Abstract
Rewilding is a novel approach to ecological restoration. Trophic rewilding in particular aims to reinstate ecological functions, especially trophic interactions, through the introduction of animals. We consider the potential for trophic rewilding to address biological invasions. In this broad review, we note some of the important conceptual and ethical foundations of rewilding, including a focus on ecosystem function rather than composition, reliance on animal agency, and an appeal to an ethic of coexistence. Second, we use theory from invasion biology to highlight pathways by which rewilding might prevent or mitigate the impacts of an invasion, including increasing biotic resistance. Third, we use a series of case studies to illustrate how reintroductions can mitigate the impacts of invasions. These include reintroductions and positive management of carnivores and herbivores including European pine martens (Martes martes), Eurasian otters (Lutra lutra), dingoes (Canis dingo), Tasmanian devils (Sarcophilus harrisii) and tule elk (Cervus canadensis nannodes). Fourth, we consider the risk that rewilding may enable a biological invasion or aggravate its impacts. Lastly, we highlight lessons that rewilding science might take from invasion biology.
This article is part of the theme issue ‘Trophic rewilding: consequences for ecosystems under global change’.
Keywords: trophic rewilding, biological invasion, coexistence
1. Introduction
Various versions of rewilding have been described since the term was first coined in the 1990s [1]. Recently, Svenning et al. ([2], p. 898) defined trophic rewilding as ‘species introductions to restore top-down trophic interactions and associated trophic cascades to promote self-regulating biodiverse ecosystems'. This and similar approaches to rewilding focus on the introduction of vertebrate populations or their functional analogues to landscapes from which they have been extirpated. The primary aim is often to restore top-down trophic interactions, although this does not preclude restoration of other functions such as physical disturbance of substrate or dispersal of seeds. In contrast to much current environmental policy [3], rewilding is focussed on ecosystem functions rather than species and assemblages. Autonomy is a guiding principle—reintroduced populations should be self-sustaining—and the restorative effects are expected to be long term and large scale.
Rewilding typically involves reintroducing species to ecosystems that have been modified from their original state. In addition to habitat modifications, species loss and climate change, many ecosystems across the globe have been severely impacted by biological invasions [4]. Invasions have been a major cause of extinctions and, in synergy with other factors, continue to threaten many species [5–7]. While rewilding has been undertaken or proposed with various goals in mind, few studies have considered rewilding in the context of biological invasions.
Our aim here is to review the potential for trophic rewilding to prevent, mitigate or enhance the impacts of biological invasions. We restrict our review to the effects of reintroducing large carnivores and herbivores, although rewilding programs could involve the introduction of other agents of change, such as pollinators, seed dispersers or ecosystem engineers. First, we note some of the important conceptual and ethical foundations of rewilding; second, we use theory from invasion biology to highlight two pathways by which rewilding might prevent or mitigate the impacts of an invasion; third, we illustrate these points through a series of case studies; and fourth, we consider the risk that rewilding could enable invasions or aggravate their impacts.
2. Ethical and conceptual considerations
Conservation is arguably becoming more interventionist. Corlett [8] has identified three current paradigms that reflect this in different ways: conservation translocations, novel ecosystems and rewilding. The latter interventions focus on ecosystem function, as opposed to composition [9], which is also reflected in recent calls for a ‘new conservation’ [10]. These paradigms sometimes distance themselves from traditional, preservationist conservation approaches, which are described, perhaps unfairly, as reactive and pessimistic [11,12].
Two key concepts that support trophic rewilding are non-human agency and coexistence. A presumption of non-human agency is evident in the rewilding literature (e.g. [11–14]), especially in the preference for autonomy in the restored ecosystem, the deployment of animals to do restoration, calls for ‘open-ended’ conservation, tolerance for unexpected outcomes and the adaptation of the word ‘wild’, which denotes, among other things, self-willed nature [15]. Various theorists have promoted non-human agency as a necessary feature of an adequate environmental ethic (e.g. [16,17]). Having regard for agency may also help reconcile conflicting obligations to environmental integrity and the welfare of other creatures. This is a key problem for environmental ethics [18] and a particularly difficult problem when managing biological invasions [19].
Agency, in particular autonomous agency, can entail an ethic of respect (e.g. [20]). Something similar is at play in rewilding: Rewilding advocacy often includes an assumption that coexistence of disparate groups is possible and even preferable to exclusion, for example, coexistence between humans and large predators [21–23]. An ethic based on coexistence may be a viable alternative to exclusion-based approaches to conservation such as the separation of large animals and humans [21], a focus on protected areas [24] or a wilderness-focussed ethic [25]. However, coexistence in this context requires more detailed explanation.
What implications does this new paradigm have for biological invasions? First, there may be moral implications. If coexistence were broadly accepted then managers would have greater warrant to reintroduce large animals. It could lead to wider acceptance that preservation is not a default conservation aim, releasing managers from an a priori obligation to return the system to some pristine state. Similarly, the mere presence of a non-native species need not be considered a failure of management. Practically speaking, managers often have insufficient resources to eradicate non-native species anyway [26] so this might seem to make little practical difference. However, thinking about ecosystems and animals in terms of function, agency and coexistence might inspire novel solutions.
Biological control programs are founded on a similar approach and involve the introduction of organisms with the purpose of altering invasive populations. Conceptually at least, trophic rewilding differs from classical biological control in several ways [27]. It focusses on large vertebrates rather than including invertebrates; values reintroduced populations for their own sake rather than as means to an end; concentrates on non-commercial ecosystems; aims for influence on many species rather than a single species; and preferences species assumed to have coevolved with the community, reducing the risk of negative influences (but see below). Beyond biological control, examples of agent- and function-oriented approaches include recent proposals to introduce Tasmanian devils to mainland Australia to regulate cat and fox impacts on small mammals (described below); behavioural and evolutionary modifications to ‘train’ species to avoid novel predators; and targeting individual predators that cause the most damage to vulnerable populations [28–30].
The discipline of invasion biology is now well established [7]. It is predicated on the fact that some populations that are new to a place cause problems, including undermining important ecological functions and threatening species with extinction. However, some concepts and language commonly associated with invasion biology have been recently criticised, notably the militaristic and xenophobic framing of problems, the seeming incommensurability with animal welfare concerns and the assumption that any influence of a new population is negative [31–33]. It is increasingly recognized that introduced or newly arrived populations can be valuable additions to an ecosystem, a view which has obvious overlap with rewilding advocacy [33,34]. Recognizing the above issues, we apply the term ‘invasive’ only to those new arrivals that cause significant negative impacts.
3. Invasion processes
Invasion biology has generated several prominent hypotheses to explain invasion processes [35]. However, theoretical generalities explain far less variation than we would like, and outcomes are strongly affected by local context, unpredictable ecological dynamics and a multiplicity of processes [36]. This precludes general statements about whether rewilding will work to prevent or mitigate biological invasions.
On the other hand, rewilding might work against invasions in specific circumstances. There are several biotic processes that constrain invasions [37]. Rewilding can increase biotic resistance, i.e. reduce the probability of successful establishment or spread of an invasive population. Invasions are often described in terms of stages, e.g. arrival, establishment and spread, and the barriers between them [38]. Biotic interactions play an important role in strengthening barriers at each stage [35,37], though it is doubtful whether communities can completely repel newcomers [37].
More specifically, once an invasive population is established, a rewilding introduction might contain, prevent or reduce impacts, for example via top-down trophic processes that suppress the invasive species and promote its competitors. Carnivores can suppress invasive populations directly by predation. Herbivores might selectively feed on exotic species, reducing their abundance; or by feeding more broadly prevent any one species (including an invasive species) from dominating. We present several case studies below, focussed on rewilding with carnivores and herbivores.
4. Rewilding with carnivores
Trophic rewilding proposals have focussed on large mammals [2]. The ecological influence of large predators and their disproportionate vulnerability to extinction have heightened their importance in conservation [39,40]. However, we are aware of no cases where a large terrestrial predator has been introduced to its former historic range to control a biological invasion and to persist as a valued part of the assemblage.
Top predators can have strong influences on ecosystems [39,40], primarily mediated through trophic cascades and resource facilitation [40]. For example, large body size in mammalian predators is associated with a narrower prey base. Owing to energy and intake constraints, most species larger than 21.5 kg feed on vertebrate prey only, typically species similar to their own body mass or larger [41]. For most taxonomic groups there are fewer large-bodied species [42]. Thus, large predators targeting large prey tend to directly impact few species.
Despite this specificity, cascading impacts can be dramatic. For example, the reintroduction of wolves (Canis lupus) into Yellowstone National Park in 1995–6, following an absence of more than seven decades, resulted in changes to Rocky Mountain elk (Cervus elaphus) abundance and spatial habitat use. This in turn may have influenced woody vegetation recruitment, species composition and biomass, along with riparian songbird and beaver populations [43], although there is uncertainty about the strength of the influence of wolves in this landscape relative to other factors, such as drought and the human hunting of elk [44–46].
Predators can dampen variability in prey abundance and may increase community resilience to climate change [47]. Predators can provide carcasses for scavengers. They are often also scavengers themselves and influence other species via resource-mediated competition [48]. These processes point to the potential for top predators, especially large carnivores, to keep invasions in check via top-down trophic pressure.
Carnivores can also prevent the establishment and spread of invasive populations. Carnivore guilds are often characterised by strong, aggressive intraguild interactions, including harassment, kleptoparasitism and intra-guild killing without consumption [49–51]. Like prey species, other predators often respond to the presence of a predator with spatial and temporal avoidance [51]. When the disrupted population is invasive, these processes represent modes of biotic resistance, i.e. preventing the establishment of new populations or their spread into new areas.
5. Case 1: Martens and squirrels in the British Isles
Following introductions around the turn of the twentieth century, grey squirrels (Sciurus carolinensis) have displaced red squirrels (Sciurus vulgaris) in Britain and elsewhere, through exploitative competition. This process has been aggravated by squirrelpox virus, a novel pathogen possibly introduced with the grey squirrels, which is lethal to red squirrels but asymptomatic in grey squirrels, resulting in disease-mediated apparent competition [52].
European pine martens (Martes martes) are generalist predators native to most of Europe [53,54]. Because of persecution and habitat loss, they are now rare in the British Isles and functionally extinct in some areas [55]. Populations in Scotland have recently increased following new legal protections, reforestation and conservation introductions [54].
Sheehy et al. [56] have shown that the recovery of the pine marten in Britain has favoured red squirrels over grey squirrels where pine marten density is sufficiently high. Predation by pine martens probably benefits red squirrels via predator-mediated apparent competition and pathogen dynamics. Predatory impacts of pine martens are much greater on grey than red squirrels [57], reducing pathogen spillover of squirrelpox from its reservoir in grey squirrels. Modelling by Sheehy et al. [56] predicted near-zero probability of grey squirrel presence in landscapes with high pine marten connectivity, indicating that predation by pine martens may be able to severely suppress grey squirrel populations and promote coexistence with red squirrels.
6. Case 2: Mink and otters in Europe and South America
American mink (Neovison vison) were introduced to Europe, the former USSR and Tierra del Fuego for fur farming, beginning in the 1920s. Subsequently, escaped animals established wild populations [58–60], which are now widely distributed across much of the European continent and continue to spread across the Tierra del Fuego archipelago [61]. American mink threaten a wide range of predator and prey species in Europe, including the critically endangered European mink (Mustela lutreola), the European polecat (Mustela putorius), voles, shrews, birds, frogs and fish [59,62–67]. In Patagonia, they prey particularly on native rodents but also on a wide variety of ground-nesting birds and fish [68,69].
Abundance of Eurasian otters (Lutra lutra) declined sharply in Britain in the middle of the twentieth century due to hunting and insecticide pollution [70]. Eurasian otters are larger than American mink, they are better swimmers, and there is evidence that they outcompete American mink for food [71].
Following the experimental release of otters into an area only occupied by mink, Bonesi and Macdonald [72] observed a reduction in the proportion of sites occupied by mink and a reduction in mink abundance, range and range integrity compared with control sites with no otter release. The authors attributed the effect to aggressive exclusion and predation by otters, i.e. interference competition. This pattern is reflected in Patagonia, where southern river otters (Lontra provocax) are unaffected by the presence of mink, can suppress their abundance and temporal activity, force dietary and habitat shifts, and potentially prevent their occupancy in sympatric areas [60,73].
7. Case 3: Top predators in Australia
Australia has lost 29 endemic land mammal species since European colonisation, the highest rate of extinction of mammals of any continent over the last 200 years [74]. A primary cause has been predation by two introduced mesopredators: red foxes (Vulpes vulpes) and feral cats (Felis catus) [74,75]. Predation pressure has been enabled and enhanced by factors such as habitat destruction, the loss of traditional burning regimes and widespread suppression of the apex predator: the dingo (Canis dingo) [76,77].
Persecution of dingoes, intended to prevent attacks on livestock, accounts for their low abundance or absence across much of their former range [78]. There is evidence that dingoes regulate mesopredators, benefitting small vertebrates threatened by cats and foxes [79,80]. Dingoes also prey on macropods and other large herbivores. Predator-control of these herbivores can result in more vegetation of greater structural complexity [50,80,81] which can, in turn, help small mammals to avoid predation [82].
The strengths of these effects may be dependent on assemblage and bioclimatic region [83] and productivity may play a significant role [84]. Several authors have proposed positive management—relaxation of lethal control of dingoes and even reintroduction——to reduce extinction risk for native fauna through suppression of cats and foxes [85]. This is unlikely to gain community support in the sheep rangelands of Australia without significant shifts in attitudes towards predators.
More speculative is the suggestion that the Tasmanian devil (‘devil’, Sarcophilus harrisii) could be introduced to the mainland of Australia to reduce the impacts of cats and foxes on threatened vertebrates [86]. Dingoes arrived in Australia at least 3500 years before present [87]. Both the thylacine (Thylacinus cynocephalus) and the devil were widespread on the mainland of Australia at the time, and persisted until about 3200 years before present [88]. Several factors have been implicated in the mainland extinction of the devil and the thylacine. These include climate, humans and dingoes, although recent modelling suggests that the influence of dingoes was not strong [89,90]. Dingoes never reached Tasmania and the thylacine was hunted to extinction there in the twentieth century, leaving the devil as the largest predator [91].
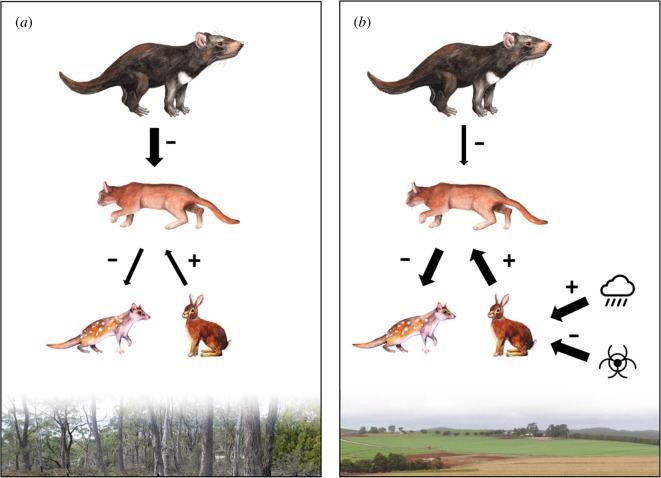
There is evidence that devils can influence the abundance and activity of cats, with positive effects on small and medium-sized mammal populations ([92–96], cf. [97]). In a long term, wide-ranging study, Hollings et al. [94] found that the strength of top-down suppression of cats varied by region. Devils appeared able to suppress cat activity and/or abundance in forested areas with few human settlements and higher rainfall. However, in drier agricultural areas this top-down effect was weaker. Factors related to food resources were more important for predicting cat occurrence. These factors included rainfall and the availability of prey, particularly the European rabbit (Oryctolagus cuniculus), a key prey species for cats in agricultural areas. Rabbit abundance was probably influenced by rainfall and outbreaks of rabbit haemorrhagic disease. Cats in Tasmania may be suppressing populations of the endangered eastern quoll (Dasyurus viverrinus), preventing their recovery by preying upon juveniles (figure 1) [95].
Figure 1.
In Tasmania, the strength of top-down control by the apex predator was found to be mediated by productivity and climate: devils suppressed cat activity and/or abundance in wet, forested areas (a). In drier, agricultural areas with more human settlements (b), bottom-up effects, including prey availability, were more important. High cat abundance, driven by rabbit abundance, may be preventing the recovery of the threatened eastern quoll [94,95]. (Online version in colour.)
The reintroduction of devils to the mainland of Australia may be a viable option to promote the coexistence of cats, foxes and threatened prey species, complementing the influence of dingoes. Modelling based on body size and diet suggests that devils could suppress the abundance and activity of cats, foxes and wallabies, benefitting small and medium-sized mammals and their habitat ([86,98], cf. [99]). The same modelling suggests that dingoes and devils could coexist. Species distribution modelling points to available habitat in south-eastern Australia, including areas where dingoes are scarce [86].
8. Rewilding with herbivores
Large herbivores influence ecosystems by removing plant biomass, changing vegetation structure, increasing light at ground level, moving large amounts of soil, dispersing nutrients, reducing fire-fuel loads and dispersing seeds over long distances [100]. These influences are often strong for the largest-bodied species (e.g. [101–103]). Some influences are species-specific, for example, hundreds of plants have propagule dispersal syndromes that reflect a strong mutualistic association with megaherbivores [104]. Others are more generalized, for example, larger bodied herbivores tend to consume a broader range of structural plant material than smaller herbivores and are generally less selective among different plant species on offer [105,106] so they have large effects on plant biomass and vegetation density. These processes are potential means of increasing biotic resistance or applying top-down trophic pressure and could inspire a range of imaginative rewilding solutions to invasion problems. We focus on the most direct means, the consumption of plant matter, and highlight two mechanisms by which introduced herbivores could reduce the abundance of invasive plant populations: by preferentially grazing invasive plants relative to native plant species, and by removing the most abundant species and preventing dominance by any one species.
Herbivory can prevent the establishment of exotic plants and reduce their performance under a range of conditions [37]. This seems to be driven in part by the biogeographical context of the plant and the consumer. A meta-analysis of manipulative field studies of mainly vertebrate herbivory found that the relative abundance of exotic plants tended to be decreased by native herbivores but was increased by exotic herbivores [107]. This pattern held for exotic plants further classified as invasive.
The above suggests that the reintroduction of a vertebrate herbivore, or the introduction of functional replacement of an extinct species, could reduce the abundance of an invasive population and mitigate its impacts through preferential feeding, that is, promote its benign coexistence in a landscape. A well-studied example is the introduction of two species of tortoise to Round Island, near Madagascar [108]. Following the eradication of goats and rabbits, the loss of grazing pressure resulted in invasive exotic plants and some fast-growing native plants dominating grasslands. Mauritian giant tortoises (Cylindraspis inepta and C. triserrata) were once grazers on the island but are extinct. Populations of two species unknown from the island, Aldabran giant (Aldabrachelys gigantea) and Madagascan radiated (Astrochelys radiata) tortoises, were introduced. These species browsed primarily on exotic plants, along with a few native species that became abundant after the eradications. In this case, coevolution of the herbivores and plants was evidently crucial. Species that coevolved with giant tortoises have traits that reduce browsing pressure relative to fast growing yet more palatable invasive plants on Round Island.
For some systems, a range of large generalist herbivores might perform a similar function and biogeographic history may be less important. In these systems, grazing by exotic generalists might prevent exotic plants from dominating. For example, grazing by cattle was found to be effective at maintaining plant diversity on vernal pools in California, substantially reducing the cover of exotic annual species and increasing the cover and richness of native plant species [109]. However, the influence of herbivory is not always so straightforward.
9. Case 4: Tule elk and velvet grass in California
Tule elk (Cervus canadensis nannodes) is a subspecies endemic to California. Hunting and land conversion in the nineteenth century drove numbers from the hundreds of thousands to a mere ten individuals. Legal protections allowed the herd to recover into the thousands through the twentieth century. Tule elk were introduced to several reserves, including at Tomales Point in 1978, a coastal grassland north of San Francisco. The site had been grazed by cattle for a century beforehand but cattle were removed as the elk were introduced. By 2003 the elk population in the 1030 ha Tomales Point Elk Reserve was approximately 500 individuals. Tule elk primarily consume herbaceous forbs and grasses but feed on shrubs during winter months [110].
In a 5-year exclusion experiment, Johnson & Cushman [110] showed that within functional groups, for the most part, tule elk affected native and exotic plants similarly. Herbivory reduced the biomass of both exotic and native perennials and increased the biomass and abundance of both exotic and native annuals [110]. These effects are of potential benefit to Californian grasslands that have been invaded by perennial shrubs [111].
Velvet grass (Holcus lanatus) is widely invasive in Californian grasslands. In Tomales Point Elk Reserve, elk reduced both the abundance and biomass of velvet grass, probably through herbivory and trampling. However, velvet grass escaped herbivory when associated with a native shrub, Baccharis pilularis [110]. Subsequent work demonstrated that tule elk were less effective in reducing velvet grass biomass in Baccharis-dominated grasslands. Soil heterogeneity (in terms of pH and moisture) was a stronger influence than herbivory on velvet grass success. Elk did not prevent the spread of the species to new areas [112]. This parallels outcomes in Californian grasslands, many of which are highly invaded, under managed livestock grazing. A meta-analysis of fifteen studies of Californian grasslands found that grazing consistently increased the cover of exotic forbs but that other responses by native and exotic species depended on factors such as precipitation, the seasonality of grazing and community type [113].
10. Causes for concern: making invasions worse
A critical lesson from studies of biological invasions is that the addition or removal of species from ecosystems can generate trophic cascades with unanticipated and sometimes unwanted consequences [114,115]. As outlined above, community dynamics are notoriously unpredictable. Moreover, there are several cases where intentionally introduced species have become invasive, with widespread negative consequences [116,117]. We highlight two important pathways by which trophic rewilding could aggravate invasion problems.
First, reintroduced or replacement species could themselves become overabundant. Despite having an ecological and evolutionary history in the recipient ecosystem, an introduced population may display novel properties: regulatory factors such as diseases or predators may no longer be operating; other novel species might support larger populations; habitat or resources may have been altered to favour the introduced populations; or slight differences between an extinct species and its analogue might interact with these factors to induce a large impact. For example, the koala (Phascolarctos cinereus) is a tree-dwelling folivore with narrow dietary preferences. Several introduced koala populations, on islands and in isolated habitats on the mainland, have become so abundant that they have defoliated trees, resulting in local koala population crashes [118]. In 1972 at Sandy Point, Victoria, twenty individuals were introduced to a reserve and began feeding on manna gums (Eucalyptus viminalis). By the mid-1980s the koalas had severely defoliated many trees and 1100 koalas were removed from a 200 ha area. Removals proved inadequate and by 1988 most of the remaining koalas had starved to death, having killed almost all the manna gums in that area [119].
Second, introduced species can facilitate other invasive populations [120]. For example, large populations of introduced rabbits and house mice (Mus musculus) in Australia can subsidise cat and fox populations, increasing predation pressure on declining species (figure 1) [121]. Effects can be indirect: Pigs (Sus scrofa) were introduced to the Channel Islands, USA in the nineteenth century. In the 1990s golden eagles (Aquila chrysaetos) colonized the islands, preying on the pigs. The pigs constituted a resource subsidy for the eagles and as a result, eagle predation almost caused extinction of an endemic fox (Urocyon littoralis). In addition, the abundance of skunks (Spilogale gracilis) increased dramatically because of release from competition with foxes [122].
11. Conclusion
Trophic rewilding can work to prevent biological invasions, mitigate their impacts and promote the coexistence of newcomer species with long-time residents. However, the conditions for success will vary from case to case. Several processes may be at play, reflected in the multiple hypotheses generated to explain invasions. Local conditions will determine which processes pertain and to what degree. This carries two implications. Firstly, that general trends may have limited application to local problems. Secondly, that local conditions and proximate causes must be understood in detail before predictions can be made about rewilding and invasions.
Predicting the outcomes of biological invasions has proven difficult [123]. Predicting the outcomes of rewilding introductions is likely to be just as demanding [124]. If scientists engaged in rewilding are to avoid criticism on this point then quantitative methods of prediction are sorely needed. Related conservation practices such as ex situ conservation and managed relocations will also need these tools. Developing predictive methods will require a multidisciplinary approach, encompassing modelling [125], life history studies [126], long-term and landscape-scale ecological experiments [127,128], palaeoecological investigation [129], climate and species distribution forecasting [128] and field trials [130] as well as input from invasion biologists.
Good predictive ability will not be enough to support decisions about rewilding. Rewilding carries risks and opportunities that range across social, legal, ethical, ecological and other domains. Many of these risks and opportunities are shared with conservation strategies such as conservation translocation and ex situ conservation [131]. Decisions about rewilding, and the development of policy and law to accommodate it, will require reflective discussion between stakeholders. It will also require the clear articulation of ethical principles [132,133].
The concepts of agency, autonomy and coexistence hold potential for guiding both rewilding and invasion biology. However, they will not generate clear and agreed rules for conduct without further theoretical work and discussion. In the meantime, they represent starting points for conversation, reflection and research, and new ground from which to view environmental problems.
Finally, there are several lessons that scientists working on rewilding problems might take from invasion biology. Some research in invasion biology is directly applicable to rewilding, for example, work on the likelihood of newly arrived species to replace the functions of missing residents, or the importance of mutualisms for establishment success [134]. Aslan et al. [130] point out that risk management procedures for the use of biological control agents could be brought to bear on rewilding proposals. More broadly, there have been several recent attempts to create a unified theoretical framework for invasion biology [38], a difficult task considering the variety of phenomena described as biological invasions. Likewise for rewilding, the plurality of rewilding practices will probably preclude a simple and unified research framework. On the other hand, rewilding will continue to encourage reflection, discussion and imaginative solutions to biological invasion problems.
Acknowledgements
We thank Tiana Pirtle, Elia Pirtle and Vishesh Diengdoh for artwork; T.P. and Calum Cunningham for comments as well as Jens-Christian Svenning, Arian Wallach and one anonymous reviewer for thoughtful suggestions.
Data accessibility
This article has no additional data.
Authors' contributions
All the authors contributed to content, drafting and critical evaluation of the manuscript.
Competing interests
We have no competing interests.
Funding
T.T.D. and C.N.J. are funded by the Australian Research Council Centre of Excellence for Biodiversity and Heritage (CABAH); T.T.D. is funded by Australian Laureate Fellowship FL160100101 (Prof. Barry Brook) and an Australian Postgraduate Award Research Training Scholarship; R.P.D is funded by grants (DP180103844; DP150101839) from the Australian Research Council. M.E.J. is funded by grants (DP110103069; LP130100949; DP170101653) and a Future Fellowship (FT100100250) from the Australian Research Council.
References
- 1.Jørgensen D. 2015. Rethinking rewilding. Geoforum 65, 482–488. ( 10.1016/j.geoforum.2014.11.016) [DOI] [Google Scholar]
- 2.Svenning JC, et al. 2016. Science for a wilder Anthropocene: synthesis and future directions for trophic rewilding research. Proc. Natl Acad. Sci. USA 113, 898–906. ( 10.1073/pnas.1502556112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormack PC. 2018. Conservation introductions for biodiversity adaptation under climate change. Transnational Environ. Law. 7, 1–23. [Google Scholar]
- 4.Vilà M, et al. 2011. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 14, 702–708. ( 10.1111/j.1461-0248.2011.01628.x) [DOI] [PubMed] [Google Scholar]
- 5.Bellard C, Cassey P, Blackburn TM. 2016. Alien species as a driver of recent extinctions. Biol. Lett. 12, 20150623 ( 10.1098/rsbl.2015.0623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellard C, Genovesi P, Jeschke J. 2016. Global patterns in threats to vertebrates by biological invasions. Proc. R. Soc. B 283, 20152454 ( 10.1098/rspb.2015.2454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson DM, Ricciardi A. 2013. Misleading criticisms of invasion science: a field guide. Divers Distrib. 19, 1461–1467. ( 10.1111/ddi.12150) [DOI] [Google Scholar]
- 8.Corlett RT. 2016. Restoration, reintroduction, and rewilding in a changing world. Trends Ecol. Evol. 31, 453–462. ( 10.1016/j.tree.2016.02.017) [DOI] [PubMed] [Google Scholar]
- 9.Callicott JB, Crowder LB, Mumford K. 1999. Current normative concepts in conservation. Cons. Biol. 13, 22–35. ( 10.1046/j.1523-1739.1999.97333.x) [DOI] [Google Scholar]
- 10.Kareiva P, Marvier M. 2012. What is conservation science? BioScience 62, 962–969. ( 10.1525/bio.2012.62.11.5) [DOI] [Google Scholar]
- 11.Donlan CJ, et al. 2006. Pleistocene rewilding: an optimistic agenda for twenty-first century conservation. Am. Nat. 168, 660–681. ( 10.2307/3873461) [DOI] [PubMed] [Google Scholar]
- 12.Sandom C, Donlan CJ, Svenning JC, Hansen D. 2013. Rewilding. Key topics in conservation biology (eds Macdonald DW, Willis KJ), vol. 2, pp. 430–451. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 13.Navarro LM, Pereira HM. 2015. Rewilding abandoned landscapes in Europe. In Rewilding European landscapes, pp. 3–23. Berlin, Germany: Springer. [Google Scholar]
- 14.Prior J, Ward KJ. 2016. Rethinking rewilding: a response to Jørgensen. Geoforum 69, 132–135. ( 10.1016/j.geoforum.2015.12.003) [DOI] [Google Scholar]
- 15.Nash R. 2001. Wilderness and the American mind. New Haven and London: Yale University Press. [Google Scholar]
- 16.Mathews F. 1991. The ecological self. London, UK: Routledge. [Google Scholar]
- 17.Plumwood V. 1993. Feminism and the mastery of nature. London, UK: Routledge. [Google Scholar]
- 18.Callicott JB. 1980. Animal liberation: a triangular affair. Environ. Ethics 2, 311–338. ( 10.5840/enviroethics19802424) [DOI] [Google Scholar]
- 19.Wallach AD, Bekoff M, Nelson MP, Ramp D. 2015. Promoting predators and compassionate conservation. Conserv. Biol. 29, 1481–1484. ( 10.1111/cobi.12525) [DOI] [PubMed] [Google Scholar]
- 20.Taylor P. 1986. Respect for nature. Princeton, NJ: Princeton University Press. [Google Scholar]
- 21.Chapron G, et al. 2014. Recovery of large carnivores in Europe's modern human-dominated landscapes. Science 346, 1517–1519. ( 10.1126/science.1257553) [DOI] [PubMed] [Google Scholar]
- 22.Boitani L, Linnell JDC. 2015. Bringing large mammals back: Large carnivores in Europe. In Rewilding European landscapes (eds Pereira H, Navarro L), pp. 67–84. Berlin, Germany: Springer International Publishing. [Google Scholar]
- 23.Carter NH, Linnell JD. 2016. Co-adaptation is key to coexisting with large carnivores. Trends Ecol. Evol. 31, 575–578. ( 10.1016/j.tree.2016.05.006) [DOI] [PubMed] [Google Scholar]
- 24.Miller TR, Minteer BA, Malan L-C. 2011. The new conservation debate: the view from practical ethics. Biol. Conserv. 144, 948–957. ( 10.1016/j.biocon.2010.04.001) [DOI] [Google Scholar]
- 25.Cronon W. 1995. Uncommon ground: toward reinventing nature. New York, NY: Norton. [Google Scholar]
- 26.Simberloff D. 2011. Non-natives: 141 scientists object. Nature 475, 36 ( 10.1038/475036a) [DOI] [PubMed] [Google Scholar]
- 27.Howarth FG. 1991. Environmental impacts of classical biological control. Annu. Rev. Entomol. 36, 485–509. ( 10.1146/annurev.en.36.010191.002413) [DOI] [Google Scholar]
- 28.Moseby KE, Blumstein DT, Letnic M. 2016. Harnessing natural selection to tackle the problem of prey naïveté. Evol. Appl. 9, 334–343. ( 10.1111/eva.12332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty TS, Ritchie EG. 2017. Stop jumping the gun: a call for evidence-based invasive predator management. Conserv. Lett. 10, 15–22. ( 10.1111/conl.12251) [DOI] [Google Scholar]
- 30.Moseby K, Peacock D, Read J. 2015. Catastrophic cat predation: a call for predator profiling in wildlife protection programs. Biol. Conserv. 191, 331–340. ( 10.1016/j.biocon.2015.07.026) [DOI] [Google Scholar]
- 31.Larson BMH. 2005. The war of the roses: demilitarizing invasion biology. Front Ecol. Environ. 3, 495–500. ( 10.1890/1540-9295(2005)0030495:TWOTRD%5D2.0.CO;2) [DOI] [Google Scholar]
- 32.Perry D, Perry G. 2008. Improving interactions between animal rights groups and conservation biologists. Cons. Biol. 22, 27–35. ( 10.1111/j.1523-1739.2007.00845.x) [DOI] [PubMed] [Google Scholar]
- 33.Schlaepfer MA, Sax DF, Olden JD. 2011. The potential conservation value of non-native species. Cons. Biol. 25, 428–437. ( 10.1111/j.1523-1739.2010.01646.x) [DOI] [PubMed] [Google Scholar]
- 34.Lundgren EJ, Ramp D, Ripple WJ, Wallach AD. 2018. Introduced megafauna are rewilding the Anthropocene. Ecography 41, 857–866. ( 10.1111/ecog.03430) [DOI] [Google Scholar]
- 35.Catford JA, Jansson R, Nilsson C. 2009. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib. 15, 22–40. ( 10.1111/j.1472-4642.2008.00521.x) [DOI] [Google Scholar]
- 36.White EM, Wilson JC, Clarke AR. 2006. Biotic indirect effects: a neglected concept in invasion biology. Divers Distrib. 12, 443–455. ( 10.1111/j.1366-9516.2006.00265.x) [DOI] [Google Scholar]
- 37.Levine JM, Adler PB, Yelenik SG. 2004. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 7, 975–989. ( 10.1111/j.1461-0248.2004.00657.x) [DOI] [Google Scholar]
- 38.Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, Wilson JR, Richardson DM. 2011. A proposed unified framework for biological invasions. Trends Ecol. Evol. 26, 333–339. ( 10.1016/j.tree.2011.03.023) [DOI] [PubMed] [Google Scholar]
- 39.Ripple WJ, et al. 2014. Status and ecological effects of the world's largest carnivores. Science 343, 1241484 ( 10.1126/science.1241484) [DOI] [PubMed] [Google Scholar]
- 40.Sergio F, Caro T, Brown D, Clucas B, Hunter J, Ketchum J, McHugh K, Hiraldo F. 2008. Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Annu. Rev. Ecol. Evol. Syst. 39, 1–19. ( 10.1146/annurev.ecolsys.39.110707.173545) [DOI] [Google Scholar]
- 41.Carbone C, Mace GM, Roberts SC, Macdonald DW. 1999. Energetic constraints on the diet of terrestrial carnivores. Nature 402, 286 ( 10.1038/46266) [DOI] [PubMed] [Google Scholar]
- 42.Gardezi T, Silva JD. 1999. Diversity in relation to body size in mammals: a comparative study. Am. Nat. 153, 110–123. ( 10.1086/303150) [DOI] [PubMed] [Google Scholar]
- 43.Beschta RL, Ripple WJ. 2016. Riparian vegetation recovery in Yellowstone: the first two decades after wolf reintroduction. Biol Conserv. 198, 93–103. ( 10.1016/j.biocon.2016.03.031) [DOI] [Google Scholar]
- 44.Kauffman MJ, Brodie JF, Jules ES. 2010. Are wolves saving Yellowstone's aspen? A landscape-level test of a behaviorally mediated trophic cascade. Ecology 91, 2742–2755. ( 10.1890/09-1949.1) [DOI] [PubMed] [Google Scholar]
- 45.David ML. 2012. Is science in danger of sanctifying the wolf? Biol. Conserv. 150, 143–149. ( 10.1016/j.biocon.2012.03.003) [DOI] [Google Scholar]
- 46.Peterson RO, Vucetich JA, Bump JM, Smith DW. 2014. Trophic cascades in a multicausal world: Isle Royale and Yellowstone. Annu. Rev. Ecol. Evol. Syst. 45, 325–345. ( 10.1146/annurev-ecolsys-120213-091634) [DOI] [Google Scholar]
- 47.Sala E. 2006. Top predators provide insurance against climate change. Trends Ecol. Evol. 21, 479–480. ( 10.1016/j.tree.2006.07.006) [DOI] [PubMed] [Google Scholar]
- 48.O'Bryan CJ, Braczkowski AR, Beyer HL, Carter NH, Watson JE. M, McDonald-Madden E. 2018. The contribution of predators and scavengers to human well-being. Nat. Ecol. Evol. 2, 229–236. ( 10.1038/s41559-017-0421-2) [DOI] [PubMed] [Google Scholar]
- 49.Palomares F, Caro TM. 1999. Interspecific killing among mammalian carnivores. Am. Nat. 153, 492–508. ( 10.1086/303189) [DOI] [PubMed] [Google Scholar]
- 50.Ritchie EG, Johnson CN. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998. ( 10.1111/j.1461-0248.2009.01347.x) [DOI] [PubMed] [Google Scholar]
- 51.Linnell JDC, Strand O. 2000. Interference interactions, co-existence and conservation of mammalian carnivores. Divers Distrib. 6, 169–176. ( 10.1046/j.1472-4642.2000.00069.x) [DOI] [Google Scholar]
- 52.Tompkins DM, Sainsbury AW, Nettleton P, Buxton D, Gurnell J. 2002. Parapoxvirus causes a deleterious disease in red squirrels associated with UK population declines. Proc. R. Soc. Lond. B 269, 529–533. ( 10.1098/rspb.2001.1897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Proulx G, et al. 2005. World distribution and status of the genus Martes in 2000. In Martens and fishers (Martes) in human-altered environments (eds Harrison DJ, Fuller AK, Proulx G), pp. 21–76. Berlin, Germany: Springer. [Google Scholar]
- 54.Croose E, Birks JDS, Schofield HW, O'Reilly C. 2014. Distribution of the pine marten (Martes martes) in southern Scotland in 2013. Scottish Natural Heritage Commissioned Report No. 740. Inverness, UK: Scottish Natural Heritage. [Google Scholar]
- 55.Davison A, Birks JD, Brookes RC, Braithwaite TC, Messenger JE. 2002. On the origin of faeces: morphological versus molecular methods for surveying rare carnivores from their scats. J. Zool. 257, 141–143. ( 10.1017/S0952836902000730) [DOI] [Google Scholar]
- 56.Sheehy E, Sutherland C, O'Reilly C, Lambin X. 2018. The enemy of my enemy is my friend: native pine marten recovery reverses the decline of the red squirrel by suppressing grey squirrel populations. Proc. R. Soc. B 285, 20172603 ( 10.1098/rspb.2017.2603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheehy E, Lawton C. 2014. Population crash in an invasive species following the recovery of a native predator: the case of the American grey squirrel and the European pine marten in Ireland. Biodivers Conserv. 23, 753–774. ( 10.1007/s10531-014-0632-7) [DOI] [Google Scholar]
- 58.Macdonald DW, Harrington LA. 2003. The American mink: the triumph and tragedy of adaptation out of context. New Zealand J. Zool. 30, 421–441. ( 10.1080/03014223.2003.9518350) [DOI] [Google Scholar]
- 59.Põdra M, Gómez A. 2018. Rapid expansion of the American mink poses a serious threat to the European mink in Spain. Mammalia ( 10.1515/mammalia-2017-0013 [DOI] [Google Scholar]
- 60.Valenzuela AE. J., Raya Rey A, Fasola L, Schiavini A. 2013. Understanding the inter-specific dynamics of two co-existing predators in the Tierra del Fuego Archipelago: the native southern river otter and the exotic American mink. Biol. Invas. 15, 645–656. ( 10.1007/s10530-012-0315-9) [DOI] [Google Scholar]
- 61.Schüttler E, Ibarra JT, Gruber B, Rozzi R, Jax K. 2010. Abundance and habitat preferences of the southernmost population of mink: implications for managing a recent island invasion. Biodivers Conserv. 19, 725–743. ( 10.1007/s10531-009-9730-3) [DOI] [Google Scholar]
- 62.Barrientos R. 2015. Adult sex-ratio distortion in the native European polecat is related to the expansion of the invasive American mink. Biol. Conserv. 186, 28–34. ( 10.1016/j.biocon.2015.02.030) [DOI] [Google Scholar]
- 63.Richards DR, Maltby L, Moggridge HL, Warren PH. 2014. European water voles in a reconnected lowland river floodplain: habitat preferences and distribution patterns following the restoration of flooding. Wetlands Ecol. Manage. 22, 539–549. ( 10.1007/s11273-014-9350-x) [DOI] [Google Scholar]
- 64.García-Díaz P, Arévalo V, Vicente R, Lizana M. 2013. The impact of the American mink (Neovison vison) on native vertebrates in mountainous streams in Central Spain. Eur. J. Wildlife Res. 59, 823–831. ( 10.1007/s10344-013-0736-5) [DOI] [Google Scholar]
- 65.Brzeziński M, Natorff M, Zalewski A, Z̈mihorski M. 2012. Numerical and behavioral responses of waterfowl to the invasive American mink: a conservation paradox. Biol. Conserv. 147, 68–78. ( 10.1016/j.biocon.2011.11.012) [DOI] [Google Scholar]
- 66.Salo P, Ahola MP, Korpimäki E. 2010. Habitat-mediated impact of alien mink predation on common frog densities in the outer archipelago of the Baltic Sea. Oecolog 163, 405–413. ( 10.1007/s00442-010-1573-9) [DOI] [PubMed] [Google Scholar]
- 67.Melero Y, Plaza M, Santulli G, Saavedra D, Gosàlbez J, Ruiz-Olmo J, Palazón S. 2012. Evaluating the effect of American mink, an alien invasive species, on the abundance of a native community: is coexistence possible? Biodivers Conserv. 21, 1795–1809. ( 10.1007/s10531-012-0277-3) [DOI] [Google Scholar]
- 68.Schüttler E, Klenke R, McGehee S, Rozzi R, Jax K. 2009. Vulnerability of ground-nesting waterbirds to predation by invasive American mink in the Cape Horn Biosphere Reserve, Chile. Biol Conserv. 142, 1450–1460. ( 10.1016/j.biocon.2009.02.013) [DOI] [Google Scholar]
- 69.Schüttler E, Cárcamo J, Rozzi R. 2008. Diet of the American mink Mustela vison and its potential impact on the native fauna of Navarino Island, Cape Horn Biosphere Reserve, Chile. Rev. Chil. Hist. Nat. 81, 585–598. ( 10.4067/S0716-078X2008000400011) [DOI] [Google Scholar]
- 70.Chanin P, Jefferies D. 1978. The decline of the otter Lutra lutra L. in Britain: an analysis of hunting records and discussion of causes. Biol. J. Linn. Soc. 10, 305–328. ( 10.1111/j.1095-8312.1978.tb00018.x) [DOI] [Google Scholar]
- 71.Bonesi L, Chanin P, Macdonald DW. 2004. Competition between Eurasian otter Lutra lutra and American mink Mustela vison probed by niche shift. Oikos 106, 19–26. ( 10.1111/j.0030-1299.2004.12763.x) [DOI] [Google Scholar]
- 72.Bonesi L, Macdonald DW. 2004. Impact of released Eurasian otters on a population of American mink: a test using an experimental approach. Oikos 106, 9–18. ( 10.1111/j.0030-1299.2004.13138.x) [DOI] [Google Scholar]
- 73.Medina-Vogel G, Barros M, Organ JF, Bonesi L. 2013. Coexistence between the southern river otter and the alien invasive North American mink in marine habitats of southern Chile. J. Zool. 290, 27–34. ( 10.1111/jzo.12010) [DOI] [Google Scholar]
- 74.Woinarski JC, Burbidge AA, Harrison PL. 2015. Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proc. Natl Acad. Sci. USA 112, 4531–4540. ( 10.1073/pnas.1417301112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson CN. 2006. Australia's mammal extinctions: a 50,000-year history. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 76.Johnson CN, VanDerWal J. 2009. Evidence that dingoes limit abundance of a mesopredator in eastern Australian forests. J. Appl. Ecol. 46, 641–646. ( 10.1111/j.1365-2664.2009.01650.x) [DOI] [Google Scholar]
- 77.Johnson CN, Isaac JL, Fisher DO. 2007. Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proc. R. Soc. B 274, 341–346. ( 10.1098/rspb.2006.3711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fleming P, Corbett L, Harden R, Thomson P. 2001. Managing the impacts of dingoes and other wild dogs. Canberra, Bureau of Rural Sciences. [Google Scholar]
- 79.Johnson CN. 2009. Ecological consequences of Late Quaternary extinctions of megafauna. Proc. R. Soc. B 276, 2509–2519. ( 10.1098/rspb.2008.1921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Letnic M, Koch F, Gordon C, Crowther MS, Dickman CR. 2009. Keystone effects of an alien top-predator stem extinctions of native mammals. Proc. R. Soc. B 276, 3249–3256. ( 10.1098/rspb.2009.0574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wallach AD, Johnson CN, Ritchie EG, O'Neill AJ. 2010. Predator control promotes invasive dominated ecological states. Ecol. Lett. 13, 1008–1018. [DOI] [PubMed] [Google Scholar]
- 82.Colman NJ, Gordon CE, Crowther MS, Letnic M. 2014. Lethal control of an apex predator has unintended cascading effects on forest mammal assemblages. Proc. R. Soc. B 281, 20133094 ( 10.1098/rspb.2013.3094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Visser RL, Watson JE, Dickman CR, Southgate R, Jenkins D, Johnson CN. 2009. A national framework for research on trophic regulation by the Dingo in Australia. Pacific Cons. Biol. 15, 209–216. ( 10.1071/PC090209) [DOI] [Google Scholar]
- 84.Oksanen L, Oksanen T. 2000. The logic and realism of the hypothesis of exploitation ecosystems. Am. Nat. 155, 703–723. ( 10.1086/303354) [DOI] [PubMed] [Google Scholar]
- 85.Newsome TM, et al. 2015. Resolving the value of the dingo in ecological restoration. Restor. Ecol. 23, 201–208. ( 10.1111/rec.12186) [DOI] [Google Scholar]
- 86.Hunter DO, Britz T, Jones M, Letnic M. 2015. Reintroduction of Tasmanian devils to mainland Australia can restore top-down control in ecosystems where dingoes have been extirpated. Biol. Conserv. 191, 428–435. ( 10.1016/j.biocon.2015.07.030) [DOI] [Google Scholar]
- 87.Smith B, Savolainen P. 2015. The origin and ancestry of the dingo. In The dingo debate: origins, behaviour and conservation (ed. Smith B.), pp. 55–80. Australia: CSIRO Publishing. [Google Scholar]
- 88.White LC, Saltré F, Bradshaw CJA, Austin JJ. 2018. High-quality fossil dates support a synchronous, Late Holocene extinction of devils and thylacines in mainland Australia. Bio. Lett. 14, 20170642 ( 10.1098/rsbl.2017.0642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brüniche–Olsen A, Jones ME, Burridge CP, Murchison EP, Holland BR, Austin JJ. 2018. Ancient DNA tracks the mainland extinction and island survival of the Tasmanian devil. J. Biogeogr. 45, 963–976. ( 10.1111/jbi.13214) [DOI] [Google Scholar]
- 90.Prowse TAA, Johnson CN, Bradshaw CJA, Brook BW. 2014. An ecological regime shift resulting from disrupted predator–prey interactions in Holocene Australia. Ecology 95, 693–702. ( 10.1890/13-0746.1) [DOI] [PubMed] [Google Scholar]
- 91.Prowse TA, Johnson CN, Lacy RC, Bradshaw CJ, Pollak JP, Watts MJ, Brook BW. 2013. No need for disease: testing extinction hypotheses for the thylacine using multi-species metamodels. J. Anim. Ecol. 82, 355–364. ( 10.1111/1365-2656.12029) [DOI] [PubMed] [Google Scholar]
- 92.Jones ME, et al. 2007. Conservation management of Tasmanian devils in the context of an emerging, extinction-threatening disease: devil facial tumor disease. EcoHealth 4, 326–337. ( 10.1007/s10393-007-0120-6) [DOI] [Google Scholar]
- 93.Lazenby BT, Dickman CR. 2013. Patterns of detection and capture are associated with cohabiting predators and prey. PLoS ONE 8, e5984 ( 10.1371/journal.pone.0059846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hollings T, Jones M, Mooney N, McCallum H. 2014. Trophic cascades following the disease-induced decline of an apex predator, the Tasmanian devil. Cons Biol 28, 63–75. ( 10.1111/cobi.12152) [DOI] [PubMed] [Google Scholar]
- 95.Fancourt BA, Hawkins CE, Cameron EZ, Jones ME, Nicol SC. 2015. Devil declines and catastrophic cascades: is mesopredator release of feral cats inhibiting recovery of the eastern quoll? PLoS ONE 10, e0119303 ( 10.1371/journal.pone.0119303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hollings T, Jones M, Mooney N, McCallum H. 2016. Disease-induced decline of an apex predator drives invasive dominated states and threatens biodiversity. Ecology 97, 394–405. ( 10.1890/15-0204.1) [DOI] [PubMed] [Google Scholar]
- 97.Fancourt BA. 2016. Avoiding the subject: the implications of avoidance behaviour for detecting predators. Behav. Ecol. Sociobiol. 70, 1535–1546. ( 10.1007/s00265-016-2162-7) [DOI] [Google Scholar]
- 98.Hunter DO, Britz T, Jones M, Letnic M. 2016. Reintroduction of Tasmanian devils to mainland Australia can restore top-down control in ecosystems where dingoes have been extirpated: a response to Baker et al. 2016 and Fancourt & Mooney 2016. Biol. Conserv. 196, 20–21. ( 10.1016/j.biocon.2016.01.021) [DOI] [Google Scholar]
- 99.Baker CM, Bode M, McCarthy MA. 2016. Models that predict ecosystem impacts of reintroductions should consider uncertainty and distinguish between direct and indirect effects. Biol. Conserv. 196, 211–212. ( 10.1016/j.biocon.2016.01.023) [DOI] [Google Scholar]
- 100.Ripple WJ, et al. 2015. Collapse of the world's largest herbivores. Sci. Adv. 1 ( 10.1126/sciadv.1400103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doughty CE, Wolf A, Malhi Y. 2013. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat. Geosci. 6, 761 ( 10.1038/ngeo1895) [DOI] [Google Scholar]
- 102.Vidal MM, Pires MM, Guimarães PR Jr. 2013. Large vertebrates as the missing components of seed-dispersal networks. Biol. Conserv. 163, 42–48. ( 10.1016/j.biocon.2013.03.025) [DOI] [Google Scholar]
- 103.Woodward G, Ebenman B, Emmerson M, Montoya JM, Olesen JM, Valido A, Warren PH. 2005. Body size in ecological networks. Trends Ecol. Evol. 20, 402–409. ( 10.1016/j.tree.2005.04.005) [DOI] [PubMed] [Google Scholar]
- 104.Guimarães PR Jr, Galetti M, Jordano P. 2008. Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS ONE 3, e1745 ( 10.1371/journal.pone.0001745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Owen-Smith RN. 1988. Megaherbivores: the influence of very large body size on ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 106.Steuer P, Südekum KH, Tütken T, Müller DW. H., Kaandorp J, Bucher M, Clauss M, Hummel J, McArthur C. 2014. Does body mass convey a digestive advantage for large herbivores? Funct. Ecol. 28, 1127–1134. ( 10.1111/1365-2435.12275) [DOI] [Google Scholar]
- 107.Parker JD, Burkepile DE, Hay ME. 2006. Opposing effects of native and exotic herbivores on plant invasions. Science 311, 1459–1461. ( 10.1126/science.1121407) [DOI] [PubMed] [Google Scholar]
- 108.Griffiths CJ, Zuel N, Jones CG, Ahamud Z, Harris S. 2013. Assessing the potential to restore historic grazing ecosystems with tortoise ecological replacements. Conserv. Biol. 27, 690–700. ( 10.1111/cobi.12087) [DOI] [PubMed] [Google Scholar]
- 109.Marty JT. 2005. Effects of cattle grazing on diversity in ephemeral wetlands. Conserv. Biol. 19, 1626–1632. ( 10.1111/j.1523-1739.2005.00198.x) [DOI] [Google Scholar]
- 110.Johnson BE, Cushman JH. 2007. Influence of a large herbivore reintroduction on plant invasions and community composition in a California grassland. Conserv. Biol. 21, 515–526. ( 10.1111/j.1523-1739.2006.00610.x) [DOI] [PubMed] [Google Scholar]
- 111.Zavaleta E, Kettley L. 2006. Ecosystem change along a woody invasion chronosequence in a California grassland. J. Arid. Environ. 66, 290–306. ( 10.1016/j.jaridenv.2005.11.008) [DOI] [Google Scholar]
- 112.Ender CL, Christian CE, Cushman JH. 2017. Native herbivores and environmental heterogeneity as mediators of an exotic grass invasion. Ecol. Evol. 7, 1561–1571. ( 10.1002/ece3.2727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stahlheber KA, D'Antonio CM. 2013. Using livestock to manage plant composition: a meta-analysis of grazing in California Mediterranean grasslands. Biol. Conserv. 157, 300–308. ( 10.1016/j.biocon.2012.09.008) [DOI] [Google Scholar]
- 114.Ruscoe WA, et al. 2011. Unexpected consequences of control: competitive vs. predator release in a four-species assemblage of invasive mammals. Ecol. Lett. 14, 1035–1042. ( 10.1111/j.1461-0248.2011.01673.x) [DOI] [PubMed] [Google Scholar]
- 115.Zavaleta ES, Hobbs RJ, Mooney HA. 2001. Viewing invasive species removal in a whole-ecosystem context. Trends Ecol. Evol. 16, 454–459. ( 10.1016/S0169-5347(01)02194-2) [DOI] [Google Scholar]
- 116.Ricciardi A, Simberloff D. 2009. Assisted colonization is not a viable conservation strategy. Trends Ecol. Evol. 24, 248–253. ( 10.1016/j.tree.2008.12.006) [DOI] [PubMed] [Google Scholar]
- 117.Simberloff D, Stiling P. 1996. How risky is biological control? Ecology 77, 1965–1974. ( 10.2307/2265693) [DOI] [Google Scholar]
- 118.Martin RW. 1985. Overbrowsing, and decline of a population of the koala, Phascolarctos cinereus, in Victoria. I. Food preference and food tree defoliation. Wildlife Res. 12, 355–365. ( 10.1071/WR9850355) [DOI] [Google Scholar]
- 119.Martin R, Handasyde KA. 1999. The koala: natural history, conservation and management, Second edition. Sydney, Australia: UNSW Press. [Google Scholar]
- 120.Simberloff D. 2006. Invasional meltdown 6 years later: important phenomenon, unfortunate metaphor, or both? Ecol. Lett. 9, 912–919. ( 10.1111/j.1461-0248.2006.00939.x) [DOI] [PubMed] [Google Scholar]
- 121.Smith AP, Quin D. 1996. Patterns and causes of extinction and decline in Australian conilurine rodents. Biol. Conserv. 77, 243–267. ( 10.1016/0006-3207(96)00002-X) [DOI] [Google Scholar]
- 122.Roemer GW, Donlan CJ, Courchamp F. 2002. Golden eagles, feral pigs, and insular carnivores: how exotic species turn native predators into prey. Proc. Natl. Acad. Sci. USA 99, 791–796. ( 10.1073/pnas.012422499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kueffer C, Pyšek P, Richardson DM. 2013. Integrative invasion science: model systems, multi-site studies, focused meta-analysis and invasion syndromes. New Phytol. 200, 615–633. ( 10.1111/nph.12415) [DOI] [PubMed] [Google Scholar]
- 124.Nogues-Bravo D, Simberloff D, Rahbek C, Sanders NJ. 2016. Rewilding is the new Pandora's box in conservation. Curr. Biol. 26, R87–R91. ( 10.1016/j.cub.2015.12.044) [DOI] [PubMed] [Google Scholar]
- 125.Baker CM, Gordon A, Bode M. 2017. Ensemble ecosystem modeling for predicting ecosystem response to predator reintroduction. Cons. Biol. 31, 376–384. ( 10.1111/cobi.12798) [DOI] [PubMed] [Google Scholar]
- 126.Tschinkel WR, Wilson EO. 2014. Scientific natural history: telling the epics of nature. BioScience 64, 438–443. ( 10.1093/biosci/biu033) [DOI] [Google Scholar]
- 127.Ripple WJ, Beschta RL. 2012. Trophic cascades in Yellowstone: the first 15 years after wolf reintroduction. Biol. Conserv. 145, 205–213. ( 10.1016/j.biocon.2011.11.005) [DOI] [Google Scholar]
- 128.Root-Bernstein M, Svenning J.-C. 2016. Prospects for rewilding with camelids. J. Arid Environ. 130, 54–61. ( 10.1016/j.jaridenv.2016.03.011) [DOI] [Google Scholar]
- 129.Fordham DA, Akçakaya HR, Alroy J, Saltré F, Wigley TM, Brook BW. 2016. Predicting and mitigating future biodiversity loss using long-term ecological proxies. Nat. Clim. Change 6, 909 ( 10.1038/nclimate3086) [DOI] [Google Scholar]
- 130.Aslan CE, Aslan A, Croll D, Tershy B, Zavaleta E. 2014. Building taxon substitution guidelines on a biological control foundation. Restor Ecol. 22, 437–441. ( 10.1111/rec.12096) [DOI] [Google Scholar]
- 131.Kreyling J, Bittner T, Jaeschke A, Jentsch A, Jonas Steinbauer M, Thiel D, Beierkuhnlein C. 2011. Assisted colonization: a question of focal units and recipient localities. Restor Ecol. 19, 433–440. ( 10.1111/j.1526-100X.2011.00777.x) [DOI] [Google Scholar]
- 132.Schwartz MW, et al. 2012. Managed relocation: integrating the scientific, regulatory, and ethical challenges. BioScience 62, 732–743. ( 10.1525/bio.2012.62.8.6) [DOI] [Google Scholar]
- 133.Richardson DM, et al. 2009. Multidimensional evaluation of managed relocation. Proc. Natl Acad. Sci. USA 106, 9721–9724. ( 10.1073/pnas.0902327106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Traveset A, Richardson DM. 2011. Mutualisms: key drivers of invasions… key casualties of invasions. In Fifty years of invasion ecology: the legacy of Charles Elton (ed. DM Richardson.), pp. 143–160. Chichester, UK: Wiley-Blackwell. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.