Summary
Interleukin‐17 (IL‐17) is a pro‐inflammatory cytokine and is involved in the development of many diseases. Recent studies have revealed that IL‐17‐producing γδ T cells (γδ17 cells) in addition to IL‐17‐producing CD4+ T cells [T helper type 17 (Th17) cells] are often the main producers of IL‐17 in mouse models of inflammatory diseases. γδ T cells are functionally committed during intra‐thymic differentiation. γδ thymocytes capable of producing IL‐17, which express the transcription factor retinoic‐acid‐receptor‐related orphan receptor γt and the signature cytokine receptor IL‐23R, leave the thymus, and produce IL‐17 rapidly by the stimulation with IL‐1β and IL‐23 in the periphery. Therefore, γδ17 cells play important roles in the early phase of host defence against pathogens and in inflammatory diseases. γδ T cells that can produce IL‐17 are also increased in the skin of patients with psoriasis and in peripheral blood of patients with ankylosing sclerosis. Indeed, the therapy targeting IL‐17 has been approved or is in clinical trials, and proved to be very efficient to treat psoriasis, psoriatic arthritis and ankylosing sclerosis. In this review, we discuss recent knowledge about the pathophysiological function of γδ17 cells in infection and inflammatory diseases and therapeutic advances targeting IL‐17.
Keywords: cytokines, inflammatory disease, pathogen clearance
Introduction
Interleukin‐17A (IL‐17A, called ‘IL‐17’ hereafter) is a member of the IL‐17 family.1 The binding of IL‐17 to the heterodimeric receptor consisting of IL‐17RA and IL‐17RC subunits transduces signals to activate a group of cytokines and chemokines such as tumour necrosis factor, IL‐1, IL‐6, granulocyte colony‐stimulating factor, CXCL1 and CXCL2 through activation of the actin related gene 1‐TNF receptor associated factor 6–nuclear factor‐κB axis in the downstream.1 The function of IL‐17 is pleiotropic. It plays a crucial role in the host defence against bacterial and fungal infection by inducing pro‐inflammatory cytokines and chemokines, recruiting neutrophils, and activating T cells and B cells.1, 2 Mice deficient for IL‐17RA are highly susceptible to Klebsiella pneumoniae 3 and IL‐17‐deficient mice are susceptible to bacterial and fungal infection.4, 5 Interleukin‐17 is also implicated in various inflammatory/autoimmune disease models such as experimental autoimmune encephalomyelitis (EAE), arthritis in IL‐1 receptor antagonist‐deficient (Il1rn –/–) mice and imiquimod‐induced psoriatic dermatitis in mice.6, 7, 8, 9, 10 Anti‐IL‐17 and anti‐IL‐17RA antibodies are effective to treat patients with psoriasis and psoriatic arthritis.11, 12, 13 Interleukin‐17 is also important for the maintenance of intestinal barrier integrity and its functional deficiency causes the development of inflammatory bowel diseases.14, 15, 16 In contrast, suppression of IL‐17F, another highly homologous member of IL‐17 family, is suggested to be beneficial for the treatment of inflammatory bowel diseases.17
Interleukin‐17 was initially found to be produced by helper CD4+ T [T helper type 17 (Th17)] cells, but subsequent studies showed that innate immune cells and innate‐like immune cells are also important sources of IL‐17 in inflamed tissues.18, 19 γδ T cells are the principal source of IL‐17 in some mouse inflammatory disease models and thereby exert considerable impact on disease development and progression.20, 21, 22, 23, 24 The IL‐17‐producing γδ T cells (γδ17 cells) share many features with Th17 cells, such as cell surface expression of IL‐23R and CCR6 and the expression of transcriptional factor retinoic‐acid‐receptor‐related orphan receptor γt (RORγt). To induce IL‐17, naive T cells have to differentiate into Th17 cells in the periphery by the stimulation with T‐cell receptor (TCR) and cytokines such as IL‐6 and transforming growth factor‐β. In contrast, the functional potential to produce IL‐17 in γδ17 cells is already established during intra‐thymic development25, 26, 27 and IL‐17 is directly induced by IL‐23 and IL‐1 without TCR stimulation in the periphery. This pre‐programming contributes to rapid IL‐17 production in peripheral tissues in the early phase of pathogen infection. In this review, we would like to introduce the roles of γδ17 cells in inflammatory diseases and recent therapeutic advances targeting IL‐17.
γδ T‐cell subsets and their development
γδ T cells and αβ T cells are generated in thymus from common progenitor cells. Unlike αβ T cells, γδ T cells are functionally committed during intra‐thymic differentiation.28, 29 In mice, the TCR‐γ locus consists of seven Vγ (Vγ1–Vγ7) genes (Heilig & Tonegawa's nomenclature30) that are closely correlated with the effector function, although Vγ3 is a pseudogene in most mouse strains.31 Production of IL‐17 is mostly limited to Vγ4+ and Vγ6+ γδ T cells,32 although Vγ1+ γδ T cells also produce IL‐17 in some cases.33 On the other hand, interferon‐γ (IFN‐γ) production is associated with Vγ1+, Vγ5+ and Vγ7+ γδ T cells. Although overall gene expression patterns are similar between Vγ4+ and Vγ6+ subsets,34 each subset has distinct features (Fig. 1). Vγ6+ γδ T cells express the invariant Vγ6/Vδ1 TCR, develop only in the late embryonic thymus and preferentially localize to the uterus, vagina, lung, dermis and peritoneal cavity.35, 36 On the other hand, Vγ4+ γδ T cells develop in both fetal and adult thymus, have more diverse TCR repertoire and reside in the dermis, lung, liver and secondary lymphoid organs.37, 38 In addition to RORγt, transcription factors such as Blk,39 Hes‐1,40 nuclear factor‐κB,41 Sox4 and Sox1342 are also important for γδ17 cell development. Transforming growth factor‐β and IL‐7 are required for γδ17 thymocyte development and expansion, respectively.43, 44, 45 Epigenetic and transcriptional regulation during γδ17 cell differentiation has been reviewed elsewhere.46 γδ thymocytes capable of producing IL‐17, which express the transcription factor RORγt and the signature cytokine receptor IL‐23R,34 leave the thymus as functionally committed cells,47 and produce IL‐17 directly by the stimulation with IL‐1β and IL‐23 in the periphery. Although IL‐23R is constitutively expressed on γδ17 cells, the expression of IL‐1R in peripheral γδ17 cells is tissue‐dependent.48 In addition to IL‐1R and IL‐23R, the expression of scavenger receptor 2 (Scart 2)49 and CCR6,27 and the lack of CD12225 and CD27 expression26 are often used as markers for γδ17 cells, with the exception for IL‐17‐producing Vγ1+ γδ T cells.33 These phenotypes, established during thymic development, distinguish γδ17 cells from IFN‐γ‐producing γδ T (γδIFN‐γ) cells (Fig. 2). γδ17 cells that develop before birth persist in adult mice as self‐renewing, long‐lived cells.50
Figure 1.
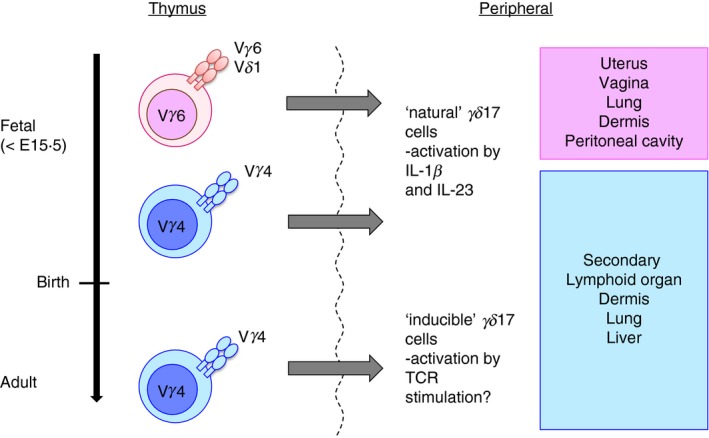
Distinct features of γδ17 cell subset and suggested model of ‘natural’ γδ17 cells versus ‘inducible’ γδ17 cells. Vγ6+ γδ T cells express the invariant Vγ6/Vδ1 T‐cell receptor (TCR), develop only in the late embryonic thymus, and preferentially localize to the uterus, vagina, lung, dermis and peritoneal cavity. On the other hand, Vγ4+ γδ T cells develop in both fetal and adult thymus and have a more diverse TCR repertoire. These cells circulate in blood and reside in the dermis, lung, liver and secondary lymphoid organs. In according to Haas et al.50 ‘natural’ γδ17 cells (Vγ6+ and part of Vγ4+) developed before birth acquire interleukin‐17 (IL‐17) ‐producing ability in thymus and produce IL‐17 stimulated by IL‐1β and IL‐23 in the periphery. Conversely, IL‐17 production induced by TCR signalling was also reported.56 Naive γδ T cells developed after birth may egress the thymus as ‘inducible’ γδ17 cells (mostly Vγ4+) and differentiate to produce IL‐17 after encounter with antigen.
Figure 2.
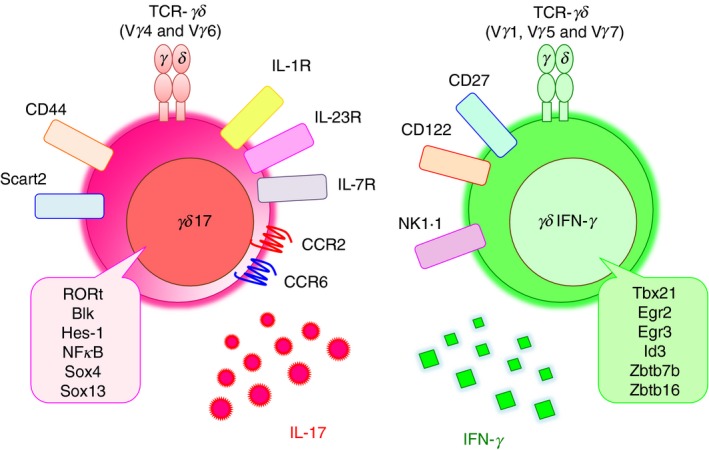
Characterization of γδ17cells. γδ17 cells have some distinct phenotypes and are distinguished from interferon‐γ (IFN‐γ)‐producing γδ T cells ; γδ17 cells express interleukin‐1 receptor (IL‐1R), IL‐23R, IL‐7R, CCR2, CCR6, CD44 and Scart2 on the cell surface. The transcriptional factors of RORγt, Blk, Hes‐1, NF‐κB, Sox4 and Sox13 are important for γδ17 development. On the other hand, IFN‐γ‐producing γδ T cells express CD27, CD122 and NK1.1. These phenotypes are established during thymic development.
The requirement of TCR signalling for γδ17 cell development is not fully understood.51 Early T‐cell precursors can produce IL‐17 before TCR recombination50 and Sox4 and Sox13 are expressed before activation with TCR signalling.42 Antigen‐naive γδ T cells in the thymus differentiate into IL‐17 producers, whereas antigen‐experienced cells make IFN‐γ.28 Furthermore, Vγ5+Vδ1+ thymocytes induce Egr3 upon recognition of Skint‐1 expressed on thymic epithelial cells, resulting in the induction of IFN‐γ expression and suppression of RORγt and Sox13 expression.52 Thus, TCR signalling seems to direct γδ thymocytes to differentiate into IFN‐γ‐producing γδ T cells by suppressing the ‘default’ IL‐17 programme.
The mechanism of IL‐17 production in γδ17 cells
γδ17 cells are functionally committed in the thymus, producing IL‐17 in the periphery after stimulation with IL‐1β and IL‐23 without additional TCR stimulation.21, 24 The ‘ready‐to‐go’ phenotype of γδ17 cells is especially efficient for early‐stage pathogen clearance. A combination of IL‐1β and IL‐23, but not IL‐1β or IL‐23 alone, is required to induce IL‐17 by γδ17 cells.20 Further study revealed that IL‐23 is required for the induction of IL‐1R, and IL‐1β is essential for the induction of IL‐17.20 However, because IL‐1β alone does not induce IL‐17 production in Il1rn −/− mouse‐derived splenic γδ T cells20 and in normal peritoneum‐ and lung‐derived γδ T cells, in which high levels of IL‐1R are expressed,48 IL‐23 may play other roles than up‐regulating IL‐1R in the induction of IL‐17 expression in γδ T cells. In this context, RORγt expression is up‐regulated by IL‐1β and IL‐23 in a synergistic manner.20 The inflammatory cytokine IL‐18,53 complement C5a,54 the ligand of Toll‐like receptors 1 and 2, and dectin‐155 also induce IL‐17 in collaboration with IL‐23. Although γδ17 cells typically behave as innate‐like immune cells, IL‐17 induction by TCR signalling is also reported. Mouse and human γδ T cells recognize an algal protein, phycoerythrin, and differentiate to IL‐17‐producing cells after immunization by this antigen.56 These studies, in combination with cell reconstitution studies,50 suggest that ‘natural’ γδ17 cells (Vγ6+ and part of Vγ4+) acquire IL‐17‐producing ability in the fetal thymus and do not require TCR stimulation in the periphery, whereas ‘inducible’ γδ17 cells (mostly Vγ4+) that develop after birth produce IL‐17 upon encounter with antigens57 (Fig. 1). Notably, phycoerythrin antigen stimulation induces IL‐1R expression on γδ T cells.56 Therefore, γδ TCR activation may make ‘inducible’ γδ17 cells respond to IL‐1β and IL‐23 to induce IL‐17. A similar activation mechanism is also suggested in Th17 cell differentiation; IL‐1R expression is increased upon Th17 differentiation from naive CD4+ T cells,58 and the polarized Th17 cells can produce IL‐17 by IL‐1 and IL‐23 in the absence of TCR stimulation.59 However, the molecular basis for the ‘inducible’ state and the difference between ‘naive’ and ‘inducible’ γδ17 cells remain to be elucidated.
The pathogenic roles of γδ17 cells in mouse inflammatory disease models
Il17 –/– mice show significantly reduced severity in various inflammatory and autoimmune disease models, such as collagen‐induced arthritis,6 Il1rn −/− mouse arthritis,7 EAE8 and imiquimod (IMQ)‐induced skin inflammation,9, 10 suggesting critical roles of IL‐17 in inflammatory/autoimmune diseases. γδ17 cells are detected in inflamed tissues of these disease models. However, the roles of γδ T cells in the development of diseases and the responsible γδ subset are different in different models (Table 1). As no conditional Il17 –/– mice in which the Il17 gene is deleted specifically in γδ17 cells are available, the pathogenicity of γδ17 cells has been analyzed using γδ T‐cell‐deficient mice (Tcrd ‐/‐ mice) or the γδ17 cell subset from these disease models.
Table 1.
γδ17 induction and function in inflammatory disease
| Disease | Disease model | Dominant subset | Induction of γδ17 cells | γδ17 function | References |
|---|---|---|---|---|---|
| Rheumatoid arthritis (RA) | collagen‐induced arthritis | Vγ4+ Vδ4+ | Mycobacterium tuberculosis components in complete Freund's adjuvant (CFA) or subsequent cytokine induction | Promote Th17 cells | 22, 24, 60 |
| Il1rn −/− mice | Vγ6+ Vδ1+ | Up‐regulation of interleuki ‐1 receptor (IL‐1R) | IL‐17‐induced hyperinflammation | 20 | |
| Spondyloarthritides (SpA) | IL‐23 overexpression | Vγ6+ | Hyper IL‐23 induction | IL‐17‐induced hyperinflammation | 65 |
| Multiple sclerosis (MS) | EAE | Vγ4+ | IL‐1β and IL‐23 from dendritic cells (DC) induced by Mycobacterium tuberculosis components in CFA | Promote Th17 cells, and suppress regulatory T cells | 21, 61 |
| Uveitis | EAU | Vγ4+ Vδ4+ | Components in CFA or subsequent cytokine induction | Promote Th17 cells | 104 |
| Psoriasis | IMQ | Vγ4+ and Vγ6+ | Imiquimod (IMQ)‐induced IL‐23 from DC | IL‐17 production and subsequent neutrophil inflammation | 9, 10, 23, 35, 42, 64 |
| IL‐23 | Hyper IL‐23 induction | IL‐17 production and subsequent neutrophil inflammation | 23 | ||
| Uveoretinitis in autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APCED) | Aire −/− mice | Vγ6+ Vδ1+ | Up‐regulation of IL‐7 in the thymus | IL‐17‐induced hyperinflammation | 44 |
| Skin graft rejection | Male to female skin transplantation | Vγ4+ | Accumulation CCR6+ γδ17 cells in skin graft | IL‐17 promotes the accumulation of DC in draining lymph nodes to subsequently activate Th17 cells | 66 |
In the collagen‐induced arthritis model, both γδ17 cells and Th17 cells are found in joints and draining lymph nodes and the majority of the γδ17 cells are Vγ4+/Vδ4+.22, 24 Vγ4+ cell depletion reduces Th17 cell number60 as well as arthritis severity,22 suggesting that the Vγ4+/Vδ4+subset aggravates disease by promoting a Th17 cell response. Some components of heat‐killed Mycobacterium tuberculosis in complete Freund's adjuvant or inflammatory cytokines induced by the adjuvant are suggested to induce Vγ4+/Vδ4+ cell expansion.24, 60 Il1rn −/− mice develop arthritis spontaneously in an IL‐17‐dependent manner.7 In these mice, however, only γδ17 cells are the IL‐17 producer in the joints, although both γδ17 cells and Th17 cells are detected in the draining lymph nodes.20 IL‐17‐GFP reporter mice reveal that the Vγ6+/Vδ1+ cells predominantly produce IL‐17 in affected joints. Adoptive transfer of Il1rn –/– T cells into scid/scid mice shows that only a mixture of γδ T and CD4+ T cells, but not γδ T cells or CD4 T cells alone, can induce arthritis. Moreover, γδ17 cells localize in joints only when γδ T cells are transferred together with CD4+ T cells. These observations suggest that CD4+ T cells are required for γδ17 cells to localize in joints, and IL‐17 from γδ17 cells drives the development of arthritis. Interestingly, Vγ6+ γδ17 cells in Il1rn −/− mice intrinsically express IL‐1R at high levels, indicating that these cells are ready for IL‐17 production. Hence, IL‐17 derived from different γδ17 cell subsets is suggested to play a crucial role in the development of arthritis in mouse models.
The mouse model of multiple sclerosis (MS), EAE is another model in which IL‐17 plays a crucial role in the pathogenesis. After induction of EAE, γδ17 cells as well as Th17 cells are found in the brain, with Vγ4+ cells as the major component.21 Tcrd −/− mice delay the onset of disease and reduce the clinical scores. γδ T cells activated with IL‐1β and IL‐23 promote IL‐17 production by CD4+ T cells in vitro and co‐transfer of CD4+ and γδ T cells promote development of EAE, suggesting that γδ17 cells act in an amplification loop for IL‐17 production by Th17 cells.21 Interleukin‐23‐activated γδ T cells also prevent regulatory T‐cell function, resulting in the enhancement of αβ T‐cell responses and EAE development.61
A pathogenic role for γδ17 cells is implicated in psoriasis. Vγ5+/Vδ1+ γδ T cells, also called dendritic epidermal T cells, uniquely residing in epidermis produce IFN‐γ and participate in immunosurveillance.62 On the other hand, dermis contains Vγ4+ and Vγ6+ subsets responsible for IL‐17 production. The pathogenicity of γδ17 cells in psoriasis is well studied in a mouse model of psoriasis, IMQ‐induced dermatitis.9, 23 Imiquimod is a Toll‐like receptor‐7/8 agonist and induces IL‐17‐dependent psoriasiform dermatitis by inducing IL‐23.63 The IMQ‐induced epidermal thickening is significantly decreased in Tcrd –/– mice, but not in Tcrb –/– mice.9 Development of dermatitis is also suppressed by the deficiency of innate lymphoid cells (ILCs), suggesting that γδ17 cells and IL‐17‐producing group 3 ILCs (ILC3s) are responsible for the development of psoriasiform dermatitis in mice.9, 23 Both Vγ4+ and Vγ6+ subsets produce IL‐17 after IMQ treatment of the skin.35 Skin inflammation after IMQ treatment is significantly attenuated in Sox4 −/− mice, in which dermal Vγ4+ but not dermal Vγ6+ γδ T cells are greatly reduced.42 Congenic CD45.1+ (B6.SJL) mice with naturally occurring Sox13 mutation, in which dermal Vγ4+ γδ17 cell development is defective, develop attenuated ear skin inflammation with less acanthosis and fewer epidermal neutrophil pustules upon treatment with IMQ.64 However, both wild‐type bone marrow cell‐reconstituted mice and neonatal thymocytes plus CD45.1+(B6.SJL) bone marrow cell‐reconstituted mice (in which Vγ4+ and Vγ6+ cells are predominant, respectively) similarly develop epidermal thickening with increased dermal γδ17 cells and neutrophil infiltration, suggesting that both γδ17 subsets induce IMQ‐induced dermatitis.35 Interleukin‐17F and IL‐22 from γδ T cells are also pathogenic in IMQ‐induced dermatitis.9 Interleukin‐23‐induced skin inflammation is another model of psoriasis. The IL‐17‐producing dermal cells are significantly reduced in Tcrd −/− mice accompanied with less skin inflammation and acanthosis, whereas Tcra −/− mice normally develop dermatitis,23 suggesting that γδ17 cells play major roles in the pathogenesis of psoriatic dermatitis in this model. The involvement of γδ17 cells in the development of psoriasiform dermatitis suggests that innate immune responses rather than an autoimmune reaction are important for the development of psoriatic dermatitis.
The involvement of Vγ6+ γδ17 cells in the pathogenesis of uveoretinitis in Aire‐deficient mice, a model of autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APCED), is suggested, because Vγ6+ γδ17 thymocytes are expanded due to high levels of IL‐7 expression in Aire −/− medullary thymic epithelial cells.44 Importantly, expansion of IL‐17‐producing Vγ9+/Vδ2+ γδ T cells is observed in APCED patients, suggesting the involvement of γδ17 cells in these patients. γδ17 cells are also implicated in the pathogenesis of spondyloarthritis; the CD27− γδ T‐cell population is increased in the Achilles tendon after over‐expression of IL‐23, which induces spondyloarthritis‐like enthesitis in mice.65 The Vγ4+ subset produces IL‐17 in the skin grafts and in the host epidermis around grafts, suggesting the involvement in skin graft rejection.66 Vγ4+cell‐derived IL‐17 promotes the accumulation of mature dendritic cells in the draining lymph nodes to subsequently increase Th17 cells after skin graft transplantation.66
Migration of γδ17 cells to inflammatory sites
Trafficking of γδ17 cells to the inflammatory sites is important for the development of inflammation. Naive αβ T cells express a chemokine receptor CCR7, which is important for homeostatic circulation; its expression is down‐regulated during differentiation and the inflammatory chemokine receptor CCR6 is induced on Th17 cells instead. CCR6+ Th17 cells are recruited by CCL20 to cause inflammation, as shown in SKG mice67 and the EAE model.68, 69 On the other hand, a gene array analysis shows that the expression of chemokine receptors such as CCR6, CCR2 and CXCR6 is already up‐regulated in γδ17 cells during thymic development.34 Recent studies have indicated that the CCL20–CCR6 axis is mainly required for γδ17 cell recruitment into homeostatic sites such as dermis,70 whereas the CCL2–CCR2 axis recruits γδ17 cells into inflammatory sites, including psoriatic skin,71 arthritic joints,20 central nervous system in EAE, infected mucosal tissues and tumours.70 Interestingly, γδ17 cells constitutively express both CCR2 and CCR6, but down‐regulate CCR6 expression after inflammation.70 This is consistent with the ‘ready‐to‐go’ nature of γδ17 cells and suggests a γδ17 cell‐recruiting mechanism in which γδ17 cells are released from the tissue‐specific harness to migrate into inflammatory sites by reducing the tissue‐specific homing receptor.
γδ17 cells in tumours
Pro‐tumour function of γδ17 cells has been demonstrated in several cancer models, including a breast cancer metastasis model72 and an ovarian cancer model,73 and the roles of γδ17 cells in the development of tumours have been reviewed elsewhere.74 Abundant γδ17 cell infiltration accompanied by immunosuppressive myeloid‐derived suppressor cells is found in human colorectal cancer with positive correlation with advanced tumour clinicopathological features, suggesting that γδ17 cells induce myeloid‐derived suppressor cell‐mediated immunosuppression.75 On the other hand, anti‐tumour function of γδ17 cells after therapeutic treatment has also been reported. γδ17 cells infiltration is observed when bladder cancer is treated by intravesical injection of Mycobacterium bovis bacillus Calmette–Guérin, (BCG) and these cells are protective against tumour development by recruiting neutrophils.76 Moreover, γδ17 cell infiltration in epithelial tumours is observed after chemotherapy and γδ17 cells enhance the recruitment of IFN‐γ‐producing CD8+ T cells that mediate the anti‐tumour function.77
γδ17 cells in pathogen clearance
Interleukin‐17 plays protective roles against bacterial and fungal infection by recruiting neutrophils, activating T cells and inducing antimicrobial peptides and inflammatory cytokines.1, 4, 5 γδ17 cells produce much more IL‐17 than Th17 cells after Mycobacterium tuberculosis 78 or Mycobacterium bovis BCG infection.79 Expression of IL‐17 is detected in lungs from the first day after infection with BCG and induces not only neutrophil‐mediated inflammation but also granuloma formation.79 Dermal γδ T cells also produce IL‐17 at the first day after intradermal BCG infection and induce neutrophil recruitment and antigen‐specific CD4+ T‐cell expansion.80 Rapid IL‐17 production by Vδ1+ T cells is also observed in the peritoneum after intraperitoneal infection with Escherichia coli, followed by neutrophil recruitment.81 γδ17 cells are also found in the liver after Listeria monocytogenes infection82 and in lungs after Klebsiella pneumoniae infection.83
γδ17 cells produce IL‐17 rapidly after infection with fungi, such as Candida albicans. γδ17 cells are observed in lungs after systemic C. albicans infection, and both Il17 −/− and Tcrd −/− mice are defective in neutrophil recruitment and fungal clearance.84 Interleukin‐17 is produced in tongue‐resident γδ T cells as well as CD3+ CD4+ CD44hi TCR‐β + CCR6+ natural Th17 cells within 1–2 days after oral C. albicans infection.85 As IL‐17 production in γδ T cells is found at the early stage after infection, γδ17 cells are suggested to play an important role in early host defence before establishment of acquired immunity. In humans, patients carrying mutations in either STAT3, IL‐17RA, or IL‐17F or producing anti‐IL‐17F autoantibodies are also highly susceptible to skin infection with Staphylococcus aureus and C. albicans.86, 87 However, the involvement of γδ17 cells in host defence in humans against these pathogens remains to be elucidated.
Recently, pathogen‐specific memory γδ T cells have been implicated in several infection models. Memory γδ T cells are elicited in mesenteric lymph nodes after oral L. monocytogenes infection and contribute to clearance of the bacteria by promptly producing IL‐17 after secondary infection.88, 89 Interestingly, both Vγ4+ and Vγ1− Vγ4− γδ (potentially Vγ6+) T cells produce IL‐17 in the lungs as early as 2 hr after Bordetella pertussis infection, whereas the exclusively Vγ4+ subset expands in lungs 14 days after infection. Moreover, lung Vγ4+ γδ T cells produce IL‐17 in response to heat‐killed B. pertussis in the presence of antigen‐presenting cells.90 These studies suggest that Vγ4+ T cells, but not Vγ6+ T cells, behave like adaptive immunological memory cells in a pathogen‐specific manner.
γδ17 cells in human inflammatory diseases
Although it has been thought that psoriasis is caused by an autoimmune mechanism,91 recent studies using mouse models suggest the involvement of innate immunity.92 High frequency of γδ T cells is detected in psoriasiform dermal lesions in mice induced by IMQ, and these cells produce IL‐17 through stimulation with IL‐23,23 indicating the innate immune nature of the disease. γδ T cells, especially Vγ9+Vδ2+ cells which can produce IL‐17, are also accumulated in the skin of patients with psoriasis.93 In this report, however, Vγ9+Vδ2+ cells were activated to produce cytokines from keratinocytes by the specific antigen, suggesting that recognition of specific antigens may be important for the development of psoriasis. Because Th17 cells94 and ILC3s95 as well as γδ17 cells91 are also detected in the psoriatic skin, the involvement of autoimmunity and the main source of IL‐17 during the development of psoriasis still remain obscure in humans.
Recently, several antibodies targeting IL‐17 and its receptor have been approved or are in clinical trials for the treatment of psoriasis and psoriatic arthritis.96 Secukinumab, an anti‐IL‐17 antibody, has been approved in Japan in 2014 and by the US Food and Drug Administration in 2015 for the treatment of psoriasis and psoriatic arthritis. Treatment with secukinumab for psoriasis patients in a phase III trial shows that more than half of the patients accomplish almost complete remission after 12 weeks of treatment, as determined by the Psoriasis Area and Severity Index (PASI 90), and show better efficacy than a tumour necrosis factor inhibitor (etanercept).11 Ixekizumab, another monoclonal antibody against IL‐17, and Brodalumab, a monoclonal antibody against IL‐17RA, are also effective for the treatment of psoriasis in phase III trials12, 13 and approved for the treatment of psoriasis. Secukinumab has also been approved for the treatment of ankylosing spondylitis.97
As described, the importance of IL‐17 in the development of rheumatoid arthritis (RA) is suggested in mouse models and γδ17 cells are accumulated in arthritic joints,7, 20, 22, 24 but the importance of γδ17 cells in patients with RA is controversial. A recent report shows that Vδ2+ γδ T cells accumulate in the synovium of patients with RA and produce high levels of IL‐17 as well as IFN‐γ.98 However, the predominance of IFN‐γ‐producing γδ T cells instead of γδ17 cells in affected joints is reported elsewhere.24 This discrepancy may result from the differences of medical treatment and/or stages of RA. Because the efficacy of anti‐IL‐17 treatment of RA is moderate except for some patients with specific HLA types,99, 100 γδ17 cells may not play crucial roles in the development of RA in humans. Further studies in patients with RA are necessary.
Elevated IL‐17 expression in γδ T cells, but not CD4+ T cells, was found in patients with systemic juvenile idiopathic arthritis,101 and IL‐17 production was detected in CD161hi CCR6+ γδ T cells in cerebrospinal fluid of patients with MS.102 Treatment of patients with MS with secukinumab non‐significantly reduced the number of combined unique active lesions and significantly reduced the number of cumulative new gadolinium‐enhancing T1 lesions by 67%.103 Further studies are necessary to elucidate the role of γδ17 cells in the development of these diseases.
Concluding remarks
In this review, we discussed the development and the function of γδ17 cells and the roles of γδ17 cells in inflammatory/autoimmune diseases and host defence against pathogens. However, several important questions still remain to be elucidated. The function of TCR signalling in the thymic development of γδ17 and the functional roles of TCR signalling in the periphery upon infection and inflammation are not completely elucidated. Elucidation of epigenetic modifications of genes in ‘natural’ and ‘inducible’ γδ17 cells may provide important information. Discovery of γδ17 cell‐specific markers may provide a more efficient approach to induce γδ17 cell‐specific dysfunction in inflammatory diseases without affecting systemic Th17 cells. Most importantly, it is not clear how well mouse disease models represent the pathogenesis of human diseases, especially those of psoriasis, MS and RA. In the case of psoriasis, inhibition of IL‐17 signalling efficiently cures the symptoms both in psoriasis patients and IMQ‐induced psoriasis models, suggesting that γδ17 cells play important roles in both humans and mice. However, in MS and RA, in which the involvement of γδ17 is suggested by mouse models, the therapeutic effects of anti‐IL‐17 are not so drastic as indicated by these models, suggesting that the pathogenic mechanisms may be different in some parts between diseases in humans and mice. Clearly, further analysis of pathogenic mechanisms in patients is necessary to explain this discrepancy.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
We thank Professor Danny Altmann, the Editor of Immunology, for the invitation to submit this review. Our laboratory is funded by Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Y.I.) and AMED (Y.I.).
Contributor Information
Aoi Akitsu, Email: aoi_akitsu@dfci.harvard.edu.
Yoichiro Iwakura, Email: iwakura@rs.tus.ac.jp.
References
- 1. Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin‐17 family members. Immunity 2011; 34:149–62. [DOI] [PubMed] [Google Scholar]
- 2. Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol 2017; 18:612–21. [DOI] [PubMed] [Google Scholar]
- 3. Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P et al Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony‐stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001; 194:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y et al Differential roles of interleukin‐17A and ‐17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 2009; 30:108–19. [DOI] [PubMed] [Google Scholar]
- 5. Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A et al Dectin‐2 recognition of alpha‐mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans . Immunity 2010; 32:681–91. [DOI] [PubMed] [Google Scholar]
- 6. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen‐induced arthritis in IL‐17‐deficient mice. J Immunol 2003; 171:6173–7. [DOI] [PubMed] [Google Scholar]
- 7. Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL‐17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL‐1 receptor antagonist. Proc Natl Acad Sci U S A 2003; 100:5986–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S et al IL‐17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 2006; 177:566–73. [DOI] [PubMed] [Google Scholar]
- 9. Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA et al Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest 2012; 122:2252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tortola L, Rosenwald E, Abel B, Blumberg H, Schäfer M, Coyle AJ et al Psoriasiform dermatitis is driven by IL‐36‐mediated DC‐keratinocyte crosstalk. J Clin Invest 2012; 122:3965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CEM, Papp K et al Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med 2014; 371:326–38. [DOI] [PubMed] [Google Scholar]
- 12. Griffiths CEM, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386:541–51. [DOI] [PubMed] [Google Scholar]
- 13. Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C et al A prospective phase III, randomized, double‐blind, placebo‐controlled study of brodalumab in patients with moderate‐to‐severe plaque psoriasis. Br J Dermatol 2016; 175:273–86. [DOI] [PubMed] [Google Scholar]
- 14. Maxwell JR, Zhang Y, Brown WA, Smith CL, Byrne FR, Fiorino M et al Differential roles for interleukin‐23 and interleukin‐17 in intestinal immunoregulation. Immunity 2015; 43:739–50. [DOI] [PubMed] [Google Scholar]
- 15. Lee JS, Tato CM, Joyce‐Shaikh B, Gulen MF, Cayatte C, Chen Y et al Interleukin‐23‐independent IL‐17 production regulates intestinal epithelial permeability. Immunity 2015; 43:727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song X, Dai D, He X, Zhu S, Yao Y, Gao H et al Growth factor FGF2 cooperates with interleukin‐17 to repair intestinal epithelial damage. Immunity 2015; 43:488–501. [DOI] [PubMed] [Google Scholar]
- 17. Tang C, Kakuta S, Shimizu K, Kadoki M, Kamiya T, Shimazu T et al Suppression of IL‐17F, but not of IL‐17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat Immunol 2018; 19:755–65. [DOI] [PubMed] [Google Scholar]
- 18. Cua DJ, Tato CM. Innate IL‐17‐producing cells: the sentinels of the immune system. Nat Rev Immunol 2010; 10:479–89. [DOI] [PubMed] [Google Scholar]
- 19. Akitsu A, Kakuta S, Saijo S, Iwakura Y. Rag2‐deficient IL‐1 receptor antagonist‐deficient mice are a novel colitis model in which innate lymphoid cell‐derived IL‐17 is involved in the pathogenesis. Exp Anim 2014; 63:235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akitsu A, Ishigame H, Kakuta S, Chung S‐H, Ikeda S, Shimizu K et al IL‐1 receptor antagonist‐deficient mice develop autoimmune arthritis due to intrinsic activation of IL‐17‐producing CCR2+Vγ6+ γδ T cells. Nat Commun 2015; 6:7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin‐1 and IL‐23 induce innate IL‐17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 2009; 31:331–41. [DOI] [PubMed] [Google Scholar]
- 22. Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen‐induced arthritis by oligoclonal, IL‐17‐producing γδ T cells. J Immunol 2007; 179:5576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cai Y, Shen X, Ding C, Qi C, Li K, Li X et al Pivotal role of dermal IL‐17‐producing γδ T cells in skin inflammation. Immunity 2011; 35:596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito Y, Usui T, Kobayashi S, Iguchi‐Hashimoto M, Ito H, Yoshitomi H et al γδ T cells are the predominant source of interleukin‐17 in affected joints in collagen‐induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum 2009; 60:2294–303. [DOI] [PubMed] [Google Scholar]
- 25. Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ γδ T cells as fetal thymus‐derived naturally occurring IL‐17 producers. J Immunol 2008; 181:5940–7. [DOI] [PubMed] [Google Scholar]
- 26. Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ et al CD27 is a thymic determinant of the balance between interferon‐γ‐ and interleukin 17‐producing γδ T cell subsets. Nat Immunol 2009; 10:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haas JD, González FHM, Schmitz S, Chennupati V, Föhse L, Kremmer E et al CCR6 and NK1.1 distinguish between IL‐17A and IFN‐γ‐producing γδ effector T cells. Eur J Immunol 2009; 39:3488–97. [DOI] [PubMed] [Google Scholar]
- 28. Jensen KDC, Su X, Shin S, Li L, Youssef S, Yamasaki S et al Thymic selection determines γδ T cell effector fate: antigen‐naive cells make interleukin‐17 and antigen‐experienced cells make interferon γ . Immunity 2008; 29:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shibata K, Yamada H, Nakamura M, Hatano S, Katsuragi Y, Kominami R et al IFN‐γ‐producing and IL‐17‐producing γδ T cells differentiate at distinct developmental stages in murine fetal thymus. J Immunol 2014; 192:2210–8. [DOI] [PubMed] [Google Scholar]
- 30. Heilig JS, Tonegawa S. Diversity of murine γ genes and expression in fetal and adult T lymphocytes. Nature 1986; 322:836–40. [DOI] [PubMed] [Google Scholar]
- 31. Pereira P, Gerber D, Regnault A, Huang SY, Hermitte V, Coutinho A et al Rearrangement and expression of Vγ1, Vγ2 and Vγ3 TCR γ genes in C57BL/6 mice. Int Immunol 1996; 8:83–90. [DOI] [PubMed] [Google Scholar]
- 32. O'Brien RL, Born WK. γδ T cell subsets: a link between TCR and function? Semin Immunol 2010; 22:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T et al Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 2008; 451:211–5. [DOI] [PubMed] [Google Scholar]
- 34. Narayan K, Sylvia KE, Malhotra N, Yin CC, Martens G, Vallerskog T et al Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat Immunol 2012; 13:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai Y, Xue F, Fleming C, Yang J, Ding C, Ma Y et al Differential developmental requirement and peripheral regulation for dermal Vγ4 and Vγ6T17 cells in health and inflammation. Nat Commun 2014; 5:3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W et al Homing of a γδ thymocyte subset with homogeneous T‐cell receptors to mucosal epithelia. Nature 1990; 343:754–7. [DOI] [PubMed] [Google Scholar]
- 37. Bonneville M, O'Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010; 10:467–78. [DOI] [PubMed] [Google Scholar]
- 38. Papotto PH, Ribot JC, Silva‐Santos B. IL‐17+ γδ T cells as kick‐starters of inflammation. Nat Immunol 2017; 18:604–11. [DOI] [PubMed] [Google Scholar]
- 39. Laird RM, Laky K, Hayes SM. Unexpected role for the B cell‐specific Src Family Kinase B lymphoid kinase in the development of IL‐17‐producing T cells. J Immunol 2010; 185:6518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T et al Notch‐Hes1 pathway is required for the development of IL‐17‐producing γδ T cells. Blood 2011; 118:586–93. [DOI] [PubMed] [Google Scholar]
- 41. Powolny‐Budnicka I, Riemann M, Tänzer S, Schmid RM, Hehlgans T, Weih F. RelA and RelB transcription factors in distinct thymocyte populations control lymphotoxin‐dependent interleukin‐17 production in γδ T cells. Immunity 2011; 34:364–74. [DOI] [PubMed] [Google Scholar]
- 42. Malhotra N, Narayan K, Cho OH, Sylvia KE, Yin C, Melichar H et al A network of high‐mobility group box transcription factors programs innate interleukin‐17 production. Immunity 2013; 38:681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Do J‐S, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ et al Cutting edge: spontaneous development of IL‐17‐producing γδ T cells in the thymus occurs via a TGF‐β1‐dependent mechanism. J Immunol 2010; 184:1675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fujikado N, Mann AO, Bansal K, Romito KR, Ferre EMN, Rosenzweig SD et al Aire inhibits the generation of a perinatal population of interleukin‐17A‐producing γδ T cells to promote immunologic tolerance. Immunity 2016; 45:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Michel M‐L, Pang DJ, Haque SFY, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL‐7) selectively promotes mouse and human IL‐17‐producing γδ cells. Proc Natl Acad Sci U S A 2012; 109:17549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmolka N, Wencker M, Hayday AC, Silva‐Santos B. Epigenetic and transcriptional regulation of γδ T cell differentiation: programming cells for responses in time and space. Semin Immunol 2015; 27:19–25. [DOI] [PubMed] [Google Scholar]
- 47. Prinz I, Silva‐Santos B, Pennington DJ. Functional development of γδ T cells. Eur J Immunol 2013; 43:1988–94. [DOI] [PubMed] [Google Scholar]
- 48. Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL‐1 receptor 1‐expressing and IL‐17‐producing γδ T cells. Cell Host Microbe 2010; 7:140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kisielow J, Kopf M, Karjalainen K. SCART scavenger receptors identify a novel subset of adult γδ T cells. J Immunol 2008; 181:1710–6. [DOI] [PubMed] [Google Scholar]
- 50. Haas JD, Ravens S, Düber S, Sandrock I, Oberdörfer L, Kashani E et al Development of interleukin‐17‐producing γδ T cells is restricted to a functional embryonic wave. Immunity 2012; 37:48–59. [DOI] [PubMed] [Google Scholar]
- 51. Muñoz‐Ruiz M, Ribot JC, Grosso AR, Gonçalves‐Sousa N, Pamplona A, Pennington DJ et al TCR signal strength controls thymic differentiation of discrete proinflammatory γδ T cell subsets. Nat Immunol 2016; 17:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Turchinovich G, Hayday AC. Skint‐1 identifies a common molecular mechanism for the development of interferon‐γ‐secreting versus interleukin‐17‐secreting γδ T cells. Immunity 2011; 35:59–68. [DOI] [PubMed] [Google Scholar]
- 53. Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KHG. Caspase‐1‐processed cytokines IL‐1β and IL‐18 promote IL‐17 production by γδ and CD4 T cells that mediate autoimmunity. J Immunol 2011; 186:5738–48. [DOI] [PubMed] [Google Scholar]
- 54. Han G, Geng S, Li Y, Chen G, Wang R, Li X et al γδ T‐cell function in sepsis is modulated by C5a receptor signalling. Immunology 2011; 133:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin‐17‐producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009; 31:321–30. [DOI] [PubMed] [Google Scholar]
- 56. Zeng X, Wei Y‐L, Huang J, Newell EW, Yu H, Kidd BA et al γδ T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen‐specific interleukin‐17 response. Immunity 2012; 37:524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chien Y‐H, Zeng X, Prinz I. The natural and the inducible: interleukin (IL)‐17‐producing γδ T cells. Trends Immunol 2013; 34:151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ikeda S, Saijo S, Murayama MA, Shimizu K, Akitsu A, Iwakura Y. Excess IL‐1 signaling enhances the development of Th17 cells by downregulating TGF‐β‐induced Foxp3 expression. J Immunol 2014; 192:1449–58. [DOI] [PubMed] [Google Scholar]
- 59. Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS et al Critical regulation of early Th17 cell differentiation by interleukin‐1 signaling. Immunity 2009; 30:576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roark CL, Huang Y, Jin N, Aydintug MK, Casper T, Sun D et al A canonical Vγ4Vδ4+ γδ T cell population with distinct stimulation requirements which promotes the Th17 response. Immunol Res 2012; 55:217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S et al γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin‐23‐dependent mechanism. Immunity 2010; 33:351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hayday AC. γδ T cells and the lymphoid stress‐surveillance response. Immunity 2009; 31:184–96. [DOI] [PubMed] [Google Scholar]
- 63. Yoshiki R, Kabashima K, Honda T, Nakamizo S, Sawada Y, Sugita K et al IL‐23 from Langerhans cells is required for the development of imiquimod‐induced psoriasis‐like dermatitis by induction of IL‐17A‐producing γδ T cells. J Invest Dermatol 2014; 134:1912–21. [DOI] [PubMed] [Google Scholar]
- 64. Gray EE, Ramírez‐Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE et al Deficiency in IL‐17‐committed Vγ4+ γδ T cells in a spontaneous Sox13‐mutant CD45.1+ congenic mouse substrain provides protection from dermatitis. Nat Immunol 2013; 14:584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdörfer L et al Interleukin‐23‐dependent γ/δ T cells produce interleukin‐17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol 2016; 68:2476–86. [DOI] [PubMed] [Google Scholar]
- 66. Li Y, Huang Z, Yan R, Liu M, Bai Y, Liang G et al Vγ4 γδ T cells provide an early source of IL‐17A and accelerate skin graft rejection. J Invest Dermatol 2017; 137:2513–22. [DOI] [PubMed] [Google Scholar]
- 67. Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N et al Preferential recruitment of CCR6‐expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med 2007; 204:2803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S et al C‐C chemokine receptor 6‐regulated entry of TH‐17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 2009; 10:514–23. [DOI] [PubMed] [Google Scholar]
- 69. Arima Y, Harada M, Kamimura D, Park J‐H, Kawano F, Yull FE et al Regional neural activation defines a gateway for autoreactive T cells to cross the blood–brain barrier. Cell 2012; 148:447–57. [DOI] [PubMed] [Google Scholar]
- 70. McKenzie DR, Kara EE, Bastow CR, Tyllis TS, Fenix KA, Gregor CE et al IL‐17‐producing γδ T cells switch migratory patterns between resting and activated states. Nat Commun 2017; 8:15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ramírez‐Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vγ4+ γδT17 memory‐like cells that travel to distant skin and accelerate secondary IL‐17‐driven responses. Proc Natl Acad Sci U S A 2015; 112:8046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau C‐S et al IL‐17‐producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015; 522:345–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rei M, Goncalves‐Sousa N, Lanca T, Thompson RG, Mensurado S, Balkwill FR et al Murine CD27– V 6+ T cells producing IL‐17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A 2014; 111:E3562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Silva‐Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol 2015; 15:683–91. [DOI] [PubMed] [Google Scholar]
- 75. Wu P, Wu D, Ni C, Ye J, Chen W, Hu G et al γδT17 cells promote the accumulation and expansion of myeloid‐derived suppressor cells in human colorectal cancer. Immunity 2014; 40:785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Takeuchi A, Dejima T, Yamada H, Shibata K, Nakamura R, Eto M, et al IL‐17 production by γδ T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette–Guérin treatment against bladder cancer. Eur J Immunol 2010; 41:246–51. [DOI] [PubMed] [Google Scholar]
- 77. Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P et al Contribution of IL‐17‐producing γδ T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011; 208:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lockhart E, Green AM, Flynn JL. IL‐17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 2006; 177:4662–9. [DOI] [PubMed] [Google Scholar]
- 79. Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K et al IL‐17‐mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette–Guérin infection. J Immunol 2007; 178:3786–96. [DOI] [PubMed] [Google Scholar]
- 80. Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL et al Cutaneous immunosurveillance by self‐renewing dermal γδ T cells. J Exp Med 2011; 208:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL‐17 production. J Immunol 2007; 178:4466–72. [DOI] [PubMed] [Google Scholar]
- 82. Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD et al IL‐17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol 2008; 181:3456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Murakami T, Hatano S, Yamada H, Iwakura Y, Yoshikai Y. Two types of interleukin 17A‐producing γδ T cells in protection against pulmonary infection with Klebsiella pneumoniae . J Infect Dis 2016; 214:1752–61. [DOI] [PubMed] [Google Scholar]
- 84. Dejima T, Shibata K, Yamada H, Hara H, Iwakura Y, Naito S et al Protective role of naturally occurring interleukin‐17A‐producing T cells in the lung at the early stage of systemic candidiasis in mice. Infect Immun 2011; 79:4503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Conti HR, Peterson AC, Brane L, Huppler AR, Hernández‐Santos N, Whibley N et al Oral‐resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. J Exp Med 2014; 211:2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK et al Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin‐17 immunity. Science 2011; 332:65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kisand K, Wolff ASB, Podkrajšek KT, Tserel L, Link M, Kisand KV et al Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17‐associated cytokines. J Exp Med 2010; 207:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sheridan BS, Romagnoli PA, Pham Q‐M, Fu H‐H, Alonzo F, Schubert W‐D et al γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity 2013; 39:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Romagnoli PA, Sheridan BS, Pham Q‐M, Lefrançois L, Khanna KM. IL‐17A‐producing resident memory γδ T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proc Natl Acad Sci U S A 2016; 113:8502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Misiak A, Wilk MM, Raverdeau M, Mills KHG. IL‐17‐producing innate and pathogen‐specific tissue resident memory γδ T cells expand in the lungs of Bordetella pertussis‐infected mice. J Immunol 2017; 198:363–74. [DOI] [PubMed] [Google Scholar]
- 91. Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C et al The antimicrobial peptide LL37 is a T‐cell autoantigen in psoriasis. Nat Commun 2014; 5:5621. [DOI] [PubMed] [Google Scholar]
- 92. Keijsers RRMC, Joosten I, van Erp PEJ, Koenen HJPM, van de Kerkhof PCM. Cellular sources of IL‐17 in psoriasis: a paradigm shift? Exp Dermatol 2014; 23:799–803. [DOI] [PubMed] [Google Scholar]
- 93. Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N et al Identification of a novel proinflammatory human skin‐homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol 2011; 187:2783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Matos TR, O'Malley JT, Lowry EL, Hamm D, Kirsch IR, Robins HS et al Clinically resolved psoriatic lesions contain psoriasis‐specific IL‐17‐producing αβ T cell clones. J Clin Invest 2017; 127:4031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Villanova F, Flutter B, Tosi I, Grys K, Sreeneebus H, Perera GK et al Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol 2014; 134:984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ritchlin CT, Krueger JG. New therapies for psoriasis and psoriatic arthritis. Curr Opin Rheumatol 2016; 28:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pavelka K, Kivitz A, Dokoupilova E, Blanco R, Maradiaga M, Tahir H et al Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double‐blind phase 3 study, MEASURE 3. Arthritis Res Ther 2017; 19:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mo W‐X, Yin S‐S, Chen H, Zhou C, Zhou J‐X, Zhao L‐D et al Chemotaxis of Vδ2 T cells to the joints contributes to the pathogenesis of rheumatoid arthritis. Ann Rheum Dis 2017; 76:2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Genovese MC, Braun DK, Erickson JS, Berclaz P‐Y, Banerjee S, Heffernan MP et al Safety and efficacy of open‐label subcutaneous ixekizumab treatment for 48 weeks in a Phase II study in biologic‐naive and TNF‐IR patients with rheumatoid arthritis. J Rheumatol 2015; 43:289–97. [DOI] [PubMed] [Google Scholar]
- 100. Burmester GR, Durez P, Shestakova G, Genovese MC, Schulze‐Koops H, Li Y et al Association of HLA‐DRB1 alleles with clinical responses to the anti‐interleukin‐17A monoclonal antibody secukinumab in active rheumatoid arthritis. Rheumatology 2016; 55:49–55. [DOI] [PubMed] [Google Scholar]
- 101. Kessel C, Lippitz K, Weinhage T, Hinze C, Wittkowski H, Holzinger D et al Proinflammatory cytokine environments can drive interleukin‐17 overexpression by γ/δ T cells in systemic juvenile idiopathic arthritis. Arthritis Rheumatol 2017; 69:1480–94. [DOI] [PubMed] [Google Scholar]
- 102. Schirmer L, Rothhammer V, Hemmer B, Korn T. Enriched CD161high CCR6+ γδ T cells in the cerebrospinal fluid of patients with multiple sclerosis. JAMA Neurol 2013; 70:345–51. [DOI] [PubMed] [Google Scholar]
- 103. Havrdová E, Belova A, Goloborodko A, Tisserant A, Wright A, Wallstroem E et al Activity of secukinumab, an anti‐IL‐17A antibody, on brain lesions in RRMS: results from a randomized, proof‐of‐concept study. J Neurol 2016; 263:1287–95. [DOI] [PubMed] [Google Scholar]
- 104. Cui Y, Shao H, Lan C, Nian H, O'Brien RL, Born WK et al Major role of γδ T cells in the generation of IL‐17+ uveitogenic T cells. J Immunol 2009; 183:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


