Summary
Mitochondrial alternative oxidase (AOX) is involved in a large number of plant physiological processes, such as growth, development and stress responses; however, the exact role of AOX in response to drought remains unclear. In our study, we provide solid evidences that the activated AOX capacity positively involved in ethylene‐induced drought tolerance, in tomato (Solanum lycopersicum), accompanied by the changing level of hydrogen peroxide (H2O2) and autophagy. In AOX1a‐RNAi plants, the ethylene‐induced drought tolerance was aggravated and associated with decreasing level of autophagy. The H2O2 level was relatively higher in AOX1a‐RNAi plants, whereas it was lower in AOX1a‐overexpressing (35S‐ AOX1a‐OE ) plants after 1‐(aminocarbonyl)‐1‐cyclopropanecarboxylic acid (ACC) pretreatment in the 14th day under drought stress. Interestingly, the accumulation of autophagosome was accompanied by the changing level of reactive oxygen species (ROS) in AOX transgenic tomato under drought stress whether or not pretreated with ACC. Pharmacological scavenging of H2O2 accumulation in AOX1a‐RNAi (aox19) stimulated autophagy acceleration under drought stress, and it seems that AOX‐dependent ROS signalling is critical in triggering autophagy. Lower levels of ROS signalling positively induce autophagy activity, whereas higher ROS level would lead to rapid programmed cell death (PCD), especially in ethylene‐mediated drought tolerance. Moreover, ethylene‐induced autophagy during drought stress also can be through ERF5 binding to the promoters of ATG8d and ATG18h. These results demonstrated that AOX plays an essential role in ethylene‐induced drought tolerance and also played important roles in mediating autophagy generation via balancing ROS level.
Keywords: ethylene, alternative oxidase, autophagy, drought, reactive oxygen species, Solanum lycopersicum
Introduction
Nowadays, the increasing demand for food and vegetables was still one of the big challenges for global population growth and economic development, especially in developing countries. Lack of water led to reduced crop yields and economic losses in drought regions. Plant drought tolerance is a meaningful measure to reduce the impact of drought on crop productivity. In order to survive in adversities, plants have evolved sophisticated mechanisms to degrade and recycle useless or valuable intracellular components when they perceive the signal of environmental deterioration. Autophagy (also known as macroautophagy), an eukaryotic cell degrading and recycling process, plays an important role in the cellular response to abiotic stresses (Bassham, 2007; Liu et al., 2009; Lv et al., 2014; Slavikova et al., 2008). In brief, the double‐membrane vesicle which is called autophagosome could enclose any cytoplasmic components and deliver them to the vacuole/lysosome; after then, the toxic components and required molecules are gradually degraded and recycled in organelles (He and Klionsky, 2009; Liu and Bassham, 2012). Autophagy‐related genes (ATG) have been found in almost all eukaryotes, and they are quite conserved through evolution (Pérez‐Pérez et al., 2012). There are more than 30 autophagy‐related (ATG) genes in Arabidopsis. These ATG genes and their homologs participate in main autophagy process which have been identified in different kinds of plants over the last 20 years (Kim et al., 2012; Kwon and Park, 2008). Recent study reported that there were more than 25 ATG genes in tomato and several ATG families were proved to take part in the process of drought and salt resistance (Liu et al., 2009; Wang et al., 2015).
Increasing studies have demonstrated reactive oxygen species (ROS) and autophagy have been associated with cell death under environmental stresses (Henry et al., 2015; Pérez‐Pérez et al., 2012). There were also some reports showing a complex crosstalk between ROS and autophagy that is partly mediated by hormone signalling cascades in the regulation of flowering, plant senescence and stress tolerance (Love et al., 2008; Shibuya et al., 2013; Slavikova et al., 2008; Yoshimoto et al., 2009). For instance, jasmonic acid, ethylene and salicylic acid play important but antagonistic roles in rapid apoptosis‐like PCD (programmed cell death) (Love et al., 2008). Evidences show that ROS also regulate starvation‐induced autophagy, which is clearly a survival pathway (Scherz‐Shouval and Elazar, 2007). Recent findings show that mitochondrial generation of ROS is a trigger for autophagy (Chen and Gibson, 2008; Lee et al., 2012; Scherz‐Shouval and Elazar, 2007). The mitochondrial electron transport chain and the peroxisomes are primary sources of ROS production in most eukaryotes. Studies indicate that plant mitochondria are at a cross‐point in the signalling pathways involving ROS, especially those concerning cell death (Jones, 2000). ROS, specifically, H2O2, are reported to participate in inducing autophagy and autophagic cell death (Scherz‐Shouval and Elazar, 2007).
In order to withstand the redundant ROS generation, plant cells have evolved sophisticated defence mechanisms to avoid organelle damage. For example, mitochondrial redox status is maintained by dissipation of excessive electron flow via several systems such as AOX, plant uncoupling proteins (UCPs) and plant mitochondrial potassium channel (Pmito‐KATP). Studies have shown that AOX functions to balance the energy stability and keep the electron transport chain (ETC) flowing through mitochondrial by limiting the formation of mitochondrial ROS (Panda et al., 2013; Wang et al., 2012; Xu et al., 2012a). It is well known that the ETC in the mitochondria could be over‐reduced, accompanying with the generation of superoxide (O2 .−), especially in stress condition (Deng et al., 2015; Minibayeva et al., 2012; Pu et al., 2015). Expression of AOX is activated not only by stress conditions but also by several phytohormone stimuli. Previous researches have reported that ethylene‐dependent pathways are required for O3‐induced up‐regulation of AOX1a (Ederli et al., 2006). Ethylene treatment could also enhance the AOX capacity under stress condition (Wang et al., 2010). Other phytohormones, like brassinosteroids, could enhance the alternative respiratory pathway in Nicotiana benthamiana in different abiotic stresses (Deng et al., 2015). In plant, AOX‐dependent defence mechanism seems to be continuous and indispensable, in particular in response to various stresses (Mittler, 2002; Xu et al., 2012a). Furthermore, evidences have shown that up‐regulated AOX pathway enhanced drought tolerance in wheat and the absence of AOX1a in Arabidopsis resulted in severe damage under drought stress (Bartoli et al., 2005; Giraud et al., 2008).
Studies have also shown that ethylene signal could also mediate induction of GmATG8i in soybean plants under starvation stress (Du et al., 2014; Okuda et al., 2011). Although ABA‐dependent pathway is important in plant drought response, an ABA‐independent pathway probably played a role in autophagy induction during drought stress (Liu et al., 2009). Until now, the roles of mitochondrial AOX and autophagy in the drought response have been investigated independently, and it was observed that both regulatory systems share some common regulatory and response genes. So we speculate the AOX‐dependent ROS signalling and autophagy pathway might have as critical roles in ethylene‐mediated tomato drought responses. Furthermore, the possible relationship between AOX and autophagy in alleviating ethylene‐induced drought tolerance was investigated.
Results
Ethylene‐induced AOX capacity and drought responses
To assess the effect of ethylene in tomato drought response, tomato seedlings were pretreated with water, various concentrations of 1‐aminocyclopropane‐1‐carboxylic acid (ACC, the precursor of ethylene) and then withholding water for 2 weeks. After 14 days, the change in relative water content (Figure 1a) and electrolyte leakage (Figure 1b) were examined. The results showed that relative higher concentration (e.g. >1 μm) of ACC‐pretreated tomato exhibited more significant drought sensitivity compared with control whereas 0.1 μm ACC showed an enhanced drought tolerance in the 14th day after withholding water. We found that ACC pretreatment caused an induction in either AOX1a transcript levels or AOX protein levels under drought (Figure 1c and d). Concentrations of ACC from 0.01 to 10 μm promoted obvious AOX accumulation, but only 0.1 μm or lower ACC concentration had significant effects on tomato drought tolerance. Based on these results, 0.1 μm ACC was used in our subsequent experiments. We found that ACC pretreatment could obviously elevate V t and V alt during the first 7 days after withholding water, whereas aminoethoxyvinylglycine (AVG, a specific inhibitor of ethylene biosynthesis) pretreated plants showed relative lower V t and V alt compared with controls (Figure 1e and f). These results demonstrated that AOX might involve in ethylene‐induced drought tolerance.
Figure 1.

Ethylene‐induced AOX capacity and drought tolerance. (a, b) Relative water content (RWC) (a) and electrolyte leakage (EL) (b) of terminal leaflets in different concentration of ACC‐pretreated tomato plants measured in the 14th day under drought stress. (c, d) Induction of SlAOX1a expression (c) and AOX protein levels (d) of the terminal leaflets were determined immediately in the 14th day of drought after mock or ACC pretreatment. Total RNA and protein were isolated from leaf samples at the same times. Ponceau S‐stained membranes are shown below the blots to indicate the amount of protein loaded per lane. (e, f) Total respiration (e) and alternative respiration (f) were measured in the indicated time points under drought condition. All data are presented as the means of at least three biological replicates (±SE). Means with the same letter did not significantly differ at P < 0.05 according to Duncan multiple range tests. Three independent experiments were performed with similar results.
AOX is essential in ethylene‐induced tomato drought tolerance
To further explore the function of AOX in ethylene‐induced drought tolerance, the transgenic tomato overexpressing AOX1a (35S‐AOX1a‐OE) and suppressing AOX (AOX1a‐RNAi) plants were generated. As shown in Figure 2b, all six 35S‐AOX1a transgenic lines were tested and showed increased levels of AOX1a. The 35S‐AOX1a‐OE‐2 and 35S‐AOX1a‐OE‐5 lines showed the greatest expression levels, while AOX1b, AOX1c and AOX2 exhibited no changes among these transgenic lines. Among all four AOX1a‐RNAi transgenic lines, aox13 and aox19 exhibited the most severe AOX reduction (90% for the AOX1a transcript and ~50% for the AOX1b and AOX2 transcripts) (Figure 2c). The respiration in transgenic plants showed the different changes compared with WT plants (Figure S2). Therefore, 35S‐AOX1a‐OE‐2, 35S‐AOX1a‐OE‐5, aox13 and aox19 transgenic lines were selected for further experiments (Figure 2a). There were also some differences among these mutants, for example the axillary bud outgrowth and the activity of antioxidant enzymes (Figures S3 and S4).
Figure 2.
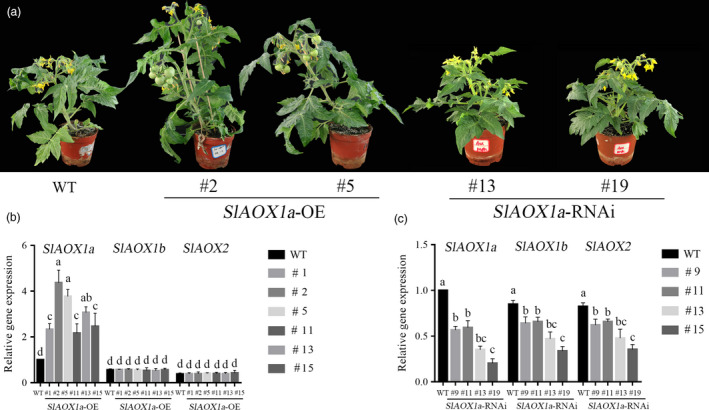
Identification of SlAOX1a transgenic lines. (a) Photographs of F2 progeny of transgenic tomato. (b, c) Expression of SlAOX1a, SlAOX1b and SlAOX2 in F2 progeny of transgenic lines. The age of the plants was 6–7 weeks. The data are presented as the means of five biological replicates (±SE). Significant differences (P < 0.05) are denoted by different lowercase letters. Three independent experiments were performed with similar results.
We further compared the drought tolerance of AOX mutants after pretreated with ACC. Of these, two AOX1a‐RNAi lines showed impaired drought tolerance compared with WT plants, as reflected by decreases in the F v/F m, relative water content and increases in electrolyte leakage. On the contrary, the OE‐2 and OE‐5 plants showed greater drought tolerance compared with WT plants (Figure 3a‐e). Moreover, we found the H2O2 content, a marker for ROS level which was generated by oxidative damage, was significantly increased by drought and was much higher in aox13 and aox19 mutants than WT plants. However, the 35S‐SlAOX1a‐OE plants showed decreased ROS generation under drought (Figure 3f). Therefore, we conclude that a pivotal role of AOX in drought tolerance could be mediated by ethylene.
Figure 3.

Drought tolerance in ACC‐pretreated transgenic SlAOX1a lines. (a) Photographs of ACC‐pretreated WT and transgenic SlAOX1a lines in the 14th day under drought stress. (b, c) Changes of the maximum PSII quantum yield (F v/F m) in ACC‐pretreated WT and transgenic SlAOX1a lines in the 14th day under drought stress. (d‐f) RWC (d), EL (e) and H2O2 content (f) of ACC‐pretreated WT and transgenic SlAOX1a lines in the 14th day under drought stress. Ten plants were used for each treatment group, and a picture of one representative leaf is shown. Bars represent mean and standard deviation of values obtained from six independent plants. Significant differences (P < 0.05) are denoted by different lowercase letters.
Autophagy plays an important role in ethylene‐mediated drought tolerance
To explore the possible influences caused by ethylene in tomato under drought stress, transmission electron (TEM) was used to monitor the changes of organelles in the leaf cell under drought stress. As shown in Figure 4a, there were little differences between water pretreated and ACC‐pretreated tomatoes under normal condition. Whereas the most intuitive scan was the twisted chloroplast with expanded starch and the collapsed mitochondria under drought condition. In addition, there were many small vesicles (autophagosome, red arrow) occurring inside the cells, especially in ACC‐pretreated tomato under drought condition. Thus, this result indicated that autophagy might be involved in this progress.
Figure 4.

Autophagosomes accumulation in tomato leaves under drought stress. (a) Representative transmission electron microscopy (TEM) images of autophagic structures in the mesophyll cells of different treatment plants. Five‐week‐old plants were exposed to dehydration by withholding water, and the mesophyll cells were visualized in the 7th day by TEM. Autophagic bodies are indicated by red arrows. Bars: 1 μm. (b) MDC‐stained autophagosomes of different treatment groups in the leaves. Five‐week‐old plants were exposed to dehydration by withholding water, and the leaves were MDC‐stained and visualized in the 7th day by fluorescence microscopy. MDC‐labelled structures are shown as blue signals. Bars: 25 μm. (c) ATG8 protein levels in mock and ACC‐pretreated plants after drought treatment. ATG8 and ATG8‐PE are the nonlipidated and lipidated forms of ATG8, respectively. The β‐actin was used as a loading control for the Western blotting analysis. Experiments were repeated three times with similar results. Means with the same letter did not significantly differ at P < 0.05 according to the Duncan multiple range tests.
Studies have shown that autophagy participated in plant drought tolerance (Liu et al., 2009), so whether autophagy participated in ethylene‐mediated drought responses need be further investigated. The monodansylcadaverine (MDC, an autophagosome‐specific autofluorescent dye) was used to directly stain the autophagosome‐like components. Anti‐ATG8 was used to detect the formation of Atg8‐phosphatidylethanolamine (PE) conjugates as a marker for autophagic activation (Wang et al., 2015). Under normal water supply conditions, we observed low numbers of punctate fluorescent signals and slight Atg8‐PE band in the plants whatever pretreated with water or ACC. After two‐week drought treatment, either increased numbers of punctate fluorescent signals or faster migration of the Atg8‐PE bands was observed. Importantly, ACC‐pretreated tomato exhibited relative higher autophagy activity than water pretreated tomato under drought (Figure 4b and c). Then, the changes in transcription level among all the ATG genes were tested and we found ATG8d and ATG18h were obviously induced after ACC pretreatment under drought stress (Figure 4d). These results demonstrated that autophagy could be further activated by ethylene under drought.
After the investigation of several ethylene response factors (ERFs) which were demonstrated to be induced by drought stress, we found that SlERF5, a typical drought responsive transcription factor, was significantly induced by ACC treatment under drought (Figure S5). To examine the possible regulation of tomato ATG genes by ERF5, we inspected 1.5‐kb sequences located upstream of the predicted transcriptional start sites of 26 tomato ATG genes. Only ATG8d and ATG18h promoters were shown to contain DRE‐binding site (ACCGAC); therefore, electrophoretic mobility shift assay (EMSA) was performed to explore whether ERF5 could directly bind these promoters (Figure 5a and Figure S10). As Figure 5b shows, probe‐protein complex was detected using ATG8d and ATG18h promoter probes. When we mutated the core DRE‐binding site of these two genes, as ATG8d‐competitor and ATG18h‐competitor, the binding to the complexes was vanished. These results suggested that the ERF5 protein specifically binds to the ATG sequences in the synthesized probes of the ATG8d and ATG18h promoters in vitro. Transient transfection assay revealed that ACC treatment or ERF5 protein induced the expression of ATG8d‐pro:LUC and ATG18h‐pro:LUC in N. benthamiana as compared to the control under drought stress (Figure 5c‐f).
Figure 5.
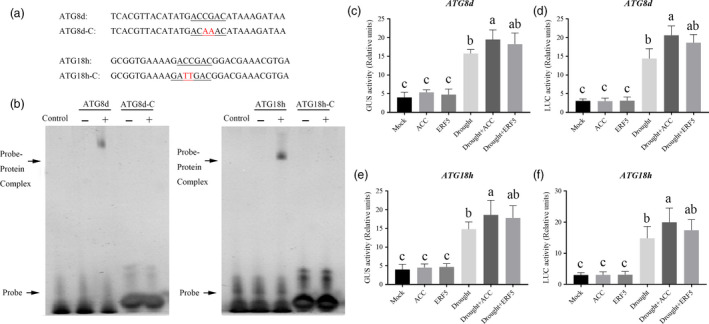
Analysis of ATG8d and ATG18h promoters. (a) Oligonucleotide used in the electrophoretic mobility shift assays (EMSA). The WT and mutated DRE sequences are underlined. The mutated bases were indicated in red. (b) EMSA showing ERF5 bound to the DRE sequences of the ATG8d or ATG18h promoters. Recombinant ERF5 was purified from E. coli cells and used for DNA binding assays. His was included as the negative control. (c‐f) Relative GUS and LUC activity of ATG8d (c,d) and ATG18h (e,f) promoters were detected under control or drought condition by different treatments. N. benthamiana plants were treated with 0.1 μm ACC for 12 h, and then, the leaves were transiently transformed with different constructs. For drought treatment, the N. benthamiana plants were first treated with 0.1 μm ACC for 12 h and then the leaves were transiently transformed with different constructs, after another 12 h, they were treated with 16% PEG6000 for 60 h. The CaMV 35S promoter was fused to GUS or LUC as a control for variation in transformation rate. Bars represent mean and standard deviation of values obtained from three biological repeats. Means with the same letter did not significantly differ at P < 0.05 according to the Duncan multiple range tests.
To investigate the roles of these two ATG genes act in ethylene‐induced drought tolerance, the method of virus‐induced gene silencing (VIGS) was used to generate ATG8d‐/ATG18h‐silenced plants and the results showed that the levels of these genes were only 30%–40% of the TRV control plants (Figure S6). After pretreated with ACC, ATG8d‐ and ATG18h‐silenced plants showed severe damage compared with ACC‐pretreated TRV control plants under drought stress (Figure 6a). Figure 6b and c showed that the photosynthetic efficiency in ACC‐pretreated TRV‐ATG8d and TRV‐ATG18h plants was obviously lower than TRV control plants under drought stress, which indicated the ATG8d and ATG18h are essential in ethylene‐induced drought resistance. The RWC and EL in ACC‐pretreated ATG8d‐ and ATG18h‐silenced plants further confirmed above result (Figure 6d and e). As shown in Figures 6f and Figure S7, autophagy activity significantly decreased in ATG8d‐ and ATG18h‐silenced plants, with or without ACC pretreatment. Furthermore, we found that ATG8d‐ and ATG18h‐silenced tomato leaf cells were earlier to be death compared with control plants under drought stress (Figure 6g), which means that autophagy is indispensable and functioning to prevent soil water deficit‐triggered rapid cell death.
Figure 6.
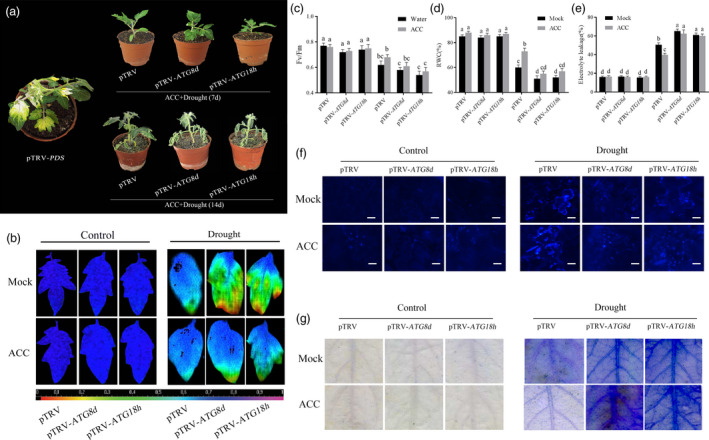
Reduced tomato drought tolerance in TRV‐ ATG8d and TRV‐ ATG18h plants. TRV‐SlPDS was used as positive control, and TRV‐mock was used as negative control. (a) Phenotypes of ACC‐induced drought tolerance in TRV‐ ATG8d and TRV‐ ATG18h plants (7d: upper; 14d: lower). (b, c) Changes of the maximum PSII quantum yield (F v/F m) in ACC‐pretreated ATG8d‐ and ATG18h‐silenced plants in the 14th day under drought conditions. (d, e) Detection of RWC and EL in ACC‐pretreated TRV‐ ATG8d and TRV‐ ATG18h plants under different condition. (d) MDC‐stained autophagosomes of different treatment groups in the leaves of TRV, TRV‐ ATG8d, and TRV‐ ATG18h plants. Five‐week‐old plants were exposed to dehydration by withholding water, and the leaves were MDC‐stained and visualized in the 7th day by fluorescence microscopy. MDC‐labelled structures are shown as blue signals. Bars: 25 μm. (e) Trypan blue staining showing cell death in the leaves of TRV, TRV‐ ATG8d and TRV‐ ATG18h plants. Means with the same letter did not significantly differ at P < 0.05 according to Duncan multiple range tests. Three independent experiments were performed with similar results.
The collaboration of AOX and autophagy are indispensable in ethylene‐mediated drought tolerance
As either AOX or autophagy participated in ethylene‐induced drought resistance, we next investigated whether the increased AOX and autophagy capacity are both essential in this process. We used VIGS technical to silence ATG8d in aox19 mutant plants for generating ATG8d‐ and AOX‐co‐silenced plants. The silence efficiency was confirmed by RT‐PCR (Figure S8). As shown in Figure 7a, even at the 7th day after withholding water, the ATG8d‐ and AOX‐co‐silenced plants exhibited badly growth condition, falling leaves, dwarf, wilting shoots. Thus, the damage level among these mutants were detected and we found that the F v/F m and RWC were significant lower whereas EL was higher in ATG8d‐ and AOX‐co‐silenced plants compared to aox19 and TRV control aox19 plants with or without ACC pretreatment, especially in drought stress (Figure 7b‐d). That means the ATG8d‐ and AOX‐co‐silenced plants were severely damaged under drought stress. Several antioxidant enzymes activity were almost collapse in ATG8d‐ and AOX‐co‐silenced plants under drought stress (Figure 7e‐g). Notably, ACC pretreatment actually alleviated the defence systems reduction. These results showed that AOX and autophagy both play positive and indispensable roles in ethylene‐induced tomato drought tolerance.
Figure 7.

The indispensable roles of AOX and autophagy in ethylene‐mediated drought tolerance. (a) Photographs of ACC‐pretreated TRV and TRV‐ ATG8d in aox19 plants under drought. (b‐d) The F v/F m(b), RWC(c) and EL(d) in different groups under drought stress. (e‐g) The antioxidant enzyme activity of SOD (e), CAT (f) and APX (g) in different groups under drought stress. Means with the same letter did not significantly differ at P < 0.05 according to Duncan multiple range tests. Three independent experiments were performed with similar results.
The relationship between AOX and autophagy in ethylene‐induced drought tolerance
To monitor the changes of autophagy activity in ACC‐pretreated AOX transgenic tomato, we used transmission electron microscopy (TEM), MDC and anti‐ATG8 to survey the autophagy capacity. As was shown in Figure 8a‐d, ACC‐pretreated OE‐2 and OE‐5 plants exhibited higher whereas aox13 and aox19 exhibited lower autophagic activity than WT plants. The TEM detection showed that the starch in the chloroplasts was inflated and almost occupied most chloroplast space under drought condition. The mitochondrial were also almost collapsed because there were almost no integrity mitochondria structures were found in aox mutants. To figure out the crosstalk between AOX and autophagy, we then checked the AOX capacity in ACC‐pretreated TRV‐ATG8d and TRV‐ATG18h plants. The total respiration rate (V t) and alternative pathway respiration rate (V alt) had no differences between TRV‐ATG8d or TRV‐ATG18h and TRV control plants with or without ACC pretreatment under normal condition (Figure 8e and f). However, V t and V alt were significant lower in ATG8d‐ and ATG18h‐silenced plants compared with TRV control plants under drought stress. Furthermore, although ACC pretreatment elevated V t and V alt under drought, silencing ATG8d and ATG18h alleviated the increasing level of V t and V alt (Figure 8e and f). Then, we examined the transcription level of AOX1a and there were no significant changes between two ATG‐silenced plants and TRV control plants (Figure 8g). To explore the reason why ethylene‐induced V alt was almost vanished in TRV‐ATG8d and TRV‐ATG18h plants under drought, we analysed the AOX protein level in these two ATG‐silenced plants. As shown in Figure 8h, AOX protein level was significantly decreased in ATG8d‐ and ATG18h‐silenced plants. So we presumed that the compromised V alt was probably due to the AOX protein degradation under drought stress. Previous studies have reported that autophagy could help to recycle any molecules that are useful for the cellular components, which might be the reason to explain why the AOX protein level was obvious lower when ATG8d/ATG18h was silenced under drought stress. In accordance with previous researches, the ROS level was higher in AOX‐silenced plants whereas lower in AOX‐overexpressing plants, it could be result from the AOX functioning to scavenge ROS (Moore and Albury, 2008; Xu et al., 2012b) and play a role in the process of programmed cell death (PCD) in plants (Vanlerberghe et al., 2002; Vanlerberghe et al., 2009; Zhang et al., 2014a). To make it clear, we compared the ROS generation and autophagic activity in different ACC‐pretreated AOX transgenic plants under drought stress. H2DCF‐DA fluorescence probe was used to detect the H2O2 generation. As Figure 9a and b shown, the ROS accumulation decreased in OE‐2 and OE‐5 plants whereas elevated in aox13 and aox19 plants when compared with the control plants. In contrast, the autophagic activity in OE‐2 and OE‐5 showed increased level but decreased in aox13 or aox19 plants when compared with the control plants.
Figure 8.

AOX and autophagy worked collaboratively in ethylene‐induced drought tolerance. (a) Representative transmission electron microscopy (TEM) images of autophagic structures in the mesophyll cells of different treatment in transgenic plants. Autophagic bodies are indicated by red arrows. Bars: 1 μm. (b‐d) MDC staining (b), autophagic activity(c) and ATG8 protein levels (d) for autophagosomes, respectively. Bars: 25 μm. (e‐h) Detection of respiration rate (e, f), AOX1a expression level (g) and AOX protein level (h) in ACC‐pretreated TRV‐ ATG8d and TRV‐ ATG18h plants. Ponceau S‐stained membranes are shown below the blots to indicate the amount of protein loaded per lane. Means with the same letter did not significantly differ at P < 0.05 according to Duncan multiple range tests. Three independent experiments were performed with similar results.
Figure 9.

Quantitative measurements of H2O2 and autophagic capacity. (a, b) H2 DCF‐DA staining showing ROS accumulation in the leaves of different treatment groups in the 14th day under drought stress. (c, d) ATG8d (c) and ATG18h (d) expression level in ACC‐pretreated transgenic tomatoes in the 14th day under drought stress. (e, f) H2O2 content and (e) NADPH activity (f) in ACC‐pretreated transgenic tomatoes in the 14th day under drought stress. Means with the same letter did not significantly differ at P < 0.05 according to Duncan multiple range tests. Three independent experiments were performed with similar results.
To confirm above results, we further checked the H2O2 content, the most stable type of ROS, NADPH oxidase activity which is a crucial source of H2O2 generation and the two key ATG genes transcription in ACC‐pretreated transgenic plants under drought stress condition. Consistent with previous results, AOX1a‐overexpressing tomato exhibited lower H2O2 accumulation and higher expression level of autophagy than WT plants. On the contrary, AOX1a‐RNAi plants showed relative higher H2O2 generation and extremely weak autophagy capacity compared with WT plants (Figure 9c‐f). These results demonstrated that both AOX‐mediated ROS signalling and autophagy might be involved in ACC‐mediated drought responses.
AOX‐dependent ROS signalling is a key factor for the induction of autophagy in response to drought stress
To further elucidate the underlying crosstalk between AOX‐mediated ROS signalling and autophagy in ACC‐mediated drought response, we measured H2O2 and autophagic activity in OE‐2 and aox19 plants with different pharmacological treatments. In OE‐2 plants, foliar application of H2O2 enhanced ROS signalling accompanying with a decreased autophagic activity under drought in response to ACC. In aox19 plants, the dimethylthiourea (DMTU, a H2O2 scavenger) treatment compromised ROS generation whereas increased the autophagic activity (Figure 10a‐c). In accordance with ROS fluorescence, the H2O2 content and NADPH activity were relative higher when applied with exogenous H2O2 compared with water control treatment (Figure S9). By the way, DMTU treatment could scavenge the H2O2 generation but enhanced autophagic activity in ACC‐pretreated aox19 plants (Figure 10d‐h). We found the autophagic activity was higher when the H2O2 level was keeping in a relative low level in these samples have been improved in AOX capacity. Therefore, it seems that the autophagy could be triggered by the relative low ROS signal and function in plants against drought stress (Figure S9).
Figure 10.

Changing ROS level contributing to different autophagic capacity. (a‐c) In vivo and vitro detection of H2O2 level in the 14th day under drought stress. (d‐e) MDC staining (d) and autophagic activity (e) in different treatments of transgenic tomatoes in the 14th day under drought condition. (f‐h) ATG8d (c), ATG18h (d) and ATG8 protein levels expression level in different treatments of transgenic tomatoes in the 14th day under drought condition. ATG8 and ATG8‐PE are the nonlipidated and lipidated forms of ATG8, respectively. The β‐actin was used as a loading control for the Western blotting analysis. Exogenous H2O2 at or DMTU was sprayed onto tomato plants every 3 d under drought condition. Means with the same letter did not significantly differ at P < 0.05 according to Duncan multiple range tests. Three independent experiments were performed with similar results.
Discussion
Drought stress has been well studied and known as a range of separate stresses, such as high vapour pressure deficit and soil water deficit, often associated with oxidative stress, decreased soil nutrient availability, increasing soil salinity and mechanical impedance to root growth in hard soil. It is widely known that ethylene played an important role in the processes of stomatal control and root elongation (Sharp and LeNoble, 2002; Tanaka et al., 2005). As shown in Figure S1, 0.1 μm ACC pretreatment could significantly enhance tomato drought resistance. It is worth mentioning that Desikan et al. (2006) reported that ethylene could induce stomatal closure via AtRBOHF‐mediated hydrogen peroxide synthesis in Arabidopsis; therefore, we monitored the stomata conductance between ACC and AVG treatments. The stomatal aperture in ACC‐pretreated tomato leaves was lower compared with controls whereas AVG treatment compromised this process in the 7th day under normal or under drought condition (Figure S1). Studies also showed that ethylene production in various plant tissues are enhanced when plants were challenged by various types of stresses (Morgan and Drew, 1997). Recent studies showed ethylene‐insensitive mutants exhibited improved drought tolerance in Arabidopsis and Maize (Shi et al., 2015). Previous studies have also shown that inhibition of ethylene‐induced maturation led to an obvious impaired drought tolerance and reduction in carbohydrate consumption, grain‐filling and spikelet fertility in rice (Du et al., 2014). Shi et al., 2006 had reported the dual role of ethylene concentration in regulating cotton fibre growth. It must also be noted, although ethylene can promote biomass accumulation, it also has a strong effect to reduce extension growth, when plants faced to stress condition (Gallie et al., 2009). Several ethylene responsive factors (ERFs) could also enhance drought tolerance in tomato and tobacco (Pan et al., 2012; Quan et al., 2010). So, combining with former reports, we speculated that the mechanism of ethylene‐induced drought tolerance is the so‐called giving up a rook to save the king. Specifically, it reflected in the following aspects: shedding of older leaves, diversion from vegetative to reproductive growth, accumulation of osmotically active solutes involved in the maintenance of cell turgor, and the synthesis of antioxidant proteins (Chaves, Maroco & Pereira 2003; Wilkinson, & Davies 2010). It is well known that mitochondrial CN‐resistant alternative pathway plays a pivotal role in plant environmental stresses resistance, as AOX located in the mitochondrial inner membrane and acts as a terminal oxidase in the plant mitochondrial electron transport chain; thus, it functions in the releasing of excess energy as heat (Millenaar and Lambers, 2003) or serve a general function by limiting mitochondrial ROS formation (Bartoli et al., 2005; Kühn et al., 2015; Wang et al., 2012). Studies also showed that ethylene could induce AOX capacity in response to abiotic stresses (Wang et al., 2010, 2012). 1‐(Aminocarbonyl)‐1‐cyclopropanecarboxylic acid (ACC) is the precursor of ethylene, which is catalysed by ACC oxidase (ACO). When ACC converted to ethylene,the coproduction of this reaction was cyanide (CN) which could trigger the generation of ROS or H2O2, thereby enhance alternative respiration and AOX capacity (Miyazaki and Yang, 1987; Siedow and Berthold, 1986). CN‐triggered ROS or H2O2 was the signal molecule which could activate the ROS‐responsive motifs in AOX1 promoter (Deng et al., 2015; Xu et al., 2012b). Therefore, the relative lower concentration of ACC could generate lower concentration of CN and H2O2 which promote AOX expression. AOX1a expression was increasing as the ACC concentration rising, but 10μM ACC showed similar effect to 1 μm ACC indicating that the AOX was sensitive to ACC which reached the maximum capacity when ACC concentration is 1 μm. It is well known that higher CN is extreme toxic to all living cells. Science ACC would induce CN production, as the above mentioned, it was not hard to imagine that higher concentration of ACC pretreatment decreased the plants drought tolerance. Accordingly, the dual role of ACC might be due to the amount of CN and H2O2 which produced with ethylene synthesis (Deng et al., 2015; Ho et al., 2008; Xu et al., 2012b). Based on the results presented in this study, we suppose that AOX is definitely participated in ethylene‐induced drought tolerance. Interestingly, we found that F2 progeny of AOX1a‐RNAi tomato seedlings exhibited more lateral shoots growth and less H2O2 accumulation than wild‐type tomato seedlings, whereas conversely in 35S‐AOX1a‐OE plants (Figure S3). As Chen et al. (2016) reported that H2O2 plays a critical role in axillary bud outgrowth in tomato plants, we speculated that the changing AOX capacity leads to altering H2O2 accumulation and thus results in the variation of lateral bud development. Surprisingly, the H2O2 content in AOX1a‐RNAi was relative lower than WT and AOX1a‐OE plants under normal growth condition. As previous studies have reported that the H2O2 concentration is governed by either synthesis or degradation, and knockdown of AOX could active multiple H2O2‐scavenging systems under normal growth conditions (Amirsadeghi et al., 2006; Giraud et al., 2008). In tobacco, this may overcompensate for the lack of AOX, in terms of H2O2 scavenging (Amirsadeghi et al., 2006; Cvetkovska et al., 2014). Conversely, overexpression of AOX in tobacco can suppress H2O2‐scavenging systems, resulting in elevated H2O2 (Pasqualini et al., 2007). So this trait of the AOX transgenic tomato is consistent with the previously researches. The results showed that SlAOX1a‐RNAi lines have suppressed SlAOX1b and SlAOX2 expressions (Figure 2c). It might due to the homologous sequence of the SlAOX1a, SlAOX1b and SlAOX2 cDNA fragment which we chose to generate a double‐stranded RNA interference (RNAi) trial to knock down the AOX capacity thereby to repress alternative respiration pathway. Furthermore, the SlAOX1a‐RNAi lines which we used may avoid the function redundancy of other SlAOX gene members. As ACC+SlAOX1a‐RNAi lines exhibited decreased drought tolerance whereas ACC+35S‐SlAOX1a‐OE lines showed enhanced drought tolerance compared with ACC+WT plants, it demonstrated that modification of SlAOX1a influenced AOX capacity thereby affected drought tolerance (Figure 3). Giraud et al., 2008 reported that AOX is necessary for drought response and the absence of AtAOX1a results in acute sensitivity to combined light and drought stress. Overexpression of AOX1a could enhance plant tolerance to aluminium phytotoxicity (Liu et al., 2014). A large number of studies indicated the indispensible role of AOX in plant drought tolerance (Bartoli et al., 2005; Dahal and Vanlerberghe, 2017; Dahal et al., 2014). So combined with these results, we can speculate the important role of AOX in plant response to drought. So we can conclude that the drought tolerance changes were caused by SlAOX1a together with its two homologs SlAOX1b and SlAOX2. Our results confirmed that ACC treatment could induce tomato resistance to drought, and AOX is involved in this progress. Furthermore, overexpressing of AOX1a alleviate drought‐induced H2O2 generation thereby amplified ACC‐induced drought tolerance (Figure 3f). Evidences showed that autophagy could regulate plant senescence or starvation‐induced chlorosis (Hanaoka et al., 2002; Liu et al., 2005). We found ACC pretreatment enhanced autophagy activity via up‐regulating the transcription level of ATG8d and ATG18h under drought condition whereas had little effect on autophagy under normal growth condition. Previous reports have indicated that ABA‐dependent pathway as well as ABA‐independent, but DREB2‐dependent pathway was essential in plants drought responses (Liu et al., 2009). Ethylene response factors (ERFs) have been shown to bind to DRE‐elements (ACCGAC) and to play a regulatory role in plant responses to abiotic stresses (Cheng et al., 2013). Therefore, several ERFs which respond to drought and ACC were examined. SlERF5, encoding a typical class III group of ERFs, is induced by abiotic stress (Figure S2). After analysing the promoters of each ATG genes, we found there was a conserved DRE‐binding sequence located in the promoters of SlATG8d and SlATG18h. Promoter analysis showed that these ATG genes might only be regulated by ethylene or ERF5 in drought condition. Thus, we speculate that ethylene pretreatment enhanced autophagy in drought probably due to the enhanced transcription activity of SlERF5. Therefore, the enhanced ERF5 induced the transcription of these two ATG genes to stimulate autophagy. Previous studies have demonstrated that autophagy is essential in leaf senescence (Hanaoka et al., 2002; Wada et al., 2009). Ethylene has been shown to be a regulator of leaf senescence in many plants (Grbić and Bleecker, 1995; John et al., 1995). However, there were few reports about ethylene and autophagy in tomato drought responses. These results showed that autophagy was not only enhanced by exogenous ethylene under drought condition but also played a critical role in ethylene‐induced tomato drought tolerance. ACC‐pretreated SlATG8d and SlATG18h knockdown plants exhibited impaired tolerance and significant cell death, accompanied by decreased photosynthetic efficiency and severe oxidative damage in tomato under drought condition. It means lacking of autophagy led to rapid cell death, indicating that autophagy plays an important role in ethylene‐induced drought resistance. To further explore the function of AOX and autophagy in ethylene‐induced drought tolerance, we knock down the SlATG8d in ACC‐pretreated WT and aox19 plants, both SlATG8d‐silenced aox19 plants exhibited serious wilting after 13d of drought stress. The ACC‐pretreated SlATG8d‐silenced aox19 plants showed the most compromised drought tolerance compared with ACC‐pretreated TRV control plants, not only reflected in the physiological parameter but also exhibited in the rapid death of elder leaves (Figure 7). These results indicating that AOX and autophagy are both necessary and play positive roles in tomato antagonizing drought. Studies have shown that AOX help to scavenge the redundant ROS in response to abiotic stress and plant senescence (Munné‐Bosch and Alegre, 2004; Xu et al., 2012b). There were other evidences demonstrating that autophagy played a critical role in plant drought tolerance and mitochondria generation of ROS is a trigger for autophagy (Chen and Gibson, 2008; Lee et al., 2012; Liu et al., 2009; Minibayeva et al., 2012). AOX protein level was obviously decreased when ATG8d and ATG18h were silenced whereas AOX1a transcription level has no changed (Figure 8g and h). Combined with the changing of AOX‐dependent H2O2 level and autophagic activity in ACC‐pretreated AOX transgenic plants under drought, these results means AOX and autophagy worked collaboratively in response to drought. To further determine the result, exogenous H2O2 and its scavenger DMTU were used to modify AOX‐dependent ROS signalling. As shown in Figure 10, ACC+H2O2 pretreatment in OE‐2 plants compromised the autophagy activity compared with ACC pretreatment in OE‐2 plants. However, ACC+DMTU pretreatment in aox19 plants exhibited increased autophagy activity. These results further confirmed that AOX‐dependent ROS signalling modified autophagy participated in ethylene‐induced drought tolerance.
In conclusion, it is not difficult to imagine that drought stress led to a gradual degradation of mitochondria and the AOX function as a ROS scavenger to avoid rapid O2 ‐ burst while the relative lower ROS level acts as a signal to trigger autophagy. Furthermore, the activated autophagy which may be also regulated by DRE‐binding elements (e.g. ERF5) delayed the processes of mitochondria collapse, thus maintaining the AOX integrity under drought condition. ERF5 might be a key regulator of autophagy which could be stimulated by ethylene under drought condition. The collaborative relationship between AOX and autophagy played critical roles in ethylene‐induced resistance to drought and led to significant survival of plants under stress conditions (Figure 11).
Figure 11.

Proposed model for ethylene‐mediated drought tolerance.
Experimental procedures
Plant materials and chemicals
The tomato Solanum lycopersicum cv. Micro Tom genotype was used in all experiments. Seeds were germinated and grown in plastic pots filled with a mixture of peat and vermiculite (2:1, v: v). The plants were grown in a controlled growth chamber at 25 °C under 16 h light/8 h darkness and watered daily. The seedlings used in the experiments were 4–5 weeks old.
To induce drought stress, plants in soil were watered to saturation with Hoagland solution or Hoagland plus 0–10 μm ACC and then exposed to dehydration by withholding water for 14 d.
ACC, AVG, H2O2 and DMTU were purchased from Sigma‐Aldrich (http://www.sigmaaldrich.com). The concentrations used are as follows: ACC, 0.1 μm; AVG, 50 μm; H2O2, 500 μm; DMTU, 500 μm. Distilled water containing 0.02% v/v Tween‐20 was used as a control treatment.
Generation and selection of transgenic plants
To generate the tomato overexpression SlAOX1a vector, the full‐length coding DNA sequence (CDS) of 1254 bp PCR product was cloned into the pBI121 vector (Clontech, Palo Alto, CA, USA). The homologous sequence of the SlAOX1a, SlAOX1b and SlAOX2 cDNA fragment was used for a double‐stranded RNA interference (RNAi) trial. The fragment and its inverted repeat fragment were inserted downstream of the CaMV 35S promoter at the BamHI and SacI restriction sites of the modified PBI121. The construct AOX1a‐RNAi was thus generated. The primers were shown in Table S1.
Transgenic plants were generated by Agrobacterium tumefaciens (strain EHA105)‐mediated transformation according to the method described previously (Xu et al., 2012b), and transformed lines were first selected for kanamycin (70 mg/L) resistance and then analysed by PCR to determine the presence of T‐DNA. The primers designed for NPTII (Kana resistance) were used as marker of PBI121 for identified of transgenic tomato (Table S1).
VIGS constructs and Agrobacterium‐mediated virus infection
TRV VIGS constructs were used to silence the tomato PDS, ATG8d and ATG18h genes. These genes were generated by PCR amplification using gene‐specific primers (Table S1), digested with the appropriate restriction enzymes and ligated into the same sites in TRV2. The constructs were transformed into Agrobacterium tumefaciens strain GV3101. The VIGS assay was performed as described previously (Zhu et al., 2016b).
Oxidative damage estimation
Leaf relative water content (RWC), electrolyte leakage (EL) and trypan blue staining were conducted as described (Zhu et al., 2016b).
Protein extraction and Western blotting analysis
Total proteins were extracted using extraction buffer (50 mm Tris/Cl, pH 6.8, 5% mercaptoethanol, 10% glycerol, 4% SDS and 4 m urea). For Western blotting analysis, approximately 10 μg of protein from each sample was subjected to SDS‐PAGE and transferred to nitrocellulose membranes. Then, the membranes were hybridized with antibody against the AOX protein (Agrisera, cat. No. AS04 054; 1:1000). Specific anti‐ATG8 (Abcam, cat. No. ab77003, 1:1000) was used in the protein blotting analysis. Anti‐β‐actin (Invitrogen cat. No. MA5‐15739, 1:10000) was used as loading control.
Respiration and chlorophyll fluorescence measurements
Respiratory rate and chlorophyll fluorescence were measured using Clark‐type electrodes (Hansatech, King's Lynn, UK) and an imaging pulse amplitude‐modulated fluorometer (IMAG‐MINI, Heinz Walz, Germany) according to Zhu et al. (2016a,b).
RNA extraction, RT‐PCR and quantitative real‐time PCR
Total RNA was purified from tomato leaves. RT‐PCR and real‐time PCR analyses were conducted as described (Zhu et al., 2016a,b). The primer sequences are shown in Table S1.
MDC staining
To visualize the accumulation of autophagosomes, tomato leaves were excised and then immediately vacuum infiltrated with 100 mm MDC (Sigma‐Aldrich) for 30 min, followed by two washes with PBS buffer (pH = 7.8) buffer. MDC‐incorporated structures were monitored under a Nikon Eclipse E600 epifluorescence microscope (Nikon, Tokyo, Japan), excited by a wavelength of 405 nm and detected at 400–580 nm.
Electron microscopy
Samples from differently treated leaves were fixed overnight at 4 °C in 3% glutaraldehyde and 0.1 m sodium cacodylate buffer (pH 6.9) and then processed for electron microscopy according to Xu et al. (2012a). Ultrathin sections, cut with an ultramicrotome (Ultracut, Reichert Jung), were observed with a transmission electron microscope (TEM 300, Itachi) operating at 75 kV.
Protein purification and EMSA assay
The tomato ERF5 recombinant protein was prepared according to Zhang et al. (2014a,b). Briefly, the full‐length ERF5 CDS PCR product was digested with BamHI and SalI and ligated into the same sites of pET‐32a vector. The recombinant vector was transformed into E. coli strain BL21 (DE3). Expression of the recombinant proteins was induced by isopropyl b‐D‐1‐thiogalactopyranoside and purified according to the instructions of the Novagen pET purification system. The probes were shown in Table S1. EMSA of the ERF5‐DNA complexes was performed according to the instructions of electrophoretic mobility shift assay (EMSA) Kit, with SYBR® Green & SYPRO® Ruby EMSA stains (Invitrogen).
Characterization of promoter activity
To determine the promoter activity, the 1200bp/1700bp fragments of ATG8d/ATG18h promoter region were amplified using specific primers and fused independently to the β‐d‐glucuronidase (GUS) or luciferase (LUC) reporter gene in the pBI121 vector. Additionally, the cauliflower mosaic virus (CaMV) 35S promoter was fused to GUS or LUC as a control for variation in transformation rate. All constructs were transformed into Agrobacterium strain EHA105. Transient expression was analysed in N. benthamiana leaves as described previously (Deng et al., 2015). After 12 h of transient transformation, the plants were treated with ACC. Three days after infiltration, GUS and LUC activity was determined as described previously (Deng et al., 2015). For drought treatment, the N. benthamiana plants were first treated with 0.1 μm ACC for 12 h, then the leaves were transiently transformed with different constructs, after another 12 h, they were treated with 16% PEG6000 for 60 h. GUS and LUC activity was determined as described previously (Deng et al., 2015).
H2O2 detection and Stomatal conductance
H2O2 fluorescence and content were conducted as described (Zhu et al., 2016a,b).
Stomatal conductance was detected according to Wang et al. (2015).
For NADPH oxidase activity analysis, leaf plasma membranes were isolated using a two‐phase aqueous polymer partition system. The NADPH‐dependent superoxide‐generating activity was determined as described previously (Deng et al., 2016).
Superoxide and H2O2 staining were visually detected with nitro blue tetrazolium (NBT) and 3, 3′‐diaminobenzidine (DAB) (Sigma‐Aldrich) according to Zhu et al. (2016a,b).
Enzyme activity assays
SOD CAT and APX activity was measured according to Zhu et al. (2016a,b). By monitoring the decrease in absorbance at 290 nm as ascorbate was oxidized.
Statistical analysis
Data from experiments with three or more mean values were statistically analysed using Duncan multiple range. The difference was considered to be statistically significant at P < 0.05.
Conflict of interests
The authors declare no conflict of interests.
Supporting information
Figure S1 Phenotype and stomatal closure in ACC pretreated tomatoes under drought stress.
Figure S2 ROS accumulation and respiration rate in transgenic lines.
Figure S3 Lateral buds length in transgenic lines.
Figure S4 SOD and CAT activity in transgenic tomatoes.
Figure S5 ACC‐induced autophagic activity and ERF5 transcription under drought stress.
Figure S6 Silence efficiency of ATG8d/ATG18h in WT plants.
Figure S7 Autophagic activity in ATG8d/ATG18h‐silenced plants.
Figure S8 Silence efficiency of ATG8d/ATG18h in aox19 plants.
Figure S9 Modified ROS signaling lead to different drought responses in ACC pre‐treated transgenic tomatoes.
Figure S10 Purified ERF5 protein for EMSA assay.
Table S1 Primers used in this assay.
Acknowledgements
The research is supported by the National Natural Science Foundation of China [31570237, 31670235 and 31400242]; the National Basic Research Program of China [973 Program (2015CB150100)]; the Development Project of Transgenic Crops of China (2016ZX08009‐003‐002); The Fundamental Research Funds for the Central Universities (2012017yjsy156).
Contributor Information
Dawei Zhang, Email: zhdawei@scu.edu.cn.
Honghui Lin, Email: hhlin@scu.edu.cn.
References
- Amirsadeghi, S. , Robson, C.A. , McDonald, A.E. and Vanlerberghe, G.C. (2006) Changes in plant mitochondrial electron transport alter cellular levels of reactive oxygen species and susceptibility to cell death signaling molecules. Plant Cell Physiol. 47, 1509–1519. [DOI] [PubMed] [Google Scholar]
- Bartoli, C.G. , Gomez, F. , Gergoff, G. , Guiamét, J.J. and Puntarulo, S. (2005) Up‐regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. J. Exp. Bot. 56, 1269–1276. [DOI] [PubMed] [Google Scholar]
- Bassham, D.C. (2007) Plant autophagy‐more than a starvation response. Curr. Opin. Plant Bio. 10, 587–593. [DOI] [PubMed] [Google Scholar]
- Chaves, M.M. , Maroco, J.P. , and Pereira, J.S. (2003) Understanding plant responses to drought‐ from genes to the whole plant. Functional Plant Biology 30, 239–264. [DOI] [PubMed] [Google Scholar]
- Chen, Y. and Gibson, S.B. (2008) Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy, 4, 246–248. [DOI] [PubMed] [Google Scholar]
- Chen, X.J. , Xia, X.J. , Guo, X. , Zhou, Y.H. , Shi, K. , Zhou, J. , Yu, J.Q. (2016) Apoplastic H2O2 plays a critical role in axillary bud outgrowth by altering auxin and cytokinin homeostasis in tomato plants. New Phytol. 211, 1266. [DOI] [PubMed] [Google Scholar]
- Cheng, M.C. , Liao, P.M. , Kuo, W.W. and Lin, T.P. (2013) The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress‐responsive gene expression by binding to different cis‐acting elements in response to different stress signals. Plant Physio. 162, 1566–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetkovska, M. , Dahal, K. , Alber, N.A. , Jin, C. , Cheung, M. and Vanlerberghe, G.C. (2014) Knockdown of mitochondrial alternative oxidase induces the ‘stress state’ of signaling molecule pools in Nicotiana tabacum, with implications for stomatal function. New Phytol. 203, 449–461. [DOI] [PubMed] [Google Scholar]
- Dahal, K. and Vanlerberghe, G.C. (2017) Alternative oxidase respiration maintains both mitochondrial and chloroplast function during drought. New Phytol. 213, 560–571. [DOI] [PubMed] [Google Scholar]
- Dahal, K. , Wang, J. , Martyn, G.D. , Rahimy, F. and Vanlerberghe, G.C. (2014) Mitochondrial alternative oxidase maintains respiration and preserves photosynthetic capacity during moderate drought in Nicotiana tabacum . Plant Physio. 166, 1560–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X.G. , Zhu, T. , Zhang, D.W. and Lin, H.H. (2015) The alternative respiratory pathway is involved in brassinosteroid‐induced environmental stress tolerance in Nicotiana benthamiana . J. Exp. Bot. 66, 6219–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X.G. , Zhu, T. , Zou, L.J. , Han, X.Y. , Zhou, X. , Xi, D.H. , Zhang, D.W. et al. (2016) Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid‐mediated systemic virus resistance in Nicotiana benthamiana . Plant J. 85, 478–493. [DOI] [PubMed] [Google Scholar]
- Desikan, R. , Last, K. , Harrett‐Williams, R. , Tagliavia, C. , Harter, K. , Hooley, R. , Hancock, J.T. et al. (2006) Ethylene‐induced stomatal closure in Arabidopsis occurs via AtrbohF‐mediated hydrogen peroxide synthesis. Plant J. 47, 907–916. [DOI] [PubMed] [Google Scholar]
- Du, H. , Wu, N. , Cui, F. , You, L. , Li, X. and Xiong, L. (2014) A homolog of ETHYLENE OVERPRODUCER, OsETOL1, differentially modulates drought and submergence tolerance in rice. Plant J. 78, 834–849. [DOI] [PubMed] [Google Scholar]
- Ederli, L. , Morettini, R. , Borgogni, A. , Wasternack, C. , Miersch, O. , Reale, L. , Ferranti, F. et al. (2006) Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone‐treated tobacco plants. Plant Physiol. 142, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie, D.R. , Geisler‐Lee, J. , Chen, J.F. and Jolley, B. (2009) Tissue specific expression of the ethylene biosynthetic machinery regulates root growth in maize. Plant Mol. Bio. 69, 195–211. [DOI] [PubMed] [Google Scholar]
- Giraud, E. , Ho, L.H.M. , Clifton, R. , Carroll, A. , Estavillo, G. , Tan, Y.‐F. , Howell, K.A. et al. (2008) The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 147, 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbić, V. and Bleecker, A.B. (1995) Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J. 8, 595–602. [Google Scholar]
- Hanaoka, H. , Noda, T. , Shirano, Y. , Kato, T. , Hayashi, H. , Shibata, D. , Tabata, S. et al. (2002) Leaf senescence and starvation‐induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol. 129, 1181–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, C. and Klionsky, D.J. (2009) Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, E. , Fung, N. , Liu, J. , Drakakaki, G. and Coaker, G. (2015) Beyond glycolysis: GAPDHs are multi‐functional enzymes involved in regulation of ROS, autophagy, and plant immune responses. PLoS Genet. 11, e1005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, L.H. , Giraud, E. , Uggalla, V. , Lister, R. , Clifton, R. , Glen, A. , Thirkettle‐Watts, D. et al. (2008) Identification of regulatory pathways controlling gene expression of stress‐responsive mitochondrial proteins in Arabidopsis. Plant Physiol. 147, 1858–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, I. , Drake, R. , Farrell, A. , Cooper, W. , Lee, P. , Horton, P. and Grierson, D. (1995) Delayed leaf senescence in ethylene‐deficient ACC‐oxidase antisense tomato plants: Molecular and physiological analysis. Plant J. 7, 483–490. [Google Scholar]
- Jones, A. (2000) Does the plant mitochondrion integrate cellular stress and regulate programmed cell death? Trends Plant Sci. 5, 225–230. [DOI] [PubMed] [Google Scholar]
- Kim, S.H. , Kwon, C. , Lee, J.H. and Chung, T. (2012) Genes for plant autophagy: Functions and interactions. Mol. Cells, 34, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn, K. , Yin, G. , Duncan, O. , Law, S.R. , Kubiszewski‐Jakubiak, S. , Kaur, P. , Meyer, E. et al. (2015) Decreasing electron flux through the cytochrome and/or alternative respiratory pathways triggers common and distinct cellular responses dependent on growth conditions. Plant Physiol. 167, 228–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, S.I. and Park, O.K. (2008) Autophagy in plants. J. Plant Bio. 51, 313–320. [Google Scholar]
- Lee, J. , Giordano, S. and Zhang, J. (2012) Autophagy, mitochondria and oxidative stress: Cross‐talk and redox signalling. Biochem. J. 441, 523–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Li, Z. , Wang, Y. and Xing, D. (2014) Overexpression of ALTERNATIVE OXIDASE1a alleviates mitochondria‐dependent programmed cell death induced by aluminium phytotoxicity in Arabidopsis. J. Exp. Bot. 65, 4465–4478. [DOI] [PubMed] [Google Scholar]
- Liu, Y. and Bassham, D.C. (2012) Autophagy: Pathways for self‐eating in plant cells. Annu. Rev. Plant Biol. 63, 215–237. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Schiff, M. , Czymmek, K. , Tallóczy, Z. , Levine, B. and Dinesh‐Kumar, S.P. (2005) Autophagy regulates programmed cell death during the plant innate immune response. Cell, 121, 567–577. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Xiong, Y. and Bassham, D.C. (2009) Autophagy is required for tolerance of drought and salt stress in plants. Autophagy, 5, 954–963. [DOI] [PubMed] [Google Scholar]
- Love, A.J. , Milner, J.J. and Sadanandom, A. (2008) Timing is everything: Regulatory overlap in plant cell death. Trends Plant Sci. 13, 589–595. [DOI] [PubMed] [Google Scholar]
- Lv, X. , Pu, X.J. , Qin, G.W. , Zhu, T. and Lin, H.H. (2014) The roles of autophagy in development and stress responses in Arabidopsis thaliana . Apoptosis, 19, 905–921. [DOI] [PubMed] [Google Scholar]
- Millenaar, F. and Lambers, H. (2003) The alternative oxidase: In vivo regulation and function. Plant Biology, 5, 2–15. [Google Scholar]
- Minibayeva, F. , Dmitrieva, S. , Ponomareva, A. and Ryabovol, V. (2012) Oxidative stress‐induced autophagy in plants: The role of mitochondria. Plant Physiol. Bioch. 59, 11–19. [DOI] [PubMed] [Google Scholar]
- Mittler, R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. [DOI] [PubMed] [Google Scholar]
- Miyazaki, J.H. and Yang, S.F. (1987) The methionine salvage pathway in relation to ethylene and polyamine biosynthesis. Physiol. Plantarum, 69, 366–370. [Google Scholar]
- Moore, A.L. and Albury, M.S. (2008) Further insights into the structure of the alternative oxidase: From plants to parasites. Biochem. Soc. T. 36, 1022–1026. [DOI] [PubMed] [Google Scholar]
- Morgan, P.W. and Drew, M.C. (1997) Ethylene and plant responses to stress. Physiol. Plantarum, 100, 620–630. [Google Scholar]
- Munné‐Bosch, S. and Alegre, L. (2004) Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 31, 203–216. [DOI] [PubMed] [Google Scholar]
- Okuda, M. , Nang, M.P.S.H. , Oshima, K. , Ishibashi, Y. , Zheng, S.H. , Yuasa, T. and Iwaya‐Inoue, M. (2011) The ethylene signal mediates induction of GmATG8i in soybean plants under starvation stress. Biosci. Biotech. Bioch. 75, 1408–1412. [DOI] [PubMed] [Google Scholar]
- Pan, Y. , Seymour, G.B. , Lu, C. , Hu, Z. , Chen, X. and Chen, G. (2012) An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep. 31, 349–360. [DOI] [PubMed] [Google Scholar]
- Panda, S.K. , Sahoo, L. , Katsuhara, M. and Matsumoto, H. (2013) Overexpression of alternative oxidase gene confers aluminum tolerance by altering the respiratory capacity and the response to oxidative stress in tobacco cells. Mol. Biotechnol. 54, 551–563. [DOI] [PubMed] [Google Scholar]
- Pasqualini, S. , Paolocci, F. , Borgogni, A. , Morettini, R. and Ederli, L. (2007) The overexpression of an alternative oxidase gene triggers ozone sensitivity in tobacco plants. Plant, Cell Environ. 30, 1545–1556. [DOI] [PubMed] [Google Scholar]
- Pérez‐Pérez, M.E. , Lemaire, S.D. and Crespo, J.L. (2012) Reactive oxygen species and autophagy in plants and algae. Plant Physiol. 160, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu, X. , Lv, X. , Tan, T. , Fu, F. , Qin, G. and Lin, H. (2015) Roles of mitochondrial energy dissipation systems in plant development and acclimation to stress. Ann. Bot. 116, 583–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, R. , Hu, S. , Zhang, Z. , Zhang, H. , Zhang, Z. and Huang, R. (2010) Overexpression of an ERF transcription factor TSRF1 improves rice drought tolerance. Plant Biotechnol. J. 8, 476–488. [DOI] [PubMed] [Google Scholar]
- Scherz‐Shouval, R. and Elazar, Z. (2007) ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 17, 422–427. [DOI] [PubMed] [Google Scholar]
- Sharp, R.E. and LeNoble, M.E. (2002) ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 53, 33–37. [PubMed] [Google Scholar]
- Shi, Y.H. , Zhu, S.W. , Mao, X.Z. , Feng, J.X. , Qin, Y.M. , Zhang, L. , Cheng, J. et al. (2006) Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. Plant Cell, 18, 651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Habben, J.E. , Archibald, R.L. , Drummond, B.J. , Chamberlin, M.A. , Williams, R.W. , Lafitte, H.R. et al. (2015) Overexpression of ARGOS genes modifies plant sensitivity to ethylene, leading to improved drought tolerance in both Arabidopsis and maize. Plant Physiol. 169, 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya, K. , Niki, T. and Ichimura, K. (2013) Pollination induces autophagy in petunia petals via ethylene. J. Exp. Bot. 64, 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedow, J.N. and Berthold, D.A. (1986) The alternative oxidase: A cyanide‐resistant respiratory pathway in higher plants. Physiol. Plantarum, 66, 569–573. [Google Scholar]
- Slavikova, S. , Ufaz, S. , Avin‐Wittenberg, T. , Levanony, H. and Galili, G. (2008) An autophagy‐associated Atg8 protein is involved in the responses of Arabidopsis seedlings to hormonal controls and abiotic stresses. J. Exp. Bot. 59, 4029–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, Y. , Sano, T. , Tamaoki, M. , Nakajima, N. , Kondo, N. and Hasezawa, S. (2005) Ethylene inhibits abscisic acid‐induced stomatal closure in Arabidopsis. Plant Physiol. 138, 2337–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe, G.C. , Cvetkovska, M. and Wang, J. (2009) Is the maintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase?. Physiol. Plant, 137, 392–406. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe, G.C. , Robson, C.A. and Yip, J.Y. (2002) Induction of mitochondrial alternative oxidase in response to a cell signal pathway down‐regulating the cytochrome pathway prevents programmed cell death. Plant Physiol. 129, 1829–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, S. , Ishida, H. , Izumi, M. , Yoshimoto, K. , Ohsumi, Y. , Mae, T. and Makino, A. (2009) Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol. 149, 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Liang, X. , Huang, J. , Zhang, D. , Lu, H. , Liu, Z. and Bi, Y. (2010) Involvement of ethylene and hydrogen peroxide in induction of alternative respiratory pathway in salt‐treated Arabidopsis calluses. Plant Cell Physiol. 51, 1754–1765. [DOI] [PubMed] [Google Scholar]
- Wang, H. , Huang, J. , Liang, X. and Bi, Y.R. (2012) Involvement of hydrogen peroxide, calcium, and ethylene in the induction of the alternative pathway in chilling‐stressed Arabidopsis callus. Planta, 235, 53–67. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Cai, S. , Yin, L. , Shi, K. , Xia, X. , Zhou, Y. , Yu, J. et al. (2015) Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy, 11, 2033–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, F. , Zhang, D.W. , Zhu, F. , Tang, H. , Lv, X. , Cheng, J. , Xie, H.F. et al. (2012a) A novel role for cyanide in the control of cucumber (Cucumis sativus L.) seedlings response to environmental stress. Plant, Cell Environ. 35, 1983–1997. [DOI] [PubMed] [Google Scholar]
- Xu, F. , Yuan, S. , Zhang, D.W. , Lv, X. and Lin, H.H. (2012b) The role of alternative oxidase in tomato fruit ripening and its regulatory interaction with ethylene. J. Exp. Bot. 63, 5705–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto, K. , Jikumaru, Y. , Kamiya, Y. , Kusano, M. , Consonni, C. , Panstruga, R. , Ohsumi, Y. et al. (2009) Autophagy negatively regulates cell death by controlling NPR1‐dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell, 21, 2914–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, S. and Davies, W. J. (2010) Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ, 33, 510–525. [DOI] [PubMed] [Google Scholar]
- Zhang, B. , Van Aken, O. , Thatcher, L. , De Clercq, I. , Duncan, O. , Law, S.R. , Murcha, M.W. et al. (2014a) The mitochondrial outer membrane AAA ATPase AtOM66 affects cell death and pathogen resistance in Arabidopsis thaliana. Plant J. 80, 709–727. [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Ye, H. , Guo, H. , Johnson, A. , Zhang, M. , Lin, H. and Yin, Y. (2014b) Transcription factor HAT1 is phosphorylated by BIN2 kinase and mediates brassinosteroid repressed gene expression in Arabidopsis. Plant J. 77, 59–70. [DOI] [PubMed] [Google Scholar]
- Zhu, T. , Deng, X.G. , Tan, W.R. , Zhou, X. , Luo, S.S. , Han, X.Y. , Zhang, D.W. et al. (2016a) Nitric oxide is involved in brassinosteroid‐induced alternative respiratory pathway in Nicotiana benthamiana seedlings’ response to salt stress. Physiol. Plantarum, 156, 150–163. [DOI] [PubMed] [Google Scholar]
- Zhu, T. , Deng, X. , Zhou, X. , Zhu, L. , Zou, L. , Li, P. , Zhang, D. et al. (2016b) Ethylene and hydrogen peroxide are involved in brassinosteroid‐induced salt tolerance in tomato. Sci. Rep. 6, 35392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Phenotype and stomatal closure in ACC pretreated tomatoes under drought stress.
Figure S2 ROS accumulation and respiration rate in transgenic lines.
Figure S3 Lateral buds length in transgenic lines.
Figure S4 SOD and CAT activity in transgenic tomatoes.
Figure S5 ACC‐induced autophagic activity and ERF5 transcription under drought stress.
Figure S6 Silence efficiency of ATG8d/ATG18h in WT plants.
Figure S7 Autophagic activity in ATG8d/ATG18h‐silenced plants.
Figure S8 Silence efficiency of ATG8d/ATG18h in aox19 plants.
Figure S9 Modified ROS signaling lead to different drought responses in ACC pre‐treated transgenic tomatoes.
Figure S10 Purified ERF5 protein for EMSA assay.
Table S1 Primers used in this assay.


