Abstract
Trophic rewilding involves adding species into ecosystems to restore extinct, top-down interactions, but limited quantitative data have prevented a systematic attempt to quantify its outcomes. Here, we exploit species introductions that have occurred for purposes other than restoration to inform trophic rewilding. We compiled 51 studies with 158 different responses of lower trophic levels to a species introduction that restored an extinct interaction, whether it intended to do so or not. Unintentional introductions were compared with checklists of extinct animals to identify potential analogues. Using the latest meta-analysis techniques, we found that the few cases of intentional rewilding had similar effects to unintentional rewilding, though there were large taxonomic and geographical biases. We also tested predictions from studies on trophic cascades about the factors that should influence rewilding. Unintentional rewilding was stronger where introduced consumers were non-invasive, but there was no effect of time that compared sites differed in introduction status, latitude or coevolution of responses with a taxonomically related analogue. Our study now shows that rewilding can reinstate extinct trophic interactions and highlights remaining data gaps that need closure to restore ecosystems across larger scales than has been previously possible.
This article is part of the theme issue ‘Trophic rewilding: consequences for ecosystems under global change’.
Keywords: conservation, extinction, invasive species, reintroduction, restoration, trophic cascades
1. Introduction
Few ideas in biology have stirred as much recent controversy with as few data as rewilding [1–3]. Although many definitions of rewilding exist [4,5], perhaps the readiest for research and practice is that of trophic rewilding [6]. Trophic rewilding theoretically involves adding species into ecosystems to restore top-down trophic interactions that can be sustained without continuous human intervention [6]. The keystone impacts of many carnivores and herbivores downwards through food webs have been lost because of human actions, with far-reaching consequences for biodiversity and ecosystem processes [7–10]. Reversing these impacts through trophic rewilding involves reintroducing species that have recently been locally extirpated, although active management is often required because of space limitations and a lack of native predators. Controversially, trophic rewilding can also involve reintroducing species that disappeared as far back as the Pleistocene and even introducing functional substitutes where the original lineages have gone globally extinct ([11], e.g. [12]). Despite the proliferation of essays and opinion pieces, few studies have measured the feasibility and success of trophic rewilding using empirical data [6,13,14].
Defining an appropriate management goal is a major challenge in quantifying the feasibility and success of trophic rewilding [5]. Historic baselines are one approach for goal setting [15], but as reference ecosystems can shift over time, a better approach may be to compare interventions against the outcomes that would arise without rewilding [16]. By definition, successful trophic rewilding should involve reducing the total biomass and/or abundance of prey species in the next lower trophic level in the food web as compared with the absence of a species (re)introduction [17]. This change in biomass and/or abundance should release two trophic levels beneath the (re)introduced consumer from top-down control, consistent with a trophic cascade [17,18]. Some species in the trophic level immediately beneath the (re)introduction may also respond positively because of indirect outcomes of rewilding, such as reduced intraguild competition [10]. Thus, depending on the trophic level that is monitored, success may appear to have a negative or positive effect on population and community dynamics, further complicating the evaluation of management goals. Finally, perhaps the greatest obstacle in assessing the potential of trophic rewilding is that most (re)introduction experiments have been conducted in the past decade [5,6]. Most learning has instead come from palaeoreconstructions and experiments excluding extant animals [10]. Given these challenges, there is consequently no data-driven synthesis, to our knowledge, of trophic rewilding efforts.
Trophic rewilding experiments have, however, been run unintentionally many times under the guise of species introductions. These introductions have been both deliberate to benefit human interests, such as from biocontrol or increasing food, trade and hunting opportunities, and accidental, including from escaping captivity and hitchhiking with vectors such as people, soils and vehicles [19,20]. Irrespective of the route, the defining feature of unintentional rewilding is that it restores an extinct top-down trophic interaction without having intended to do so [6,21]. Unintentional rewilding therefore involves introducing a species that either went locally extinct or acts as an analogue for an extinct functional type. The frequency with which such species introductions have occurred worldwide offers an opportunity to synthesize additional evidence about the factors that influence the success of trophic rewilding [22].
Invasiveness will be a key determinant of whether species introductions offer an analogy to intentional trophic rewilding. Although many introductions have detrimentally impacted other species [23,24], negative impacts on prey and plants at lower trophic levels are entirely predicted by trophic rewilding. Of more importance will be whether species introductions become widespread and/or numerically dominant, defined as invasive irrespective of whether they are native or not [25]. Invasive species have impacts beyond suppressing the abundance or altering the behaviour of the organisms they consume. For example, in New Zealand, the seven species of deer (Cervidae) introduced during the twentieth century share similar diet and habitat use as the extinct avian megafauna [26]. However, deer attain much higher densities and biomass over larger areas than the avian herbivores they replaced, arresting forest and grassland development prematurely compared with the climax communities that historically developed in their absence before megafaunal extinctions [26]. By contrast, large and giant tortoises from the Testudinidae family have been introduced worldwide to restore lost grazing and seed dispersal interactions without necessarily becoming numerically dominant or geographically widespread [27]. Thus, introductions may only be analogous to intentional rewilding where species do not become invasive. Invasive species should generally have stronger effects on lower trophic levels.
Considerable theory predicts that other factors in addition to invasiveness also influence the effects of higher trophic levels downwards through food webs [28]. These factors include many organismal traits and environmental conditions (table 1). For example, in a meta-analysis of 114 studies, Borer et al. [28] found that trophic cascades onto plant community biomass strengthened as metabolic efficiency increased in herbivores and was generally greater with endotherm predators that had larger metabolic demands. Mathematical modelling of feeding interactions based on physiological demands of differentially sized organisms also predicts that high metabolic efficiency should strengthen trophic cascades from predators to herbivores to plants [34]. Evolutionary history is another important consideration. For example, prey that are co-adapted with the introduced mode of predation may suffer fewer losses than populations exposed to very different predators and selection pressures [35]. This naiveté may partly explain why more evolutionarily distinct species are more negatively affected by introduced predators [36].
Table 1.
Factors influencing variation in trophic rewilding. For each factor, we predicted the change in the strength of a trophic interaction (TI) between two adjacent trophic levels. Interactions could include changes in behaviour, life history (e.g. reproduction, survival), abundance and/or total standing biomass of prey. The list is not meant to be exhaustive.
| factor | ΔTI | mechanism | reference |
|---|---|---|---|
| consumer invasiveness | ↑ | numerically dominant and widespread consumers will intake more prey/plants | n.a. |
| consumer body size | ↑ | larger organisms need to consume more biomass | [29] |
| consumer richness | ↓ | more intra-trophic-level interactions (e.g. competition, predation) can reduce prey/plant consumption | ([30], but see [31]) |
| consumer foraging efficiency | ↑ | high efficiency will remove more prey/plants | [28] |
| prey/plant generation time | ↓ | faster turnover will mitigate losses in population size | [28] |
| prey/plant spatial heterogeneity | ↓ | high heterogeneity reduces the search efficiency of consumers | [32] |
| prey/plant quantity and quality | ↑ | high quantity and quality can promote consumption rates | [18,33] |
Here, we aimed to inform future trophic rewilding projects by identifying general explanations for why some introductions have stronger effects than others on lower trophic levels. Our approach involved delivering the first meta-analysis of trophic rewilding and identifying the biases in empirical knowledge. We then compared intentional rewilding studies with those where extinct top-down interactions were restored without having intended to do so. Finally, we tested how the effects of unintentional rewilding on lower trophic levels varied with factors such as the focal biome, time and evolutionary history. Using studies that have synthesized evidence around the factors that determine the strength of trophic cascades [28,37], we formulated and tested four hypotheses that predicted unintentional rewilding would have stronger effects when:
-
(i)
lower trophic levels had not coevolved with introduced consumers or their close relatives;
-
(ii)
introduced consumers were invasive;
-
(iii)
sites had greater productivity and/or energy availability and
-
(iv)
there was less time for lower trophic levels to compensate for introduced consumers.
Critically, there is no effect size that defines when trophic rewilding has occurred. The effect of trophic rewilding—either unintentional or intentional—should simply be discernible relative to the absence of trophic interactions, allowing us to quantify the associated impacts by drawing on the rich literature comparing areas with and without species introductions. Moreover, our approach forgoes the difficulties in defining absolute restoration success by comparing unintentional and intentional rewilding studies and then separately trying to understand variation within each of these groups.
2. Methods
(a). Data assembly
We used keyword searches from 18 January to 21 February 2018 to identify articles that contained quantitative data on the impacts of (re)introduced primary to tertiary consumers on lower trophic levels. Search terms focused either on identifying intentional rewilding for restoring trophic interactions or introductions of consumers. The exact search terms and databases are given in electronic supplementary material, Text S1. In total, we identified 1169 relevant papers after duplicates were removed.
One of the authors then read the abstract of each paper to identify whether it involved the introduction or reintroduction (hereafter simply ‘introduction’) of a consumer and contained quantitative data on the responses of lower trophic levels to the species introduction. Typically, this meant that we retained studies that employed animal exclosures or comparisons of invaded versus uninvaded areas/islands and measured the responses of lower trophic levels and soil properties.
There were 191 papers remaining after the initial filtering step that we screened to extract the (i) identity and trophic level of the introduced species; (ii) date of introduction; (iii) geographical coordinates; (iv) study biome; (v) mean, error and sample size associated with responses of lower trophic levels with and without the introduced species; and (vi) time the two treatments differed in introduction status. Studies could have measured different responses at the same trophic level, and we collated all responses that were accessible from raw statistics and display items. Where the same response was measured on different organisms in the same trophic level (e.g. across different plant species), we summed responses and propagated the associated uncertainty into error estimates. We also differentiated between non-native and native responses, and discarded measurements that reported combining the two groups but did not provide separate values. For studies measuring responses across different years, we generally took the last time point, whereas we averaged responses across different seasons, always propagating errors. We excluded temporal comparisons of before and after species introductions, even when studies employed such a design, because differences could be confounded by background successional changes [16].
We then collated lists of Quaternary animal extinctions from published sources [38–41] to identify whether studies could be classed as unintentional rewilding, i.e. introducing a species that acts as an analogue for an extinct functional type without having intended to do so [6]. We classed introduced species as potential analogues for an extinct consumer if they occupied a similar ecological role. Evidence for similar roles came from literature searches in Google Scholar for studies on the diet and habitat use of extinct animals, which are summarized in electronic supplementary material, table S1. In practice, this exercise required asking questions such as ‘do deer fill the role of moa in New Zealand?’ or ‘are dingoes like thylacines in Australia?’. We accept that these comparisons are controversial, but we aimed to minimize this by focusing on the trophic interactions that the species introductions provided and their evolutionary history. Although evolutionary isolation may preclude likening introduced species to extinct animals in some parts of the world, even biotas traditionally considered relicts (e.g. New Zealand) have recently been shown to experience ongoing colonization [42]. We also used the extinction information to determine whether lower trophic levels might have coevolved with taxonomically related species to those that were introduced, e.g. the same Order. In total, 51 papers were included in our final dataset with both quantitative data associated with a species introduction and potential analogues identified (electronic supplementary material, table S2 and Data S1).
(b). Effect size calculations
We calculated the effect size associated with each observation i of each response variable within each study using the log response ratio Ri [43]. Ri was equal to the log of the ratio between the mean response with the species introduction and the mean response without the introduction. Thus, negative Ri values indicated a reduction in the response due to the species introduction, such as might be expected for the abundance of the next lowest trophic level, whereas positive values indicated an increased response with a species introduction, such as might be expected for the abundance of two trophic levels beneath the introduction due to a trophic cascade. This interpretation of effects was inappropriate for a handful of responses associated with vigilance time, stress hormones and zoochory dispersal distance (n = 11 of 158 in our dataset). We took the inverse of the response ratios in these cases to ensure consistency in the direction of effects, i.e. increased response due to species introduction. Variances Vi for each Ri were calculated by summing the squared standard deviation (SD) divided by product between the sample size and mean response for each treatment after Gurevitch et al. [43]. For the three studies without corresponding error estimates (n = 4 response observations), we imputed variances after Hedges & Olkin [44] from Ri and sample sizes.
(c). Statistical modelling
We used meta-regression to compare the effects of intentional and unintentional trophic rewilding and test our study hypotheses. This approach involved assuming that Ri was sampled with error from a normal distribution centred on a ‘true’ but unobserved effect size θi and SD equal to the square root of the pooled variances [45]. We could then sample θi from a normal distribution described by an estimated SD σ and estimated mean that we related to our variables of interest. These variables included the following:
-
(1)
The j number of trophic levels between the introduced species and the response tj[i] (0, 1, 2 or 3). For responses that were 0 or 1 trophic level beneath the introduced species, we also considered whether these were indirect interactions rather than direct consumption, such as arising from reduced intraguild competition or changes in bottom-up processes associated with the main cascade (i.e. ‘knock-on’ effects, [18]). The presence of indirect interactions was denoted by a binary variable ni.
-
(2)
Whether the introduced species were exclusively herbivores, carnivores or omnivores (including if a guild of introduced species spanned herbivores and carnivores) oj[i].
-
(3)
Whether the responses had coevolved with species from the same Order as those that were introduced ci (binary value) based on our assignment of extinct analogues. We also considered whether responses coevolved with species from the same Family, even though an analysis at this level risked overlooking suitable comparisons, such as between New and Old World pigs (Tayassuidae versus Suidae, respectively).
-
(4)
Whether the introduced species were on the IUCN list of the world's worst invaders (http://www.iucngisd.org/gisd/) or classed as locally invasive vi (binary value) in the associated study. Sometimes the same species could be invasive in one study site but not another.
-
(5)
Absolute latitude ui of the study site. We interpreted sites closer to the poles as having lower energy [46].
-
(6)
Net primary productivity pi extracted from the corresponding 0.25′ × 0.25′ grid cell wherein each site was located [47].
-
(7)
Log study duration di, which assessed the time that the two treatments have differed in introduction status.
We also accounted for variation in effect sizes from repeatedly observing the same k studies sk[i] and l study biomes bl[i], some of which were overly represented in the dataset. Finally, we accounted for random variation among the m types of response measurements rm[i] that were categorized as related to either biomass, life history (e.g. reproduction, dispersal, survival), behaviour (e.g. feeding and escape rates), species richness or ecosystem processes and services. The importance of top-down trophic interactions for the last two responses has only recently been appreciated [7,48]. Moreover, rm[i] allowed some response types to show opposite effects from those of biomass, such as if herbivory and predation increased richness of lower trophic levels.
We expected different signs for the effects associated with the number of trophic levels between the introduced species and the response tj[i]. This expectation comes from trophic cascade theory because consumers should reduce the next lowest trophic level in the food web (i.e. negative Ri with t1) and release two trophic levels down from predation (i.e. positive Ri with t2). Any predictor of trophic interactions should subsequently maintain the different signs of the tj[i] categories either by dampening or heightening their effects on Ri. For example, shorter studies might increase Ri when the introduced consumer is one trophic level above the response and decrease Ri when it is two trophic levels higher, rather than always changing the effect of tj[i] on Ri by the same amount, i.e. additively. To estimate a single effect for each level of tj[i] on Ri that could vary with different predictors while keeping the same sign, we scaled interactions proportionally on an exponential scale. We also varied Ri with the other predictors independently of tj[i]. Our final model took the form of
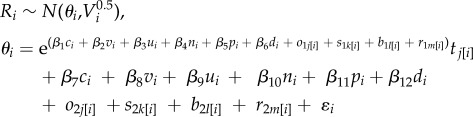 |
2.1 |
where β1–β6 were separately estimated effects that heightened or dampened the effects of trophic level differences on Ri, the j values of tj were each separately estimated effects that described average effects of trophic level differences on Ri, β7–β12 accounted for variation in Ri due to study characteristics, o, s, b and r parameters were each sampled from zero-mean normal distributions with separately estimated SDs, and ɛi was estimated normally distributed error. The formulation meant that it was straightforward to determine when tj[i] was unmodified, as the predictors would be 0 and the exponent would simplify to 1.
We simultaneously fitted our model to all observations of unintentional and intentional rewilding. For the latter, 18 of the 19 observations involved a one-trophic-level difference between the introduced species and the response, so we dropped the one extraneous measurement from the analysis. We did so because we wanted to estimate separate effects for tj[i] with intentional rewilding as compared with unintentional rewilding and could not do so with a single observation.
(d). Model estimation
We fitted equation (2.1) to our data using Hamiltonian Monte Carlo sampling by calling Stan v.2.16 from R v. 3.4 [49]. Full details are given in electronic supplementary material, text S2, and model code is given in Data S2. To infer effects, we calculated posterior means and 95% credible intervals (CIs) for each parameter by drawing a subset of 800 simulations. We did not reject hypotheses if 95% CIs for associated effects excluded 0. All covariates were standardized to a common scale with a mean of 0 and SD of 1 to compare the relative importance of different effects.
Finally, we tested for bias in the estimated effect sizes using residuals from our meta-regression model. Results from studies with a small sample size will have lower precision (i.e. reciprocal of the SD) and thus relatively more variation in their effects. Consequently, in the absence of bias, the spread in effect sizes should decrease with increasing precision. Selection biases (e.g. smaller studies with no statistically significant effect remaining unpublished), ‘true’ heterogeneity between studies (e.g. if studies estimate different underlying effects) and other irregularities can result in deviations from this pattern [50], and we tested for such biases by modelling weighted residuals as a linear function of the associated precision [51]. Bias was inferred if the resulting intercept excluded zero [52].
3. Results
(a). Geographical, taxonomic and publication biases
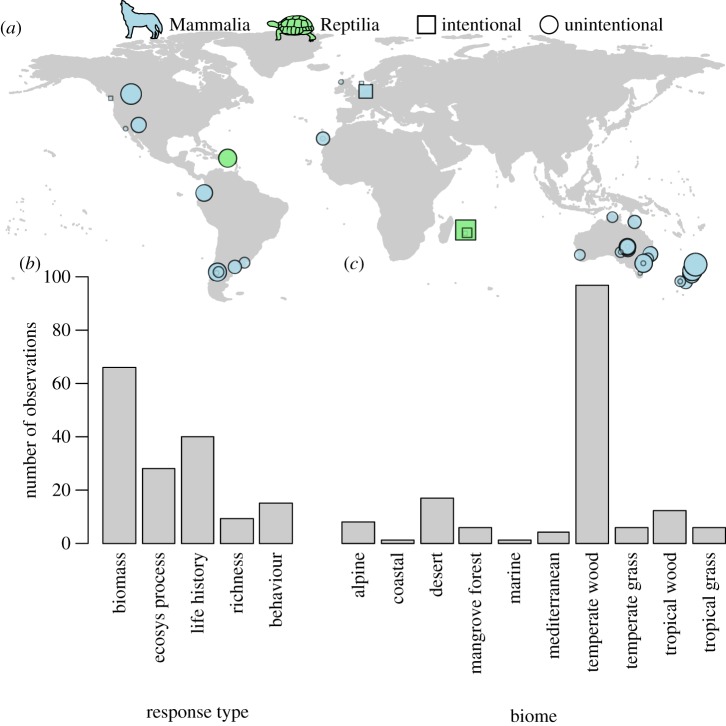
Our final database showed strong geographical and taxonomic biases (figure 1). We amassed 158 observations from six studies of intentional trophic rewilding and 45 studies of unintentional rewilding. Most (78%) of these observations came from the Southern Hemisphere (figure 1a), corresponding to the biomass (i.e. height, abundance, cover, density) and life history (e.g. reproduction, dispersal, survival) of lower trophic levels (42% and 25% of all observations, respectively), with relatively fewer observations (less than or equal to 10%) monitoring species richness and behavioural responses (figure 1b). Data mostly (61%) were recorded in temperate forests and woodlands, with no other biome represented by more than 11% of the observations (figure 1c). Responses were mostly measured one trophic level beneath the (re)introduced species (65%), with fewer observations between two and three trophic levels (18% and 10%, respectively), and were evenly split among introductions of carnivores (32%), herbivores (35%) and omnivores (33%). The introduced species were overwhelmingly mammals (88%), with the remaining observations involving reptiles (figure 1a). Modelled effect sizes showed no evidence of bias (95% CI for intercept: −0.18 to 0.13; electronic supplementary material, figure S1). Overall, our meta-regression model fitted the data reasonably well (95% CI for Bayesian R2: 0.65–0.81; electronic supplementary material, figure S2).
Figure 1.
Rewilding responses were dominated by unintentional introductions of mammals in the Southern Hemisphere and temperate woodlands. (a) Global distribution of compiled studies (n = 51) in relation to the Order of the introduced species and whether introductions were intentional or unintentional. Symbols are scaled to the number of responses recorded in each study (total n = 139 and 19 for unintentional and intentional rewilding, respectively). (b) Number of observations associated with five response types: biomass, ecosystem processes and services, life history (e.g. reproduction, dispersal, survival), species richness and behaviour (e.g. feeding and escape rates). (c) Number of observations associated with ten biome types. Wood, woodland; grass, grassland.
(b). Are species introductions a form of unintentional rewilding?
Unintentional rewilding had similar effects on lower trophic levels as the few cases where introductions were intended to restore top-down interactions (figure 2), suggesting that it could be used to understand the factors that generally influence trophic rewilding. Responses that were one trophic level beneath introduced species—all cases of intentional rewilding in our dataset—were reduced, on average, by 79% (95% CI: 24 to 99%) by unintentional rewilding as compared with 77% (95% CI: 13 to 99%) for intentional rewilding. There was no difference between these two values (95% CI for difference: −63 to 61%).
Figure 2.
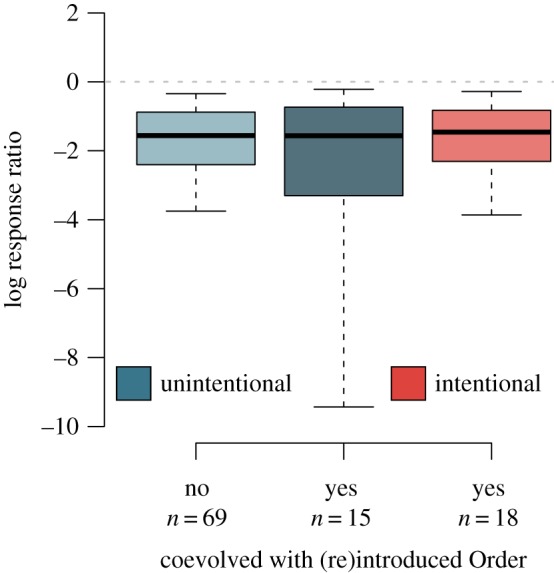
Intentional and unintentional rewilding have similar effects. Estimated log response ratios across all response types in the absence of species being invasive and with or without responses having coevolved with a taxon from the same Order as the introduced species. Solid lines are mean ratios ± 95% CIs, with boxes denoting interquartile range.
(c). How do the effects of unintentional rewilding vary across trophic levels?
Unintentional rewilding had effects consistent with those of trophic cascades. In addition to the strong negative effect on the next lowest trophic level (figure 2), we found that responses were positive when they were measured two trophic levels beneath the introduced species (figure 3; 95% CI for effect on Ri: 0.02–0.92). There was no effect at either the same trophic level (95% CI: −4.09 to 3.59) or three levels lower (95% CI: −3.06 to 3.15), which entirely comprised measurements of ecosystem processes, e.g. soil nutrients (figure 3).
Figure 3.
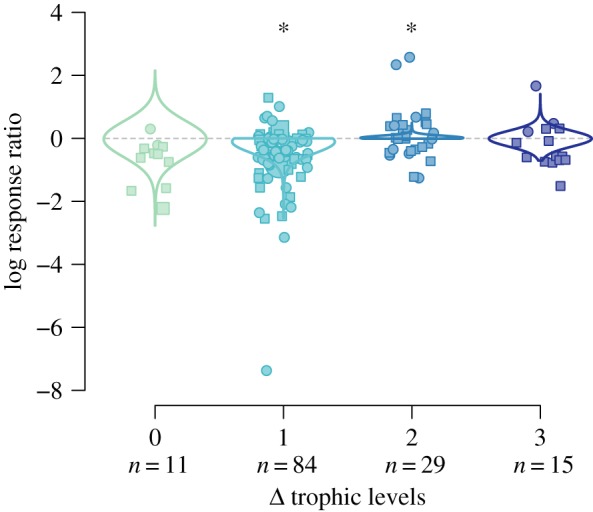
Intentional rewilding causes trophic cascades. Points are estimated log response ratios at 0 to 3 trophic levels beneath introduced species that are either non-invasive (circle symbols) or invasive (square symbols). Symbol size was scaled to precision of the observation (i.e. reciprocal of SD). Lines are posterior densities for responses estimated for invasive introduced species, which account for most of the observations in the unintentional rewilding dataset (n = 107 of 139). * = estimated effects excluded zero.
We found support only for the prediction that the invasive status of introduced species influences the responses of different trophic levels to rewilding. The effect of the number of trophic levels between the response and introduced species on the response ratios was surprisingly dampened in the presence of invasive species (95% CI for β2 in equation (2.1): −6.02 to −0.91). For example, an invasive species that was not introduced for rewilding managed to reduce responses in the next lowest trophic level by a mean of only 5% (95% CI: 0.2 to 57%) as compared with 79% (95% CI: 24 to 99%) by a non-invasive species. Coevolution of a response with a similar Order as an extinct species, study duration, indirect interactions, net primary productivity and absolute latitude all had no effect on the response ratios either individually or by changing how they varied at different trophic levels beneath the introduced species (electronic supplementary material, table S3). Repeating our analysis with coevolution measured at the Family level did not change our results (electronic supplementary material, table S4). Similarly, dietary breadth, biome, response type and study explained about two to eight times less variation in response ratios, on average, as compared with the residual error, suggesting that they were of little importance, and only study identity weakly influenced how the response ratios varied across different trophic levels (electronic supplementary material, table S3 and figure S3).
4. Discussion
With this year marking the 20th anniversary of the term rewilding entering the conservation lexicon [53], there remain few empirical data about its outcomes. This knowledge gap has restricted efforts to advance the practice of rewilding and incorporate it into policy [5]. Here, we found that unintentional rewilding influenced lower trophic levels similarly to where top-down trophic interactions were intentionally restored. Our analysis also showed that most quantitative measurements of intentional rewilding have focused on the introduction of tortoises on oceanic islands, highlighting incompleteness in our understanding. Nonetheless, our study offers a new perspective on the few cases of intentional rewilding by synthesizing their effects alongside species introductions that have occurred over large spatio-temporal scales, charting a course to inform interventions across more ambitious taxonomic and spatial scales.
(a). Why do the effects of rewilding vary? Lessons for intentional introductions
The many studies of unintentional rewilding allowed us to explore the factors that might influence the responses of lower trophic levels to intentional rewilding with larger datasets than would otherwise be possible. In doing so, we found that introduced species that were invasive had smaller effects on lower trophic levels, contrary to our initial hypothesis. We offer three potential explanations for this result. First, most species classed as invasive in our dataset (e.g. Rattus spp. and Vulpes vulpes) have broad diets that include omnivory and scavenging as compared with the species that were not classified as invasive (e.g. Bos taurus). Trophic cascades are predicted to be weaker in such cases because interactions among trophic levels are more diffuse [54,55]. Although we did not find a separate effect of dietary breadth, our classification could have been too coarse to detect such differences. Second, most of the invasive species were mesopredators as compared with the non-invasive species that were mostly large herbivores or modern apex predators (e.g. Canis lupus). Relatively few individuals of an apex predator are sufficient to trigger strong responses across multiple trophic levels [7]. For example, the trophic cascades facilitated by wolves on Isle Royale, USA, were attributed to just two or three packs [56]. Similar outcomes have been reported over the considerably larger Yellowstone National Park [57]. Therefore, numerical abundance may be a poorer predictor of rewilding outcomes than trophic position. Additionally, high competition among mesopredators, as well as their control by extant apex predators [58,59], may limit their effects on lower trophic levels. Finally, the effect of invasiveness may reflect biases in our data. Nearly 45% of the responses to invasive species were associated with biomass, behaviour and ecosystem processes as compared with 15% for the non-invasive responses. These response types had relatively weaker effects (electronic supplementary material, figure S3). Importantly, our finding that invasive species caused relatively weaker trophic cascades should not be interpreted as supporting the view that they do not merit control. Although some invasive consumers can eventually fill an important ecological role in their communities (e.g. dingoes in Australia), they have many undesirable effects, such as displacing competitors [59], and our analysis ignores the identity of their prey, such as if they are critically endangered [36].
The lack of support for our other hypotheses was consistent with effects observed elsewhere. First, we found no support for the prediction that sites with greater productivity and/or energy availability would have stronger responses to rewilding. Borer et al. [27] similarly found no effect of plant standing crop or nutrient fertilization on the strength of trophic cascades across 45 studies of aquatic and terrestrial ecosystems. One explanation is that prey/plants are better defended in more resource-rich environments, offsetting any effect of greater consumption. For example, plant defences have been reported to increase nearer the Tropics [60]. Latitudinal defence gradients are less studied in animals, but some work suggests that predators may be more selective with respect to anti-predator adaptations in more energetically favourable environments [61]. A related explanation is that predation can alleviate competitive exclusion in resource-rich environments, thereby offsetting any direct negative effects on lower trophic levels [62]. Large herbivores can instead have more negative effects in low productivity environments [62,63]. Second, we found no evidence that shorter studies respond more strongly to species introductions. While raw response ratios were negatively correlated with the amount of time that treatments differed in introduction status (weighted Pearson's r = −0.58), as reported in a synthesis of plant biomass responses to large mammalian herbivores [64], there was no discernible effect in our meta-regression model. Others have similarly found that raw correlations disappear when accounting for predictors such as biome type or response generation time [28,37]. This finding suggests that long timescales (i.e. years) may not necessarily be required to observe an effect of trophic rewilding. Finally, the lack of an effect of coevolution with a taxonomically related species may be unsurprising because trophic interactions ultimately depend upon function. In some cases, such as a niche space with a limited number of dimensions, species can converge towards similar functions despite being distantly related. For example, canids and the marsupial thylacine (Thylacinus cynocephalus) are morphologically and ecologically similar despite last sharing a common ancestor over 160 Ma [65] (electronic supplementary material, table S1).
(b). Show me the data: empirical gaps that need closure
The field of rewilding is arguably caught in a circle: it needs empirical evidence to convince decision makers that it will not have unintended consequences, but it cannot gather those data without being implemented. Most intentional rewilding studies have thus been restricted in scale and focused on animals that pose relatively little potential economic and cultural conflict (after [66]). Rather than continue to repeat the call for more experimental evidence [6,13,14], our study narrows the geographical and taxonomic gaps in quantitative data that need to be closed by future research. South America may be a particularly important area to focus upon. It had disproportionately more Late Quaternary extinctions associated with human impacts than elsewhere [67,68], and many are still ongoing [8,69]. There are also few quantitative data associated with rewilding in South America, particularly from biodiverse eastern regions (figure 1), which are highly suitable for rewilding with low risk solutions (e.g. [70]). Partly, this gap may have arisen because relevant studies were published in non-English-language journals and missed by our literature search. Finally, much of what we can learn from unintentional rewilding comes from the Southern Hemisphere, mirroring geographical patterns in global species transport [20], and suggesting there may be opportunities to measure impacts elsewhere.
An additional bias that may arise in rewilding studies is the focus on species-level cascades that involve a subset of taxa in lower trophic levels rather than community-level cascades that influence aggregate properties of entire systems [18]. Top-down control of aggregate responses may be harder to detect if there are compensatory trade-offs between species at lower trophic levels, such as in biomass [54,71]. We dealt with this by aggregating observations at the community-level, where possible, and separating response types associated with species-level measures, such as behaviour, from more community-centric responses, such as richness and biomass. Future rewilding studies should bear in mind these different scales of trophic responses when measuring outcomes.
(c). Conclusions: moving beyond the frontiers of rewilding
Controversial or not, rewilding is already happening. Our work reveals that species introductions for purposes other than rewilding can restore trophic interactions and offer a rich evidence base about what interventions work. Certainly, some cases of unintentional rewilding have been disasters for the conservation of endemic wildlife, such as the introduction of mammalian predators on insular oceanic islands [36]. Care therefore needs to be taken in balancing restoration with other conservation goals. Palaeoecological data will be particularly invaluable in helping to identify opportunities for rewilding by reconstructing known diets and interaction networks of extinct species (e.g. [72]), so as to avoid unwanted consequences.
Supplementary Material
Supplementary Material
Supplementary Material
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
Both authors designed the study. B.R.S. performed the literature search and collected data with A.J.T. A.J.T. analysed and interpreted the data and wrote the paper with input from B.R.S.
Competing interests
We have no competing interests.
Funding
A.J.T. is supported by the Gatsby Charitable Trust (GAT2962).
References
- 1.Carey J. 2016. Core concept: rewilding. Proc. Natl Acad. Sci. USA 113, 806–808. ( 10.1073/pnas.1522151112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nogués-Bravo D, Simberloff D, Rahbek C, Sanders NJ. 2016. Rewilding is the new Pandora's box in conservation. Curr. Biol. 26, R87–R91. ( 10.1016/j.cub.2015.12.044) [DOI] [PubMed] [Google Scholar]
- 3.Svenning J-C, et al. 2016. Reply to Rubenstein and Rubenstein: time to move on from ideological debates on rewilding. Proc. Natl Acad. Sci. USA 113, E2–E3. ( 10.1073/pnas.1521891113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jørgensen D. 2015. Rethinking rewilding. Geoforum 65, 482–488. ( 10.1016/j.geoforum.2014.11.016) [DOI] [Google Scholar]
- 5.Pettorelli N, et al. 2018. Making rewilding fit for policy. J. Appl. Ecol. 55, 1114–1125. ( 10.1111/1365-2664.13082) [DOI] [Google Scholar]
- 6.Svenning J-C, et al. 2016. Science for a wilder Anthropocene: synthesis and future directions for trophic rewilding research. Proc. Natl Acad. Sci. USA 113, 898–906. ( 10.1073/pnas.1502556112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 8.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 9.Gill JL. 2014. Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 201, 1163–1169. ( 10.1111/nph.12576) [DOI] [PubMed] [Google Scholar]
- 10.Bakker ES, Gill JL, Johnson CN, Vera FWM, Sandom CJ, Asner GP, Svenning J-C. 2016. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl Acad. Sci. USA 113, 847–855. ( 10.1073/pnas.1502545112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seddon PJ, Griffiths CJ, Soorae PS, Armstrong DP. 2014. Reversing defaunation: restoring species in a changing world. Science 345, 406–412. ( 10.1126/science.1251818) [DOI] [PubMed] [Google Scholar]
- 12.Tanentzap AJ, Lee WG, Monks A. 2013. Increased nitrogen cycling facilitates native forest regeneration: potential for restoring extinct ecological processes? Ecol. Appl. 23, 36–45. ( 10.1890/12-0247.1) [DOI] [PubMed] [Google Scholar]
- 13.Lorimer J, Sandom C, Jepson P, Doughty C, Barua M, Kirby KJ. 2015. Rewilding: science, practice, and politics. Annu. Rev. Environ. Resour. 40, 39–62. ( 10.1146/annurev-environ-102014-021406) [DOI] [Google Scholar]
- 14.Rubenstein DR, Rubenstein DI. 2016. From Pleistocene to trophic rewilding: a wolf in sheep's clothing. Proc. Natl Acad. Sci. USA 113, E1 ( 10.1073/pnas.1521757113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svenning J-C, Faurby S. 2017. Prehistoric and historic baselines for trophic rewilding in the Neotropics. Perspect. Ecol. Conserv. 15, 282–291. ( 10.1016/j.pecon.2017.09.006) [DOI] [Google Scholar]
- 16.Corlett RT. 2016. Restoration, reintroduction, and rewilding in a changing world. Trends Ecol. Evol. 31, 453–462. ( 10.1016/j.tree.2016.02.017) [DOI] [PubMed] [Google Scholar]
- 17.Ripple WJ, et al. 2016. What is a trophic cascade? Trends Ecol. Evol. 31, 842–849. ( 10.1016/j.tree.2016.08.010) [DOI] [PubMed] [Google Scholar]
- 18.Polis GA. 1999. Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos 86, 3–15. ( 10.2307/3546565) [DOI] [Google Scholar]
- 19.Hulme PE, et al. 2008. Grasping at the routes of biological invasions: a framework for integrating pathways into policy. J. Appl. Ecol. 45, 403–414. ( 10.1111/j.1365-2664.2007.01442.x) [DOI] [Google Scholar]
- 20.Turbelin AJ, Malamud BD, Francis RA. 2017. Mapping the global state of invasive alien species: patterns of invasion and policy responses. Glob. Ecol. Biogeogr. 26, 78–92. ( 10.1111/geb.12517) [DOI] [Google Scholar]
- 21.Wilder BT, Betancourt JL, Epps CW, Crowhurst RS, Mead JI, Ezcurra E. 2014. Local extinction and unintentional rewilding of bighorn sheep (Ovis canadensis) on a desert island. PLoS ONE 9, e91358 ( 10.1371/journal.pone.0091358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundgren EJ, Ramp D, Ripple WJ, Wallach AD. 2017. Introduced megafauna are rewilding the Anthropocene. Ecography 41, 857–866. ( 10.1111/ecog.03430) [DOI] [Google Scholar]
- 23.Clavero M, García-Berthou E. 2005. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 20, 110 ( 10.1016/j.tree.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 24.Mollot G, Pantel JH, Romanuk TN. 2017. The effects of invasive species on the decline in species richness: a global meta-analysis. Adv. Ecol. Res. 56, 61–83. ( 10.1016/bs.aecr.2016.10.002) [DOI] [Google Scholar]
- 25.Colautti RI, MacIsaac HJ. 2004. A neutral terminology to define ‘invasive’ species. Divers. Distrib. 10, 135–141. ( 10.1111/j.1366-9516.2004.00061.x) [DOI] [Google Scholar]
- 26.Forsyth DM, Wilmshurst JM, Allen RB, Coomes DA. 2010. Impacts of introduced deer and extinct moa on New Zealand ecosystems. New Zealand J. Ecol. 34, 48–65. [Google Scholar]
- 27.Hansen DM, Donlan CJ, Griffiths CJ, Campbell KJ. 2010. Ecological history and latent conservation potential: large and giant tortoises as a model for taxon substitutions. Ecography 33, 272–284. ( 10.1111/j.1600-0587.2010.06305.x) [DOI] [Google Scholar]
- 28.Borer ET, Seabloom EW, Shurin JB, Anderson KE, Blanchette CA, Broitman B, Cooper SD, Halpern BS. 2005. What determines the strength of a trophic cascade? Ecology 86, 528–537. ( 10.1890/03-0816) [DOI] [Google Scholar]
- 29.DeLong JP, et al. 2015. The body size dependence of trophic cascades. Am. Nat. 185, 354–366. ( 10.1086/679735) [DOI] [PubMed] [Google Scholar]
- 30.Finke DL, Denno RF. 2004. Predator diversity dampens trophic cascades. Nature 429, 407–410. ( 10.1038/nature02554) [DOI] [PubMed] [Google Scholar]
- 31.Byrnes J, Stachowicz JJ, Hultgren KM, Randall Hughes A, Olyarnik SV, Thornber CS. 2006. Predator diversity strengthens trophic cascades in kelp forests by modifying herbivore behaviour. Ecol. Lett. 9, 61–71. ( 10.1111/j.1461-0248.2005.00842.x) [DOI] [PubMed] [Google Scholar]
- 32.Polis GA, Sears ALW, Huxel GR, Strong DR, Maron J. 2000. When is a trophic cascade a trophic cascade? Trends Ecol. Evol. 15, 473–475. ( 10.1016/S0169-5347(00)01971-6) [DOI] [PubMed] [Google Scholar]
- 33.Leibold MA. 1989. Resource edibility and the effects of predators and productivity on the outcome of trophic interactions. Am. Nat. 134, 922–949. ( 10.1086/285022) [DOI] [Google Scholar]
- 34.Shurin JB, Seabloom EW. 2005. The strength of trophic cascades across ecosystems: predictions from allometry and energetics. J. Anim. Ecol. 74, 1029–1038. ( 10.1111/j.1365-2656.2005.00999.x) [DOI] [Google Scholar]
- 35.Cox JG, Lima SL. 2006. Naiveté and an aquatic–terrestrial dichotomy in the effects of introduced predators. Trends Ecol. Evol. 21, 674–680. ( 10.1016/j.tree.2006.07.011) [DOI] [PubMed] [Google Scholar]
- 36.Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR. 2016. Invasive predators and global biodiversity loss. Proc. Natl Acad. Sci. USA 113, 11 261–11 265. ( 10.1073/pnas.1602480113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS. 2002. A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 5, 785–791. ( 10.1046/j.1461-0248.2002.00381.x) [DOI] [Google Scholar]
- 38.Cole FR, Reeder DM, Wilson DE. 1994. A synopsis of distribution patterns and the conservation of mammal species. J. Mammal. 75, 266–276. ( 10.2307/1382545) [DOI] [Google Scholar]
- 39.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. 2004. Assessing the causes of late Pleistocene extinctions on the continents. Science 306, 70–75. ( 10.1126/science.1101476) [DOI] [PubMed] [Google Scholar]
- 40.IUCN. 2016. The IUCN Red List of Threatened Species See http://www.iucnredlist.org.
- 41.Seddon PJ, Moehrenschlager A, Ewen J. 2014. Reintroducing resurrected species: selecting deextinction candidates. Trends Ecol. Evol. 29, 140–147. ( 10.1016/j.tree.2014.01.007) [DOI] [PubMed] [Google Scholar]
- 42.Goldberg J, Trewick SA, Paterson AM. 2008. Evolution of New Zealand's terrestrial fauna: a review of molecular evidence. Phil. Trans. R. Soc. B 363, 3319–3334. ( 10.1098/rstb.2008.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurevitch J, Curtis PS, Jones MH. 2001. Meta-analysis in ecology. Adv. Ecol. Res. 32, 199–247. ( 10.1016/S0065-2504(01)32013-5) [DOI] [Google Scholar]
- 44.Hedges LV, Olkin I. 1985. Statistical methods for meta-analysis. San Diego, CA: Academic Press. [Google Scholar]
- 45.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. [Google Scholar]
- 46.Hawkins BA, et al. 2003. energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117. ( 10.1890/03-8006) [DOI] [Google Scholar]
- 47.Imhoff ML, Bounoua L, Ricketts T, Loucks C, Harriss R, Lawrence WT. 2004. Global patterns in human consumption of net primary production. Nature 429, 870–873. ( 10.1038/nature02619) [DOI] [PubMed] [Google Scholar]
- 48.Terborgh JW. 2015. Toward a trophic theory of species diversity. Proc. Natl Acad. Sci. USA 112, 11 415–11 422. ( 10.1073/pnas.1501070112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carpenter B, et al. 2017. Stan: a probabilistic programming language. J. Stat. Softw. 76, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sterne JAC, Becker BJ, Egger M. 2006. The funnel plot. In Publication bias in meta-analysis, pp. 73–98. New York, NY: Wiley-Blackwell. [Google Scholar]
- 51.Egger M, Smith GD, Schneider M, Minder C. 1997. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315, 629–634. ( 10.1136/bmj.315.7109.629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakagawa S, Santos ESA. 2012. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26, 1253–1274. ( 10.1007/s10682-012-9555-5) [DOI] [Google Scholar]
- 53.Soulè M, Noss R. 1998. Rewilding and biodiversity: complementary goals for continental conservation. Wild Earth 8, 18–28. [Google Scholar]
- 54.Schmitz OJ, Hambäck PA, Beckerman AP. 2000. Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am. Nat. 155, 141–153. ( 10.1086/303311) [DOI] [PubMed] [Google Scholar]
- 55.Bascompte J, Melián CJ, Sala E. 2005. Interaction strength combinations and the overfishing of a marine food web. Proc. Natl Acad. Sci. USA 102, 5443–5447. ( 10.1073/pnas.0501562102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLaren BE, Peterson RO. 1994. Wolves, moose, and tree rings on Isle Royale. Science 266, 1555–1558. ( 10.1126/science.266.5190.1555) [DOI] [PubMed] [Google Scholar]
- 57.Ripple WJ, Larsen EJ, Renkin RA, Smith DW. 2001. Trophic cascades among wolves, elk and aspen on Yellowstone National Park's northern range. Biol. Conserv. 102, 227–234. ( 10.1016/S0006-3207(01)00107-0) [DOI] [Google Scholar]
- 58.Crooks KR, Soulé ME. 1999. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563–566. ( 10.1038/23028) [DOI] [Google Scholar]
- 59.Wallach AD, Ripple WJ, Carroll SP. 2015. Novel trophic cascades: apex predators enable coexistence. Trends Ecol. Evol. 30, 146–153. ( 10.1016/j.tree.2015.01.003) [DOI] [PubMed] [Google Scholar]
- 60.Anstett DN, Nunes KA, Baskett C, Kotanen PM. 2016. Sources of controversy surrounding latitudinal patterns in herbivory and defense. Trends Ecol. Evol. 31, 789–802. ( 10.1016/j.tree.2016.07.011) [DOI] [PubMed] [Google Scholar]
- 61.Chatelain M, Halpin CG, Rowe C. 2013. Ambient temperature influences birds' decisions to eat toxic prey. Anim. Behav. 86, 733–740. ( 10.1016/j.anbehav.2013.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bakker ES, Ritchie ME, Olff H, Milchunas DG, Knops JMH. 2006. Herbivore impact on grassland plant diversity depends on habitat productivity and herbivore size. Ecol. Lett. 9, 780–788. ( 10.1111/j.1461-0248.2006.00925.x) [DOI] [PubMed] [Google Scholar]
- 63.Pringle RM, Young TP, Rubenstein DI, McCauley DJ. 2007. Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc. Natl Acad. Sci. USA 104, 193–197. ( 10.1073/pnas.0609840104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanentzap AJ, Coomes DA. 2012. Carbon storage in terrestrial ecosystems: do browsing and grazing herbivores matter? Biol. Rev. 87, 72–94. ( 10.1111/j.1469-185X.2011.00185.x) [DOI] [PubMed] [Google Scholar]
- 65.Feigin CY, et al. 2018. Genome of the Tasmanian tiger provides insights into the evolution and demography of an extinct marsupial carnivore. Nat. Ecol. Evol. 2, 182–192. ( 10.1038/s41559-017-0417-y) [DOI] [PubMed] [Google Scholar]
- 66.Donlan J. 2005. Re-wilding North America. Nature 436, 913–914. ( 10.1038/436913a) [DOI] [PubMed] [Google Scholar]
- 67.Sandom C, Faurby S, Sandel B, Svenning J-C. 2014. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc. R. Soc. B 281, 20133254 ( 10.1098/rspb.2013.3254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Araujo BBA, Oliveira-Santos LGR, Lima-Ribeiro MS, Diniz-Filho JAF, Fernandez FAS. 2017. Bigger kill than chill: the uneven roles of humans and climate on late Quaternary megafaunal extinctions. Quat. Int. 431, 216–222. ( 10.1016/j.quaint.2015.10.045) [DOI] [Google Scholar]
- 69.Benítez-López A, Alkemade R, Schipper AM, Ingram DJ, Verweij PA, Eikelboom JAJ, Huijbregts MAJ. 2017. The impact of hunting on tropical mammal and bird populations. Science 356, 180–183. ( 10.1126/science.aaj1891) [DOI] [PubMed] [Google Scholar]
- 70.Sobral-Souza T, Lautenschlager L, Morcatty TQ, Bello C, Hansen D, Galetti M. 2017. Rewilding defaunated Atlantic Forests with tortoises to restore lost seed dispersal functions. Perspect. Ecol. Conserv. 15, 300–307. ( 10.1016/j.pecon.2017.08.005) [DOI] [Google Scholar]
- 71.Schmitz OJ, Krivan V, Ovadia O. 2004. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163. ( 10.1111/j.1461-0248.2003.00560.x) [DOI] [Google Scholar]
- 72.Wood JR, Perry GLW, Wilmshurst JM. 2017. Using palaeoecology to determine baseline ecological requirements and interaction networks for de-extinction candidate species. Funct. Ecol. 31, 1012–1020. ( 10.1111/1365-2435.12773) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.