Abstract
Background
Type 1 diabetes is associated with significant mortality and economic cost. Management of type 1 diabetes involves completing multiple daily adherence behaviors, and many adolescents struggle with self-management and show poor glycemic control.
Purpose
The purpose was to conduct an unblinded pilot randomized controlled parallel-group study of a web-delivered multicomponent intervention targeting self-monitoring of blood glucose, working memory, and parent supervision of diabetes care among adolescents with type 1 diabetes. Intervention components included high magnitude incentives for adolescents and parents, motivational and cognitive behavioral therapy and working memory training for adolescents, and training in contingency contracting for parents.
Methods
Adolescents (N = 114) with poorly controlled type 1 diabetes were screened, and N = 61 were randomized using minimum likelihood allocation to usual care (usual care, N = 31) or to a 25-week/15-session web-delivered intervention (WebRx, N = 30).
Results
At the end of treatment, adolescents in WebRx had higher self-monitoring of blood glucose (d = 0.58) (primary outcome), better visual spatial working memory (d = 0.48) and inhibition (d = 0.98), and lower HbA1c (d = 0.45) than those in usual care. WebRx parents reported more frequent review of the adolescent’s glucometer (d = 1.30) and reduced family conflict (d = 0.56). Between-condition differences were maintained 6 months later in self-monitoring of blood glucose (d = 0.42), visual spatial working memory (d = 0.76), family conflict (d = 0.50), and HbA1c (d = 0.44).
Conclusions
Results showing sustained effects on self-monitoring of blood glucose and HbA1c support moving forward with a larger trial to test this innovative web-delivered and multicomponent intervention.
ClinicalTrials.gov Number (NCT01722643)
Keywords: Type 1 diabetes, Behavioral intervention, Adolescents, Working memory
Teens with type 1 diabetes showed sustained improved glycemic control compared to usual care following a web-delivered intervention that included incentives for blood glucose checks, working memory training, and counseling for teens and parents.
Introduction
Type 1 diabetes is associated with significant morbidity, mortality, and economic cost [1]. Unfortunately, adolescents have much poorer outcomes than adults, with nearly 77% of adolescents with type 1 diabetes failing to meet current recommendations for glycemic control [2]. Management of type 1 diabetes involves the completion of multiple daily adherence behaviors that may be complex and often disruptive to daily life (e.g., blood glucose checking at least four times per day, correctly calculating and administering insulin doses). Key predictors of poor glycemic control (elevated glycated hemoglobin, HbA1c) among adolescents include less frequent self-monitoring of blood glucose [3] as well as other factors affecting diabetes self-management behaviors. These factors include parental supervision and high levels of family conflict, as well as problems in cognitive functioning [4–6]. Interventions to improve outcomes among adolescents with type 1 diabetes have included coping skills, motivational, cognitive behavioral, and family systems components and have typically shown only small-to-moderate improvements in adherence behaviors and HbA1c, and effects generally do not persist over time [7].
One novel intervention that has been explored to improve self-monitoring of blood glucose adherence is the use of incentives to increase glucose checks among adolescents with type 1 diabetes [8, 9]. These studies chose self-monitoring of blood glucose as the target behavior because of its strong predictive relations with HbA1c, the limited success of prior interventions in improving glucose checking, and the ability to objectively monitor glucose checking remotely using existing technology used by all patients with type 1 diabetes (electronic glucometers). These studies used incentives to target self-monitoring of blood glucose as the only intervention among samples restricted to youth with low levels of self-monitoring of blood glucose at baseline (<4 times per day on average). They showed significant pre–post positive effects of incentives on the frequency of glucose checks [8, 9]. The randomized study (N = 41) provided incentives for a 20-day period, with maximum earnings of $11 per day ($220 total) [8]. Mean earnings were $65 per participant. The non-randomized pilot study (N = 10) provided incentives for a 12-week period, with maximum earnings of ~$250 [9]. Mean earnings were $122 per participant. These studies suggest that using incentives to target glucose checking frequency among adolescents with type 1 diabetes holds promise; however studies with larger sample sizes and adequate follow-up periods testing effects on HbA1c are needed.
In addition, working memory is an executive function that involves goal-oriented active monitoring or manipulation of information [10]. Working memory may be important for adolescent type 1 diabetes outcomes based on studies showing that executive skills including working memory are significantly related to adherence and glycemic control [11, 12]. Working memory training, which aims to improve executive function by strengthening working memory neurocognitive processes through practice, was also included. Commercially available computerized working memory training programs can reliably enhance cognitive function in diverse populations, including adolescents [13–15]. Of note, studies of working memory training typically include incentives for completing the training. Although not without controversy and limitations [16], working memory training reliably improves working memory performance and may generalize to enhance performance on other cognitive tasks that have not been trained, including those measuring inhibitory control (e.g., Stroop tasks) [17, 18]. Therefore, working memory training may be an effective adjunct to evidence-based interventions to improve type 1 diabetes outcomes.
Some recent studies among adolescents with type 1 diabetes have focused on web-based approaches to promote self-management [19–21]. Such approaches are critical because many families must travel long distances to receive care at regional pediatric endocrinology clinics. For example, about one third of U.S. children do not have access to an endocrinologist within a 20-mile radius, and in rural areas, about one third have no endocrinologist within a 50-mile radius [22]. Evidence-based approaches targeting the adolescent [23] and family [24] have been implemented on the web, reporting similar effect sizes compared with face-to-face formats. These results suggest that web-based approaches may enhance dissemination without sacrificing efficacy.
To improve clinical outcomes among adolescents with type 1 diabetes, we developed a web-delivered intervention that targeted multiple predictors of poor outcomes (self-monitoring of blood glucose, parent monitoring, and executive function). Intervention components included (a) individual counseling that combined motivational enhancement therapy with cognitive behavioral therapy, two evidence-based approaches to improve outcomes among teens with type 1 diabetes [23, 25]; (b) high magnitude incentives for teens to increase and maintain self-monitoring of blood glucose; (c) high magnitude incentives for parents to increase parental monitoring of adolescents’ glucose checking behaviors, plus instruction for parents in how to use contingency contracting, a common approach to a wide range of child and adolescent behaviors in family behavioral therapy that has been used to improve adherence among adolescents with diabetes [26]; and (d) a working memory training program for adolescents (Cogmed-RM, Pearson, Inc), a commercially available program.
The rationale for selecting these components was as follows. Other studies have combined individual motivational enhancement therapy/cognitive behavioral therapy with family-based interventions for adolescents with type 1 diabetes but have shown limited sustained efficacy [27]. To improve outcomes, we added additional components. Incentives have the advantage of potentially increasing efficacy in both the short and long term without increasing patient burden (although they do increase intervention costs) [28]. We chose to test high magnitude incentives in this pilot study to ensure that a lack of effect would not be due to inadequate incentives. Working memory training has not been previously integrated with other psychosocial interventions. However, we would not expect that working memory training would be effective in the absence of an evidence-based intervention focused on behavior change.
This intervention was tested in a pilot parallel-group randomized controlled trial. Participants were randomized to usual care or to a web-delivered intervention (WebRx). The intervention comprised an active treatment phase when behavioral economic incentives and therapy were delivered weekly for 11 weeks, followed by a 14-week maintenance treatment phase during which the time between sessions and incentive payments was lengthened. Two prior uncontrolled pilot studies were conducted in developing this intervention model [29, 30]. The initial 14-week pilot study included all components except working memory training and was delivered through face-to-face sessions. The second 25-week pilot study was web-delivered and included all components. These pilot studies indicated improvement in self-monitoring of blood glucose, parenting, cognitive function, and HbA1c.
For this study, we hypothesized that WebRx would show better outcomes than usual care on the following outcomes directly targeted by intervention components: adolescent self-monitoring of blood glucose frequency (primary outcome), frequency of parent review of the adolescent’s glucometer, and adolescent working memory. We further hypothesized greater improvement in glycemic control (HbA1c) for WebRx than usual care. We also hypothesized improved inhibitory control and family conflict for WebRx than usual care. Improving adolescent self-monitoring of blood glucose, providing parents a structured method for daily monitoring of glucose checking adherence (and blood glucose values), and providing prespecified rules (self-monitoring of blood glucose frequency targets for teens and strategies for parents to communicate with teens about self-monitoring of blood glucose and to respond to self-monitoring of blood glucose frequency with prespecified rewards and consequences) were expected to reduce family conflict about the regimen. We also explored maintenance of these effects at a 12-month follow-up (6 months after the end of the intervention).
Methods
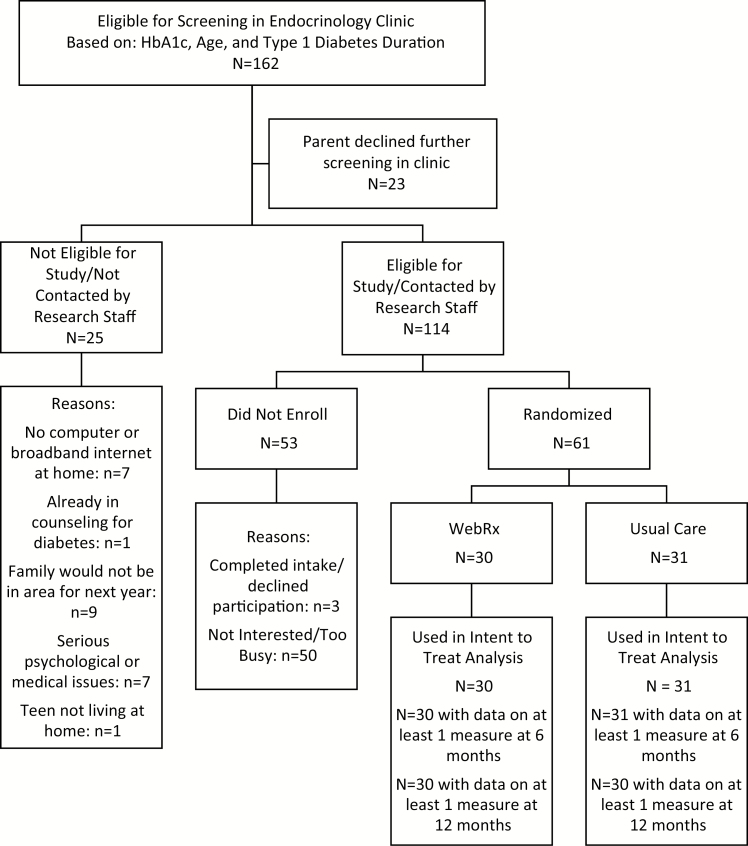
Sixty-one adolescents were recruited from endocrinology clinics affiliated with a regional children’s hospital. Inclusion criteria were as follows: age 13–17, average HbA1c ≥8% for past 6 months, most recent HbA1c ≥8%, type 1 diabetes duration >18 months, at least one parent/guardian participant living with the adolescent, and a computer with broadband Internet at home. Exclusion criteria were as follows: pregnancy, severe medical or psychiatric illness, plans to leave the area within 12 months, and concurrent counseling focused on diabetes regimen adherence. As shown in Fig. 1, 162 adolescents were identified by the electronic medical record as eligible based on HbA1c, age, and diagnosis duration and were screened by medical staff at regular clinic visits. Of those, 23 declined further screening and 25 were ineligible. Of the 114 eligible for the study, 61 were randomized. Comparisons of those randomized to those eligible but not randomized showed no significant differences in HbA1c or type of insurance. Computerized minimum likelihood allocation was used to randomly assign participants while balancing across conditions on gender, age (<16 vs. ≥16 years), ethnicity (minority), clinic location (Lebanon, NH vs. Bedford, NH), HbA1c (≤9% vs. >9%), and mean daily self-monitoring of blood glucose in the 7 days before intake (≤3.5 vs. >3.5). In order to balance conditions on baseline HbA1c and self-monitoring of blood glucose, we used the uncontrolled pilot study median to establish these cutoffs. Research staff conducting assessments were not blinded. However, all questionnaires were completed online in private by teens and parents, cognitive assessments were computerized, and objective measures (e.g., meter downloads and HbA1c tests) were also used. Recruitment ended when the sample size initially proposed as sufficient to detect the effects on the primary outcomes was reached. The Institutional Review Board at Dartmouth College approved the study (ClinicalTrials.gov # NCT01722643; CPHS protocol # 23559).
Fig. 1.
Consort table.
Web sessions were completed and recorded using WebEx, an online Health Insurance Portability and Accountability Act of 1996 (HIPAA)-compliant web conference system. Families assigned to WebRx were loaned a web camera if needed. All adolescents in WebRx were required to use a Carelink compatible meter and testing strips provided by the project during the 25-week intervention (Abbott FreeStyle Lite). Meters were provided to 28 adolescents, and 2 already used the FreeStyle. Adolescents in usual care were offered the same meter and free strips, and meters were provided to 16 usual care adolescents, and 3 usual care adolescents already using the FreeStyle were provided strips. All WebRx and any interested usual care participants used Carelink Personal software, available from Medtronic as a free download. The Carelink Personal portal was accessed by study therapists during web sessions, and data were reviewed with participants by sharing screens. Participants were enrolled between January 2014 and September 2015, and follow-up assessments were completed by September 2016.
Intake and follow-up assessments were conducted in the pediatric endocrinology department or a study office centrally located in the region. At intake, the study was explained to parents or guardians and adolescents, and parental or guardian consent and adolescent assent were obtained for all participants. Parents and adolescents completed tasks and questionnaires, and a glycated hemoglobin (HbA1c) blood test was conducted. After the baseline assessment, a computerized program (https://sourceforge.net/projects/minimpy/, last accessed 31 January 2018) was used to randomize participants. At follow-up assessments, parents and adolescents completed tasks and questionnaires and HbA1c blood tests. Parents and adolescents were each compensated $50 for each assessment, plus an additional $100 each for completing both follow-up assessments.
Usual Care
Participants randomly assigned to usual care received ongoing treatment from their current pediatric endocrinologist and diabetes care team, consistent with the Standards of Medical Care in Diabetes [31]. This standard treatment includes telephone consultations with a nurse/certified diabetes educator in the treating clinic as necessary between clinic visits.
Web-Delivered Intervention (WebRx)
WebRx included incentives, brief motivational interviewing/cognitive behavioral therapy and parent contingency contracting sessions, and working memory training all delivered over the internet. For adolescents, incentives involved a 2-week baseline phase with $10 per week for uploading glucometers to Carelink. From weeks 3–7, incentives were earned for meeting the self-monitoring of blood glucose goal, testing ≥5 times daily (>2 hr apart), on 1 day more than the prior week, up to 5 days per week. Weekly incentives escalated $5 per week from $10 to $30 plus a weekly $5 bonus for exceeding the weekly goal. In weeks 8–25, the target was set at meeting the daily goal 5 days per week. Incentives were awarded weekly in weeks 1–11, and then each payment was delayed by 1 more week, resulting in payments in weeks 13, 16, 20, and 25.
Parents earned incentives for making a daily report regarding their adolescent’s daily self-monitoring of blood glucose frequency to encourage parents to review the adolescent’s glucometer daily. Parents were asked to call, text, or email the number of times the previous day their adolescent had checked their glucose level, and the reward or consequence delivered. Parent reporting goals were always set at 5 days per week, with the same incentive schedule as adolescents. To encourage independent weekly family review of self-monitoring of blood glucose throughout the maintenance phase, adolescents and parents each earned $5 per weekly meter upload during weeks 12–25.
As described in Stanger et al. [29] and Lansing et al. [30], in weeks 1–11, adolescents also received weekly 20-min web-delivered manualized motivational enhancement therapy/cognitive behavioral therapy sessions, which coincided with awarding of incentives. Motivational enhancement therapy included a menu of intervention components [25]. Therapists reviewed self-monitoring of blood glucose and other self-care behaviors using motivational exercises designed to build awareness of costs and benefits of change, identify and weigh alternatives, choose alternative behaviors, and set goals while avoiding confrontation. Cognitive behavioral therapy components (adapted from Webb et al. [32]) included functional analysis (identify antecedents/consequences of missed/skipped glucose checks and other self-care behaviors), increasing social support, effective communication, problem-solving, mood management, and anger management. Adolescents were asked to practice skills and complete assignments between sessions. Self-monitoring of blood glucose was reviewed at each session in the context of the incentive intervention. Adolescents also selected diabetes management or other goals that might impact diabetes management (e.g., stress reduction or mood management). In general, self-monitoring of blood glucose was discussed by therapists as a foundation upon which other aspects of self-management could be added.
Additional sessions were held during weeks 13, 16, 20, and 25 to review and problem solve as necessary. Parents participated in 20-min sessions focused on developing and implementing a contingency contract that identified a daily home reward for meeting the daily self-monitoring of blood glucose goal (≥5 checks per day, spaced at least 2 hr apart) and a small daily consequence for failing to meet the goal. Parents and teens also received instruction in problem-solving in week 11 and were encouraged to complete a review of the teen’s meter data in a weekly family meeting during the maintenance phase, identifying diabetes management concerns (e.g., periods with infrequent self-monitoring of blood glucose such as during or after school, times of persistently high glucose readings such as early morning or after meals), selecting a target management behavior to work on, brainstorming possible solutions or strategies to try, evaluating the pros and cons of each solution, and selecting a solution, then implementing that solution and following up in subsequent weeks to assess the outcome, based on the meter review. Therapists assessed family meeting compliance and outcomes, providing support and feedback in each maintenance session.
Beginning in week 3 of WebRx, adolescents completed 25 sessions of working memory training (five sessions per week; eight training tasks per session), via Cogmed-RM v. 2. Adolescents earned $5 for completing a session in a single day and a $5 bonus for improving or maintaining performance on three of eight training tasks. Weekly coaching calls from research staff provided feedback and motivational support.
The maximum incentive earnings for adolescents and parents across the 25-week intervention were $845 each, plus adolescents could earn an additional $245 for working memory training. Incentives were remotely loaded onto a study-provided debit card. The study protocol is available from the first author.
Fidelity
Therapists were two female master’s level clinicians. Therapists were trained and supervised weekly by the first author, and trainings included watching videos of the intervention delivery from the in-person pilot study, readings, and role-plays. Structured checklists were used during each session. In addition, the randomized controlled trial therapists treated the pilot participants before the randomized controlled trial. Fidelity was assessed using two measures: the Contingency Management Competence Scale [33] and the Contingency Management/Cognitive Behavioral Therapy Therapist Adherence Measure [34]. Bachelor level raters, who were trained to ≥80% agreement with doctoral level staff, rated two randomly selected videotaped sessions for each family (100% of families had at least one rated session and 93% had two). Approximately 25% of sessions were rated by two raters with ≥90% agreement. Mean fidelity scores were 5.51 (SD = 0.46) for adolescent incentives and 5.31 (SD = 0.35) for parent contingency contracting on a seven-point scale, reflecting scores in the “good” to “very good” range. Mean fidelity scores for adolescent motivational interviewing/cognitive behavioral therapy were 3.33 (SD = 0.31) on a five-point scale, indicating fidelity between “some” and “pretty much.” These ratings for incentives and cognitive behavioral therapy/motivational enhancement therapy fidelity are comparable to or slightly higher than other published reports [33, 35, 36].
Measures
Primary outcome
To assess self-monitoring of blood glucose frequency, the target behavior of the adolescent incentive program, the total number of times a day teens monitored their blood glucose during the 14 days before each assessment point was recorded from all glucometers used by the adolescent and used to calculate an average daily frequency. At each assessment, participants were asked to bring all meters to the assessment. If meters were forgotten, assistants collected information after the visit via a remote download or over the phone (teen or parent reading the information to the staff member) after the visit.
Other intervention targets
To assess parental review of their child’s glucometer, the target behavior of the parent incentive program, a single five-point Likert scaled item indexing this behavior was used. The item read “How often did you look at the readings in your child’s blood glucose meter?” and was drawn from the Revised Parental Monitoring of Diabetes Care questionnaire [37]. To assess visual spatial working memory, the primary target of working memory training, a computerized task based on Rapport et al. [38] was administered. This task involved three conditions (24-trial sequences of three-, four-, and six-dots). Participants were asked to replicate the sequence of dots on a 9 × 9 grid. A ceiling effect was observed on three- and four-dot trials; therefore, the proportion of correctly replicated sequences on the six-dot task was used in analyses.
Family conflict, inhibitory control, and glycemic control were also assessed, as they were potential secondary benefits not directly targeted by the intervention components. Family conflict was measured via the Revised-Diabetes Family Conflict Scale [39] completed by parents. The 19 items are rated on a three-point Likert scale, with total scores ranging from 19 to 57. This measure showed good reliability in the current sample at baseline and all follow-up assessments (alpha ≥ 0.84). To assess inhibitory control, an untrained executive function, a computerized Stroop Color-Word test was administered. The color-word interference task measures inhibitory processes involved in suppressing an automatic, prepotent response (putatively reflecting executive function that plays a role in adherence). It also has demonstrated sensitivity to working memory training in adolescents [14]. The color-word interference score was used as the dependent variable. Glycemic control (HbA1c) was obtained for research purposes and assessed via a blood draw and laboratory test (Roche immunoassay), all analyzed in the same laboratory.
Sample size
This study was designed to detect differences between arms at 6 months adjusting for baseline value. Enrollment of 60 participants was planned anticipating a 10% drop-out rate by 6 months. With this sample size, there is 80% power to detect a difference between arms that is 0.78 times a SD. Using prior study estimates for SDs and within-individual correlations, this standardized effect size is equivalent to mean differences of 1.5 for self-monitoring of blood glucose and 1.4% for HbA1c.
Statistical Analyses
Descriptive analyses were conducted to characterize treatment completion in the WebRx condition. Intent-to-treat longitudinal mixed-effects models were used to model the primary and secondary outcomes at 6 and 12 months. These models included fixed effects for time point (6 or 12 months), treatment condition (WebRx vs. usual care), and a treatment condition by time point interaction. These models also included as adjusting covariates the baseline level of each outcome, duration of diabetes diagnosis, and whether the participant used an insulin pump. Random individual-level intercepts were also included to account for repeated assessments within individual. The primary effects of interest in these analyses were the main effects of treatment condition, as these effects reflect between-condition differences at the 6-month assessment, adjusting for baseline values. The treatment × time interaction term tested between-condition differences in changes from the end of treatment (6 months) to the 12-month follow-up assessment. Interactions were not expected to be statistically significant, as we hypothesized maintenance of between-condition differences from 6 to 12 months.
Least-square means from the adjusted models are also presented for each treatment and time point. To test whether outcomes were better for WebRx than usual care at the end of treatment and whether improvements were sustained after treatment ended, between-condition parameter estimate contrasts were performed at 6 and 12 months. Analyses were completed using SAS (version 9.4). Follow-up rates were as follows: 61 and 58 participants provided self-monitoring of blood glucose data at 6 and 12 months, respectively, and 56 and 54 adolescents completed cognitive assessments at 6 and 12 months, respectively. At both 6 and 12 months, 60 provided HbA1cs (three were point of care values), and 57 parents completed measures. Two participants were removed from the longitudinal models for specific outcomes because of improbable baseline values on variables that were adjusting covariates in the models. One participant had scores below chance on the Stroop, and one participant had no correct responses for the visual spatial working memory task for any number of dots. One additional participant was excluded from the 6-month Stroop analysis because of no correct responses on the Stroop at 6 months, but this participant was included in the 12-month Stroop analysis. No missing data were estimated, and all available data were used in analyses.
Results
Baseline Comparisons
Table 1 shows comparisons between the usual care and WebRx participants on demographic variables, baseline diabetes duration and insulin pump usage, and baseline scores on outcome variables. There were no significant differences between the conditions on any of these variables. On average, participants were of middle-class socioeconomic status (M = 5.4 on the nine-point Hollingshead scale for parental occupation), and 38% had Medicaid insurance. On average, youth were about 15 years old, and 43% were female, and almost all were White, non-Hispanic. They had had type 1 diabetes for about 6 years, with mean HbA1c of about 9.1%. Baseline self-monitoring of blood glucose was between 4 and 5 times per day, higher than might be expected based on the literature [40]; however, these data were collected at a scheduled intake assessment after a recent clinic visit, possibly resulting in increased testing in the days before the assessment. Participating parents were mostly female, with one third having a high school education or less. The only between-condition difference in demographic or outcome variables was found for sex of participating parent, with more female primary parents in the WebRx than usual care conditions (97% vs. 79%, p < 0.05).
Table 1.
Baseline characteristics of usual care and web intervention condition participants
| Measures | Usual care (n = 31) Mean (SD) | WebRx (n = 30) N (%) | Test statistic (df) t value or X2 |
|---|---|---|---|
| Demographics | |||
| Youth age | 14.9 (1.5) | 15.2 (1.4) | −0.76 (59) |
| SES | 5.4 (2.4) | 5.4 (2.6) | −0.02 (59) |
| Duration of diabetes in years | 6.5 (3.9) | 5.9 (3.2) | 0.69 (59) |
| Use of insulin pump | 17 (55%) | 23 (78%) | 3.21 (1) |
| Youth sex (female) | 15 (48%) | 11 (37%) | 0.86 (1) |
| Race and ethnicity | |||
| Non-white or Hispanic | 1 (3%) | 1 (3%) | 0.0006 (1) |
| White and non-Hispanic | 30 (98%) | 29 (97%) | |
| Insurance | 2.54 (1) | ||
| Medicaid | 7 (27%) | 13 (48%) | |
| Private | 19 (73%) | 14 (52%) | |
| Parent agea | 47.6 (8.0) | 46.0 (6.0) | 0.83 (54) |
| Parent sex (female)b | 22 (79%) | 28 (97%) | 4.28 (1)c |
| Parent education | 0.60 (2) | ||
| High school or less | 10 (32%) | 11 (37%) | |
| Some college | 11 (35%) | 12 (40%) | |
| Bachelor’s degree or higher | 10 (32%) | 7 (23%) | |
| Parent married | 23 (74%) | 21 (70%) | 0.13 (1) |
| Outcome measures | |||
| SMBG mean times per day (past 14 days) | 4.5 (2.1) | 4.8 (2.8) | −0.46 (59) |
| Parent monitoring | 3.04 (0.60) | 2.97 (0.63) | 0.42 (59) |
| VSWM percentage correctd | 46.1% (23.4%) | 47.4% (21.1%) | −0.22 (58) |
| HbA1c | 9.2% (0.9%) | 9.1% (1.0%) | 0.55 (59) |
| DFCS total score | 27.1 (5.5) | 28.4 (5.6) | −0.92 (59) |
| Stroop color-word interference time (msec)e | 2.2 (2.8) | 1.10 (2.3) | 1.62 (58) |
Note: All comparisons were nonsignificant except sex of participating parent. Parent monitoring is a single item scored 1–5 reflecting frequency of parent reviewing adolescent’s glucometer.
aMissing parent age for five participants, n = 56.
bMissing parent sex for four participants, n = 57.
c p < 0.05.
dExcludes one participant with 0% correct on all VSWM task blocks (three, four, and six dots).
eExcludes one participant with <25% correct (worse than chance responding).
DFCS = Diabetes Family Conflict Scale, SES = socioeconomic status (Hollingshead [45] nine-step parental occupation scale), SMBG = self-monitoring of blood glucose, VSWM = visual spatial working memory scale (six-dot condition).
Treatment Adherence and Incentive Earnings
Among participants assigned to WebRx, almost all (28 of 30) adolescents and parents completed at least 12 of 15 of the incentives + motivational enhancement therapy/cognitive behavioral therapy/parent sessions (adolescent M = 14.1 [SD = 2.4]; parent M = 14.0 [SD = 2.4]). For working memory training, 25 of 30 participants completed at least 20 of 25 sessions. Adolescents trained an average of 3.35 times per week (SD = 1.45) and improved an average of 62% of tasks relative to prior performance on the same task. On average, adolescents met their self-monitoring of blood glucose goal on 16.0 (SD = 7.8) of the possible 23 weeks. On average, adolescents earned $507 (SD = $286) of $845 possible from incentives and $197 (SD = $63) of $245 possible from working memory training. Parents met their self-monitoring of blood glucose and contract reporting goal on 18.3 (SD = 5.7) weeks, and families held the meter review meeting on 7.3 (SD = 4.0) weeks of the possible 14 weeks. Parents earned on average $587 (SD = $223) of the $845 maximum.
Between-Condition Outcome Comparisons
In mixed models, we examined effects of treatment condition. All outcomes showed a significant main effect of treatment condition adjusting for baseline value (Table 2). Table 3 shows the least-square means adjusting for baseline and all model terms, plus the contrasts comparing the two treatment conditions (usual care vs. WebRx) at 6 and 12 months. In terms of the primary outcome, there was a significant difference at 6 months, adjusting for baseline value, favoring the WebRx condition on self-monitoring of blood glucose frequency (d = 0.58, mean difference [95% CI] = 1.54 [0.61, 2.46]). Between-condition differences were also observed for parent monitoring of the adolescent’s glucometer use (d = 1.30, mean difference [95% CI] = 1.50 [0.88, 2.12]) and visual spatial working memory (d = 0.48, mean difference [95% CI] = 13.3% [1.9%, 24.8%]). At 12 months, consistent with the lack of significant treatment × time interactions, between-condition differences, adjusting for baseline value, were found on self-monitoring of blood glucose frequency (d = 0.42, mean difference [95% CI] = 1.19 [0.25, 2.13]) and visual spatial working memory (d = 0.76, mean difference [95% CI] = 18.2% [6.6%, 29.7%]). Consistent with the significant treatment × time interactions, differences were not significant at 12 months for parent monitoring.
Table 2.
Mixed model results for effects of time (end-of-treatment and 12-month follow-up), treatment condition, and treatment × time on each outcome, controlling for pump status, duration of diagnosis, and baseline value of each outcome
| Model results | Outcomes | |||||
|---|---|---|---|---|---|---|
| SMBG B(SE) | Monitoring B(SE) | VSWM B(SE) | HBA1c B(SE) | DFCS B(SE) | Stroop B(SE) | |
| Intercept | 0.21 (0.56) | 1.40 (0.39)** | 0.15 (0.07)* | 1.32 (1.27) | 13.64 (3.49)** | 1.28 (0.55)* |
| Main effect of time | −0.41 (0.33) | 0.20 (0.24) | 0.04 (0.04) | 0.19 (0.17) | 0.36 (1.13) | −0.73 (0.42) |
| Main effect of treatment | 1.54 (0.46)** | 1.50 (0.31)** | 0.13 (0.06)* | −0.52 (0.26)* | −3.34 (1.55)* | −2.00 (0.52)** |
| Treatment × time | −0.35 (0.47) | −1.18 (0.34)** | 0.05 (0.06) | −0.04 (0.25) | −0.08 (1.59) | 1.42 (0.60)* |
| Pump | 0.23 (0.46) | 0.05 (0.29) | 0.01 (0.06) | 0.02 (0.27) | 0.24 (1.49) | 0.53 (0.47) |
| Diabetes duration | −0.04 (0.06) | 0.02 (0.04) | 0.003 (0.007) | 0.0007 (0.03) | −0.17 (0.20) | 0.05 (0.06) |
| Baseline value | 0.83 (0.08)** | 0.33 (0.09)** | 0.66 (0.11)** | 0.85 (0.13)** | 0.53 (0.11)** | 0.23 (0.08)** |
Parent monitoring is a single item scored 1–5 reflecting frequency of parent reviewing adolescent’s glucometer.
DFCS = Diabetes Family Conflict Scale, SMBG = self-monitoring of blood glucose, VSWM = visual spatial working memory scale, six-dot condition.
*p < 0.05, **p < 0.01.
Table 3.
Least-square means for each treatment condition at each time point, adjusting for baseline and between-condition comparisons at 6 and 12 months after baseline
| 6 months | 12 months | |||||
|---|---|---|---|---|---|---|
| Measures | LSMeans Usual care | LSMeans WebRx | t(df) | LSMeans Usual care | LSMeans WebRx | t(df) |
| Primary (targeted) outcomes | ||||||
| SMBG mean times per day (past 14 days) | 3.97 (0.32) | 5.51 (0.32) | −3.34 (56)** | 3.56 (0.33) | 4.75 (0.33) | −2.55 (56)* |
| Parent monitoring | 2.28 (0.21) | 3.78 (0.22) | −4.84 (53)** | 2.48 (0.21) | 2.80 (0.21) | −1.04 (53) |
| VSWM percentage correct | 48.77 (3.87) | 62.10 (4.00) | −2.34 (49)* | 52.82 (3.91) | 70.96 (4.01) | −3.16 (49)** |
| Secondary outcomes | ||||||
| HbA1c | 9.10 (0.18) | 8.57 (0.18) | 2.05 (57)* | 9.29 (0.18) | 8.73 (0.18) | 2.19 (57)* |
| DFCS total score | 27.39 (1.07) | 24.06 (1.08) | 2.15 (53)* | 27.75 (1.08) | 24.34 (1.07) | 2.21 (53)* |
| Stroop color-word interference (msec) | 2.30 (0.35) | 0.30 (0.36) | 3.38 (50)** | 1.57 (0.36) | 0.99 (0.36) | 1.10 (50) |
Parent monitoring is a single item scored 1–5 reflecting frequency of parent reviewing adolescent’s glucometer.
DFCS = Diabetes Family Conflict Scale, SMBG = self-monitoring of blood glucose, VSWM = visual spatial working memory scale, six-dot condition.
*p < 0.05, **p < 0.01.
In terms of the more distal outcomes, there were significant differences favoring WebRx at 6 months, adjusting for baseline, on HbA1c (d = 0.45, mean difference [95% CI] = −0.52% [−1.14%, −0.01%]), family conflict (d = 0.56, mean difference [95% CI] = −3.34 [−6.45, −0.22]), and Stroop color-word interference (d = 0.98, mean difference [95% CI] = –2.00 [−3.05, −0.95]). At 12 months adjusting for baseline, significant differences were maintained on HbA1c (d = 0.44, mean difference [95% CI] = −0.56% [−1.07%, −0.05%]) and family conflict (d = 0.50, mean difference [95% CI] = −3.42 [−6.52, −0.31]). Consistent with the significant treatment × time interactions, differences were not significant at 12 months for inhibition.
Discussion
Results from this initial randomized controlled trial indicated that this web-delivered intervention showed significant impacts on self-monitoring of blood glucose frequency, parent review of the adolescent’s glucometer, and visual spatial working memory after treatment. These improvements in self-monitoring of blood glucose and working memory were maintained at the 12-month follow-up. More distal to targeted outcomes, but important benefits were observed at the end of the intervention on HbA1c, family conflict, and inhibitory control (Stroop color-word interference time), and effects on both HbA1c and family conflict remained significant at the 12-month follow-up. All effects sizes were larger than d = 0.40 at all time points on all measures and highly similar at the end of treatment and at the 12-month follow-up, reflecting good maintenance of intervention effects on multiple outcomes. Overall, compliance was excellent with this complex intervention, evidenced through high rates of completion of web counseling sessions as well as working memory training. These results suggest that this multicomponent intervention was acceptable for the families and provides initial support for the acceptability of utilizing working memory training in conjunction with individual and parent counseling in adolescents with poorly controlled type 1 diabetes.
The intervention showed sustained positive effects on self-monitoring of blood glucose. At the end of the 6-month intervention period, which included a 3-month period of fading incentive delivery and clinical contact (with the gap between the final two sessions lasting 5 weeks), adolescents in the WebRx condition were conducting self-monitoring of blood glucose almost six times per day on average, compared with about four times per day in usual care. Although for WebRx participants the self-monitoring of blood glucose means at baseline (Table 1) does not appear different from 12-month least-square means in Table 3, those in usual care were checking less at 12 months than they were at baseline (4.5 vs. 3.6 times per day). Those in the WebRx condition had lower self-monitoring of blood glucose at 12 months than 6 months, similar to baseline. This combination of between-condition differences at 12 months similar in magnitude to those at 6 months, combined with the observed means, might result from a baseline self-monitoring of blood glucose level that is not reflective of typical checking (“white coat adherence” [41]). Inflated baseline self-monitoring of blood glucose might indicate that 12-month self-monitoring of blood glucose for usual care participants actually reflected a return to baseline (not deterioration) and that 12-month self-monitoring of blood glucose for WebRx reflected maintenance of some (but not all) of their improved self-monitoring of blood glucose. Or, the observed pattern might reflect an actual worsening in self-monitoring of blood glucose in the usual care condition that is ameliorated in the WebRx condition.
Overall, improved self-monitoring of blood glucose as a result of the intervention may not only help adolescents more effectively manage their daily blood glucose levels but provide endocrinologists and diabetes educators with sufficient data to assist families in making appropriate changes, if needed, to their insulin dosing. Not all studies targeting adherence among adolescents with type 1 diabetes report effects on self-monitoring of blood glucose, but when such effects are reported, they have generally been small and nonsignificant [7]. Two recent studies [8, 9] using a single-component incentive intervention solely targeting self-monitoring of blood glucose, among samples restricted to youth with low levels of glucose checking at baseline (<4 times per day on average), have shown significant pre–post positive effects of incentives, similar to this study. An intensive multisystemic family-based intervention for diabetes management has also shown a significant effect on self-monitoring of blood glucose at the end of the intervention; however it was not maintained at a 12-month follow-up [27].
There are no other studies that reported effects on the specific parental meter review behavior targeted in our intervention. Effects on this behavior were the largest across the assessed outcomes at the end of treatment but were no longer significant at the 12-month follow-up. The secondary family conflict outcome showed smaller effects at the end of treatment, but those effects were well maintained at the follow-up. Similar results showing improved family conflict at the end of treatment were obtained with behavioral systems family therapy; however those effects were not sustained at the 12-month follow-up [42]. These results suggest that family conflict improvements may result from the broader set of intervention components and not simply improved parental supervision. Furthermore, they suggest that maintained improvements in parental supervision may not be necessary for stable improvement in adolescent self-monitoring of blood glucose and HbA1c.
This is the first randomized controlled trial to our knowledge to utilize cognitive training in youth with type 1 diabetes. The results showing sustained improvements in visual spatial working memory in the WebRx condition relative to the usual care condition suggest that the Cogmed intervention succeeded in improving working memory among these adolescents with type 1 diabetes. Better performance on Stroop color-word interference after treatment indicates improvements in inhibition that were not directly trained in the working memory training tasks. Such improvements are consistent with a near transfer of executive skill to domains other than working memory [17] and provide support for the possible utility of delivering working memory training to address executive functioning and self-regulation in adolescents with poorly controlled type 1 diabetes. Also, because the improvements in inhibition were not maintained at the 12-month follow-up, this suggests that improving inhibition may not have been the critical mechanism related to sustained improvements in self-monitoring of blood glucose or HbA1c. This study was not designed to test the unique impact of working memory training on adherence or glycemic outcomes, and thus it is not possible to assess the specific role such training played in the positive outcomes observed for the WebRx condition. However, supplemental correlational analyses found that there was no significant correlation between change in visual spatial working memory and change in either self-monitoring of blood glucose or HbA1c, suggesting that working memory improvement may not have been a key intervention mechanism.
With regards to improving HbA1c, this intervention also shows promise. There was a moderate effect size for differences between treatment conditions at both the end of treatment and the 12-month follow-up. Similar to the pattern observed for self-monitoring of blood glucose, the trend for those in usual care was worsening in mean HbA1c over time. This pattern is also observed in longitudinal studies across adolescence [43]. Therefore, although the improvement in individual HbA1c appears to be less than 0.5% within the WebRx condition, it is not clear that the within-condition difference in HbA1c is the most appropriate or sole metric by which to judge clinical significance. The between-condition effects on HbA1c on average are approximately 0.5% at both 6 and 12 months, a promising sustained effect relative to comparably intensive and expensive interventions delivered in the home (e.g., multisystemic therapy [27]). The average effect size on HbA1c for active versus control conditions across intervention studies completed before 2010 was 0.11 [7]. Since that 2010 review, many trials targeting outcomes among youth with type 1 diabetes have focused on technology-based interventions [19, 20]. These interventions have demonstrated feasibility, but have shown nonsignificant effects on HbA1c, or have not tested such effects.
Our findings provide support for the use of a web-delivery model of a multicomponent family intervention, which is important as web-delivery might increase access to care among youth who must travel long distances to receive care at regional pediatric endocrinology clinics. Among family-based interventions for youth with type 1 diabetes, only one has been translated into a web-delivered format, behavioral family systems therapy for diabetes [24]. In that study, effect sizes for changes in adherence and glycemic control were similar to face-to-face delivered formats, as were parent- and adolescent-reported working alliance with the therapist [24]. These results suggest that web delivery of family-based interventions holds promise for enhancing dissemination without sacrificing intervention efficacy.
Of note, this pilot randomized controlled trial has several important limitations. First, the sample size was small and was powered to detect primarily medium to large effects. It was not powered to test mechanistic effects of the intervention on improvements in HbA1c via targeted primary outcomes. Adequately powered mechanistic analyses will be critical to inform future research on this multicomponent mechanistic model and can help identify the impact of the distinct incentives, counseling, and cognitive components. The sample was largely white and middle class, and it will also be important to test potential moderators such as age, gender, ethnicity, family status, or initial HbA1c. Only about one half of those reached by research staff enrolled in the study, suggesting additional intervention approaches will be needed to reach the full population of youth in need. The intervention is fairly complex and expensive, using high magnitude incentives with both teens and parents, and it will be important in the future to address the cost-effectiveness of the intervention and the impact of individual components and varying incentive amounts in achieving desired clinical outcomes. Furthermore, an attention control condition or alternative active intervention was not included. In addition, the majority of treated adolescents remained above the clinically recommended HbA1c cutoffs at the end of the intervention period. Ideally, adaptive intervention models should be developed to provide extended interventions to adolescents who fail to achieve target HbA1c levels at the end of the initial intervention period.
In conclusion, this pilot randomized controlled trial of WebRx provides support for the sustained efficacy of this approach across multiple primary and secondary outcomes, including glycemic control. Given the high cost of hospital admissions for hyper- and hypoglycemic events and the long-term costs associated with vascular disease into adulthood, this multicomponent intervention, despite its cost, may reduce overall expenditures of care. Efficacy might be enhanced by using incentives to target other self-care behaviors in addition to self-monitoring of blood glucose. Potential targets include carbohydrate counting, insulin dosing, and increasing the percentage of blood glucose values in a healthy range, all challenging for adolescents with poorly controlled type 1 diabetes. Furthermore, some emerging research suggests that domain-specific cognitive training may have a greater effect on modifying executive functioning and self-regulation for specific health behaviors than the general working memory training used here [44]. The development of diabetes-specific cognitive training interventions where the training tasks include stimuli that are associated with diabetes such as meters, insulin injections, pumps, and carbohydrate counting may help improve the efficacy of cognitive training interventions targeting adherence. Web-delivered intervention models that target adolescent adherence and parent involvement with incentives and counseling and that include cognitive training for adolescents show promise for improving outcomes for adolescents with poorly controlled type 1 diabetes.
Acknowledgments
Cogmed and Cogmed Working Memory Training are trademarks, in the USA and/or other countries, of Pearson Education, Inc. or its affiliate(s). This study was supported by a grant from the National Institute of Child Health and Human Development (DP3 HD076602). Drs. Stanger and Budney were also supported by a grant from NIDA-P30 DA029926 (PI: Lisa A. Marsch).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards All authors declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Author Contributions Dr. Stanger was the project Principal Investigator, and she designed the intervention and the study and wrote the manuscript. All co-authors participated in writing and editing the manuscript. Dr. Hughes Lansing was responsible for the study data, and Dr. Scherer conducted the data analyses.
Ethical Approval All procedures performed in this study were in accordance with the ethical standards of the institutional review board, the American Psychological Association, and with the 1964 Helsinki Declaration.
Informed Consent Informed consent was obtained for all individual participants included in the study.
References
- 1. Centers for Disease Control and Prevention. National diabetes statistics report: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 2. Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA; T1D Exchange Clinic Network The T1D Exchange clinic registry. J Clin Endocrinol Metab. 2012; 97(12): 4383–4389. [DOI] [PubMed] [Google Scholar]
- 3. Campbell MS, Schatz DA, Chen V et al. ; T1D Exchange Clinic Network. A contrast between children and adolescents with excellent and poor control: The T1D Exchange clinic registry experience. Pediatr Diabetes. 2014; 15(2): 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butler DA, Zuehlke JB, Tovar A, Volkening LK, Anderson BJ, Laffel LM. The impact of modifiable family factors on glycemic control among youth with type 1 diabetes. Pediatr Diabetes. 2008; 9(4 Pt 2): 373–381. [DOI] [PubMed] [Google Scholar]
- 5. Duke DC, Harris MA. Executive function, adherence, and glycemic control in adolescents with type 1 diabetes: A literature review. Curr Diab Rep. 2014; 14(10): 532. [DOI] [PubMed] [Google Scholar]
- 6. Ellis DA, Podolski CL, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: Impact on regimen adherence in youth with type 1 diabetes. J Pediatr Psychol. 2007; 32(8): 907–917. [DOI] [PubMed] [Google Scholar]
- 7. Hood KK, Rohan JM, Peterson CM, Drotar D. Interventions with adherence-promoting components in pediatric type 1 diabetes: Meta-analysis of their impact on glycemic control. Diabetes Care. 2010; 33(7): 1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raiff BR, Barrry VB, Ridenour TA, Jitnarin N. Internet-based incentives increase blood glucose testing with a non-adherent, diverse sample of teens with type 1 diabetes mellitus: A randomized controlled trial. Transl Behav Med. 2016; 6(2): 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petry NM, Cengiz E, Wagner JA, Weyman K, Tichy E, Tamborlane WV. Testing for rewards: A pilot study to improve type 1 diabetes management in adolescents. Diabetes Care. 2015; 38(10): 1952–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jurado MB, Rosselli M. The elusive nature of executive functions: A review of our current understanding. Neuropsychol Rev. 2007; 17(3): 213–233. [DOI] [PubMed] [Google Scholar]
- 11. McNally K, Rohan J, Pendley JS, Delamater A, Drotar D. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010; 33(6): 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohmann S, Popow C, Rami B et al. Cognitive functions and glycemic control in children and adolescents with type 1 diabetes. Psychol Med. 2010; 40(1): 95–103. [DOI] [PubMed] [Google Scholar]
- 13. Holmes J, Gathercole SE, Place M, Dunning DL, Hilton KA, Elliott JG. Working memory deficits can be overcome: Impacts of training and medication on working memory in children with ADHD. Appl Cogn Psychol. 2010; 24(6): 827–836. [Google Scholar]
- 14. Klingberg T, Fernell E, Olesen PJ et al. Computerized training of working memory in children with ADHD—a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005; 44(2): 177–186. [DOI] [PubMed] [Google Scholar]
- 15. Løhaugen GC, Antonsen I, Håberg A et al. Computerized working memory training improves function in adolescents born at extremely low birth weight. J Pediatr. 2011; 158(4): 555–561.e4. [DOI] [PubMed] [Google Scholar]
- 16. Melby-Lervåg M, Redick TS, Hulme C. Working memory training does not improve performance on measures of intelligence or other measures of “Far Transfer”: Evidence from a meta-analytic review. Perspect Psychol Sci. 2016; 11(4): 512–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bigorra A, Garolera M, Guijarro S, Hervás A. Long-term far-transfer effects of working memory training in children with ADHD: A randomized controlled trial. Eur Child Adolesc Psychiatry. 2016; 25(8): 853–867. [DOI] [PubMed] [Google Scholar]
- 18. von Bastian CC, Oberauer K. Effects and mechanisms of working memory training: A review. Psychol Res. 2014; 78(6): 803–820. [DOI] [PubMed] [Google Scholar]
- 19. Mulvaney SA, Rothman RL, Wallston KA, Lybarger C, Dietrich MS. An internet-based program to improve self-management in adolescents with type 1 diabetes. Diabetes Care. 2010; 33(3): 602–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grey M, Whittemore R, Jeon S, Murphy K, Faulkner MS, Delamater A; TeenCope Study Group Internet psycho-education programs improve outcomes in youth with type 1 diabetes. Diabetes Care. 2013; 36(9): 2475–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jaser SS, Patel N, Rothman RL, Choi L, Whittemore R. Check it! A randomized pilot of a positive psychology intervention to improve adherence in adolescents with type 1 diabetes. Diabetes Educ. 2014; 40(5): 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malasanos T, Ramnitz MS. Diabetes clinic at a distance: Telemedicine bridges the gap. Diabetes Spectr. 2013; 26:226–231. [Google Scholar]
- 23. Whittemore R, Jaser SS, Jeon S et al. An internet coping skills training program for youth with type 1 diabetes: Six-month outcomes. Nurs Res. 2012; 61(6): 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris MA, Freeman KA, Duke DC. Seeing is believing: Using Skype to improve diabetes outcomes in youth. Diabetes Care. 2015; 38(8): 1427–1434. [DOI] [PubMed] [Google Scholar]
- 25. Channon SJ, Huws-Thomas MV, Rollnick S et al. A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care. 2007; 30(6): 1390–1395. [DOI] [PubMed] [Google Scholar]
- 26. Carroll AE, DiMeglio LA, Stein S, Marrero DG. Contracting and monitoring relationships for adolescents with type 1 diabetes: A pilot study. Diabetes Technol Ther. 2011; 13(5): 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ellis DA, Templin T, Naar-King S et al. Multisystemic therapy for adolescents with poorly controlled type I diabetes: Stability of treatment effects in a randomized controlled trial. J Consult Clin Psychol. 2007; 75(1): 168–174. [DOI] [PubMed] [Google Scholar]
- 28. Sutherland K, Christianson JB, Leatherman S. Impact of targeted financial incentives on personal health behavior: A review of the literature. Med Care Res Rev. 2008; 65(6 Suppl):36S–78S. [DOI] [PubMed] [Google Scholar]
- 29. Stanger C, Ryan SR, Delhey LM et al. A multicomponent motivational intervention to improve adherence among adolescents with poorly controlled type 1 diabetes: A pilot study. J Pediatr Psychol. 2013; 38(6): 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lansing AH, Stanger C, Budney A, Christiano AS, Casella SJ. Pilot study of a web-delivered multicomponent intervention for rural teens with poorly controlled type 1 diabetes. J Diabetes Res. 2016; 2016:7485613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. American Diabetes Association. American diabetes association standards of medical care in diabetes—2016. Diabetes Care. 2018; 41(Supplement 1): S1–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Webb C, Scudder M, Kaminer Y, Kadden R.. The Motivational Enhancement Therapy and Cognitive Behavioral Therapy for Adolescent Cannabis Users. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2001. [Google Scholar]
- 33. Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the contingency management competence scale. Drug Alcohol Depend. 2010; 109(1–3): 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chapman JE, Sheidow AJ, Henggeler SW, Halliday-Boykins C, Cunningham PB. Developing a measure of therapist adherence to contingency management: An application of the many-facet Rasch model. J Child Adolesc Subst Abuse. 2008; 17(3): 47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holth P, Torsheim T, Sheidow AJ, Ogden T, Henggeler SW. Intensive quality assurance of therapist adherence to behavioral interventions for adolescent substance use problems. J Child Adolesc Subst Abuse. 2011; 20(4): 289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. J Consult Clin Psychol. 2012; 80(2): 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ellis DA, Templin TN, Moltz K, Naar-King S, Dekelbab B, Carcone AI. Psychometric properties of the revised parental monitoring of diabetes care questionnaire in adolescents with type 1 diabetes. J Adolesc Health. 2012; 50(3): 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rapport MD, Alderson RM, Kofler MJ, Sarver DE, Bolden J, Sims V. Working memory deficits in boys with attention-deficit/hyperactivity disorder (ADHD): The contribution of central executive and subsystem processes. J Abnorm Child Psychol. 2008; 36(6): 825–837. [DOI] [PubMed] [Google Scholar]
- 39. Hood KK, Butler DA, Anderson BJ, Laffel LM. Updated and revised Diabetes Family Conflict Scale. Diabetes Care. 2007; 30(7): 1764–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wysocki T, Green L, Huxtable K. Blood glucose monitoring by diabetic adolescents: Compliance and metabolic control. Health Psychol. 1989; 8(3): 267–284. [DOI] [PubMed] [Google Scholar]
- 41. Driscoll KA, Wang Y, Bennett Johnson S et al. White coat adherence in pediatric patients with type 1 diabetes who use insulin pumps. J Diabetes Sci Technol. 2016; 10(3): 724–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wysocki T, Harris MA, Buckloh LM et al. Randomized trial of behavioral family systems therapy for diabetes: Maintenance of effects on diabetes outcomes in adolescents. Diabetes Care. 2007; 30(3): 555–560. [DOI] [PubMed] [Google Scholar]
- 43. Clements MA, Foster NC, Maahs DM et al. ; T1D Exchange Clinic Network. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes. 2016; 17(5): 327–336. [DOI] [PubMed] [Google Scholar]
- 44. Lopez RB, Vohs KD, Wagner DD, Heatherton TF. Self-regulatory strength: Neural mechanisms and implications for training. In: Gendolla GHE, Tops M and Koole SL, editors. Handbook of Biobehavioral Approaches to Self-Regulation. New York: Springer; 2015:43–54. [Google Scholar]
- 45. Hollingshead AB. Four factor index of social status. New Haven, CT: Department of Sociology, Yale University; 1975. [Google Scholar]