Abstract
Trophic rewilding maintains that large mammals are functionally important to resource subsidies and nutrient repletion, yet this prediction is understudied. Here, I report on the potential magnitude and variability of nitrogen that moose populations move from aquatic to terrestrial ecosystems. My aim is to provide justified approximations of the role of moose in the flux of a limiting nutrient across ecotones and to illustrate how this role is linked to wolf predation and climate warming. Using Isle Royale and northeastern Minnesota, USA as contrasting focal systems, I found that the long-term annual N gain for riparian forests likely ranges from 1 to 10 kg N ha–1 yr–1, depending on the heterogeneity of moose movements. For these systems, this range is equivalent to approximately 4–30% of net annual N mineralization, approximately 62–625% of annual N runoff, approximately 28–333% of annual atmospheric N deposition and approximately 31–312% of the N sequestered by trees. The N flux approximation is most sensitive to moose population levels and, as such, is influenced by wolves, climate warming and disease. The potential for other terrestrial ungulates that feed on aquatic plants to provide significant nutrient repletion across ecotones is unknown but important to examine in the context of trophic rewilding. The extent to which predators influence ungulate abundance indirectly impacts this nutrient repletion.
This article is part of the theme issue ‘Trophic rewilding: consequences for ecosystems under global change’.
Keywords: boreal, climate change, moose (Alces alces), resource subsidy, ungulate, wolves (Canis lupus)
1. Background
(a). Biotic nutrient vectors can flow against gravity
Nutrient flux across ecotones fundamentally links aquatic and terrestrial systems [1,2]. Physical forces as significant as gravity entrain nutrient flow downslope, but biotic forces can create nutrient vectors that flow upslope against gravity. Marine nutrients are captured by salmon, grizzly bears, seabirds and sea turtles via consumption and are released above sea level via excretion and reproduction at levels that often double local availability despite historic declines in populations [3–6]. Clouds of emerging midges lift significant amounts of freshwater nutrients from lake bottoms to grasses, elevating riparian N deposition three to five times above background rates [7]. Additional examples abound, and although biotic nutrient vectors are ultimately ephemeral, the annual life cycles that generate upslope nutrient flow are as perpetual as Earth's orbit if conserved.
When not conserved, the loss or depletion of biotic nutrient vectors impacts species interactions, the stability of ecosystems and human livelihoods [8–10]. For example, when anadromous fish migrations are obstructed, then marine nutrients cannot reach upper watershed waters or their riparian food webs via biotic vectors, nor can they reach subsistence, recreational and commercial fishing communities [11,12]. While biotic nutrient vectors naturally exhibit variation in magnitude (e.g. ±10%), global change that leads to species decline, loss or thwarted species movement increasingly eliminates these vectors; for example, nutrients derived from spawning pacific salmon have been eliminated or declined 90% in many cases [12]. Such changes can cause rippling effects that drive the transformation among stable states [13,14]. Rewilding is a conservation strategy that can restore and protect the functional role of species that create the upslope nutrient flow, thereby maintaining ecological and evolutionary links between aquatic and terrestrial systems that are important to ecosystem processes, food webs and human communities [15].
(b). Ungulates as nutrient vectors
Resources flow through consumers via consumption, then egestion, excretion and growth, reproduction and lastly mortality. For mobile consumers, such as ungulates, this flow is highly dynamic and the nutrient vector is defined by a species' physiology and the spectrum of movement from local habitat selection to global migrations. Ungulates are selective consumers, generally preferring to ingest relatively nutritious plant species and nitrogen-rich plant parts [16]. Ungulate nitrogen elimination toggles between urea and faecal pathways depending on forage nitrogen concentration that varies seasonally [17]. Nutrient deposition via egestion and excretion pathways depends on species-specific movements from daily activity budgets to seasonal migrations. Nutrients synthesized into biomass are allocated to reproduction, enter food webs via predation and scavenging, or return to systems via direct carrion decomposition.
Nutrient flow via ungulate vectors is best understood in grassland systems. Grazing ungulates track green waves of grassland vegetation across landscapes and move nutrients via seasonal migrations [18–20]. Nutrient flow via browsing forest ungulates is comparatively less understood, perhaps because seasonal migrations are absent, less pronounced or less observed. In both systems, how ungulates affect the magnitude and direction of nutrient flow across aquatic-terrestrial ecotones has been largely understudied but recent reviews emphasize the likely importance of such links [21–23]. In the context of trophic rewilding [15], how ungulates serve as nutrient vectors and, in turn, how ungulate predators may indirectly impact this functional aspect is a key focal area.
Among ungulates, moose (Alces alces) typify aquatic-terrestrial ecotone specialists, with physiological adaptations that facilitate foraging on aquatic macrophytes and that indicate an evolutionary history linking water bodies and land [24]. Because moose feed extensively on aquatic macrophytes but ruminate, egest, excrete and die most frequently on land, they are a vector for aquatic-derived nutrients and energy to flow upslope into boreal forests globally. The importance of moose to nutrient repletion via this vector is worth examination because boreal forests are circumpolar and cover approximately 17% of Earth's land surface area [25]. Also, moose distribution largely overlaps boreal forests, but moose populations are increasingly impacted by climate warming. As moose populations decline or recover, what is the effect on the nutrient flux that results from their unique foraging and what are the potential implications for recipient riparian forests and supplier water bodies?
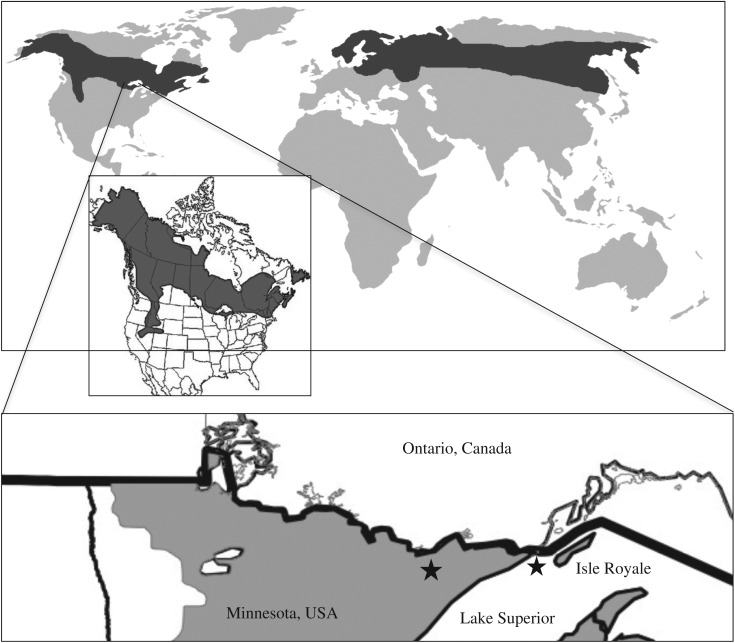
I explore the magnitude and ecological relevance of aquatic-derived nitrogen (N) that moose can move into forests in two systems with nearly the same moose habitat but with different moose population dynamics. The Isle Royale, USA moose population is one and the northeastern Minnesota, USA population is the other. Both populations occur along the southern limit of moose range in North America (approx. 48° N; figure 1), in systems that are characterized by similar features: numerous small lakes and mostly southern boreal forests, with interspersed northern temperate hardwoods. Wolves (Canis lupus) are the primary predator in both systems, but these two moose populations currently have dissimilar trajectories. The Isle Royale moose population is increasing, with a growth rate greater than 20% over the past 6 years [26]. Since 2005, the northeastern Minnesota moose population has declined by approximately 50% [27]. Currently, two wolves remain on Isle Royale and the northeastern Minnesota wolf density has average approximately 30 per 1000 km2 recently. These contrasts in population trends allow me to examine how nutrient repletion across ecotones is indirectly affected by factors that drive large ungulate populations (e.g. predation, climate change) and to evaluate how trophic rewilding might influence the functional role of moose in nutrient repletion across ecotones (e.g. wolf restoration on Isle Royale).
Figure 1.
Map of boreal forests (shaded) globally, in North America, and in the study areas (stars). Both study areas occur along the southern limit of moose (Alces alces) range in North America in systems that are characterized by numerous small lakes, mostly southern boreal forests interspersed with some northern temperate hardwoods, and wolves (Canis lupus) as predators. Moose populations currently have dissimilar trajectories, with Isle Royale's increasing and Minnesota's decreasing.
2. Material and methods
I focused on nitrogen (N) repletion because it is commonly a limiting nutrient in temperate and boreal forests [28]. To estimate the moose N vector from aquatic to terrestrial systems requires approximating (i) the excretory N that is derived from an aquatic origin per moose and (ii) the total moose population size. I brought together data from studies focused on individual foraging and long-term population dynamics to account for the impact of moose on N flux from aquatic to terrestrial systems within boreal and northern hardwood forest ecosystems.
(a). Moose N vector
An estimate of mean (±s.d.) daily excretory N (Ndaily aquatic) derived from aquatics (78 ± 12 g N d–1 moose–1) is available based on research that synthesized N urinary and faecal excretion dynamics, intake rates, plant N content, body mass, the proportion of aquatic plants in the diet and time spent foraging in aquatic habitats [29]. This measure is possible because the amount of N that cervids (Cervidae) return to the environment via excretion and egestion can be expressed per day as function of body mass and forage quality in terms of N content [17] and the proportion of N excreted and egested that is of aquatic origin was calculated using a measurement of the aquatic proportion of moose diets (95% CI = 0.09–0.57) that was based on stable isotope analyses [29]. Summer N flux was determined by summing the daily excretory N derived from aquatics for the total days (D) spent aquatic foraging through summer:
with D equal to 108, as determined from activity budgets derived from direct observation [30]. I used local sensitivity analysis (one-at-a-time technique) to examine the effect of variation of each input factor on the moose N vector estimate. The influence of each input parameter on N flux was analysed separately, keeping the other parameters fixed at mean values.
(b). Annual flux and dispersion
Annual moose population estimates on Isle Royale (1958–2018) are based on cohort analysis and aerial surveys ([31]; www.isleroyalewolf.org). In 2017, the Isle Royale moose population estimate was 1600 (95% CI 1077–2238 [32]). Moose population estimates for northeastern Minnesota (2005–2018) are also based on aerial surveys [33]. In 2018, the Minnesota moose population estimate was 3030 (95% CI 1077–2238 [33]).
Nutrient fluxes are often expressed and most comparable when expressed on an area basis (e.g. N kg ha–1 yr–1). This was done by dividing the annual excretory N derived from an aquatic origin per moose by measures of the mean (±s.d.) summer core-area size (0.4 ± 0.1 km2 [34]). This assumes moose egestion and excretion occur terrestrially and returns to aquatic systems while foraging are negligible. For approximation, this is reasonable given that aquatic feeding bouts average about 42 min d–1 in our study systems, digestive retention times typically exceed 12 h and moose in summer spend 5× more time per day on land than aquatic foraging [30]. In 65 h of viewing moose foraging in water shallow enough to permit detection, defecation was never observed, while in 48 h of terrestrial observation, defecation was frequently observed [30].
3. Results
Annual excretory N that is derived from aquatic forage is estimated to average (±s.d.) 8.4 ± 1.3 kg moose–1. When dispersed over the summer core area that individual moose typically occupy, the mean moose N vector is approximately 0.21 ± 0.03 kg ha–1 yr–1 moose–1. At the landscape scale, Isle Royale and Minnesota moose populations could be moving, respectively, an average of 8514 kg and 41 799 kg of N from aquatic foraging areas into terrestrial habitat annually. The approximate annual N gain for riparian habitats ranged from 1 to 4 kg N ha–1 yr–1 and depends on local moose densities and aggregations. Approximation of the moose N vector is most sensitive to variation in aquatic plant N content and aquatic diet fraction at the individual scale and population size at the landscape scale.
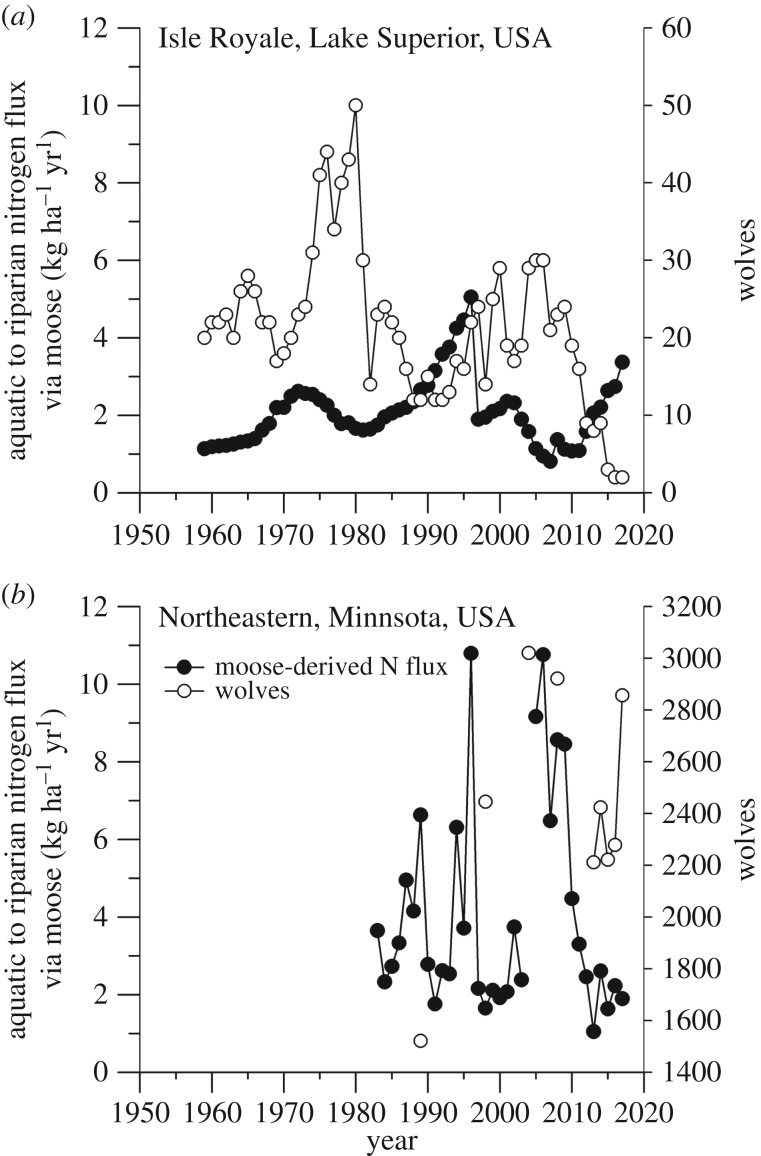
Over the past six decades, the moose N vector from aquatic to terrestrial systems on Isle Royale has exhibited significant fluctuations and increased markedly in the past 8 years (figure 2). Since 2005, the northeastern Minnesota moose N vector from aquatic to terrestrial systems decreased with a declining moose population (figure 2).
Figure 2.
Nitrogen of aquatic origin transferred to riparian zones via moose (Alces alces; closed symbols) and wolf (Canis lupus) abundance (right axes; open symbols) on Isle Royale National, Lake Superior, USA (a) and in northeastern Minnesota, USA (b). Nitrogen flux for 2004 in Minnesota was not calculated because a reliable moose population estimate was not available.
4. Discussion
These results indicate that moose are an important nutrient vector in forests and can move ecologically significant amounts of aquatic-derived N to terrestrial systems. Given that studies have documented the growth-stimulating effects of ungulate excreta, particularly urea nitrogen [28,35], N repletion across the aquatic-terrestrial ecotone by moose likely increases N availability and heterogeneity in soils. Spatial heterogeneity of soil resources, particularly nitrogen availability, affects plant and soil microbial diversity in boreal and north temperate hardwood forest ecosystems [36,37]. The functional role of moose as a nutrient vector is unaccounted for in discussions of what is to be gained or lost with growing or declining populations. This expanded quantification of the functional role of moose informs the theory behind trophic rewilding, which often assumes a net functional benefit with large ungulate restoration.
To put this role in perspective, in forests typified by the Isle Royale and Minnesota examples, net N mineralization is 23–38 kg N ha–1 yr–1 [38,39], the reported maximum for dissolved inorganic N runoff is 1.6 kg kg N ha–1 yr–1 [40], atmospheric deposition is 3–4 kg N ha–1 yr–1 [41] and the amount of N sequestered by trees amounts to 3.2 kg N ha–1 yr–1 [41]. The estimated N flux in these forests of 1–10 kg N ha–1 yr–1 created by moose populations is therefore approximately 4–30% of net annual N mineralization, approximately 62–625% of annual N runoff, approximately 28–333% of annual atmospheric N deposition and approximately 31–312% of the N sequestered by trees [42]. By any of these comparisons, it is likely that moose are moving ecologically relevant amounts of highly available N upslope.
Variation in moose density is the most important determinant of the magnitude of moose-derived N flux and largely subsumes the uncertainty associated with excretion and foraging parameters. Large variations do exist in the extent to which moose forage on aquatic plants. Some moose populations do not appear to use aquatic resources at all, while others appear to use aquatic resources year-round [43,44]. As such, these results should not be viewed as precise calculations, but rather first approximations of the N repletion by moose from aquatic to terrestrial systems that apply to globally extensive boreal forests (1.89 × 106 km2 of North America [25]).
Predators, disease and climate are key regulators of the moose N vector because of their influence on moose population dynamics. While a large proportion (approx. 0.4) of the variability in the population growth rate of Isle Royale moose is unexplained, wolves were a significant regulator until 1981 when an outbreak of human-introduced disease (canine parvovirus) caused wolf numbers to drop and doubled the influence of climate on moose [32,45]. Wolves and parasites each cause about a third of the mortalies in recent analyses of the northeastern Minnesota moose population [27,33]. To the extent that wolves or their absence influence moose population levels and their aquatic resource selection, then wolves have a cascading impact on nutrient repletion across aquatic-terrestrial ecotones via moose, which may have important implications for trophic cascades across ecosystems ([46]; figure 3).
Figure 3.

Photograph progression of the same large herbivore exclosure on Isle Royale, USA (photo credit: JK Bump). Moose herbivory has significantly decreased aquatic macrophytes (e.g. Nymphaea sp. and Potamogeton sp.) outside the exclosure [47]. This illustrates trophic rewilding effects that can be caused directly by aquatic herbivory by moose (Alces alces) and indirectly influenced by wolves (Canis lupus) via trophic cascades.
(a). Relevance to trophic rewilding
Climate warming and wolf–moose relationships are important drivers of the functional roles of moose and therefore each is of key importance to understanding the effects of moose restoration or conservation, e.g. a beneficial rewilding component. For example, average temperatures in northern Minnesota from 1901 to 1960 compared to 1991 to 2012 have warmed 2–3°C, which is faster than warming rates (0–1°C) in southern regions of the state and among the fastest warming areas in the USA [48]. Evidence indicates that the boreal-temperate forest ecotone is shifting northward [49]. The northwestern moose population in Minnesota (approx. 3500–4000 individuals) was extirpated from the state by 2012, and the loss of that population is thought to be linked to heat stress. Hence, given that annual excretory N that is derived from aquatic forage is estimated to average (±s.d.) 8.4 ± 1.3 kg moose–1, then a warming climate is linked to a loss of N repletion equivalent to approximately 29 000–33 600 kg of N annually in former moose range in northwestern Minnesota. If deer replace moose with climate warming, as is predicted in Minnesota, they will not duplicate the N vector functional of moose because deer do not feed on aquatic plants to the same extent. This highlights the importance of considering the consequences of climate warming in the context of trophic rewilding efforts that restore species or the functional role of species via surrogates.
Without significant wolf predation, moose on Isle Royale have increased at historically high rates [32]. This trend contrasts with Minnesota moose populations and illustrates what can potentially be achieved with a growing large ungulate population, which is often a trophic rewilding goal. Currently, the US National Park Service is considering restoring the Isle Royale wolf population, which is currently only two, highly inbred individuals that are not reproducing. If carried out, restoration will constitute an intentional trophic rewilding experiment in which it will be possible to better quantify how predator restoration influences nutrient repletion by ungulates. The decision to restore wolves not only has important implications for moose-driven nutrient vectors but also for aquatic ecosystem nutrient dynamics and plant communities (figure 3 [47,50]).
In conclusion, my estimates serve as plausible approximations to evaluate the functional potential for moose to provide a biotic vector for the flow of N from aquatic to terrestrial systems along the boreal-temperate forest ecotone and more generally in boreal forests globally. As with the functional relationship between many species and ecosystem processes, the strength of moose as N vectors is likely context dependent and heterogeneous. The potential for other terrestrial ungulates that feed on aquatic plants, such as marsh deer (Blastocerus dichotomus), swamp deer (Cervus duvaucelii) and wild water buffalo (Bubalus arnee) [21,51,52], to provide a similar vector of significant nutrients is unknown but important to examine in the context of trophic rewilding. Such an examination ought to also include the role of predation and humans.
Acknowledgements
This research was made possible because of the efforts of the Wolves and Moose of Isle Royale project and the Minnesota Department of Natural Resources.
Data accessibility
The datasets supporting this article are publically available.
Authors' contributions
J.K.B. conceived the study, synthesized the data parameters, conducted the analysis and drafted the manuscript.
Competing interests
I declare I have no competing interests.
Funding
This effort was partially supported by US National Science Foundation grants to J.K.B. (NSF ID#1545611, NSF ID#1556676).
References
- 1.Bartels P, Cucherousset J, Steger K, Eklöv P, Tranvik LJ, Hillebrand H. 2012. Reciprocal subsidies between freshwater and terrestrial ecosystems structure consumer resource dynamics. Ecology 93, 1173–1182. ( 10.1890/11-1210.1) [DOI] [PubMed] [Google Scholar]
- 2.Leroux SJ, Loreau M. 2012. Dynamics of reciprocal pulsed subsidies in local and meta-ecosystems. Ecosystems 15, 48–59. ( 10.1007/s10021-011-9492-0) [DOI] [Google Scholar]
- 3.Hocking MD, Reynolds JD. 2011. Impacts of salmon on riparian plant diversity. Science 331, 1609–1612. ( 10.1126/science.1201079) [DOI] [PubMed] [Google Scholar]
- 4.Naiman RJ, Bilby RE, Schindler DE, Helfield JM. 2002. Pacific salmon, nutrients, and the dynamics of freshwater and riparian ecosystems. Ecosystems 5, 399–417. ( 10.1007/s10021-001-0083-3) [DOI] [Google Scholar]
- 5.Moss B. 2017. Marine reptiles, birds and mammals and nutrient transfers among the seas and the land: an appraisal of current knowledge. J. Exp. Mar. Biol. Ecol. 492, 63–80. ( 10.1016/j.jembe.2017.01.018) [DOI] [Google Scholar]
- 6.Bouchard SS, Bjorndal KA. 2000. Sea turtles as biological transporters of nutrients and energy from marine to terrestrial ecosystems. Ecology 81, 2305–2313. ( 10.1890/0012-9658(2000)081%5B2305:STABTO%5D2.0.CO;2) [DOI] [Google Scholar]
- 7.Dreyer J, Townsend PA, James CH III, Hoekman D, Vander Zanden MJ, Gratton C. 2015. Quantifying aquatic insect deposition from lake to land. Ecology 96, 499–509. ( 10.1890/14-0704.1) [DOI] [PubMed] [Google Scholar]
- 8.Schmitz OJ, Hawlena D, Trussell GC. 2010. Predator control of ecosystem nutrient dynamics. Ecol. Lett. 13, 1199–1209. ( 10.1111/j.1461-0248.2010.01511.x) [DOI] [PubMed] [Google Scholar]
- 9.DeAngelis DL. 2012. Dynamics of nutrient cycling and food webs. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 10.Marczak LB, Thompson RM, Richardson JS. 2007. Meta-analysis: trophic level, habitat, and productivity shape the food web effects of resource subsidies. Ecology 88, 140–148. ( 10.1890/0012-9658(2007)88%5B140:MTLHAP%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 11.Willson MF, Halupka KC. 1995. Anadromous fish as keystone species in vertebrate communities. Conserv. Biol. 9, 489–497. ( 10.1046/j.1523-1739.1995.09030489.x) [DOI] [Google Scholar]
- 12.MacAvoy SE, Garman GC, Macko SA. 2009. Anadromous fish as marine nutrient vectors. Fish. Bull. 107, 165–174. [Google Scholar]
- 13.DeAngelis DL, Mulholland PJ, Palumbo AV, Steinman AD, Huston MA, Elwood JW. 1989. Nutrient dynamics and food-web stability. Annu. Rev. Ecol. Syst. 20, 71–95. ( 10.1146/annurev.es.20.110189.000443) [DOI] [Google Scholar]
- 14.Weber MJ, Brown ML. 2013. Continuous, pulsed and disrupted nutrient subsidy effects on ecosystem productivity, stability, and energy flow. Ecosphere 4, 1–3. ( 10.1890/ES12-00354.1) [DOI] [Google Scholar]
- 15.Svenning JC, et al. 2016. Science for a wilder Anthropocene: synthesis and future directions for trophic rewilding research. Proc. Natl Acad. Sci. USA 113, 898–906. ( 10.1073/pnas.1502556112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray BD, Webster CR, Bump JK. 2013. Broadening the ecological context of ungulate–ecosystem interactions: the importance of space, seasonality, and nitrogen. Ecology 94, 1317–1326. ( 10.1890/12-1582.1) [DOI] [PubMed] [Google Scholar]
- 17.Hobbs NT. 1996. Modification of ecosystems by ungulates. J. Wildl. Manage. 60, 695–713. ( 10.2307/3802368) [DOI] [Google Scholar]
- 18.Frank DA, McNaughton SJ, Tracy BF. 1998. The ecology of the earth's grazing ecosystems. BioScience 48, 513–521. ( 10.2307/1313313) [DOI] [Google Scholar]
- 19.Aikens EO, Kauffman MJ, Merkle JA, Dwinnell SP, Fralick GL, Monteith KL. 2017. The greenscape shapes surfing of resource waves in a large migratory herbivore. Ecol. Lett. 20, 741–750. ( 10.1111/ele.12772) [DOI] [PubMed] [Google Scholar]
- 20.Mueller T, Olson KA, Fuller TK, Schaller GB, Murray MG, Leimgruber P. 2008. In search of forage: predicting dynamic habitats of Mongolian gazelles using satellite-based estimates of vegetation productivity. J. Appl. Ecol. 45, 649–658. ( 10.1111/j.1365-2664.2007.01371.x) [DOI] [Google Scholar]
- 21.Bakker ES, Pagès JF, Arthur R, Alcoverro T. 2016. Assessing the role of large herbivores in the structuring and functioning of freshwater and marine angiosperm ecosystems. Ecography 39, 162–179. ( 10.1111/ecog.01651) [DOI] [Google Scholar]
- 22.Bakker ES, Wood KA, Pagès JF, Veen GC, Christianen MJ, Santamaría L, Nolet BA, Hilt S. 2016. Herbivory on freshwater and marine macrophytes: a review and perspective. Aquat. Bot. 135, 18–36. ( 10.1016/j.aquabot.2016.04.008) [DOI] [Google Scholar]
- 23.Moss B. 2015. Mammals, freshwater reference states, and the mitigation of climate change. Freshw. Biol. 60, 1964–1976. ( 10.1111/fwb.12614) [DOI] [Google Scholar]
- 24.Clifford AB, Witmer LM. 2004. Case studies in novel narial anatomy: 2. The enigmatic nose of moose (Artiodactyla: Cervidae: Alces alces). J. Zool. 262, 339–360. ( 10.1017/S0952836903004692) [DOI] [Google Scholar]
- 25.Hansen MC, et al. 2013. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853. ( 10.1126/science.1244693) [DOI] [PubMed] [Google Scholar]
- 26.Hoy SR, Peterson RO, Vucetich JA. 2018. Climate warming is associated with smaller body size and shorter lifespans in moose near their southern range limit. Glob. Change Biol. 24, 2488–2497. ( 10.1111/gcb.14015) [DOI] [PubMed] [Google Scholar]
- 27.Mech LD, Fieberg J, Barber-Meyer S. 2018. An historical overview and update of wolf–moose interactions in northeastern Minnesota. Wildl. Soc. Bull. 42, 40–47. ( 10.1002/wsb.844) [DOI] [Google Scholar]
- 28.LeBauer DS, Treseder KK. 2008. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89, 371–379. ( 10.1890/06-2057.1) [DOI] [PubMed] [Google Scholar]
- 29.Bump JK, Tischler KB, Schrank AJ, Peterson RO, Vucetich JA. 2009. Large herbivores and aquatic–terrestrial links in southern boreal forests. J. Anim. Ecol. 78, 338–345. ( 10.1111/j.1365-2656.2008.01498.x) [DOI] [PubMed] [Google Scholar]
- 30.Belovsky GE, Jordan PA. 1978. The time-energy budget of a moose. Theor. Popul. Biol. 14, 76–104. ( 10.1016/0040-5809(78)90006-0) [DOI] [PubMed] [Google Scholar]
- 31.Vucetich JA, Peterson RO. 2004. The influence of top–down, bottom–up and abiotic factors on the moose (Alces alces) population of Isle Royale. Proc. R. Soc. B 271, 183–189. ( 10.1098/rspb.2003.2589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson RO, Vucetich JA. 2017. Ecological studies of the wolves on Isle Royale. Annual Report 2016–2017. See http://www.isleroyalewolf.org/sites/default/files/annual-report-pdf/Annual%20Report%202016-2017_0.pdf.
- 33.DelGiudice G. 2018. Aerial moose survey. St Paul, MN: Minnesota Department of Natural Resources. [Google Scholar]
- 34.Dussault C, Courtois R, Ouellet JP, Girard I. 2005. Space use of moose in relation to food availability. Can. J. Zool. 83, 1431–1437. ( 10.1139/z05-140) [DOI] [Google Scholar]
- 35.Peek MS, Forseth IN. 2003. Enhancement of photosynthesis and growth of an aridland perennial in response to soil nitrogen pulses generated by mule deer. Environ. Exp. Bot. 49, 169–180. ( 10.1016/S0098-8472(02)00068-0) [DOI] [Google Scholar]
- 36.Masse J, Prescott CE, Renaut S, Terrat Y, Grayston SJ. 2017. Plant community and nitrogen deposition as drivers of alpha and beta diversities of prokaryotes in reconstructed oil sand soils and natural boreal forest soils. Appl. Environ. Microbiol. 83, e03319-16 ( 10.1128/AEM.03319-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett JA, Maherali H, Reinhart KO, Lekberg Y, Hart MM, Klironomos J. 2017. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355, 181–184. ( 10.1126/science.aai8212) [DOI] [PubMed] [Google Scholar]
- 38.Scott NA, Binkley D. 1997. Foliage litter quality and annual net N mineralization: comparison across North American forest sites. Oecologia 111, 151–159. ( 10.1007/s004420050219) [DOI] [PubMed] [Google Scholar]
- 39.Stottlemyer R, Travis B, Toczydlowski D. 1995. Nitrogen mineralization in boreal forest stands of Isle Royale, northern Michigan. Water Air Soil Pollut. 82, 191–202. ( 10.1007/BF01182833) [DOI] [Google Scholar]
- 40.Wetzel RG. 2001. Limnology: lake and river ecosystems. San Diego, CA: Gulf Professional Publishing. [Google Scholar]
- 41.Schwede DB, Lear GG. 2014. A novel hybrid approach for estimating total deposition in the United States. ASTM Spec. Tech. Publ. 92, 207–220. ( 10.1016/j.atmosenv.2014.04.008) [DOI] [Google Scholar]
- 42.Talhelm AF, Pregitzer KS, Burton AJ, Zak DR. 2012. Air pollution and the changing biogeochemistry of northern forests. Front. Ecol. Environ. 10, 181–185. ( 10.1890/110007) [DOI] [Google Scholar]
- 43.MacCracken JG, Ballenberghe VV, Peek JM. 1993. Use of aquatic plants by moose: sodium hunger or foraging efficiency? Can. J. Zool. 71, 2345–2351. ( 10.1139/z93-329) [DOI] [Google Scholar]
- 44.MacCracken JG, Van Ballenberghe V, Peek JM. 1997. Habitat relationships of moose on the Copper River Delta in coastal south-central Alaska. Wildl. Monogr. 136, 3–52. [Google Scholar]
- 45.Wilmers CC, Post E, Peterson RO, Vucetich JA. 2006. Predator disease out-break modulates top-down, bottom-up and climatic effects on herbivore population dynamics. Ecol. Lett. 9, 383–389. ( 10.1111/j.1461-0248.2006.00890.x) [DOI] [PubMed] [Google Scholar]
- 46.Leroux SJ, Loreau M. 2008. Subsidy hypothesis and strength of trophic cascades across ecosystems. Ecol. Lett. 11, 1147–1156. ( 10.1111/j.1461-0248.2008.01235.x) [DOI] [PubMed] [Google Scholar]
- 47.Bergman BG, Bump JK. 2015. Experimental evidence that the ecosystem effects of aquatic herbivory by moose and beaver may be contingent on water body type. Freshw. Biol. 60, 1635–1646. ( 10.1111/fwb.12595) [DOI] [Google Scholar]
- 48.USGCRP. 2017. Climate science special report: fourth national climate assessment, vol. I (eds Wuebbles DJ, et al.), p. 470 Washington, DC: U.S. Global Change Research Program. [Google Scholar]
- 49.Evans P, Brown CD. 2017. The boreal–temperate forest ecotone response to climate change. Environ. Rev. 25, 423–431. ( 10.1139/er-2017-0009) [DOI] [Google Scholar]
- 50.Bump JK, Bergman BG, Schrank AJ, Marcarelli AM, Kane ES, Risch AC, Schütz M. 2017. Nutrient release from moose bioturbation in aquatic ecosystems. Oikos 126, 389–397. ( 10.1111/oik.03591) [DOI] [Google Scholar]
- 51.Ceacero F, Landete-Castillejos T, Miranda M, García AJ, Martínez A, Gallego L. 2014. Why do cervids feed on aquatic vegetation? Behav. Processes 103, 28–34. ( 10.1016/j.beproc.2013.10.008) [DOI] [PubMed] [Google Scholar]
- 52.Sitters J, Bakker ES, Veldhuis MP, Veen GF, Olde Venterink H, Vanni MJ.. 2017. The stoichiometry of nutrient release by terrestrial herbivores and its ecosystem consequences. Front. Earth Sci. 5, 32 ( 10.3389/feart.2017.00032) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting this article are publically available.