Highlights
-
•
Reduced diffusion is an early and important imaging to evaluate patients with acute encephalopathic syndromes.
-
•
The presence of diffuse cortical injury (DCI) on diffusion-weighted imaging (DWI) can help narrow the differential diagnosis.
-
•
“CRUMPLED” is a convenient acronym for the categorization of a diverse range of acute etiologies associated with DCI on DWI.
Keywords: Acute encephalopathy, Diffuse cortical injury, DWI
Abbreviations: AHE, Acute Hepatic/Hyperammonemic Encephalopathy; DCI, Diffuse cortical injury; CJD, Creutzfeldt-Jakob disease; MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes; PRES, Posterior reversible encephalopathy syndrome; RCVS, Reversible cerebral vasoconstriction syndrome; UCD, Urea cycle disorders
Abstract
Purpose
Acute encephalopathic syndromes can present a diagnostic challenge due to the wide range of possible etiologies, which also can have vastly different outcomes. The presence of diffuse cortical injury (DCI) on diffusion-weighted imaging (DWI) can help narrow the differential diagnosis. The aim of this review is to categorize the range of possible etiologies of DCI into a useful acronym, "CRUMPLED".
Methods
A review of the PACS system was completed to find a characteristic example of patients with DCI on DWI from different etiologies. The diagnosis was confirmed for each example via a subsequent review of the electronic medical record used to assess for data such as biopsy results, laboratory values, and clinical correlation. The electronic exhibit intends to demonstrate several sample cases of each letter within the acronym, and to demonstrate which types of DCI are potentially reversible or irreversible.
Findings/Discussion
The possible etiologies of DCI on DWI can be organized using the acronym "CRUMPLED": 'C' = Creutzfeldt-jakob disease, 'R' = reversible cerebral vasoconstriction syndrome; 'U' = urea cycle disorders (hyperammonemia) and Uremia; 'M' = mitochondrial (cytopathy/encephalopathy); 'P' = prolonged seizure and posterior reversible encephalopathy (PRES); 'L' = laminar necrosis (hypoxic-ischemic encephalopathy) and liver disease (acute hepatic encephalopathy); 'E' = encephalitis (infectious meningoencephalitis); 'D' = diabetes mellitus (hypoglycemia). Other secondary imaging findings (outside of DWI) can be used to help differentiate between the aforementioned etiologies, such as the use of ADC maps, FLAIR imaging, intravenous contrast.
Conclusion
"CRUMPLED" is proposed as a convenient acronym for the categorization of a diverse range of acute etiologies associated with DCI on DWI, arising from varying degrees of cytotoxic edema. These etiologies can range from being potentially reversible (e.g. hyperammonemia or prolonged seizures) to irreversible (e.g. hypoxic-ischemic injury).
1. Introduction
Acute encephalopathic syndromes can be a diagnostic challenge due to the wide range of possible etiologies, which also can have vastly different outcomes. Accordingly, the presence of diffuse cortical injury (DCI) on diffusion-weighted imaging (DWI) in an encephalopathic patient can narrow the differential diagnosis, as reduced diffusion has been traditionally recognized in acute thromboembolic infarction, but may also occur with other disorders, some of which may be reversible [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10]]. Secondary imaging findings, outside of DCI on DWI, can be helpful to differentiate between the aforementioned etiologies, such as concomitant areas of anatomic involvement, the use of FLAIR, and intravenous contrast. Therefore, possible etiologies of DCI on DWI are presented here as a useful acronym, “CRUMPLED”: ‘C’ = Creutzfeldt-jakob disease, ‘R’ = reversible cerebral vasoconstriction syndrome (RCVS); ‘U’ = urea cycle disorders (hyperammonemia) and Uremia; 'M'= mitochondrial (cytopathy/encephalopathy); ‘P’ = prolonged seizures and posterior reversible encephalopathy syndrome (PRES); ‘L’ = laminar necrosis (hypoxic-ischemic encephalopathy) and liver-related (acute hepatic or hyperammonemic encephalopathy); ‘E’ = encephalitis (infectious meningoencephalitis); ‘D’ = diabetes mellitus (hypoglycemia) [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10]].
2. ‘C’ = Creutzfeldt-jakob disease
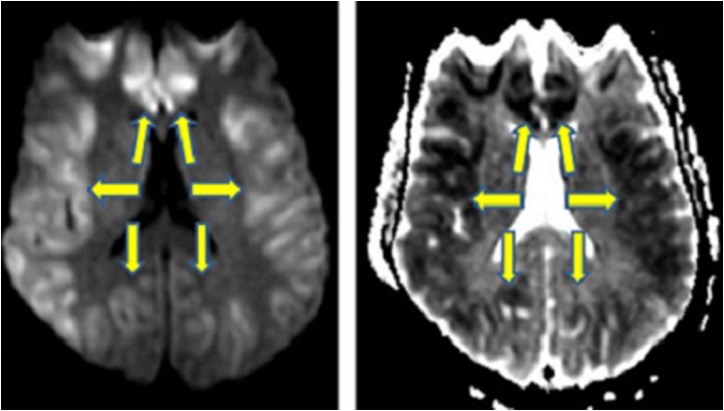
Magnetic Resonance Imaging (MRI), especially DWI is an important noninvasive diagnostic tool in early stage of Creutzfeldt-jakob disease (CJD). Cortical (superior frontal gyri and cortical areas near midline) and basal ganglia (especially the corpus striatum) involvement, hyperintensity on DWI in the insula and cingulate (limbic lobe) are the most common MRI findings in the most common type, sporadic CJD (Fig. 1); however, isolated limbic involvement is not found while the perirolandic area is usually spared in this subtype [11]. High-signal-intensity abnormalities both within the caudate nuclei and the cerebral cortex can be characteristic even in the early stages of the disease, and thus radiologists should be alert to the possibility that in the early stages of disease, high signal on DWI can be confined to the cerebral cortex [12]. In addition, the presence of abnormal hyperintensity solely on FLAIR, but not in DWI, in cortical gray matter is an unexpected finding that should prompt one to consider an alternative diagnosis [1].
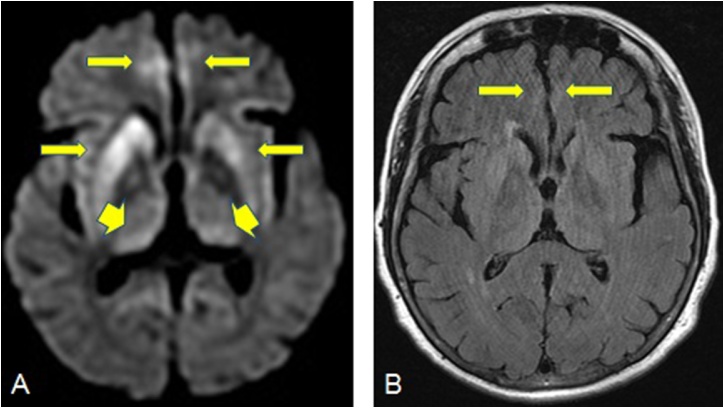
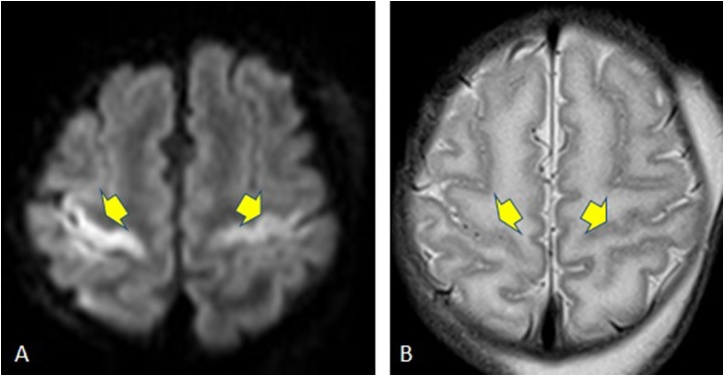
Fig. 1.
A 58-year-old female patient with sporadic CJD. 1A: Reduced diffusion is noted in the parasagittal cortices bilaterally as well as the basal ganglia, with additional involvement of the thalami, specifically pulvinar nuclei (arrowheads). 1B: On FLAIR, hyperintensity is noted only in the bilateral parasagittal cerebral cortices.
3. ‘R’ = reversible cerebral vasoconstriction syndrome
RCVS is a unifying term proposed in 2007 that applies to a mostly idiopathic group of disorders characterized by reversible narrowing of the cerebral arteries on imaging, and typically presenting with thunderclap headaches [13]. The role of neuroimaging in patients with RCVS is to demonstrate the characteristic multifocal cerebral vasoconstriction on vascular imaging; MRA is the preferred non-invasive diagnostic tool in RCVS-related vasoconstriction, while catheter angiography should be reserved for questionable cases (Fig. 2) [2]. However, imaging can be normal, particularly if imaging is delayed up to one week after the onset of the symptoms. On MRI, the combination of magnetic resonance angiography (MRA) combined with brain MRI is valuable in evaluating for an alternative diagnoses, especially primary angiitis of the CNS, and in monitoring for potential complications such as intracerebral haemorrhage (20%), posterior reversible encephalopathy syndrome (PRES, 38%), or cerebral infarction (39%) [14]. Hence, DCI-type of insults on DWI may occur in a minority.
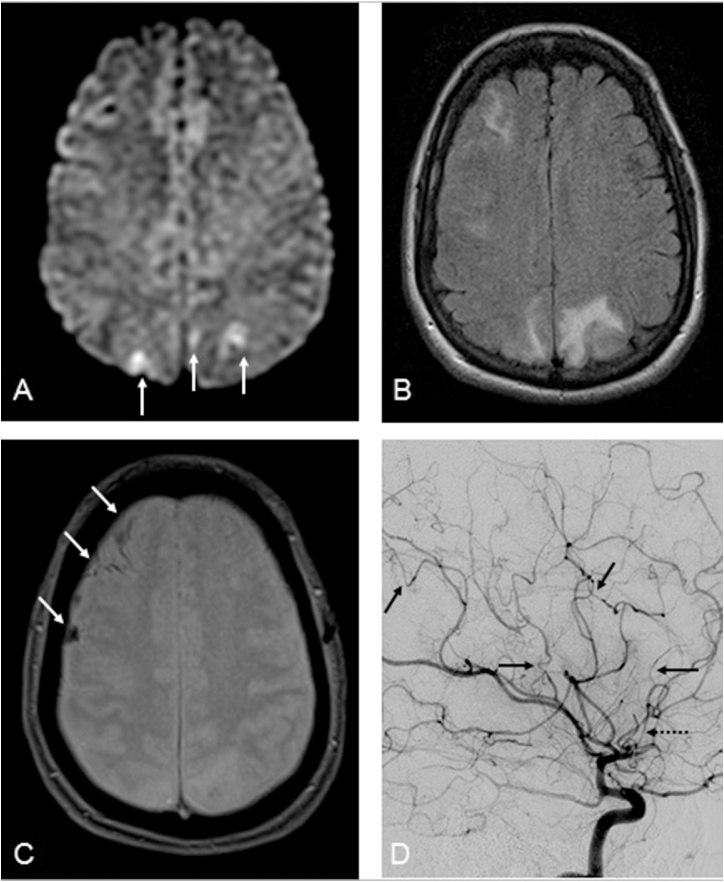
Fig. 2.
A 57-year-old female with RCVS presenting with “thunderclap headache”. 2A-C: several small foci of gyriform reduced diffusion were noted in the parietooccipial cortices (arrows, 2A), but less prominent than the amount of edema on FLAIR (2B), indicative of concomitant PRES, with small amounts of subarachnoid hemorrhage on T2*WI as well (arrows, 2C); MRA (not shown) suggestive mild multifocal segmental narrowing. 2D: On catheter DSA of the right ICA, multifocal areas of narrowing of ACA and MCA segments are noted (arrows), with focal narrowing of the A2 (dotted arrow) and A1 (not shown) segments as well.
4. ‘U’ = urea cycle disorders (hyperammonemia) and uremia
Urea Cycle Disorders (UCD) typically exhibit DCI-type edema scattered throughout the cerebral cortex, particularly involving the peri-insular cortices and basal ganglia, with multifocal cortical diffusion abnormalities being characterically noted in UCD’s [15]. The thalami are typically spared, and the findings in UCD’s may be reversible. In adult-onset UCD’s, involvement of the perirolandic and occipital cortices are not expected unless involvement of the disease is severe (Fig. 3) [3].
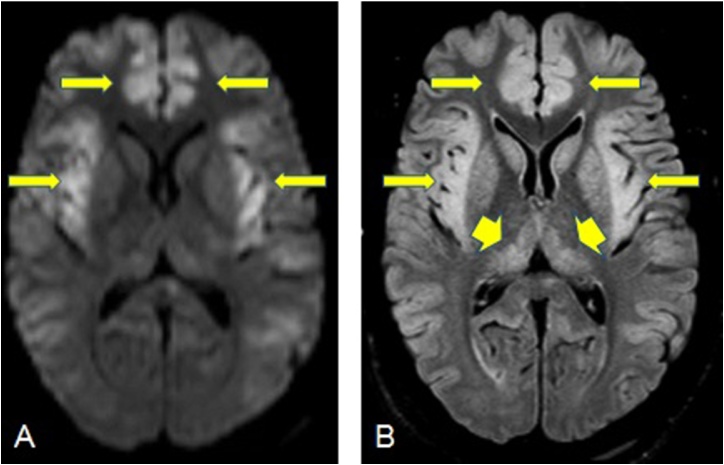
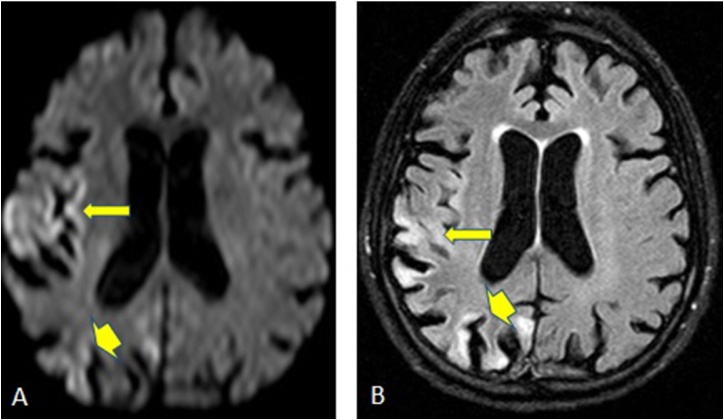
Fig. 3.
A 38-year-old male patient with Ornithine transcarbamylase deficiency, has reduced diffusion in the bilateral insular and cingulate cortices on DWI (arrows, 3A) and FLAIR images (arrows, 3B). In addition, bilateral thalamic involvement seen in FLAIR images without restricted diffusion (arrowheads 3B).
Regarding the metabolic disorder uremic encephalopathy, it typically occurs in patients with renal failure and anion gap metabolic acidosis [15]. The imaging findings that have been described may be controversial, but DCI have been noted in addition to basal ganglia edema in preliminary reports of small numbers of patients having lesions on DWI and FLAIR [15,16]. In the authors’ experience, such cases of uremic encephalopathy can be subtle due to only mild edema within the insula and basal ganglia, and thus may overlap somewhat in appearance with acute hepatic encephalopathy, although laboratory testing for liver function in uremic encephalopathy is typically normal (Fig. 4).
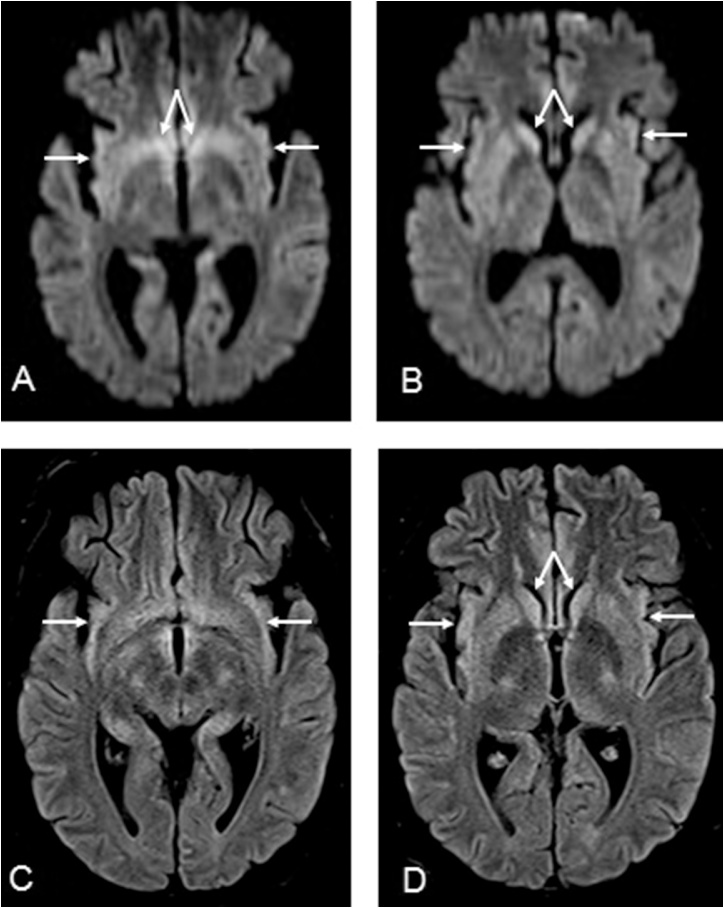
Fig. 4.
A 20-year-old male with altered mental status from uremic encephalopathy, having mildly reduced diffusion in the bilateral insular and caudate nuclei on DWI (arrows, 4A-B), with subtle edema on FLAIR (arrows, 4C-D). Follow-up imaging was not obtained, as the patient’s symptoms improved with correction of the uremia and metabolic acidosis.
5. ‘M’ = mitochondrial disorders
MELAS (myopathy, encephalopathy with lactic acidosis, and stroke-like episodes) is the most common subtype of mitochondrial disorders, and may have imaging findings of DCI, particularly involving the occipital, parietal and temporal lobes; areas of cortical involvement are usually not confined to a particular vascular distribution (Fig. 5) [17]. The DWI findings of stroke-like episodes in MELAS patients can be confused with acute ischemic stroke, as both disorders may have diffuse or gyriform abnormalities in a multifocal pattern with a cortical-subcortical distribution. Meanwhile the reduction of ADC values in this disorder are still controversial, where some studies have reported increased ADC (representing vasogenic oedema), whereas others have reported a decrease in ADC due to cytotoxic edema [[18], [19], [20]]. Hence, a mitochondrial disorder is in the differential diagnosis for a multifocal appearance of DCI in a child or infant in a non-vascular distribution with acute, stroke-like onset, and an uneventful gestation and birth.
Fig. 5.
A 1-week-old term female with MELAS, has reduced diffusion in the bilateral perirolandic area on DWI (arrows, 5A) and delayed myelination pattern is seen on T2*WI (arrows, 5B).
6. ‘P’ = prolonged seizures and posterior reversible encephalopathy syndrome (PRES)
The hippocampus is a classic location for the abnormalities on MRI that have been described with prolonged seizures, while other areas of DCI on DWI may be visualized as well, such as the subcortical white matter, callosal splenium, and even the basal ganglia, thalami, and cerebellum, which can all be reversible [21,22]. Among these areas, isolated or mulifocal cortical involvement seems most common, as some studies have shown that nearly 80% of patients have cortical involvement in a DCI-like pattern (Fig. 6) [5,22].
Fig. 6.
A 58-years-old male with confusion from status epilepticus has reduced diffusion in the right temparoparietal and occipital cortices seen on DWI (arrows, 6A) and FLAIR image (arrows, 6B).
PRES is a clinicoradiologic syndrome characterized by multifocal cortical-subcortical edema on FLAIR that is potentially reversible, whereas larger imaging-based studies that utilized DWI have described that focal or multifocal gyriform reduced diffusion (cytotoxic edema) occurs in a minority (<20%) (Fig. 7) [6,23]. Notably, foci of “gyriform” reduced diffusion can be difficult to discern, as the ADC values may not be visibly low, given the presence of larger and more diffuse areas of surrounding vasogenic edema. It is important for radiologists to report such areas of DCI-like multifocal reduced diffusion in PRES, as the presence of cytotoxic edema on DWI has been correlated with a poorer outcome in PRES, [6,23,24].
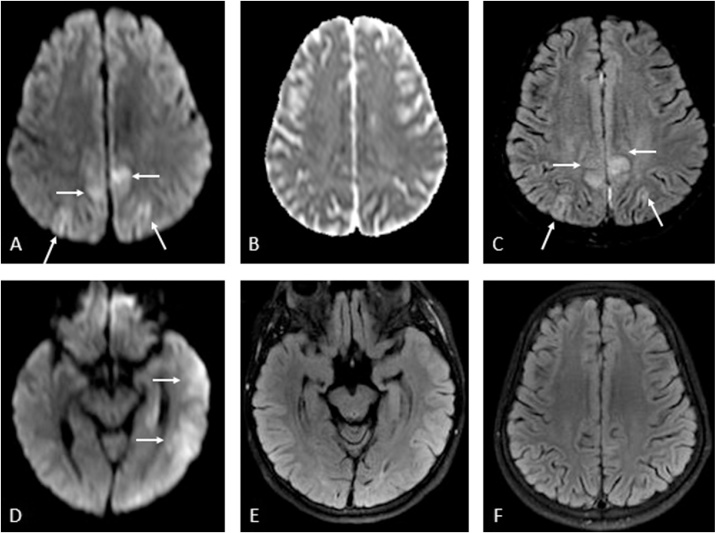
Fig. 7.
A 22-years-old female with seizures from PRES due to chronic renal failure and hypertension. 7A-E: Multifocal mildly reduced diffusion and edema is noted on DWI and FLAIR (arrows, 7A, 7C), not having bright vasogenic edema on ADC map (7B); there is also cytotoxic edema within the left temporal lobe on DWI (arrows, 7D), but less visible on FLAIR (7E). After therapy with subsequently resolved seizures, a follow-up FLAIR MRI 40 days later showed resolved multifocal abnormalities (7F).
7. ‘L’ = laminar necrosis (hypoxic-ischemic encephalopathy) and liver insufficiency (acute hepatic or hyperammonemic encephalopathy)
Hypoxic-ischemic encephalopathy (HIE) may affect any brain region, but typically involves the deep gray matter nuclei, cortices, hippocampi, or cerebellum in older children and adults, where cortical hyperintensity of the parietooccipital regions and borderzone areas predominates. The sometimes-visualized hyperintensity on nocontrast T1WI in the late subacute and chronic phases is compatible with the histologic findings of cortical laminar necrosis, while in the acute or early subacute phase the multifocal gray matter abnormalities are usually quite evident on DWI; this variably affects the basal ganglia in the more severe cases (Fig. 8). Notably, also occurring in the late subacute phase, the cortex may no longer be bright on DWI as white matter abnormalities may abound, having a striking appearance on DWI and ADC maps that is quite different and near-reversed from the initial DCI-type cortical injury [[25], [26], [27]]. As studies of HIE in adults have shown that the presence of diffuse, uniform hyperintensity in the cortex in the acute phase on DWI typically results in severe outcomes (such as a persistent vegetative state or brain death), it is important to recognize this appearance in the appropriate clinical setting, given the particularly poor prognosis [7,28].
Fig. 8.
A 42-years-old male with hypoxic-ischemic encephalopathy due to cardiac arrest, has reduced diffusion in perirolandic area on DWI (arrows, 8A), also bilateral basal ganglia, posterior insular and visual cortices involvement are seen (arrows, 8B), with subtle findings on FLAIR (arrows, 8C).
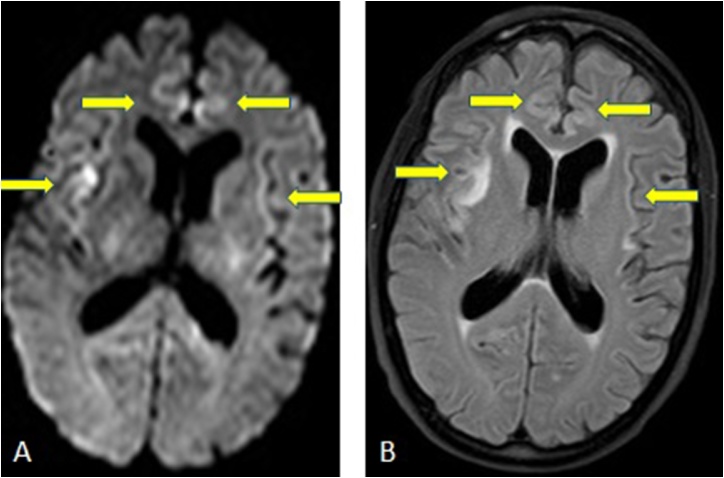
Acute hepatic, or hyperammonemic, encephalopathy (AHE) is another clinicoradiologic syndrome that is usually reversible with treatment, while its severity is usually (but not always) related to the degree of hyperammonemia in the setting of hepatic insufficiency [29,30]. Recognition of the MRI findings and correction of hyperammonemia may be essential to avoid significant brain injury and long term sequelae in more severely affected patients; the insulae, thalami, and cingulate cortices are particularly areas of involvement commonly noted in adults with acute hepatic encephalopathy (Fig. 9). Other areas may include abnormalities in the internal capsules, posterior limbs, periventricular white matter, and dorsal brain stem [8]. Notably, a solely DCI-type of cortical involvement may occur without the involvement of other regions, which has a relatively worse prognosis, although this entity may be reversible [8,[29], [30], [31]]. Additionally, the degree of involvement on DWI and plasma ammonia levels correlate with the clinical outcome. Hence, in the setting of DCI on DWI, the radiologist should be alert to the early diagnosis of AHE, where recognition of this appearance is critical in the setting of an elevated plasma ammonia level [8].
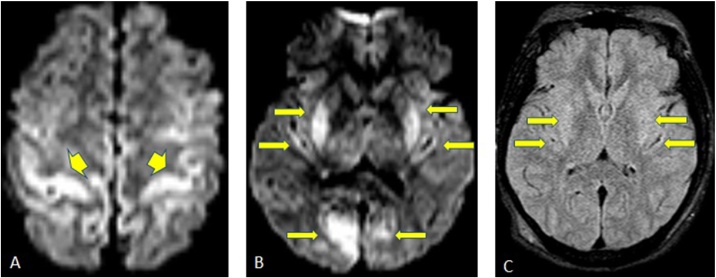
Fig. 9.
A 48-years-old male with end-stage liver disease, presented with myoclonic jerking, has an ammonium level of 226 μg/dl. He has restricted diffusion in frontal, parietal and occipital cortices seen on DWI and ADC (arrows, 9A-9B).
8. ‘E’ = encephalitis
Encephalitides can be infectious or autoimmune; the infectious types are most commonly either bacterial or viral. While the most common viral agent, herpes simplex virus (HSV) encephalitis, in an immunocompetent adult usually has bilateral involvement of the medial temporal lobes, insular cortices, and inferolateral frontal lobes, temporal involvement does not have to occur [9]. DWI is superior to spin-echo or FLAIR imaging in demonstrating encephalitic lesions and also may allow for earlier detection of lesions [32]. Cortical involvement on DWI can be also seen in other viral etiologies, such as varicella virus, and in bacterial etiologies as well; additionally, DCI on DWI may be present subjacent to epidural empyemas, being induced by inflammation [33]. Autoimmune causes of encephalitis are many, being generally characterized as “paraneoplastic” or “non-paraneoplastic”, most of which have a predilection for the temporal lobe (although not always), and can present with a DCI-type of appearance with reduced diffusion [33]. In such patients, the radiologist’s role can be critical to suggest an infectious or autoimmune cause of encephalitis (Fig. 10). Enhancement on postcontrast T1WI or FLAIR may solidify this suspicion, and an infectious versus autoimmune cause can often be further determined by lumbar puncture and serum testing [34,35].
Fig. 10.
A 66-years-old male diagnosed with HSV encephalitis has reduced diffusion and edema on DWI and FLAIR image (arrows, 10A, 10B) in the parasagittal and temporal cortices bilaterally.
9. ‘D’ = diabetes mellitus (hypoglycemic encephalopathy)
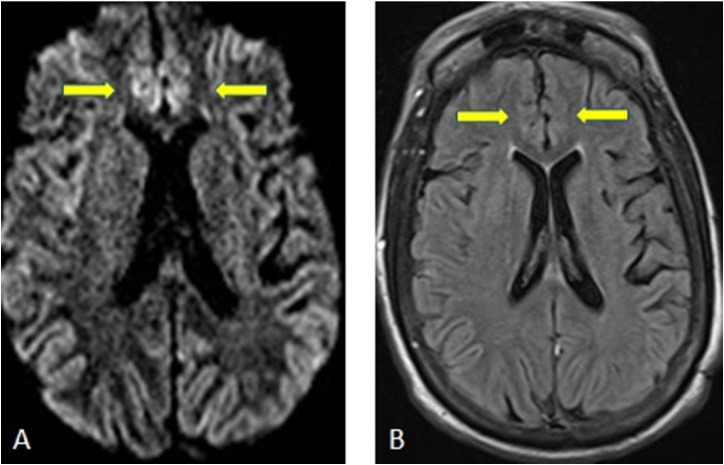
The gray matter is considered to be more vulnerable to hypoglycemic injury than white matter, with the mostly commonly affected sites being the cerebral cortices, hippocampi, and basal ganglia; the cerebellum and brainstem are typically spared. These lesions on DWI lesions often exhibit ADC reduction, especially in the cerebral cortices, temporal lobes, hippocampi, and the basal ganglia (Fig. 11) [36]. Preliminary results in studies with smaller numbers of patients suggest that DWI may also be useful to predict outcomes, as lesions in the white matter are more likely to recover, while lesions in the cerebral cortex, basal ganglia, or hippocampi may not regress on follow-up, perhaps associated with a poor outcome [10]. Hence, it is important to recognize the MRI findings of DCI and suggest the diagnosis of hypoglycemic encephalopathy if there is an appropriate clinical history.
Fig. 11.
A 66-years-old female with loss of consciousness due to hypoglycemic coma has reduced diffusion and edema on DWI (arrows, 11A) and FLAIR images (arrows, 11B) in the bilateral parasagittal cortices.
10. Conclusion
“CRUMPLED” is proposed as a convenient acronym for the categorization of a diverse range of acute etiologies associated with DCI on DWI, arising from varying degrees of cytotoxic edema. These etiologies can range from being potentially reversible (e.g. hyperammonemia/AHE or prolonged seizures) to irreversible (e.g. laminar necrosis/hypoxic-ischemic injury). Upon recognizing the appearance of DCI, this acronym may enable the radiologists to suggest further testing or clinical history, which can help cement the diagnosis for most of these disorders.
Conflicts of interest/disclosures
None for any of the authors.
Footnotes
The review was presented as an electronic educational poster at ASNR 56th Annual Meeting. June 2–7, 2018 Vancouver Convention Centre East. Vancouver, BC, Canada.
References
- 1.Young G.S., Geschwind M.D., Fischbein N.J., Martindale J.L., Henry R.G., Liu S., Lu Y., Wong S., Liu H., Miller B.L., Dillon W.P. Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: high sensitivity and specificity for diagnosis. AJNR Am. J. Neuroradiol. 2005;26:1551–1562. [PMC free article] [PubMed] [Google Scholar]
- 2.Miller T.R., Shivashankar R., Mossa-Basha M., Gandhi D. Reversible cerebral vasoconstriction syndrome, part 2: diagnostic work-up, imaging evaluation, and differential diagnosis. AJNR Am. J. Neuroradiol. 2015;36:1580–1588. doi: 10.3174/ajnr.A4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takanashi J., Barkovich A.J., Cheng S.F., Kostiner D., Baker J.C., Packman S. Brain MR imaging in acute hyperammonemic encephalopathy arising from late-onset ornithine transcarbamylase deficiency. AJNR Am. J. Neuroradiol. 2003;24:390–393. [PMC free article] [PubMed] [Google Scholar]
- 4.Malhotra K., Liebeskind D.S. Imaging of MELAS. Curr. Pain Headache Rep. 2016;20:54. doi: 10.1007/s11916-016-0583-7. [DOI] [PubMed] [Google Scholar]
- 5.Kim J.A., Chung J.I., Yoon P.H., Kim D.I., Chung T.S., Kim E.J., Jeong E.K. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. AJNR Am. J. Neuroradiol. 2001;22:1149–1160. [PMC free article] [PubMed] [Google Scholar]
- 6.McKinney A.M., Short J., Truwit C.L., McKinney Z.J., Kozak O.S., SantaCruz K.S., Teksam M. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am. J. Roentgenol. 2007;189:904–912. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 7.McKinney A.M., Teksam M., Felice R., Casey S.O., Cranford R., Truwit C.L., Kieffer S. Diffusion-weighted imaging in the setting of diffuse cortical laminar necrosis and hypoxic-ischemic encephalopathy. AJNR Am. J. Neuroradiol. 2004;25:1659–1665. [PMC free article] [PubMed] [Google Scholar]
- 8.McKinney A.M., Lohman B.D., Sarikaya B., Uhlmann E., Spanbauer J., Singewald T., Brace J.R. Acute hepatic encephalopathy: diffusion-weighted and fluid-attenuated inversion recovery findings, and correlation with plasma ammonia level and clinical outcome. AJNR Am. J. Neuroradiol. 2010;31:1471–1479. doi: 10.3174/ajnr.A2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koeller K.K., Shih R.Y. Viral and prion infections of the central nervous system: radiologic-pathologic correlation: from the radiologic pathology archives. Radiographics. 2017;37:199–233. doi: 10.1148/rg.2017160149. A review publication of the Radiological Society of North America, Inc. [DOI] [PubMed] [Google Scholar]
- 10.Kang E.G., Jeon S.J., Choi S.S., Song C.J., Yu I.K. Diffusion MR imaging of hypoglycemic encephalopathy. AJNR Am. J. Neuroradiol. 2010;31:559–564. doi: 10.3174/ajnr.A1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fragoso D.C., Goncalves Filho A.L., Pacheco F.T., Barros B.R., Aguiar Littig I., Nunes R.H., Maia A.C., Junior, da Rocha A.J. Imaging of creutzfeldt-jakob disease: imaging patterns and their differential diagnosis. Radiographics. 2017;37:234–257. doi: 10.1148/rg.2017160075. A review publication of the Radiological Society of North America, Inc. [DOI] [PubMed] [Google Scholar]
- 12.Ukisu R., Kushihashi T., Tanaka E., Baba M., Usui N., Fujisawa H., Takenaka H. Diffusion-weighted MR imaging of early-stage Creutzfeldt-Jakob disease: typical and atypical manifestations. Radiographics. 2006;26(Suppl 1):191–204. doi: 10.1148/rg.26si065503. A review publication of the Radiological Society of North America, Inc. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese L.H., Dodick D.W., Schwedt T.J., Singhal A.B. Narrative review: reversible cerebral vasoconstriction syndromes. Ann. Intern. Med. 2007;146:34–44. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 14.Singhal A.B., Hajj-Ali R.A., Topcuoglu M.A., Fok J., Bena J., Yang D. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch. Neurol. 2011;68:1005–1012. doi: 10.1001/archneurol.2011.68. [DOI] [PubMed] [Google Scholar]
- 15.Kim D.M., Lee I.H., Song C.J. Uremic encephalopathy: MR imaging findings and clinical correlation. AJNR Am. J. Neuroradiol. 2016;37:1604–1609. doi: 10.3174/ajnr.A4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada J., Yoshikawa K., Matsuo H., Kanno K., Oouchi M. Reversible MRI and CT findings in uremic encephalopathy. Neuroradiology. 1991;33:524–526. doi: 10.1007/BF00588046. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzoni P.J., Werneck L.C., Kay C.S., Silvado C.E., Scola R.H. When should MELAS (Mitochondrial myopathy, Encephalopathy, Lactic Acidosis, and Stroke-like episodes) be the diagnosis? Arq. Neuropsiquiatr. 2015;73:959–967. doi: 10.1590/0004-282X20150154. [DOI] [PubMed] [Google Scholar]
- 18.Oppenheim C., Galanaud D., Samson Y., Sahel M., Dormont D., Wechsler B., Marsault C. Can diffusion weighted magnetic resonance imaging help differentiate stroke from stroke-like events in MELAS? J. Neurol. Neurosurg. Psychiatr. 2000;69:248–250. doi: 10.1136/jnnp.69.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohshita T., Oka M., Imon Y., Watanabe C., Katayama S., Yamaguchi S., Kajima T., Mimori Y., Nakamura S. Serial diffusion-weighted imaging in MELAS. Neuroradiology. 2000;42:651–656. doi: 10.1007/s002340000335. [DOI] [PubMed] [Google Scholar]
- 20.Kolb S.J., Costello F., Lee A.G., White M., Wong S., Schwartz E.D., Messe S.R., Ellenbogen J., Kasner S.E., Galetta S.L. Distinguishing ischemic stroke from the stroke-like lesions of MELAS using apparent diffusion coefficient mapping. J. Neurol. Sci. 2003;216:11–15. doi: 10.1016/s0022-510x(03)00218-1. [DOI] [PubMed] [Google Scholar]
- 21.Cianfoni A., Caulo M., Cerase A., Della Marca G., Falcone C., Di Lella G.M., Gaudino S., Edwards J., Colosimo C. Seizure-induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur. J. Radiol. 2013;82:1964–1972. doi: 10.1016/j.ejrad.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Jabeen S.A., Cherukuri P., Mridula R., Harshavardhana K.R., Gaddamanugu P., Sarva S., Meena A.K., Borgohain R., Jyotsna Rani Y. A prospective study of diffusion weighted magnetic resonance imaging abnormalities in patients with cluster of seizures and status epilepticus. Clin. Neurol. Neurosurg. 2017;155:70–74. doi: 10.1016/j.clineuro.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Schweitzer A.D., Parikh N.S., Askin G., Nemade A., Lyo J., Karimi S., Knobel A., Navi B.B., Young R.J., Gupta A. Imaging characteristics associated with clinical outcomes in posterior reversible encephalopathy syndrome. Neuroradiology. 2017;59:379–386. doi: 10.1007/s00234-017-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastide L., Legros B., Rampal N., Gilmore E.J., Hirsch L.J., Gaspard N. Clinical Correlates of Periodic Discharges and Nonconvulsive Seizures in Posterior Reversible Encephalopathy Syndrome (PRES) Neurocrit. Care. 2018 doi: 10.1007/s12028-018-0548-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Takahashi S., Higano S., Ishii K., Matsumoto K., Sakamoto K., Iwasaki Y., Suzuki M. Hypoxic brain damage: cortical laminar necrosis and delayed changes in white matter at sequential MR imaging. Radiology. 1993;189:449–456. doi: 10.1148/radiology.189.2.8210374. [DOI] [PubMed] [Google Scholar]
- 26.Huang B.Y., Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics. 2008;28:417–439. doi: 10.1148/rg.282075066. A review publication of the Radiological Society of North America, Inc. [DOI] [PubMed] [Google Scholar]
- 27.Arbelaez A., Castillo M., Mukherji S.K. Diffusion-weighted MR imaging of global cerebral anoxia. AJNR Am. J. Neuroradiol. 1999;20:999–1007. [PMC free article] [PubMed] [Google Scholar]
- 28.Wijman C.A., Mlynash M., Caulfield A.F., Hsia A.W., Eyngorn I., Bammer R., Fischbein N., Albers G.W., Moseley M. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann. Neurol. 2009;65:394–402. doi: 10.1002/ana.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.JM U.K.-I., Yu E., Bartlett E., Soobrah R., Kucharczyk W. Acute hyperammonemic encephalopathy in adults: imaging findings. AJNR Am. J. Neuroradiol. 2011;32:413–418. doi: 10.3174/ajnr.A2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinney A.M., Kieffer S.A., Paylor R.T., SantaCruz K.S., Kendi A., Lucato L. Acute toxic leukoencephalopathy: potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imaging. AJR Am. J. Roentgenol. 2009;193:192–206. doi: 10.2214/AJR.08.1176. [DOI] [PubMed] [Google Scholar]
- 31.Arnold S.M., Els T., Spreer J., Schumacher M. Acute hepatic encephalopathy with diffuse cortical lesions. Neuroradiology. 2001;43:551–554. doi: 10.1007/s002340000461. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya K., Katase S., Yohino A., Hachiya J. Diffusion-weighted MR imaging of encephalitis. AJR Am. J. Roentgenol. 1999;173:1097–1099. doi: 10.2214/ajr.173.4.10511186. [DOI] [PubMed] [Google Scholar]
- 33.McKinney A., Palmer C., Short J., Lucato L., Truwit C. Utility of fat-suppressed FLAIR and subtraction imaging in detecting meningeal abnormalities. Neuroradiology. 2006;48:881–885. doi: 10.1007/s00234-006-0145-5. [DOI] [PubMed] [Google Scholar]
- 34.Kelley B.P., Patel S.C., Marin H.L. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR. 2017;38:1070–1078. doi: 10.3174/ajnr.A5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Splendiani A., Puglielli E., De Amicis R., Necozione S., Masciocchi C., Gallucci M. Contrast-enhanced FLAIR in the early diagnosis of infectious meningitis. Neuroradiology. 2005;47:591–598. doi: 10.1007/s00234-005-1383-7. [DOI] [PubMed] [Google Scholar]
- 36.Fujioka M., Okuchi K., Hiramatsu K.I., Sakaki T., Sakaguchi S., Ishii Y. Specific changes in human brain after hypoglycemic injury. Stroke. 1997;28:584–587. doi: 10.1161/01.str.28.3.584. [DOI] [PubMed] [Google Scholar]