Abstract
Ecosystem engineers can increase biodiversity by creating novel habitat supporting species that would otherwise be absent. Their more routine activities further influence the biota occupying engineered habitats. Beavers are well-known for transforming ecosystems through dam building and are therefore increasingly being used for habitat restoration, adaptation to climate extremes and in long-term rewilding. Abandoned beaver ponds (BP) develop into meadows or forested wetlands that differ fundamentally from other terrestrial habitats and thus increase landscape diversity. Active BP, by contrast, are superficially similar to other non-engineered shallow wetlands, but ongoing use and maintenance might affect how BP contribute to aquatic biodiversity. We explored the ‘within-habitat’ effect of an ecosystem engineer by comparing active BP in southern Sweden with coexisting other wetlands (OW), using sedentary (plants) and mobile (water beetles) organisms as indicators. BP differed predictably from OW in environmental characteristics and were more heterogeneous. BP supported more plant species at plot (+15%) and site (+33%) scales, and plant beta diversity, based on turnover between plots, was 17% higher than in OW, contributing to a significantly larger species pool in BP (+17%). Beetles were not differentiated between BP and OW based on diversity measures but were 26% more abundant in BP. Independent of habitat creation beaver are thus significant agents of within-habitat heterogeneity that differentiates BP from other standing water habitat; as an integral component of the rewilding of wetlands re-establishing beaver should benefit aquatic biodiversity across multiple scales.
This article is part of the theme issue ‘Trophic rewilding: consequences for ecosystems under global change’.
Keywords: beaver, wetland, aquatic plants, beetle, richness, heterogeneity
1. Introduction
Ecosystem engineers alter the supply of resources to other organisms and probably influence all ecosystems in some way [1]. Ecosystem engineers generally have positive effects on biodiversity [2], via a variety of mechanisms including the creation of novel habitat, modification of existing habitat, reduction in patch size or increase in patch turnover. However, increased habitat heterogeneity is a unifying theme. Heterogeneity itself is a cornerstone of the maintenance of biodiversity but restoring heterogeneity at biologically relevant scales through direct human intervention is challenging; re-establishing populations of the larger key ecosystem engineering species, a common element of trophic rewilding, might therefore prove effective in achieving a step change in the restoration of biodiversity. However, to justify this rationale the mechanisms by which such ecosystem engineers affect biodiversity and the scale at which these mechanisms operate require better understanding.
Freshwaters are crucial for the conservation of global biodiversity and functioning of ecosystems, as well as contributing ecosystem services essential to society [3]. Despite this, most freshwater habitats have been impacted by multiple anthropogenic stressors for many centuries and are losing biodiversity at unprecedented rates and faster than terrestrial habitats [4]. Various ‘hard engineering’ options are available to restore freshwater habitats, ranging from river meandering and excavating new clean water ponds, to diversion of external nutrient loading and sediment removal to reduce internal loading. However, softer, lower cost, ‘biotic’ options that exploit the distribution or behaviour of organisms or trophic interactions, can also be effective, ranging from use of donor seedbanks, biomanipulation of fish stocks in lakes, to the reintroduction of keystone species [5].
The Eurasian beaver (Castor fiber) has been widely reintroduced throughout its native range in recent decades. Initial motivations for reintroduction were to reverse a severe decline caused mainly by hunting, which had reduced C. fiber to a few small and highly fragmented populations [6]. Since the status of the species has been secured, emphasis has shifted to exploiting the ecosystem engineering activities of beaver; the use of beavers (C. fiber and C. canadensis) to restore rivers and increase landscape heterogeneity [7,8], or adapt to floods and droughts [9,10] is gaining traction. Beaver are also increasingly featuring in long-term rewilding projects where targets are broadly unscripted [11], but empirical evidence of effects is often lacking. In this article, we explore how reintroduction of beavers might benefit freshwater biodiversity in impacted landscapes. We use a cross-sectional comparison of active beaver ponds (BP) and other wetlands (OW) in an area of Sweden where beaver have long been established. We also discuss if the observed spatial differences mirror the temporal changes observed in Scotland following beaver reintroduction after a prolonged (400 year) absence.
Beaver dam streams to raise and stabilize water levels, thus maintaining a submerged lodge entrance and increasing access to resources, while reducing exposure to terrestrial predators [12]. Existing aquatic habitats such as lakes and lowland rivers, where abundant, are also commonly used by Eurasian beaver, sometimes with minimal hydrological alteration. Damming converts land into aquatic habitat (beaver ponds) with largely predictable effects on nutrient cycling and decomposition dynamics that are expressed via water chemistry and downstream transport [13]. The recovery of Northern Hemisphere beaver populations from near extinction in the early 1900s to approximately 30 million animals has been associated with the creation of an estimated 9500–42 000 km2 of shallow aquatic habitat [14]. The successional changes in BP following abandonment are well described [15] and can yield novel habitats such as beaver meadows that further enhance landscape diversity [16]. In wetland-poor environments, BP may represent a novel habitat in their own right. The combination of novel engineered and non-engineered habitats, including the coexistence of engineered sites covering successional states from newly formed to long-abandoned, enhances landscape diversity for many groups of organisms, e.g. aquatic plants [15], other herbaceous plants [16], invertebrates [17], fish [18], amphibians [19], bats [20] and waterfowl [21].
However, alongside these classical ‘between-habitat’ effects it is increasingly recognized that ecosystem engineers can exert important effects at finer spatial scales—nested within habitats—that may also influence biodiversity at coarser scales. These effects can also apply through both non-engineering (e.g. consumptive) and engineering (e.g. burrowing) pathways [22]. In the case of beaver, herbivory alone (i.e. in the absence of dam building) has major effects on wetland vegetation richness and biomass [23,24]. Dam maintenance and its effect on water level regime [25], together with inputs of felled or windblown deadwood [26] and canals dug to transport felled material [27], should further differentiate active BP from OW in the wider landscape. A simple test of the importance of these within-habitat effects would be to compare active BP with nearby superficially similar OW to identify how and at what scale these habitats differ. In the absence of strong differences, the effects of beaver might, in theory, be recreated by conventional approaches, such as pond creation, or restoring existing water bodies (e.g. [28,29]). Beaver bring their own specific management challenges (e.g. damage to forestry, flooding of agricultural land, threats to infrastructure integrity) and pose important dilemmas (e.g. benefits for aquatic biodiversity, flood attennuation, storage of nutrients and sediment [30,31] versus the potential impact of increased global methane emissions [14]). It is, therefore, pertinent to ask if we actually need ecosystem engineers such as beaver to achieve ‘beaver-like’ biodiversity benefits and what, specifically, beavers offer to the rewilding of wetlands.
Few studies question how ecosystem engineering by beaver affects disparate biota in parallel, or in comparison to superficially similar wetlands formed by independent processes. Scales finer than the water body are also commonly ignored. Plants (submerged, floating-leaved and emergent taxa) and water beetles found in wetlands are taxonomically diverse, easily sampled, indicative of particular environmental conditions and differ in their dispersal ability, making them ideal subjects for a comparative study of biodiversity in BP and OW at different spatial scales. Here, we test the following hypotheses: (i) BP contain greater wetland plant and water beetle richness at contrasting spatial scales (plot or sample versus site) than OW in the landscape, and (ii) spatial turnover in biota is systematically higher within individual BP than OW, reflecting increased habitat heterogeneity and active use, making active BP unique in the hydroscape.
2. Material and methods
(a). Field sites
The study was conducted in July 2012 within a 100 × 100 km area between Örebro and Skinnskatteberg, in central southern Sweden (59°30′ N, 15°10′ W, elevation range: 28–156 m). Within this area, wetlands that were created by beaver, i.e. beaver ponds (henceforth BP; figure 1a), were identified by the presence of well-maintained beaver dams that impounded an area of shallow standing water. Given the regional topography, all of the BP studied were valley wetlands as opposed to small cascade dam systems. The minimum age of BP sampled was estimated at 5 years based on age and extent of standing dead wood and aerial imagery from 2006 to 2010 (Google Earth 7.1.2.2041). All BP supported active beaver colonies as indicated by freshly grazed herbaceous plants or coppiced trees, canal creation, dam maintenance and lodge construction. Other (i.e. non-beaver) wetlands (henceforth OW; figure 1b) were permanent, shallow, standing freshwaters (i.e. ponds or non-eroding lake margins and associated minerotrophic wetlands), formed naturally by river migration or during the last glacial retreat, enlarged in one case through artificial impoundment. Their hydrological regime was uninfluenced by beaver dams, although most sites showed evidence of occasional use by beaver. OW were located in close proximity (less than 5 km) to sampled BP, but were not paired with specific sites. A total of 10 BP (1.3 ± 0.5 ha, 0.6–2.1 ha; mean ± s.d., range) and 10 OW (0.9 ± 0.4 ha, 0.3–1.7 ha) were sampled. All sites were situated in areas dominated by managed forestry or low-intensity agriculture.
Figure 1.
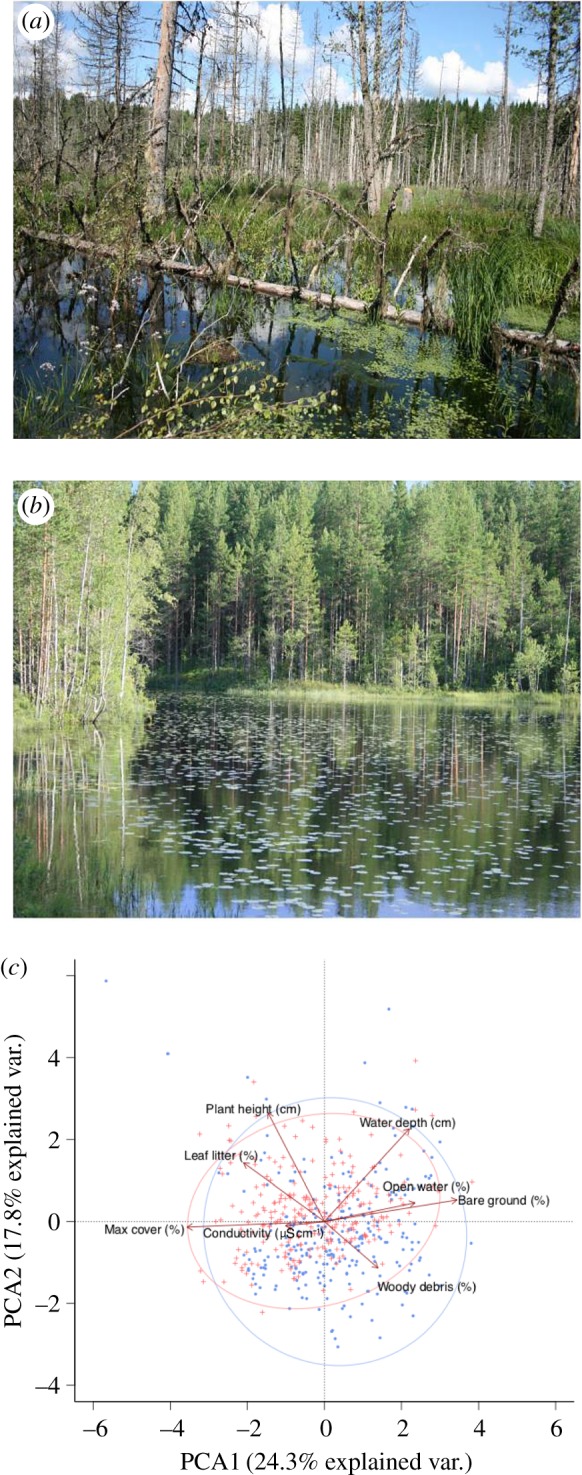
Examples of a beaver pond (a) and other wetland (b) within the study area. Principal components analysis (PCA) of plot level environmental data collected in beaver ponds (blue) and other wetlands (red). Ellipses cover 95% of the variation in scores for each wetland type on the PCA axes shown (c). (Online version in colour.)
(b). Methods
To compare wetlands of varying size we applied a fixed sampling effort per site, with samples distributed over an area of less than 1.5 ha at all sites. For plants, 25 plots of 2 × 2 m were located a minimum of 10 m apart but otherwise randomly in each of the 20 wetlands (n = 500). Wetland plants (i.e. all submerged, floating-leaved, emergent and marginal plants, including tree saplings and bryophytes) were identified to the highest feasible taxonomic level. Nomenclature followed Karlsson & Agestam [32]. Species cover was estimated visually and assigned a score on a scale of 1–5 (1 ≤ 2%; 2 = 3–10%; 3 = 11–25%; 4 = 26–50%; 5 ≥ 51%). Water beetles were sampled in shallow water (less than 0.75 m deep) and associated vegetation using a D-framed net (1 mm mesh) swept continuously through the water column, over the bed and through aquatic vegetation within an area of 2 × 2 m for approximately 1 min. Five sweep samples were taken in each of the 20 wetlands (n = 100). Beetles were sorted and counted in the field. Specimens of adults and larvae were preserved in 80% methylated spirit and later identified by light microscopy to the highest taxonomic level feasible. We considered only true water beetles, i.e. those ‘at least partly submerged for most of the time in their adult stage’ [33]. Beetle nomenclature followed Nilsson [34].
For each sample (plot or sweep), the mean plant height and water depth were determined from six replicate measurements. The extent of leaf litter, open water, woody debris, bare ground and grazing was scored visually on a 1–5 scale. Water conductivity was measured using a multi-range conductivity meter (Hanna instruments HI 9033) calibrated to 25°C.
(c). Treatment of data and statistical analyses
Alpha diversity was assessed as numbers of species per sample (plot for plants, sweep for beetles), each sample being equivalent to 2 × 2 m. To estimate beta diversity we calculated the mean Bray–Curtis dissimilarity index (BCI) between all pairwise combinations of plot-level plant composition data or sweep-level beetle data from a site. This provided a measure of within-site heterogeneity. We used two measures of gamma diversity: (i) the species pool per site, derived from an aggregation of the plots or sweeps, and (ii) the estimated overall species pool per wetland type derived from species accumulation curves. A sample-based species accumulation curve was computed for vegetation based on a maximum sample size of 250 plots per wetland type (25 plots in each of 10 sites) without replacement. An individual-based rarefaction curve [35] was used for beetles due to the pronounced variation in number of individuals per sample. Both curves were extrapolated to observe the effects of doubling plot number or abundance. Expected species richness was calculated using Chao's species estimator based on a species abundance matrix [36]. Finally, we compared beetle abundance between wetlands based on individuals per sample, or per site (based on aggregating five samples).
The effects of wetland type (i.e. BP versus OW) and specific environmental variables on sample- or site-level plant richness, beetle richness and beetle density were tested using generalized linear mixed-effect models with a log-link Poisson error distribution (R library lme4 [37]). Plant alpha diversity, maximum plant coverage per plot (%) and plant height were additionally included in the beetle model. Site was included in all sample-level models as a random effect. Continuous environmental variables were standardized to zero mean and unit standard. Since no environmental variables were found to be collinear based on checking using the corvif function in the R library AED [38] all were retained in the models. Beta diversity values (BCI) were not normally distributed and failed to meet parametric requirements after transformation so non-parametric Kruskal–Wallis tests were used to test the effect of wetland type.
All statistical analyses and graphics were generated using R Studio v. 2.15.0 (R Development Core Team, 2013) with the additional packages vegan [39], fossil [40], plyr [41], reshape2 [42] and iNEXT [43]. All model outputs are given in the electronic supplementary material, table S1.
3. Results
(a). Environmental conditions
The typical conditions prevailing in BP and OW are illustrated in figure 1. The plot level environmental data collected during vegetation sampling is summarized in figure 1c via a PCA, the first two axes of which captured 42% of the observed variation. The conditions found in OW are essentially a subset of those recorded in BP, with BP demonstrating greater overall variation in the range of local conditions present across a common number of wetland sites (10 per wetland type) and samples (25 per wetland). BP were characterised by more woody debris, bare ground and open water, while OW were characterised by taller, more extensive vegetation and associated litter, and deeper water.
(b). Alpha diversity
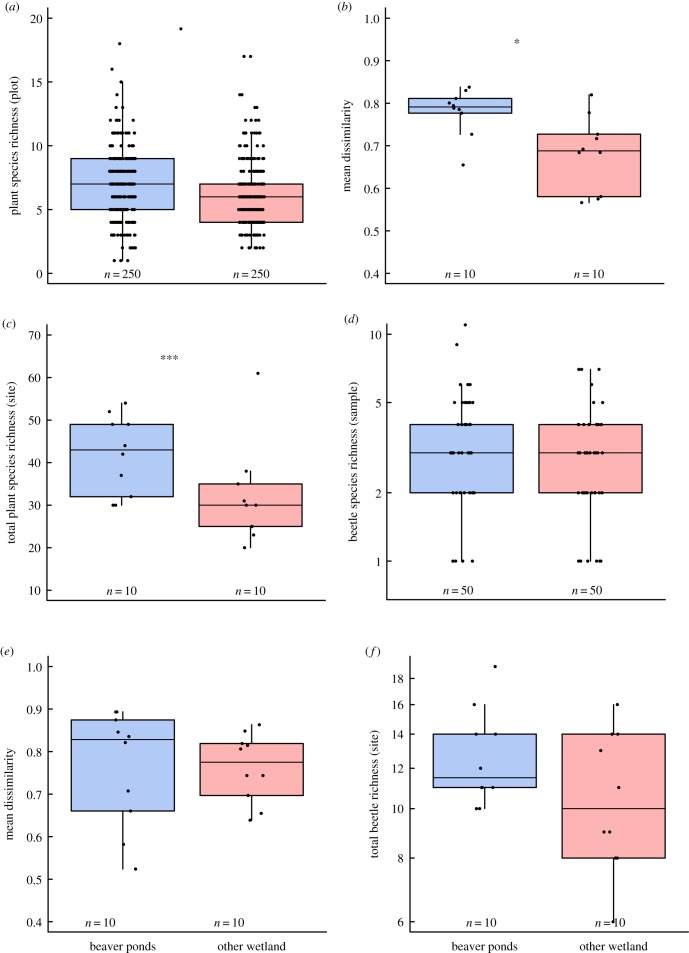
BP were more plant species-rich (+15%) at the plot scale in comparison to OW (figure 2a), although this difference was marginal (p = 0.06). BP also had a higher richness of beetles per sample (+16%) than OW (figure 2d) but this difference was not significant (p = 0.19).
Figure 2.
Boxplots comparing beaver ponds (blue) and other wetlands (red) in terms of (a) alpha diversity of plants (richness per plot); (b) beta diversity of plants (Bray Curtis dissimilarity); (c) gamma diversity of plants (richness per site); (d) alpha diversity of beetles (richness per sweep); (e) beta diversity of beetles; (f) gamma diversity of beetles. Boxes show median and enclose interquartile range, whiskers show 10th and 90th percentiles. Dots show individual data points. ▪ p < 0.01; *p < 0.05; ***p < 0.001. (Online version in colour.)
A generalized linear mixed effects model revealed that three variables, water depth, extent of litter and bare ground, all had highly significant (p < 0.001) negative effects on plot-level plant species richness (electronic supplementary material, figure S1). Having accounted for the variation explained by these variables the term wetland type was the only remaining significant variable (p = 0.015), with richness being higher in plots in BP. This indicates that there is direct residual effect of wetland type on plant richness that is not captured via the measured environmental variables.
Based on the same modelling approach none of the measured environmental variables (including plant height, cover and richness) could explain a significant amount of variation in the sample-level beetle richness (range of p = 0.11–0.65; electronic supplementary material, figure S1). The effect of wetland type was marginal (p = 0.07).
(c). Beta diversity
Within BP, vegetation plots were significantly more dissimilar (+17%; p = 0.013) from each other than in OW, indicating higher within-site beta diversity (figure 2b). However, turnover in beetle composition between samples did not differ between wetland types (p = 0.5) (figure 2e).
(d). Gamma diversity
Site level richness for plants was significantly higher (+33%; p < 0.001) in BP than OW (figure 2c), consistent with the marginally higher alpha diversity and significantly higher plant beta diversity in BP. By contrast, there was no significant difference (p = 0.21) in site-level richness of beetles between BP and OW (figure 2f; p = 0.25). Wetland area had no effect on site-scale plant (F1,18 = 0.66, p = 0.42) or beetle (F1,18 = 0.01, p = 0.96) species richness.
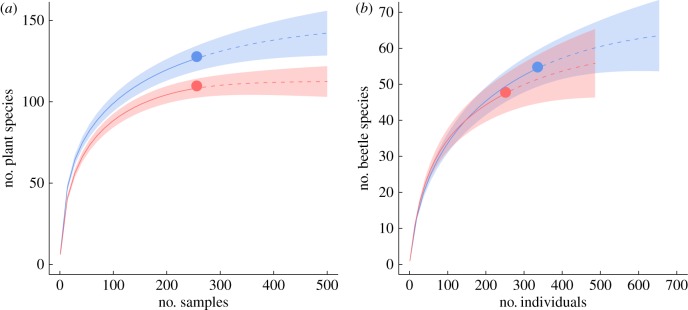
For plants, the estimated total species pool in BP (128 species) was 17% higher than in OW (109 species) for the same sampling effort. Species accumulation curves for both wetland types (figure 3a) illustrate the higher rate of plant species accumulation in BP and the larger overall species pool (based on non-overlapping 95% confidence intervals). Rarefaction indicated that for an equivalent level of sampling effort (i.e. the same number of individuals) the number of beetle species was marginally higher (+10%) in BP but not significantly so (figure 3b). Although accumulation curves were not fully asymptotic, the total number of plant species recorded was 98% of the Chao estimator value confirming that sampling was satisfactory. For water beetles, it was evident that greater un-sampled richness exists within both wetland types (87% of expected value).
Figure 3.
Species accumulation curves for beaver ponds (blue, dashed lines) and other wetlands (red, dotted lines) for (a) plants (sample-based accumulation at plot scale) and (b) beetles (individual-based rarefaction at sweep scale). In both cases, lines are extrapolated to estimate the effect of doubling sampling effort. Shaded polygons indicate 95% confidence intervals. (Online version in colour.)
(e). Beetle abundance
The number of individuals per sample was higher in BP than OW (+26%; p = 0.034). Wetland type was the only variable that significantly explained variation in sample beetle density despite inclusion of a range of sample-specific environmental variables as predictors. At the site level, BP contained higher numbers of beetles, though at this level the effect was marginal (p = 0.086).
4. Discussion
The creation of wetlands by beaver through the damming of streams is unique among global fauna and has the potential to create, modify, restore and rewild habitat [8,11,13] and thus benefit freshwater biodiversity. Our study reveals some more nuanced effects, namely that our focal biota respond differently to wetland creation by beaver and that marked differences exist between active BP and superficially similar OW that coexist in the same landscape.
For plants, alpha diversity was marginally higher in BP than OW but turnover between patches (beta diversity) was much higher in BP and consequently both site richness and the overall plant species pool was larger in BP than OW. In BP, fluctuations in water level can be rapid due to changes in dam height or integrity, and, due to limited storage capacity and a shallow bank profile, small changes in depth can expose extensive marginal habitat [25,44]. This increases the area of bare ground and favours recruitment of annuals which may be excluded from deeper water or continuous tall vegetation. Smaller scale disturbances that are unique to BP also enhance habitat complexity, e.g. canal building and felled or wind-blown trees, plus lodges and cached material contributing to woody debris accumulations [26]. Together these characteristics render high internal habitat heterogeneity a defining feature of BP that differentiates them from OW and probably promotes coexistence through reduced interspecific competition (as indicated by reduced plant height and litter in BP). The importance of wetland type in explaining patterns of local richness over and above the effect of variables such as water depth and litter extent, testifies to the added direct effects of beaver, such as selective plant foraging and herbivory [23,24], on wetland vegetation. This suite of direct and indirect effects imposes an ongoing dynamic unique to BP that promotes plant species diversity at a range of spatial scales. Aside from being ecosystem engineers (sensu [1]) beaver can therefore justifiably also be regarded as agents of within-habitat heterogeneity. Wetlands used, but not created by beavers, might be expected to show similar but less pronounced differences from OW (neither used or created by beavers), with herbivory alone then being the main source of differences.
BP supported a higher abundance of beetles, but none of the diversity measures differed between BP and OW. The response of beetles was also uncoupled from that of vegetation (i.e. there was no link between beetle richness or abundance and vegetation-based predictors). These findings suggest that for beetles the diversity of microhabitats is either similar across wetland types (even if the habitats themselves differ), the regional species pool lacks the specialists that might exploit additional novel microhabitats in BP, or the mobility of beetles within and between wetlands blurs any relationships between richness and environment. However, given the higher densities of beetles in samples from BP the overall quality of habitat is evidently superior. Since beaver will not affect beetles directly several factors might contribute to this improved habitat quality. These include: (i) increased availability of vegetated shallow edge habitats and canal building [27], which have been shown to enhance beetle abundance [45]; (ii) increased availability of invertebrate prey [30] and nutrients derived from decomposition of former terrestrial vegetation; (iii) reduced exposure to fish predation compared to OW of a similar age due to a combination of reduced fish access or habitat suitability [46], or higher water colour in BP due to elevated dissolved organic carbon [47]; and (iv) high volumes of submerged felled or windblown deadwood acting as a dietary resource or refugia [26,48]. The relevance of these factors might be pond age-dependent (e.g. sparsely vegetated young ponds are preferentially colonised by dytiscids [46]) but we focussed intentionally on mature BP to provide a balanced comparison with OW established in the landscape, rather than introduce age as a confounding variable.
(a). Implications
BP are a natural component of Northern Hemisphere landscapes and have increased greatly in extent following recovery of beaver populations over the past century, albeit probably short of their historic extent [14]. BP are now commonly managed to maintain drainage, restrict forestry losses or to protect the integrity of transport infrastructure [49]. Conversely, beaver are increasingly being reintroduced for their value as creators of wetland habitat, to restore incised stream ecosystems [8], or where adaptation to hydrological extremes is required due to depleted wetland resources [9]. Can spatial differences in biodiversity in wetlands associated with forested landscapes, such as Sweden, where beaver already occur naturally at high densities [50], offer a useful guide to the temporal changes in wetland biodiversity that occur when beaver are reintroduced to other, more intensively agricultural countries?
Isolation has weak effects on the wetland biota of BP under natural conditions [51] but in human-impacted landscapes greater inter-wetland distances might limit dispersal. The absence of a diverse and highly connected regional species pool might also constrain colonisation and local heterogeneity. However, long-term monitoring of the ecological effects of habitat engineering by beaver in Scotland, to which they have recently been reintroduced [52], reveals that responses closely emulate the differences between BP and OW we observed in Sweden. Thus, Law et al. [23] found that after 9 years exposure to beaver foraging alpha diversity of plants in a swamp more than doubled and gamma diversity of plants in swamp and quaking bog habitat tripled. Spatial turnover in composition between plots also increased significantly, rising by 20% in swamp and fivefold in quaking bog habitats. In a separate study of an agriculturally degraded fen to which beaver were reintroduced Law et al. [11] found that after 12 years plant alpha diversity had increased on average by 46%, whilst gamma diversity increased by 148%. Heterogeneity, measured by dissimilarity of plot composition, increased by 71%. The strong similarity in findings with the present spatial study suggests that it is reasonable to extrapolate the patterns reported here to situations where beaver are reintroduced after prolonged absence.
5. Conclusion
Ecosystem engineers create unique habitats and thus benefit wider biodiversity at multiple spatial scales. This study illustrates that use or maintenance of engineered habitats by beaver also enhances landscape scale diversity by creating lentic habitat that is distinctively heterogeneous in terms of habitat and vegetation, rather than being novel per se. Thus, benefits of ecosystem engineering also accrue during the phase of active pond creation and maintenance by beaver, (and probably also to a lesser extent when beaver colonise pre-existing water bodies), not solely as a consequence of the genuinely novel habitats (e.g. [16]) that follow beaver pond abandonment. Beaver are textbook ecosystem engineers and much-studied, but, like other large aquatic herbivores [53,54], their importance as agents of finer scale heterogeneity within habitats is largely overlooked. As one of very few large(ish) herbivorous mammals strictly associated with freshwater beaver represent an integral component of both trophic rewilding and the improved ecological status of freshwaters; in those parts of their native range from which they have long been absent population expansion and reintroduction is gradually reinstating their influence. Provided they can be accommodated beaver may yet prove most valuable in landscapes artificially deficient in wetlands where the processes that would naturally drive heterogeneity have long been lost or tamed. It is tempting to assume that any natural process or feature can be replicated through human intervention. However, while anyone can make a pond there is only one way to make a beaver pond.
Supplementary Material
Supplementary Material
Ethics
The research was undertaken in compliance with the research ethics requirements of the University of Stirling.
Data accessibility
All data referred to in this article and code used in analyses are deposited in DataSTORRE—the University of Stirling research data repository at https://datastorre.stir.ac.uk/handle/11667/39.
Authors' contributions
N.J.W. developed the project methodology and obtained principle funding. Primary data collection was carried out by N.J.W. and A.L. F.E. and O.L. provided information on beaver activity and distribution for central Sweden and advised on choice of sites using database collated by O.L. Identification of beetle specimens was carried out by A.L. with the assistance of G.F. Data analyses were undertaken by A.L. with input from N.J.W. Manuscript was written by N.J.W. with input from A.L. and additional comments from G.F. and F.E.
Competing interests
The authors declare that they have no conflicts of interest in presenting this work for publication.
Funding
The Swedish Research Council Formas (grant no. 2010-1647) financed the contributions of O.L. and F.E. We thank the Carnegie Trust for a grant to cover travel costs and a University of Stirling Horizon studentship for funding of A.L.
References
- 1.Jones CG, Lawton JH, Shachak M. 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78, 1946–1957. ( 10.1890/0012-9658(1997)078%5B1946:PANEOO%5D2.0.CO;2) [DOI] [Google Scholar]
- 2.Romero GQ, Goncalves-Souza T, Vieira C, Koricheva J. 2015. Ecosystem engineering effects on species diversity across ecosystems: a meta-analysis. Biol. Rev. 90, 877–890. ( 10.1111/brv.12138) [DOI] [PubMed] [Google Scholar]
- 3.Vigerstol KL, Aukema JE. 2011. A comparison of tools for modelling freshwater ecosystem services. J. Environ. Manage. 92, 2403–2409. ( 10.1016/j.jenvman.2011.06.040) [DOI] [PubMed] [Google Scholar]
- 4.Dudgeon D, et al. 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182. ( 10.1017/S1464793105006950) [DOI] [PubMed] [Google Scholar]
- 5.Nienhuis PH, Bakker JP, Grootjans AP, Gulati RD, de Jonge VN. 2002. Ecological restoration of aquatic and semi-aquatic ecosystems in the Netherlands. Hydrobiologia 478, 219–233. ( 10.1023/A:1021090900341) [DOI] [Google Scholar]
- 6.Halley DJ, Rosell F. 2002. The beaver's reconquest of Eurasia: status, population development and management of a conservation success. Mamm. Rev. 32, 153–178. ( 10.1046/j.1365-2907.2002.00106.x) [DOI] [Google Scholar]
- 7.Burchsted D, Daniels M, Thorson R, Vokoun J. 2010. The river discontinuum: applying beaver modifications to baseline conditions for restoration of forested headwaters. Bioscience 60, 908–922. ( 10.1525/bio.2010.60.11.7) [DOI] [Google Scholar]
- 8.Pollock MM, Beechie TJ, Wheaton JM, Jordan CE, Bouwes N, Weber N, Volk C. 2014. Using beaver dams to restore incised stream ecosystems. Bioscience 64, 279–290. ( 10.1093/biosci/biu036) [DOI] [Google Scholar]
- 9.Hood GA, Bayley SE. 2008. Beaver (Castor canadensis) mitigate the effects of climate on the area of open water in boreal wetlands in western Canada. Biol. Conserv. 141, 556–567. ( 10.1016/j.biocon.2007.12.003) [DOI] [Google Scholar]
- 10.Gibson PP, Olden JD. 2014. Ecology, management, and conservation implications of North American beaver (Castor canadensis) in dryland streams. Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 391–409. ( 10.1002/aqc.2432) [DOI] [Google Scholar]
- 11.Law A, Gaywood MJ, Jones KC, Ramsay P, Willby NJ. 2017. Using ecosystem engineers as tools in habitat restoration and rewilding: beaver and wetlands. Sci. Total Environ. 605, 1021–1030. ( 10.1016/j.scitotenv.2017.06.173) [DOI] [PubMed] [Google Scholar]
- 12.Hartman G. 1996. Habitat selection by European beaver (Castor fiber) colonizing a boreal landscape. J. Zool. 240, 317–325. ( 10.1111/j.1469-7998.1996.tb05288.x) [DOI] [Google Scholar]
- 13.Naiman RJ, Johnston CA, Kelley JC. 1988. Alteration of North American streams by beaver. Bioscience 38, 753–762. ( 10.2307/1310784) [DOI] [Google Scholar]
- 14.Whitfield CJ, Baulch HM, Chun KP, Westbrook CJ. 2015. Beaver-mediated methane emission: the effects of population growth in Eurasia and the Americas. Ambio 44, 7–15. ( 10.1007/s13280-014-0575-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMaster RT, McMaster ND. 2001. Composition, structure, and dynamics of vegetation in fifteen beaver-impacted wetlands in western Massachusetts. Rhodora 103, 293–320. [Google Scholar]
- 16.Wright JP, Jones CG, Flecker AS. 2002. An ecosystem engineer, the beaver, increases species richness at the landscape scale. Oecologia 132, 96–101. ( 10.1007/s00442-002-0929-1) [DOI] [PubMed] [Google Scholar]
- 17.Rolauffs P, Hering D, Lohse S. 2001. Composition, invertebrate community and productivity of a beaver dam in comparison to other stream habitat types. Hydrobiologia 459, 201–212. ( 10.1023/A:1012507613952) [DOI] [Google Scholar]
- 18.Kemp PS, Worthington TA, Langford TEL, Tree ARJ, Gaywood MJ. 2011. Qualitative and quantitative effects of reintroduced beavers on stream fish. Fish Fish. 13, 158–181. ( 10.1111/j.1467-2979.2011.00421.x) [DOI] [Google Scholar]
- 19.Dalbeck L, Luscher B, Ohlhoff D. 2007. Beaver ponds as habitat of amphibian communities in a central European highland. Amphibia-Reptilia 28, 493–501. ( 10.1163/156853807782152561) [DOI] [Google Scholar]
- 20.Nummi P, Kattainen S, Ulander P, Hahtola A. 2011. Bats benefit from beavers: a facilitative link between aquatic and terrestrial food webs. Biodivers. Conserv. 20, 851–859. ( 10.1007/s10531-010-9986-7) [DOI] [Google Scholar]
- 21.Nummi P, Holopainen S. 2014. Whole-community facilitation by beaver: ecosystem engineer increases waterbird diversity. Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 623–633. ( 10.1002/aqc.2437) [DOI] [Google Scholar]
- 22.Prugh LR, Brashares JS. 2012. Partitioning the effects of an ecosystem engineer: kangaroo rats control community structure via multiple pathways. J. Anim. Ecol. 81, 667–678. ( 10.1111/j.1365-2656.2011.01930.x) [DOI] [PubMed] [Google Scholar]
- 23.Law A, Jones KC, Willby NJ. 2014. Medium vs. short-term effects of herbivory by Eurasian beaver on aquatic vegetation. Aquat. Bot. 116, 27–34. ( 10.1016/j.aquabot.2014.01.004) [DOI] [Google Scholar]
- 24.Parker JD, Caudill CC, Hay ME. 2007. Beaver herbivory on aquatic plants. Oecologia 151, 616–625. ( 10.1007/s00442-006-0618-6) [DOI] [PubMed] [Google Scholar]
- 25.Gurnell AM. 1998. The hydrogeomorphological effects of beaver dam-building activity. Prog. Phys. Geogr. 22, 167–189. ( 10.1191/030913398673990613) [DOI] [Google Scholar]
- 26.France RL. 1997. The importance of beaver lodges in structuring littoral communities in boreal headwater lakes. Can. J. Zool. 75, 1009–1013. ( 10.1139/z97-121) [DOI] [Google Scholar]
- 27.Hood GA, Larson DG. 2013. Beaver-created habitat heterogeneity influences aquatic invertebrate assemblages in boreal Canada. Wetlands 34, 19–29. ( 10.1007/s13157-013-0476-z) [DOI] [Google Scholar]
- 28.Biggs J, Williams P, Whitfield M, Nicolet P, Weatherby A. 2005. 15 years of pond assessment in Britain: results and lessons learned from the work of Pond Conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 15, 693–714. ( 10.1002/aqc.745) [DOI] [Google Scholar]
- 29.Thiere G, Milenkovski S, Lindgren PE, Sahlen G, Berglund O, Weisner SEB. 2009. Wetland creation in agricultural landscapes: biodiversity benefits on local and regional scales. Biol. Conserv. 142, 964–973. ( 10.1016/j.biocon.2009.01.006) [DOI] [Google Scholar]
- 30.Law A, McLean F, Willby NJ. 2016. Habitat engineering by beaver benefits aquatic biodiversity and ecosystem processes in agricultural streams. Freshw. Biol. 61, 486–499. ( 10.1111/fwb.12721) [DOI] [Google Scholar]
- 31.Puttock A, Graham HA, Carless D, Brazier RE. 2018. Sediment and nutrient storage in a beaver-engineered wetland. Earth Surf. Process. Landf. 43, 2358–2370. ( 10.1002/esp.4398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlsson T, Agestam M. 2013. Checklista över Nordens kärlväxter See http://www.euphrasia.nu/checklista/.
- 33.Jäch MA, Balke M. 2007. Global diversity of water beetles (Coleoptera) in freshwater. Hydrobiologia 595, 419–442. ( 10.1007/s10750-007-9117-y) [DOI] [Google Scholar]
- 34.Nilsson AN. 2014. Catalogue of Palaearctic Coleoptera: Noteridae and Dytiscidae See http://www2.emg.umu.se/projects/biginst/andersn/Cat_main.htm.
- 35.Colwell RK, Mao CX, Chang J. 2004. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85, 2717–2727. ( 10.1890/03-0557) [DOI] [Google Scholar]
- 36.Chao A. 1987. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43, 783–791. ( 10.2307/2531532) [DOI] [PubMed] [Google Scholar]
- 37.Bates D, Maechler M, Bolker B, Walker S. 2013. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0-4 See http://CRAN.R-project.org/package=lme4.
- 38.Zuur AF, Hilbe JM, Ieno EN. 2013. A beginner's guide to GLM and GLMM with R: a frequentist and Bayesian perspective for ecologists. Newburgh, UK: Highland Statistics. [Google Scholar]
- 39.Oksanen J, et al. 2013. Vegan: community ecology package. R package version 2.0-9 See http://CRAN.R-project.org/package=vegan.
- 40.Vavrek MJ. 2011. fossil: palaeoecological and palaeogeographical analysis tools. Palaeontol. Electronica 14, 1T See http://palaeo-electronica.org/2011_1/238/index.html. [Google Scholar]
- 41.Wickham H. 2016. plyr: Tools for splitting, applying and combining data. R package version 1.8.4. See https://cran.r-project.org/web/packages/plyr/index.html. [Google Scholar]
- 42.Wickham H. 2007. Reshaping data with the reshape package. J. Stat. Softw. 21, 1–20. ( 10.18637/jss.v021.i12) [DOI] [Google Scholar]
- 43.Hsieh TC, Ma KH, Chao A. 2016. iNEXT: iNterpolation and EXTrapolation for species diversity. R package version 2.0.12 See http://chao.stat.nthu.edu.tw/blog/software-download/.
- 44.Pollock MM, Naiman RJ, Hanley TA. 1998. Plant species richness in riparian wetlands—a test of biodiversity theory. Ecology 79, 94–105. [Google Scholar]
- 45.Bloechl A, Koenemann S, Philippi B, Melber A. 2010. Abundance, diversity and succession of aquatic Coleoptera and Heteroptera in a cluster of artificial ponds in the North German Lowlands. Limnol. Ecol. Manag. Inl. Waters 40, 215–225. ( 10.1016/j.limno.2009.08.001) [DOI] [Google Scholar]
- 46.Fairchild GW, Faulds AM, Matta JF. 2000. Beetle assemblages in ponds: effects of habitat and site age. Freshw. Biol. 44, 523–534. ( 10.1046/j.1365-2427.2000.00601.x) [DOI] [Google Scholar]
- 47.Correll D, Jordan T, Weller D. 2000. Beaver pond biogeochemical effects in the Maryland coastal plain. Biogeochemistry 49, 217–239. ( 10.1023/A:1006330501887) [DOI] [Google Scholar]
- 48.Thompson S, Vehkaoja M, Nummi P. 2016. Beaver-created deadwood dynamics in the boreal forest. Forest Ecol. Manag. 360, 1–8. ( 10.1016/j.foreco.2015.10.019) [DOI] [Google Scholar]
- 49.Törnblom J, Angelstam P, Hartman G, Henrikson L, Sjoberg G. 2011. Toward a research agenda for water policy implementation: knowledge about beaver (Castor fiber) as a tool for water management with a catchment perspective. Baltic Forestry 17, 154–161. [Google Scholar]
- 50.Hartman G. 2011. The beaver (Castor fiber) in Sweden. In Restoring the European beaver: 50 years of experience (eds Sjöberg G, Ball JP), pp. 13–17. Sofia, Bulgaria: Pensoft. [Google Scholar]
- 51.Wright JP, Gurney WSC, Jones CG. 2004. Patch dynamics in a landscape modified by ecosystem engineers. Oikos 105, 336–348. ( 10.1111/j.0030-1299.2004.12654.x) [DOI] [Google Scholar]
- 52.Gaywood MJ. (ed.). 2015. Beavers in Scotland: a report to the Scottish Government. Inverness, UK: Scottish Natural Heritage. [Google Scholar]
- 53.Bakker ES, Pagès JF, Arthur R, Alcoverro T. 2016. Assessing the role of large herbivores in the structuring and functioning of freshwater and marine angiosperm ecosystems. Ecography 39, 162–179. ( 10.1111/ecog.01651) [DOI] [Google Scholar]
- 54.Moss B. 2015. Mammals, freshwater reference states, and the mitigation of climate change. Freshw. Biol. 60, 1964–1976. ( 10.1111/fwb.12614) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data referred to in this article and code used in analyses are deposited in DataSTORRE—the University of Stirling research data repository at https://datastorre.stir.ac.uk/handle/11667/39.