Abstract
The right ventricular (RV) response to pulmonary arterial hypertension (PAH) is heterogeneous. Most patients have maladaptive changes with RV dilation and RV failure, whereas some, especially patients with PAH secondary to congenital heart disease, have an adaptive response with hypertrophy and preserved systolic function. Mechanisms for RV adaptation to PAH are unknown, despite RV function being a primary determinant of mortality. In our congenital heart disease ovine model with fetally implanted aortopulmonary shunt (shunt lambs), we previously demonstrated an adaptive physiological RV response to increased afterload with hypertrophy. In the present study, we examined small noncoding microRNA (miRNA) expression in shunt RV and characterized downstream effects of a key miRNA. RV tissue was harvested from 4-wk-old shunt and control lambs (n = 5), and miRNA, mRNA, and protein were quantitated. We found differential expression of 40 cardiovascular-specific miRNAs in shunt RV. Interestingly, this miRNA signature is distinct from models of RV failure, suggesting that miRNAs might contribute to adaptive RV hypertrophy. Among RV miRNAs, miR-199b was decreased in the RV with eventual downregulation of nuclear factor of activated T cells/calcineurin signaling. Furthermore, antifibrotic miR-29a was increased in the shunt RV with a reduction of the miR-29 targets collagen type A1 and type 3A1 and decreased fibrosis. Thus, we conclude that the miRNA signature specific to shunt lambs is distinct from RV failure and drives gene expression required for adaptive RV hypertrophy. We propose that the adaptive RV miRNA signature may serve as a prognostic and therapeutic tool in patients with PAH to attenuate or prevent progression of RV failure and premature death.
NEW & NOTEWORTHY This study describes a novel microRNA signature of adaptive right ventricular hypertrophy, with particular attention to miR-199b and miR-29a.
Keywords: congenital heart disease, microRNA, pulmonary hypertension, right ventricular hypertrophy
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare but devastating disease. The prognosis for untreated PAH is dismal, with a mean survival of 2.8 yr (8). Although both PAH research and clinical evaluation of patients with PAH have focused on abnormal pulmonary vascular reactivity and remodeling, mortality in patients with PAH is ultimately due to right ventricular (RV) failure (RVF), and indexes of RV function in patients with PAH, including right atrial pressure and cardiac index, correlate most closely with survival and functional class (34). Indeed, the prognostic significance of RV function across a diverse group of cardiovascular diseases, including PAH (35), left heart failure (14), and congenital heart disease (CHD), has only recently been recognized (30). Furthermore, as survival for patients with CHD improves, there is a growing population of adult patients with CHD; 10% of adults with CHD develop PAH, a proportion that is expected to rise (23). Understanding the molecular mechanism of RV adaptation to PAH and CHD continues to be an urgent clinical need.
The RV response to PAH is heterogeneous and unpredictable, even among patients with equivalent hemodynamic afterload and advanced pulmonary vasculopathy (7). Some patients with adaptive RV hypertrophy (RVH) remain clinically stable for decades, whereas others with maladaptive RVH rapidly progress to RVF, resulting in morbidity (29). This adaptive RV phenotype is particularly notable in PAH secondary to CHD, similar to our shunt model (17). The morphological and functional characteristics of adaptive RVH (concentric hypertrophy and preserved ejection fraction and cardiac output), unlike maladaptive RVH (fibrosis, RV dilation, and decreased ejection fraction and cardiac output), are well known, but the mechanisms underlying these differences are poorly understood. Moreover, the potential of translating mechanisms underlying adaptive RVH into therapeutic interventions remains unrealized.
The focus of the present study was to identify the microRNA (miRNA) expression pattern in a model of CHD with fetally implanted aortopulmonary shunt with adaptive RVH and to compare that expression pattern with other animal models of RVH and RVF due to PAH or pulmonary stenosis as well as human RVF. Although there are some commonalities of miRNA expression in cardiac hypertrophy regardless of the inciting mechanism, it is increasingly recognized that each disease state has a unique “miRNA signature” (21). By comparing miRNA expression levels in our model with other animal models of RVF, those targets that are differentially regulated may prove important for distinguishing the adaptive from maladaptive RV phenotype. Furthermore, we investigated specific miRNA signaling pathways and their consequences on RVH and pathobiology, including nuclear factor of activated T cell (NFAT)-mediated transcription as well as cardiac fibrosis. Using unbiased bioinformatics techniques with this unique physiology, our investigations may yield insights that eventually alter therapeutic paradigms for both PAH and RVF in CHD.
METHODS
Animal surgical preparations.
The Committee on Animal Research of the University of California (San Francisco,CA) approved all protocols and procedures. A total of nine pregnant mixed-breed Western ewes (137−141 days gestation, full term = 145 days) were anesthetized. Fetal exposure was obtained through the horn of the uterus, and a left lateral thoracotomy was performed on the fetal lamb. With the use of side-biting vascular clamps, an 8.0-mm vascular graft was anastomosed between the ascending aorta and main pulmonary artery of the fetal lambs, as previously described in detail (28). Control lambs were provided by twin gestation (n = 3) or age matched (n = 3). Control lambs did not undergo a lateral thoracotomy. Left ventricular (LV) and RV tissues were harvested, and hemodynamic measurements were performed when control and shunt lambs were 4–6 wk old.
At the time of hemodynamic experiment, lambs were anesthetized and catheters were placed into the right and left atrium and main pulmonary artery, as previously described (28). An ultrasonic flow probe (Transonics Systems, Ithaca, NY) was placed around the left pulmonary artery to measure pulmonary blood flow.
At the end of the study, all lambs were euthanized with a lethal injection of pentobarbital sodium (150 mg/kg) followed by bilateral thoracotomy, as described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Sample collection and general molecular methodologies.
At the conclusion of the study, heart tissue was harvested and divided into chambers. A portion of the tissue was snap frozen and stored at −80°C. Snap-frozen tissue was homogenized for protein experiments according to the usual methods. Protein levels were determined using Western blot analysis.
Quantitative real-time PCR.
RNA was extracted from sheep hearts using an RNeasy fibrous tissue mini kit (Qiagen, Toronto, ON, Canada) according to the manufacturer’s protocols. Identical amounts of purified total RNA were used as the starting material. First-strand cDNA was synthesized from each sample and subjected to reverse transcription using an RNA-to-cDNA ecoDry premix [oligo(dT)] kit (Clontech Laboratories) according to the manufacturer’s protocol. cDNA templates were mixed with gene-specific primers and FastStart Universal SYBR green master mix with ROX reference dye (Roche). Primers were designed with the public OligoPerfect Designer software (Life Technologies). Reactions were completed with the iCycler iQ real-time PCR detection system (Bio-Rad).
MiRNA quantitative real-time PCR.
RNA was extracted as described above. Reverse transcription was performed using the miRCURY LNA universal RT miRNA PCR, polyadenylation, and cDNA synthesis kit (Exiqon). cDNA was prepared according to the protocol for miRCURY LNA Universal RT microRNA PCR; amplification was then performed in a LightCycler 480 real-time PCR system (Roche). Amplification curves were analyzed using Roche LightCycler software, both for the determination of the quantification cycle and for melting-curve analysis. ΔΔCT (where CT is threshold cycle) was calculated using let-7a-5p, which was shown by Normfinder software (Aarhus University Hospital) to have the lowest stability value among six potential candidate reference genes (2), as a reference and normalized to compare relative miRNA expression between shunt and control tissue; UniSp6 was used as a loading control per protocol.
Immunohistochemistry.
Sections from the RV and LV of juvenile control and shunt lambs were fixed and prepared for immunohistochemistry staining as previously described (5) and incubated with Masson’s trichrome according to the usual protocol for fibrosis quantification. Samples were sent to a blinded collaborator (University of California, Davis, CA), where interstitial myocardial fibrosis was assessed by stereological analysis according to published techniques (3, 22).
Western blot and densitometry analysis.
Protein was determined by Western blot analysis, as previously described (4). Primary antibodies against dual-specificity tyrosine phosphorylation-regulated kinase 1a (Dyrk1a), NFATc2, phosphorylated (Ser326) NFAT, collagen type 1A (Col1A), and collagen type 3A (Col3A) were obtained from Santa Cruz Biotechnology. Anti-β-actin was used as the reference protein for loading controls. Quantification of protein band density in X-ray films from ECL Western blots was quantified by a public domain Java image-processing program (ImageJ, National Institutes of Health). Immunoprecipitation experiments were performed to examine protein interactions.
Chromatin immunoprecipitation (ChIP) was performed in RVs and LVs from juvenile control and shunt sheep using the Upstate Biotechnology ChIP assay kit according to the manufacturer’s instructions; rabbit polyclonal antibody against NFATc2 (Santa Cruz Biotechnology) and IgG (Millipore) were used as negative controls. Equal amounts of soluble chromatin were immunoprecipitated with antisera, and immunocomplexes were immobilized and subjected to reverse cross-linking. The associated DNA was purified, and PCR was carried out using specific primers directed toward NFATc2-binding regions within the promoter regions of Rho family GTPase 1 (RND1), regulator of calcineurin 1 (RCAN1)/Down syndrome critical region (DSCR), and nuclear receptor subfamily 4 group A (NR4A1 and NR4A2) genes. ChIP output was normalized using primers directed against the sheep input quantitative PCR fold change values. Quantitative real-time PCRs were performed with a single-color real-time PCR detection system (MyiQ, Bio-Rad). Data were analyzed with IQ5 real-time PCR detection system software (Bio-Rad). Promoter pairs are shown in Table 1.
Table 1.
Primers for NFATc2 chromatin immunoprecipitation-quantitative PCR: promoter regions for sheep RND1, RCAN1/DSCR, NR4A1, and NR4A2 genes
| Primers |
||
|---|---|---|
| Gene | Forward | Reverse |
| RCAN1 promoter | 5′-CCGGACCTCGTTGCTTTAT-3′ | 5′-CACAGTGAGAGCTACAGCGACT-3′ |
| RND1 promoter | 5′-CTGGCCTCTTTCTCAGTTGG-3′ | 5′-GGGACCTGGACTCGGATT-3′ |
| NR4A1 promoter | 5′-TCCTGGGTTCTGTTGTGG-3′ | 5′-GGCCTGAAGCCTGATCTCTA-3′ |
| NR4A2 promoter | 5′-CCCTAAGGCTTCCTGTGTCTT-3′ | 5′-GGTGGACAGTGTCGTAATTCAA-3′ |
NFAT, nuclear factor of activated T cells; RND1, Rho family GTPase 1; RCAN1, regulator of calcineurin 1; DSCR, Down syndrome critical region; NR4A, nuclear receptor subfamily 4 group A.
Statistical analysis.
For Western blot analysis, means ± SD were calculated. Results for quantitative PCR are shown as means ± SE. ΔΔCT was calculated with GAPDH as the reference for mRNA and let-7a-5p for miRNA and normalized to compare relative mRNA expression between control and shunt PAECs. Control and shunt samples were compared by an unpaired t-test. P < 0.05 was considered statistically significant.
RESULTS
As previously demonstrated, pulmonary blood flow and pulmonary arterial pressures were significantly higher in juvenile shunt than control lambs (20). Given the presence of a fetally implanted unrestrictive shunt at the main pulmonary artery, the aortopulmonary shunt maintains fetal RV afterload through postnatal life. The fetal RV is exposed to both elevated pulmonary vascular resistance and systemic afterload (through the patent ductus), and our unrestrictive shunt perpetuates this afterload.
Using quantitative PCR analysis, we determined expression levels of 93 miRNAs relevant to cardiovascular biology in LV and RV tissue from juvenile shunt and control lambs. Forty miRNAs were differentially expressed in the control RV versus sham RV (Table 2). We then compared miRNA expression patterns in our animal model of adaptive RVH with other animal models of RVH (Table 3). Although some similarities exist among models, important differences in our model warrant highlighting. Among the shunt RV miRNAs, miR-199b was downregulated in adaptive RVH but upregulated in maladaptive RVH (26). miR-199b has been demonstrated to be a critical mediator of pathological cardiac hypertrophy by regulating NFAT (9). miR-29a was increased in adaptive RVH but decreased in maladaptive RVH (26). Schlosser and colleagues demonstrated differences among animal models and tissues (heart, lung, and blood) within a model (32); however, instead of being a limitation, these differences may be a key in differentiating between maladaptive and adaptive RVH. Given the role of miRNAs as posttranscriptional modulators of mRNAs, the alterations in miRNA expression can provide insights into functional differences translating to our previously observed global physiological differences in superior response to afterload stimulus (18).
Table 2.
MicroRNAs with significant differences between control and shunt RVs
| Target | Fold Change (Relative to Control) | P Value |
|---|---|---|
| miR-378a-3p | 0.38* | 0.024* |
| miR-208a-3p | 0.41* | 0.016* |
| miR-101-3p | 0.44* | 0.008* |
| miR-199b-5p | 0.60* | 0.042* |
| miR-28-5p | 1.43 | 0.024 |
| miR-25-3p | 1.44 | 0.033 |
| miR-152-3p | 1.56 | 0.012 |
| let-7e-5p | 1.61 | 0.042 |
| miR-23a-3p | 1.76 | 0.043 |
| miR-22-3p | 1.80 | 0.018 |
| miR-143-3p | 1.83 | 0.029 |
| miR-379-5p | 1.88 | 0.044 |
| miR-99b-5p | 1.91 | 0.018 |
| miR-106b-5p | 1.92 | 0.048 |
| miR-92a-3p | 2.12 | 0.009 |
| miR-27b-3p | 2.18 | 0.016 |
| miR-7-5p | 2.33 | 0.018 |
| miR-374a-5p | 2.47 | 0.006 |
| miR-191-5p | 2.58 | 0.003 |
| miR-221-3p | 2.58 | 0.036 |
| miR-208b-3p | 2.59 | 0.004 |
| miR-15a-5p | 2.69 | 0.035 |
| miR-128-3p | 2.78 | 0.001 |
| miR-99a-5p | 2.85 | 0.041 |
| miR-185-5p | 2.95 | 0.002 |
| miR-543 | 3.32 | 0.012 |
| miR-222-3p | 3.47 | 0.009 |
| miR-379-3p | 3.50 | 0.042 |
| let-7f-5p | 5.52 | 0.037 |
| miR-18a-5p | 7.33 | 0.027 |
| miR-338-3p | 7.46 | 0.045 |
| miR-212-5p | 9.23 | 0.033 |
| miR-29a-3p | 10.98 | 0.016 |
| miR-664a-3p | 15.72 | 0.016 |
| miR-382-5p | 19.19 | 0.008 |
| miR-1248 | 20.93 | 0.015 |
| miR-191-3p | 103.10 | 0.010 |
| miR-200b-3p | 133.40 | 0.007 |
| miR-34c-5p | 461.97 | 0.004 |
A total of 40 (of 93 tested) microRNA (miRNA) targets were significantly different (P < 0.05) between control and shunt right ventricular (RV) tissue. *Downregulated compared with control. ΔΔCT (where CT is threshold cycle) was calculated using let-7a-5p as a reference and normalized to compare relative miRNA expression between shunt and control tissue (n = 5); UniSp6 was used as a loading control per protocol.
Table 3.
MicroRNAs in our ovine model of congenital heart disease. with adaptive RV hypertrophy and other animal models of RV hypertrophy and RV failure as well as human RV failure
| RV Failure |
||||
|---|---|---|---|---|
| MicroRNA | Shunt RV | Pulmonary artery banding | Hypoxia and Sugen | Monocrotaline |
| miR-29a | Increased | Decreased | Unchanged | |
| miR-30a-5p | Increased | Decreased | Decreased | |
| miR-92a | Increased | Decreased | ||
| miR-126 | Unchanged | |||
| miR-127-3p | Increased | Increased | Decreased | |
| miR-155 | Unchanged | Increased | ||
| miR-199b-5p | Decreased | Increased | ||
| miR-200b | Increased | Decreased | ||
| miR-208a-3p | Decreased | Decreased | Decreased | |
| miR-379-5p | Increased | Increased | Decreased | |
| miR-338-3p | Increased | Decreased | ||
Selected microRNAs (miRNAs) were compared with other notable published models of right ventricular (RV) hypertrophy and RV failure. Antifibrotic miR-29a was increased in our model but decreased in RV failure secondary to pulmonary artery banding. Additionally, miR-199b-5p, which was increased in our model, was also decreased in pulmonary artery banding RV failure as well as human RV failure. Pulmonary artery banding RV failure data are from Ref. 26, hypoxia and Sugen RVF data from Ref. 10, and monocrotaline RVF data from Refs. 25 and 32.
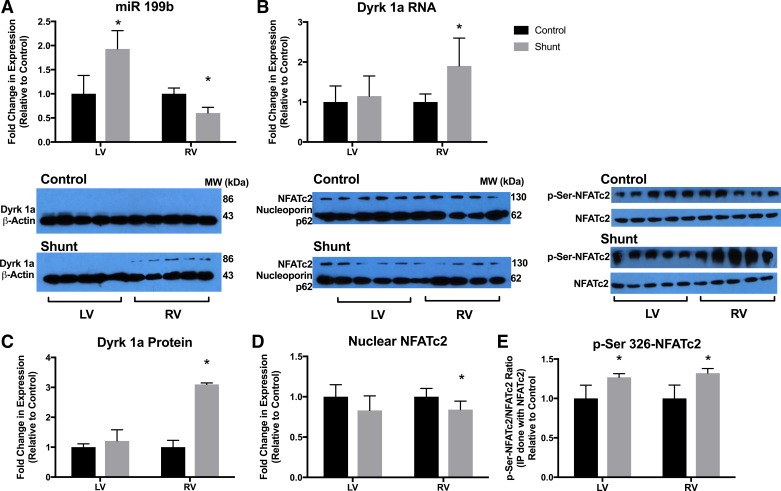
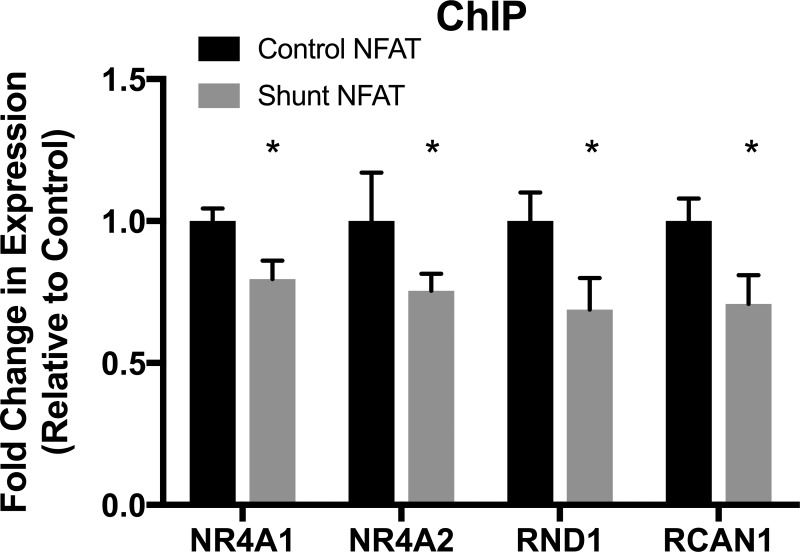
Expression of miR-199b was increased 1.93 ± 0.37-fold in the shunt LV compared with control LV, whereas it was decreased 0.60 ± 0.12-fold in the shunt RV compared with the control RV (Fig. 1A). miR-199b was increased in an animal model of RVH due to pulmonary artery banding (26) and is classically associated with maladaptive hypertrophy in LV animal models (21). One target of miR-199b, the mRNA of Dyrk1a, was increased 2.1 ± 0.71-fold (Fig. 1B), and Dyrk1a protein (Fig. 1C) was increased 3.1 ± 0.05-fold compared with control. Dyrk1a is known to be a negative regulator of NFAT signaling (9). Consistently, we found increased cytosolic NFAT phosphorylation (Fig. 1E) and decreased nuclear NFAT (Fig. 1D) in shunt RV tissue [phosphorylated (Ser326) NFAT increased 1.3 ± 0.6-fold and nuclear NFAT increased 0.8 ± 0.1-fold compared with control]. Using the ChIP assay, we also confirmed the decreased binding of NFAT at the promoter region of the target genes NR4A1, NR4A2, RCAN1, and RND1 (Fig. 2). Negative control was performed with IgG antibody and no-antibody control, and output results were the same for IgG and no-antibody control. These results suggest that the reduction of miR-199b in the shunt RV results in the decrease in the NFAT-calcineurin pathway and maladaptive RVH in the ovine model of CHD.
Fig. 1.
The microRNA (miR)-199b dual-specificity tyrosine phosphorylation-regulated kinase 1a (Dyrk1a)-nuclear factor of activated cell (NFAT) axis. A: miR-199b expression was increased in the shunt left ventricle (LV) and decreased in the shunt right ventricle (RV) compared with the control LV and RV. B: Dyrk1a RNA expression was increased in the shunt RV compared with control RV. MiRNA and mRNA expression was quantified by PCR. Values are means ± SE. C: Dyrk1a protein expression was increased in the shunt RV compared with the control RV. There was no difference in Dyrk1a protein between shunt and control LVs. D: the nuclear fraction of cells was separated from LV and RV homogenates, and NFATc2 was quantified. There was significantly less NFATc2 in the nuclear isolate from the shunt RV compared with control RV. There was no difference in LV nuclear NFATc2. E: the cytosolic fraction of LV and RV homogenates was immunoprecipitated using NFATc2 and then immunoblotted using phosphorylated (Ser326) NFATc2 (p-Ser326-NFATc2) and NFATc2 for quantification. p-Ser326-NFATc2 did not translocate to the nucleus to promote transcription and was more abundant in the LV and RV of shunt lambs compared with control lambs. In C–E, protein expression was quantified by Western blot analysis. For each protein, immunoblots of different membranes were performed in parallel using identical aliquots of the same protein homogenate. Bar graphs and results represent densitometry performed on individual samples (n = 5 in all groups). *P < 0.05 vs. control.
Fig. 2.
Nuclear factor of activated T cell (NFATc2) chromatin binding. Chromatin derived from control and shunt right ventricular (RV) tissue was immunoprecipitated using NFATc2 antibody with IgG antibody as a negative control, and PCR was carried out using specific primers directed toward NFATc2-binding regions within the promoter regions of Rho family GTPase 1 (RND1), regulator of calcineurin 1 (RCAN1)/Down syndrome critical region (DSCR), and nuclear receptor subfamily 4 group A (NR4A1 and NR4A2) genes. In the shunt RV, NFATc2 binding to promoters of downstream targets RND1, RCAN1/DSCR, NR4A1, and NR4A2 was significantly decreased. ChIP, chromatin immunoprecipitation. Values are means ± SE; n = 5 in all groups. *P < 0.05 vs. control NFAT.
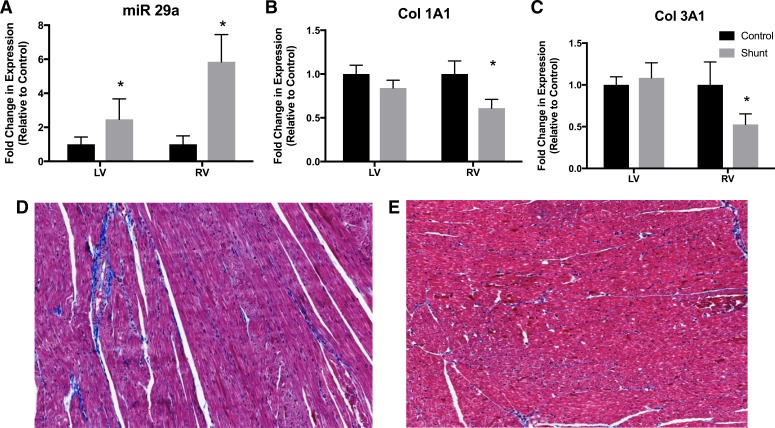
Unlike miR-199b, miR-29a was increased 5.9 ± 1.6-fold in the shunt RV (Fig. 3A). Col1A1 (0.6 ± 0.1-fold decrease compared with control) and Col3A1 (0.5 ± 0.1-fold decrease compared with control) were significantly decreased in the shunt RV (Fig. 3, B and C). Furthermore, there was no induction of fibrosis in the RV of shunt lambs, as demonstrated by collagen staining (Fig. 3, D and E). Thus, we conclude that the induction of miR-29a plays an antifibrotic role.
Fig. 3.
MicroRNA (miR)-29a expression and fibrosis. A: antifibrotic miR-29a was significantly elevated in the shunt left ventricle (LV) and right ventricle (RV) compared with the control LV and RV. Values are means ± SE; n = 5 in all groups. B and C: downstream targets of miR-29a, collagen type 1A1 (Col1A1) and collagen type 3A1 (Col3A1), were significantly decreased in shunt RV tissue compared with control RV tissue, as determined by Western blot analysis. Values are means ± SD; n = 5 in all groups. *P < 0.05 vs. control. D and E: collagen fibrosis in the control RV (D) and shunt RV (E) was visualized using Masson’s trichrome staining, which stains collagen blue, muscle fibers red, and nuclei black. Stereological analysis of fibrosis was performed in a blinded fashion and demonstrated no increase in fibrosis in shunt RV tissue, despite previously demonstrated RV hypertrophy.
Interestingly, miR-29a expression was increased in both shunt LVs and RVs compared with control LVs and RVs. Transforming growth factor (TGF)-β has been demonstrated to be an upstream regulator of miR-29a expression (36), and, in a model of chronic pulmonary artery banding, Friedberg and colleagues demonstrated that maladaptive fibrotic changes were not confined to the RV but extended to the LV as well (13). In their model, these changes were associated with a decrease in TGF-β expression. In our model, we demonstrated increased TGF-β mRNA expression, which may explain the increase in miR-29a expression.
DISCUSSION
Multiple distinct animal models of RVH exist, either consequent to experimentally induced PAH or due to pulmonary artery banding. However, in all these RVH models, the RV eventually progresses to RVF. In the most commonly used animal models of PAH, the RV and pulmonary vasculature are typically subjected to an endothelial toxin, such as monocrotaline (15) or the VEGF receptor type 2 inhibitor SU5416 (Sugen) in combination with hypoxia (1); these models cause advanced pulmonary vasculopathy and consequent RVH and RVF (16, 37). Similarly, animal models of pressure-induced RVH due to pulmonary artery banding typically show a period of compensated hypertrophy that ultimately leads to RVF (7, 27). This early, compensated RVH is likely fundamentally different from adaptive hypertrophy, given inexorable progression to RVF with the same hemodynamic stimulus. Thus, although PAH models and pulmonary artery banding have yielded valuable understanding of the pathophysiology of RVF, models of adaptive RVH are lacking. Using fetal cardiac surgical techniques, we have previously developed an animal model of PAH secondary to CHD with increased pulmonary blood flow (shunt) that mimics the clinical and morphological aspects of the human disease (28). Importantly, we recently demonstrated an adaptive RV Anrep effect in response to acute RV afterload in these shunt lambs (18). Consequently, our model of CHD with RVH and adaptive responses to increased afterload holds particular promise as a tool to provide insights into mechanisms distinguishing adaptive RVH. As upstream mediators of expression in multiple different gene pathways, miRNAs are a tantalizing target to better understand these global effects on RV function.
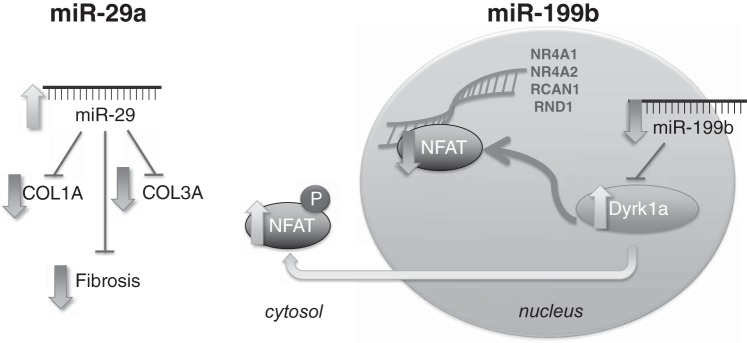
The role of noncoding genomic transcripts, including miRNA, as regulators of cardiovascular physiological and pathological processes has increasingly been appreciated (6). Each individual miRNA can alter the transcription and transcript stability of hundreds of genes (21). Given the differences in global RV function in our shunt model of adaptive RVH, we determined differential expression of miRNA and found important differences in miRNA expression between our adaptive RVH model and human and animal maladaptive RVH. Finally, we investigated downstream effects of miR-199b and miR-29a expression as they relate to NFAT-calcineurin-mediated cardiac hypertrophy and fibrosis. Alterations in miR-199b and miR-29a and downstream effects are shown in Fig. 4. These changes yield valuable insights into the one of the potential mechanisms behind adaptive RVH in our model of ovine CHD.
Fig. 4.
Diagram with schematic representation of downstream effects of microRNAs miR-199b and miR-29a in shunt right ventricular (RV) hypertrophy. In the shunt RV, miR-29a expression was increased, downstream expression of the miR-29a targets collagen type 1A1 (Col1A) and collagen type 3A1 (Col3A1) was decreased, and fibrosis was similarly decreased. Also, in shunt RV tissue, expression of miR-199b was decreased and expression of the miR-199b target dual-specificity tyrosine phosphorylation-regulated kinase 1a (Dyrk1a) was increased, which led to increased phosphorylation (P) of nuclear factor of activated T cell (NFAT) and its exclusion from the nucleus. Decreased NFAT activity was demonstrated by decreased binding to promoter regions of nuclear receptor subfamily 4 group A (NR4A1 and NR4A2), regulator of calcineurin 1 (RCAN1), and Rho family GTPase 1 (RND1).
Increased miR-199b has been linked to pathological NFAT/calcineurin signaling in cardiac hypertrophy, and antagomiR-induced inhibition of miR-199b signaling abrogates pathological hypertrophy in response to increased calcineurin expression or aortic banding (9). As previously discussed, the NFAT-miR-199b-Dyrk1a pathway represents a positive feedback loop perpetuating pathological hypertrophic signaling (9). In contrast to this pathological process in maladaptive hypertrophy, the shunt RV demonstrated increased miR-199b expression, which was ultimately associated with decreased NFAT transcriptional activity, as quantified by ChIP. NFAT/calcineurin signaling participates in pathological, but not physiological, hypertrophy. The distinction between decreased miR-199b expression in adaptive RVH and increased miR-199b expression in maladaptive RVH may have important implications for leveraging mediators of physiological versus pathological hypertrophy in response to increased RV afterload.
Members of the miR-29 family are mediators of antifibrotic signaling pathways, and increased miR-29 expression has further been demonstrated in physiological hypertrophy in animal exercise models (i.e., swim training), which has a distinct molecular signature compared with pathological hypertrophy. Our data suggest a shared semiology between exercise-induced hypertrophy and the adaptive RVH in our CHD model (12, 24). Similar to miR-199b, further investigations into the differential role of miR-29 family members in physiological RVH in response to increased afterload may offer a new paradigm of therapeutic strategies for PAH.
This study has several important limitations. Most importantly, these findings are correlative and do not prove causation of miRNA expression alterations in the global phenotype. Also, our selection of miRNA targets for analysis was focused on 93 targets based on potential implications for cardiovascular development and heart failure based on a literature search of PubMed for miRNA associated with cardiovascular disease. Thus, this data set is an incomplete instrument for full interrogation of the role of miRNA in regulation of adaptive RVH. Despite these limitations, these investigations provide valuable insights into the mechanisms guiding differential phenotypic responses to increased RV afterload in PAH that are ultimately more revelatory than traditional approaches targeting single gene pathways, as changes in miRNA expression potentially modulate many different downstream targets.
Together, these data provide strong preliminary evidence that the miRNA expression signature in our model of CHD is a promising determinant of downstream molecular pathways and a mediator of the adaptive RV phenotype. Our model of CHD with RVH and adaptive responses to increased afterload (18) holds particular promise as a tool to provide insights into mechanisms distinguishing adaptive RVH from maladaptive hypertrophy. Once the unique miRNA transcriptome of adaptive RVH has been identified and its downstream effects have been validated in animal models, there are potential applications to manipulation of miRNA expression as a therapeutic strategy. One advantage of this strategy compared with typical drug treatments is the ability of a single miRNA to modulate expression of multiple different genes. This theoretical benefit may soon be realized in certain diseases: phase I and II clinical trials of inhibitors of miR-122 in patients with hepatitis C are underway (31, 33), and a phase I clinical trial of miR-34 mimic treatment of patients with primary liver cancer or other hematological malignancies is ongoing. Although there are multiple preclinical models using miRNA as therapeutic targets in cardiovascular disease (11), there are no current clinical trials with cardiovascular miRNA-based therapeutics. However, this therapeutic application is the focus of intensive biomedical research and may provide a novel mechanism to support adaptive RV responses in pulmonary hypertension and CHD, where traditional therapies have proven disappointing.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-61284 (to J. R. Fineman), American Heart Association Grant 14FTF19670001 (to R. J. Kameny), a Pulmonary Hypertension Association Robyn J. Barst Pediatric Pulmonary Hypertension Research and Mentoring Grant (to R. J. Kameny), and an Entelligence MD Young Investigator Grant (to R. J. Kameny).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.K., G.W.R., A.H., and J.R.F. conceived and designed research; R.J.K., T.Z., W.G., G.W.R., C.J.C., S.A.D., and J.B. performed experiments; R.J.K., Y.H., T.Z., W.G., C.J.C., and S.A.D. analyzed data; R.J.K., Y.H., S.A.D., J.B., and A.H. interpreted results of experiments; R.J.K. and Y.H. prepared figures; R.J.K. drafted manuscript; R.J.K., A.H., and J.R.F. edited and revised manuscript; R.J.K. and J.R.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Linda Talken and the University of California (Davis, CA) veterinary staff for excellent animal care and Michael Johengen for assistance with surgical preparation.
REFERENCES
- 1.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 2.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250, 2004. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 3.Ardeshir A, Oslund KL, Ventimiglia F, Yee J, Lerche NW, Hyde DM. Idiopathic microscopic colitis of rhesus macaques: quantitative assessment of colonic mucosa. Anat Rec (Hoboken) 296: 1169–1179, 2013. doi: 10.1002/ar.22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azakie A, Fineman J, He Y. Differential responses of the right ventricle to abnormal loading conditions in vivo: possible pathophysiologic mechanisms. J Thorac Cardiovasc Surg 145: 1335–1344, 2013. doi: 10.1016/j.jtcvs.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Azakie A, Fineman JR, He Y. Sp3 inhibits Sp1-mediated activation of the cardiac troponin T promoter and is downregulated during pathological cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol 291: H600–H611, 2006. doi: 10.1152/ajpheart.01305.2005. [DOI] [PubMed] [Google Scholar]
- 6.Batkai S, Bär C, Thum T. MicroRNAs in right ventricular remodelling. Cardiovasc Res 113: 1433–1440, 2017. doi: 10.1093/cvr/cvx153. [DOI] [PubMed] [Google Scholar]
- 7.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 120: 1951–1960, 2009. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 8.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, . et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115: 343–349, 1991. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 9.da Costa Martins PA, Salic K, Gladka MM, Armand A-S, Leptidis S, el Azzouzi H, Hansen A, Coenen-de Roo CJ, Bierhuizen MF, van der Nagel R, van Kuik J, de Weger R, de Bruin A, Condorelli G, Arbones ML, Eschenhagen T, De Windt LJ. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol 12: 1220–1227, 2010. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- 10.Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol 45: 1239–1247, 2011. doi: 10.1165/rcmb.2010-0412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duygu B, de Windt LJ, da Costa Martins PA. Targeting microRNAs in heart failure. Trends Cardiovasc Med 26: 99–110, 2016. doi: 10.1016/j.tcm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes T, Barauna VG, Negrao CE, Phillips MI, Oliveira EM. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am J Physiol Heart Circ Physiol. In press. doi: 10.1152/ajpheart.00899.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedberg MK, Cho M-Y, Li J, Assad RS, Sun M, Rohailla S, Honjo O, Apitz C, Redington AN. Adverse biventricular remodeling in isolated right ventricular hypertension is mediated by increased transforming growth factor-β1 signaling and is abrogated by angiotensin receptor blockade. Am J Respir Cell Mol Biol 49: 1019–1028, 2013. doi: 10.1165/rcmb.2013-0149OC. [DOI] [PubMed] [Google Scholar]
- 14.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 37: 183–188, 2001. doi: 10.1016/S0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol 302: L363–L369, 2012. doi: 10.1152/ajplung.00212.2011. [DOI] [PubMed] [Google Scholar]
- 16.Hessel MHM, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol 291: H2424–H2430, 2006. doi: 10.1152/ajpheart.00369.2006. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins WE. Right ventricular performance in congenital heart disease: a physiologic and pathophysiologic perspective. Cardiol Clin 30: 205–218, 2012. doi: 10.1016/j.ccl.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RC, Datar SA, Oishi PE, Bennett S, Maki J, Sun C, Johengen M, He Y, Raff GW, Redington AN, Fineman JR. Adaptive right ventricular performance in response to acutely increased afterload in a lamb model of congenital heart disease: evidence for enhanced Anrep effect. Am J Physiol Heart Circ Physiol 306: H1222–H1230, 2014. doi: 10.1152/ajpheart.01018.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kameny RJ, He Y, Morris C, Sun C, Johengen M, Gong W, Raff GW, Datar SA, Oishi PE, Fineman JR. Right ventricular nitric oxide signaling in an ovine model of congenital heart disease: a preserved fetal phenotype. Am J Physiol Heart Circ Physiol 309: H157–H165, 2015. doi: 10.1152/ajpheart.00103.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latronico MVG, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol 6: 419–429, 2009. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 22.Luciw PA, Oslund KL, Yang X-W, Adamson L, Ravindran R, Canfield DR, Tarara R, Hirst L, Christensen M, Lerche NW, Offenstein H, Lewinsohn D, Ventimiglia F, Brignolo L, Wisner ER, Hyde DM. Stereological analysis of bacterial load and lung lesions in nonhuman primates (rhesus macaques) experimentally infected with Mycobacterium tuberculosis. Am J Physiol Lung Cell Mol Physiol 301: L731–L738, 2011. doi: 10.1152/ajplung.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 115: 163–172, 2007. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 24.Melo SFS, Fernandes T, Baraúna VG, Matos KC, Santos AAS, Tucci PJF, Oliveira EM. Expression of microRNA-29 and collagen in cardiac muscle after swimming training in myocardial-infarcted rats. Cell Physiol Biochem 33: 657–669, 2014. doi: 10.1159/000358642. [DOI] [PubMed] [Google Scholar]
- 25.Paulin R, Sutendra G, Gurtu V, Dromparis P, Haromy A, Provencher S, Bonnet S, Michelakis ED. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res 116: 56–69, 2015. doi: 10.1161/CIRCRESAHA.115.303910. [DOI] [PubMed] [Google Scholar]
- 26.Reddy S, Zhao M, Hu D-Q, Fajardo G, Hu S, Ghosh Z, Rajagopalan V, Wu JC, Bernstein D. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics 44: 562–575, 2012. doi: 10.1152/physiolgenomics.00163.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy S, Osorio JC, Duque AM, Kaufman BD, Phillips AB, Chen JM, Quaegebeur J, Mosca RS, Mital S. Failure of right ventricular adaptation in children with tetralogy of Fallot. Circulation 114, Suppl: I37–I42, 2006. doi: 10.1161/CIRCULATIONAHA.105.001248. [DOI] [PubMed] [Google Scholar]
- 28.Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. In utero placement of aortopulmonary shunts. A model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation 92: 606–613, 1995. doi: 10.1161/01.CIR.92.3.606. [DOI] [PubMed] [Google Scholar]
- 29.Rich S, Pogoriler J, Husain AN, Toth PT, Gomberg-Maitland M, Archer SL. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest 138: 1234–1239, 2010. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche SL, Redington AN. The failing right ventricle in congenital heart disease. Can J Cardiol 29: 768–778, 2013. doi: 10.1016/j.cjca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Niño MD, Ortiz A. HCV infection and miravirsen. N Engl J Med 369: 877–878, 2013. doi: 10.1056/NEJMc1307787. [DOI] [PubMed] [Google Scholar]
- 32.Schlosser K, Taha M, Deng Y, Jiang B, Stewart DJ. Discordant regulation of microRNA between multiple experimental models and human pulmonary hypertension. Chest 148: 481–490, 2015. doi: 10.1378/chest.14-2169. [DOI] [PubMed] [Google Scholar]
- 33.van der Ree MH, van der Meer AJ, de Bruijne J, Maan R, van Vliet A, Welzel TM, Zeuzem S, Lawitz EJ, Rodriguez-Torres M, Kupcova V, Wiercinska-Drapalo A, Hodges MR, Janssen HLA, Reesink HW. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antiviral Res 111: 53–59, 2014. doi: 10.1016/j.antiviral.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB; National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure . Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883–1891, 2006. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 35.Vonk Noordegraaf A, Galiè N. The role of the right ventricle in pulmonary arterial hypertension. Eur Respir Rev 20: 243–253, 2011. doi: 10.1183/09059180.00006511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Li C, Jian Z, Ou Y, Ou J. TGF-β1 reduces miR-29a expression to promote tumorigenicity and metastasis of cholangiocarcinoma by targeting HDAC4. PLoS One 10: e0136703, 2015. doi: 10.1371/journal.pone.0136703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Schreier DA, Hacker TA, Chesler NC. Progressive right ventricular functional and structural changes in a mouse model of pulmonary arterial hypertension. Physiol Rep 1: e00184, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]