Summary
Kawasaki disease (KD) is an immune‐mediated vasculitis with symptoms that mimic febrile illness; the immune origin has been suggested but never proved. First, cell chip technology was used to screen immune targets cells. With the indirect immunofluorescence assay we found that the HeLa cell chip could be recognized by KD patient serum samples. The target cell proteome was extracted as antigens, and antigen recognition reaction was performed using the patients’ serum as antibodies and the detected target protein was detected and identified as phosphoglycerate kinase 1 (PGK1). Then PGK1 was produced and tested with enzyme‐linked immunosorbent assay (ELISA), Western blotting, immunoprecipitation and competitive inhibition immunofluorescence assay. Immunoglobulin (Ig)G against PGK1 was detected in 46% (23 of 50) sera of KD patients, 13% (five of 38) sera in febrile non‐KD patients (FC) and 2·6% (one of 38) sera in healthy donors. As an immune target, PGK1 not only helps understanding of the pathogenesis, it also has potential value in facilitating the laboratory diagnosis of KD.
Keywords: antigenicity analysis, immune target, Kawasaki disease, PGK1
Introduction
Kawasaki disease (KD), also known as paediatric mucocutaneous lymph node syndrome, is an acute vascular inflammatory disease that occurs predominantly in infants and young children from the age of 6 months to 5 years 1. Currently, the diagnosis of KD relies on clinical symptoms, such as fever persisting for 5 days or longer, changes on the lips, oral cavity and peripheral extremities, etc. 2, as the main symptoms of Kawasaki disease do not have a significant clinical specificity and a higher proportion of atypical cases of KD. Conversely, due to lack of adequate clinical experience, some primary‐level physicians have insufficient knowledge of the disease and cannot observe the condition in a timely and dynamic fashion, which may lead to the increase of clinical misdiagnosis of KD and even the risk of coronary arterial lesions 3. Therefore, the development of Kawasaki disease‐specific laboratory early diagnostic methods, screening and identification of specific targets for clinical diagnosis has become the focus of KD research. Most scholars believe that KD is involved in a combination of genetic factors, genetic susceptibility, infection and immune responses 4. In all these factors immune responses may be implicated in the pathogenesis of KD, and an imbalance in the immune system induced by external infection may be involved in the pathogenesis and development of KD. In the persistent acute febrile phase of KD, the production of cytokines which associate with innate immune response is deemed as the defence of foreign pathogens 5. The immunoglobulin (Ig)A antibodies infiltrate into vascular tissue, suggesting that lymphocytes are responding to certain antigens 6, 7, which is suggestive of an acquired immune response. In general, these researches indicated that KD may be trigged by abnormal immune responses which may result in the production of antibodies. However, reports about immune responses of KD are relatively scarce; antibodies to peroxiredoxin 2 (PRDX2) and 4‐trimethylaminobutyraldehyde dehydrogenase (TMABA‐DH) have been described in KD patient serum 8, 9, and anti‐endothelial cell antibodies (AECA) have also been found in serum of KD patients 10. However, the mechanisms of TMABA‐DH and PRDX2 in causes of KD are not yet known.
Comprehensive utilization of bioinformatics, immunology and molecular biology methods to screen and identify potential biomarkers of disease efficiently is a universally recognized disease target discovery strategy. In our previous studies, immunological analyses were used to make a contribution to the identification of potential diagnostic biomarkers specific to immune‐mediated diseases and other vasculitis‐associated diseases 11. As is widely known in the early diagnosis of many vasculitis and autoimmune diseases, there are some specific immune attack targets, and the related detection of antibody levels in the serum of patients has important reference value in the diagnosis of diseases 12. As a typical systemic vasculitis disease, we speculate that there should be some potential targets for autoantibody attack; therefore, an in‐depth study of antibody levels in the serum of KD patients can not only help to reveal the cause of KD, but is also useful to establish a new method of differential diagnosis of KD.
Material and methods
Serum samples
A total of 186 serum samples from Beijing Children’s Hospital Affiliated of Capital Medical University were selected, including 80 Kawasaki disease patients [the KD serum samples were taken after intravenous Ig (IVIG) administration], 68 febrile non‐KD patients and 38 age‐matched healthy donors. The detailed clinical information of patients of KD, febrile controls (FC) and healthy controls (HC) is described in the Supporting information Tables. The subjects were selected according to the following criteria: KD was diagnosed based on the fifth revised edition of guidelines issued by the Japan Kawasaki Disease Research Committee in 2002 13; febrile non‐KD patients were selected among patients with fever lasting longer than 3 days and with symptoms mimicking KD. The study was approved by the Ethics Committee of Beijing Children’s Hospital Affiliated of Capital Medical University. All subjects signed informed consent.
Cell line culture and immunofluorescence assays
HeLa cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s high glucose medium (DMEM) containing 10% fetal bovine serum (HyClone, South Logan, UT, USA) and 1% penicillin/streptomycin (PAA Laboratories GmbH, Pasching, Austria) in 5% carbon dioxide at 37°C at a constant temperature in the incubator.
For immunofluorescence assay pretreatment, HeLa cells were plated on sterile slides, incubated in a 5% CO2 incubator at 37°C for 10 h, then fixed with 4% paraformaldehyde for 10 min at room temperature. The cell membrane was then treated by 0·2% Triton X‐100 for 10 min. After incubation, the cell slips were blocked with 5% goat serum at 37ºC for 2 h.
An indirect immunofluorescence assay was performed as follows: using serum samples from KD patients, FC and HC were diluted 1 : 20 in phosphate‐buffered saline (PBS) and incubated with the blocked cell slides for 1 h at 37°C. Coverslips were washed afterwards three times with 0·2% PBS Tween (PBST) and then incubated with fluorescein isothiocyanate (FITC)‐conjugated goat‐anti human IgG secondary antibodies (diluted 1 : 100 in PBS; ImmunoHunt, Beijing, China) at 37°C for 1 h in the dark. Finally, imaging analysis was performed using a confocal laser scanning microscope (Olympus, Tokyo, Japan).
Western blotting of HeLa cell extracts
HeLa cells were solubilized with RIPA solution (Beyotime, Jiangsu, China) containing 1 mM PMSF (Sigma‐Aldrich, St Louis, MO, USA) and 1% protease inhibitor cocktail (Sigma‐Aldrich). Cell extracts were separated on 12% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE), and the proteins in the gel were transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Burlington, MA, USA) and blocked with 5% non‐fat milk at 37°C for 2 h. Serum samples of KD, FC and HC (1 : 1000 dilution in 1% skimmed milk) were then incubated with the PVDF membrane overnight at 4°C and washed with 0·1% PBST to remove non‐specifically bound antibodies and incubated with horseradish peroxidase‐conjugated (HRP) goat anti‐human IgG antibodies (diluted 1 : 10 000 in TBS with 1% non‐fat milk; ImmunoHunt, Beijing, China) at 37°C for 1 h. After washing, enhanced chemiluminescence (ECL; Applygen, Beijing, China) detection was performed and the positive band was cut and recovered for mass spectrometry analysis.
Protein expression and purification
First, total RNA of HeLa cells was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), then reverse transcription–polymerase chain reaction (RT–PCR) was carried out (Fermentas, Hanover, MD, USA). Human PGK1 gene was amplified by PCR and separated by agarose gel electrophoresis. The PCR products and pET‐28a (+) vector were both cut by EcoRI and XhoI restriction endonuclease. The expression of recombinant human PGK1 protein (rhPGK1) was induced in Escherichia coli BL21 (DE3) cultured at 28°C for 12 h with shaking at 200 rpm and recombinant human (rh)PGK1 was purified with nickel‐nitrilotriacetic acid (Ni‐NTA) resin (CWBIO, Beijing, China). Finally, the purity and sequence of PGK1 were confirmed with SDS‐PAGE (the gel was stained by Coomassie Blue; Sigma‐Aldrich) and liquid chromatography tandem mass spectrometry time‐of‐flight (LC‐MALDI‐TOF/TOF) mass spectrometry (protocols were consistent with our previous studies 14), respectively.
Enzyme‐linked immunosorbent assay (ELISA)
First, rhPGK1 protein (500 ng/ml, dissolved in 0·05 M carbonate‐bicarbonate buffer, pH 9·6) was diluted and added to a 96‐well microplate (Coring, New York, NY, USA) at 4ºC for 12 h. The 96‐well microplate was then blocked with 10% goat serum at 37ºC for 2 h. Serum samples of KD, FC and HC (diluted 1 100 in PBS) were added to the 96‐well microplate and incubated at 37ºC for 2 h with a constant temperature. After washing with 0·3% PBST, goat anti‐human IgG/HRP antibodies (diluted 1 10 000 in 0·05% PBST) were added and incubated at 37ºC for 1 h. After rinsing with 0·3% PBST, 50 μl of tetramethylbenzidine (TMB)‐A and 50 μl of TMB‐B were added to the 96‐well microplate, respectively, then incubated at room temperature for 5 min in the dark. Finally, the reaction was terminated with 2 M H2SO4. The optical density (OD) 450 nm value of the reference absorbance at 620 nm was measured with a microplate reader (Tecan, Hombrechtikon, Switzerland).
PGK1‐based Western blotting
RhPGK1 protein was separated by electrophoresis in 12% SDS‐PAGE gel and transferred onto PVDF membranes. Afterwards, PVDF membranes were blocked with 5% non‐fat milk at 37ºC for 2 h. PVDF membranes were cut into strips and incubated with KD, FC and HC serum samples (diluted 1 : 1000 in TBS containing 1% non‐fat milk) at 4ºC overnight. After washing, goat anti‐human IgG/HRP antibodies (diluted 1 : 10 000 in 1% non‐fat milk) were incubated with PVDF membrane strips at 37ºC for 2 h, then positive target bands were detected with the ECL kit.
Immunoprecipitation
RhPGK1 protein (5 μg total protein per well) was incubated with 2 μl serum samples of KD, FC and HC overnight at 4°C on a rotator. Afterwards, 40 μl of protein A Sepharose beads (Sigma‐Aldrich) were added and incubated at 4°C for 4 h on a rotator. The immune complex was obtained by centrifuge and analysed with 10% SDS‐PAGE electrophoresis. Finally, anti‐PGK1 polyclonal antibodies (Sangon Biotech, Shanghai, China) were used to verify whether the immune complex contained rhPGK1.
Competitive inhibition immunofluorescence assay
The serum samples were preincubated with rhPGK1 at 37°C for 1 h and the next processes were consistent with indirect immunofluorescence analysis.
Antigenic determinant prediction
As aforementioned, TMABA‐DH and PRDX2 were identified as antigens of KD; thus, Bepipred Linear Epitope Prediction was performed for prediction of the epitopes 15. Potential common epitopes between PGK1 and TMABA‐DH, PRDX2 proteins were selected with the following standards: amino acid lengths were no less than 6 and occurred simultaneously in PGK1, TMABA‐DH and PRDX2.
Statistical analysis
Statistical analysis was performed using spss software (version 21; SPSS Inc., Chicago, IL, USA). The Mann–Whitney U‐test was used for comparison between the two groups. P < 0·05 was considered statistically significant. Receiver operating characteristic (ROC) analysis was carried out with MedCalc (version 9.2.0.1; MedCalc Software, Mariakerke, Belgium) and the diagnostic performance was evaluated. The fluorescence intensity of the cell chip was analysed semiquantitatively using Image J software (NIH, MD, USA).
Results
HeLa is potential target cell
The HeLa cells were cultured and used for the production of a cell chip for indirect immunofluorescence microarray. The results of the incubation of the cell chip with the serum samples are shown in Fig. 1. Obvious fluorescence staining was observed in the KD group, whereas no significant fluorescence staining was observed in the FC and HC groups. The fluorescence intensity ratio of cell chips in the KD group was analysed semiquantitatively by Image J software (Supporting information, Appendix Fig. S1), and was significantly higher than that in the FC and HC groups (P‐values were all less than 0·05). The fluorescence intensity difference between each group was statistically significant, indicating that the proteins on the cell chip can be recognized by antibodies in the serum of KD patients, and HeLa cells can be used as target cells for screening KD immune targets.
Figure 1.
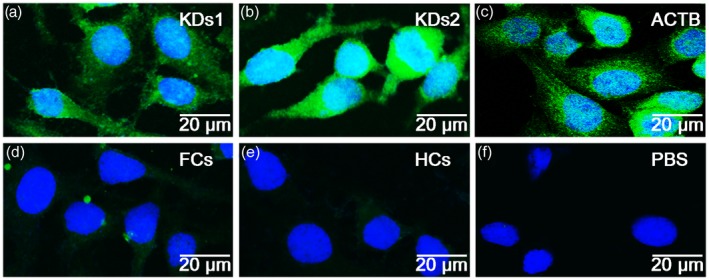
Indirect immune fluorescence assay with HeLa cell chips. HeLa cells were utilized to perform an indirect immunofluorescence assay. The strong positive staining was observed in Kawasaki disease (KD) and positive control groups (a–c), whereas no obvious staining was showed in the febrile non‐KD patients (FC), age‐matched healthy donors (HC) and phosphate‐buffered saline (PBS) control groups (d–f). KDs1: mixture of serum samples from six patients with KD group 1. KDs2: mixture of serum samples from six patients with KD group 2. FCs: mixture of six serum samples from febrile controls. HCs: mixture of six serum samples from healthy controls. ACTB: positive control, serum samples was replaced by anti‐β actin antibody. Phosphate‐buffered saline (PBS): negative control, serum samples was replaced by PBS.
Identification of novel antigens in HeLa
Proteome of HeLa cells was extracted and used as antigens, separated by SDS‐PAGE electrophoresis before being transferred onto PVDF. Then Western blot analysis was performed using serum samples as the primary antibodies. Sheep anti‐human IgG/HRP was used as secondary antibody to detect serum samples. As a result, one of 12 KD serum samples showed a clear band at 45 kDa (Fig. 2); no positive bands were detected in 12 FC controls and 12 HC control serum samples. The 45 kDa protein was then excised from polyacrylamide gel followed by analysis with mass spectrometry. Finally, the amino acid sequence of the target protein was compared with the protein in the mascot bioinformatics database (Supporting information, Appendix Table S1) and shared high sequence homology with human PGK1 protein (Fig. 2), indicating that the positive band at 45 kDa was PGK1 protein, and the KD immune target of PGK1 was recognized by antibodies in serum samples.
Figure 2.
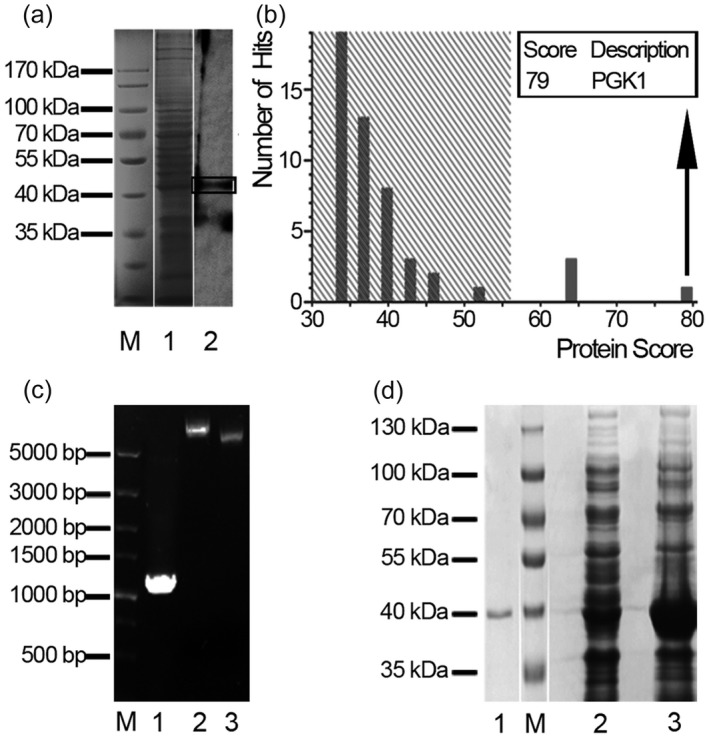
Detection and identification of phosphoglycerate kinase 1 (PGK1). (a) Western blotting of HeLa cell lysates (line 1) using sera from Kawasaki disease (KD) patients demonstrated a positive band around 45 kDa (line 2). (b) Amino acid sequence of the 45 kD target band was identified as PGK1 by mass spectrometry. (c,d) Gene amplification, expression, purification and identification of recombinant human (rh)PGK1. (c) Construction of recombinant plasmid PGK1‐pET/28a(+). M, 1, 2, 3 lanes represent DNA marker, polymerase chain reaction (PCR)‐amplified PGK1 gene sequence, recombinant plasmid and pET‐28a(+) vector, respectively. (d) Expression and purification of recombinant human (rh)PGK1 protein. M, 1, 2, 3 lanes represent protein marker, purified PGK1 protein, protein over‐expression without and with isopropyl β‐D‐1‐thiogalactopyranoside (IPTG) induction, respectively.
Expression and purification of rhPGK1 protein
The human PGK1 protein was produced by recombinant DNA technology for further use in our study. Human PGK1 gene was amplified with PCR and verified by running 0·8% agarose gel electrophoresis (Fig. 2c). Then, recombinant plasmid pET‐28a(+)/PGK1 was constructed successfully and transformed into the E. coli BL21 (DE3) strain. After inducing, the E. coli extracts were analysed by SDS‐PAGE electrophoresis and a strongly over‐expressed protein band appeared between 40 kDa and 50 kDa. Then, protein containing his‐tag was purified with Ni‐NTA resin (Fig. 2d) and its sequence was verified with LC‐MALDI‐TOF/TOF (Supporting information, Appendix Table S2).
Prevalence of the anti‐PGK1 antibodies in sera of KD patients
The levels of anti‐PGK1 antibodies in 126 serum samples were detected by ELISA. The OD value of anti‐PGK1 antibodies in KD serum was 0·2582 ± 001830999 [mean ± standard deviation (s.d.)]. The ROC curve was used to evaluate the performance of PGK1 as a KD immune target in clinical diagnosis (Fig. 3a). The area under the curve was 0·863 [95% confidence interval (CI) = 0·773–0·927; P < 0·01]. The critical point of positive reaction was calculated (healthy controls, mean ± 2 s.d., cut‐off value = 0·243). In the KD serum sample, the anti‐PGK1 antibody‐positive rate was 46% (23 of 50), while in the corresponding FC and HC groups the positive rate of anti‐PGK1 antibodies was 13% (five of 38) and 2·6% (one of 38), respectively. The statistical analysis of ELISA results by spss software showed that there was a significant difference between KD group and FC and HC (Fig. 3b). The levels of anti‐PGK1 antibodies in serum of KD patients were significantly higher than those of FC and HC. In addition, the prevalence of anti‐PGK1 antibodies in KD was validated in another group series of samples; 19 of 30 patients with KD (63·3%), 10 of 30 patients with FC (33·3%) and one of 30 HC (3·3%) serum samples had a positive reaction (Supporting information, Appendix Fig. S2). As shown in Supporting information, Appendix Table S3, there was no significant difference in the clinical and laboratory dates between the KD groups with and without anti‐PGK1 IgG antibodies.
Figure 3.
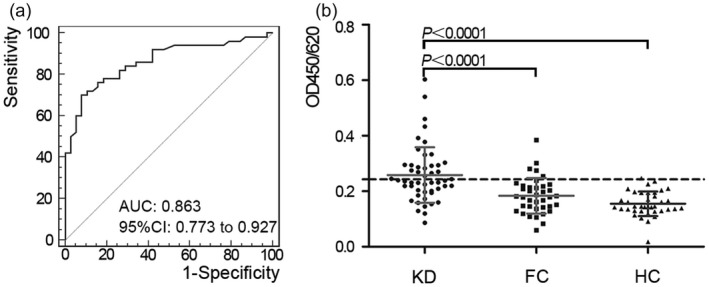
Reactivity of antibodies against phosphoglycerate kinase 1 (PGK1) in serum of Kawasaki disease (KD). (a) Receiver operating characteristic (ROC) curve analysis of anti‐PGK1 antibody reactivity in KD with area under the curve (AUC) values of 0·863. (b) Scatter‐plot of results of enzyme‐linked immunosorbent assay (ELISA) demonstrated the difference between KD, febrile non‐KD patients (FC) and age‐matched healthy donors (HC) groups. Anti‐PGK1 antibodies were detected in 23 of 50 KD patients (46%), five of 38 FC (13%) and one of 38 HC (2·6%). The reactivity of KD serum immunoglobulin (Ig)G antibodies against PGK1 was found to be significantly higher than that of FC (P < 0·0001) and HC (P < 0·0001). The cut‐off value was 0·243 [healthy controls mean ± 2 standard deviations (s.d.)].
PGK1 protein is a novel antigen of KD
To further test the binding capacity of KD serum antibodies to PGK1 protein, we performed Western blotting, immunoprecipitation and competitive inhibiting immunofluorescence assay.
First, rhPGK1‐based Western blotting was performed with 15 KD, five FC and five HC serum samples. It was shown that rhPGK1 protein could be recognized successfully by sera from KD patients (three of 15), whereas all FC and HC demonstrated a negative antigen–antibody reaction (Fig. 4).
Figure 4.
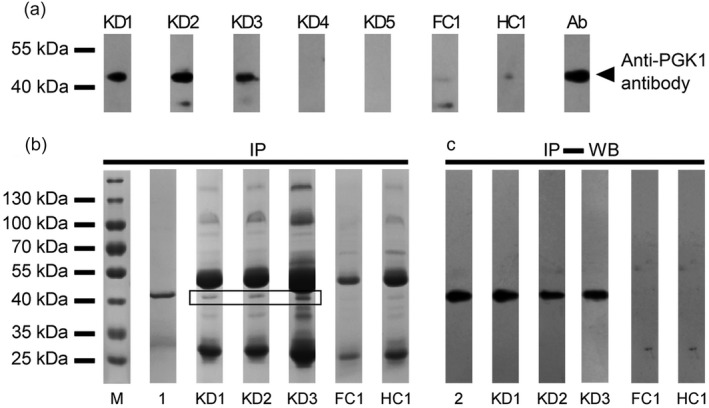
Identification of phosphoglycerate kinase 1 (PGK1) antigenicity in serum of Kawasaki disease (KD). (a) Results of Western blotting. Three positive results among 15 KD patients are displayed as lanes KD1, KD2, KD3. A 45‐kDa band was observed in all three cases. Two negative results were selected randomly among 12 negative results, and are displayed as lanes KD4, KD5. All FC and healthy control (HC) serum samples showed negative results. Ab: positive control (using anti‐PGK1 polyclonal antibodies instead of serum sample). (b) Immunoprecipitation of PGK1 protein with the sera from KD patients was performed. A 45‐kDa band can be clearly observed in the immune complexes. Lane M represents protein marker. (c) Validation of immune complexes contained rhPGK1 by Western blotting. Lanes 1 and 2 represent purified PGK1 serving as positive control.
Secondly, immunoprecipitation was performed to confirm further whether the PGK1 was a real antigen of KD. As shown in Fig. 4, a 45‐kDa protein band was clearly present in the immune complex (Fig. 4b), which was identified as rhPGK1 by Western blotting with anti‐PGK1 polyclonal antibodies (Fig. 4c).
Furthermore, a competitive inhibiting immunofluorescence assay was performed to analyse the inhibitory effect of rhPGK1 on KD patient serum bond with HeLa. As a result, the bonding activity of KD serum in vitro was reduced by rhPGK1 (Fig. 5a+, b+, c+), the fluorescence intensity ratio was significantly different between the experimental group (preincubated serum with rhPGK1) and the control group (Supporting information, Appendix Fig. S3).
Figure 5.
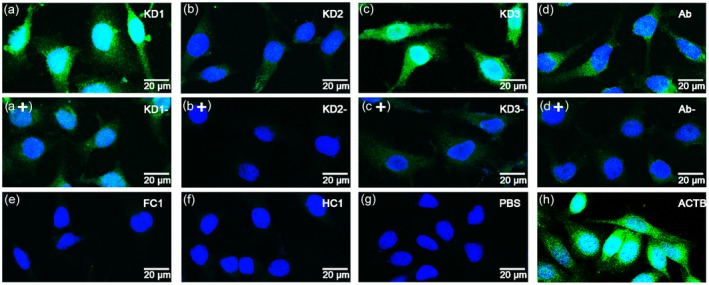
Competitive inhibit immunofluorescence assay. (a–c) HeLa staining patterns of Kawasaki disease (KD)1, KD2 and KD3 serum samples in competitive immunofluorescence assay were used to analyse the inhibitory activity of phosphoglycerate kinase 1 (PGK1). (a+, b+, c+) Preincubation of KD serum samples with PGK1 resulted in inhibition of staining. (d) HeLa cells incubated with anti‐PGK1 antibodies. (d+) Preincubation of anti‐PGK1 antibodies with PGK1 resulted in inhibition of staining. (e–h) HeLa cells incubated with FC, HC serum, phosphate‐buffered saline (PBS) and anti‐ACTB antibodies.
Epitopes sequence similarities between PGK1 and other antigens of KD
According to antigenic determinant prediction analysis, epitope peptides of PGK1 (15), TMABA‐DH (19) and PRDX2 (seven) were shown (Fig. 6 and Supporting information, Appendix Table S4) and three predicted epitopes of PGK1 were similar to epitopes of TMABA‐DH and PRDX2, respectively (Fig. 6d). A more interesting discovery is that one predicted epitope (133–146, ‐KDASGNKVKAEPAK‐) of PGK1 co‐occurred in TMABA‐DH and PRDX2 proteins.
Figure 6.
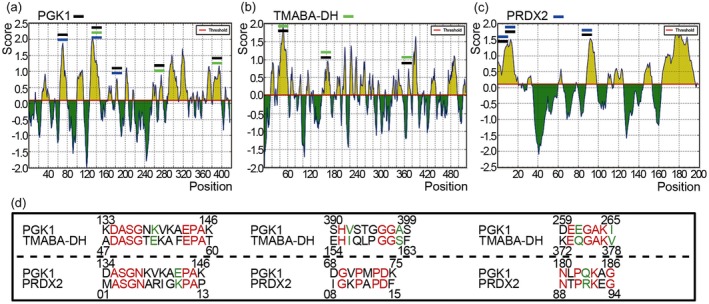
Antigenic determinant prediction analysis. (a–c) Result of antigenic determinant prediction of phosphoglycerate kinase 1 (PGK1), 4‐trimethylaminobutyraldehyde dehydrogenase (TMABA‐DH) and peroxiredoxin 2 (PRDX2), respectively. (d) Sequence alignment of epitopes. The similarity of potential epitopes between PGK1, TMABA‐DH and PRDX2 is displayed, which had similar properties is marked in green and the same amino acid is marked in red.
Discussion
As an immune‐mediated disease of KD, its aetiology was associated with infection and abnormal immune responses. According to studies on clinical and epidemiological data, the occurrence of KD is related closely to factors such as superantigen 16 and a T cell‐mediated immune response that is triggered by bacterial infection 17. In the course of the research on KD, although there were some related reports of infection and autoimmunity, the corresponding infection sources and immune targets were poorly understood. Cytokines as immune markers were detected in KD, such as interleukin (IL)‐1 beta and IL‐10 et al. 18, 19; studies in serum immune level are relatively scarce. Fujieda et al. examined the production of anti‐PRDX2 antibodies in 43·3% sera of KD patients 8, and Karasawa et al. found up‐regulated secretion of cytokines and chemokines such as IL‐6, IL‐8 and granulocyte colony‐stimulating factor (G‐CSF), which was produced by stimulating human umbilical vein endothelial cells (HUVEC) with rabbit anti‐PRDX2 antibodies 20, suggesting that anti‐PRDX2 antibodies may initiate vasculitis of KD. In addition, Matsunaga et al. discovered that the anti‐TMABA‐DH antibodies were increased significantly in sera of KD patients 9. Interestingly, the PRDX2 proteins that have powerful peroxidase activity and TMABA‐DH, which catalyzes the oxidation of aliphatic and aromatic aldehydes 21, 22, are both involved in oxidation. PGK1 protein identified as an antigen of KD in our study was concerned primarily with glucose oxidation. Therefore, oxidation may inter‐relate with the cause of KD. Is there any similarity in sequence or structure between PGK1, TMABA‐DH and PRDX2? In our antigenic determinant prediction assays, significant homologous antigen epitope pairs between PGK1, TMABA‐DH and PRDX2 were found (Supporting information, Appendix Fig. S4); in addition, one predicted epitope (133–146, ‐KDASGNKVKAEPAK‐) of PGK1 co‐occurred in TMABA‐DH and PRDX2. We speculate that this similar antigen epitope co‐occurred in three proteins and might cause antigen‐activation in KD.
In KD, endothelial cells have been described as immune target cells 10; nonetheless, few researches concerning anti‐endothelial cell antibodies in KD have been reported. Endothelial cells may be not the best candidates due to the variation of protein abundance in different conditions 23. In our other study, HUVEC cell proteome was extracted as antigen and antigen recognition reaction was performed using the serum samples as antibodies; target protein was detected and identified, but no PGK1 was detected: PGK1 cannot be detected in HUVEC cells. We found that HeLa cells were also suitable for antibody screening. The novel antigen of KD discovered in HeLa cells, i.e. PGK1, was confirmed to be expressed in cytoplasm but absent in nucleus, and is involved in several important biological processes, including angiogenesis and glycolysis 24. PGK1 has also been described in a number of diseases, including prostate cancer 25. Immune response that attacks normal host cells by antibodies is a common feature in immune diseases. Some of these antibodies are pathogenic, whereas others act as indicators of specific organ involvement 26. As the presence of anti‐PGK1 antibodies may bring an abnormal immune response, we speculate that abundant antibodies against PGK1 might cross‐link crucial signalling molecules and disrupt normal angiogenesis functions and the glycolytic pathway in humans, which lead to vascular inflammation in KD. To determine if the results are related to IVIG administration, the immunofluorescence HeLa‐based Western blot and PGK1‐based Western blot were repeated with IVIG [5% human Ig (pH4) for intravenous injection; Taibang Health, China] as a control. The HeLa cell chip could be recognized by IVIG without being diluted, but cannot be recognized by IVIG, which was diluted 1 : 100 (Supporting information, Appendix Fig. S4). HeLa cell proteome was extracted as antigens and the Western blot assay was performed using different concentrations of IVIG as antibodies. No positive bands were detected between 40 and 55 kDa. Furthermore, when recombinant human PGK1 protein‐based Western blotting was performed with IVIG, it was shown that PGK1 protein cannot be recognized by IVIG (Supporting information, Appendix Fig. S5). In addition, with a more detailed analysis of the clinical date, there was no significant difference in the overall inflammation between the KD and FC groups (Supporting information, Appendix Fig. S6). The anti‐PGK1 antibody level is not related to degree of inflammation (Supporting information, Appendix Fig. S7), suggesting that the differences in PGK1 antibodies are not due to a polyclonal stimulation in KD. Furthermore, there were no greater antibody titres comparing with the KD group with coronary artery lesions (CAL) and without CAL (Supporting information, Appendix Fig. S8).
In this study, only a proportion of the KD patient serum recognized PGK1; IgG against PGK1 was detected in 46% sera in KD patients, but it is possible that PGK1 has its value in facilitating the laboratory diagnosis of KD. However, the limitations of our work should be taken into account. Further research is necessary to elucidate the role of anti‐PGK1 antibodies in the pathogenesis of KD, and more clinical data need to be obtained.
Disclosures
No financial or commercial conflict of interest to declare.
Author contributions
J. C., Y. Z., H. H. and L. Z. performed the experiments; J. C., Y. Z., Z. D. and H. D. designed the study; J. C. wrote the paper.
Supporting information
Table S1. Mass spectrometry (MS) results of 45 kDa‐band protein
Table S2. Mass spectrometry results of PGK1 protein
Table S3. The clinical and laboratory dates between the KD groups with and without anti‐PGK1 IgG antibodies
Table S4. Antigenic epitopes prediction of PGK1, TMABA‐DH and PRDX2
Figure S1. Quantification of Immunofluorescence assay with HeLa cell chips
Figure S2. The prevalence of anti‐PGK1 antibodies in serum of KD patients (Verification group)
Figure S3. Quantification of competitive inhibition immunofluorescence assay with HeLa cell chip
Figure S4. Indirect immunofluorescence assay with HeLa cell chips
Figure S5. The Western blotting of HeLa cell lysates (A) and PGK1 (B) using IVIG as antibodies
Figure S6. The inflammation overall between the KD groups and FC groups
Figure S7. The differences of CRP between KD group with and without anti‐PGK1 IgG antibodies
Figure S8. The differences of antibody titers between KD group with CAL and without CAL
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81571592), and the National S&T Major Project of China (no. 2018ZX10301201).
Contributor Information
Z. Du, Email: duzhongdong@bch.com.cn
H. Du, Email: hwdu93@126.com
References
- 1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967; 16:178–222. [PubMed] [Google Scholar]
- 2. Dajani AS, Taubert KA, Gerber MA et al Diagnosis and therapy of Kawasaki disease in children. Circulation 1993; 87:1776–80. [DOI] [PubMed] [Google Scholar]
- 3. Kato H, Sugimura T, Akagi T et al Long‐term consequences of Kawasaki disease. A 10‐ to 21‐year follow‐up study of 594 patients. Circulation 1996; 94:1379–85. [DOI] [PubMed] [Google Scholar]
- 4. Newburger JW, Takahashi M, Gerber MA et al Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, council on cardiovascular disease in the young, American Heart Association. Pediatrics 2004; 114:1708–33. [DOI] [PubMed] [Google Scholar]
- 5. Maeno N, Takei S, Masuda K et al Increased serum levels of vascular endothelial growth factor in Kawasaki disease. Pediatr Res 1998; 44:596–9. [DOI] [PubMed] [Google Scholar]
- 6. Rowley AH, Eckerley CA, Jack HM, Shulman ST, Baker SC. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol 1997; 159:5946–55. [PubMed] [Google Scholar]
- 7. Rowley AH, Shulman ST, Spike BT, Mask CA, Baker SC. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol 2001; 166:1334–43. [DOI] [PubMed] [Google Scholar]
- 8. Fujieda M, Karasawa R, Takasugi H et al A novel anti‐peroxiredoxin autoantibody in patients with Kawasaki disease. Microbiol Immunol 2012; 56:56–61. [DOI] [PubMed] [Google Scholar]
- 9. Matsunaga A, Harita Y, Shibagaki Y et al Identification of 4‐trimethylaminobutyraldehyde dehydrogenase (TMABA‐DH) as a candidate serum autoantibody target for Kawasaki Disease. PLOS ONE 2015; 10:128–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujieda M, Oishi N, Kurashige T. Antibodies to endothelial cells in Kawasaki disease lyse endothelial cells without cytokine pretreatment. Clin Exp Immunol 1997; 107:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen P, Yan H, Tian Y et al Annexin A2 as a target endothelial cell membrane autoantigen in Behcet’s disease. Sci Rep 2015; 5:8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramos‐Remus C, Castillo‐Ortiz JD, Aguilar‐Lozano L et al Autoantibodies in prediction of the development of rheumatoid arthritis among healthy relatives of patients with the disease. Arthritis Rheumatol 2015; 67:2837–44. [DOI] [PubMed] [Google Scholar]
- 13. Karasawa K, Harada K, Kato H et al Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease. Pediatr Int 2005; 47:711–32. [DOI] [PubMed] [Google Scholar]
- 14. Sun S, Ma H, Han G, Wu R, Zou H, Liu Y. Efficient enrichment and identification of phosphopeptides by cerium oxide using on‐plate matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometric analysis. Rapid Commun Mass Spectrom 2011; 25:1862–8. [DOI] [PubMed] [Google Scholar]
- 15. Larsen JE, Lund O, Nielsen M. Improved method for predicting linear B‐cell epitopes. Immunome Res 2006; 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meissner HC, Leung DY. Superantigens, conventional antigens and the etiology of Kawasaki syndrome. Pediatr Infect Dis J 2000; 19:91–4. [DOI] [PubMed] [Google Scholar]
- 17. Iemura M, Ishii M, Sugimura T, Akagi T, Kato H. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart 2000; 83:307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maury CP, Salo E, Pelkonen P. Circulating interleukin‐1 beta in patients with Kawasaki disease. N Engl J Med 1988; 319:1670–1. [DOI] [PubMed] [Google Scholar]
- 19. Kim DS, Lee HK, Noh GW, Lee SI, Lee KY. Increased serum interleukin‐10 level in Kawasaki disease. Yonsei Med J 1996; 37:125–30. [DOI] [PubMed] [Google Scholar]
- 20. Karasawa R, Kurokawa MS, Yudoh K, Masuko K, Ozaki S, Kato T. Peroxiredoxin 2 is a novel autoantigen for anti‐endothelial cell antibodies in systemic vasculitis. Clin Exp Immunol 2010; 161:459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 2000; 407:211–5. [DOI] [PubMed] [Google Scholar]
- 22. Lin SW, Chen JC, Hsu LC, Hsieh CL, Yoshida A. Human gamma‐aminobutyraldehyde dehydrogenase (ALDH9): cDNA sequence, genomic organization, polymorphism, chromosomal localization, and tissue expression. Genomics 1996; 34:376–80. [DOI] [PubMed] [Google Scholar]
- 23. Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 2003; 4:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lay AJ, Jiang XM, Kisker O et al Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature 2000; 408:869–73. [DOI] [PubMed] [Google Scholar]
- 25. Jung Y, Shiozawa Y, Wang J et al Expression of PGK1 by prostate cancer cells induces bone formation. Mol Cancer Res 2009; 7:1595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee SJ, Kavanaugh A. 4. Autoimmunity, vasculitis, and autoantibodies. J Allergy Clin Immunol 2006; 117:S445–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mass spectrometry (MS) results of 45 kDa‐band protein
Table S2. Mass spectrometry results of PGK1 protein
Table S3. The clinical and laboratory dates between the KD groups with and without anti‐PGK1 IgG antibodies
Table S4. Antigenic epitopes prediction of PGK1, TMABA‐DH and PRDX2
Figure S1. Quantification of Immunofluorescence assay with HeLa cell chips
Figure S2. The prevalence of anti‐PGK1 antibodies in serum of KD patients (Verification group)
Figure S3. Quantification of competitive inhibition immunofluorescence assay with HeLa cell chip
Figure S4. Indirect immunofluorescence assay with HeLa cell chips
Figure S5. The Western blotting of HeLa cell lysates (A) and PGK1 (B) using IVIG as antibodies
Figure S6. The inflammation overall between the KD groups and FC groups
Figure S7. The differences of CRP between KD group with and without anti‐PGK1 IgG antibodies
Figure S8. The differences of antibody titers between KD group with CAL and without CAL


