Abstract
The aim of the present study was to serially track how myocardial infarction (MI) impacts regional myocardial strain and mechanical properties of the left ventricle (LV) in a large animal model. Post-MI remodeling has distinct regional effects throughout the LV myocardium. Regional quantification of LV biomechanical behavior could help explain changes in global function and thus advance clinical assessment of post-MI remodeling. The present study is based on a porcine MI model to characterize LV biomechanics over 28 days post-MI via speckle-tracking echocardiography (STE). Regional myocardial strain and strain rate were recorded in the circumferential, radial, and longitudinal directions at baseline and at 3, 14, and 28 days post-MI. Regional myocardial wall stress was calculated using standard echocardiographic metrics of geometry and Doppler-derived hemodynamic measurements. Regional diastolic myocardial stiffness was calculated from the resultant stress-strain relations. Peak strain and phasic strain rates were nonuniformly reduced throughout the myocardium post-MI, whereas time to peak strain was increased to a similar degree in the MI region and border zone by 28 days post-MI. Elevations in diastolic myocardial stiffness in the MI region plateaued at 14 days post-MI, after which a significant reduction in MI regional stiffness in the longitudinal direction occurred between 14 and 28 days post-MI. Post-MI biomechanical changes in the LV myocardium were initially limited to the MI region but nonuniformly extended into the neighboring border zone and remote myocardium over 28 days post-MI. STE enabled quantification of regional and temporal differences in myocardial strain and diastolic stiffness, underscoring the potential of this technique for clinical assessment of post-MI remodeling.
NEW & NOTEWORTHY For the first time, speckle-tracking echocardiography was used to serially track regional biomechanical behavior and mechanical properties postmyocardial infarction (post-MI). We found that changes initially confined to the MI region extended throughout the myocardium in a nonuniform fashion over 28 days post-MI. Speckle-tracking echocardiography-based evaluation of regional changes in left ventricular biomechanics could advance both clinical assessment of left ventricular remodeling and therapeutic strategies that target aberrant biomechanical behavior post-MI.
Keywords: myocardial infarction, myocardial remodeling, myocardial stiffness, speckle-tracking echocardiography
INTRODUCTION
The postmyocardial infarction (post-MI) period is accompanied by temporal changes in inflammatory signaling proteolytic cascades within the MI region, which, in turn, give rise to changes in left ventricular (LV) geometry, composition, and function in a process termed post-MI remodeling (28, 31). Global changes in LV geometry [e.g., increased end-diastolic volume (EDV)] and pump function [e.g., reduced ejection fraction (EF)] are commonly used to assess the relative extent of post-MI remodeling (14, 39a, 42). However, this is a spatially nonuniform process, as myocardial thinning (within the MI region) occurs concomitantly with hypertrophy (within the viable remote region) (6, 12). As such, improved regional assessment of temporal changes throughout the myocardium may enhance clinical evaluation of the rate and extent of the post-MI remodeling process.
In vivo regional mechanical behavior has been assessed post-MI in both basic scientific and clinical studies using techniques such as sonomicrometry, cardiac magnetic resonance imaging, and tissue Doppler-derived imaging (16, 33, 34, 37, 40, 41, 45). Although these and other approaches have yielded critical information on the post-MI remodeling process, they have not been uniformly applied to assess LV regional function across the entire chamber and in a serial fashion because of several factors. In large animal studies using sonomicrometry, high spatial and temporal resolution is offset by a limited field of view (16, 33). In clinical studies, noninvasive approaches such as cardiac magnetic resonance imaging can provide high-fidelity regional assessment of LV function, but lengthy acquisition and postprocessing times make it impractical to collect repeated measurements (45). Tissue Doppler-derived imaging has also been applied in both large animal models and clinical settings to quantify regional myocardial strain with reported limitations stemming from a significant dependency on the angle of the ultrasound beam and low signal-to-noise ratio (37, 40, 41).
Speckle-tracking echocardiography (STE), an ultrasound-based technique that tracks the movement of intrinsic acoustic markers of the LV, has the potential to address limitations of tissue Doppler approaches. Past large animal and clinical studies have demonstrated the feasibility of STE in terms of post-MI assessment of LV regional function (3, 7, 8, 15, 19, 32, 36, 38, 39). It has been established that longitudinal strain patterns can be used to identify regions of ischemic injury and predict remodeling outcomes (8, 36). Measures of global radial and circumferential strains have shown correlation with MI size and can enable differentiation between subendocardial and transmural infarction (7, 15, 32). STE can also capture the heterogeneous and temporal changes in LV strain in the minutes immediately after MI and over more extensive time intervals post-MI (3, 38, 39). Despite these advancements, there is a paucity of studies that have applied STE to serially assess the extent and magnitude of changes in regional strain over the critical early remodeling period (up to 28 days post-MI) in a large animal model. Furthermore, no studies to date have extended STE to track regional mechanical property changes post-MI. The present study utilizes a porcine model of an acute coronary syndrome and subsequent MI based on an intracoronary ischemia-reperfusion (I/R) approach; STE is then performed up to 28 days post-MI. We postulate that this combinatorial approach to the in vivo quantification of LV mechanical behavior post-MI will yield novel insights into the progression of the post-MI remodeling process and thereby motivate the development of therapeutic strategies, which aim to mitigate these adverse effects.
METHODS
I/R protocol.
Mature pigs (n = 8, Yorkshire, male, 22.0 ± 0.9 kg) were administered amiodarone (200 mg po) and aspirin (81 mg po) for 3 days preoperatively and a broad-spectrum antibiotic [Draxxin (2.5 mg/kg im)] at least once preoperatively. On the day of surgery, pigs were sedated [ketamine-acepromazine-atropine (22.00/1.10/0.04 mg/kg im)], intubated, and then maintained on 1.5–2.0% isoflurane delivered in an oxygen/nitrous mixture (3:1 l/min, respectively). Buprenorphine (0.05 mg/kg im) was administered as presurgery analgesia. An intravenous infusion (via ear vein cannula) of Benadryl (25 mg) in conjunction with a continuous lidocaine infusion (4 mg·kg−1·h−1) and lactated Ringer solution (10 mg·kg−1·h−1) was initiated. The region encompassing the right femoral artery was prepared in a sterile fashion, the main branch of the femoral artery was surgically exposed, and a catheter introducer (6F Input Introducer Sheath, Medtronic) positioned and stabilized in the artery. A bolus of heparin was administered (4,000 units iv) before placement of the guide catheter followed by an additional bolus every hour (1,000 units iv). Under fluoroscopic guidance (GE OEC 9600), a coronary angiography catheter/launcher (5F Launcher guiding catheter, 0.058-in. HSI, Medtronic) was placed in the left coronary ostia, and an angioplasty balloon catheter containing an injection lumen (3 × 10-mm Sprinter OTW balloon catheter, Medtronic) was positioned in the lower portion of the left anterior descending artery (LAD; defined as 1 cm below the first diagonal). The LAD was occluded by balloon inflation (12 atm balloon inflation pressure, Everest 30 Disposable inflation device, Medtronic) and maintained for 90 min. The balloon was then deflated, and the catheter system was disengaged and removed. The femoral artery was ligated, and the incision was closed. A transdermal fentanyl patch was placed for 3 days for postoperative analgesia. Prior studies have demonstrated that this technique produces a highly reproducible anteroapical ischemic region resulting in an MI, which constitutes ~8.7 ± 1.3% of the entire LV at 28 days post-MI (4, 26).
All experimental protocols were approved by the Institutional Animal Care and Use Committees of the University of South Carolina and performed according to the National Institutes of Health Guidelines for Care and Use of Laboratory Animals.
Standard echocardiography.
The day before animals underwent the I/R protocol, they were sedated [diazepam (200 mg po), Barr Laboratories], placed in a custom-designed sling, and echocardiography was performed [GE Vivid E9 with XDclear Ultrasound System M5S (1.5–4.6 MHz) transducer probe]. Cardiac dimensions and function were assessed by two-dimensional echocardiographic experiments. Transthoracic images were acquired from a right parasternal approach, and the LV was imaged in long-axis and short-axis views. The short-axis views were taken at the level of the papillary muscles. EDV and EF were calculated using the biplane method of disks. Pulse-wave Doppler, using a sample volume placed at the tips of the mitral valve leaflets, was used to determine the peak early mitral inflow velocity (E). Tissue Doppler assessment was used to calculate the peak early mitral annular velocity (E′) with a sample volume positioned at the lateral site of the mitral annulus. Pulmonary capillary wedge pressure (PCWP) was calculated using the method proposed by Nagueh et al. in 1997 (29a). Additionally, wall thickness was collected at early and end diastole for each of the six conventional echocardiogram LV segments in the parasternal long axis and papillary level short axis. Pigs were returned to the laboratory under identical sedation/study conditions at 3, 14, and 28 days post-MI.
Strain imaging.
Digital loops of the two-dimensional echocardiography for the long axis and papillary short axis (acquired at 40 Hz) were stored on a hard disk and transferred to a workstation (EchoPac, Vingmed, General Electric) for postprocessing. For each digital loop, a region of interest (ROI) was defined at the onset of the R wave by manually identifying the endocardial and epicardial borders. The ROI was then discretized with a spatial mesh of acoustic clusters to be tracked on a frame-to-frame basis throughout a single cardiac cycle (25). The end of systole was defined as the point at which the LV cross-sectional area was at a minimum. After the semiautomated grouping of acoustic clusters in accordance with six anatomic locations (Fig. 1A), regional tracking quality was assessed, and the ROI was manually adjusted by the operator to improve tracking quality where necessary. Successful tracking of the ROI allows for the definition of segmental lengths, which were computed at end diastole (L0) and continuously throughout the cardiac cycle (L). Local segmental strains (ε) and strain rates (γ) were then computed as follows:
| (1) |
where Δt is the relative time in the cardiac cycle. Peak strain, systolic strain rate, and diastolic strain rate were quantified in the three normal directions, circumferential (papillary short axis), radial (papillary short axis), and longitudinal (long axis). Circumferential and longitudinal segmental strains/strain rates were quantified at the epicardial, midmyocardial, and endocardial surfaces. Global strain values were calculated as the mean segmental strain observed in the 18 myocardial segments in the circumferential and longitudinal directions and 6 segments in the radial direction. Obtained segmental strain and strain rate data were assessed for intraobserver variance by calculating the intraclass correlation coefficient for a single operator under the assumption that systematic differences are relevant. The intraclass correlation coefficient for all measures of strain and strain rate ranged from 0.94 to 0.99.
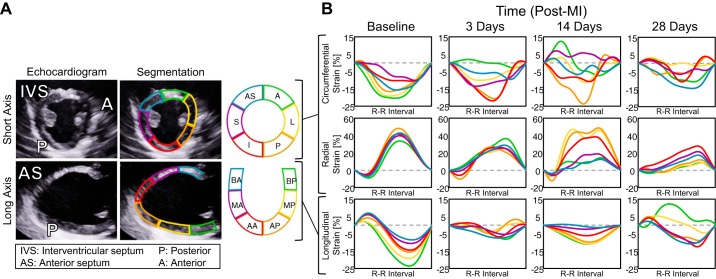
Fig. 1.
Speckle-tracking echocardiography enables quantification of myocardial segmental strain. A: representative echocardiographic images obtained 28 days post-myocardial infarction (post-MI) (at end diastole) divided into six anatomic zones for speckle tracking. B: representative segmental strain curves of the left ventricular (LV) midwall over one cardiac cycle at 28 days post-MI in the circumferential, radial, and longitudinal directions. AS, anterior septum; S, septal; I, inferior; P, posterior; L, lateral; A, anterior; BA, basal anterior septum; MA, mid anterior septum; AA, apical anterior septum; BP, basal posterior; MP, mid posterior; AP, apical posterior.
Aggregate regional definitions.
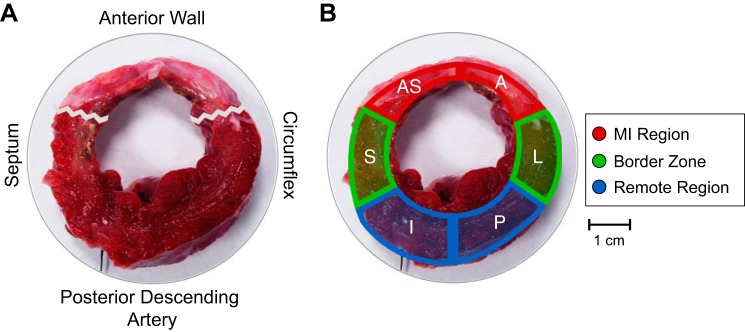
In this study, each pig had a total occlusion of the LAD. To facilitate data analysis, we defined the following aggregate regions: the MI region is all anterior and anteroseptum walls; the border zone is the apical and mid posterior, basal septal, and basal lateral walls; and the remote region is the basal posterior and inferior walls. Although the size of the MI region, and thus the size and location of the border zone, change over time post-MI, the use of consistent regions at all study time points allows for the continuous monitoring of local biomechanical changes throughout the myocardium.
Mean wall stresses.
We modified the thick-walled ellipsoidal model proposed by Janz (23) to compute mean wall stresses. Mean circumferential wall stress (σC) was computed as follows:
| (2) |
where r is the inner radius, P is LV pressure, and t is the wall thickness. For the purposes of this study, P was assumed to equal 0 at the onset on diastole and the PCWP at end diastole.
Mean longitudinal wall stress (σL) was computed as follows:
| (3) |
where Φ is the angle between the normal vector from the endocardium at the ROI and the axis of revolution in the truncated ellipsoid model.
Regional diastolic myocardial stiffness.
Obtained values for mean wall stresses and segmental strains at the onset of diastole and end diastole enabled calculation of regional diastolic myocardial stiffness in both the circumferential and longitudinal directions. The slope of the line between these two points in the stress-strain plane, developed in either the circumferential or longitudinal direction and referenced to the defined anatomic locations, was used to compute the regional diastolic myocardial stiffness (κMR) as follows:
| (4) |
where σED and εD0 are the end-diastolic mean wall stress and segmental strain at the onset of diastole, respectively.
Statistical analysis.
Data are reported as means ± SE. A post hoc analysis of baseline (pre-MI) global stiffness values with an assumption of a 30% effect and sample size of 8 yielded a statistical power of >80%. The comparative analysis was performed with either one-way or two-way ANOVA followed by pairwise comparisons using the least-significant-difference post hoc study. All statistical analyses were performed with SPSS software (version 24.0, SPSS). P values of <0.05 were considered statistically significant.
RESULTS
LV function and geometry.
Assessments of LV function and geometry at baseline and at specific post-MI time points are shown in Table 1. At 28 days post-MI, LV EF fell by over 40%, LV EDV more than doubled, and PCWP increased by 80% relative to baseline values.
Table 1.
Indexes of left ventricular pump function, geometry, and filling at baseline and at 3, 14, and 28 days postmyocardial infarction
| Baseline | 3 Days | 14 Days | 28 Days | |
|---|---|---|---|---|
| Ejection fraction, % | 69.1 ± 0.8 | 49.4 ± 2.7* | 37.8 ± 2.3*† | 37.5 ± 3.4*† |
| Left ventricular end-diastolic volume, ml | 37.2 ± 1.5 | 59.3 ± 5.1* | 77.2 ± 6.5*† | 83.6 ± 6.4*† |
| Pulmonary capillary wedge pressure, mmHg | 7.6 ± 0.6 | 9.3 ± 0.6* | 12.2 ± 0.9*† | 13.9 ± 1.1*† |
Values are means ± SE; n = 8. One-way ANOVA with post hoc least-significant-difference comparisons was used.
P < 0.05 vs. the respective baseline value;
P < 0.05 vs. the respective 3-day value.
STE-based strain measurements.
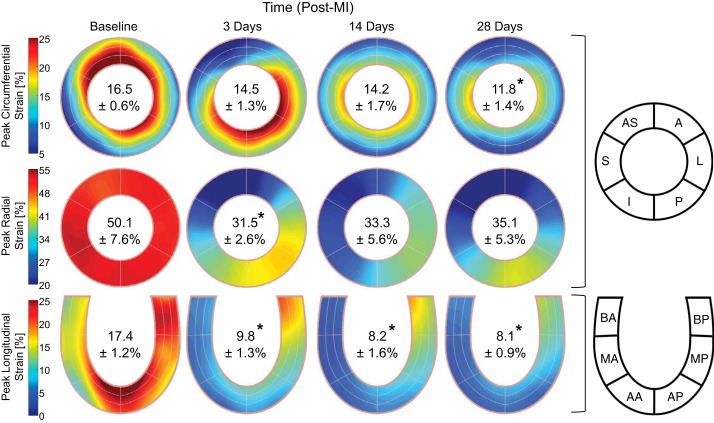
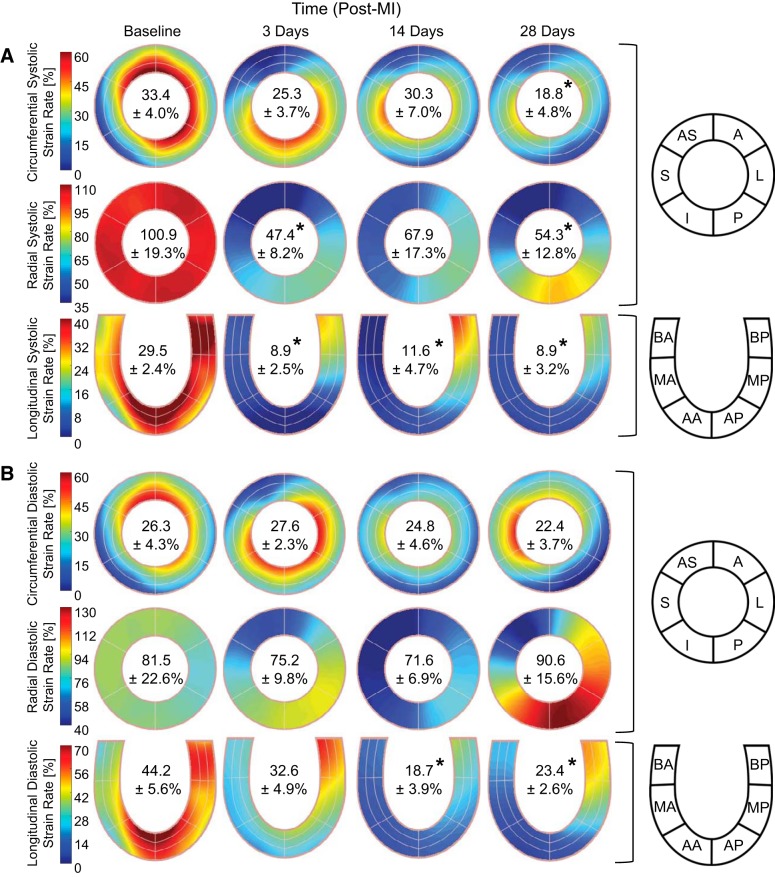
Representative plots of midmyocardial segmental strains (Fig. 1B) demonstrated that STE provided a continuous strain measurement over the cardiac cycle at all specified anatomic locations. As depicted by a representative LV circumferential section, the MI region, border zone, and remote region were contained within the designated myocardial regions at 28 days post-MI, thus supporting the aggregate definition of these regions throughout the study (Fig. 2). Spatial maps of peak segmental strains qualitatively show that post-MI changes varied throughout the myocardium and exhibited obvious directional dependence (Fig. 3). At 3 days post-MI, peak global strain in the radial and longitudinal directions decreased by ~40%, whereas reduction in the circumferential direction was not significant until 28 days post-MI.
Fig. 2.
Definition of aggregate regions based on myocardial infarction (MI) location. A and B: representative left ventricular (LV) circumferential section cut across the central portion of the MI and stained with triphenyltetrazolium chloride showing the agreement between the designated aggregate MI region, border zone, and remote region at 28 days post-MI. The jagged line represents the edges of the MI region. AS, anterior septum; S, septal; I, inferior; P, posterior; L, lateral; A, anterior.
Fig. 3.
Peak myocardial strain. Spatial maps of the absolute value of peak myocardial segmental strain in the circumferential (top), radial (middle), and longitudinal (bottom) directions at baseline and at 3, 14, and 28 days post-myocardial infarction (post-MI) are shown. Inscribed values indicate the peak global strain reported as mean absolute values ± SE. *P < 0.05 vs. the respective baseline value (n = 8, one-way ANOVA with post hoc least-significant-difference comparisons). AS, anterior septum; S, septal; I, inferior; P, posterior; L, lateral; A, anterior; BA, basal anterior septum; MA, mid anterior septum; AA, apical anterior septum; BP, basal posterior; MP, mid posterior; AP, apical posterior.
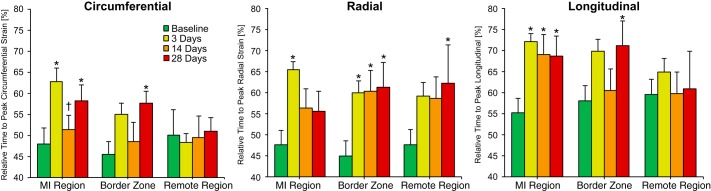
The relative time at which peak strain occurred in the MI region increased by ~30–40% with respect to baseline at 3 days post-MI in all directions, with these early changes in the circumferential and longitudinal directions largely maintained throughout the study observation period (Fig. 4). Within the border zone, an ~30% increase in the relative time to peak strain occurred at all post-MI times in the radial direction, but significant increases in the circumferential and longitudinal directions were only recorded at 28 days post-MI. The remote region exhibited the least pronounced changes, as the only significant deviations from baseline were found in the radial direction at 28 days post-MI.
Fig. 4.
Relative time to peak myocardial strain. The relative time at which peak myocardial strain occurred for each region at baseline and at 3, 14, and 28 days post-myocardial infarction (post-MI) is shown, with relative time defined as percent completion of one cardiac cycle; *P < 0.05 vs. the respective baseline value; †P < 0.05 vs. the respective 3-day value (n = 8, one-way ANOVA with post hoc least-significant-difference comparisons).
Phasic strain rates.
Spatial maps of segmental strain rates show that MI impacted both phases of the cardiac cycle and that the greatest reductions in strain rates primarily occurred within the MI region (Fig. 5). Reductions of over 50% in global systolic strain rate were observed at 3 days post-MI in the radial and longitudinal directions but were not significant until 28 days post-MI in the circumferential direction. Global diastolic strain rates were relatively preserved post-MI with significant reductions observed only at 14 and 28 days post-MI in the longitudinal direction.
Fig. 5.
Phasic myocardial strain rate. Spatial maps of the absolute value of the systolic (A) and diastolic (B) myocardial segmental strain rates in the circumferential (top), radial (middle), and longitudinal (bottom) directions at baseline and at 3, 14, and 28 days post-myocardial infarction (post-MI) are shown. Inscribed values indicate the global strain rate reported as mean absolute values ± SE. *P < 0.05 vs. the respective baseline value (n = 8, one-way ANOVA with post hoc least-significant-difference comparisons). AS, anterior septum; S, septal; I, inferior; P, posterior; L, lateral; A, anterior; BA, basal anterior septum; MA, mid anterior septum; AA, apical anterior septum; BP, basal posterior; MP, mid posterior; AP, apical posterior.
Mean wall stresses and diastolic myocardial stiffness.
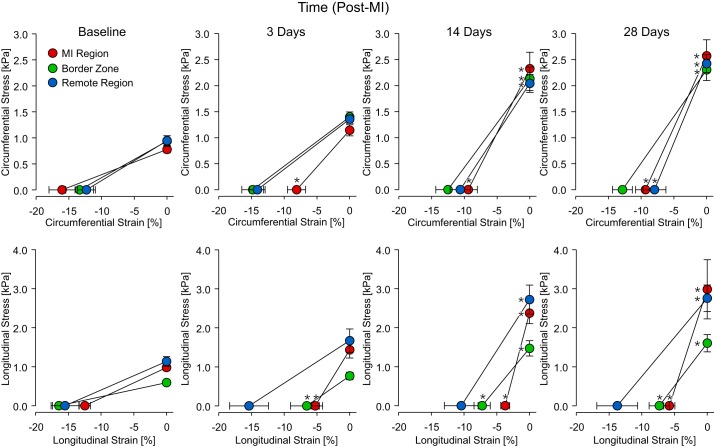
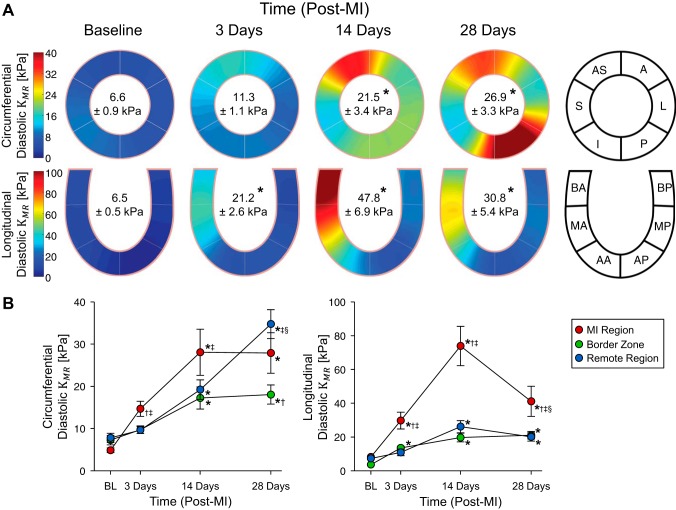
End-diastolic mean wall stress monotonically increased throughout the 28-day observation period in both the circumferential and longitudinal directions with all regional values approximately doubling with respect to baseline by 28 days post-MI (Fig. 6). Spatial maps of diastolic myocardial stiffness show that mechanical heterogeneity evidently increased post-MI (Fig. 7A). Global diastolic myocardial stiffness underwent a greater than threefold elevation by 14 days post-MI in the circumferential direction and threefold elevation by 3 days post-MI in the longitudinal direction. In the longitudinal direction, significant diastolic myocardial stiffness elevations occurred in the MI region and border zone by 3 days post-MI and by 14 days post-MI in the remote region (Fig. 7B). All three regions exhibited significantly elevated diastolic myocardial stiffness by 14 days post-MI in the circumferential direction. In the MI region, diastolic myocardial stiffness in both directions was maximal at 14 days post-MI. In the longitudinal direction, an approximate 40% decrease in stiffness from this maximal value occurred between 14 and 28 days post-MI.
Fig. 6.
Stress-strain relations. Regional [myocardial infarction (MI) region, border zone, and remote region] myocardial stress-strain relations were computed at early and end diastole in the circumferential and longitudinal directions at baseline and at 3, 14, and 28 days post-MI. *P < 0.05 vs. the respective baseline stress or strain value (n = 8, one-way ANOVA with post hoc least-significant-difference comparisons).
Fig. 7.
Diastolic myocardial stiffness. A: spatial maps of diastolic myocardial stiffness in the circumferential and longitudinal directions at baseline and at 3, 14, and 28 days post-myocardial infarction (post-MI). Inscribed values indicate the myocardial stiffness reported as means ± SE. B: region-specific change in myocardial stiffness post-MI. *P < 0.05 vs. the respective baseline value; †P < 0.05 vs. the respective remote region value; ‡P < 0.05 vs. the respective border zone value; §P < 0.05 vs. the respective 14-day post-MI value (n = 8, two-way ANOVA with post hoc least-significant-difference comparisons). AS, anterior septum; S, septal; I, inferior; P, posterior; L, lateral; A, anterior; BA, basal anterior septum; MA, mid anterior septum; AA, apical anterior septum; BP, basal posterior; MP, mid posterior; AP, apical posterior.
DISCUSSION
Study overview.
Changes in LV geometry and function invariably occur after MI in a multifactorial process termed post-MI remodeling (28, 31). Given the inherent spatial nonuniformity of post-MI remodeling, approaches for quantifying LV function in a global context can be problematic, and therefore assessment of regional biomechanics may provide improved insights into this process. The present study addressed this issue by serially examining LV strain and strain rates, indexes of myocardial function, in defined myocardial regions post-MI. With the use of a large animal model of post-MI remodeling and STE-derived indexes, the significant findings from this study were twofold. First, myocardial strain and strain rate fell within the MI region at 3 days post-MI, consistent with a loss of viable myocardium and hence contractile capacity. However, although the reduction in strain patterns was confined initially within the MI region, this reduction nonuniformly extended into the viable LV myocardial regions over 1 mo post-MI. Second, when regional myocardial diastolic stiffness was computed using local stress-strain relationships, a biphasic change within the MI region was observed. Specifically, diastolic myocardial stiffness initially increased within the MI region but at later post-MI time points remained constant in the circumferential direction and significantly fell in the longitudinal direction. Despite these regional and directional differences, diastolic myocardial stiffness was significantly elevated throughout the entire myocardium by 28 days post-MI. Thus, serial assessment of regional LV function post-MI identified that time- and region-dependent changes in myocardial mechanical properties occur over 28 days post-MI, potentially providing more sensitive response variables when evaluating disease progression and therapeutic interventions.
Previous supportive findings.
STE-based strain measures in our study were comparable to previous large animal and clinical studies. Specifically, our baseline global strain values fell within normal ranges defined by a meta-analysis of over 2,500 human subjects (44). Additionally, directional changes in peak strain observed in the MI region, border zone, and remote region track well with those reported in other large animal models (38, 39). Our data also support previous studies that found elevated MI region stiffness (20, 21, 30, 35), including via sonomicrometry (33) and three-dimensional magnetic resonance (2). Moreover, results from planar biaxial mechanical testing on freshly excised square samples of remote and infarcted myocardium showed that MI region stiffness peaked at 1–2 wk post-MI followed by a period of decreasing stiffness (18).
Mechanistic implications.
The present study identified unique spatial, directional, and temporal variations in regional function during post-MI remodeling, which are associated with increased mechanical heterogeneity throughout the LV myocardium. We posit that this mechanical heterogeneity may be the biophysical stimulus that is translated into cellular and extracellular changes within the MI and surrounding region, ultimately contributing to MI expansion and eventually LV failure. For example, during the first 14 days post-MI, the diastolic myocardial stiffness of the MI region and the neighboring border zone became increasingly disparate, exemplified by the nearly order-of-magnitude greater MI region stiffness in the longitudinal direction. Such abrupt spatial gradients in mechanical stiffness that emerge between the MI and viable regions likely induce localized elevations in myocardial wall stress and dyskinesis throughout the cardiac cycle, which, in turn, would stimulate further remodeling and the progressive expansion of the MI region (17). The reduction in stiffness of the MI region observed at 28 days post-MI relative to 14 days post-MI may facilitate the continued expansion of the MI region as evidenced by the increase in LV EDV over that period. Furthermore, relative values of border zone/MI region stiffness at 14 and 28 days post-MI suggest that, at the terminal time point, mechanical properties throughout the myocardium become more homogeneous in both the circumferential and longitudinal directions. Such property homogenization may in turn suggest an approach to mechanical homeostasis by 28 days post-MI, commensurate with the plateau in MI region expansion reported in a previous study (34). Conversely, the progressive stiffening of the hypertrophied remote region between 14 and 28 days may play a pivotal role in driving the elevations in PCWP, which may in turn facilitate the LV dilation. To promote further association of regional mechanical property changes with global LV function, we consider as an index of LV chamber stiffness the ratio between PCWP and LV EDV. When computed using our data, this index at baseline is valued as 0.24 ± 0.04 and is found to be consistently reduced by 40% relative to baseline at 14 and 28 days post-MI. Antithetical changes in diastolic myocardial stiffness of the MI region and remote region between 14 and 28 days post-MI may explain the relatively constant index of LV chamber stiffness over this post-MI period. The coincidence between the divergence of regional changes in myocardial mechanical properties and the development of congestive symptoms suggests that regional stiffness variations may be a key factor, and thus therapeutic target, in heart failure progression.
These biomechanical changes are also seemingly synchronized with deterministic biological processes that occur in and around the MI region. These changes include fibroblast proliferation and collagen deposition, resulting in the formation of a mature scar composed of extracellular matrix by 14 days post-MI (24). This intermediate phase of post-MI remodeling also concurs with the highest disparity in fibrillar collagen content between the MI region and border zone and dynamic changes in protein expression profiles, which directly modulate the reparative response (10). Furthermore, there is evidence that many of these regional biophysical changes are modulated by mechanosensitive cardiac cells that sense and respond to their local mechanical environment (5). To that end, mechanical heterogeneity that emerges post-MI represents a biophysical response variable that can explain temporal and spatial variations in key biological processes that occur during post-MI remodeling.
Therapeutic implications.
Although cause-and-effect relationships are difficult to discern with respect to synchronized biological versus biomechanical changes, our data support the regional modulation of myocardial mechanical properties as a potential therapeutic target. As suggested above, the increase in EDV from 14 to 28 days may be facilitated by a decrease in MI region stiffness, suggesting that treatment to maintain or increase MI region stiffness may blunt dilation. Similarly, the increase in PCWP from 14 to 28 days may be facilitated by an increase in remote region stiffness, suggesting that treatment to decrease remote region stiffness may also blunt dilation. Indeed, previous studies show that biomaterials directly injected into the MI region acutely stiffen the myocardium and effectively curtail the adverse effects of post-MI remodeling (11, 29). Regional biomechanical assessment can be used to advance local biomaterial injection strategies, including the optimization of biomaterial mechanical properties, total amount, and spatial distribution upon injection, such that mechanical heterogeneity is limited in the early stages of post-MI remodeling. This general concept is supported by previous results obtained with finite element simulations, which predict that biomaterial injections concentrated within the border zone (as opposed to uniformly delivered throughout the MI region) attenuate myocardial wall stress and promote efficient pump function (43).
STE has been previously used to discriminate between infarcted and contractile myocardium post-MI (9). Moreover, many studies have shown the utility of quantifying global values of STE-derived strain and strain rate with good feasibility, reproducibility, and accuracy in clinical and large animal populations (3, 7, 8, 15, 19, 32, 36, 38, 39). In accordance with these prior studies, we found significant reductions in global myocardial strain and strain rate post-MI. Although global strain has been extensively quantified in the post-MI context, regional measurements in our study suggest that the initially antithetical changes in the MI and remote regions likely weaken the sensitivity and diagnostic strength of traditional global metrics of function. Furthermore, this study demonstrates that STE-derived myocardial strain combined with traditional echocardiography and Doppler-derived hemodynamic parameters can be used to compute a regional diastolic myocardial stiffness. Although an analogous technique has been applied to aortic aneurysms (1), we are the first to noninvasively quantify regional diastolic stiffness using STE in the context of post-MI remodeling.
Study limitations.
Several study limitations must be considered for careful interpretation of the obtained results. First, all metrics of LV function are dependent on load. To that end, the findings from this study only account for patient-specific variance in hemodynamics through the estimation of LV PCWP and an assumption of negligible LV pressure at the onset of diastole. Additionally, as with all echocardiographic techniques, successful quantification of myocardial strain via STE is highly dependent on image quality. To translate this technology into a clinical setting, one must consider that the ability to capture echocardiographic images of sufficient quality is markedly impaired in obese patients or patients with respiratory disorders. However, we expect that the clinical utility of the regional tracking of segmental strains will markedly increase as the underlying technology becomes more reproducible and reliable. Furthermore, these findings were for an anteroapical MI location in a porcine model and thus may not hold true with different MI locations, severity of myocardial ischemia, and/or across species. Finally, biomechanical changes were only assessed over 28 days post-MI in our study. Although this period has been shown to exhibit significant collagen deposition in a porcine model (22), care must be taken when extrapolating these findings into long-term effects, given evidence that there are changes in LV strain and strain rate that continue through 1–3 mo post-MI (27).
Conclusions.
Post-MI remodeling induced progressive changes in LV biomechanics that manifest as regional differences in myocardial strain and strain rate over 28 days post-MI. For the first time, the utility of STE was extended in a large animal model to quantify regional diastolic myocardial stiffness in a serial fashion post-MI. Finally, this study identified the biphasic and at times opposing nature of regional changes in myocardial diastolic stiffness, which in turn is a likely contributing factor for a feed-forward mechanism for adverse LV post-MI remodeling. Future therapeutic strategies that mitigate this significant regional stiffness gradient between the MI and adjacent viable myocardium may be a potential target for attenuating post-MI remodeling.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 5-R01-HL-111090 and R01-HL-130792-01A1S1 (Supplement) as well as Veterans Affairs Health Administration Merit Award BX000168-06.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.M.T., J.J., H.D., S.C.B., and F.G.S. performed experiments; W.M.T., M.R.Z., T.S., and F.G.S. analyzed data; W.M.T., M.R.Z., T.S., and F.G.S. interpreted results of experiments; W.M.T. and T.S. prepared figures; W.M.T. and T.S. drafted manuscript; W.M.T., T.S., and F.G.S. edited and revised manuscript; W.M.T., T.S., and F.G.S. approved final version of manuscript; F.G.S. conceived and designed research.
REFERENCES
- 1.Alreshidan M, Shahmansouri N, Chung J, Lash V, Emmott A, Leask RL, Lachapelle K. Obtaining the biomechanical behavior of ascending aortic aneurysm via the use of novel speckle tracking echocardiography. J Thorac Cardiovasc Surg 153: 781–788, 2017. doi: 10.1016/j.jtcvs.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 2.Arunachalam SP, Arani A, Baffour F, Rysavy JA, Rossman PJ, Glaser KJ, Lake DS, Trzasko JD, Manduca A, McGee KP, Ehman RL, Araoz PA. Regional assessment of in vivo myocardial stiffness using 3D magnetic resonance elastography in a porcine model of myocardial infarction. Magn Reson Med 79: 361–369, 2018. doi: 10.1002/mrm.26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachner-Hinenzon N, Malka A, Barac Y, Meerkin D, Ertracht O, Carasso S, Shofti R, Leitman M, Vered Z, Adam D, Binah O. Strain analysis in the detection of myocardial infarction at the acute and chronic stages. Echocardiography 33: 450–458, 2016. doi: 10.1111/echo.13079. [DOI] [PubMed] [Google Scholar]
- 4.Barlow SC, Doviak H, Jacobs J, Freeburg LA, Perreault PE, Zellars KN, Moreau K, Villacreses CF, Smith S, Khakoo AY, Lee T, Spinale FG. Intracoronary delivery of recombinant TIMP-3 after myocardial infarction: effects on myocardial remodeling and function. Am J Physiol Heart Circ Physiol 313: H690–H699, 2017. doi: 10.1152/ajpheart.00114.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxter SC, Morales MO, Goldsmith EC. Adaptive changes in cardiac fibroblast morphology and collagen organization as a result of mechanical environment. Cell Biochem Biophys 51: 33–44, 2008. doi: 10.1007/s12013-008-9013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capasso JM, Li P, Zhang X, Anversa P. Heterogeneity of ventricular remodeling after acute myocardial infarction in rats. Am J Physiol Heart Circ Physiol 262: H486–H495, 1992. doi: 10.1152/ajpheart.1992.262.2.H486. [DOI] [PubMed] [Google Scholar]
- 7.Chan J, Hanekom L, Wong C, Leano R, Cho GY, Marwick TH. Differentiation of subendocardial and transmural infarction using two-dimensional strain rate imaging to assess short-axis and long-axis myocardial function. J Am Coll Cardiol 48: 2026–2033, 2006. doi: 10.1016/j.jacc.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 8.Choi JO, Cho SW, Song YB, Cho SJ, Song BG, Lee SC, Park SW. Longitudinal 2D strain at rest predicts the presence of left main and three vessel coronary artery disease in patients without regional wall motion abnormality. Eur J Echocardiogr 10: 695–701, 2009. doi: 10.1093/ejechocard/jep041. [DOI] [PubMed] [Google Scholar]
- 9.Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol 69: 1043–1056, 2017. doi: 10.1016/j.jacc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol 48: 504–511, 2010. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorsey SM, McGarvey JR, Wang H, Nikou A, Arama L, Koomalsingh KJ, Kondo N, Gorman JH III, Pilla JJ, Gorman RC, Wenk JF, Burdick JA. MRI evaluation of injectable hyaluronic acid-based hydrogel therapy to limit ventricular remodeling after myocardial infarction. Biomaterials 69: 65–75, 2015. doi: 10.1016/j.biomaterials.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feild BJ, Russell RO Jr, Dowling JT, Rackley CE. Regional left ventricular performance in the year following myocardial infarction. Circulation 46: 679–689, 1972. doi: 10.1161/01.CIR.46.4.679. [DOI] [PubMed] [Google Scholar]
- 14.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol 58: 1733–1740, 2011. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Gjesdal O, Helle-Valle T, Hopp E, Lunde K, Vartdal T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. Noninvasive separation of large, medium, and small myocardial infarcts in survivors of reperfused ST-elevation myocardial infarction: a comprehensive tissue Doppler and speckle-tracking echocardiography study. Circ Cardiovasc Imaging 1: 189–196, 2008. doi: 10.1161/CIRCIMAGING.108.784900. [DOI] [PubMed] [Google Scholar]
- 16.Gorman JHI III, Gupta KB, Streicher JT, Gorman RC, Jackson BM, Ratcliffe MB, Bogen DK, Edmunds LHJ Jr. Dynamic three-dimensional imaging of the mitral valve and left ventricle by rapid sonomicrometry array localization. J Thorac Cardiovasc Surg 112: 712–726, 1996. doi: 10.1016/S0022-5223(96)70056-9. [DOI] [PubMed] [Google Scholar]
- 17.Guccione JM, Moonly SM, Moustakidis P, Costa KD, Moulton MJ, Ratcliffe MB, Pasque MK. Mechanism underlying mechanical dysfunction in the border zone of left ventricular aneurysm: a finite element model study. Ann Thorac Surg 71: 654–662, 2001. doi: 10.1016/S0003-4975(00)02338-9. [DOI] [PubMed] [Google Scholar]
- 18.Gupta KB, Ratcliffe MB, Fallert MA, Edmunds LH Jr, Bogen DK. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation 89: 2315–2326, 1994. doi: 10.1161/01.CIR.89.5.2315. [DOI] [PubMed] [Google Scholar]
- 19.Haugaa KH, Grenne BL, Eek CH, Ersbøll M, Valeur N, Svendsen JH, Florian A, Sjøli B, Brunvand H, Køber L, Voigt JU, Desmet W, Smiseth OA, Edvardsen T. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging 6: 841–850, 2013. doi: 10.1016/j.jcmg.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Hayashida W, Van Eyll C, Rousseau MF, Pouleur H; The SOLVD Investigators . Regional remodeling and nonuniform changes in diastolic function in patients with left ventricular dysfunction: modification by long-term enalapril treatment. J Am Coll Cardiol 22: 1403–1410, 1993. doi: 10.1016/0735-1097(93)90550-K. [DOI] [PubMed] [Google Scholar]
- 21.Hess OM, Koch R, Bamert C, Krayenbuehl HP, Policlinic M. Regional wall stiffness during acute myocardial ischaemia in the canine left ventricle. Eur Heart J 1: 435–443, 1980. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa K, Kawase Y, Ladage D, Chemaly ER, Tilemann L, Fish K, Sanz J, Garcia MJ, Hajjar RJ. Temporal changes of strain parameters in the progress of chronic ischemia: with comparison to transmural infarction. Int J Cardiovasc Imaging 28: 1671–1681, 2012. doi: 10.1007/s10554-012-0010-z. [DOI] [PubMed] [Google Scholar]
- 23.Janz RF. Estimation of local myocardial stress. Am J Physiol Heart Circ Physiol 242: H875–H881, 1982. doi: 10.1152/ajpheart.1982.242.5.H875. [DOI] [PubMed] [Google Scholar]
- 24.Jugdutt BI, Joljart MJ, Khan MI. Rate of collagen deposition during healing and ventricular remodeling after myocardial infarction in rat and dog models. Circulation 94: 94–101, 1996. doi: 10.1161/01.CIR.94.1.94. [DOI] [PubMed] [Google Scholar]
- 25.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 17: 1021–1029, 2004. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Lindsey ML, Bolli R, Canty JM Jr, Du X-J, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGarvey JR, Mojsejenko D, Dorsey SM, Nikou A, Burdick JA, Gorman JH III, Jackson BM, Pilla JJ, Gorman RC, Wenk JF. Temporal changes in infarct material properties: an in vivo assessment using magnetic resonance imaging and finite element simulations. Ann Thorac Surg 100: 582–589, 2015. doi: 10.1016/j.athoracsur.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKay RG, Pfeffer MA, Pasternak RC, Markis JE, Come PC, Nakao S, Alderman JD, Ferguson JJ, Safian RD, Grossman W. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation 74: 693–702, 1986. doi: 10.1161/01.CIR.74.4.693. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee R, Zavadzkas JA, Saunders SM, McLean JE, Jeffords LB, Beck C, Stroud RE, Leone AM, Koval CN, Rivers WT, Basu S, Sheehy A, Michal G, Spinale FG. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann Thorac Surg 86: 1268–1276, 2008. doi: 10.1016/j.athoracsur.2008.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 30: 1527–1533, 1997. doi: 10.1016/S0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 30.Park TH, Nagueh SF, Khoury DS, Kopelen HA, Akrivakis S, Nasser K, Ren G, Frangogiannis NG. Impact of myocardial structure and function postinfarction on diastolic strain measurements: implications for assessment of myocardial viability. Am J Physiol Heart Circ Physiol 290: H724–H731, 2006. doi: 10.1152/ajpheart.00714.2005. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172, 1990. doi: 10.1161/01.CIR.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 32.Reant P, Labrousse L, Lafitte S, Bordachar P, Pillois X, Tariosse L, Bonoron-Adele S, Padois P, Deville C, Roudaut R, Dos Santos P. Experimental validation of circumferential, longitudinal, and radial 2-dimensional strain during dobutamine stress echocardiography in ischemic conditions. J Am Coll Cardiol 51: 149–157, 2008. doi: 10.1016/j.jacc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 33.Romito E, Doviak H, Logdon C, Freels P, Shazly T, Spinale FG. Sonomicrometry-based analysis of post-myocardial infarction regional mechanics. Ann Biomed Eng 44: 3539–3552, 2016. doi: 10.1007/s10439-016-1694-3. [DOI] [PubMed] [Google Scholar]
- 34.Romito E, Shazly T, Spinale FG. In vivo assessment of regional mechanics post-myocardial infarction: a focus on the road ahead. J Appl Physiol 123: 728–745, 2017. doi: 10.1152/japplphysiol.00589.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross J., Jr Is there a true increase in myocardial stiffness with acute ischemia? Am J Cardiol 63: 87E–91E, 1989. doi: 10.1016/0002-9149(89)90237-3. [DOI] [PubMed] [Google Scholar]
- 36.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2: 356–364, 2009. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 37.Strotmann JM, Hatle L, Sutherland GR. Doppler myocardial imaging in the assessment of normal and ischemic myocardial function−past, present and future. Int J Cardiovasc Imaging 17: 89–98, 2001. doi: 10.1023/A:1010679522539. [DOI] [PubMed] [Google Scholar]
- 38.Sun JP, Niu J, Chou D, Chuang HH, Wang K, Drinko J, Borowski A, Stewart WJ, Thomas JD. Alterations of regional myocardial function in a swine model of myocardial infarction assessed by echocardiographic 2-dimensional strain imaging. J Am Soc Echocardiogr 20: 498–504, 2007. doi: 10.1016/j.echo.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Sun QW, Zhen L, Wang Q, Sun Y, Yang J, Li YJ, Li RJ, Ma N, Li ZA, Wang LY, Nie SP, Yang Y. Assessment of retrograde coronary venous infusion of mesenchymal stem cells combined with basic fibroblast growth factor in canine myocardial infarction using strain values derived from speckle-tracking echocardiography. Ultrasound Med Biol 42: 272–281, 2016. doi: 10.1016/j.ultrasmedbio.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 39a.Taylor GJ, Humphries JO, Mellits ED, Pitt B, Schulze RA, Griffith LS, Achuff SC. Predictors of clinical course, coronary anatomy and left ventricular function after recovery from acute myocardial infarction. Circulation 62: 960–970, 1980. doi: 10.1161/01.CIR.62.5.960. [DOI] [PubMed] [Google Scholar]
- 40.Thomas G. Tissue Doppler echocardiography−a case of right tool, wrong use. Cardiovasc Ultrasound 2: 12, 2004. doi: 10.1186/1476-7120-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation 102: 1158–1164, 2000. doi: 10.1161/01.CIR.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 42.Verma A, Meris A, Skali H, Ghali JK, Arnold JMO, Bourgoun M, Velazquez EJ, McMurray JJV, Kober L, Pfeffer MA, Califf RM, Solomon SD. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging 1: 582–591, 2008. doi: 10.1016/j.jcmg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation 114: 2627–2635, 2006. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 44.Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 26: 185–191, 2013. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging–a method for noninvasive assessment of myocardial motion. Radiology 169: 59–63, 1988. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]