Summary
Emerging evidence has linked the exosomes to many immunological disorders, including infectious diseases. However, knowledge regarding the role of exosomes in Helicobacter pylori infection is limited. Here, we show that serum exosomes from chronic gastritis patients with H. pylori infection (Hp exosomes) stimulate the expression of the soluble interleukin (IL)‐6 receptor (sIL‐6R), which is involved in IL‐6 trans‐signalling in gastric epithelial cells. Interestingly, sIL‐6R up‐regulates expression of the proinflammatory cytokine IL‐1α, and the neutralization of sIL‐6R suppresses IL‐1α secretion. Thus, Hp exosomes regulate IL‐1α expression via sIL‐6R‐mediated IL‐6 trans‐signaling. Altogether, this study reveals a novel perspective in which exosomes play a vital role in immunological mechanisms during H. pylori infection.
Keywords: exosomes, Helicobacter pylori, IL‐1α, IL‐6, sIL‐6R
Introduction
Helicobacter pylori (H. pylori, Hp) infection is globally widespread. Almost half of the population has been estimated to be carriers of H. pylori 1] The prevalence of infection varies and depends on several factors, such as age, ethnicity, geography and socioeconomic status 2, 3, 4. H. pylori causes chronic gastric mucosal inflammation that leads to peptic ulcer disease in 5–10% of infected people 5. Inflammation in acute or chronic H. pylori infection is a major determinant of peptic ulceration and gastric malignancy 6. Many studies have revealed the relationship between H. pylori infection and certain diseases localized outside the stomach, including oesophageal diseases, inflammatory bowel diseases, allergic disease and hepatobiliary disease 7, 8, 9. Extensive studies investigating the molecular pathogenesis of the extragastric effects of H. pylori infection are ongoing. Recently, the role of extracellular vesicles, such as exosomes, has been shown to explain the specific mechanisms 10.
Extracellular vesicles (EVs) are membrane‐bound vesicles constitutively released by many cells. EVs are subdivided into exosomes, microvesicles and apoptotic bodies. Exosomes are the smallest vesicles (40–150 nm) released from many cell types, including cancer cells, endothelial cells and immune cells, and are generated by the fusion of multi‐vesicular bodies containing intraluminal vesicles with the plasma membrane 11, 12, 13, 14. Recently, many studies have focused on the role of EVs in the regulation of different pathophysiological conditions, such as cancer 15, immunological disorders 16 and other systematic disorders in different organs. Exosomes contain substantial amounts of RNA (including mRNA, microRNA and tRNA) and proteins and are involved in immune regulatory mechanisms in many human diseases.
Exosomes can act as nanocarriers for the systemic delivery of related signal molecules, which produces gastroduodenal and extragastric effects. MicroRNA‐155 from exosomes regulates the expression of cytokines and inflammatory signalling pathway proteins involved in the inflammatory response of H. pylori‐infected macrophages 17. To date, only one study has investigated the role of exosomes in Hp‐induced extragastric diseases 10. The study indicates that plasma‐derived and gastric epithelial cell‐derived exosomes could carry the Hp virulence factor cytotoxin‐associated gene A (CagA) and enter into the blood circulation. These exosomes could be transported to other organs and tissues to participate in the pathogenesis of Hp‐induced extragastric diseases. Thus, exosomes may play a vital role in the abnormal immune response induced by H. pylori infection. However, relevant studies are scarce, and the specific mechanism remains poorly understand. In this study, we screened for cytokines induced by serum exosomes from chronic gastritis patients infected with H. pylori (Hp exosomes) by performing an antibody array analysis and found that interleukin (IL)‐1α and the soluble IL‐6 receptor (sIL‐6R) were up‐regulated by Hp exosomes in human gastric epithelial cells (GES‐1). IL‐6 trans‐signalling via sIL‐6R exhibited a proinflammatory effect, including the promotion of inflammatory cytokine expression 18, 19. In addition, IL‐1α exerted a proinflammatory effect as the initiator of several human diseases, such as inflammation 20. Based on these results, in this study we investigated the role of serum exosomes in IL‐6‐mediated inflammatory cytokine regulation. Our data are the first, to our knowledge, to reveal a previously unidentified regulatory mechanism involving exosomes, sIL‐6R and IL‐1α in H. pylori infection.
Materials and methods
Patient samples
Gastric mucosa samples were collected for an immunohistochemistry analysis and (haematoxylin and eosin H&E) staining from 58 children with chronic gastritis who were recruited randomly from the Department of Pediatrics at Ruijin Hospital and Ruijin Hospital North between March 2016 and October 2017. All the patients were diagnosed by gastroscopy and pathological examination. The sample comprised 22 female and 36 male patients aged between 2 and 17 years with a mean age of 10·47 ± 0·137 years. Based on the Sydney system (definition and grading of chronic inflammation were on the basis of histological features, such as lymphocytes and plasma cells infiltration and loss of specialized glands), the patients were classified into ‘mild’, ‘moderate’ and ‘severe’ subgroups (detected by H&E staining). The patients were divided into the H. pylori‐negative group (n = 20) or H. pylori‐positive group (n = 38) according to the Sydney classification system. Blood samples were collected from five H. pylori‐positive gastritis patients and three healthy volunteers without H. pylori infection or gastritis. The serum samples were prepared following a standard venous blood sampling protocol. The collected blood was centrifuged at 3000g for 10 min at 4°C, and the serum was transferred to a clean tube and stored at –80°C until use. Several tissue specimens were frozen at –80°C for the protein extraction and Western blotting analysis. This study was approved by the ethics committee of Shanghai Jiao Tong University School of Medicine, and written informed consent was obtained from all participants.
Cell culture
Human gastric epithelial cells (GES‐1) were purchased from the Shanghai Institute for Life Science, Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI‐1640 medium (Gibco, USA) supplemented with 10% Fetal Bovine Serum (gibco, Carlsbad, CA, USA), 100 mg/ml penicillin G and 50 μg/ml streptomycin (Life Technologies, Carlsbad, CA, USA) at 37°C in a humidified atmosphere containing 5% CO2. Lipopolysaccharide (LPS) (Escherichia coli 0111:B4; Sigma, St Louis, MO, USA), a tumour necrosis factor (TNF)‐α protease inhibitor (TAPI; Santa Cruz Biotechnology, Santa Cruz, CA, USA), exosomes from serum (100 μg/ml Hp or control exosomes), human sIL‐6R recombinant protein (rhsIL‐6R; Abeomics, Newmarket, UK) and the human IL‐6R alpha antibody (R&D Systems, Minneapolis, MN, USA) were used to stimulate the cells at suitable concentrations depending on the experimental needs.
Exosome isolation and labelling
The serum exosomes were isolated using ExoQuick Exosome Precipitation Solution (SBI, Palo Alto, CA, USA) according to the manufacturer’s instructions. Briefly, 200 μl serum was mixed with 50 μl ExoQuick solution and incubated at 4°C overnight. The mixtures were centrifuged at 10 000g for 30 min at 4°C, and the exosome pellets were resuspended in diluent C (Sigma) for the fluorescent labelling, resuspended in phosphate‐buffered saline (PBS) for the nanoparticle tracking analysis or in‐vitro stimulation of GES‐1, or lysed immediately in radioimmunoprecipitation assay buffer (RIPA) buffer for Western blot analysis. The exosomes are designated ‘con’ and ‘exo(Hp)’ (or Hp exosomes) for simplicity.
Identification of serum exosomes
Particle size was measured using the Nanosight LM10 HS‐BF instrument (Nanosight Ltd, Salisbury, UK) on the basis of nanoparticle tracking analysis (NTA). Briefly, exosome pellets resuspended in PBS were diluted to a concentration of 3 μg/μl after protein quantification, then exosome suspensions were further diluted 100‐fold and analysed following the manufacturer’s protocol. Transmission electron microscopy (TEM) was performed to detect the exosomes morphology. Briefly, exosomes suspended in 2% glutaraldehyde were loaded onto a copper grid and stained negatively with 3% (w/v) aqueous phosphotungstic acid for 1 min. The grid was then examined using an FEI Tecnai G2 Sprit Twin transmission electron microscope (JEM‐1230; Jeol Ltd, Tokyo, Japan). Moreover, for further identification of the exosomes, CD63 (a surface protein marker of exosome) and calnexin (an integral protein of the endoplasmic reticulum) were detected by using Western blotting.
Immunohistochemistry
The gastric mucosa samples were fixed, dehydrated, embedded in paraffin and sliced (4 µm). Immunohistochemical staining was performed according to standard protocols. The IL‐1α antibody (1 : 200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added, and the sections were incubated overnight at 4°C. The images were acquired under an Olympus microscope. The cells were considered positive if brown granules were clearly present in the cytoplasm or nuclei of the epithelial cells. The immunohistochemistry scores were based on the percentage of positive cells (< 10% = 0; 10–30% = 1; 31–50% = 2; 51–75% = 3; and > 75% = 4) multiplied by the stain intensity (0 = negative, 1 = weak, 2 = moderate and 3 = strong) in five different high‐power fields of each section. A score of 4+ was considered positive.
Western blot analysis
Western blot analysis was performed as described previously 21. Total protein from the cell lysates and exosomes was harvested using RIPA cell lysis buffer (Beyotime Institute of Biotechnology, Haimen, China). The membrane proteins were isolated using a Mem‐PER Plus Membrane Protein Extraction Kit (Thermo Scientific, Fremont, CA, USA). A BCA protein assay kit (Yeasen, Shanghai, China) was used to quantify and standardize the protein concentrations in the exosomes and cell lysates. The following specific primary antibodies were used: anti‐human IL‐6R (1 : 1000; R&D Systems), anti‐IL‐1α (1 : 1000; Santa Cruz), anti‐calnexin (1 : 1000; Cell Signaling Technology, Danvers, MA, USA), anti‐CD63 (1:1000; Abcam, Cambridge, MA, USA) and anti‐glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (1 : 1000; Sangon Biotech, Shanghai, China). The anti‐Na+/K+‐ATPase α1 polyclonal (1 : 500; Abbkine, Waltham, MA, USA) and horseradish peroxidase (HRP)‐conjugated secondary antibodies were purchased from Cell Signaling Technology. The neutralizing antibodies (NAb) against sIL‐6R were purchased from R&D Systems.
Immunofluorescence assay
Immunofluorescence assays were performed to verify that the exosomes could be incorporated by GES‐1. The exosomes (100 µl) were naturally dissolved after removal from –80°C and labelled with PKH67 (Sigma) following the manufacturer’s procedure. The PKH67‐labelled exosomes were co‐cultured with GES‐1 for 12 h. Then, the cells were fixed in 4% formaldehyde and permeabilized with 0·1% Triton. The cells were observed under a confocal microscope (Leica, Wetzlar, Germany).
Quantitative real‐time–polymerase chain reaction (qRT–PCR)
Total RNA was extracted from the cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. PCR amplification was performed on a LightCycler 480 system (Roche, Indianapolis, IN, USA) using SYBER Green Supermix (Takara, Dalien, China). Quantification was performed in quadruplicate, and the experiments were repeated independently three times using the following primers: IL‐1α (5GGCAACACCATTGAAGGC3/5CGGGAGGTATGCGTAAGG3) and GAPDH (5TTCACCACCATGGAGAAGGC3/5 CACACCCATCACAAACATGGG 3).
Enzyme‐linked immunosorbent assay (ELISA)
The cell culture supernatants were collected, and the concentrations of human soluble IL‐6 receptor (Invitrogen) and TNF‐α (Anogen, Missassauga, ON, Canada) were measured using ELISA kits according to the manufacturer’s instructions. The absorbance of each well was read at 450 nm using a microplate spectrophotometer. The experiments were repeated independently three times.
Screening for secreted cytokines using a protein array
A human inflammation antibody array (RayBiotech, Inc., Norcross, GA, USA) was used to detect various cytokines in the cell culture supernatants according to the manufacturer’s protocol. The fluorescent signals were detected using a laser scanner (GenePix 4000B Microarray Scanner; Axon, Boston, MA, USA). The densities of the individual spots were measured using ImageJ software to determine the relative cytokine concentrations. The intensities were normalized to internal positive controls for comparison.
Statistical analysis
The statistical analysis was performed using spss version 16.0 software (SPSS Inc., Chicago, IL, USA). The data are presented as the mean ± standard deviation (s.d) or mean ± standard error (s.e.). The statistical significance of the differences was calculated by performing Student’s t‐test or one‐way analysis of variance (anova). The enumeration data were evaluated by performing χ2 test or Fisher’s exact test as appropriate. P < 0·05 was considered statistically significant.
Results
Identification and characterization of serum exosomes from chronic gastritis patients with H. pylori infection
To study the effects of exosomes in H. pylori infection, ExoQuick exosome precipitation solution was used to isolate the exosomes from the serum samples; the characteristics of gastritis patients and healthy volunteers chosen for exosome extraction are presented in Table 1; then, we verified the isolated pellets. A nanoparticle tracking analysis was performed to confirm the successful isolation and purity of the exosomes. The peak size of the particles in the exosome preparations was approximately 139 nm (Fig. 1a), which is within the expected size range for exosomes. Representative transmission electron microscope images of exosomes obtained from serum samples are shown in Fig. 1b. Homogeneous populations of small cup‐shaped circular vesicles (50–140 nm in diameter) were observed. Cellular markers of exosomes were abundant, such as CD63, TSG101, CD81, CD9 and Hsc70. Among them, CD63 is an evolutionarily conserved protein in exosomes and widely used as a biomarker for exosomes. Calnexin, is a integral protein of the endoplasmic reticulum (ER) that exists in the cell, which should not appear in exosomes. Thus, to further confirm that the isolated pellets were exosomes, we detected CD63 and calnexin expression by Western blot. The results showed the presence of CD63 protein in exosome fraction, but not in GES‐1 whole cell lysate, and calnexin protein presented in GES‐1 cell lysate, but not in exosome fraction (Fig. 1c).These results support that the isolated pellets are true exosomes.
Table 1.
Characteristics of the gastritis patients and healthy volunteers selected for serum exosomes extraction
| No. | Age (years) | Gender | Dyspeptic symptoms | Chronic gastritis | H. pylori infection | Treatment before diagnosis | |
|---|---|---|---|---|---|---|---|
| Gastritis patients | 1 | 6 | F | Yes | Severe | Positive | No |
| 2 | 7 | M | Yes | Severe | Positive | No | |
| 3 | 6 | F | Yes | Severe | Positive | No | |
| 4 | 7 | M | Yes | Severe | Positive | No | |
| 5 | 8 | M | Yes | Severe | Positive | No | |
| Healthy volunteers | 1 | 6 | M | No | No | Negative | No |
| 2 | 7 | F | No | No | Negative | No | |
| 3 | 7 | M | No | No | Negative | No |
M = male; F = female.
Figure 1.
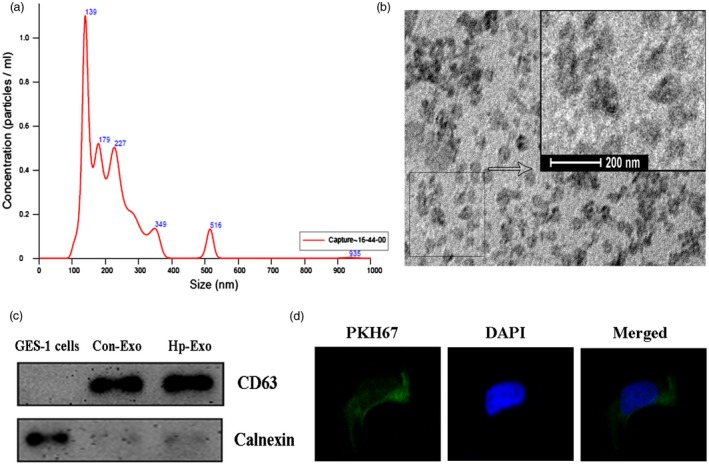
Characterization and validation of exosomes isolated from serum samples of Helicobacter pylori‐positive chronic gastritis patients. (a) The nanoparticle tracking analysis of the exosome preparations was performed using a Nanosight system, and a peak was observed at approximately 139 nm. (b) Electron micrograph of Hp exosomes revealing the typical morphology and size (50–140 nm). (c) CD63 and calnexin expression in gastric epithelial cells (GES)‐1 cells and exosomes were assessed by Western blotting. Con‐Exo = exosomes isolated from serum of healthy volunteers; Hp‐Exo = exosomes isolated from serum of Hp‐positive patients. (d) An immunofluorescence assay verified the GES‐1 cellular uptake of Hp exosomes. Green fluorescence represents PKH67‐labelled exosomes, and blue fluorescence represents cell nuclei. Images of exosomes taken up by GES‐1 cells and the location of exosomes in the cytoplasm around the nucleus.
Exosome uptake by human GES‐1
The internalization of exosomes is a mechanism of cargo delivery to recipient cells. In this study, to determine whether the exosomes could enter GES‐1 cells, an immunofluorescence assay was performed using PKH67, which is a green fluorescent cell linker compound that becomes incorporated into the cell membrane by selective partitioning. First, the Hp exosomes were labelled with PKH67; then, the exosomes were co‐cultured with GES‐1 for 2 h. We observed the internalization of the exosomes under fluorescent and confocal microscopes. In the confocal images, the green fluorescence emission localized in the cytoplasm, indicating the successful internalization of the PKH26‐labelled Hp exosomes by GES‐1 (Fig. 1d).
Screening for secreted cytokines induced by serum exosomes in GES‐1 cells
Exosomes are heavily involved in the regulation of numerous fundamental biological functions, including the immune response 22, and exosomes may function as endogenous inflammation regulators 23. Therefore, a human inflammation antibody array was performed to detect secreted cytokines in GES‐1 cell supernatants treated with serum exosomes and investigate the role of serum exosomes from chronic gastritis patients in the inflammatory response. As shown in Fig. 2a, 40 human cytokines were detected simultaneously and, interestingly, among these cytokines, only sIL‐6R and IL‐1α were increased by more than 1·5 times in the exo(Hp) treatment group compared with those in the control group (Fig. 2b,c).
Figure 2.
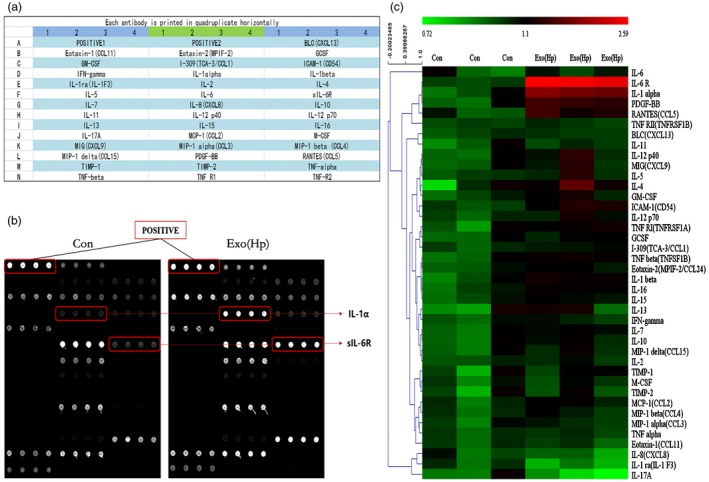
Secreted cytokines induced by Helicobacter pylori (Hp) exosomes using a protein array. (a) Map of antibodies against cytokines on the RayBiotech human inflammation antibody array. (b) Cell culture supernatants from gastric epithelial cells (GES)‐1 cells treated with exosomes were used in the array. Bound cytokines were recognized by a pool of anti‐cytokine antibodies corresponding to the antibodies spotted on the array. The Hp exosome group revealed increased expression of IL‐1α and sIL‐6R. (c) Semi‐quantification of scanned antibody arrays. The levels were normalized to internal positive controls present in the membrane. Semiquantitative levels are represented in the heat map.
Exosomes activate sIL‐6R expression via differential splicing in GES‐1 cells
As mentioned previously, using a protein array, we first discovered that the protein level of sIL‐6R was increased dramatically in GES‐1 cells treated with Hp exosomes.Therefore, we investigated whether exosomes regulated sIL‐6R expression and compared the sIL‐6R cytokine levels in the GES‐1 cell supernatants following the exosome treatment using ELISA. The culture supernatants of the GES‐1 cells were harvested at 6, 12, 24 and 48 h post‐treatment (100 µg/ml Hp or control exosomes). The sIL‐6R levels increased as the treatment time increased and peaked at 24 h in the GES‐1 cells (Fig. 3a). IL‐6 binds sIL‐6R, and membrane‐bound IL‐6R initiates intracellular signalling. Therefore, we examined the membrane‐bound IL‐6R protein levels by performing Western blot analysis. The total membrane proteins were isolated using a membrane protein extraction kit. No changes were observed in the membrane‐bound IL‐6R protein levels in the GES‐1 cells after the Hp exosome treatment (Fig. 3b). Thus, Hp exosomes promote sIL‐6R expression in gastric epithelial cells.
Figure 3.
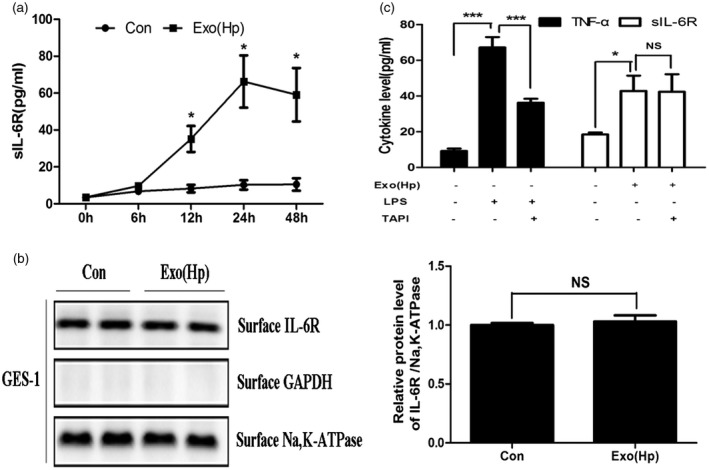
Helicobacter pylori (Hp) exosomes activate sIL‐6R expression via differential splicing in gastric epithelial cells (GES)‐1 cells. (A) The levels of secreted soluble interleukin‐6 receptor (sIL‐6R) were determined using enzyme‐linked immunosorbent assay (ELISA) in GES‐1 cells at the indicated times. Con = group treated with serum exosomes from healthy volunteers. Exo(Hp) = group treated with serum exosomes from chronic gastritis patients infected with H. pylori. *P < 0·05. (b) Detection of membrane‐bound IL‐6R in GES‐1 cells following exosome treatment by Western blotting; n.s. = not significant. (c) Left: GES‐1 cells were incubated with or without TAPI (20 nM) for 8 h and treated with lipopolysaccharide (LPS) for 12 h. The protein levels of tumour necrosis factor (TNF)‐α in the culture supernatants were measured using ELISA. Right: GES‐1 cells were incubated with or without TAPI (20 nM) for 8 h and treated with exosomes for 24 h. The levels of sIL‐6R were measured using ELISA. TAPI = TNF‐α protease inhibitor. *P < 0·05, ***P < 0·001; n.s. = not significant.
sIL‐6R is generated via two independent mechanisms, i.e. translation from an alternatively spliced mRNA and limited proteolysis of the membrane‐bound protein 24. A TNF‐α protease inhibitor (TAPI) was used as a shedding inhibitor to block the proteolytic cleavage of the membrane‐bound IL‐6R 25 and elucidate the mechanism of the Hp exosome influence on sIL‐6R. TNF‐α, which is a known target of TAPI, was used as a positive control 26. In our protein array analysis, the Hp exosomes did not alter TNF‐α expression. Therefore, we used LPS to increase the TNF‐α expression, which was verified in our previous study. TAPI did not inhibit Hp exosome‐induced sIL‐6R expression in the GES‐1 cells, but the TNF‐α release was blocked. Thus, the Hp exosome‐induced soluble IL‐6R protein was generated primarily by the differential splicing of IL‐6R mRNA rather than shedding from the cell surface via the proteolytic cleavage of membrane‐bound IL‐6R.
Identification of cytokines regulated by sIL‐6R
IL‐6 trans‐signalling via sIL‐6R has been suggested to act in a proinflammatory manner via diverse mechanisms, including the recruitment of mononuclear cells, inhibition of the differentiation of regulatory T cells and promotion of inflammatory cytokine expression 27. We investigated whether the increased sIL‐6R levels played a key role in the immune response induced by H. pylori infection and identified that the inflammatory cytokines were regulated by sIL‐6R in the GES‐1 cells. The GES‐1 cells were treated with human sIL‐6R recombinant protein (40 ng/ml) and neutralizing antibodies (NAb) against sIL‐6R (40 ng/ml) with Hp exosomes, and the supernatants were harvested. An inflammation antibody array of 17 inflammation‐related interleukins was performed, and the sIL‐6R recombinant protein up‐regulated the expression of the inflammatory cytokines IL‐5, IL‐10, IL‐11 and IL‐1α (Fig. 4a). However, the neutralization of sIL‐6R only suppressed the IL‐1α levels in the GES‐1 cells (Fig. 4b). Thus, the inducible expression of IL‐1α was specific to sIL‐6R regulation in gastric epithelial cells treated with Hp exosomes.
Figure 4.
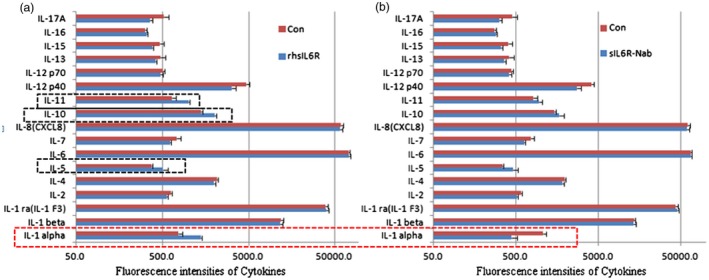
Identification of cytokines regulated by soluble interleukin‐6 receptor (sIL‐6R) using an antibody array. (a) Inflammation‐related interleukins were detected using an inflammation antibody array after treatment with human sIL‐6R recombinant protein (40 ng/ml) and Helicobacter pylori (Hp) exosomes. rhsIL6R = recombinant human soluble IL‐6 receptor. (b) Neutralizing antibodies (40 ng/ml) were used to weaken the effect of sIL‐6R. sIL6R‐Nab = neutralizing antibodies against sIL‐6R.
Hp exosomes activate IL‐1α expression via sIL‐6R, which is associated with the H. pylori‐induced inflammatory response
The Hp exosomes promoted the expression of IL‐1α and sIL‐6R, and sIL‐6R regulated IL‐1α secretion in the Hp exosome‐treated GES‐1 cells. Therefore, we hypothesized that the Hp exosome‐induced activation of IL‐1α expression depended upon sIL‐6R. We detected the Hp exosome‐induced IL‐1α mRNA and protein levels following the sIL‐6R neutralizing antibody treatment using real‐time RT–PCR and Western blotting. The sIL‐6R neutralization suppressed the Hp exosome‐induced IL‐1α mRNA expression significantly (Fig. 5a). Similar changes were observed in the IL‐1α protein levels in the Western blotting analysis (Fig. 5b). Thus, the Hp exosome‐triggered IL‐1α production was dependent upon sIL‐6R.
Figure 5.
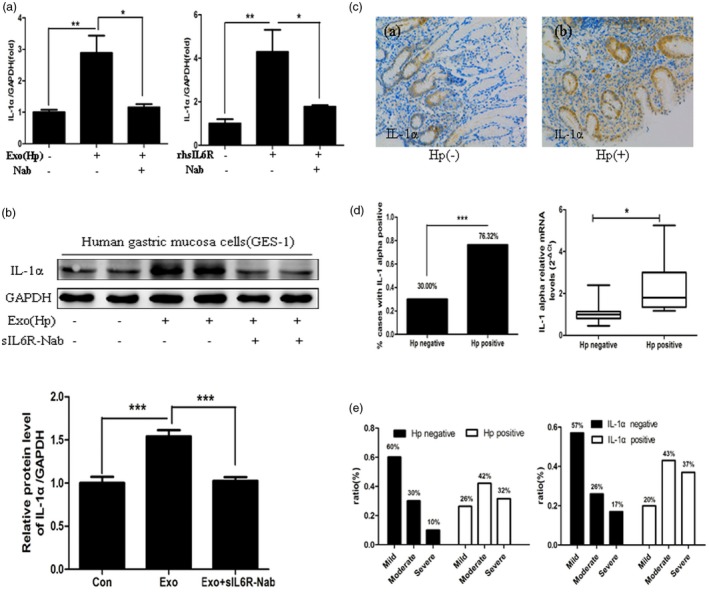
Interleukin (IL)‐1α expression is activated by Helicobacter pylori (Hp) exosomes via soluble IL‐6 receptor (sIL‐6R) and correlates with the inflammatory response induced by H. pylori. (a) gastric epithelial cells (GES)‐1 cells were incubated with exosomes, recombinant human IL‐6R (40 ng/ml) or neutralizing antibodies (40 ng/ml), and the mRNA level of IL‐1α was examined using real‐time reverse transcription–polymerase chain reaction (RT–PCR). rhsIL6R = recombinant human soluble IL‐6 receptor; Nab = neutralizing antibodies against soluble IL‐6 receptor. *P < 0·05, **P < 0·01. (B) GES‐1 cells were incubated with serum exosomes with or without sIL6R neutralizing antibodies. The protein levels of IL‐1α were measured using Western blotting. sIL6R‐Nab = neutralizing antibodies against soluble IL‐6 receptor. ***P < 0·001. (c) Expression of IL‐1α was determined using immunohistochemical (IHC) staining in the gastric mucosa of chronic gastritis patients. Original magnification ×200. Hp(–) = chronic gastritis without H. pylori infection; Hp(+): chronic gastritis with H. pylori infection. (d) Left: quantification of IL‐1α expression using IHC analysis. ***P < 0·001. Right: increased mRNA expression of IL‐1α in chronic gastritis patients with H. pylori infection. The data are shown as 2‐ΔCt. *P < 0·05. (e) The ratios of mild, moderate and severe inflammation in H. pylori‐negative and H. pylori‐positive group and IL‐1α‐negative and IL‐1α‐positive group.
In previous studies, IL‐1α was shown to be proinflammatory during the initiation of several major human diseases 20, including chronic H. pylori infection 28. Therefore, gastric mucosa samples from 58 children with chronic gastritis were analysed to verify the association between IL‐1α and inflammation in human gastric mucosa with H. pylori infection. The baseline characteristics of all patients are shown in Table 2. Positive IL‐1α staining was observed in 76·32% (29 of 38) of the Hp‐positive chronic gastritis patients and 30% (six of 20) of the negative patients (P < 0·05). We performed qRT‐–PCR to detect the IL‐1α mRNA levels in 24 patients with chronic gastritis and found that the patients with H. pylori infection exhibited higher IL‐1α mRNA levels than the negative patients (P < 0.05) (Fig. 5c,d). The ratios of mild, moderate and severe inflammation in the H. pylori‐positive group were 26, 42 and 32%, respectively, and 60, 30 and 10%, respectively in the negative group. The ratios of mild, moderate and severe inflammation in the IL‐1α‐positive group were 20, 43 and 37%, respectively, and 57, 26 and 17%, respectively, in the negative group (Fig. 5e). Thus, the IL‐1α expression in gastric mucosa was associated strongly with H. pylori‐induced inflammation, and the Hp exosomes were related closely to the inflammatory response in the H. A hylori‐infected gastric mucosa. Hypothetical schematic of the regulation of IL‐1α by sIL‐6R in GES‐1 cells is shown in Fig. 6.
Table 2.
Baseline characteristics of all patients with chronic gastritis
| Characteristic | Hp negative (n = 20) | Hp positive (n = 38) | P‐value |
|---|---|---|---|
| Age (years)a | 9·80 ± 0·807 | 9·05 ± 0·513 | 0·419 |
| Gender | |||
| Male | 70%(14/20) | 57.9%(22/38) | 0·408 |
| Female | 30%(6/20) | 42.1%(16/38) | 0·408 |
| Chronic inflammation | |||
| Mild | 60%(12/20) | 26.3%(10/38) | 0·022* |
| Moderate | 30%(6/20) | 42.1%(16/38) | 0·408 |
| Severe | 10%(2/20) | 31.6%(12/38) | 0·106 |
| Major | |||
| dyspeptic symptoms | |||
| Epigastric pain | 90%(18/20) | 94.7%(36/38) | 0·602 |
| Acid regurgitation | 85%(17/20) | 86.8%(33/38) | 0·847 |
| Postprandial fullness | 90%(18/20) | 92.1%(35/38) | 0·786 |
| Nausea | 80%(16/20) | 94.7%(36/38) | 0·168 |
| Treatment before diagnosis | No | No |
Data are presented as mean ± standard error (s.e.).*P < 0·05. Hp = Helicobacter pylori.
Figure 6.
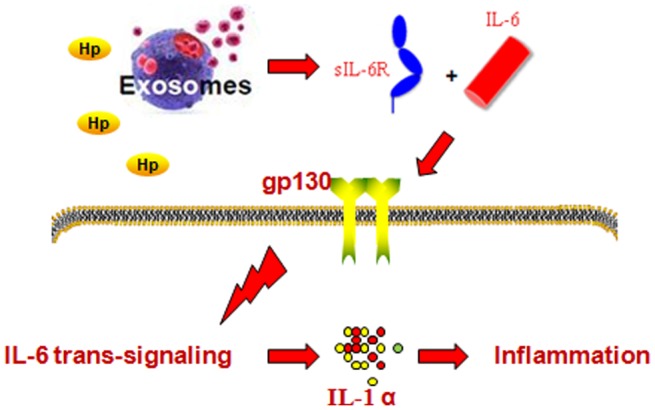
Hypothetical schematic of the regulation of interleukin (IL)‐1α by soluble interleukin‐6 receptor (sIL‐6R) in gastric epithelial cells (GES)‐1 cells in response to Helicobacter pylori (Hp) exosome treatment.
Discussion
Intercellular communication is performed via a variety of mechanisms, such as direct cell‐to‐cell contact, transfer of secreted molecules and the intercellular transfer of EVs. Exosomes are small vesicles (40–150 nm) produced by many cell types, including immune cells, tumour cells and epithelial cells, under physiological and pathological conditions. Exosomes play a vital role in inflammation‐associated pathologies 29. In a recent study, serum‐derived exosomes from CagA‐positive H. pylori‐infected patients were shown to be involved in the pathogenesis of H. pylori infection, particularly in the development of extragastric disorders in these patients 10. In this study, we investigated the effect of circulating serum exosomes on the production of inflammatory cytokines during the mucosal inflammatory response after H. pylori infection.
We observed that the serum exosomes from the patients with H. pylori infection were taken up by the gastric epithelial cells and that the exosomes activated sIL‐6R expression. IL‐6 is a four‐helical cytokine that is involved in the co‐ordination of the innate and acquired immune response 30. IL‐6 binds IL‐6 receptors (IL‐6R) on target cells. The soluble form of IL‐6R (sIL‐6R) comprises the extracellular portion of the receptor that binds IL‐6 with a similar affinity to the membrane‐bound IL‐6R. This process is called trans‐signalling. Soluble IL‐6R is generated via the proteolytic cleavage of the membrane‐bound protein or translation from an alternatively spliced mRNA. The IL‐6R in human circulation is derived predominantly from the former mechanism, and only a small amount is generated from alternative splicing 31, 32. However, in our study, the Hp exosome‐induced soluble IL‐6R was generated primarily via differential splicing by an unknown mechanism.
The classic signalling by the membrane‐bound IL‐6R is regenerative and protective, and IL‐6 trans‐signalling by sIL‐6R is proinflammatory 19. We identified the cytokines regulated by sIL‐6R following exosome treatment in GES‐1 cells. sIL‐6R exerted a strong effect on IL‐1α expression and secretion. Furthermore, sIL‐6R affected the secretion of other cytokines, including IL‐5, IL‐10 and IL‐11. However, the neutralization of sIL‐6R did not suppress the over‐expression of these cytokines. In conclusion, sIL‐6R may be a multi‐functional cellular factor in the immune response. In the protein array, the Hp exosomes only up‐regulated IL‐1α secretion but did not influence IL‐5, IL‐10 or IL‐11 expression. Therefore, the Hp exosome‐induced IL‐1α upregulation was specific to sIL‐6R regulation in gastric epithelial cells. Our study is the first report, to our knowledge, to demonstrate the relationship between IL‐1α and sIL‐6R in gastric epithelial cells during H. pylori infection.
The interleukin (IL)‐1 family comprises the following 11 members that exhibit pleiotropic functions and apical regulation of inflammation: IL‐1α, IL‐1β, IL‐18, IL‐33, IL‐36α, IL‐36, IL‐36γ, IL‐1R antagonist (IL‐1Ra), IL‐36Ra, IL‐37 and IL‐38 20, 33. IL‐1α is expressed constitutively in immune, epithelial and stromal cells, and numerous stimuli regulate its expression in haematopoietic and non‐haematopoietic cells 34, 35, 36, 37. IL‐1α exhibits a proinflammatory effect as the initiator of many human diseases, including inflammation and cancer. IL‐1α up‐regulation leads to consequences during the early stages of H. pylori infection in cats, and the response is similar to that in infected people, particularly children 28. Furthermore, IL‐1α over‐expression in the gastric mucosa correlated with the H. pylori‐induced inflammation in children.
In summary, our results are the first to demonstrate that serum exosomes derived from chronic gastritis patients infected with H. pylori promote the expression of the proinflammatory cytokine IL‐1α via IL‐6/sIL‐6R trans‐signalling in gastric epithelial cells. These results improve our understanding of exosomes and IL‐6 signalling and provide new insight into the immunological mechanisms of H. pylori‐associated inflammatory responses.
Disclosure
The authors declare no competing financial interests.
Author contributions
C. X. and P. L. conceived the idea and directed the whole project. Y. C., X. W. and Y. Y. carried out most of the experiments. Y. X., X. C. and T. Z. provided technical assistance for performing the experiment. J. H. and Z. Y. collected the clinical samples. Y. C., X. W. and J. H. performed the analysis and interpretation of the results. Y. C. wrote the paper, and all authors were involved in editing the manuscript.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81570508, 81470941, 81400588, 81741103 and 81372187).
Contributor Information
P. Li, Email: nfkb@sohu.com
C. Xu, Email: chundixu55@163.com
References
- 1. Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer 2010;10:403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mentis A, Lehours P, Megraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter 2015; 20 (Suppl 1):1–7. [DOI] [PubMed] [Google Scholar]
- 3. Laszewicz W, Iwanczak F, Iwanczak B for the Task Force of the Polish Society of Gastroenterology . Seroprevalence of Helicobacter pylori infection in Polish children and adults depending on socioeconomic status and living conditions. Adv Med Sci 2014;59:147–50. [DOI] [PubMed] [Google Scholar]
- 4. Jafri W, Yakoob J, Abid S, Siddiqui S, Awan S, Nizami SQ. Helicobacter pylori infection in children: population‐based age‐specific prevalence and risk factors in a developing country. Acta Paediatr 2010;99:279–82. [DOI] [PubMed] [Google Scholar]
- 5. Yin M, Hu Z, Tan D, Ajani JA, Wei Q. Molecular epidemiology of genetic susceptibility to gastric cancer: focus on single nucleotide polymorphisms in gastric carcinogenesis. Am J Transl Res 2009;1:44–54. [PMC free article] [PubMed] [Google Scholar]
- 6. Robinson K, Argent RH, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Pract Res Clin Gastroenterol 2007;21:237–59. [DOI] [PubMed] [Google Scholar]
- 7. Kyburz A, Muller A. Helicobacter pylori and extragastric diseases. Curr Top Microbiol Immunol 2017;400:325–47. [DOI] [PubMed] [Google Scholar]
- 8. Rabelo‐Goncalves EM, Roesler BM, Zeitune JM. Extragastric manifestations of Helicobacter pylori infection: possible role of bacterium in liver and pancreas diseases. World J Hepatol 2015;7:2968–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris PR, Serrano CA, Villagran A et al Helicobacter pylori‐associated hypochlorhydria in children, and development of iron deficiency. J Clin Pathol 2013;66:343–7. [DOI] [PubMed] [Google Scholar]
- 10. Shimoda A, Ueda K, Nishiumi S et al Exosomes as nanocarriers for systemic delivery of the Helicobacter pylori virulence factor CagA. Sci Rep 2016;6:18346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma A, Khatun Z, Shiras A. Tumor exosomes: cellular postmen of cancer diagnosis and personalized therapy. Nanomedicine (Lond) 2016;11:421–37. [DOI] [PubMed] [Google Scholar]
- 12. Tang XJ, Sun XY, Huang KM et al Therapeutic potential of CAR‐T cell‐derived exosomes: a cell‐free modality for targeted cancer therapy. Oncotarget 2015;6:44179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bovy N, Blomme B, Freres P et al Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget 2015;6:10253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc 2015;2015:319–23. [DOI] [PubMed] [Google Scholar]
- 15. Muralidharan‐Chari V, Clancy JW, Sedgwick A, D'Souza‐Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 2010;123:1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014;14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, Deng Z, Wang Z et al MicroRNA‐155 in exosomes secreted from helicobacter pylori infection macrophages immunomodulates inflammatory response. Am J Transl Res 2016;8:3700–9. [PMC free article] [PubMed] [Google Scholar]
- 18. Rose‐John S. IL‐6 trans‐signaling via the soluble IL‐6 receptor: importance for the pro‐inflammatory activities of IL‐6. Int J Biol Sci 2012;8:1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rose‐John S. The soluble interleukin 6 receptor: advanced therapeutic options in inflammation. Clin Pharmacol Ther 2017;102:591–8. [DOI] [PubMed] [Google Scholar]
- 20. Malik A, Kanneganti TD. Function and regulation of IL‐1alpha in inflammatory diseases and cancer. Immunol Rev 2018;281:124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu J, Chen X, Wang X et al . ERp19 contributes to tumorigenicity in human gastric cancer by promoting cell growth, migration and invasion. Oncotarget 2015;6:11794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Selmaj I, Mycko MP, Raine CS, Selmaj KW. The role of exosomes in CNS inflammation and their involvement in multiple sclerosis. J Neuroimmunol 2017;306:1–10. [DOI] [PubMed] [Google Scholar]
- 23. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schumacher N, Meyer D, Mauermann A et al . Shedding of endogenous interleukin‐6 receptor (IL‐6R) is governed by a disintegrin and metalloproteinase (ADAM) proteases while a full‐length IL‐6R isoform localizes to circulating microvesicles. J Biol Chem 2015;290:26059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhan M, Jin B, Chen SE, Reecy JM, Li YP. TACE release of TNF‐alpha mediates mechanotransduction‐induced activation of p38 MAPK and myogenesis. J Cell Sci 2007;120:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mohler KM, Sleath PR, Fitzner JN et al . Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature 1994;370:218–20. [DOI] [PubMed] [Google Scholar]
- 27. Scheller J, Chalaris A, Schmidt‐Arras D, Rose‐John S. The pro‐ and anti‐inflammatory properties of the cytokine interleukin‐6. Biochim Biophys Acta 2011;1813:878–88. [DOI] [PubMed] [Google Scholar]
- 28. Straubinger RK, Greiter A, McDonough SP et al Quantitative evaluation of inflammatory and immune responses in the early stages of chronic Helicobacter pylori infection. Infect Immun 2003;71:2693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maravillas‐Montero JL, Martinez‐Cortes I. Regulation of immune responses by exosomes derived from antigen presenting cells. Rev Alerg Mex 2017;64:463–76. [DOI] [PubMed] [Google Scholar]
- 30. Kishimoto T. IL‐6: from its discovery to clinical applications. Int Immunol 2010;22:347–52. [DOI] [PubMed] [Google Scholar]
- 31. Dimitrov S, Lange T, Benedict C et al Sleep enhances IL‐6 trans‐signaling in humans. FASEB J 2006;20:2174–6. [DOI] [PubMed] [Google Scholar]
- 32. Horiuchi S, Ampofo W, Koyanagi Y et al High‐level production of alternatively spliced soluble interleukin‐6 receptor in serum of patients with adult T‐cell leukaemia/HTLV‐I‐associated myelopathy. Immunology 1998;95:360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garlanda C, Dinarello CA, Mantovani A. The interleukin‐1 family: back to the future. Immunity 2013;39:1003–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weber A, Wasiliew P, Kracht M. Interleukin‐1 (IL‐1) pathway. Sci Signal 2010;3:cm1. [DOI] [PubMed] [Google Scholar]
- 35. Itoh Y, Hayashi H, Miyazawa K, Kojima S, Akahoshi T, Onozaki K. 17beta‐estradiol induces IL‐1alpha gene expression in rheumatoid fibroblast‐like synovial cells through estrogen receptor alpha (ERalpha) and augmentation of transcriptional activity of Sp1 by dissociating histone deacetylase 2 from ERalpha. J Immunol 2007;178:3059–66. [DOI] [PubMed] [Google Scholar]
- 36. Tynan GA, Hearnden CH, Oleszycka E et al Endogenous oils derived from human adipocytes are potent adjuvants that promote IL‐1alpha‐dependent inflammation. Diabetes 2014;63:2037–50. [DOI] [PubMed] [Google Scholar]
- 37. Freigang S, Ampenberger F, Weiss A et al Fatty acid‐induced mitochondrial uncoupling elicits inflammasome‐independent IL‐1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol 2013;14:1045–53. [DOI] [PubMed] [Google Scholar]


