Summary
Antiretroviral therapy (ART) for the treatment of human immunodeficiency virus (HIV) infection represents a major breakthrough in the treatment of HIV/acquired immune‐deficiency syndrome. However, it remains unclear how ART influences virus‐specific immune responses and understanding this is important for developing novel cure and eradication interventions for HIV‐1. In the present study, we evaluate how ART impacts T‐cell and antibody responses in simian immunodeficiency virus (SIV) ‐infected rhesus macaques. We evaluated CD4 and CD8 T‐cell responses by multiparameter flow cytometry, viral loads by quantitative RT‐PCR by a two‐step process using SIV‐specific primers and antibody neutralization function by luciferase‐based TZM‐bl assays. We demonstrate that macaques treated with ART exhibit phenotypic and qualitative effects on T‐cell and antibody responses. Macaques on ART exhibited low numbers of virus‐specific T‐cell responses, and these responses appeared to be partially biased towards central memory subsets. More importantly, there were significantly reduced neutralizing antibody responses in macaques treated with ART. Collectively, these data improve the understanding of how virus‐specific immune responses are generated during ART, and suggest the potential importance of therapeutic vaccines to maintain adaptive immunity during treated infection.
Keywords: antiretroviral therapy, central memory T cell, effector memory T cell, neutralizing antibody, simian immunodeficiency virus
Introduction
Human immunodeficiency virus (HIV) affects > 35 million people worldwide. Although antiretroviral therapy (ART) has substantially reduced disease progression and mortality, virus rebounds rapidly following treatment interruption. This is due to the rapid and permanent establishment of the viral reservoir, composed mainly of resting memory CD4 T cells and other quiescent long‐lived cells.1, 2, 3, 4, 5 We recently demonstrated using the simian immunodeficiency virus (SIV) model in rhesus macaques that the viral reservoir is rapidly established within the first 3 days of challenge. Administration of ART shortly after a mucosal viral challenge renders the virus undetectable, but this also results in decreased levels of SIV‐specific T‐cell responses, demonstrating that high viral antigen levels during uncontrolled SIV infection are necessary to prime virus‐specific immune responses.
Since uncontrolled viral replication results in accelerated disease progression, various novel approaches have been tested to provide antigen stimulation in the context of ART. Previous studies have investigated the efficacy of therapeutic vaccines to prime virus‐specific immune responses in the setting of ART.6, 7 A therapeutic vaccine approach composed of an adenovirus serotype 26 prime and a modified vaccinia Ankara boost (Ad26/MVA) has been previously shown to elicit robust virus‐specific T‐cell responses and modest antibody responses. This regimen induced improved virological control after ART discontinuation, highlighting the critical role of cytotoxic T cells in controlling viral rebound.6 It is not well understood which specific memory T‐cell subsets are necessary for controlling viral rebound after treatment discontinuation. CD8 T cells can be divided into various subsets, including effector memory and central memory subsets, which differ in their phenotype, proliferative capacity and cytotoxic ability. Effector memory T cells are characterized by their rapid degranulation, whereas central memory T cells exhibit high proliferative function.8, 9 A previous study suggested that effector memory CD8 T‐cell responses may be critical for controlling viral replication during chronic SIV infection,10, 11 providing a rationale for evaluating T‐cell subset differentiation during ART.
It is not clear how adaptive immune responses are induced during ART, constituting a major gap in our understanding of how therapy affects the host antiviral response. In this report, we show that infection of Indian rhesus macaques with SIVmac251 followed by treatment with ART results in quantitative and qualitative changes in SIV‐specific T‐cell and B‐cell responses. These findings provide an improved understanding of how adaptive immune responses develop during ART, and may provide a framework for future HIV cure and eradication strategies.
Materials and methods
Macaques, infections and treatments
Outbred, Indian, young adult, male and female rhesus macaques (Macaca mulatta) were major histocompatibility complex typed to select those that were negative for the protective Mamu‐A*01, Mamu‐B*08 and Mamu‐B*17 alleles. Macaques were housed at Bioqual (Rockville, MD). Rhesus macaques received 500 TCID50 of SIVmac251 intrarectally12, 13, 14 and were bled longitudinally for viral load quantification. All animal studies were approved by the Institutional Animal Care and Use Committee.
Antiretroviral regimen
The antiretroviral regimen was composed of two reverse transcriptase inhibitors, 20 mg/ml of tenofovir and 50 mg/ml of emtricitabine, plus 2·5 mg/ml of integrase inhibitor dolutegravir diluted with 25% (v/v) polyethylene glycol 400, 15% (w/v) Captisol and 0·075 m NaOH in H2O. The ART cocktail was administered daily at 1 ml/kg subcutaneously. The mixture was clear, at pH 6 and was sterile‐filtered and frozen at −20° until each use.
Cellular immune assays
The SIV‐specific T‐cell responses were assessed by intracellular cytokine staining assays, using Aqua green‐fluorescent reactive dye for live–dead exclusion (Invitrogen, Carlsbad, CA; L23101) and using pre‐titred antibodies from Becton Dickinson (Franklin Lakes, NJ) against CD3 (SP34; Alexa Fluor 700), CD4 (OKT4; BV711; BioLegend, San Diego, CA), CD8 (SK1; allophycocyanin–cyanine 7), CD28 (L293; BV610), CD95 (DX2; allophycocyanin), CD69 (TP1.55.3; phycoerythrin‐Texas red (energy‐coupled dye); Beckman Coulter), interferon‐γ (B27; phycoerythrin‐cyanine 7 and programmed cell death protein 1 (PD‐1) (EH21.1; peridinin chlorophyll‐A‐cyanine 5.5).
TZM‐bl neutralization assays
Neutralization assays were performed in 96‐well plates using TZM‐bl cells that expressed a Tat‐induced luciferase reporter. TZM‐bl cells are HeLa cells that express human CD4, CXCR4 and CCR5. We used a laboratory adapted, Tier 1, easy to neutralize SIV pseudotype. This pseudotype contained the HIV‐1 SG3 backbone and an SIV envelope derived from SIVmac251TCLA.15, provided in trans. The luciferase signal was detected with a Victor luminometer (Perkin Elmer, Waltham, MA). The neutralization assay protocol was performed as described previously.15, 16
Viral RNA assays
Viral RNA was extracted from infected plasma using a viral RNA extraction kit from Qiagen (Hilden, Germany). RNA was isolated by phenol–chloroform purification and ethanol precipitation. Quantitative reverse transcription–polymerase chain reaction (RT–PCR) was conducted by a two‐step process. First, RNA was reverse transcribed using the gene‐specific primer sGag‐R 5′‐CACTAGGTGTCTCTGCACTATCTGTTTTG‐3′. Samples were then treated with RNase H (Stratagene, La Jolla, CA) for 20 min at 37°. We used the forward primer sGag‐F: 5′‐P: 5′‐CTTCCTCAGTGTGTTTCACTTTCTCTTCTGCG‐3′, linked to Fam and BHQ (Invitrogen). Quantitative RT‐PCRs were carried out on a 7300 ABI Real‐Time PCR system (Applied Biosystems, Foster City, CA) in triplicate according to the manufacturer's protocols and using SIV gag RNA standard for viral load calculations. Limit of detection is 50 copies/ml.
Statistical analyses
Statistical analyses were performed using two‐tailed non‐parametric Mann–Whitney U‐tests (unless indicated otherwise) in graphpad prism software version 7 (GraphPad, San Diego, CA).
Results
Effects of ART on T‐cell differentiation
We first infected six rhesus macaques intrarectally with 500 TCID50 of SIVmac251 and followed the kinetics of viraemia and T‐cell responses longitudinally. Our data show that the peak of SIV replication is normally around day 14 post‐challenge, followed by set‐point viraemia after day 35 (Fig. 1a). Following infection, there was a gradual induction of CD4 and CD8 T cells, which expressed PD‐1 (Fig. 1b–d).
Figure 1.

T‐cell responses following uncontrolled simian immunodeficiency virus (SIV) replication. (a) Plasma viral loads by quantitative polymerase chain reaction (PCR). (b) Representative FACS plots showing the frequencies of CD4 or CD8 T cells that are SIV Gag‐specific. (c) Summary of SIV Gag‐specific CD4 T‐cell responses (gated from total CD4 T cells). (d) Summary of SIV Gag‐specific CD8 T‐cell responses (gated from total CD8 T cells). Data from panels b–d are from peripheral blood mononuclear cells. Data are from six rhesus macaques infected intrarectally with 500 TCID50 of heterologous SIVmac251.
We then evaluated the phenotype of T‐cell responses following ART using CD28 and CD95 as cell markers to distinguish effector versus memory T cells. Starting ART on day 10 post‐infection resulted in an overall reduction in the frequencies of SIV‐specific CD4 T‐cell responses (Fig. 2a), consistent with our previous findings,1 but we now make the novel observation that ART also has partial effects on T‐cell subset differentiation. Rhesus monkeys that received ART on day 10 after challenge exhibited enhanced central memory CD4 T‐cell differentiation, characterized by higher expression of CD28 and CD95 on Gag‐specific CD4 T cells (Fig. 2b–e), although this qualitative observation may have been partially influenced by the low levels of virus‐specific T cells in ART‐treated macaques. Conversely, uncontrolled infection seemed to exhibit enhanced effector memory CD4 T‐cell differentiation (Fig. 2b–e).
Figure 2.

Antiretroviral therapy (ART) results in biased central memory CD4 T‐cell differentiation. (a) Plasma viral loads by quantitative polymerase chain reaction. Limit of detection (LOD) is 50 copies/ml. (b) Representative FACS plots showing the frequencies of total CD4 T cells that express CD95 and CD28 and the percentages that are simian immunodeficiency virus (SIV) Gag‐specific. (c) Representative FACS plots showing the frequencies of Gag‐specific CD4 T cells that express CD95 and CD28. (d) Summary of effector memory CD4 T‐cell responses. (e) Summary of central memory CD4 T‐cell responses. (f) Representative FACS plots showing the frequencies of total CD8 T cells that express CD95 and CD28 and the percentages that are SIV Gag‐specific. (g) Representative FACS plots showing the frequencies of Gag‐specific CD8 T cells that express CD95 and CD28. (h) Summary of effector memory CD8 T‐cell responses. (i) Summary of central memory CD8 T‐cell responses. We included CD28 and CD95 gating because these markers are typically used to identify central and memory T‐cell subsets. See top right diagram for a key to this phenotypic characterization. Data are from peripheral blood mononuclear cells at day 140 post‐infection. Data are from four rhesus macaques infected intrarectally with 500 TCID50 of heterologous SIVmac251 and treated with ART daily after day 10 post‐infection. Error bars represent SEM.
A similar pattern of T‐cell differentiation was observed for CD8 T‐cell responses (Fig. 2f‐i). It is important to note that the ART‐treated groups at earlier time‐points (ART starting at day 3 or day 7 post‐infection) exhibited very low frequencies of interferon‐γ‐expressing T cells, rendering it difficult to phenotype T‐cell subsets (due to low events in the flow cytometric gates). In addition, the CD8 T cells of monkeys that received ART at day 10 showed decreased PD‐1 expression relative to untreated infection (Fig. 3a,b). Altogether, these findings suggest that ART‐treated SIV infection is associated with a biased central memory T‐cell differentiation and decreased levels of inhibitory PD‐1 receptor relative to untreated SIV infection.
Figure 3.

Antiretroviral therapy (ART) results in reduced programmed cell death protein 1 (PD‐1) up‐regulation by virus‐specific T cells. (a) Representative histograms showing PD‐1 expression by Gag‐specific CD8 T cells. (b) Summary of PD‐1 expression by Gag‐specific CD8 T cells. Data are indicated as mean fluorescence intensity (MFI). Data are from peripheral blood mononuclear cells at day 140 post‐infection. Data are from four rhesus macaques infected intrarectally with 500 TCID50 of heterologous simian immunodeficiency virus mac251 (SIVmac251) and treated with ART daily after day 10 post‐infection. *P = 0·002. Error bars represent SEM.
Effects of ART timing on neutralizing antibody responses
To determine whether ART has an effect on the functional quality of the antibody response in terms of neutralization capacity, we performed luciferase‐based TZM‐bl assays (Tier 1 pseudovirus, SIVmac251 TCLA.15) using plasma samples from macaques that initiated ART at early times following intrarectal SIVmac251 challenge. We initiated ART at days 3, 7, 10 and 14 post‐challenge to model hyperacute, acute and post‐acute events. ART was interrupted in all groups at day 168 post‐infection to examine neutralizing antibody dynamics after viral rebound. Interestingly, untreated macaques generated robust neutralizing antibody responses after 70 days of infection, with ID50 titres > 106 (Fig. 4a).
Figure 4.
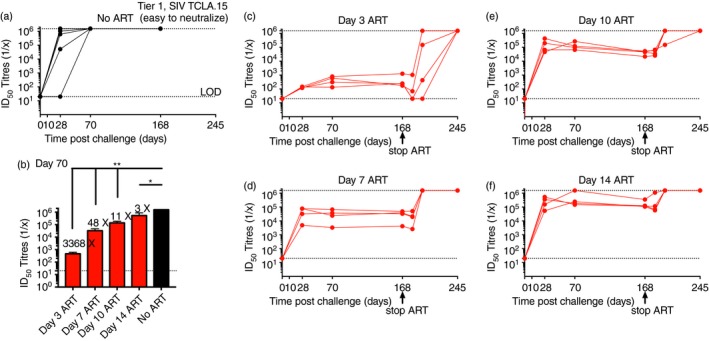
Timing of antiretroviral therapy (ART) initiation correlates directly with neutralizing antibody responses against Tier 1 pseudovirus. (a) Longitudinal analyses of neutralizing antibody responses during an untreated simian immunodeficiency virus (SIV) infection. (b) Comparison of neutralizing antibody responses following early ART initiation at various times post‐challenge (data shown are from day 70 post‐challenge for all groups). (c) Longitudinal analyses of neutralizing antibody responses following ART initiation at day 3 post‐challenge. (d) Longitudinal analyses of neutralizing antibody responses following ART initiation at day 7 post‐challenge. (e) Longitudinal analyses of neutralizing antibody responses following ART initiation at day 10 post‐challenge. (f) Longitudinal analyses of neutralizing antibody responses following ART initiation at day 14 post‐challenge. Note that samples from untreated macaques reach the upper limit of detection, which is indicated by the top dashed line in panel (a). Data are from six untreated rhesus macaques, or from four rhesus macaques (four animals per ART group). Macaques were infected intrarectally with 500 TCID50 of heterologous SIVmac251 and treated with ART daily starting on days 3, 7, 10 or 14. ART was interrupted at day 168 post‐infection. *P = 0·0002; **P = 0·0001 (analysis of variance multiple comparisons). Error bars represent SEM.
In contrast, all ART‐treated macaques exhibited significant delay in their neutralizing antibody response, which appeared to be more exaggerated in animals that started very early ART (Fig. 4b–f). Macaques that initiated ART at day 3 post‐challenge exhibited 3368‐fold lower neutralizing antibody responses, whereas macaques that started ART at day 14 post‐challenge exhibited only threefold lower neutralizing antibody responses, relative to untreated macaques. However, after treatment interruption (day 168 post‐infection), neutralizing antibody responses rapidly increased, and by later times post‐infection, all animals developed neutralizing antibody responses that were comparable to untreated controls and that had reached the upper limit of detection (Fig. 4). An interesting aspect from the day 14 ART group is that robust neutralizing antibody responses can be generated within a month of uncontrolled infection and these responses exhibit long‐term persistence with only a short period of uncontrolled viral replication. Collectively, these data demonstrated that the induction of robust neutralizing antibody responses is antigen‐dependent and more potent in the context of uncontrolled viral replication. We also evaluated neutralization for a difficult‐to‐neutralize Tier 2 pseudovirus (SIV mac251.30, primary isolate from Norm Letvin's laboratory). However, these data were partially inconclusive, as ART resulted in substantial ART‐related background levels (Fig. 5). After ART interruption (when the levels of ART decline), the levels of Tier 2 neutralizing activity were also not significantly different between the different experimental groups, and approximately half of all the macaques from each group exhibited no neutralizing activity (lower level of detection). These results highlight the difficulty of generating neutralizing antibodies against Tier 2 viruses.
Figure 5.

Antiretroviral therapy (ART) ‐related background when evaluating neutralizing antibody responses against Tier 2 pseudovirus. (a) Longitudinal analyses of neutralizing antibody responses during an untreated simian immunodeficiency virus (SIV) infection. (b) Longitudinal analyses of neutralizing antibody responses following ART initiation at day 3 post‐challenge. (c) Longitudinal analyses of neutralizing antibody responses following ART initiation at day 7 post‐challenge. (d) Longitudinal analyses of neutralizing antibody responses following ART initiation at day 10 post‐challenge. (e) Longitudinal analyses of neutralizing antibody responses following ART initiation at day 14 post‐challenge. For the untreated group, we used data from macaques that were part of another study. Data are from 29 untreated rhesus macaques, or from four rhesus macaques (four animals per ART group). Macaques were infected intrarectally with 500 TCID50 of heterologous SIVmac251 and treated with ART daily starting on days 3, 7, 10 or 14. ART was interrupted at day 168 post‐infection. These responses were not statistically different between the groups (analysis of variance multiple comparisons).
Discussion
Antiretroviral therapy has significantly improved disease outcomes in HIV‐infected individuals. Notwithstanding, the rapid seeding of the viral reservoir represents a critical challenge in HIV eradication strategies. Following ART interruption, the virus inevitably rebounds to high levels.17 Latent infection of CD4 T cells is thought to be a major contributor to the lifelong persistence of HIV.18 Substantial efforts have been aimed at understanding the nature of the vial reservoir during ART, but little is known about how adaptive immune responses develop during ART. In this study, we analysed various components of adaptive immunity and how they are influenced following ART during an SIV infection in macaques.
It is well established that CD8 T cells play a critical role in controlling viral replication following infection with SIV and other viruses.19, 20, 21, 22, 23 Recently, it was reported that CD8 T cells are important for controlling also the viral reservoir in the setting of ART.24 In the present study, we wanted to expand on these observations to understand how CD8 T cells differentiate during ART, and whether these CD8 T cells exhibit a specific memory phenotype. Interestingly, we observed that uncontrolled SIV infection induced mostly an activated effector memory T‐cell response, whereas controlled SIV infection in the setting of ART induced a more quiescent central memory T‐cell response. It must be noted that these qualitative observations may have been at least partly influenced by the low number of events in the flow cytometry gates of peripheral blood mononuclear cells from ART‐treated macaques. The phenotypic differentiation of memory T cells may have profound functional effects. Effector memory T cells exhibit an activated phenotype characteristic of recent TCR stimulation and are poised for rapid target cell killing, homing to non‐lymphoid tissues and constitutive glycolytic metabolism, whereas central memory T cells develop after acutely controlled antigen challenges and are characterized by their delayed cytotoxic capacity, homing to lymphoid tissues and improved ability to undergo homoeostatic proliferation.8, 9, 25, 26, 27 In light of this phenotypic distinction, previous data have suggested that effector memory T cells, which can be induced by replicating cytomegalovirus vaccine vectors, are effective at controlling HIV/SIV challenges,11, 28, 29 by a mechanism that is dependent on rapid control of virus in tissues immediately upon challenge. Other studies demonstrated that the magnitude of effector T‐cell responses in lymph nodes, induced by attenuated SIV vaccines, predicted immune control following SIV infection.30
Our previous data in a vaccination model of SIV also demonstrate that high antigen loads favour effector memory T‐cell differentiation. In one such previous study, we showed that macaques immunized with high vaccine doses exhibit a biased induction of effector memory T‐cell response compared with macaques immunized with lower vaccine doses.31 This suggests that high antigen stimulation during natural infection or vaccination has an impact on the phenotype of T‐cell responses, specifically favouring effector memory T‐cell differentiation. Whether high vaccine doses, which favour effector memory T‐cell differentiation, can result in improved immune protection following SIV challenge (relative to low vaccine doses) remains to be determined.
Our data suggest that by reducing the amount of viral antigen, ART promotes the differentiation of a more quiescent long‐lived central memory subset, but this could potentially come at a cost to the host, especially after treatment interruption, given that central memory T cells are not poised for rapid degranulation and cytotoxic killing of infected cells. This suggests the potential importance of therapeutic vaccination strategies to maintain effector T‐cell function during ART. Consistent with this, a previous study showed that vaccination with Ad26/MVA and Toll‐like receptor stimulation during the onset of ART resulted in a significant delay in viral rebound following treatment interruption.6 Whether this improved viral control after treatment interruption is caused by the preferential expansion of effector memory T‐cell subsets over central memory T‐cell subsets remains to be determined.
In the present study, we have evaluated the induction of T‐cell responses, including CD8 and CD4 T‐cell responses following ART. We then analysed neutralizing antibody responses following ART to achieve a better understanding of how other adaptive immune responses develop during therapy. A previous clinical study in HIV‐infected individuals demonstrated a profound increase in HIV‐specific antibody responses by enzyme‐linked immunosorbent assay after analytical treatment interruption.32 We have demonstrated a similar increase in antibody responses following analytical treatment interruption in the SIV model, but until now, it was not clear if the timing of ART initiation influences the quality of neutralizing antibody responses. Interestingly, our data showed that ART impinged on the neutralizing function of antibody responses. We show that early ART initiation during the hyperacute phase of infection (day 3 ART) resulted in a greater than 3000‐fold reduction in neutralizing antibody responses. Nevertheless, these responses became similar to untreated controls after treatment interruption. Previous studies have demonstrated that a subset of HIV‐infected individuals develops broadly neutralizing antibodies against heterologous viral variants, induced following multiple rounds of germinal centre reactions after many years of uncontrolled HIV infection.33, 34, 35, 36, 37, 38, 39 Such broadly neutralizing antibody responses that recognize heterologous viruses cannot be induced by candidate HIV vaccines, probably because of the limited antigen diversity and antigen load present in candidate HIV vaccines.31, 40, 41, 42 Nevertheless, we have previously demonstrated that autologous viral neutralization can be enhanced by increasing vaccine dose.31 Immunization of macaques with escalating doses of SIV vaccines resulted in a significant dose‐dependent increase in autologous SIV‐specific neutralizing antibody responses, suggesting a direct correlation between antigen load and induction of autologous neutralization.31 Notwithstanding, we did not observe further increase in autologous neutralizing antibody responses after repetitive boosting with the same vaccine vector, whereas repetitive boosting did result in an increase in binding antibodies by enzyme‐linked immunosorbent assay. This demonstrated that antibody neutralization titres and antibody binding titres are not always directly correlated. Similar to our T‐cell data, our antibody data suggest the benefit of therapeutic vaccination approaches to provide the necessary antigen stimulation to induce and maintain virus‐specific immune responses in the setting of a suppressed viral infection.
Taken together, these data suggest some of the limitations of current ART for the treatment of chronic HIV infection. Although ART can result in undetectable viral loads, preservation of immune function and improved clinical outcomes, it could also affect the differentiation and function of virus‐specific immune responses. Our data are important for the development of HIV cure and eradication strategies. Future studies will address how therapeutic vaccination administered during ART influences the quality of the virus‐specific immune response, and how these changes correlate with enhanced control or clearance of the viral reservoir.
Disclosures
The authors declare that no financial conflict of interests exist.
Acknowledgements
This work was supported by an NIH grant to PPM (1K22AI118421), the Chicago Third Coast CFAR grant to PPM (P30 AI117943), the Bill and Melinda Gates Foundation (OPP1033091 to DHB) and the Ragon Institute of MGH, MIT and Harvard. We thank Shana Gregory, Yidan Wang and Erik Smoy for assistance.
References
- 1. Whitney JB, Hill AL, Sanisetty S, Penaloza‐MacMaster P, Liu J, Shetty M et al Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014; 512:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE et al Identification of a reservoir for HIV‐1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 3. Persaud D, Zhou Y, Siliciano JM, Siliciano RF. Latency in human immunodeficiency virus type 1 infection: no easy answers. J Virol 2003; 77:1659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV‐1 infection. Proc Natl Acad Sci U S A 1998; 95:8869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yukl SA, Shergill AK, Ho T, Killian M, Girling V, Epling L et al The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV‐positive patients on ART: implications for viral persistence. J Infect Dis 2013; 208:1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Ng'ang'a D et al Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV‐infected rhesus monkeys. Nature 2016; 540:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barouch DH, Deeks SG. Immunologic strategies for HIV‐1 remission and eradication. Science 2014; 345:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R et al Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 2003; 4:225–34. [DOI] [PubMed] [Google Scholar]
- 9. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22:745–63. [DOI] [PubMed] [Google Scholar]
- 10. Hansen SG, Piatak M Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC et al Immune clearance of highly pathogenic SIV infection. Nature 2013; 502:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne‐Johnson L et al Profound early control of highly pathogenic SIV by an effector memory T‐cell vaccine. Nature 2011; 473:523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A et al Low‐dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol 2010; 84:10406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM et al Vaccine protection against acquisition of neutralization‐resistant SIV challenges in rhesus monkeys. Nature 2012; 482:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM et al Immune control of an SIV challenge by a T‐cell‐based vaccine in rhesus monkeys. Nature 2009; 457:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M et al Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine‐elicited neutralizing antibodies. J Virol 2005; 79:10108–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A et al Tiered categorization of a diverse panel of HIV‐1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 2010; 84:1439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chun TW, Davey RT Jr, Engel D, Lane HC, Fauci AS. Re‐emergence of HIV after stopping therapy. Nature 1999; 401:874–5. [DOI] [PubMed] [Google Scholar]
- 18. Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T et al Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV‐1, even in patients on effective combination therapy. Nat Med 1999; 5:512–7. [DOI] [PubMed] [Google Scholar]
- 19. Chowdhury A, Hayes TL, Bosinger SE, Lawson BO, Vanderford T, Schmitz JE et al Differential impact of in vivo CD8+ T lymphocyte depletion in controller versus progressor simian immunodeficiency virus‐infected macaques. J Virol 2015; 89:8677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klatt NR, Shudo E, Ortiz AM, Engram JC, Paiardini M, Lawson B et al CD8+ lymphocytes control viral replication in SIVmac239‐infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog 2010; 6:e1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA et al Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 1999; 283:857–60. [DOI] [PubMed] [Google Scholar]
- 22. Penaloza‐MacMaster P, Barber DL, Wherry EJ, Provine NM, Teigler JE, Parenteau L et al Vaccine‐elicited CD4 T cells induce immunopathology after chronic LCMV infection. Science 2015; 347:278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wherry EJ, Ahmed R. Memory CD8 T‐cell differentiation during viral infection. J Virol 2004; 78:5535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cartwright EK, Spicer L, Smith SA, Lee D, Fast R, Paganini S et al CD8+ lymphocytes are required for maintaining viral suppression in SIV‐infected macaques treated with short‐term antiretroviral therapy. Immunity 2016; 45:656–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pepper M, Jenkins MK. Origins of CD4+ effector and central memory T cells. Nat Immunol 2011; 12:467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708–12. [DOI] [PubMed] [Google Scholar]
- 27. Phan AT, Doedens AL, Palazon A, Tyrakis PA, Cheung KP, Johnson RS et al Constitutive glycolytic metabolism supports CD8+ T cell effector memory differentiation during viral infection. Immunity 2016; 45:1024–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hansen SG, Vieville C, Whizin N, Coyne‐Johnson L, Siess DC, Drummond DD et al Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 2009; 15:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Picker LJ. Are effector memory T cells the key to an effective HIV/AIDS vaccine? EMBO Rep 2014; 15:820–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N et al Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med 2012; 18:1673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Penaloza MacMaster P, Shields JL, Alayo QA, Cabral C, Jimenez J, Mondesir J et al Development of novel replication‐defective lymphocytic choriomeningitis virus vectors expressing SIV antigens. Vaccine 2017; 35:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stephenson KE, Neubauer GH, Bricault CA, Shields J, Bayne M, Reimer U et al Antibody responses after analytic treatment interruption in human immunodeficiency virus‐1‐infected individuals on early initiated antiretroviral therapy. Open Forum Infect Dis 2016; 3:ofw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD et al Co‐evolution of a broadly neutralizing HIV‐1 antibody and founder virus. Nature 2013; 496:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK et al Broad HIV‐1 neutralization mediated by CD4‐binding site antibodies. Nat Med 2007; 13:1032–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J et al Broad diversity of neutralizing antibodies isolated from memory B cells in HIV‐infected individuals. Nature 2009; 458:636–40. [DOI] [PubMed] [Google Scholar]
- 36. Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez‐Rodriguez BM et al Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV‐1‐infected individuals. PLoS ONE 2010; 5:e8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL et al The neutralization breadth of HIV‐1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol 2011; 85:4828–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL et al Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV‐1‐infected individuals. J Virol 2011; 85:11502–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bonsignori M, Montefiori DC, Wu X, Chen X, Hwang KK, Tsao CY et al Two distinct broadly neutralizing antibody specificities of different clonal lineages in a single HIV‐1‐infected donor: implications for vaccine design. J Virol 2012; 86:4688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP et al HIV vaccine design and the neutralizing antibody problem. Nat Immunol 2004; 5:233–6. [DOI] [PubMed] [Google Scholar]
- 41. Hoot S, McGuire AT, Cohen KW, Strong RK, Hangartner L, Klein F et al Recombinant HIV envelope proteins fail to engage germline versions of anti‐CD4bs bNAbs. PLoS Pathog 2013; 9:e1003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS et al Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV‐1 vaccine efficacy trials. J Infect Dis 2012; 206:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


