Abstract
Gene electrotransfer upregulate DNA Pattern Recognition Receptors (PRRs) or DNA sensors, which are part of the innate immune system. In this study, we tested if addition of the cocktail of innate immune system inhibitors to the cells during gene electrotransfer (GET) can increase transfection efficiency and cell survival. The results indicate that this cocktail can decrease cytosolic DNA sensors expression after GET, and consequently increase cell survival and transfection efficiency in B16 cells, but only in highly metastatic B16F10 subtype. We demonstrated that DNA sensors expression during the transfection methods, needs to be down regulated if higher transfection efficiency and better cells’ survival is needed. The inhibition of the receptors of the innate immune system can improve the transfection efficiency also for GET of malignant melanoma B16 cells, but only of highly metastatic subtype.
Keywords: Gene electrotransfer, innate immune system inhibitors, DNA sensors
Introduction
In recent years the importance of gene therapy in biomedical research and applications is increasingly growing (Naldini 2015). Efficient delivery of genetic material into the cells represents crucial step. It can be mediated by viral or non-viral delivery methods. While certain viral vector delivery can strongly activate innate immune system and is therefore questionable for in vivo use, non-viral delivery methods exert lower toxicity, are more stable and able to transfer larger size of nucleic acids. Nevertheless their clinical application is still limited by low transfection efficiency (Wang and Gao 2014).
Plasma membrane with its complex structure is the the first obstacle that must be overcome for successful transfection. The permeability of the cell membrane can be increased using either physical or chemical methods (Ibraheem et al. 2014). One of the commonly used physical method for in vitro and in vivo transfection of cells and tissues is electroporation (EP) that can be utilized also for gene electrotransfer (GET) (Yarmush et al. 2014). Electroporation utilizes transient increase in the permeability of cell membrane achieved by exposing the cells to short pulses of an intense electric field (Golzio et al. 2010). GET was introduced in the 1980s and is nowadays, due to its ease of application and efficiency, a routine method for introducing genes into prokaryotic and eukaryotic cell in vitro and into different tissues in vivo and has already reached clinical evaluation (https://clinicaltrials.gov) (Campana et al. 2016). While GET is very promising gene transfection method for in vivo application, for in vitro research applications lipofection is still the most commonly used nonviral transfection method that has not been fully translated to in vivo applications (Koynova and Tenchov 2011). Lipofection or liposome transfection is based on inclusion of the DNA in liposomes, i.e. vesicles made of a phospholipid bilayer that can merge with the cell membrane (Rao 2010).
Once the DNA is in the cell, the next obstacle for the successful transfection is detection of the foreign DNA through cytosolic DNA Pattern Recognition Receptors (PRRs) or DNA sensors, which are part of the innate immune system. The concept of cytosolic PRRs was originally elucidated in non-immune thyroid cells, but was recently demonstrated to be important also in tumor cells (Suzuki et al. 1999; Znidar et al. 2016). Foreign genetic material can activate the signal transduction cascade that ultimately triggers the production of type 1 interferons like interferon 1β (IFN 1β) (Dempsey and Bowie 2015). Among many other roles IFN 1β also inhibit cell growth and control apoptosis (Li et al. 2013). In vivo it can further activate immune system (Biron 1998).
Lipofection based K2® Transfection System (Biontex) can increases transfection efficiency by inhibiting several different factors involved in signalling cascade after DNA detection. It incorporates a cocktail of innate immune system inhibitors; antibodies and antagonists. In this study, we tested this cocktail in combination with GET with the aim to improve cell survival and transfection efficiency. We compared the transfection efficiency, cell survival and activation of selected cytosolic DNA sensors after K2® Transfection System, GET and GET combined with cocktail of innate immune system inhibitors in two melanoma cell lines, B16F1 and B16F10.
Materials and Methods
Cell lines and plasmid
Two subtypes of murine melanoma cell line; B16F1 with low- and B16F10 with high metastatic potential (American Type Culture Collection, Manassas, VA, USA) were cultured in advanced minimum essential medium (AMEM, Gibco, Life Technologies, Grand Island, NY, USA) supplemented with 5 % Fetal Bovine Serum (FBS, Life Technologies), 10 mM/l L-glutamine (Life Technologies), 100 U/ml penicillin (Grünenthal, Aachen, Germany) and 50 mg/ml gentamicin (Krka, Novo mesto, Slovenia) in a 5 % CO2 humidified incubator at 37°C.
Plasmid EGFP-N1 (pEGFP, BD Biosciences Clontech, Palo Alto, CA), encoding enhanced green fluorescent protein under the control of the CMV promoter, was used in the study. It was amplified in a competent Escherichia coli (TOP10; Life Technologies, Carlsbad, CA, USA) and then isolated and purified with JetStar Endotoxin-free Plasmid Purification Kit (Genomed, FL, USA) according to the manufacturer’s protocol. Quantity and quality of purified pEGFP were determined using spectrophotometer (Epoch Microplate spectrophotometer, Take3™ microvolume plate, Biotek, Bad Friedrichshall, Germany) and by agarose gel electrophoresis, respectively. Plasmid was diluted in endotoxin free water to concentration of 1 mg/ml.
Transfection protocols
Transfection with K2® Transfection System (K2)
One day before transfection with K2® Transfection System (Biontex Laboratories GmbH, Martinsried, Germany) cells were trypsinized and centrifuged (1500 rpm/470 g, 5 min) and 8 × 104 B16F1 and B16F10 cells were plated on 24-well plates (Corning Incorporated Corning, NY, USA) in order to reach 90 % confluence. Next day 9.5 μL of Multiplier® (cocktail of innate immune system inhibitors) were added into each well and incubated for 2 h at 37°C in a 5 % CO2. In the meantime, two separate solutions were prepared, first containing 28.5 μL serum free AMEM medium and 0.5 μg DNA, and the second containing 28.5 μL serum free medium and 2.28 μL K2® Transfection Reagent (Biontex), a cationic lipid. Solutions were mixed together and incubated for 15 min to form a lipoplex. Immediately after this incubation period, the mixture was added to the cells, which were then incubated without any further manipulations for further analyses.
Multiplier® contains (i) an antibody to TLR 1, TLR 2, TLR 3, TLR 4, TLR 5, TLR 6, TLR 7, TLR 8, TLR 9, TLR 10, TLR 11, TLR 12 or TLR 13, (ii) antibody to a cytokine receptor or a cytokine receptor antagonist, an inhibitor of kinase MEK1 and/or MEK2 (iii) an agonist for TLR7 and/or TLR8, selected from the group comprising bropirimine (2-amino-5-bromo-6-phenyl-4-pyrimidinone), imidazoquinolines, thiazoloquinolines and guanosine analogues. Its role is to partially suppress and/or activate the innate intracellular and/or intercellular immunity (US patent application, US20110045001A1) (Klosel and Konig 2011).
Transfection with GET
Monolayer of murine melanoma cell line was trypsinized, collected and washed with ice-cold electroporation buffer (EP buffer: 125 mM sucrose; 10 mM K2HPO4; 2.5mM KH2PO4; 2 mM MgCl2·6H2O) (Dolinsek et al. 2016). The pH of EP buffer was 7.2, osmolality 160 mOsm/kg and conductivity 2.1 mS/cm. Cell suspension for the electroporation was prepared in ice-cold EP buffer (25 × 106 cells/ml) and divided into aliquots of 44 μl for each parallel. To each aliquot 11 μl of plasmid pEGFP were added and 50 μl of the mixture (1 × 106 cells and 10 μg plasmid) were pipetted into 2 mm gap between two stainless-steel plate electrodes. Eight square wave electric pulses, with amplitude over distance ratio 600 V/cm, pulse duration 5 ms and frequency 1 Hz were generated by electric pulse generator GT-01 (Faculty of Electrical Engineering, University of Ljubljana, Slovenia). After GET cells were incubated at room temperature for 5 min in 100 μl of FBS and then plated on 24 well plate or 6 cm Petri dish plate for further assays.
Transfection with GET combined with Multiplier®
For studying the effect of Multiplier® (Biontex), a cocktail of innate immune system inhibitors combined with EP a minor modification of the GET method was performed. Before EP, 55 μl of the mixture (1,1 × 106 cells and 11 μg plasmid) was first incubated with 10 μL of Multiplier® in ultra-low attachment 24 well plates (Corning) for 2 hours at 37°C in a 5 % CO2. Then, 60 μl of the mixture (1 × 106 cells, 10 μg of plasmid and 10 μg of Multiplier®) was exposed to electric pulses and incubated as described above.
Cell survival assay
After GET, 1.5 × 103 cells were plated in 0.1 ml of AMEM in 96-well plates (Corning) and incubated at 37°C in a 5 % CO2 in humidified incubator. K2 transfected cells were already plated on 24 well plates. Cell survival was measured 24 h after the treatment by Presto Blue viability assay (Life Technologies). Presto Blue (10 μl/well for 96 well and 20 μl/well for 24 well plate) was added to the cells and 30 min thereafter the fluorescence intensity (excitation 560 nm and emission 590 nm) was measured by microplate reader (Genios, Tecan, Männedorf, Switzerland). Viability of the cells in each experimental group was expressed as a percentage of cells’ viability normalized to control untreated group, for each transfection procedure respectively.
Transfection efficiency
One day after transfection procedure the transfection efficiency of each transfection protocol was determine by flow cytometry. The samples were trypsinized and resuspended in 400 μl of phosphate buffered saline (PBS, Gibco) for flow cytometry analysis. The measurements were performed with FACSCanto II flow cytometer (BD Biosciences, San Jose, CA), where a 488-nm laser (air-cooled, 20 mW solid state) and 530/30-nm band-pass filter were used for the excitation and detection of enhanced green fluorescent protein fluorescence, respectively. Cells were first gated to eliminate debris and histogram of gated cells against their fluorescence intensity was recorded (software: BD FACSDiva V6.1.2). The fraction of transfected cells was multiplied by number of viable cells only (cytotoxicity data) to get the absolute number of transfected cells for each experimental group.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA extraction and qRT-PCR analysis were performed 4 hours after transfection to determine activation of cytosolic DNA sensors DDX 60, DAI and P204 and cytokine IFN 1β. Cells were trypsinized and centrifuged then total RNA was extracted with the TRIzol Plus RNA Purification System (Life Technologies) according to the manufacturer’s instructions. Concentrations and purity of RNA were determined spectrophotometrically (Epoch). 250 ng of total RNA was transcribed into cDNA using the SuperScript VILO™ cDNA Synthesis Kit (Life Technologies), according to the manufacturer’s instructions. qRT-PCR was performed with 10 fold diluted mixtures of transcribed cDNA using SYBR Green Master Mix (Applied Biosystems, Thermo Fisher Scientific Waltham, MA, USA) and custom primers (Integrated DNA technologies, Coralville, IA, USA) designed to amplify the fragment of murine DDX 60 (forward (F): ACTGGAACACTCGCTTTGG and reverse (R): GAAGTAGACATCACCCAACAGG), DAI (F: TGCTTTCTAGAGGACGCCACCATT and R: TGGCTTCAGAGCTTGTACCTGTGT), p204 (F: CCAGTCACCAATACTCCACAG and R: GAGCACCATCACTGTCAGG) and IFN 1β (F: TGGCCATCCAAGAGATGCTCCAGA and R: AGAAACACTGTCTGCTGGTGGAGT) as target gene or murine β-actin (F: GAAGTGTGACGTTGACATCC and R: ACTCATCGTACTCCTGCTTG) and GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) (F: TTCACCACCATGGAGAAGGC and R: GGCATGGACTGTGGTCATGA) as an reference genes. qPCR was performed on Quant Studio 3 thermocycler (Applied Biosystems). The thermal cycler protocol consisted of activation of Uracil-DNA Glycosylase (2 min at 50°C), hot start activation of AmpliTaq Gold Enzyme (10 min at 95°C), 40 cycles of denaturation (15 s at 95°C), annealing and extension (1 min 60°C). Relative quantification of the qPCR data was performed by the 2−ΔΔCt method (Livak and Schmittgen 2001).
Statistical analysis
For statistical analysis Sigma Plot software (Systat software, London, United Kingdom) was used. Significance was determined by Student t-test or one-way analysis of variance (ANOVA) followed by Holm-Sidak test. Statistical significance was assumed at P < 0.05. The values were expressed as arithmetic mean (AM) ± standard error of the mean (SEM).
Results
Cell survival and transfection efficiency
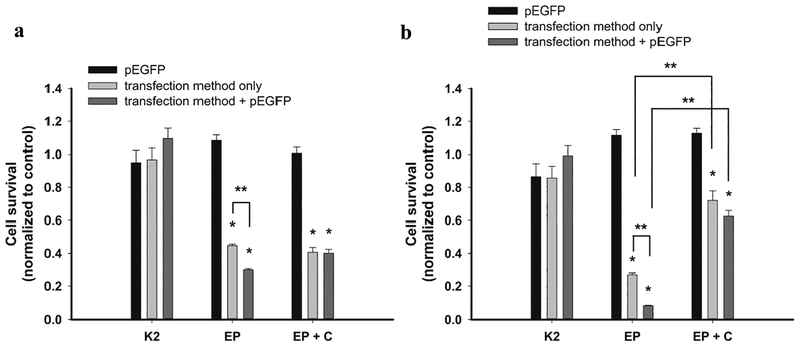
First, we studied the effect of both transfection methods, K2 and GET on cell survival. In order to see if addition of cocktail of innate immune system inhibitors affects cell survival we tested it in combination with GET.
Cell survival was not statistically affected when using K2® Transfection System alone or in combination with pEGFP in both tested cell lines, B16F1 and B16F10. In contrast, EP resulted in significant reduction of cell survival already after exposure of cells to EP alone and was even more pronounced after GET of pEGFP into cells (Figure 1). When cells were exposed to electric pulses in presence of cocktail of innate immune system inhibitors without addition of pEGFP, cell survival was statistically significantly increased in B16F10 cells, while in B16F1 it stayed in the same level as after GET. The same was observed also after GET of pEGFP (Figure 1) combined with the cocktail. These data indicate on protective effect of innate immune system inhibitors on cells survival after EP and GET, but only in B16F10 cells.
Fig. 1. Survival of B16F1 (a) and B16F10 cells (b) after K2 transfection method (K2), electroporation (EP) and electroporation in combination with the cocktail (EP+C).
*statistically significant difference compared to control groups (p<0.05), **statistically significant difference between two marked groups (p<0.05).
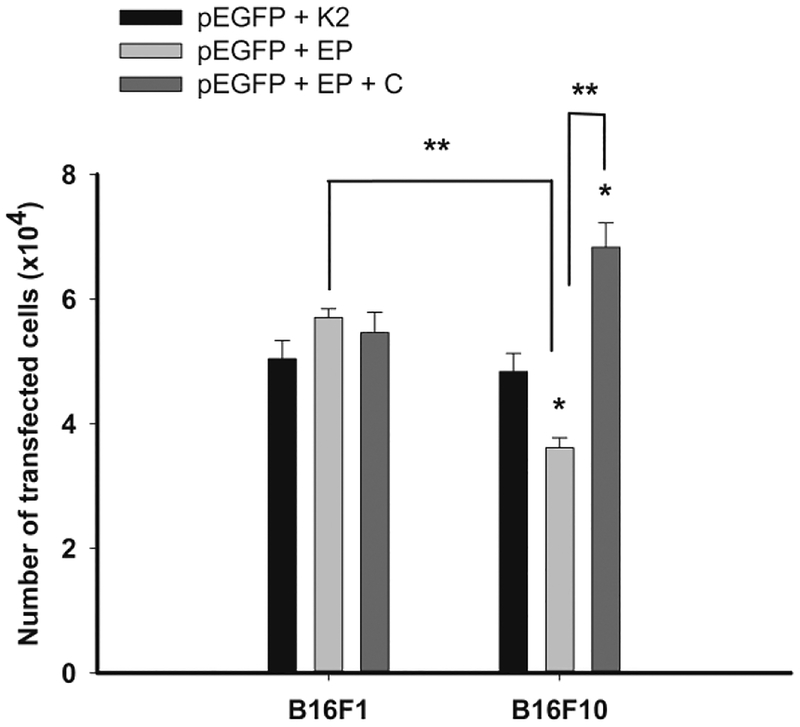
Next, we compared the transfection efficiency of the tested transfection methods, K2 and GET. The number of the transfected cells was equal by K2® Transfection System in B16F1 and B16F10 cells, whereas the GET transfection method was B16 cells’ subtype dependent, being more effective in B16F1 than B16F10 melanoma cells (p<0.05). GET of pEGFP in combination with the cocktail did not increase the number of transfected B16F1cells, however it significantly increased the number of B16F10 transfected cells (Figure 2).
Fig. 2. Transfection efficiency of plasmid pEGF transfected with K2 transfection method (K2), electroporation (EP) and electroporation in combination with the cocktail (EP+C) in B16F1 and B16F10 cells.
*statistically significant difference compared to K2 (p<0.05); **statistically significant difference between two marked groups (p<0.05).
Different transfection methods increased expression of cytosolic DNA sensors mRNA to the different level
In B16F10 cells, three specific DNA sensors have been previously observed to be upregulated after GET of non-therapeutic plasmids: DDX60, DAI and p204 (10). Therefore, after transfection with both transfection methods the expression of mRNA for those three DNA sensors and the downstream IFN-1β was determined.
In both cell lines, we confirmed the presence of these DNA sensors. After transfection with K2® the mRNA expression of cytosolic sensors and IFN-1β remain unchanged or were even lower than in control cells (Table 1). On the contrary, GET significantly increased the expression of all three tested sensors and IFN-1β (Table 1). GET of both B16 cell lines in the presence of cocktail of innate immune system inhibitors, reduced the expression of cytosolic DNA sensors and IFN-1β compared to GET only (Table 1). The addition of cocktail reduced the expression of the cytosolic DNA sensors and IFN-1β to the level in the untreated control cells.
Table 1.
Expression of cytosolic DNA sensors mRNA after transfection of B16 cells with pEGFP with two different transfection methods; K2® Transfection System (K2) and electroporation (EP). Combination of EP with the cocktail was also tested.
| B16F1 | B16F10 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | DDX60 | DAI | p204 | IFN 1β | N | DDX60 | DAI | p204 | IFN 1β | |
| CONTROL | 4 | 1.33±0.44 | 1.13±0.24 | 1.11±0.21 | 2.22±1.05 | 4 | 1.00±0.04 | 1.00±0.01 | 1.03±0.16 | 1.04±0.20 |
| pEGFP+K2 | 5 | 0.5±0.12 | 0.89±0.24 | 0.18±0.04* | 0.75±0.23 | 6 | 0.50±0.08* | 1.60±0.59 | 0.61±0.09* | 2.20±1.18 |
| CONTROL | 4 | 1.02±0.12 | 1.00±0.04 | 1.02±0.11 | 1.01±0.07 | 4 | 1.01±0.01 | 1.03±0.14 | 1.09±0.26 | 1.37±0.59 |
| pEGFP+EP | 5 | 15.63±3.43* | 42.77±11.08* | 45.79±12.20* | 15.30±7.24* | 5 | 4.08±0.55* | 5.83±0.92* | 3.80±1.07* | 15.30±7.29* |
| pEGFP+EP+cocktail | 6 | 3.42±1.20 | 2.17±0.47 | 3.03±0.31 | 7.22±3.22 | 6 | 0.75±0.43 | 0.69±0.15 | 0.89±0.12 | 0.97±0.49 |
Control, untreated cells; pEGFP+K2, pEGFP combined with K2 transfection system; pEGFP+EP, pEGFP combined with electroporation (EP); DAI, DNA-dependent activator of interferon regulator factor (Takaoka et al. 2007); DDX60, DEAD (Asp-Glu-Ala-Asp) box polypeptide 60 (Miyashita et al. 2011); p204 (Unterholzner et al. 2010); IFN 1β, Type I interferon; Arithmetic Mean±SEM,
statistically significant difference compared to control groups (p<0.05), N=number of samples in two independent experiment.
Discussion
In this study, we tested the cocktail of innate immune system inhibitors, for the purpose of increasing transfection efficiency of GET and cell survival after it. The results indicate that this cocktail can increase cell survival and transfection efficiency in B16 cells, however it’s action is selective, because the increased was obtained only in high metastatic malignant melanoma cells B16F10.
Two subtypes of B16 cell line were used in the experiments, B16F1 cells with low metastatic potential and B16F10 cells with high metastatic potential. The transfection efficiency of K2® Transfection System was very comparable between the two subtypes of cells. K2® Transfection System has already been tested and proven to be effective in several different cell lines (Carozzo et al. 2015; Chen and Foldvari 2016). In this study we confirmed its effectiveness also in melanoma B16 cells. Compared to transfection efficiency of GET, the transfection efficiency after K2 was similar in B16F1 cells and higher in B16F10 cells. The survival of both subtypes of cells after K2 was also less reduced than after GET, confirming the suitability of liposome based transfection methods for the in vitro use, if high transfection efficiency and high survival of the cells is needed (Felgner et al. 1987; Nguyen et al. 2007).
The addition of cocktail of innate immune system inhibitors to GET had a beneficial effect on transfection efficiency, correlating with the improved cell survival that was especially evident in B16F10 cell line. Interestingly, in B16F10 cells, the cocktail prevented the electrically-mediated cell death. The beneficial effect of the cocktail after EP is probably due to the broad spectrum of inhibitors (antibodies and antagonists) which are present in it. Namely, response to foreign DNA shares multiple pathways with response to different stress signals that are produced during the transfection procedure; for instance osmotic stress, oxidative stress, ROS, which were all previously documented after EP (Markelc et al. 2012). Therefore, although the effectiveness of innate immune system inhibitors is primarily based on the suppression of DNA recognition, it can also reduce the responses triggered by the transfection procedure itself.
Next, we tested the expression of selected cytosolic DNA sensors after both transfection methods. The expression of cytosolic DNA sensors after gene transfection was initially described after lipid transfection reagent (Takaoka et al. 2007; Unterholzner et al. 2010; Kondo et al. 2013; Sun et al. 2013). Our most recent research demonstrated that specific DNA sensors, namely DDX, DAI and p204, are up-regulated after GET in B16F10 melanoma cells (Znidar et al. 2016). Also, in the current study, all three sensors were statistically significantly increased after GET in both subtypes of B16 cells. This not only confirmed our previous results but also demonstrated that increased expression of DNA sensors is independent of the plasmid DNA sequence. Namely, in the previous study, non-coding plasmid DNA was used, while in the current study we used plasmid encoding EGFP (Znidar et al. 2016). In both studies, we demonstrated increased expression of IFN-1β, however, it is known that activation of DAI and p204 lead to translocation of NF-κB to nucleus, where genes for proinflammatory cytokines are transcribed. Thus, the type I IFNs as well as proinflammatory cytokines pathways are induced by cytosolic DNA sensors (Desmet and Ishii 2012).
After K2 transfection system, on the other hand, the expression of DNA sensors and IFN-1β was not induced. These results confirmed the efficiency of the cocktail to inhibit foreign DNA sensing. The expression of DNA sensors was also inhibited when we combined the cocktail with GET. GET upregulated the expression of sensors in both B16 cell lines, but in B16F1 cell line the levels of mRNAs were higher. After addition of the cocktail, the expression of DNA sensor was lower in both cell lines, although still detectable in B16F1 cells. Thus, in terms of cell survival, highly metastatic B16F10 cells seems to be more sensitive to foreign plasmid DNA than low metastatic B16F1 cells. This could be explained by the fact that B16F10 cells are genetically less stable than B16F1 and also express an unstable metastatic phenotype (Dick and Ling 1987). The survival of B16F10 cells after GET was increased for more than 6-fold after addition of cocktail to cell suspension, while in B16F1 it was increased for only 1.3-fold. Therefore, the addition of the cocktail had the protective effect on the viability of B16F10 cells. One of the reason for this observation in B16F10 can be stronger inhibition of IFN-1β mRNA expression after combining GET with the cocktail compared to B16F1 cells (~15-fold and 2-fold, respectively, Table 1). Namely, type I IFNs are cytokines that lead to induction of cell death (Parker et al. 2016). Nevertheless, the expression of proinflammatory cytokines, also inducing cell death, although not measured in the present study, could contribute to the unchanged cell survival of B16F1 cells after GET in combination with the cocktail. We can presume this, because the levels of mRNA of DNA sensors DAI and p204 were still increased in B16F1 cells, while completely abrogated in B16F10.
Therefore, the activation of immune system can be beneficial in some applications of gene transfer and agonists of DNA sensing can even be added to heighten the immune response for instance for the purpose of vaccination (Herrada et al. 2012). But when the aim of gene transfer is to obtain high and durable expression of the therapeutic gene, with minimal cell death, such as in the case of treatment of monogenic diseases, addition of the antagonists of DNA sensing is appropriate.
In conclusion, this study shows the expression of DNA sensors during the transfection methods, needs to be reduced, if higher transfection efficiency and better cells’ survival is needed. The cocktail of innate immune system inhibitors can improve also the transfection efficiency for GET of malignant melanoma cells, however it is cell type specific and thus should be evaluated for its positive effect prior to the use in experiments with therapeutic plasmid DNAs.
Acknowledgements
The authors acknowledge the financial support from the state budget by the Slovenian Research Agency (program no. P3–0003). The research was conducted in the scope of LEA EBAM (French-Slovenian European Associated Laboratory: Pulsed Electric Fields Applications in Biology and Medicine) and is a result of networking efforts within COST TD1104 Action. We would like to thank Mira Lavric (Institute of Oncology Ljubljana, Ljubljana, Slovenia) for all the valuable work she contributed to this research.
References
- Biron CA (1998) Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin Immunol 10:383–390. doi: papers3://publication/doi/10.1006/smim.1998.0138 [DOI] [PubMed] [Google Scholar]
- Campana LG, Clover AJP, Valpione S, et al. (2016) Recommendations for improving the quality of reporting clinical electrochemotherapy studies based on qualitative systematic review. Radiol. Oncol 50:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carozzo A, Diez F, Gomez N, et al. (2015) Dual role of cAMP in the transcriptional regulation of Multidrug Resistance-Associated Protein 4 (MRP4) in pancreatic adenocarcinoma cell lines. PLoS One. doi: 10.1371/journal.pone.0120651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DW, Foldvari M (2016) In vitro bioassay model for screening non-viral neurotrophic factor gene delivery systems for glaucoma treatment. Drug Deliv Transl Res. doi: 10.1007/s13346-016-0324-9 [DOI] [PubMed] [Google Scholar]
- Dempsey A, Bowie AG (2015) Innate immune recognition of DNA: A recent history. Virology 479–480:146–152. doi: 10.1016/j.virol.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet CJ, Ishii KJ (2012) Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol 12:479–491. doi: 10.1038/nri3247 [DOI] [PubMed] [Google Scholar]
- Dick JE, Ling V (1987) Generation of Drug-resistant Variants in Metastatic B16 Mouse Melanoma Cell Lines. Cancer Res 47:2604–2608. [PubMed] [Google Scholar]
- Dolinsek T, Prosen L, Cemazar M, et al. (2016) Electrochemotherapy with bleomycin is effective in BRAF mutated melanoma cells and interacts with BRAF inhibitors. Radiol Oncol 50:274–279. doi: 10.1515/raon-2016-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner PL, Gadek TR, Holm M, et al. (1987) Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A 84:7413–7. doi: 10.1073/pnas.84.21.7413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio M, Escoffre J-M, Portet T, et al. (2010) Observations of the mechanisms of electromediated DNA uptake--from vesicles to tissues. Curr Gene Ther 10:256–66. doi: 10.2174/156652310791823461 [DOI] [PubMed] [Google Scholar]
- Herrada AA, Rojas-Colonelli N, González-Figueroa P, et al. (2012) Harnessing DNA-induced immune responses for improving cancer vaccines. Hum. Vaccines Immunother. 8:1682–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraheem D, Elaissari A, Fessi H (2014) Gene therapy and DNA delivery systems. Int. J. Pharm 459:70–83. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kobayashi J, Saitoh T, et al. (2013) DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci U S A 110:2969–74. doi: 10.1073/pnas.1222694110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koynova R, Tenchov B (2011) Recent patents in cationic lipid carriers for delivery of nucleic acids. Recent Pat DNA Gene Seq 5:8–27. doi: 10.2174/187221511794839255 [DOI] [PubMed] [Google Scholar]
- Li Y, Zhu H, Zeng X, et al. (2013) Suppression of autophagy enhanced growth inhibition and apoptosis of interferon-beta in human glioma cells. Mol Neurobiol 47:1000–1010. doi: 10.1007/s12035-013-8403-0 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Markelc B, Tevz G, Cemazar M, et al. (2012) Muscle gene electrotransfer is increased by the antioxidant tempol in mice. Gene Ther 19:312–320. doi: 10.1038/gt.2011.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L (2015) Gene therapy returns to centre stage. Nature 526:351–360. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Atobe K, Barichello JM, et al. (2007) Complex formation with plasmid DNA increases the cytotoxicity of cationic liposomes. Biol Pharm Bull 30:751–757. doi: 10.1248/bpb.30.751 [DOI] [PubMed] [Google Scholar]
- Parker BS, Rautela J, Hertzog PJ (2016) Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer 16:131–144. doi: 10.1038/nrc.2016.14 [DOI] [PubMed] [Google Scholar]
- Rao NM (2010) Cationic lipid-mediated nucleic acid delivery: beyond being cationic. Chem. Phys. Lipids 163:245–252. [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, et al. (2013) Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science (80-) 339:786–791. doi: 10.1126/science.1229963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Mori a, Ishii KJ, et al. (1999) Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc Natl Acad Sci U S A 96:2285–2290. doi: 10.1073/pnas.96.5.2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Wang Z, Choi MK, et al. (2007) DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501–505. doi: 10.1038/nature06013 [DOI] [PubMed] [Google Scholar]
- Unterholzner L, Keating SE, Baran M, et al. (2010) IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11:997–1004. doi: 10.1038/ni.1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Gao G (2014) State-of-the-art human gene therapy: part I. Gene delivery technologies. Discov Med 18:67–77. [PMC free article] [PubMed] [Google Scholar]
- Yarmush ML, Golberg A, Serša G, et al. (2014) Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu Rev Biomed Eng 16:295–320. doi: 10.1146/annurev-bioeng-071813-104622 [DOI] [PubMed] [Google Scholar]
- Znidar K, Bosnjak M, Cemazar M, Heller LC (2016) Cytosolic DNA Sensor Upregulation Accompanies DNA Electrotransfer in B16.F10 Melanoma Cells. Mol Ther Acids 5:e322. doi: 10.1038/mtna.2016.34 [DOI] [PMC free article] [PubMed] [Google Scholar]