomyopathy. Circ Heart Fail 8: 57–63, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42a.National Center for Biotechnology Information MDK midkine [Homo sapiens (human)]. https://www.ncbi.nlm.nih.gov/gene/4192 [2 September 2018].
- 43.Nogami A. Trigger elimination of polymorphic ventricular tachycardia and ventricular fibrillation by catheter ablation: trigger and substrate modification. J Biomed Res 29: 44–51, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nony P, Boissel JP, Lievre M, Leizorovicz A, Haugh MC, Fareh S, de Breyne B. Evaluation of the effect of phosphodiesterase inhibitors on mortality in chronic heart failure patients. A meta-analysis. Eur J Clin Pharmacol 46: 191–196, 1994. doi: 10.1007/BF00192547. [DOI] [PubMed] [Google Scholar]
- 45.Ogrodnik J, Niggli E. Increased Ca2+ leak and spatiotemporal coherence of Ca2+ release in cardiomyocytes during beta-adrenergic stimulation. J Physiol 588: 225–242, 2010. doi: 10.1113/jphysiol.2009.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overholser BR, Zheng X, Pell C, Blickman A. Sudden death in the presence of overt beta-adrenergic receptor activation in guinea pigs immediately following isoflurane anesthesia. Vet Anaesth Analg 37: 273–279, 2010. doi: 10.1111/j.1467-2995.2010.00533.x. [DOI] [PubMed] [Google Scholar]
- 47.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK, DeMets DL; The PROMISE Study Research Group . Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med 325: 1468–1475, 1991. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 48.Packer M, Medina N, Yushak M. Hemodynamic and clinical limitations of long-term inotropic therapy with amrinone in patients with severe chronic heart failure. Circulation 70: 1038–1047, 1984. doi: 10.1161/01.CIR.70.6.1038. [DOI] [PubMed] [Google Scholar]
- 49.Pahl E, Sleeper LA, Canter CE, Hsu DT, Lu M, Webber SA, Colan SD, Kantor PF, Everitt MD, Towbin JA, Jefferies JL, Kaufman BD, Wilkinson JD, Lipshultz SE; Pediatric Cardiomyopathy Registry Investigators . Incidence of and risk factors for sudden cardiac death in children with dilated cardiomyopathy: a report from the Pediatric Cardiomyopathy Registry. J Am Coll Cardiol 59: 607–615, 2012. doi: 10.1016/j.jacc.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol 4: 441–447, 2011. doi: 10.1161/CIRCEP.110.960658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res 88: 1159–1167, 2001. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 52.Reddy YS. Phosphorylation of cardiac regulatory proteins by cyclic AMP-dependent protein kinase. Am J Physiol 231: 1330–1336, 1976. [DOI] [PubMed] [Google Scholar]
- 53.Rhee EK, Canter CE, Basile S, Webber SA, Naftel DC. Sudden death prior to pediatric heart transplantation: would implantable defibrillators improve outcome? J Heart Lung Transplant 26: 447–452, 2007. doi: 10.1016/j.healun.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY; Pediatric Carvedilol Study Group . Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA 298: 1171–1179, 2007. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 55.Shaddy RE, Curtin EL, Sower B, Tani LY, Burr J, LaSalle B, Boucek MM, Mahony L, Hsu DT, Pahl E, Burch GH, Schlencker-Herceg R. The pediatric randomized carvedilol trial in children with chronic heart failure: rationale and design. Am Heart J 144: 383–389, 2002. doi: 10.1067/mhj.2002.124402. [DOI] [PubMed] [Google Scholar]
- 56.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 93: 592–594, 2003. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 57.Siontis KC, Kim HM, Sharaf Dabbagh G, Latchamsetty R, Stojanovska J, Jongnarangsin K, Morady F, Bogun FM. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Heart Rhythm 14: 1487–1493, 2017. doi: 10.1016/j.hrthm.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Soltysinska E, Olesen SP, Osadchii OE. Myocardial structural, contractile and electrophysiological changes in the guinea-pig heart failure model induced by chronic sympathetic activation. Exp Physiol 96: 647–663, 2011. doi: 10.1113/expphysiol.2011.058503. [DOI] [PubMed] [Google Scholar]
- 59.Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin 8: 143–164, 2012. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sucharov CC, Hijmans JG, Sobus RD, Melhado WF, Miyamoto SD, Stauffer BL. β-Adrenergic receptor antagonism in mice: a model for pediatric heart disease. J Appl Physiol 115: 979–987, 2013. doi: 10.1152/japplphysiol.00627.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sucharov CC, Mariner P, Long C, Bristow M, Leinwand L. Yin Yang 1 is increased in human heart failure and represses the activity of the human alpha-myosin heavy chain promoter. J Biol Chem 278: 31233–31239, 2003. doi: 10.1074/jbc.M301917200. [DOI] [PubMed] [Google Scholar]
- 62.Sutherland EW, Rall TW. Formation of adenosine-3,5-phosphate (cyclic adenylate) and its relation to the action of several neurohormones or hormones. Acta Endocrinol Suppl (Copenh) 34, Suppl 50: 171–174, 1960. [DOI] [PubMed] [Google Scholar]
- 63.Tatman PD, Woulfe KC, Karimpour-Fard A, Jeffrey DA, Jaggers J, Cleveland JC, Nunley K, Taylor MR, Miyamoto SD, Stauffer BL, Sucharov CC. Pediatric dilated cardiomyopathy hearts display a unique gene expression profile. JCI Insight 2: 94249, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terentyev D, Györke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, Carcache de Blanco E, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Györke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 103: 1466–1472, 2008. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 296: 1867–1876, 2006. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 66.Venetucci LA, Trafford AW, O’Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res 77: 285–292, 2008. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med 15: 380–383, 2009. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe H, Knollmann BC. Mechanism underlying catecholaminergic polymorphic ventricular tachycardia and approaches to therapy. J Electrocardiol 44: 650–655, 2011. doi: 10.1016/j.jelectrocard.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 69.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113: 829–840, 2003. doi: 10.1016/S0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 70.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res 94: e61–e70, 2004. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 71.Woulfe KC, Siomos AK, Nguyen H, SooHoo M, Galambos C, Stauffer BL, Sucharov C, Miyamoto S. Fibrosis and fibrotic gene expression in pediatric and adult patients with idiopathic dilated cardiomyopathy. J Card Fail 23: 314–324, 2017. doi: 10.1016/j.cardfail.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res 77: 432–441, 2008. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 73.Yang Q, Padrini R, Piovan D, Ferrari M. Cardiac effects of quinidine on guinea-pig isolated perfused hearts after in vivo quinidine pretreatment. Br J Pharmacol 122: 7–12, 1997. doi: 10.1038/sj.bjp.0701318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoo B, Lemaire A, Mangmool S, Wolf MJ, Curcio A, Mao L, Rockman HA. β1-Adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol 297: H1377–H1386, 2009. doi: 10.1152/ajpheart.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H, Makarewich CA, Kubo H, Wang W, Duran JM, Li Y, Berretta RM, Koch WJ, Chen X, Gao E, Valdivia HH, Houser SR. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circ Res 110: 831–840, 2012. doi: 10.1161/CIRCRESAHA.111.255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J, Xiao H, Shen J, Wang N, Zhang Y. Different roles of β-arrestin and the PKA pathway in mitochondrial ROS production induced by acute β-adrenergic receptor stimulation in neonatal mouse cardiomyocytes. Biochem Biophys Res Commun 489: 393–398, 2017. doi: 10.1016/j.bbrc.2017.05.140. [DOI] [PubMed] [Google Scholar]
- 77.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71: 310–321, 2006. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]