Abstract
Background:
The search for potent radioprotective agents for the amelioration of radiation side effect is an important aim in radiobiology. The present study aimed to evaluate the effects of curcumin and seleno-L-methionine against radiation-induced micronucleus formation in rat bone marrow.
Methods:
In total, 40 male rats were divided into 8 groups (n=5 each), including control, curcumin or seleno-L-methionine treated alone or in combination, 2 Gy irradiation, irradiation of treated groups with curcumin or seleno-L-methionine or their combination. Curcumin was administrated orally and seleno-L-methionine was injected intraperitoneally 24 hours before irradiation. The frequency of micronucleated normochromatic erythrocytes (MnNCEs) and micronucleated polychromatic erythrocytes (MnPCEs) was scored in 5,000 polychromatic erythrocytes (PCEs) and the cell proliferation ratio [(PCE/(PCE+NCE); NCE=normochromatic erythrocytes] was calculated for each treatment group. Data were analyzed by the SPSS software version 16.0 and P<0.05 was considered as statistically significant differences.
Results:
Pretreatment with curcumin and seleno-L-methionine before irradiation reduced the frequency of MnPCEs and MnNCEs (P=0.01) and increased the cell proliferation ratio. Moreover, the results showed that this pretreatment reduced the frequency of MnPCEs with a protection factor (PF) of 1.2 and 1.6, respectively. The combination of curcumin and seleno-L-methionine in reducing MnPCEs and MnNCEs was not more effective than each agent alone, while improved cell proliferation ratio.
Conclusion:
Both curcumin and seleno-L-methionine showed potent protection against radiation induced MN in bone marrow cells. The combination of the two agents further ameliorates this activity, thus leading to improve bone marrow protection.
Keywords: Radiation protection, Curcumin , Seleno-L-methionine selenoxide , Micronuclei , Gamma rays
What’s Known
Radiation-induced genotoxicity can be expressed as short- or long-term effects depending on the amount of radiation dose.
To prevent induction of radiation-induced genetic effects, numerous chemicals including antioxidants have been investigated for their radioprotective capacity. However, a suitable compound or compounds has not been achieved yet.
What’s New
Radioprotective effects of curcumin combined with selenium on radiation-induced genetic damages, expressed as micronucleus in rat bone marrow erythrocytes, have not been reported previously.
Curcumin with antioxidant property and selenium are necessary for radical scavenging effects of glutathione. When the combination is used, they exhibit a profound radioprotective potential.
Introduction
Ionizing radiation has an important role in the human life, such as medicine, industry, agriculture, and power generation.1 Although the use of radiation technology is useful for human life, exposure to ionizing radiation during medical applications or radiation accidents may lead to adverse effects such as cancer, heart diseases, and some other side effects.2 One of the main purposes of radiobiology is to investigate an appropriate radioprotector to protect people against radiation side effects. Radioprotector agents may be useful for people under the risk of radiation exposure, including patients undergoing radiotherapy, soldiers exposed to nuclear radiation, radiation workers, and people involved in nuclear accidents.3,4 After the discovery of cysteine as a radioprotector agent in 1949,5 researchers investigated the radioprotective properties of many compounds, such as aminothiols. Amifostine is the only radioprotector that was clinically approved to palliate side effects in patients undergoing radiotherapy. Amifostine has a good protective effect, but is relatively toxic and has some inappropriate effects such as nausea, vomiting, and hypotension. Hence, it is required to search for less toxic and more potent radioprotectors which can be easily administered.6
Bone marrow is the most sensitive organ in response to cytotoxic effects of ionizing radiation. Ionizing radiation induces different types of DNA damage in bone marrow cells that may remain unrepaired. In this situation, unrepaired DNA damage may lead to cell death or genomic instability.7 Therefore, the risk of death or hematopoietic malignancies threatens the exposed people. Curcumin is a major bioactive agent which has a wide spectrum of anti-inflammatory, antioxidant, anti-mutagenic, and anti-carcinogenic properties.8 In response to ionizing radiation, curcumin has shown to be able to sensitize cancer cells to ionizing radiation while protecting normal tissues.9 The toxicity of curcumin is low compared to several radioprotectors. In a clinical trial study, curcumin has shown no toxicity up to doses of 8-12 gram per day.10 It was shown that pretreatment with curcumin can accelerate healing of skin damage following irradiation and be a substantial therapeutic strategy in the management of irradiation-related wounds.11
Seleno-L-methionine as an important micronutrient has broad effects on biological systems as antioxidant effects, cancer prevention, and anti-inflammatory effect. Seleno-L-methionine in animal tissues incorporates particularly to the structure of glutathione peroxidase, as the main functional form of seleno-L-methionine. Glutathione peroxidase has an important role in the removal of free radicals, thereby protects DNA, proteins, and unsaturated phospholipids from the deleterious effects of free radicals.12,13 Also, it is proposed that seleno-L-methionine can stimulate DNA repair responses.14
According to the appropriate radioprotective effect of both agents, the present study aimed to investigate the radioprotective effects of combined curcumin and seleno-L-methionine against radiation-induced micronucleus in rat bone marrow erythrocytes.
Materials and Methods
Animal Pretreatment
The present experimental study was carried out in accordance with the guidelines for the care and use of laboratory animals as adopted by the Ethics Committee of the School of Allied Medicine, Tehran University of Medical Sciences (Tehran, Iran). All rats were kept in the animal facility maintained at 20-22 ºC temperature, relative humidity of 50-70%, and 15 air exchanges per hour. Also, a time-controlled system provided 08:00-20:00 hour light and 20:00-08:00 hour dark cycles. All rats were given standard rodent chow diets and water from a sanitized bottle fitted with a stopper and sipper tubes.
Experimental Design and Irradiation
In the present experimental-interventional study, 40 male Wistar rats weighing 180-200 g were divided into 8 groups (n=5 each) using the simple random sampling method. The groups were categorized as follows:
1. Group 1: Control
2. Group 2: Curcumin treatment (200 mg/kg body weight)
3. Group 3: Seleno-L-methionine (4 mg/kg body weight)
4. Group 4: Curcumin+seleno-L-methionine
5. Group 5: 2 Gy radiation
6. Group 6: 2 Gy radiation+curcumin
7. Group 7: 2 Gy radiation+seleno-L -methionine
8. Group 8: 2 Gy radiation+curcumin+seleno -L-methionine
Following 24 hours of treatment, all rats were anesthetized by an IP injection of ketamine (60 mg/kg) and xylazine (20 mg/kg). Then, the rats in groups 5-8 were exposed to 2 Gy cobalt-60 gamma rays as whole-body irradiation (Theratron-II 780C, Kanata, ON, Canada) at a dose rate of 109 cGy/min with a source to surface distance (SSD) of 60 cm and fixed field size of 25×25 cm at 22±2 ºC temperature. The selection of 24 hours intervals between curcumin and seleno-L-methionine injection and exposure to γ-ray radiation was entirely based on previous studies.15 Additionally, the selected dose of curcumin and seleno-L-methionine and γ-ray radiation was based on other studies.4,15,16
The Micronucleus Assay
The rat bone marrow micronucleus test was carried out according to the method described by Mozdarani and Gharbali.17 All rats were sacrificed by cervical dislocation 24 hours after irradiation. Also, rats in the control group sacrificed 48 hours after injection. Their femoral bone marrow was disembogued by fetal calf serum and a cell suspension was prepared. The suspension was centrifuged for 6 minutes at 1,500 rpm. After centrifugation, the supernatant was removed and cells were re-suspended in the remaining serum. The resultant smear was fixed in methanol and stained with May-Grunewald-Giemsa (Merck, Darmstadt, Germany). In this staining method, polychromatic erythrocytes (PCEs) are stained blue-violet while norm chromatic erythrocytes (NCEs) are stained yellow-orange.
Microscopic Analysis
Following slide preparation and staining, the slides were randomized and coded for blind analysis by the same scorer. An Olympus BX43 microscope (Tokyo, Japan) with 100 objective lenses was used to score the cells. A total of 1,000 PCEs were scored for the presence of micronuclei in each sample. Based on a similar study, the ratio of PCE/(PCE+NCE) was calculated to determine the cytotoxic effects of γ-radiation on the proliferation of the bone marrow cells. This ratio is an index of the proliferation rate. A decline in the post-irradiation ratio is an expression of the known early effects of radiation on cell cycle, which indicates a mutagen-induced bone marrow cytotoxicity or suppression of erythropoiesis. The ratio of PCEs to total erythrocytes (PCEs+NCEs) has already been evaluated to determine the cytotoxic effects of gamma-radiation in the femoral bone marrow.
Statistical Analysis
Statistical analysis was performed using the SPSS software, version 16.0. Data were analyzed by one-way analysis of variance (ANOVA) method and Tukey’s post hoc test. P<0.05 was considered as statistically significant differences.
Results
MNPCE/1000PCE After 24 Hours
The results of MNPCE/1000PCE are illustrated in figure 1. Exposure to 2 Gy resulted in a significant increase in MN frequency compared to the control group (P=0.001). Pre-administration of seleno-L-methionine resulted in a significant decrease in the number of MN compared to the irradiated group (P=0.001). Pre-administration with curcumin (P=0.011) and curcumin plus seleno-L-methionine (P=0.001) resulted in a significant reduction in MN frequency compared to irradiation without drug treatment. Moreover, treatment with combined curcumin and seleno-L-methionine before irradiation decreased MN frequency compared to pretreatment with curcumin alone (P=0.001), but not seleno-L-methionine.
Figure1.
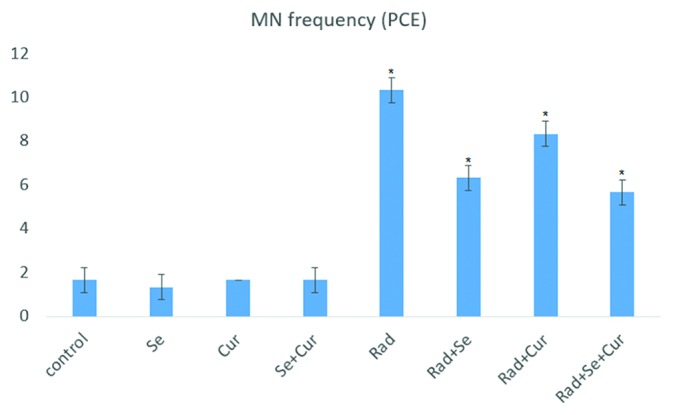
Frequency of MnPCEs in gamma-irradiated (2 Gy) rat bone marrow in the absence or presence of curcumin, seleno-L-methionine, or combined. A total number of 5,000 PCEs were scored for each treatment group. Error bars indicate standard errors of mean values obtained from 5 animals. *Significant compared to the control or RAD group.
MNNCE/1000NCE After 24 Hours
As shown in figure 2, pretreatment with seleno-L-methionine, curcumin, or seleno-L-methionine plus curcumin did not cause any significant increase in MN frequency. Irradiation with 2Gy caused a significant increase in MN frequency compared to the control or drug-treated groups (P=0.001). Administration of seleno-L-methionine before irradiation resulted in a significant decrease in MN numbers compared to the irradiated group (P=0.001). Also, a significant difference for pre-administration with curcumin (P=0.017) and the combined form of curcumin and seleno-L-methionine (P=0.001) were investigated compared to the irradiated group. Administration of curcumin plus seleno-L-methionine did not affect the MN frequency compared to pre-treatment with curcumin or seleno-L-methionine alone.
Figure2.
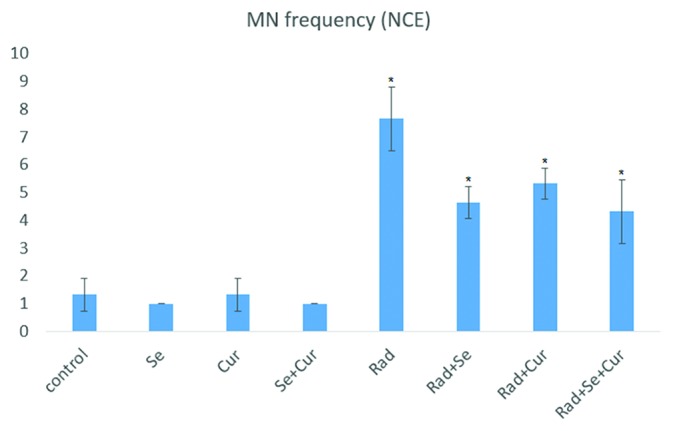
Frequency of MnNCEs in gamma-irradiated (2 Gy) rat bone marrow in the absence or presence of curcumin, seleno-L-methionine, or combined. A total number of 5,000 PCEs were scored for each treatment group. Error bars indicate standard errors of mean values obtained from 5 animals. *Significant compared to control or RAD group.
Cell Proliferation Ratio
PCE/PCE+NCE is an index for the evaluation of cell proliferation following exposure to ionizing radiation or radiation modifiers. The statistical analysis showed a significant difference in cell proliferation ratio between the groups. Pretreatment with seleno-L-methionine, curcumin or the combined form did not cause any significant effect on cell proliferation ratio. Exposure to ionizing radiation resulted in a significant decrease in PCE/PCE+NCE index. Treatment with seleno-L-methionine (P=0.001), curcumin (P=0.002) and the combined form (P=0.001) increased this index significantly. Moreover, pre-administration with the combined form increased PCE/PCE+NCE index compared to pretreatment with curcumin alone (P=0.005), but not seleno-L-methionine (figure 3).
Figure3.
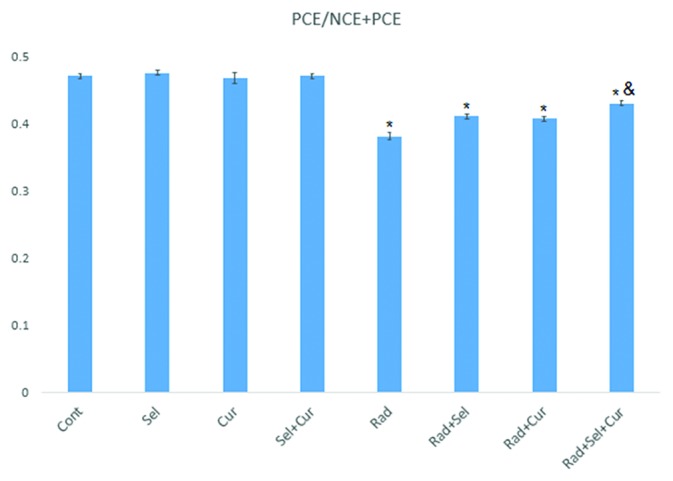
Cell proliferation ratio in gamma-irradiated (2Gy) rat bone marrow in the absence or presence of curcumin, seleno-L-methionine, or combined. A total number of 5,000 PCEs and the number of NCEs observed in the field of the 5,000 PCEs and were scored for each sample. Error bars are standard errors of mean values obtained from 5 animals. *Significant compared to control or RAD group; &Significant compared to the RAD+Cur and RAD+Sel groups.
Protection Factor (PF)
The PF was calculated as the ratio of the MnPCEs frequency induced by radiation alone to the MnPCEs frequency induced by radiation in the presence of curcumin and seleno-L-methionine or their combined form. A PF of 1.8 was achieved for the combined form of curcumin and seleno-L-methionine, 1.2 for curcumin, and 1.6 for seleno-L-methionine.
Discussion
In the present study, the radioprotective effect of curcumin, seleno-L-methionine alone, and the combined form was evaluated against radiation induced micronuclei formation in rat bone marrow erythrocytes. The cell proliferation ratio was calculated using the formula PCE/(PCE+NCE). Similar to previous studies, we showed an increased micronuclei formation in PCE and NCE in the bone marrow cells following irradiation.4 Also, the PCE/PCE+NCE significantly reduced which indicates the cytotoxic effect of ionizing radiation on the cell proliferation rate.
It is proposed that potent antioxidative effect of seleno-L-methionine in combination with the anti-inflammatory effect of curcumin can ameliorate DNA damage in bone marrow cells. The results showed that pre-administration of seleno-L-methionine or curcumin do not produce any toxic effect on bone marrow cells. Development of non-toxic and potent radioprotectors is one of the main aims in radiation biology. These properties can help to manage the detrimental effects of ionizing radiation during radiation disaster or cancer radiotherapy with acceptable side effects.
Pretreatment with seleno-L-methionine before irradiation significantly reduced micronuclei formation in both PCE and NCE and increased the PCE/PCE+NCE ratio. Similar to seleno-L-methionine, pretreatment with curcumin 24 hours before exposure resulted in an obvious reduction in micronuclei formation in both PCE and NCE. Moreover, results indicated that combining seleno-L-methionine with curcumin is more efficient compared to curcumin administration in PCE cells.
So far, several herbal products have been proposed as radioprotector compounds.18,19 It is very important to limit the toxicity caused by ionizing radiation on normal tissues as well as increase the response of malignant cells to radiotherapy. Natural and herbal agents have suitable properties compared to chemical radioprotectors, such as less toxicity, lower costs, and oral administration.6 It has been shown that curcumin compared to different types of herbal agents has many radioprotective effects. Curcumin has shown its ability to reduce the toxic effects of ionizing radiation on normal tissues, such as highly sensitive bone marrow cells. On the other hand, curcumin has shown that it can sensitize tumor cells to radiation.20 These effects reduce the total radiation dose for tumor control which increases therapeutic ratio and alleviates both early and late normal tissue toxicity.
Studies have revealed that inflammatory responses and continuous ROS production following exposure to ionizing radiation play key roles in bone marrow toxicity and genomic instability. It seems that TGF-β, NADPH oxidase activity, and mitochondrial ROS have central roles in this pathway.21 Both curcumin and seleno-L-methionine have shown good anti-inflammatory and antioxidative effects.22 Brown et al. showed that selenium and seleno-L-methionine are able to scavenge free radical production after irradiation and ameliorate DNA damage and apoptosis in bone marrow cells. The results indicated that activation of some immune responses after exposure to radiation was involved in bone marrow cell death.23
Abraham et al. showed that pretreatment with different doses of curcumin (5, 10, and 20 mg/kg) reduced the incidence of MnPCEs in mice bone marrow cells after exposure to ionizing radiation.24 This result is confirmed by the evaluation of the protective effect of curcumin against toxic effects of chemotherapy agents such as cisplatin.25 Curcumin has shown the ability to ameliorate inflammation induced ROS production through suppression of iNOS, COX-2, and NADPH oxidase gene expression.26
Similar to curcumin, selenium compounds have shown anti-tumor and synergistic effect with radiation therapy. These results increase possible application of selenium compounds for the amelioration of toxic effect of ionizing radiation in clinical oncology. Studies have proposed some mechanisms for the protective effect of seleno-L-methionine against toxic agents. Fischer et al. showed that seleno-L-methionine can enhance DNA damage response in a p53 dependent mechanism.27 Another study by these researchers showed that seleno-L-methionine stimulates BRCA1, which interacts with the RAD51, resulting in accelerated double-strand breaks repair in DNA.28
In the present study, seleno-L-methionine (as a potent antioxidant and enhancer of DNA damage response) was used in combination with curcumin (as a potent anti-inflammatory and antioxidant agent). The use of the combined form of radioprotectors for a better management of radiation side effects has been studied. Mozdarani et al. examined the radioprotective effect of single and combined form of famotidine, vitamin C, and cimetidine on radiation-induced bone marrow micronuclei formation. They showed that the combined form of these agents provides no additional protective effect.29 In another study, it is shown that the combined form of famotidine and vitamin C is more effective in reducing MnPCEs compared to the individual agent. Also, they showed that the combined form of famotidine and vitamin C increases cell proliferation ratio. The authors recommended that the combination of these compounds may further improve bone marrow protection.4
Kopjar et al. evaluated the radioprotective effect of a combined regime, including melatonin and amifostine on sister chromatid exchange (SCE) and cytokinesis-block micronucleus (CBMN) in human peripheral blood lymphocytes. They showed that the best result is obtained when the combination of both melatonin and amifostine is used before exposure to radiation.30 Although the combination of these agents has no synergistic effect on radiation-induced epiphyseal injury.31 These results may indicate that the synergic effect of radioprotector agents is highly dependent on radioprotector agents as well as being tissue specific.
In the present study, it is hypothesized that using the combined form of seleno-L-methionine and curcumin before exposure to radiation may ameliorate the detrimental effects of ionizing radiation and subsequent micronuclei formation in bone marrow cells. Dai et al. showed that curcumin has a synergistic antioxidant effect with other antioxidants like alpha-tocopherol against free-radical-induced peroxidation.32 On the other hand, it is confirmed that seleno-L-methionine is absorbed early and neutralizes free radicals in cells.33 This compound acts directly and through stimulation of glutathione peroxidase expression. Moreover, methionine elevates cell antioxidant capacity, such as glutathione peroxidase and glutathione reductase activity.34 Previous studies have demonstrated that both selenium and methionine have radioprotective and mitigatory effects and they can stimulate DNA repair responses.35 Although in the present study we did not show a synergistic effect of the seleno-L-methionine and curcumin for the reduction of micronuclei formation, the results showed a more potent effect on cell proliferation ratio. The use of other drug doses may lead to further improvement in the synergistic effect of these compounds. The results of the present study indicated that the combination of seleno-L-methionine and curcumin probably further enhances radioprotection, thus results in a higher bone marrow protection.
Conclusion
It is shown that pretreatment with seleno-L-methionine, curcumin alone, and in combination ameliorates genotoxicity of ionizing radiation in rat bone marrow cells. Based on the results of the present study, the radioprotective effect of the combined form of these compounds has a significant effect compared to the single form usage. Since ionizing radiation applications are increasing in human life (e.g. radiotherapy, nuclear energy, and industry), it is required to develop effective and non-toxic compounds that can reduce genotoxicity induced by ionizing radiation. We showed that the combination of seleno-L-methionine and curcumin might be a promising agent for such goals. We evaluated single-dose administration to detect the radioprotective effect of the combined form of seleno-L-methionine and curcumin. However, better results could be obtained if the effect of different doses at different times before and after exposure is studied.
Acknowledgement
This work was partly supported by AJA University of Medical Science, Tehran, Iran.
Conflict of Interest:None declared.
References
- 1.Galland-Girodet S, Maire JP, De-Mones E, Benech J, Bouhoreira K, Protat B, et al. The role of radiation therapy in the management of head and neck paragangliomas: impact of quality of life versus treatment response. Radiother Oncol. 2014;111:463–7. doi: 10.1016/j.radonc.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Bruheim K, Guren MG, Skovlund E, Hjermstad MJ, Dahl O, Frykholm G, et al. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76:1005–11. doi: 10.1016/j.ijrobp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Yamini K, Gopal V. Natural radioprotective agents against ionizing radiation–an overview. Int J Pharmtech Res. 2010;2:1421–6. [Google Scholar]
- 4.Zangeneh M, Mozdarani H, Mahmoudzadeh A. Potent radioprotective effects of combined regimens of famotidine and vitamin C against radiation-induced micronuclei in mouse bone marrow erythrocytes. Radiat Environ Biophys. 2015;54:175–81. doi: 10.1007/s00411-015-0586-5. [DOI] [PubMed] [Google Scholar]
- 5.Patt HM, Tyree EB, Straube RL, Smith DE. Cysteine Protection against X Irradiation. Science. 1949;110:213–4. doi: 10.1126/science.110.2852.213. [DOI] [PubMed] [Google Scholar]
- 6.Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15:360–71. doi: 10.1634/theoncologist.2009-S104. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao X, Wu X, Frassica D, Yu B, Pang L, Xian L, et al. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci U S A. 2011;108:1609–14. doi: 10.1073/pnas.1015350108. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res. 2010;173:590–601. doi: 10.1667/RR1522.1. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128–36. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 10.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- 11.Jagetia GC, Rajanikant GK. Effect of curcumin on radiation-impaired healing of excisional wounds in mice. J Wound Care. 2004;13:107–9. doi: 10.12968/jowc.2004.13.3.26589. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Bian W, Liu S, Huang K. Selenium protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation by suppressing oxidative stress and ERK signaling pathway. Biol Trace Elem Res. 2012;150:441–50. doi: 10.1007/s12011-012-9488-4. [DOI] [PubMed] [Google Scholar]
- 13.Ferencik M, Ebringer L. Modulatory effects of selenium and zinc on the immune system. Folia Microbiol (Praha) 2003;48:417–26. doi: 10.1007/BF02931378. [DOI] [PubMed] [Google Scholar]
- 14.Smith ML, Kumar MA. Seleno-L-Methionine Modulation of Nucleotide Excision DNA Repair Relevant to Cancer Prevention and Chemotherapy. Mol Cell Pharmacol. 2009;1:218–21. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh PP, Devi KR, Devi MM, Thokchom DS, Sharma GJ. Protection of low let radiation-induced DNA damage in rat bone marrow cells by free radical scavenger curcumin. Int J Pharm Sci Res. 2016;7:1168.. doi: 10.13040/IJPSR.0975-8232.7(3).1168-78. [DOI] [Google Scholar]
- 16.Schrauzer GN. Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr. 2000;130:1653–6. doi: 10.1093/jn/130.7.1653. [DOI] [PubMed] [Google Scholar]
- 17.Mozdarani H, Gharbali A. Radioprotective effects of cimetidine in mouse bone marrow cells exposed to gamma-rays as assayed by the micronucleus test. Int J Radiat Biol. 1993;64:189–94. doi: 10.1080/09553009314551291. [DOI] [PubMed] [Google Scholar]
- 18.Rezaeyan A, Haddadi GH, Hosseinzadeh M, Moradi M, Najafi M. Radioprotective effects of hesperidin on oxidative damages and histopathological changes induced by X-irradiation in rats heart tissue. J Med Phys. 2016;41:182–91. doi: 10.4103/0971-6203.189482. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddadi GH, Rezaeyan A, Mosleh-Shirazi MA, Hosseinzadeh M, Fardid R, Najafi M, et al. Hesperidin as Radioprotector against Radiation-induced Lung Damage in Rat: A Histopathological Study. J Med Phys. 2017;42:25–32. doi: 10.4103/jmp.JMP_119_16. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagetia GC. Radioprotection and radiosensitization by curcumin. Adv Exp Med Biol. 2007;595:301–20. doi: 10.1007/978-0-387-46401-5_13. [DOI] [PubMed] [Google Scholar]
- 21.Najafi M, Shirazi A, Motevaseli E, Geraily G, Norouzi F, Heidari M, et al. The melatonin immunomodulatory actions in radiotherapy. Biophys Rev. 2017;9:139–48. doi: 10.1007/s12551-017-0256-8. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP, et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. 2013;180:34–43. doi: 10.1667/RR3255.1. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown SL, Kolozsvary A, Liu J, Jenrow KA, Ryu S, Kim JH. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat Res. 2010;173:462–8. doi: 10.1667/RR1716.1. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham SK, Sarma L, Kesavan PC. Protective effects of chlorogenic acid, curcumin and beta-carotene against gamma-radiation-induced in vivo chromosomal damage. Mutat Res. 1993;303:109–12. doi: 10.1016/0165-7992(93)90022-N. [DOI] [PubMed] [Google Scholar]
- 25.Antunes LM, Araujo MC, Darin JD, Bianchi ML. Effects of the antioxidants curcumin and vitamin C on cisplatin-induced clastogenesis in Wistar rat bone marrow cells. Mutat Res. 2000;465:131–7. doi: 10.1016/S1383-5718(99)00220-X. [DOI] [PubMed] [Google Scholar]
- 26.Huang SL, Chen PY, Wu MJ, Tai MH, Ho CT, Yen JH. Curcuminoids Modulate the PKCdelta/NADPH Oxidase/Reactive Oxygen Species Signaling Pathway and Suppress Matrix Invasion during Monocyte-Macrophage Differentiation. J Agric Food Chem. 2015;63:8838–48. doi: 10.1021/acs.jafc.5b04083. [DOI] [PubMed] [Google Scholar]
- 27.Fischer JL, Mihelc EM, Pollok KE, Smith ML. Chemotherapeutic selectivity conferred by selenium: a role for p53-dependent DNA repair. Mol Cancer Ther. 2007;6:355–61. doi: 10.1158/1535-7163.MCT-06-0472. [DOI] [PubMed] [Google Scholar]
- 28.Fischer JL, Lancia JK, Mathur A, Smith ML. Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex. Anticancer Res. 2006;26:899–904. [PubMed] [Google Scholar]
- 29.Naeeji A, Mozdarani H, Shabestani Monfared A, Faeghi F, Ahmadi AA, Gholami M, et al. Oral Administration of Vitamin C, Cimetidine and Famotidine on Micronuclei Induced by Low Dose Radiation in Mouse Bone Marrow Cells. J Biomed Phys Eng. 2017;7:117–26. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopjar N, Miocic S, Ramic S, Milic M, Viculin T. Assessment of the radioprotective effects of amifostine and melatonin on human lymphocytes irradiated with gamma-rays in vitro. Arh Hig Rada Toksikol. 2006;57:155–63. [PubMed] [Google Scholar]
- 31.Topkan E, Tufan H, Yavuz AA, Bacanli D, Onal C, Kosdak S, et al. Comparison of the protective effects of melatonin and amifostine on radiation-induced epiphyseal injury. Int J Radiat Biol. 2008;84:796–802. doi: 10.1080/09553000802389678. [DOI] [PubMed] [Google Scholar]
- 32.Dai F, Chen WF, Zhou B, Yang L, Liu ZL. Antioxidative effects of curcumin and its analogues against the free-radical-induced peroxidation of linoleic acid in micelles. Phytother Res. 2009;23:1220–8. doi: 10.1080/09553000802389678. [DOI] [PubMed] [Google Scholar]
- 33.Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev. 2006;15:804–10. doi: 10.1158/1055-9965.EPI-05-0950. [DOI] [PubMed] [Google Scholar]
- 34.Kruglikova AA, Shtutman Ts M. [Glutathione peroxidase and glutathione reductase activity in rat liver following administration of vitamin E and methionine] Ukr Biokhim Zh. 1976;48:739–42. [PubMed] [Google Scholar]
- 35.Sieber F, Muir SA, Cohen EP, Fish BL, Mader M, Schock AM, et al. Dietary selenium for the mitigation of radiation injury: effects of selenium dose escalation and timing of supplementation. Radiat Res. 2011;176:366–74. doi: 10.1667/rr2456.1. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


