Abstract
Frequent Pap testing is recommended among women living with HIV (WLWH) due to their elevated risk for cervical cancer. However, there are few recent longitudinal evaluations of utilization and determinants of Pap testing among WLWH. Medical and pathology records of WLWH seen at Johns Hopkins Hospital between 2005 and 2014 were assessed using Prentice, Williams, Peterson models.
Of 554 WLWH in care for ≥ 18 months, 79% received Pap testing, however only 11% consistently received Pap testing at the recommended interval. Some women (5%) were consistently under-screened (tested at longer intervals) and 21% did not receive any Pap testing at during follow-up.
WLWH with decreased likelihood of screening included older women, injection drug users, whites and those who had lived for longer with HIV. In contrast, only women with a prior abnormal Pap result were more likely to receive Pap testing. CD4 cell count and health insurance were not significant determinants.
Although many WLWH in care received Pap testing, some WLWH were unscreened or underscreened. Determinants of Pap testing for WLWH include socio-demographic factors and a prior abnormal result; these present potential targets in an urban HIV care setting for closer monitoring and directed interventions to improve utilization among WLWH.
Keywords: Cancer screening; Pap testing; Cervical cancer; HIV; Prentice, William, Peterson models; Adherence
1. Introduction
In 2012, the Centers for Disease Control estimated about 284, 500 women were living with HIV (WLWH) in the United States (US) [1]. Even with tremendous improvements in clinical management of HIV, WLWH still represent an important high-risk subgroup for cervical cancer with the current incidence of cervical cancer among WLWH being 4-fold higher than that of the general population [2]. Additionally, treatment response and survival rates are much lower among WLWH diagnosed with cervical cancer than for women in the general population [3], [4], [5]. Thus, routine Pap testing is recommended and is important for all WLWH.
Until recently, screening guidelines for WLWH were similar to those for the general population, suggesting annual Pap testing, except for women newly diagnosed with HIV who were recommended to receive a Pap test twice in the first year post HIV diagnosis [6], [7]. More recently, guidelines in the general population have changed to suggest Pap testing every three years after a normal Pap [8], [9]. Guidelines in WLWH also changed in 2015 to suggest a three-year follow-up interval among women with three consecutive normal Pap test results [10]. However, by virtue of their higher risk, annual Pap testing is still recommended for WLWH with one or two consecutive normal Pap tests results [10].
The United States Preventive Services Task Force, in its 2012 report to congress, identified the limited evidence base on utilization of cervical cancer screening as one of the barriers to effective prevention and control in the US [11]. Indeed, among women diagnosed with cervical cancer in the US, most women were enrolled and regularly accessing healthcare services but were never screened (50%) or underscreened (10%) in the years prior to diagnosis [12], [13], [14], [15]. Further, despite the recommendation for frequent Pap testing among WLWH, some previous studies suggest that Pap testing remains underutilized even for WLWH enrolled in clinical care [16], [17], [18], [19], [20]. Initial studies exploring barriers to Pap testing among WLWH identified older age, blacks and other racial minorities, low socioeconomic status, low CD4 cell count and low level of education to be associated with the under utilization of Pap test services by WLWH [16], [17], [19], [21].
In one of the largest studies to date of Pap test utilization among WLWH, a cross-sectional survey of WLWH across 18 US states reported a sizable proportion (23%) of women did not receive Pap testing [17]. Focus group interviews on utilization of gynecologic services by WLWH at the Moore clinic of Johns Hopkins in 2008 suggested 22% of women never received a Pap test in the preceding year [18]. Relying solely on electronic records a study of WLWH in care at the Boston Medical Center reported an even higher proportion (47%) of women not receiving annual Pap testing [16]. These previous studies of Pap test utilization among WLWH have however, been largely cross-sectional in nature, relied on self-reports or followed WLWH for a relatively short period of time [16], [18], [19], [22], [23], [24], [25]. Thus, to address these limitations, this current study evaluated the utilization and determinants of cervical Pap testing among WLWH enrolled in care at Johns Hopkins Hospital (JHH) over the previous 10-years.
2. Materials and methods
2.1. Study design and population
A longitudinal study of WLWH enrolled in clinical care within a retrospective 10-year period, from January 1, 2005 to December 31, 2014 was performed. This study utilized data collected as part of the Moore Clinic cohort, which is comprised of people living with HIV in Baltimore, who are followed up longitudinally through medical record based abstraction of demographic, behavioral and clinical data at regular six months intervals [26]. The clinic funds care for uninsured and under insured people living with HIV through the Congressional Ryan White Care Act Title I, II and IV Programs. Among 669 women in the cohort and in care during this timeframe, the analysis was restricted to 554 WLWH who were: at least 18 years old, had basic demographic information available in their medical records and had clinical follow-up for at least 18 months (to allow an opportunity to be screened). For 54 women who met the inclusion criteria and were newly diagnosed with HIV, only data collected on or after their HIV diagnosis were utilized in this study, data prior to their diagnosis was excluded.
2.2. Outcome
The primary outcome of interest for this study, receipt of a Pap test, was defined using a binary indicator (yes or no). As this was a recurring outcome, women could experience multiple Pap tests during clinical follow-up. After every Pap test each woman was considered to be at risk for her next event (Pap test) until the end of time spent in clinical care or until the end of the 10-year period.
Although within the period studied all WLWH were recommended to have at least an annual Pap test; to be specific in our definition of underscreening we allowed a window of 18 months before considering a participant as not having received an annual Pap. Further, to ensure accuracy of data used in this study, individual medical records were linked to JHH pathology data using unique patient identifiers. Our study period, January 2005 to December 2014, preceded the September 2015 change in guideline recommendations for WLWH.
2.3. Determinants of Pap testing
Information assessed at entry and treated as fixed variables included: smoking status (current or never/former), injection drug use (yes or no), race (black, white or other), date of HIV diagnosis, and body mass index (BMI) calculated using recorded weight and height (underweight/normal: ≤ 24.9, overweight: 25–29.9 or obese≥ 30.0). Time-varying variables evaluated at entry and at each screening visit, included: type of health insurance (public {Medicare/Medicaid}, private, Ryan White health insurance and uninsured/out of pocket payment) and current CD4 cell count. At screening visits where information on time varying variables was not available, information about those variables from a preceding visit was utilized. Results of the preceding Pap test were also assessed at each screening visit and categorized as: normal (“negative for intraepithelial lesion or malignancy”), atypical squamous cells of undetermined significance (ASC-US) or atypical glandular cells of undetermined significance (AGUS), low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL) or atypical squamous cells- cannot exclude HSIL (ASC-H) [27].
2.4. Statistical analyses
Participants were described at entry using median values with corresponding interquartile range (IQR) estimates for continuous variables and percentages for categorical variables. The total number of Pap tests received during follow-up and the median time interval between each Pap test were also assessed. A Kruskal–Wallis test was used to compare median Pap test intervals by order of Pap test received and by results of the preceding Pap test. Linear trends were assessed using a nonparametric test for trend.
Prentice, Williams, Peterson gap time models (PWP-GT) were used to evaluate the determinants of Pap testing and follow-up for each woman was divided into multiple sets of time between Pap tests. These PWP-GT models are multiple failure time to event models that use a conditional risk set approach of time since each prior event to account for the multiple time periods within the same women at risk for Pap testing [28], [29]. PWP-GT are variance corrected models, which adjust the covariance matrix of estimators to account for the dependent nature of the outcome [28].
The unadjusted hazard ratio (HR) and 95% confidence interval (95% CI) for each factor were assessed. Factors observed to be significant in the unadjusted analyses and those identified by previous studies were included in the adjusted analysis. The adjusted model from which adjusted hazard ratios (aHR) were obtained included age, race, injection drug use, number of years since HIV diagnosis and preceding Pap test results, CD4 cell count and type of health insurance. Goodness of fit for the adjusted model was evaluated using Cox-Snell residuals [30].
All statistical tests were two sided with a significance threshold of p-value <0.05 and all continuous measures were rescaled using multiples of ten to allow for easy interpretation of the observed associations. All data management and analyses were carried out in STATA 14 [31]. The institutional review board of Johns Hopkins Medical Institutions approved this study.
3. Results
3.1. Study population
There were 554 WLWH who contributed a total of 3177 person-years to the study and these participants were followed for a median of 5.7 years (IQR:3.7–7.9). Forty-two percent of women were in care and entered our analysis in the first year of the 10-year study period (2005–06), another 25% entered the study in the 2007–2008 time period while 21% entered the study in the 2009–2010 time period (Table 1). At entry, participants had a median age of 41 years (IQR:34–48), were predominantly black (79%), and many reported a current smoking status (57%) (Table 1). Median CD4 count at entry was 307 cells/μL (IQR:127–510) and median HIV viral load at entry was 7712 copies/mL (IQR:400–53693).
Table 1.
Characteristics of 554 women were living with HIV at their first clinic visit within the study period (2005–2014).
| Characteristic | n (%) |
|---|---|
| Age (years): Median (IQR) | 41 (34–48) |
| <30 | 79 (14.3) |
| 30–39 | 162 (29.2) |
| 40–49 | 201 (36.3) |
| ≥ 50 | 112 (20.2) |
| Race | |
| Black | 440 (79.4) |
| White | 92 (16.6) |
| Other | 22 (4.0) |
| Baseline CD4 cell count (cells/μL): Median (IQR) | 307 (127–510) |
| < 200 | 180 (32.5) |
| 200–499 | 229 (41.3) |
| ≥ 500 | 144 (26.0) |
| Unknown | 1 (0.2) |
| Baseline HIV viral load (copies/mL): Median (IQR) | 7712 (400–53693) |
| Unknown | 5 (0.9) |
| Time since HIV diagnosis (years): Median (IQR) | 2.1 (0.1–8.9) |
| 0–4 | 326 (58.8) |
| 5–14 | 158 (28.5) |
| ≥ 15 | 63 (11.4) |
| Unknown | 7 (1.3) |
| BMI (kg/m3): Median (IQR) | 27.43 (23.6–33.1) |
| Underweight: < 18.5 | 15 (2.7) |
| Normal: 18.5–24.9 | 119 (21.6) |
| Overweight: 25–29.9 | 118 (21.5) |
| Obese: ≥ 30.0 | 149 (27.1) |
| Unknown | 149 (27.1) |
| Smoking status | |
| Current | 315 (56.9) |
| Former | 33 (6.0) |
| Never/ Unknown | 206 (37.2) |
| Injection drug use | |
| Yes | 146 (26.4) |
| No | 408 (73.7) |
| Calendar years of study entry | |
| 2005–2006 | 234 (42.2) |
| 2007–2008 | 142 (25.6) |
| 2009–2010 | 118 (21.3) |
| 2011–2015 | 60 (10.8) |
| Type of health insurance | |
| Public (Medicaid/Medicare) | 89 (16.1) |
| Private | 42 (7.6) |
| Ryan White | 49 (8.8) |
| Uninsured/Out of pocket | 6 (1.1) |
| Unknown | 368 (66.4) |
| Total amount of follow-up in clinical care (years): Median (IQR) | 5.7 (3.7–7.9) |
3.2. Utilization of Pap testing
Although a large proportion of WLWH received Pap testing (79%), a sizeable proportion of women (21%) never received Pap testing during study follow-up (Fig. 1a). Among those who received at least one Pap test some women received only one or two (31%) Paps, while others had three (12%), four (10%) and five or more (26%) Pap tests during follow-up (Fig. 1a).
Fig. 1.
a. Percent of women receiving 0, 1, 2, 3, 4, and 5 or more Pap tests during their follow-up 1b. Total time in care (number of years) within study period, by total number of Pap tests received by each participant.
As expected, women who were in care for a longer period were more likely to receive Pap testing than women with a shorter time in care (Fig. 1b). Median time in care for women who received at least one Pap test during follow-up was 5.1 years (IQR:3–6.9) compared to 3.8 years (IQR:2.3–5.7) among women who never received a Pap test during clinical follow-up at JHH. The total number of Pap tests received increased linearly with a longer median time in care (Fig. 1b; np trend < 0.001).
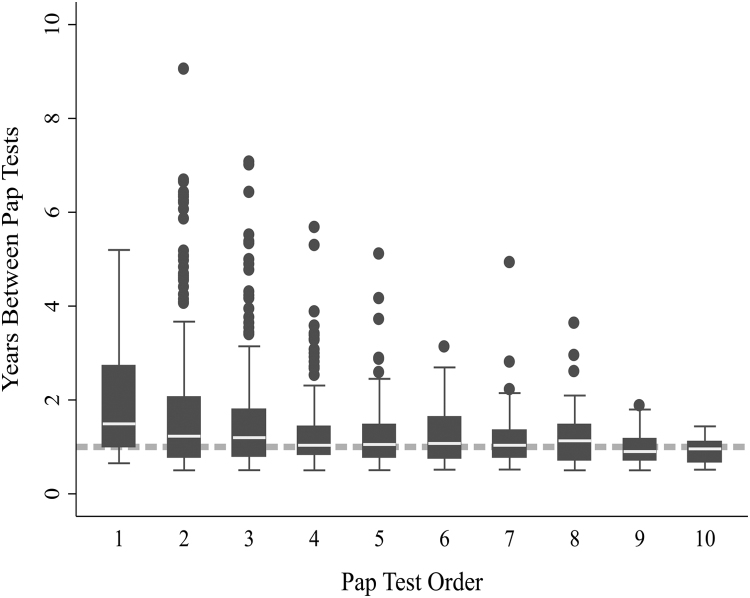
The overall median interval between successive Pap tests during follow-up was 11.3 months (IQR:6.2–17.2). However, median time from entry to the first Pap was longer (17.9 months; IQR:12.0–32.6, Fig. 2) than the recommended annual interval and women who had only one Pap had a longer median time from entry to Pap than women who had 2 or more Pap tests (19.6 vs 7.6 months). After the first Pap, median interval remained fairly consistent between subsequent Pap tests and with guideline recommendations (Fig. 2).
Fig. 2.
Among women screened, time interval (number of years) between current Pap and previous Pap test, by Pap test order. In this graph, category 1 represents the 1st Pap within the study, which is time from entry until first Pap. Category 2 represents the time to the 2nd Pap (from the first Pap), among women who had at least 2 Paps, etc. Median and interquartile range is shown. Dotted line represents recommended screening interval of 12 months.
Using a more stringent definition of screening based on the recommended annual interval, only 11% of women consistently received Pap test according to guideline recommendations. This was 19% of women who received two or more Paps during follow-up, highlighting the potential for underscreening. Also of note, 5% of women consistently received Pap test at intervals longer than 18 months.
Results of 60% of the 1410 Pap tests conducted during follow-up were normal. Abnormal Pap results included ASC-US/AGUS (13% of all Paps), LSIL (13%), and HSIL/ASC-H (7%). Nine (< 1%) of the Pap tests received did not have documented results.
Median interval between consecutive Pap tests varied significantly (p < 0.001) by results of the preceding Pap test. For women with a normal result in the preceding Pap test, median interval to the subsequent Pap was 14.3 months (IQR: 10.7–20.5) (Fig. 3). However, for women with an abnormal Pap result, this interval was shorter. Specifically, median interval for women with a preceding Pap test result of ASC-US/AGUS, LSIL or HSIL/ASC-H was 12.0 months (IQR:8.0–20.1), 11.1 months (IQR:7.2–16.5) and 11.0 months (IQR:8.7–16.5) respectively. For women with an unknown Pap test result this interval was 13.8 months (IQR:10.8–18.2).
Fig. 3.
Among women screened, time interval (number of years) between previous and current Pap test, by the preceding Pap test results. In this graph, category 1 represents the time interval from a normal Pap test result until the next Pap test. Category 2 represents the time to the next Pap test, among women with an abnormal Pap test result of ASC-US/AGUS, etc. Median and interquartile range is shown. Dotted line represents recommended screening interval of 12 months.
3.3. Determinants of Pap testing
Several determinants of Pap test utilization were identified (Table 2). In the unadjusted analysis older age (per decade, HR = 0.90, 95%CI:0.85, 0.95), white women (HR = 0.84, 95%CI:0.72, 0.99), injection drug users (HR = 0.79, 95%CI: 0.68, 0.91), women diagnosed with HIV for a longer time (per decade, HR = 0.93, 95%CI:0.79, 0.96) and those with a higher CD4 cell count (per 100 cells/ μL, HR = 0.98, 95%CI: 0.96, 1.00) were all significantly less likely to receive a Pap test, each p < 0.05. In contrast, women with Ryan White health insurance coverage compared to women with Medicaid/Medicare (HR = 1.27, 95%CI:1.06, 1.52) and those with a previous abnormal Pap test result (compared to a normal) of either ASC-US/AGUS (HR = 1.47, 95%CI:1.22, 1.76), LSIL (HR = 1.75, 95%CI:1.45, 2.10) or HSIL/ASC-H (HR = 2.22, 95%CI:1.55, 3.17) were more likely to receive a Pap test. Compared to current smokers, never and former smokers were also more likely to receive a Pap test (HR = 1.14, 95%CI:1.00, 1.30).
Table 2.
Factors associated with utilization of Pap Testing by women were living with HIV enrolled in care at the Moore Clinic of JHH, 2005–2014.
| Variable | Hazard Ratio | 95% Confidence Interval | p-value | Adjusted Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|---|---|---|
| Age, per 10 years | 0.90 | (0.85, 0.95) | < 0.01 | 0.93 | (0.89, 0.98) | 0.01 |
| < 30 (ref) | 1.00 | 1.00 | ||||
| 30–39 | 0.97 | (0.80, 1.16) | 0.72 | 1.01 | (0.84, 1.21) | 0.94 |
| 40–49 | 0.93 | (0.78, 1.11) | 0.43 | 0.98 | (0.82, 1.16) | 0.79 |
| ≥ 50 | 0.72 | (0.59, 0.88) | < 0.01 | 0.81 | (0.67, 0.99) | 0.04 |
| p for trend | < 0.01 | 0.02 | ||||
| Race | ||||||
| Black (ref) | 1.00 | 1.00 | ||||
| White | 0.84 | (0.72, 0.99) | 0.03 | 0.85 | (0.72, 1.00) | 0.04 |
| Other | 0.87 | (0.60, 1.24) | 0.44 | 0.77 | (0.53, 1.11) | 0.16 |
| Injection drug use | 0.79 | (0.68, 0.91) | < 0.01 | 0.80 | (0.70, 0.93) | < 0.01 |
| Time since HIV diagnosis, per 10 years | 0.93 | (0.89, 0.98) | < 0.01 | 0.90 | (0.81, 0.99) | 0.03 |
| 0–4 (ref) | 1.00 | 1.00 | ||||
| 5–14 | 0.78 | (0.69, 0.89) | < 0.01 | 0.84 | (0.74, 0.95) | 0.01 |
| ≥ 15 | 0.84 | (0.72, 0.99) | 0.04 | 0.88 | (0.74, 1.05) | 0.17 |
| p for trend | 0.15 | 0.08 | ||||
| Results of preceding Pap test | ||||||
| Normal (ref) | 1.00 | 1.00 | ||||
| Abnormal | 1.66 | (1.45,1.90) | < 0.01 | 1.66 | (1.44, 1.92) | < 0.01 |
| ASC-US or AGUS | 1.47 | (1.22, 1.76) | < 0.01 | 1.40 | (1.14, 1.72) | < 0.01 |
| LSIL | 1.75 | (1.45, 2.10) | < 0.01 | 1.76 | (1.45, 2.13) | < 0.01 |
| HSIL or ASC-H | 2.22 | (1.55, 3.17) | < 0.01 | 1.93 | (1.40, 2.66) | < 0.01 |
| Never received Pap test / Unknown | 1.18 | (0.91, 1.53) | 0.22 | 1.21 | (0.93, 1.55) | 0.15 |
| CD4 cell count, cells/100 μL | 0.98 | (0.96, 1.00) | 0.04 | 0.99 | (0.97, 1.01) | 0.29 |
| ≥ 500 (ref) | 1.00 | 1.00 | ||||
| 200–499 | 1.09 | (0.94, 1.27) | 0.25 | 0.97 | (0.85, 1.09) | 0.59 |
| < 200 | 1.09 | (0.94, 1.26) | 0.26 | 1.00 | (0.86, 1.16) | 0.98 |
| p for trend | 0.27 | 0.90 | ||||
| Type of health insurance | ||||||
| Public (ref) | 1.00 | |||||
| Private | 1.11 | (0.94, 1.32) | 0.23 | 1.04 | (0.87, 1.25) | 0.67 |
| Ryan White | 1.27 | (1.06, 1.52) | 0.01 | 1.16 | (0.96, 1.40) | 0.13 |
| Uninsured/Out of Pocket | 1.32 | (0.85, 2.06) | 0.22 | 1.20 | (0.77, 1.87) | 0.43 |
| Unknown | 0.94 | (0.82, 1.07) | 0.34 | 0.87 | (0.76, 0.99) | 0.04 |
| BMI, kg/per 100 m3 | 1.03 | (0 0.96,1.11) | 0.38 | |||
| Underweight / Normal: ≤ 24.9 (ref) | 1.00 | |||||
| Overweight: 25–29.9 | 1.03 | (0.87, 1.23) | 0.70 | |||
| Obese: ≥ 30.0 | 1.09 | (0.93, 1.28) | 0.30 | |||
| Unknown | 0.98 | (0.84, 1.16) | 0.85 | |||
| p for trend | 0.76 | |||||
| Smoking status | ||||||
| Current (ref) | 1.00 | |||||
| Never / Former | 1.14 | (1.00,1.30) | 0.05 | |||
| Unknown | 1.31 | (1.11, 1.55) | < 0.01 |
In the adjusted analysis, older age (aHR = 0.93, 95%CI:0.89, 0.98), white race (aHR = 0.85, 95%CI:0.72, 1.00), injection drug users (aHR = 0.80, 95%CI:0.70, 0.93) and women who had lived with HIV for a longer time (aHR = 0.90, 95%CI:0.81, 0.99) remained significantly less likely to receive a Pap test. Conversely, women with a previous abnormal Pap test result of ASC-US/AGUS (aHR = 1.40, 95%CI:1.14, 1.72), LSIL (aHR = 1.76, 95%CI:1.45, 2.13) or HSIL/ASC-H (aHR = 1.93, 95%CI:1.40, 2.66) remained significantly more likely to receive a Pap test. After adjusting for other variables, CD4 cell count and insurance were not predictors of Pap testing (Table 2).
4. Discussion
In our longitudinal assessment of Pap testing among WLWH enrolled in clinical care, we observed that 21% of WLWH did not receive Pap testing during follow-up. Additionally, only 11% of women consistently received a Pap test at the recommended annual interval and 5% were consistently underscreened. The likelihood of receiving a Pap test for WLWH enrolled in clinical care was associated with some of the socio-demographic, clinical and behavioral factors examined. Older women, white women, intravenous drug users and women who had lived with HIV for a longer time were less likely to receive a Pap test. These findings contribute to the existing evidence base on Pap test utilization among WLWH enrolled in clinical care. They suggest groups that may need to be closely monitored to ensure receipt of Pap testing as recommended.
The associations observed between older age, injection drug use and a decreased likelihood of receiving a Pap test are consistent with findings of previous studies that have also examined these demographic and behavioral risk factors [16], [18], [32]. Even though Pap testing is recommended throughout a WLWH's life [10], older women are often perceived to have a lower risk of cervical cancer making them less likely to receive Pap testing. Compared to non-users, injection drug users living with HIV have been noted to only partially engage in care [33] and as such may not have the opportunity to receive all the required medical services. This might explain the decreased association observed between injection drug use and the receipt of Pap testing. Although several previous studies have reported white WLWH are more likely of receiving Pap testing compared to black WLWH, we observed a marginally lower likelihood of screening among white WLWH. This finding is consistent with a previous study conducted in an urban health facility, which reported a twofold increased odds of non-adherence among white WLWH compared to black WLWH [16]. This may in part reflect differential healthcare access and the demographic profile of urban black WLWH compared to black WLWH residing in other settings [34], [35].
Our finding of a decreased likelihood of Pap testing among women with a longer time since HIV diagnosis, independent of older age, has not been previously reported. This decrease is potentially a result of the greater number of competing health priorities that develop as people live longer with HIV, which decreases their likelihood of receiving Pap testing. Similar to what we found, some studies have identified a prior abnormal Pap result as a predictor of future screening in both the general population [36], [37] and WLWH [16], [38]. We could not in this study differentiate whether the increased screening was driven by provider or patient motivations for screening.
The proportion of unscreened women in our study is higher than that reported for the general US population [39] and falls well above the healthy people 2020 target of 7% [40]. However, the proportion of unscreened women observed in our study is consistent with previous studies reporting 19–25% of WLWH in the United States do not receive Pap testing [16], [17], [19], [25]. Our findings suggest that even when WLWH are in care for long periods of time, some WLWH remain underscreened or unscreened. This study could not however evaluate whether women were refusing screening when offered.
Pap test intervals (i.e. the time interval between Pap tests) for WLWH have not been widely assessed and reported on. To our knowledge this is one of the few evaluations of time intervals between Pap test for WLWH. Although the median interval between Pap test for the entire follow-up period was fairly consistent with the guideline recommendations within our study period [7], the proportion of women consistently screened throughout clinical follow-up at or around the recommended annual interval was low. Some women were consistently underscreened and the time interval between Pap tests varied widely for the first few Paps. This suggests that although a sizeable proportion of WLWH receive Pap testing regularly while in care, there are WLWH who are infrequently screened and have much longer Pap tests intervals than is recommended.
This study has several strengths including its use of a comprehensive longitudinal history of Pap testing, confirmed using pathology records. Unlike previous studies, the 10-year period evaluated in this study allowed description of screening patterns and the time between Pap tests to be assessed. However, this study may not have captured Pap test received outside of JHH, we cannot exclude the possibility that some women were screened elsewhere and therefore the prevalence of underscreening may have been overestimated. To minimize the potential for this to occur, our analyses were restricted to participants who were enrolled in primary care for at least 18 months. Additionally, most (~85%) of the cohort participants in this study are known to access all of their healthcare needs through the Johns Hopkins network [26]. This study could not evaluate variables not routinely captured in medical records on factors such as patient understanding of the importance of Pap testing or their personal or physical barriers to accessing screening.
5. Conclusions
Our findings provide additional evidence that although most WLWH receive Pap testing, some women in clinical care are not screened and some women receive Pap testing at intervals longer than recommended. Characteristics of WLWH least likely to receive Pap testing include older women, women with a longer time since HIV diagnosis, injection drug users and white women. These present potential targets in an urban HIV care setting for closer monitoring and directed interventions to improve Pap screening in WLWH.
Funding
Sally Peprah was supported by the United States National Cancer Institute [T32 CA009314].
Declarations of interest
None.
References
- 1.Centers for Disease C. HIV Among Women. 〈http://www.cdc.gov/hiv/group/gender/women/〉.
- 2.Abraham A.G., Strickler H.D., Jing Y., Gange S.J., Sterling T.R., Silverberg M. Invasive cervical cancer risk among HIV-infected women: a North American multi-cohort collaboration prospective study. J. Acquir. Immune Defic. Syndr. 2013;62:405. doi: 10.1097/QAI.0b013e31828177d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maiman M., Fruchter R.G., Guy L., Cuthill S., Levine P., Serur E. Human immunodeficiency virus infection and invasive cervical carcinoma. Cancer. 1993;71:402–406. doi: 10.1002/1097-0142(19930115)71:2<402::aid-cncr2820710222>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Dryden-Peterson S., Bvochora-Nsingo M., Suneja G., Efstathiou J.A., Grover S., Chiyapo S. HIV infection and survival among women with cervical cancer. J. Clin. Oncol. 2016;34:3749–3757. doi: 10.1200/JCO.2016.67.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gichangi P., Bwayo J., Estambale B., Rogo K., Njuguna E., Ojwang S. HIV impact on acute morbidity and pelvic tumor control following radiotherapy for cervical cancer. Gynecol. Oncol. 2006;100:405–411. doi: 10.1016/j.ygyno.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Service USPH, Infectious Diseases Society of A, Prevention of Opportunistic Infections Working G. 2001 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Infectious diseases in obstetrics and gynecology;10:3, 2002. [DOI] [PMC free article] [PubMed]
- 7.L.M. Mofenson, M.T. Brady, S.P. Danner, K.L. Dominguez, R. Hazra, E. Handelsman, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV-Exposed and HIV-Infected Children: Recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recommendations and reports : morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control;58:1, 2009. [PMC free article] [PubMed]
- 8.Wilt T.J., Harris R.P., Qaseem A. Screening for cancer: advice for high-value care from the American College of Physicians. Ann. Intern. Med. 2015;162:718–725. doi: 10.7326/M14-2326. [DOI] [PubMed] [Google Scholar]
- 9.Moyer V.A. Screening for cervical cancer: US preventive services task force recommendation statement. Ann. Intern. Med. 2012;156:880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 10.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. 〈http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf〉.
- 11.V. Moyer, M. LeFevre, A. Siu Second Annual Report to Congress on High-Priority Evidence Gaps for Clinical Preventive Services. Rockville, MD: US Preventive Services Task Force. [PubMed]
- 12.Leyden W.A., Manos M.M., Geiger A.M., Weinmann S., Mouchawar J., Bischoff K. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J. Natl. Cancer Inst. 2005;97:675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 13.Freeman H.P., Wingrove B.K. Vol. 5. National Cancer Institute, Center to reduce cancer health disparities; Rockville, MD: 2005. p. 5282. (Excess Cervical Cancer Mortality: A Marker for Low Access to Health Care in Poor Communities). [Google Scholar]
- 14.Spence A.R., Goggin P., Franco E.L. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev. Med. 2007;45:93–106. doi: 10.1016/j.ypmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Sung H.Y., Kearney K.A., Miller M., Kinney W., Sawaya G.F., Hiatt R.A. Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer. 2000;88:2283–2289. [PubMed] [Google Scholar]
- 16.Baranoski A.S., Horsburgh C.R., Cupples L.A., Aschengrau A., Stier E.A. Risk factors for nonadherence with Pap testing in HIV-infected women. J. Women's Health. 2011;20:1635–1643. doi: 10.1089/jwh.2010.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oster A.M., Sullivan P.S., Blair J.M. Prevalence of cervical cancer screening of HIV-infected women in the United States. JAIDS J. Acquir. Immune Defic. Syndr. 2009;51:430–436. doi: 10.1097/QAI.0b013e3181acb64a. [DOI] [PubMed] [Google Scholar]
- 18.Tello M.A., Jenckes M., Gaver J., Anderson J.R., Moore R.D., Chander G. Barriers to recommended gynecologic care in an urban United States HIV clinic. J. Women's Health. 2010;19:1511–1518. doi: 10.1089/jwh.2009.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logan J.L., Khambaty M.Q., D'Souza K.M., Menezes L.J. Cervical cancer screening among HIV-infected women in a health department setting. AIDS Patient care STDs. 2010;24:471–475. doi: 10.1089/apc.2009.0295. [DOI] [PubMed] [Google Scholar]
- 20.Blair J.M., Fagan J.L., Frazier E.L., Do A., Bradley H., Valverde E.E. Behavioral and clinical characteristics of persons receiving medical care for HIV infection—medical monitoring project, United States, 2009. MMWR Surveill. Summ. 2014;63:1–22. [PubMed] [Google Scholar]
- 21.Lambert C.L.C. Factors influencing cervical cancer screening in women infected with HIV: a review of the literature. J. Assoc. Nurses AIDS Care. 2013;24:189–197. doi: 10.1016/j.jana.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Williams M., Moneyham L., Kempf M.-C., Chamot E., Scarinci I. Structural and sociocultural factors associated with cervical cancer screening among HIV-infected African American women in Alabama. AIDS Patient Care STDs. 2015;29:13–19. doi: 10.1089/apc.2014.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher F.E., Buchberg M., Schover L.R., Basen-Engquist K., Kempf M.-C., Arduino R.C. Perceptions of barriers and facilitators to cervical cancer screening among low-income, HIV-infected women from an integrated HIV clinic. AIDS Care. 2014;26:1229–1235. doi: 10.1080/09540121.2014.894617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonsen S.E., Kepka D., Thompson J., Warner E.L., Snyder M., Ries K.M. Preventive health care among HIV positive women in a Utah HIV/AIDS clinic: a retrospective cohort study. BMC Women's Health. 2014;14:1. doi: 10.1186/1472-6874-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein M.D., Cunningham W.E., Nakazono T., Turner B.J., Andersen R.M., Bozzette S.A. Screening for cervical cancer in HIV-infected women receiving care in the United States. JAIDS J. Acquir. Immune Defic. Syndr. 2001;27:463–466. doi: 10.1097/00126334-200108150-00007. [DOI] [PubMed] [Google Scholar]
- 26.Moore R.D. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. JAIDS J. Acquir. Immune Defic. Syndr. 1998;17:S38–S41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 27.Apgar B.S., Zoschnick L., Wright T.C., Jr The 2001 Bethesda system terminology. Am. Fam. Physician. 2003;68:1992–1998. [PubMed] [Google Scholar]
- 28.Cleves M. Analysis of multiple failure-time data with Stata. Stata Tech. Bull. 2000:9. [Google Scholar]
- 29.Prentice R.L., Williams B.J., Peterson A.V. On the regression analysis of multivariate failure time data. Biometrika. 1981;68:373–379. [Google Scholar]
- 30.Cox D.R., Snell E.J. A general definition of residuals. J. R. Stat. Soc. Ser. B. 1968:248–275. [Google Scholar]
- 31.L.P. Stata Corp, Stata Statistical Software Release 14: Stata Press Publication, 2015.
- 32.Tello M.A., Yeh H.-C., Keller J.M., Beach M.C., Anderson J.R., Moore R.D. HIV women's health: a study of gynecological healthcare service utilization in a US urban clinic population. J. Women's Health. 2008;17:1609–1614. doi: 10.1089/jwh.2008.0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westergaard R.P., Hess T., Astemborski J., Mehta S.H., Kirk G.D. Longitudinal changes in engagement in care and viral suppression for HIV-infected injection drug users. AIDS. 2013;27:2559. doi: 10.1097/QAD.0b013e328363bff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaVeist T., Pollack K., Thorpe R., Fesahazion R., Gaskin D. Place, not race: disparities dissipate in southwest Baltimore when blacks and whites live under similar conditions. Health Aff. 2011;30:1880–1887. doi: 10.1377/hlthaff.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaVeist T.A. Disentangling race and socioeconomic status: a key to understanding health inequalities. J. Urban Health. 2005;82:iii26–iii34. doi: 10.1093/jurban/jti061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirovich B.E., Welch H.G. The frequency of Pap smear screening in the United States. J. Gen. Int. Med. 2004;19:243–250. doi: 10.1111/j.1525-1497.2004.21107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paskett E.D., Phillips K.C., Miller M.E. Improving compliance among women with abnormal Papanicolaou smears. Obstet. Gynecol. 1995;86:353–359. doi: 10.1016/0029-7844(95)00176-R. [DOI] [PubMed] [Google Scholar]
- 38.Dal Maso L., Franceschi S., Lise M., de'Bianchi P.S., Polesel J., Ghinelli F. Self-reported history of Pap-smear in HIV-positive women in Northern Italy: a cross-sectional study. BMC Cancer. 2010;10:1. doi: 10.1186/1471-2407-10-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith R.A., Brooks D., Cokkinides V., Saslow D., Brawley O.W. Cancer screening in the United States, 2013. CA Cancer J. Clin. 2013;63:87–105. doi: 10.3322/caac.21174. [DOI] [PubMed] [Google Scholar]
- 40.U. S. Department of Health Human SOoD, Prevention Health, Promotion. Healthy people 2020, 2010.