Summary
Rheumatoid arthritis (RA) is a chronic inflammatory disease where serum analysis of anti‐citrullinated peptide/protein antibodies (ACPA) is an important diagnostic/prognostic tool. Levels and changes of ACPA in RA patients have been studied previously in relation to disease course and therapy response, but less is known regarding ACPA isotype changes in early RA. Hence, recent‐onset RA patients (n = 231) were subjected to a 3‐year clinical and radiological follow‐up. Serum samples were serially collected and ACPA isotypes were analysed using the second‐generation cyclic citrullinated peptide (CCP) as capture antigen. Changes in ACPA isotype levels and status were related to disease course and pharmacotherapy. At inclusion, 74% of the patients tested positive for ACPA IgG; 55% for immunoglobulin (Ig)A, 37% for secretory IgA (SIgA) and 35% for IgM. The proportion of positive patients decreased significantly at follow‐up regarding ACPA SIgA, IgM and IgA. During the initial 3 months, reduction of the 28‐joint disease activity score (DAS28) correlated with reduced levels of ACPA IgG (Rho = 0·242, P = 0·003), IgA (Rho = 0·260, P = 0·008), IgM (Rho = 0·457, P < 0·001) and SIgA (Rho = 0·402, P < 0·001). Levels of ACPA SIgA (P = 0·008) and IgM (P = 0·021) decreased significantly among patients with good response to treatment, which was not seen regarding ACPA IgA or IgG. Changes in ACPA isotype levels were not associated with radiographic damage. In conclusion, ACPA SIgA and IgM declined rapidly upon anti‐rheumatic therapy and correlated with decreased disease activity in recent‐onset RA. This may indicate that down‐regulation of mucosal immunity to citrullinated proteins/peptides and recruitment of new B cells are key features of therapy responses in early RA.
Keywords: anti‐citrullinated protein antibodies (ACPA), disease course, immunoglobulin (Ig) isotypes, longitudinal analyses, rheumatoid arthritis (RA)
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease, where genetic as well as environmental factors are of importance regarding disease development. RA patients are commonly subdivided according to the presence or absence of circulating immunoglobulin (Ig)G anti‐citrullinated protein antibodies (ACPA). The presence of ACPA identifies patients at increased risk of a severe disease course [1] and there is solid evidence that both environmental and genetic associations differ between ACPA‐positive and ‐negative cases [2, 3, 4]. In clinical routine, typically only serum ACPA IgG is analysed, but serum ACPA of IgM‐ and IgA‐class are also present in 30–60% of early RA cases [5, 6].
ACPA may contribute to RA pathogenesis in different ways depending on isotype. ACPA IgG may trigger inflammation by Fc‐gamma receptor ligation and/or classical complement activation, which may be enhanced by altered glycosylation patterns of ACPA IgG [7]. ACPA IgM has been reported to induce cell‐activating properties following complement activation [8]. The half‐life of circulating IgM is short, and the fact that ACPA IgM has been detected in patients with long‐standing RA suggests continuous recruitment of naive B cells [9]. ACPA IgA does not activate the classical complement pathway, but may exert dual effects after ligation to Fc‐alpha receptors expressed on cell‐surfaces [10, 11]. We previously reported that patients with recent‐onset RA testing double‐seropositive for IgA‐ and IgG‐class ACPA at baseline suffered from a more severe disease course compared to patients positive for ACPA IgG alone [5, 12].
Circulating IgA is produced mainly in the bone marrow, whereas secretory IgA (SIgA) is produced at mucosal surfaces, where it serves as a first line of defence [13, 14]. In humans, circulating IgA is mainly monomeric, while SIgA is a dimeric or polymeric molecule linked together by a joining chain (J‐chain) and complexed with secretory component (SC) [14]. We have shown recently that ACPA SIgA can be detected not only in mucosal fluids [15], but also in sera from recent‐onset RA patients [15, 16].
Changes in ACPA levels and specificities have been studied extensively in relation to disease course and therapeutic response in established RA [17], but the associations to clinical outcomes have not been strongh enough to justify repeated analyses in clinical routine. The temporal resolution of ACPA isotype dynamics has not previously been characterized thoroughly in early RA, despite possible associations with disease induction, course and outcome. Hence, in the present study, we investigated ACPA of IgG‐, IgA‐, IgM‐ and SIgA‐class in serially collected serum samples from a well‐defined cohort of recent‐onset RA patients and related changes in ACPA levels to treatment and disease course during 3 years.
Materials and methods
Subjects
The TIRA‐2 cohort (Swedish acronym for the 2nd ‘Early Intervention in Rheumatoid Arthritis’ cohort) enrolled patients with recent‐onset RA (< 12 months since first observed joint‐swelling according to the patient’s judgement) at six rheumatology clinics in southeast/mid‐Sweden between 2006 and 2009 [16]. Patients fulfilled either the American College of Rheumatology 1987 RA‐classification criteria (ACR‐87) [18] or a more liberal set of criteria to enable inclusion of earlier RA cases [morning stiffness ≥ 60 min, symmetrical palpable synovitis and arthritis in hands or feet (wrists, metacarpophalangeal, proximal interphalangeal, metatarsophalangeal joints)].
The patients had not received disease‐modifying anti‐rheumatic drugs (DMARDs) prior to the baseline visit. Serum samples were taken at inclusion (0 months), 3, 12 and 36 months, and stored at −70°C until analysed. To study the longitudinal response of ACPA isotypes (IgG, IgA, IgM and SIgA) we identified only the patients who had serum samples available from all visits (n = 231, 46% of the entire TIRA‐2 cohort). Baseline patient characteristics are summarized in Tables 1 and 2. Follow‐up visits including 28‐joint disease activity score (DAS28) [19], and self‐reported pain on a 100‐mm visual analogue scale (VAS) was scheduled at 3, 12 and 36 months (patient characteristics on clinical parameters shown in Supporting information, Fig. S1). Therapy during follow‐up was instituted as found appropriate by the treating physician at the baseline visit, according to current practice in Sweden. Response to therapy was categorized according to the European League Against Rheumatism (EULAR) response criteria [20]. The study protocol was approved by the regional ethics review board (Linköping, Sweden; decision number M168‐05), and all participating subjects gave written informed consent.
Table 1.
Baseline patient characteristics (n = 231) and treatment regimen started at the inclusion visit
| Women, n (%) | 162 (70·1) |
| Mean age (years ± s.d.) | 57·4 ± 13·0 |
| Rheumatoid factor‐positive, n (%) | 150 (64·9) |
| IgG anti‐CCP‐positive, n (%) | 172 (74·4) |
| DAS28 (mean ± s.d.)a | 5·1 ± 1·2 |
| HAQ (mean ± s.d.)b | 1·0 ± 0·6 |
| Mean IgG anti‐CCP level (U/ml ± s.e.m.)c | 180·2 ± 9·7 |
| Mean IgA anti‐CCP level (µg/ml ± s.e.m.)d | 7·5 ± 0·9 |
| Mean IgM anti‐CCP level (AU/ml ± s.e.m.)e | 9950·2 ± 1262 |
| Mean SIgA anti‐CCP level (AU/ml ± s.e.m.)f | 3929·4 ± 389·7 |
| Oral corticosteroids, n (%)h | 138 (59·7) |
| csDMARD single therapy, n (%) | 196 (84·8) |
| csDMARD combination therapy, n (%) | 20 (8·7) |
| bDMARD therapy, n (%) | 1 (0·4) |
DAS28 = disease activity score 28; HAQ = health assessment questionnaire; csDMARD = conventional synthetic disease‐modifying anti‐rheumatic drug (single therapy including methotrexate (MTX), salazopyrin (SSZ), anti‐malaria or leflunomide. Combination therapy including different combinations of MTX, SSZ, anti‐malarial and imurel) and bDMARD = biological disease‐modifying anti‐rheumatic drug; s.e.m. = standard error of the mean.
Data available from 215 patients;
data available from 206 patients;
cut‐off: ACPA IgG 7U/ml;
anti‐citrullinated protein antibodies (ACPA) immunoglobulin (Ig)A 2 µg/l;
ACPA IgM 6032 AU/ml;
ACPA SIgA 3089 AU/ml;
data available from 230 patients.
Table 2.
Patient characteristics (n = 231) at month 36
| Larsen score (mean ± s.d.)a | 4·2 ± 4·4 |
|---|---|
| Larsen progress (mean ± s.d.)a | 1·8 ± 2·8 |
| Oral corticosteroids, n (%) | 109 (47·2) |
| csDMARD single therapy, n (%) | 138 (59·7) |
| csDMARD combination therapy, n (%) | 42 (18·2) |
| bDMARD, n (%) | 28 (12·1) |
csDMARD = conventional synthetic disease‐modifying anti‐rheumatic drug (single therapy including methotrexate (MTX), salazopyrin (SSZ), antimalaria or leflunomide. combination therapy including different combinations of MTX, SSZ, anti‐malarial and imurel) and bDMARD = biological disease‐modifying anti‐rheumatic drug. a Data available from 155 patients; s.d. = standard deviation.
ACPA detection
All ACPA isotypes were detected by immunoassays using the second‐generation cyclic citrullinated peptide (CCP) as antigen. ACPA SIgA and IgM were measured by modifying commercially available anti‐CCP enzyme‐linked immunosorbent assay (ELISA) kits (Euro‐Diagnostica, Malmö, Sweden). Serum samples were diluted 1 : 25, added to precoated CCP microtitre plates and incubated for 1 h. Following washing, horseradish peroxidase (HRP)‐conjugated polyclonal goat anti‐human SIgA (which detects free and bound secretory component) and IgM (which detects the heavy chain) antibodies, respectively (Nordic Biosite, Täby, Sweden), were used to detect SIgA anti‐CCP (dilution 1 : 2000) and IgM (1 : 10 000). Incubation was arrested with 0·5 M H2SO4 and read at 450 nm with 650 nm as reference wavelength (Tecan Sunrise software, Magellan version 7.1; Tecan Nordic AB, Mölndal, Sweden). A seven‐step serial dilution was used for standard curve calculations using patient sera with high levels of anti‐CCP SIgA and anti‐CCP IgM, respectively. Samples were analysed in duplicate using the mean value. The intra‐ and interassay variations in the ACPA SIgA ELISA were 1 and 10%, respectively, and 2 and 17% in the ACPA IgM ELISA, which was determined using three samples with the assay repeated three times. Control serum samples from healthy blood donors (50 females, 50 males; mean age 46 years) were collected in 2010 and used to determine the cut‐off for positive tests. Healthy controls often display low levels of the analysed antibodies, but by setting the cut‐off values using the 99th percentile among the controls [anti‐CCP SIgA 3089 arbituary units (AU)/ml and anti‐CCP IgM 6032 AU/ml] this problem does not apply.
Serum ACPA IgA and IgG were analysed by a fluoroenzyme immunoassay (EliATM; Thermo Fisher Scientific/PhaDia AB, Uppsala, Sweden), as described previously [21]. The cut‐off level for a positive ACPA IgA test was set at the 99th percentile of healthy blood donors (2 µg/l). For ACPA IgG analyses, we used the cut‐off point suggested by the manufacturer (7U/ml).
Radiographic analyses
Baseline and 3‐year follow‐up radiographs of hand and feet were available from 155 of the 231 patients selected for the present study. The 155 patients with radiographs available were slightly younger than those without (mean age = 56 versus 60 years, P = 0·014), while the remaining baseline characteristics (Table 1) were not significantly different. Joint damage was evaluated in chronological order by one experienced reader (M.Z.), according to the Larsen‐Dahle method [22].
Statistical analyses
Statistical analyses were performed using spss statistics version 23 (IBM, Armonk, NY, USA) and Prism version 6.0c (Graphpad Software Inc., La Jolla, CA, USA). P‐values < 0·05 were regarded as statistically significant. Differences in proportions were analysed using the McNemar test. Differences within isotype levels during the follow‐up were analysed using Friedman’s test. The Kruskal–Wallis test was used to test differences between the relative changes in antibody level across isotypes and Dunn’s multiple comparisons test was used as post‐hoc analysis. When investigating the impact of the change in antibody levels among the four isotypes in relation to disease course, we selected only patients who were isotype‐positive at inclusion. Spearman’s test was used to compare changes in relative antibody levels with changes in DAS28 and its components, VAS pain and radiographic damage, respectively. The Mann–Whitney U‐test was used to analyse differences in DAS28 change and radiographic damage compared to patients displaying one versus two or more ACPAs. Differences in the score for radiographic damage between patients remaining ACPA positive and patients becoming ACPA‐negative were tested using the Mann–Whitney U‐test.
Differences in relative mean antibody levels change across European League Against Rheumatism (EULAR) response criteria and differences in relative mean antibody change versus treatment were tested using the Mann–Whitney U‐test.
Results
Dynamics of ACPA isotypes in early RA
At inclusion, 74% of the patients tested positive for ACPA IgG and 55% for ACPA IgA (Fig. 1), and the proportion of positive patients did not differ significantly between inclusion and any time‐point during follow‐up.
Figure 1.
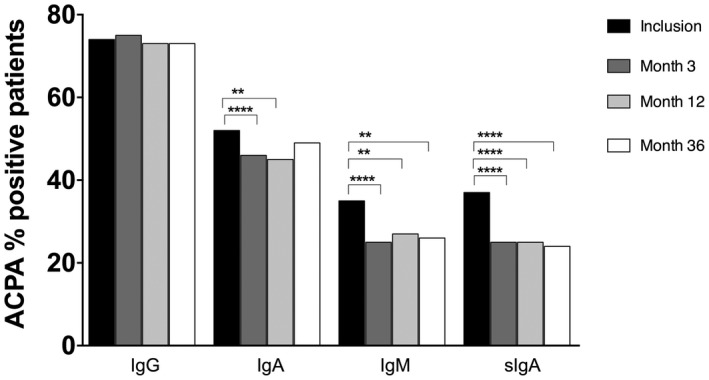
Percentage of positive anti‐citrullinated protein antibodies (ACPA) immunoglobulin (Ig)G, IgA, IgM and secretory IgA (SIgA) rheumatoid arthritis (RA) patients at inclusion and during the 36 months of follow‐up. *P < 0·05 and **P < 0·01 (McNemar test).
Regarding ACPA SIgA, 37% of the patients tested positive at inclusion, and 33% (28 of 85) of these became negative during follow‐up, resulting in a significantly lower proportion of positive patients (P < 0·0001 for baseline versus months 3, 12 and 36, respectively) (Fig. 1). Thirty‐five per cent tested positive at baseline for ACPA IgM and 30% (24 of 81) of these had become negative at month 3, resulting in a significantly lower proportion of positive patients (P < 0·0001). This remained similar at months 12 and 36, and months 12 and 36 were significantly different from baseline (P = 0·003 and P = 0·002, respectively; Fig. 1). Fifty‐two per cent of the patients were ACPA IgA‐positive at baseline, which was a significantly higher percentage compared to month 3, when 11% (13 of 120) of these patients had become negative (P = 0·007). At month 12, 13% had become ACPA IgA‐negative (16 of 120, P = 0·002). Conversely, the percentage of patients remaining ACPA IgG‐positive also remained similar during the follow‐up (Fig. 1).
Fifty‐three per cent of the ACPA SIgA‐positive patients showed high antibody levels (defined as two times above the cut‐off level) and in ACPA IgM and IgA these percentages were 61 and 70%, respectively. For ACPA IgG, as many as 98% of the positive patients showed high antibody levels. Eighteen per cent were positive in one ACPA isotype, 20% were positive in two different ACPAs, 9% in three different ACPAs and 29% displayed all four ACPAs (Supporting information, Fig. S2).
Antibody levels of all the four isotypes had decreased at months 3, 12 and 36 compared with baseline (P = 0·0001) (Fig. 2). There were no significant differences in ACPA isotype levels between months 3, 12 and 36 of follow‐up.
Figure 2.
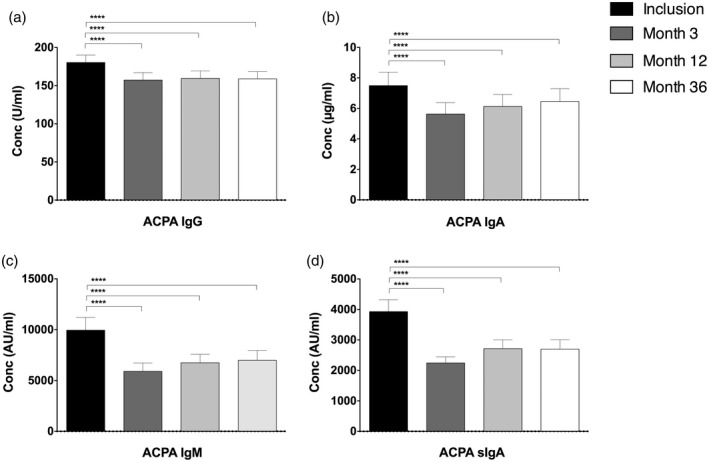
Mean levels of anti‐citrullinated protein antibodies (ACPA) immunoglobulin (Ig)G, IgA, IgM and secretory IgA (SIgA) in rheumatoid arthritis (RA) patients at inclusion and during the 36 months of follow‐up. ****P = 0·0001 (Friedman’s test). Error bars represent standard error of the mean (s.e.m.) (n = 231).
Among patients testing negative for ACPA IgG, IgA, SIgA and IgM at inclusion, 1% turned positive during the 36 months of follow‐up regarding ACPA IgG, 2% became SIgA‐ or IgM‐positive and 3% became IgA‐positive.
When comparing the relative change in antibody levels between ACPA isotypes, levels differed significantly between inclusion and month 3 (P < 0·0001). As illustrated in Fig. 3, post‐hoc analyses showed that the relative change in antibody levels between inclusion and month 3 were most pronounced regarding ACPA SIgA and IgM levels compared to ACPA IgG (P < 0·0001 for both, Fig. 3). ACPA SIgA and IgM relative antibody change were also more pronounced compared to ACPA IgA (P < 0·001 and P < 0·05, respectively). Relative changes between ACPA SIgA and IgM levels did not differ significantly, while ACPA IgA showed a greater decrease in relative antibody level compared to ACPA IgG (P < 0·05) (Fig. 3). The relative change in ACPA antibody level differed neither between months 3 and 12 nor months 12 and 36.
Figure 3.
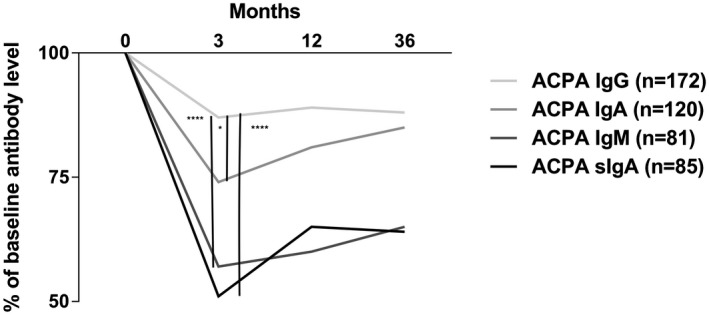
Relative change in levels of anti‐citrullinated protein antibodies (ACPA) immunoglobulin (Ig)G, IgM and secretory IgA (SIgA) in early rheumatoid arthritis (RA) compared with relative change in ACPA IgG levels (****represent P < 0·0001 and *P < 0·05, Kruskal–Wallis test followed by Dunn’s multiple comparisons test).
Relative changes in ACPA isotypes levels and disease course
During the initial 3 months, the relative decrease in ACPA IgG, IgA, IgM and SIgA levels correlated significantly with reduction in DAS28 (Table 3). DAS28 reduction did not differ between patients displaying one ACPA isotype compared to patients displaying two or more ACPA isotypes (P = 0·972). After month 12, only ACPA IgG remained correlated significantly with changes in DAS28 (Table 3). As shown in Fig. 3, changes in antibody levels were more pronounced between inclusion and month 3. We therefore analysed the change in DAS28 components during the first 3 months. The decrease in ACPA SIgA and IgM levels during the first 3 months showed weak to moderate statistically significant correlations with all DAS28 components and self‐reported pain (Table 4). The change in ACPA IgA and IgG levels correlated with change in swollen joint count. In addition, the change in ACPA IgA levels correlated with the change in C‐reactive protein (CRP) and with the change in patient’s global assessment. Conversely, the change in ACPA IgG levels correlated with the change in tender joint count and VAS pain (Table 4). Of the ACPA isotypes analysed, only the change in ACPA IgG and SIgA levels between inclusion and month 12 correlated with the change in HAQ (ACPA IgG rho = 0·227, P = 0·006 and ACPA IgA, rho = 0·245, P = 0·041).
Table 3.
Spearman’s correlation analysis of relative change in antibody levels in relation to DAS28 change in recent‐onset RA patients (n = 231)
| ACPA IgG | ACPA IgA | ACPA IgM | ACPA SIgA | |||||
|---|---|---|---|---|---|---|---|---|
| Months after inclusion | Rho | P | Rho | P | Rho | P | Rho | P |
| 0–3 | 0·242 | 0·003 | 0·260 | 0·008 | 0·457 | 0·001 | 0·402 | 0·001 |
| 3–12 | 0·271 | 0·001 | 0·171 | 0·079 | 0·276 | 0·021 | 0·240 | 0·036 |
| 12–36 | 0·232 | 0·003 | 0·056 | 0·555 | 0·210 | 0·069 | 0·018 | 0·875 |
DAS28 = disease activity score 28; ACPA = anti‐citrullinated protein antibodies; Ig = immunoglobulin; SIgA = secretory IgA.
Bold values display statistically significant differences.
Table 4.
Spearman correlation analysis of relative change in antibody levels during the first 3 months in relation to change in DAS28 components and VAS pain in recent‐onset RA patients at inclusion positive for the respective antibody
| ACPA isotype | Swollen joints | Tender joints | CRP | ESR | Patient’s global assessment | VAS pain | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho | P | Rho | P | Rho | P | Rho | P | Rho | P | Rho | P | |
| SIgA | 0.355 | 0.001 | 0.426 | 0.001 | 0.304 | 0.006 | 0.356 | 0.001 | 0.228 | 0.043 | 0.322 | 0.004 |
| IgM | 0.344 | 0.002 | 0.488 | 0.001 | 0.395 | 0.001 | 0.387 | 0.001 | 0.228 | 0.049 | 0.266 | 0.020 |
| IgG | 0.201 | 0.010 | 0.200 | 0.010 | 0.105 | 0.185 | 0.093 | 0.243 | 0.148 | 0.062 | 0.197 | 0.013 |
| IgA | 0.243 | 0.009 | 0.130 | 0.165 | 0.265 | 0.005 | 0.175 | 0.068 | 0.223 | 0.019 | 0.132 | 0.168 |
DAS28 = disease activity score 28; ACPA = anti‐citrullinated protein antibodies; VAS = visual analogue scale; RA = rheumatoid arthritis, Ig = immunoglobulin; SIgA = secretory IgA; CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate.
Bold values display statistically significant differences.
During the first 3 months, we also categorized the DAS28 change according to the EULAR response criteria. The mean change of ACPA SIgA decreased more significantly among patients (n = 46) with a good EULAR response compared to those patients (n = 19) with no EULAR response (50 versus 31%; P = 0·008). A similar difference was seen regarding ACPA IgM, where good responders (n = 45) had a mean decrease of 45% compared to 31% among those patients (n = 14) with no EULAR response, P = 0·021). There were no differences in the mean change of ACPA SIgA and IgM levels between patients with moderate and no EULAR response. The mean change in ACPA IgA and IgG levels did not differ according to EULAR response (data not shown).
Changes in ACPA isotypes levels in relation to radiographic joint damage
For each ACPA isotype, the 3‐year radiographic joint damage, as graded by the Larsen score, was compared between patients remaining ACPA positive at 3 months and patients becoming ACPA‐negative at 3 months. The Larsen score at month 36 did not differ (P = 0·57) between patients remaining ACPA IgG‐positive (4·7 ± 4·3 [mean ± standard deviation (s.d.)], n = 120) and patients becoming negative (2·0 ± 0, n = 1). This was also the case for ACPA IgA (4·3 ± 3·6 versus 3·7 ± 4·8, n = 72 versus n = 14, P = 0·31), ACPA IgM (4·4 ± 3·6 versus 2·9 ± 3·5, n = 37 versus n = 18, P = 0·11) and ACPA SIgA (4·1 ± 3·7 versus 4·8 ± 5·9, n = 34 versus n = 22, P = 0·82). The change in Larsen score during the initial 36 months did not differ between patients remaining positive or becoming negative for any of the ACPA isotypes [ACPA IgG (2·1 ± 2·9 versus 2·0 ± 0, n = 116 versus n = 1, P = 0·66 (mean ± s.d., positive versus negative)] ACPA IgA (1·8 ± 2·6 versus 1·6 ± 3·1, n = 72 versus n = 13, P = 0·45), ACPA IgM (2·0 ± 3·0 versus 1·6 ± 2·7, n = 37 versus n = 18, P = 0·46) and ACPA SIgA (1·9 ± 3·1 versus 1·5 ± 2·4, n = 34 versus n = 22, P = 0·65). Similarly, changes in ACPA isotype levels did not correlate significantly with radiographic joint damage at month 36, nor with 36‐month progression of joint damage (data not shown). However, the Larsen score at month 36 showed a trend towards being significant higher in patients positive for one single ACPA isotype compared to patients displaying two or more (P = 0·056) and the score for Larsen progression at 36 months was significantly different in single‐positive patients compared to patients with two or more ACPA isotypes (P = 0·040). However, when adjusting for multiple comparisons this significant difference disappeared.
Changes in relative ACPA isotype levels in relation to treatment
Forty‐nine of the 84 (58%) patients positive for ACPA SIgA started treatment with oral corticosteroids at the baseline visit (mean prednisolone dose of 8·9 mg, range = 1–20 mg), and in this group the mean change in ACPA SIgA levels between months 0 and 3 were greater compared to non‐treated ACPA SIgA‐positive patients (n = 35; 55 versus 30% AU/ml; P < 0·0001). Similarly, there was a more pronounced decrease in the mean change of ACPA IgA levels (30 versus 22%; P = 0·029) among corticosteroid‐treated patients (n = 70 of 120), as well as regarding the mean change of ACPA IgM levels in corticosteroid‐treated patients (41 versus 22%; n = 45 of 81); P = 0·01). Corticosteroid treatment did not associate significantly with change in ACPA IgG levels. DMARD monotreatment was compared with DMARD combination treatment in patients positive for the respective ACPA isotype. For ACPA SIgA (77 versus 48%; n = 9/80, P = 0·005) and IgA (47 versus 25%; n = 10 of 114), P = 0·022) a combination of DMARDs treatment induced a greater decrease in antibody levels between months 0 and 3, but the change in ACPA IgM and IgG levels were not affected significantly. The effect of biological DMARDs (bDMARDs) could not be analysed, as this treatment was extremely rare within the first year.
Discussion
This observational study presents a detailed longitudinal characterization of ACPA isotypes in relation to disease course in ‘real‐world’ early RA. We found clear differences in ACPA isotype responses over time, both in terms of antibody levels and status. The proportion of patients testing positive regarding ACPA SIgA and IgM decreased significantly after start of anti‐rheumatic treatment, whereas the status of ACPA IgA and IgG remained similar during follow‐up. Regarding antibody levels, all tested isotypes decreased during the first 3 months, but remained stable thereafter. Interestingly, levels of ACPA SIgA and IgM showed a more pronounced decrease than ACPA IgG, which remained significant after correction for multiple comparisons. ACPA SIgA also showed a more substantial decline than ACPA IgA, as did ACPA IgM, but following correction for multiple comparisons only ACPA SIgA showed a greater decrease compared to ACPA IgA. The half‐life of different isotypes in the circulation differs markedly (21–25 days for IgG compared to 5–6 days for IgA and IgM) which, of course, could be relevant to the dynamics of ACPA isotypes during the initial 3 months. However, we find this an unlikely explanation to the observed patterns, as ACPA IgA and IgM (where IgA and IgM generally show similar isotype half‐lives) displayed differences, whereas ACPA IgA and IgG (where IgA and IgG typically differs in isotype half‐lives) did not. Our results are partly in line with results from a smaller cohort reporting decreasing ACPA levels of IgA and IgM, as well as the IgG subclasses apart from IgG1 at 7 years follow‐up [6].
In order to identify ACPA isotypes of particular clinical and/or pathogenic interest, we correlated changes of antibody levels to a variety of clinical outcomes. During the initial 3 months, improvement in disease activity was correlated moderately with decrease in ACPA SIgA and IgM levels. Although the ACPA levels overall decreased during the follow‐up, the type of treatment appeared to influence the magnitude of the decline. Patients treated with oral corticosteroids or a combination of DMARDs displayed a greater reduction in ACPA SIgA and ACPA IgA levels, whereas a greater change in ACPA IgM levels were associated only with oral corticosteroid treatment. In contrast, the change in ACPA IgG levels was not influenced by either treatment. In RA cases with longer disease duration, it has been shown that add‐on treatment primary targeting the adaptive immune system (i.e. anti‐CD20 treatment with rituximab or T‐cell modulation with abatacept) reduces ACPA levels, which associates with reduced disease activity and improved health [17, 23, 24]. Also, Cambridge et al. showed that both ACPA IgG and IgA decreased after rituximab treatment [25]. After intracellular synthesis, SC is transported to the cell membrane and is then available to bind IgA [26]. Thus, reduced SC expression could lead to lower levels of SIgA. The expression of SC is dependent upon tumour necrosis factor (TNF) [27]‐induced nuclear factor kappa B (NF‐αB) activation [28]. TNF inhibition seems to associate with ACPA reductions in early RA [29], but not in established disease [24, 29]. Hence, it would be relevant to study the impact of anti‐TNF therapy regarding ACPA SIgA levels, but in the current study the statistical power was insufficient for investigation of any specific impact of bDMARDs on ACPA isotype response. In addition, the reduction of ACPA SIgA and IgA levels appeared greater in patients receiving more aggressive treatment, i.e. conventional synthetic DMARD (csDMARD) in combination or addition of low‐dose oral corticosteroids. The glucocorticoid receptor (GR) can act as an enhancer of SC transcription [30]. Speculatively, the reduction of ACPA SIgA in corticosteroid‐treated RA patients could be due to ligation of the corticosteroid with the GR followed by a lower SC transcription. In fact, reduced SC levels in bronchoalveolar lavage (BAL) have been shown in a model for asthma where mice were treated with corticosteroids [31].
We found no associations between radiographic joint damage and change in ACPA levels. As expected, baseline ACPA IgG positivity associated with radiographic progression, but ACPA baseline levels did not. Although not investigating antibody levels over time, a previous Dutch study reported that baseline ACPA IgA predicted radiographic progression in RA during a 4‐or 10‐year follow‐up [32]. In contrast, we did not find any significant association between baseline ACPA IgA and radiographic joint damage, but substantial differences in symptom duration and anti‐rheumatic therapy between the cohorts could have contributed to the disparate findings. In the present study, radiographic data were only available from 155 patients. Although this is a limitation, basic characteristics and clinical outcomes were similar between patients with radiographic data and those without. Also, the low proportion of patients treated with bDMARDs did not allow analysis of bDMARds effect on ACPA responses over time, and although the study design included additional time‐points for sample collection, these time‐points could not be used due to dropout.
We conclude that ACPA SIgA and IgM levels in serum decline rapidly in recent‐onset RA patients during the 3 first months, and that this correlates with improved disease activity and more intense pharmacotherapy. This finding is relevant regarding pathogenesis but the lack of correlation with radiographic damage, and with disease activity beyond 3 months, reduces the value of analysing these isotypes in clinical routine. When comparing the change in ACPA levels upon anti‐rheumatic treatment, the change in ACPA SIgA and IgA were more pronounced among patients taking oral corticosteroids or csDMARD in combination. By using CCP as antigen in the current study, we analysed multiple ACPA isotype reactivities. Therefore, forthcoming studies should address whether certain ACPA specificities of SIgA, IgA and IgM isotypes may be related more intimately to disease course.
Disclosures
The authors declare that they have no conflicts of interest.
Supporting information
Acknowledgements
We thank the clinical immunology unit at Linköping University Hospital for performing the ACPA IgG and IgA analyses. We are also most grateful to all TIRA co‐workers and participating patients. This project was funded by grants from the Swedish Society of Medicine, the Swedish research council, the Medical research council of south‐east Sweden, Reinhold Sund foundation, King Gustaf V 80‐year foundation, the Swedish rheumatism association and the Östergötland county council.
References
- 1. Willemze A, Trouw LA, Toes RE, Huizinga TW. The influence of ACPA status and characteristics on the course of RA. Nat Rev Rheumatol 2012; 8:144–52. [DOI] [PubMed] [Google Scholar]
- 2. Lundberg K, Bengtsson C, Kharlamova N et al Genetic and environmental determinants for disease risk in subsets of rheumatoid arthritis defined by the anticitrullinated protein/peptide antibody fine specificity profile. Ann Rheum Dis 2013; 72:652–8. [DOI] [PubMed] [Google Scholar]
- 3. Padyukov L, Seielstad M, Ong RT et al A genome‐wide association study suggests contrasting associations in ACPA‐positive versus ACPA‐negative rheumatoid arthritis. Ann Rheum Dis 2011; 70:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Willemze A, van der Woude D, Ghidey W et al The interaction between HLA shared epitope alleles and smoking and its contribution to autoimmunity against several citrullinated antigens. Arthritis Rheum 2011; 63:1823–32. [DOI] [PubMed] [Google Scholar]
- 5. Svard A, Kastbom A, Reckner‐Olsson A, Skogh T. Presence and utility of IgA‐class antibodies to cyclic citrullinated peptides in early rheumatoid arthritis: the Swedish TIRA project. Arthritis Res Ther 2008; 10:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verpoort KN, Jol‐van der Zijde CM, Papendrecht‐van der Voort EA et al Isotype distribution of anti‐cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum 2006; 54:3799–808. [DOI] [PubMed] [Google Scholar]
- 7. Scherer HU, van der Woude D, Ioan‐Facsinay A et al Glycan profiling of anti‐citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum 2010; 62:1620–9. [DOI] [PubMed] [Google Scholar]
- 8. Anquetil F, Clavel C, Offer G, Serre G, Sebbag M. IgM and IgA rheumatoid factors purified from rheumatoid arthritis sera boost the Fc receptor‐ and complement‐dependent effector functions of the disease‐specific anti‐citrullinated protein autoantibodies. J Immunol 2015; 194:3664–74. [DOI] [PubMed] [Google Scholar]
- 9. Pratesi F, Panza F, Paolini I et al Fingerprinting of anti‐citrullinated protein antibodies (ACPA): specificity, isotypes and subclasses. Lupus 2015; 24:433–41. [DOI] [PubMed] [Google Scholar]
- 10. Olas K, Butterweck H, Teschner W, Schwarz HP, Reipert B. Immunomodulatory properties of human serum immunoglobulin A: anti‐inflammatory and proinflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol 2005; 140:478–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu J, Ji C, Xie F et al FcalphaRI (CD89) alleles determine the proinflammatory potential of serum IgA. J Immunol 2007; 178:3973–82. [DOI] [PubMed] [Google Scholar]
- 12. Svard A, Kastbom A, Soderlin MK, Reckner‐Olsson A, Skogh T. A comparison between IgG‐ and IgA‐class antibodies to cyclic citrullinated peptides and to modified citrullinated vimentin in early rheumatoid arthritis and very early arthritis. J Rheumatol 2011; 38:1265–72. [DOI] [PubMed] [Google Scholar]
- 13. Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 2011; 4:603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol 2006; 208:270–82. [DOI] [PubMed] [Google Scholar]
- 15. Svard A, Kastbom A, Sommarin Y, Skogh T. Salivary IgA antibodies to cyclic citrullinated peptides (CCP) in rheumatoid arthritis. Immunobiology 2013; 218:232–7. [DOI] [PubMed] [Google Scholar]
- 16. Roos K, Martinsson K, Ziegelasch M et al Circulating secretory IgA antibodies against cyclic citrullinated peptides in early rheumatoid arthritis associate with inflammatory activity and smoking. Arthritis Res Ther 2016; 18:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Modi S, Soejima M, Levesque MC. The effect of targeted rheumatoid arthritis therapies on anti‐citrullinated protein autoantibody levels and B cell responses. Clin Exp Immunol 2013; 173:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arnett FC, Edworthy SM, Bloch DA et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31:315–24. [DOI] [PubMed] [Google Scholar]
- 19. Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38:44–8. [DOI] [PubMed] [Google Scholar]
- 20. van Gestel AM, Prevoo ML, van’t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996; 39:34–40. [DOI] [PubMed] [Google Scholar]
- 21. Svard A, Skogh T, Alfredsson L et al Associations with smoking and shared epitope differ between IgA‐ and IgG‐class antibodies to cyclic citrullinated peptides in early rheumatoid arthritis. Arthritis Rheumatol 2015; 67:2032–7. [DOI] [PubMed] [Google Scholar]
- 22. Larsen A. How to apply Larsen score in evaluating radiographs of rheumatoid arthritis in long‐term studies. J Rheumatol 1995; 22:1974–5. [PubMed] [Google Scholar]
- 23. Mikuls TR, O’Dell JR, Stoner JA et al Association of rheumatoid arthritis treatment response and disease duration with declines in serum levels of IgM rheumatoid factor and anti‐cyclic citrullinated peptide antibody. Arthritis Rheum 2004; 50:3776–82. [DOI] [PubMed] [Google Scholar]
- 24. Wunderlich C, Oliviera I, Figueiredo CP, Rech J, Schett G. Effects of DMARDs on citrullinated peptide autoantibody levels in RA patients‐A longitudinal analysis. Semin Arthritis Rheum 2017; 46:709–14. [DOI] [PubMed] [Google Scholar]
- 25. Cambridge G, Leandro MJ, Lahey LJ, Fairhead T, Robinson WH, Sokolove J. B cell depletion with rituximab in patients with rheumatoid arthritis: multiplex bead array reveals the kinetics of IgG and IgA antibodies to citrullinated antigens. J Autoimmun 2016; 70:22–30. [DOI] [PubMed] [Google Scholar]
- 26. Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol 2008;1:11–22. [DOI] [PubMed] [Google Scholar]
- 27. Liu DY, Wang XL, Liu P. Tumor necrosis factor‐alpha upregulates the expression of immunoglobulin secretory component. J Invest Allergol Clin Immunol 2007; 17:101–6. [PubMed] [Google Scholar]
- 28. Takenouchi‐Ohkubo N, Takahashi T, Tsuchiya M, Mestecky J, Moldoveanu Z, Moro I. Role of nuclear factor‐kappaB in the expression by tumor necrosis factor‐alpha of the human polymeric immunoglobulin receptor (plgR) gene. Immunogenetics 2000; 51:289–95. [DOI] [PubMed] [Google Scholar]
- 29. Kastbom A, Forslind K, Ernestam S et al Changes in the anticitrullinated peptide antibody response in relation to therapeutic outcome in early rheumatoid arthritis: results from the SWEFOT trial. Ann Rheum Dis 2016; 75:356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Resendiz‐Albor AA, Reina‐Garfias H, Rojas‐Hernandez S et al Regionalization of pIgR expression in the mucosa of mouse small intestine. Immunol Lett 2010; 128:59–67. [DOI] [PubMed] [Google Scholar]
- 31. Zhao J, Yeong LH, Wong WS. Dexamethasone alters bronchoalveolar lavage fluid proteome in a mouse asthma model. Int Arch Allergy Immunol 2007; 142:219–29. [DOI] [PubMed] [Google Scholar]
- 32. van der Woude D, Syversen SW, van der Voort EI et al The ACPA isotype profile reflects long‐term radiographic progression in rheumatoid arthritis. Ann Rheum Dis 2010; 69:1110–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


