Abstract
The loss of megafauna at the terminal Pleistocene has been linked to a wide range of Earth-system-level changes, such as altered greenhouse gas budgets, fire regimes and biome-level vegetation changes. Given these influences and feedbacks, might part of the solution for mitigating anthropogenic climate change lie in the restoration of extant megafauna to ecosystems? Here, we explore the potential role of trophic rewilding on Earth's climate system. We first provide a novel synthesis of the various ways that megafauna interact with the major drivers of anthropogenic climate change, including greenhouse gas storage and emission, aerosols and albedo. We then explore the role of rewilding as a mitigation tool at two scales: (i) current and near-future opportunities for national or regional climate change mitigation portfolios, and (ii) more radical opportunities at the global scale. Finally, we identify major knowledge gaps that complicate the complete characterization of rewilding as a climate change mitigation strategy. Our perspective is urgent since we are losing the Earth's last remaining megafauna, and with it a potential option to address climate change.
This article is part of the theme issue ‘Trophic rewilding: consequences for ecosystems under global change’.
Keywords: megafauna, ecosystem function, Earth system, megaherbivores, conservation, large herbivores and carnivores
1. Introduction
The ongoing collapse of the world's remaining large mammalian consumers [1] is probably leading to restructuring of Earth's ecosystems [2]. Recent work on the legacy effects of the megafaunal extinctions at the terminal Pleistocene (approximately 13 ka) suggests these resulted in significant Earth-system-level consequences, including climate warming [3], reductions in the availability and transport of nutrients [4], large-scale vegetation shifts [5], reduced carbon sequestration [6] and changes in the global methane budget [7]. If the loss of Late Pleistocene megafauna had such measurable consequences for the Earth's climate system, it is tempting to speculate whether the conservation and restoration of existing megafauna could contribute towards mitigating ongoing anthropogenic climate changes. This adds a new dimension to the original idea of rewilding: ‘the restoration of missing ecological functions and evolutionary potential of lost megafauna’ (sensu Donlan et al. [8]). While these original ideas focused strictly on biodiversity conservation, here we explore the influence of megafauna on the Earth's climate system and then explore the potential role of rewilding as a climate change (CC) mitigation tool. Our focus is on trophic rewilding (sensu Svenning et al. [9]; rather than Pleistocene rewilding, i.e. ‘returning elephants to North America’) and the restoration of recently lost or diminished megafaunal populations [10] to restore top-down trophic interactions and associated trophic cascades.
2. Mechanisms through which megafauna may affect climate
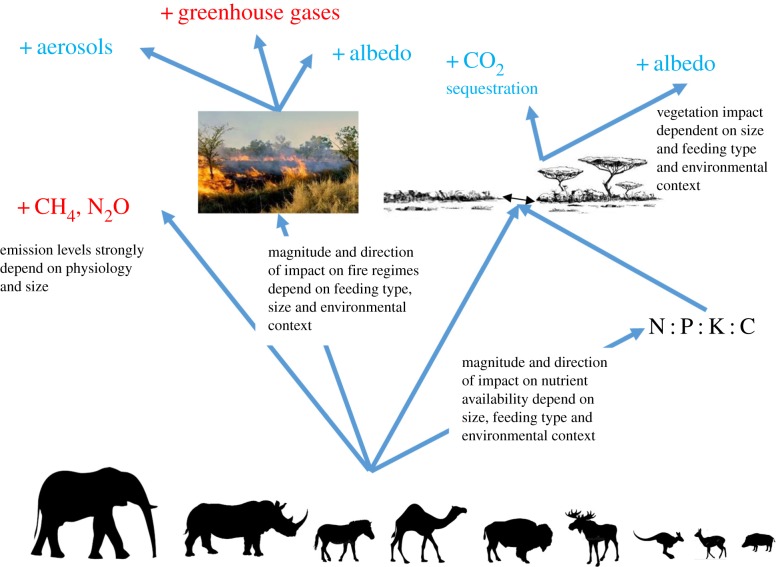
The commonly recognized drivers of anthropogenic CC include emission of greenhouse gases (e.g. CO2, CH4, N2O, halocarbons), increased production of aerosols (mineral dust, sulfate, nitrate, organic carbon and black carbon), and changes in albedo due to alterations of land cover (figure 1). We discuss how herbivory may influence these drivers through four main pathways: (i) emission of greenhouse gases, (ii) impacts on fire regimes, (iii) effects on nutrient cycling and transport, and (iv) direct impacts on vegetation and soil (figure 2). We also discuss the role of herbivore functional traits, such as digestive physiology, body mass and feeding type, in these pathways.
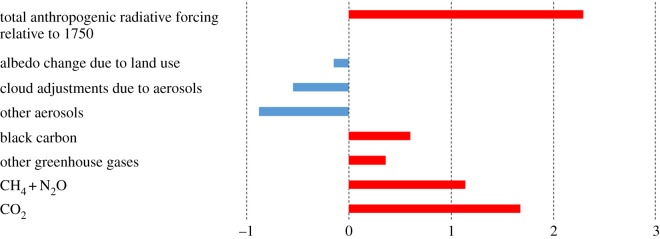
Figure 1.
Breakdown of contribution of different drivers of anthropogenic CC as global average radiative forcing (W m−2) estimates relative to the year 1750. The top bar gives the total anthropogenic radiative forcing estimate. Radiative forcing is a common currency used to estimate the contribution to global warming of diverse drivers and reflects the difference between energy from the sun absorbed by the Earth and energy radiated back to space. A positive forcing thus reflects a positive effect on global warming. (Data from [11]).
Figure 2.
Simplified scheme of the different mechanisms through which megafauna may influence drives of anthropogenic CC.
(a). Herbivores and greenhouse gas emissions
The anaerobic microbial fermentation of plant materials in the rumen, colon or caecum by herbivores results in the production of several greenhouse gases. The most important of these is methane. Although approximately 200 times less abundant than atmospheric CO2, methane's greater efficiency in trapping radiation, and its reactions with other trace gases, leads to a significant role in the radiative forcing of climate ([11]; figure 1). Today, domestic mammals are a major contributor of methane to the global budget [11], but wild mammals were a major source prior to the Late Pleistocene megafaunal extinction [7] and the historical collapse of large herds of wild ungulates in Africa and North America [12]. Methane production by mammalian herbivores is mediated by their digestive physiology. Ruminants, such as bovids and cervids, produce much more methane than hindgut fermenters, such as equids and rhinoceroses, or non-ruminant foregut-fermenters, such as macropods and sloths. Camelids, with a digestive system similar to that of ruminants, emit much less methane than similar-sized ruminants because of lower relative food intake [13]. Physiology also interacts with body mass. Ruminants produce disproportionately more methane from enteric fermentation as they increase in body size, while hindgut (and non-ruminant foregut) fermenters produce relatively less. This means that a single 100 kg ruminant releases 16.2 kg CH4 yr−1, whereas two 50 kg ruminants collectively produce 15.5 kg CH4 yr−1 [14]. In contrast, a 100 kg hindgut fermenter releases only 2.4 kg CH4 yr−1.
The effects of digestive physiology and body mass on methane emissions have clear implications for rewilding. Historically, the largest herbivorous wild mammals in ecosystems were hindgut fermenters [15,16]. Their replacement by domestic ruminants probably led to an increase in methane emissions. Indeed, Hempson et al. [17] estimated that the replacement of wild ungulates by livestock in Africa more than doubled methane emissions from 3.4 to 8.9 Tg yr−1. How megafaunal restoration might influence methane output depends on whether a reintroduction is accompanied by a concomitant reduction of livestock. Replacing domestic livestock by an equivalent biomass of hindgut megaherbivores reduces overall methane emissions, as does, to a lesser extent, replacing cattle with a similar biomass of smaller wild ruminant species.
(b). Herbivores and fire
Herbivores have an important and complicated influence on fire regimes, which is influenced by feeding type [18]. Grazers can reduce fire frequency and intensity by lowering the amount of grass fuel or by favouring less flammable grass species [18]. The effect of grazing on fire regimes is mediated by herbivore body mass and environmental conditions. In Hluhluwe-iMfolozi Park, South Africa, only the largest grazers, white rhinoceros, shaped the grass layer and fire regimes under high rainfall conditions [19]. In contrast, browsers may actually promote fires by increasing fuel in the form of woody debris or by opening up woody vegetation and reducing woody plant recruitment, thus promoting flammable grasses [18]. Unfortunately, empirical studies on the impact of browsers on fires are largely absent, as are studies on the effect of herbivores on forest fires. In such systems, intense browsing may reduce fuel in the form of, for example, litter [20]. However, browsing may also shift broadleaved communities towards more flammable conifers, promoting fires [21]. Several regional palaeo-ecological studies have linked the loss of Pleistocene megafauna with increased fire at regional to continental scales [5]. For extant systems, savannah fire prevalence across the African continent declines with grazer biomass, particularly at high levels (greater than 1500 kg km−2) and in regions with less than 1000 mm rain annually [17].
By changing fire regimes, herbivores can influence climate in a number of ways. First, biomass burning releases greenhouse gases. Such emissions have increased over the past century particularly in (sub)tropical systems, promoting warming [22]. Second, fire-emitted aerosols can lead to both warming and cooling: (i) black carbon deposits on snow and ice darken surfaces and enhance snow melt (warming effect), (ii) in the atmosphere black carbon absorbs radiation (warming effect) while sulfates, organic carbon and nitrates reflect radiation (cooling effect), and (iii) aerosols affect cloud formation and cloud properties (cooling effect) [23]. Finally, fire directly changes surface albedo. In the short term, post-fire land surfaces have a strongly reduced albedo (warming effect) due to the dark charcoal, but in the long run, fire may increase albedo (cooling effect) if the opening of dark forests leads to greater snow exposure or to an increase in more reflective grassy systems [24]. Although certain recent studies conclude that the net radiative forcing effect of all fire–climate feedbacks has a net cooling effect at global scales [25], others maintain that strong negative consequences of frequent fires on ecosystem carbon storage make the net effect of wildfires on climate remain uncertain [26].
Through strong effects on fire regimes, rewilding, particularly with the largest grazers, could have material consequences for the effects of fire regimes on CC drivers. However, since the net effect of wildfires on climate remains uncertain, so does the exact potential of rewilding. This uncertainty is exacerbated by ignorance of the effect of browsers on fire regimes, particularly in forests.
(c). Herbivores and nutrients
Mammalian herbivores alter the availability of nutrients by influencing cycling rates, transport, stoichiometry and, indirectly, fire regimes.
(i). Nutrient cycling rates
In the short term, both browsing and grazing accelerate nutrient cycling by adding nutrients to soil in a readily decomposable form (dung and urine), stimulating microbial activity [27]. In the long term, herbivory may alter plant community structure and composition, and thus the litter available to decomposers [27]. Until recently, it was thought that generalist grazers accelerate cycling by promoting the proportion of palatable species, while selective browsers slow cycling by removing high-quality plants and plant parts and shifting the plant community towards unpalatable species with low litter quality [27]. This view, however, is increasingly questioned [28], with examples of browsers accelerating and grazers decelerating cycling [29]. Instead, contrasts in accelerating and decelerating cycles are now explained by effects of herbivore trampling on soil properties [29] and by whether plant communities are dominated by traits that enhance tolerance (leading to accelerating cycles) or resistance (decelerating) to herbivory [30]. Whether plant communities are dominated by tolerance or resistance traits can in part be explained by the underlying productivity of the system where tolerance traits are more likely to dominate in resource-rich environments [28].
(ii). Nutrient transport
Large mammals alter nutrient availability through the lateral transport of nutrients against gradients of accumulation. For example, wide-ranging large mammals can move nutrients into continental interiors [31] and deep-diving whales can transport nutrients back up to ocean surface layers [4]. They may also transport nutrients across terrestrial and aquatic boundaries, such as hippopotamuses that feed in terrestrial grasslands yet defecate in waterbodies [4]. The magnitude of lateral nutrient transport depends on both body mass and the social system: large-bodied species and migratory animals have the strongest impact. This has changed with the loss of biodiversity at the terminal Pleistocene; Doughty et al. [4] estimated that modern systems retain less than 10% of the pre-extinction nutrient transport capacity. However, empirical data on contemporary megafaunal nutrient transport are scarce.
(iii). Nutrient stoichiometry
Herbivores may shape N : P stoichiometry thereby affecting plant growth potential [32]. This stoichiometric effect varies with feeding type and body mass. Since dung/urine N : P is positively related to food N : P, megafauna species with N-rich diets (such as green grass) are predicted to distribute disproportionately more N through their excreta [32]. In contrast, dung/urine N : P is hypothesized to be positively related to body mass because larger-bodied animals need to invest more P in their disproportionately large skeletons [32]. Thus the prediction is that larger mammals, and grazers focusing on fresh grass, would transport relatively more N, leading to P limitation, while small species, and browsers that consume N-poor plant items, would transport more P and lead to N limitation. Few studies have tested these patterns for mammalian herbivores (but see [33]).
(iv). Nutrients and fire
Herbivores may conserve soil nutrient pools by suppressing wildfires, since significant amounts of C, N and P may be lost from local ecosystems through short and long-term effects of fires [26].
The implications for CC mitigation of herbivore–nutrient interactions are indirect. By affecting nutrient availability and stoichiometry, herbivores may influence plant productivity positively or negatively and thus the potential for C sequestration by plants. Recent simulation studies suggest that carbon sequestration projections may be greatly overestimated if stoichiometric constraints on carbon cycling are not considered. For example, Goll et al. [34] show that simulations of terrestrial carbon cycle models that include N and P limitation predict 25% less carbon uptake compared with simulations where nutrient limitation is omitted. Rewilding focused on restoring a mix of large and small species of different feeding types may facilitate lateral nutrient transport and maintain stoichiometric ratios that are optimal for plant growth. However, this synthesis has highlighted that herbivores may increase or decrease ecosystem and plant productivity, and thus carbon storage, depending on their stoichiometric effects and whether they have accelerating or decelerating effects on nutrient cycling. This in turn depends on a complex set of interactions between herbivore traits, system productivity and environmental drivers (e.g. rainfall).
(d). Direct effects of herbivores on vegetation
Mammalian herbivores have strong direct effects on vegetation structure and composition. This is particularly true of megaherbivores (≥1000 kg) because their very large size allows them to use even the lowest quality vegetation and to escape population control by predation [15]. Elephant and black rhinoceros may significantly reduce the woody component at a landscape scale [35,36]. Megagrazers, such as white rhinoceros and hippopotamus, can convert tall, caespitose grasslands into short, prostrate-growing, grazing lawns [37] that have very limited woody recruitment [38]. Through similar mechanisms, mesograzers may also indirectly limit woody encroachment but only at high densities and/or in less productive environments [19]. However, intense grazing by mesograzers can also promote the encroachment of woody species by reducing competition within the grass layer [39]. Mesobrowsers, on the other hand, may strongly limit woody recruitment through browsing on seedlings and saplings [40]. In addition, through selective foraging [41] and seed dispersal (box 1), herbivores shape plant species composition. Functional complementarity is crucial; for example Asian tapirs cannot replace Asian elephants and rhinoceroses as dispersers of the largest seeds [53]. Finally, mammalian herbivores shape vegetation not only through foraging but also through physical impacts, such as elephants toppling trees [54] and reindeer trampling reducing shrub cover [55].
Box 1. Megafauna, seed dispersal and carbon sequestration.
Many of the tree species in the world's tropical rainforests bear large, fleshy fruits that depend on dispersal by large mammals, so-called megafaunal fruits [42]. For long, it was unclear why many Neotropical trees have such large fruits until Janzen & Martin [43] suggested that they used to be dispersed by megafrugivores such as the extinct Pleistocene gomphotheres. The loss of these megafaunal dispersers strongly reduced long-distance seed dispersal [44], tree recruitment [45] and geographical ranges of many tree species [6]. Moreover, because tree species with megafaunal fruits have a higher wood density and become taller, modelling studies indicate that the megafaunal loss resulted in a significant drop in the carbon storage potential of Neotropical rainforests [6,46]. The rapid disappearance of the last remaining megafrugivores, such as African and Asian forest elephants [47,48] and American tapirs [49], will have major consequences for recruitment of hardwood species [50]. According to a recent simulation, this loss of megafrugivores would lead to carbon losses as large as 2–12% in the majority of the world's tropical forests [51], and more diverse megafaunal communities are associated with increased carbon storage [52]. Large-scale megafrugivore rewilding programmes in these forests should therefore be seen, and financed, as carbon sequestration programmes that, in the long run, may be more effective than tree planting schemes [46].
Herbivore-driven shifts in vegetation structure and species composition lead to different biogeophysical and biogeochemical feedbacks on local to regional climates. Shifts in tree–grass ratios may influence carbon stocks and albedo (box 2). Whereas an increase in woody plants generally reduces albedo, it may lead to increased carbon storage under some conditions, but to carbon losses under others [64]. The net radiative forcing of such shifts remains unclear and probably varies across the world. Shifts in plant species composition (e.g. change in the proportion of hardwood species) may also affect carbon stocks (box 1). Effects of rewilding on CC mitigation are thus probably strongly context-dependent and will vary among systems.
Box 2. Cooling the tundra through reindeer grazing?
In recent decades the northern parts of Eurasia and North America have experienced a thickening of woody vegetation due to warming summers [56], which induces further warming through reducing winter and summer albedo [57]. Importantly, in these regions, the warming from reduced albedo overrides cooling from increased carbon sequestration by encroaching shrubs [58]. Several studies have shown that reindeer limit woody encroachment of tundra ecosystems [59] and thus counteract the negative effect of shrubs on albedo, both during the period of snow-melt [60] and during the snow-free period by maintaining graminoid vegetation [61]. This suggests that reindeer, by controlling the woody encroachment of tundra, limit further warming in these regions [60,61]. Similarly, the Late Pleistocene extinction of mammoths has been associated with the replacement of the grassy mammoth steppe, once the Earth's most extensive biome, by shrub tundra leading to regional warming due to reduced albedo [3]. Studies by Zimov et al. in a rewilding project in the Russian Arctic suggest even more far-reaching effects. Trampling by abundant herds of species such as Przewalski's horse, reindeer and muskox reduces insulation by snow in the winter, leading to increased freezing of permafrost [62]. They suggest that large-scale rewilding of the far north may be a particularly effective strategy to mitigate the effects of woody encroachment and loss of methane due to thawing permafrost [63].
(e). Direct effects of herbivores on soil
Through their foraging and physical effects (trampling), mammalian herbivores can shape soils across large scales and the extent of trampling increases with body mass [65]. Intense herbivory may lead to erosion and desertification, with examples worldwide, although mostly from livestock and not wild ungulate systems [39]. Trampling may also increase soil compaction, which reduces oxygen in wet soils, and water availability in dry soils, leading to reduced mineralization and nutrient cycling rates [29]. This may erode soil carbon stocks [39]. Large-scale erosion will also influence the biophysical feedbacks on climate through increasing or reducing albedo, depending on soil colour. However, because hooves loosen the soil in combination with the addition of dung and mulch, high-intensity short- duration grazing may also increase carbon sequestration in soils, and restore the grass layer in degraded rangelands [66]. This positive effect on soil carbon, and general soil fertility, has been linked to large mobile herds of mesograzers having strong, localized impacts for a few days a year as they move around extensive landscapes [67]. Trampling may also promote nutrient cycling, particularly at high latitudes, by removing the insulating effect of vegetation and increasing soil temperature [55]. However, such effects of trampling may be season-dependent, particularly in the far north, where trampling may reduce snow cover and its insulating effects, leading to reduced soil temperatures and thawing of permafrost, increasing the carbon storage capacity [63]. Whether herbivore-mediated soil impacts have positive or negative effects on CC mitigation thus depends on herbivore characteristics (population density, mobility of herds and herbivore body mass) and environmental and seasonal context.
3. The importance of functional diversity
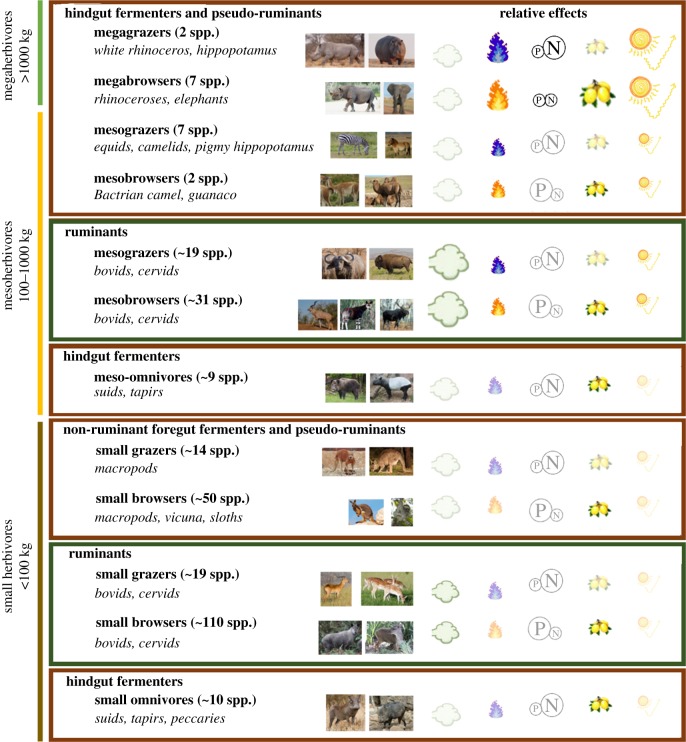
We have highlighted the important role of diversity in body mass, digestive physiology and feeding guild in determining the effects of herbivores on the climate system. Based on these traits, we identify at least 12 different functional groups (figure 3). For example, a mix of differently sized browsers ensures dispersal of both the smaller and largest tree seeds while a balance in the grazer–browser community may be crucial for avoiding N or P limitation. What figure 3 highlights is that the largest, non-ruminating herbivores play pivotal roles; it is these species that have the strongest per capita potential to shape CC drivers through effects on nutrient transport, fire regimes and landscape-scale vegetation changes, while having the lowest methane emissions. If we lose those functional groups, opportunities for megafauna to influence the Earth-climate system will be lost. Humans, however, have downgraded the body size distribution of mammal communities for at least the past 100 000 years and, if the current trends continue, only the smaller herbivore groups will remain [68]. The downgrading of body size has also resulted in a disproportionate loss of non-ruminant species, since the largest herbivorous mammals are hindgut fermenters [16]. Currently, large-bodied non-ruminant herbivores constitute 18 of the roughly 300 remaining megafaunal herbivores, while 179 are meso-ruminants (figure 3). Hence, to conserve the broadest possible diversity in herbivore functionality, rewilding should focus on the largest, non-ruminant species. Some of these may need special attention. For example, the white rhinoceros is the only remaining megagrazer with impacts across the landscape (effects of hippopotamus are limited to relatively narrow bands near waterbodies; figure 3). However, because of the current white rhinoceros poaching crisis, the world may soon lose its last, functionally relevant, megagrazer [69]. Some other, less known, key species of large non-ruminant ungulates that are currently (critically) endangered include the Bactrian camel, Przewalski's horse, African wild ass and kulan [70].
Figure 3.
Overview of different functional groupings of megafaunal herbivores based on body mass, digestive physiology and feeding type. For simplification reasons the browser category includes mixed-feeders. Giraffe has been included as mesobrowser. The symbols show, qualitatively, the relative per capita contribution of each functional group to the five drivers of anthropogenic CC; methane emission (cloud), fire regimes (flame), nutrient availability (N/P), seed dispersal (fruits) and changes in albedo (sun). Large clear symbols mean strongest per capita impact, small clear symbols mean medium impact and small transparent symbols mean least impact. For the fire, orange symbols mean promoting fire, while blue means limiting fire. For nutrients, the size of N or P suggests whether nutrient transport is biased towards N (large N) or P (large P). See the text for further details on the direction of these effects.
Our review also highlights other important aspects besides functional diversity. First, certain social behaviours, particularly the formation of megaherds and migratory behaviour, may result in species having a larger impact on CC drivers than anticipated from their body mass; see for example the impacts of migratory reindeer herds in the tundra (box 2). Megaherds and migratory species may, in some ways, be functional equivalents of megaherbivores. Both phenomena are highly endangered, and rewilding efforts may want to focus on restoring them. Second, it is important to consider abundance; smaller herbivores can under certain conditions functionally replace megaherbivores if densities are high enough. Here, it is important to consider potential trade-offs between effects on CC drivers. A high biomass of mesoruminants may have similar positive effects on vegetation and fire regimes as megaherbivores, but emit more greenhouse gasses and may not transport nutrients as evenly across the landscape. Finally, the effects of herbivory are strongly dependent on the environmental and seasonal context. For example, we show how impacts on fire regimes depend on the productivity of the system [18], that impacts on nutrient cycling may be contingent on the underlying soil physical properties [29] and that effects of trampling may vary across seasons.
4. Contribution of trophic rewilding to CC mitigation
Given the approximately 10 Gt of carbon that humans annually pump into the atmosphere, a reasonable question is whether rewilding could really contribute to mitigation efforts. We approach this question at two scales: the immediate contributions to mitigation portfolios at local to regional scales, and a more radical perspective on mitigation services of rewilding at large scales.
(a). Immediate contributions of rewilding to CC mitigation schemes at national to regional scales
Rewilding alone cannot mitigate CC, nor is it likely to be a major solution. However, this is true of most proposed mitigation strategies. Reducing our anthropogenic influence on climate will take a portfolio of complementary strategies (or CC mitigation wedges [71]), which will vary among countries and regions. We argue here that rewilding has the potential to form part of such regional mitigation portfolios. One of the most powerful examples is the role of megafaunal seed dispersers for the carbon storage of forests (see box 1 for mechanisms). The current bushmeat crisis, however, is increasingly leading to ‘empty’ forests [10]. Recent work suggests that the loss of megafaunal frugivores may be responsible for as much as a 10% reduction in carbon storage in the world's tropical forests. Rewilding with megafaunal frugivores may thus carry significant carbon sequestration benefits for tropical rainforest range states. Considering the role of megafauna in CC mitigation has other surprising consequences; for example, Brancalion et al. [72] show how large-seeded, animal-dispersed tree species, which contribute more to carbon stocking, are underrepresented in tree planting schemes owing to higher seed prices.
Another major example is the role of abundant megafaunal herds in maintaining ecosystem productivity, and associated carbon storage, in grassy biomes (savannahs, grasslands and steppes). The Serengeti in Tanzania demonstrates how protected grassy biomes may contribute hugely to regional climate mitigation. Irruption of wildebeest numbers in this area since the 1960s strongly reduced fires and increased ecosystem productivity and carbon storage in the soil [73]. This effect was so strong that it turned the Serengeti from a carbon source into a carbon sink; roughly equivalent to East Africa's annual fossil fuel emissions [74]. There are many examples of regions with extensive grassy biomes in protected areas or similar state: for example, extensive protected savannah areas in southern and east Africa (often more than 20% of land surface), and the immense steppes of central Asia. However, unlike the Serengeti, most of our conservation areas are severely depleted in terms of megafaunal numbers [75]. On average, megafaunal populations across African protected areas declined by 60% between 1970 and 2005 alone [76] and the majority of African savannah elephant populations are at densities less than 25% of levels predicted based on environmental factors [77]. It is possibly even worse for the extensive Asian grasslands and steppes where once immense populations of saiga, gazelle spp. and equids have almost vanished [78]. Following the Serengeti example, consequences of this collapse probably include strongly reduced productivity and carbon storage of protected grassy biomes. There is thus great potential for regions in the grassy biome range to rewild and better protect their conservation areas [75] as a significant carbon sequestering strategy. Such rewilding should focus on restoring the large, non-ruminant herbivores at historic baseline densities, such as African and Asian rhinoceroses and elephants, and Eurasian equids and camelids, but also the once abundant migratory herds (e.g. saiga and Mongolian gazelle in central Asia).
(b). The political agenda: rewilding as part of ‘carbon offsetting schemes’
But how do we attract the financial and political support to turn the ongoing depletion of protected areas into successful rewilding efforts? One way is to make rewilding pay for itself (box 3). However, there are also major opportunities in more effectively communicating rewilding as an important, and effective, part of regional CC mitigation portfolios; in particular, those parts of the portfolios aimed at increasing carbon storage in natural sinks. This could open opportunities for rewilding to tap into the well-funded intergovernmental CC mitigation financing schemes that are being developed under the Paris Agreement. The majority of such schemes now invest in tree planting, such as AFR100, REDD+ and the growing number of carbon offsetting schemes of the world's main airline companies [86]. The effectiveness of tree-planting carbon-offsetting schemes for mitigating carbon loss through burning fossil fuels is increasingly questioned [87]. Why are these programmes not investing in fighting the bushmeat crisis and restocking our empty tropical forests with megafaunal frugivores (box 1), or the rewilding of the tundra (box 2) or stopping the current onslaught on African and Asian megafauna, such as elephants and rhinoceros [74]?
Box 3. Achieving rewilding on a national scale: South Africa as a model.
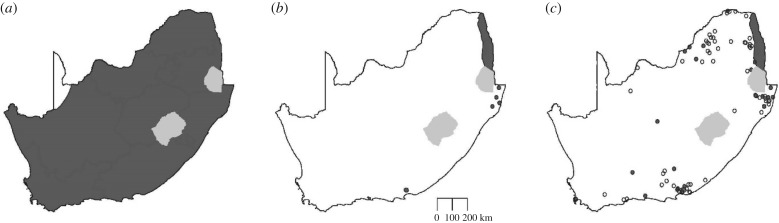
The advent of firearms, commercial markets for wildlife products and large-scale agriculture led to the virtual collapse of South African megafauna, starting in the 1600s [79]. The collapse of this fauna is reflected in the loss of the large, migratory herds of the interior [80] and the contraction of the distribution of megaherbivores (figure 4). The historical number of large mammals is unknown, but must have been at least in the range of 50 million to 100 million, and a 1964 estimate [81] of only 575 000 head of wildlife illustrates the extent of the decline. Changing legislation that allows landowners legal ownership of wildlife on their properties (The Game Theft Act, Act 105 of 1991), and hence gain benefits in terms of harvests (live sales and wildlife products), conferred value to wildlife. This led to a growth in game ranching across South Africa, with an estimated 9000 wildlife farms covering an area of more than 170 000 km2 [82]. The growth in ecotourism added value to the megaherbivores, leading to extensive rewilding with megaherbivores (and the associated mesoherbivores) of large areas (figure 4). The socio-economic benefits of this rewilding are direct: such operations employ up to twice as many staff at four times the salary as a comparably sized pastoral farming operation [83]. This rewilding led to a remarkable increase from only 0.5 million heads of wildlife in the mid-1960s to 18 million heads of wildlife just half a century later [84]. The private sector has been a key driver in this rewilding paradigm shift, with private landowners responsible for 76% of the elephant populations and 60% of the area rewilded with elephant (excluding the Kruger National Park [85]). The lessons on rewilding from South Africa therefore include the need for enabling legislation, the ability of landowners to benefit from wildlife and economic incentives to support wildlife. Probably the most important lesson is the fact that national paradigms may shift from one of squandering wildlife to massive large-scale rebuilding of wildlife populations as a national resource.
(c). A more radical perspective on rewilding as climate change mitigation strategy
According to recent studies, Earth may be more rapidly approaching an unsustainable point of no return than previously assumed [88,89]. These studies suggest we may only be able to steer back towards a trajectory of a sustainable planet if ‘collective human action’ leads to ‘widespread and fundamental transformations’ in how we use the planet [88]. Obviously, the most influential changes will have to come from actions aimed at reducing carbon emissions and consumption patterns, including control of human population growth. However, here we philosophize about more radical approaches towards rewilding that could contribute to CC mitigation at global scales as part of fundamentally transformed world views. One powerful opportunity lies in the use of rewilding to mitigate the emerging disaster of massive carbon emissions from the thawing of permafrost across the world's tundra. Conservative estimates suggest that this may lead to emissions similar to that of Russia, and 10% of emissions from China and the USA. In addition to this carbon emission problem, rapid woody encroachment across the Arctic is further accelerating warming by reducing albedo (box 2). Studies from a rewilding project in the Russian Arctic by Zimov and colleagues show that restoring the megafauna of the far north may freeze permafrost and reduce woody encroachment, strongly increasing carbon storage and albedo [62]. Their calculations suggest that restoration of abundant herds across the tundra biome would have globally significant CC mitigation effects [62,74]. But there is more to this opportunity than rewilding the Arctic. Large parts of the Northern Hemisphere, particularly across the former Soviet Union and mountainous regions of Europe, experience massive land abandonment, where as much as 40–70% of former agricultural land has been left fallow [90]. The resulting large-scale forest expansion [91] reduces albedo, which in these northern areas may overwhelm the effects of carbon sequestration and induce further warming [58]. Trophic rewilding may not only contribute towards reducing the woody expansion and maintaining the reflective properties of more grassy habitats, but also offer an alternative land use with a basket of socio-economic benefits (box 3) [92]. Rewilding as a land use strategy on, at least part of, these abandoned lands would prevent them from being used for alternative land uses with larger CC impacts. There would be an even greater CC mitigation benefit if rewilding across the Arctic and former agricultural lands were used to replace part of Eurasia's meat production by harvesting the rewilded populations of species such as reindeer, bison and muskox.
This leads us to another major, arguably more radical, CC mitigation opportunity: the transformation of the livestock industry. Since humans started domesticating animals, wild herbivore biomass has been replaced with a similar biomass of a few, largely ruminant, livestock species [93]. This has led to an intensive livestock system that is now a strong contributor to CC, particularly through its high greenhouse gas emissions [11]. More generally, the shift from wild to managed grazing has led to severe degradation of many rangelands, including large-scale soil erosion, reduced soil organic carbon, woody encroachment with associated reductions in albedo and negative effects on hydrology [39,93]. There are massive opportunities to reform the current livestock industry and rewild our rangelands [94] by replacing, at least a proportion of, domestic ruminants with native megafaunal communities (box 4). Even a shift from meso-sized (cows) to small ruminants can lead to significant reductions in methane emission [14]. Such a shift is currently in fact ongoing in large parts of Africa, where sheep and goats are replacing cattle in response to increased frequency of droughts, but the consequences of this shift are unknown. The rewilding of rangelands requires a major paradigm shift and rethinking of the world's meat consumption and provisioning (box 4). It would also face major obstacles, including current international legislative and policy schemes, such as the EU's Common Agricultural Policy (CAP) and vested interests that protect domestic herbivore meat industries and hamper the use of wild game meat: for example, through strict food safety protocols limiting game meat from entering markets [97]. We therefore realize that the rewilding of rangelands is currently at best a vision for the future. However, for certain parts of the world it could already be a realistic alternative, including large stretches of communal grazing areas in Africa [94] and Asia and of abandoned land in Eurasia (see above). This could also include mixed strategies, where part of the domestic stock is replaced with wild megafauna. Rewilding rangelands could not only lead to very significant reductions in greenhouse gas emissions (box 4) but also help restore degraded grassy biomes.
Box 4. Rewilding rangelands.
Livestock and extensive grazing land have increased rapidly during recent decades, with severe environmental consequences [93]. There is much to gain, especially in terms of greenhouse gas emissions, if we are able to replace livestock with native wildlife and, particularly, more non-ruminants [94]. A decade ago, Wilson & Edwards [95] estimated that replacing cattle with native megafauna (low-emission kangaroos) in amounts with equivalent meat production would reduce annual country-wide and the agricultural sector's greenhouse gas emissions in Australia by 3% and 28%, respectively. They also suggest higher profitability of kangaroo farming over livestock farming. Something similar has been shown for African rangelands, where native wildlife ranching may, particularly in more arid and less productive conditions, be more profitable than livestock farming [96]. Rewilding rangelands would not only come with economic and greenhouse emission benefits. It could, for example, also have major advantages for human health since game meat contains higher proportions of unsaturated fatty acids [97]. In fact, a central aspect of rewilding our rangelands should include rewilding our diet, where the focus should be on eating less meat and eating proportionally more non-ruminant meat (kangaroos, suids, equids, camelids). Moreover, it could contribute towards restoring degraded rangelands, including issues with soil degradation and hydrology [94]. Cloete et al. [96] highlighted that there may be high capital investments involved with converting a traditional farm into a game farm. However, if this leads to major reductions in greenhouse emissions, governments should consider subsidizing such capital investments through ‘green subsidies’ or green tax benefits. Rewilding the farm could also reduce perceived conflicts with increasing ungulate populations and agriculture across the Northern Hemisphere. By integrating the use of these ungulates in the land use/farming system, where ungulates are harvested and sold as game meat, farmers could compensate their crop losses due to ungulate damage with income from meat.
Finally, across significant parts of the Northern Hemisphere, spectacular increases in megafauna occur spontaneously without strong conservation management, such as the strong revival of ungulate and carnivore populations in Europe [98]. Interestingly, here we often see an emphasis on the disservices rather than the services delivered by this recovering wildlife [99]. However, recent work suggests that large-scale restoration of wolves across North America, for example, would increase net carbon storage, by suppressing ecosystem effects of their main ungulate prey, to levels similar to the annual emissions of 6–20 million cars [100]. This puts the passive rewilding in Europe and North America in a novel CC mitigation perspective that deserves further exploration.
5. Knowledge gaps and challenges
A key lesson from our synthesis is that effects of rewilding on CC mitigation are complex and highly context-dependent. We are only starting to understand this complexity. This is nicely illustrated by above-mentioned study on North American wolves and carbon storage [100]. While in boreal forests wolf restoration would increase carbon sequestration, as described above, in grasslands it reduces carbon sequestration since reduced elk numbers lead to reduced nutrient cycling. This example also illustrates that rewilding may sometimes induce further warming, instead of mitigating CC. High moose densities across North America's boreal forests reduce the sequestering of carbon, by decreasing nutrient cycling and net primary productivity. According to Schmitz and colleagues, reducing moose density (from 1.5 to 0.5 km−2) through active population management leads to a carbon sink equivalent of at least 40% of Canada's fossil-fuel emissions [74]. However, as they also state, full rewilding, including restoration of wolf populations, may have similar effects.
Trophic rewilding as a CC mitigation strategy is a particularly novel concept with major knowledge gaps. This makes it hard to predict the outcomes of rewilding for CC mitigation and to suggest the way forward. Here, we highlight some of the major knowledge gaps.
-
—
We showed that a major opportunity for rewilding and CC mitigation lies in the world's grassy biomes, which cover more than 25% of Earth's land surface. These biomes are currently rapidly invaded by woody plants and rewilding could help reduce this invasion [36]. However, the consequences of these shifts in tree–grass ratios for net radiative forcing, particularly the relative effects of woody invasion on carbon sequestration versus changes in albedo, are uncertain for many parts of the world, partly because of a scarcity of studies in ecosystems where fire and herbivory play a dominant role.
-
—
Similarly, most studies still only consider effects of megafauna on carbon storage, ignoring possible effects on biophysical processes (albedo). Our synthesis highlights the urgent need for studies that go beyond carbon and look at the net effects of biochemical and biophysical processes in the same study system. Similarly, CC mitigation should not only focus on carbon sequestration but also on maintaining or increasing Earth's capacity to reflect sunlight. Conservation and restoration of the world's grassy biomes deserves much more attention in a CC mitigation community dominated by forest thinking [101].
-
—
The net effects of fire regimes on climate are still highly uncertain, as is the net effect of replacing fire- with herbivore-driven systems on climate forcing. Moreover, fire–herbivore interactions are still poorly understood, particularly for browsers and for forest systems.
-
—
There are large uncertainties around the effects of different megafaunal communities on N and P cycling, and on nutrient stoichiometry, despite increasingly robust theoretical frameworks [29,32]. More generally, the consequences of such effects for ecosystem carbon storage are still poorly understood.
-
—
We have a relatively poor understanding of methane emissions by wild ungulates, particularly variation in methane emission among different feeding guilds and seasonal and regional variation within species because of variation in diet.
-
—
Megafaunal effects are missing from mainstream climate- and Earth-system models and are hardly recognized by the Intergovernmental Panel on Climate Change (IPCC) [74].
The existence of such large knowledge gaps should be particularly worrying since we are on the brink of losing our largest wild herbivores. With the impending collapse of megafaunal populations across South America, Africa and Asia, we may be squandering the last opportunity of restoring the world's largest mammal populations as part of mitigating the effects of global change.
Acknowledgements
We thank Celesté Maré for assisting with creating the maps in figure 4.
Figure 4.
Diagrammatic representation of the collapse in the distribution of the megaherbivore guild (elephant, hippopotamus, giraffe, and black and white rhinoceros) in South Africa (dark shaded areas represent Lesotho and Swaziland) (a) from the start of the historical period around 1500, (b) to the approximated nadir around 1880, and then (c) the current situation following the rewilding with elephant, black rhinoceros and hippopotamus (white rhinoceros data not available for security reasons and giraffe data too diffuse to capture). In (c), open dots reflect privately protected areas whereas shaded dots and areas reflect public, governmentally protected areas. Sources: [79,85] (G. Kerley, 2018, personal observation).
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Ripple WJ, et al. 2015. Collapse of the world's largest herbivores. Sci. Adv. 1, e1400103 ( 10.1126/sciadv.1400103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith FA, Doughty C, Malhi Y, Svenning JC, Terborgh J. 2016. Megafauna in the Earth system. Ecography 39, 99–108. ( 10.1111/ecog.02156) [DOI] [Google Scholar]
- 3.Doughty CE, Wolf A, Field CB. 2010. Biophysical feedbacks between the Pleistocene megafauna extinction and climate: the first human-induced global warming? Geophys. Res. Lett. 37, L15703 ( 10.1029/2010GL043985) [DOI] [Google Scholar]
- 4.Doughty CE, Roman J, Faurby S, Wolf A, Haque A, Bakker ES, Malhi Y, Dunning JB, Svenning J-C. 2016. Global nutrient transport in a world of giants. Proc. Natl Acad. Sci. USA 113, 868–873. ( 10.1073/pnas.1502549112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill JL. 2014. Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 201, 1163–1169. ( 10.1111/nph.12576) [DOI] [PubMed] [Google Scholar]
- 6.Doughty CE, et al. 2016. Megafauna extinction, tree species range reduction, and carbon storage in Amazonian forests. Ecography 39, 194–203. ( 10.1111/ecog.01587) [DOI] [Google Scholar]
- 7.Smith FA, Elliott SM, Lyons SK. 2010. Methane emissions from extinct megafauna. Nat. Geosci. 3, 374–375. ( 10.1038/ngeo877) [DOI] [Google Scholar]
- 8.Donlan JC, et al. 2006. Pleistocene rewilding: an optimistic agenda for twenty-first century conservation. Am. Nat. 168, 660–681. ( 10.2307/3873461) [DOI] [PubMed] [Google Scholar]
- 9.Svenning J-C, et al. 2016. Science for a wilder Anthropocene: synthesis and future directions for trophic rewilding research. Proc. Natl Acad. Sci. USA 113, 898–906. ( 10.1073/pnas.1502556112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galetti M, Pires AS, Brancalion PH, Fernandez FA. 2017. Reversing defaunation by trophic rewilding in empty forests. Biotropica 49, 5–8. ( 10.1111/btp.12407) [DOI] [Google Scholar]
- 11.IPCC. 2013. Climate change 2013: the Physical science basis. Contribution of Working Group I to the Fifth assessment report of the Intergovernmental Panel on Climate Change (eds Stocker TF, et al.). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.Kelliher FM, Clark H. 2010. Methane emissions from bison—an historic herd estimate for the North American Great Plains. Agric. For. Meteorol. 150, 473–477. ( 10.1016/j.agrformet.2009.11.019) [DOI] [Google Scholar]
- 13.Dittmann MT, Runge U, Lang RA, Moser D, Galeffi C, Kreuzer M, Clauss M. 2014. Methane emission by camelids. PLoS ONE 9, e94363 ( 10.1371/journal.pone.0094363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith FA, Elliott SM, Lyons SK, Wagner P. 2015. The importance of considering animal body mass in IPCC greenhouse inventories and the underappreciated role of wild herbivores. Glob. Change Biol. 21: 3880–3888. ( 10.1111/gcb.12973) [DOI] [PubMed] [Google Scholar]
- 15.Owen-Smith RN. 1988. Megaherbivores: the influence of very large body size on ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 16.Clauss M, Frey R, Kiefer B, Lechner-Doll M, Loehlein W, Polster C, Rossner GE, Streich WJ. 2003. The maximum attainable body size of herbivorous mammals: morphophysiological constraints on foregut and adaptations of hindgut fermenters. Oecologia 136, 14–27. ( 10.1007/s00442-003-1254-z) [DOI] [PubMed] [Google Scholar]
- 17.Hempson GP, Archibald S, Bond WJ. 2017. The consequences of replacing wildlife with livestock in Africa. Sci. Rep. 7, 17196 ( 10.1038/s41598-017-17348-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Archibald S, Hempson GP. 2016. Competing consumers: contrasting the patterns and impacts of fire and mammalian herbivory in Africa. Phil. Trans. R. Soc. B 371: 20150309 ( 10.1098/rstb.2015.0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waldram MS, Bond WJ, Stock WD. 2008. Ecological engineering by a mega-grazer: white rhino impacts on a South African savanna. Ecosystems 11, 101–112. ( 10.1007/s10021-007-9109-9) [DOI] [Google Scholar]
- 20.Persson IL, Pastor J, Danell K, Bergström R. 2005. Impact of moose population density on the production and composition of litter in boreal forests. Oikos 108, 297–306. ( 10.1111/j.0030-1299.2005.13844.x) [DOI] [Google Scholar]
- 21.Pastor J, Naiman RJ, Dewey B, McInnes P. 1988. Moose, microbes, and the boreal forest. Bioscience 1, 770–777. ( 10.2307/1310786) [DOI] [Google Scholar]
- 22.Mouillot F, Narasimha A, Balkanski Y, Lamarque JF, Field CB. 2006. Global carbon emissions from biomass burning in the 20th century. Geophys. Res. Lett. 33, L01801 ( 10.1029/2005GL024707) [DOI] [Google Scholar]
- 23.Bond TC, et al. 2013. Bounding the role of black carbon in the climate system: a scientific assessment. J. Geophys. Res. Atmos. 118, 5380–5552. ( 10.1002/jgrd.50171) [DOI] [Google Scholar]
- 24.Archibald S, et al. 2018. Biological and geophysical feedbacks with fire in the Earth system. Environ. Res. Lett. 13, 033003 ( 10.1088/1748-9326/aa9ead) [DOI] [Google Scholar]
- 25.Landry J-S, Partanen A-I, Matthews HD. 2017. Carbon cycle and climate effects of forcing from fire-emitted aerosols. Environ. Res. Lett. 12, 25002 ( 10.1088/1748-9326/aa51de) [DOI] [Google Scholar]
- 26.Pellegrini AF, et al. 2018. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 553, 194–198. ( 10.1038/nature24668) [DOI] [PubMed] [Google Scholar]
- 27.Pastor J, Cohen Y, Hobbs NT. 2006. The role of large herbivores in ecosystem nutrient cycles. In Large herbivore ecology, ecosystem dynamics and conservation (eds Dannell K, Bergstrom R, Duncan P, Pastor J), pp. 289–319. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 28.Cromsigt JPGM, Kuijper DPJ. 2011. Revisiting the browsing lawn concept: evolutionary interactions or pruning herbivores? Perspect. Plant Ecol. Evol. Syst. 13, 207–215. ( 10.1016/j.ppees.2011.04.004) [DOI] [Google Scholar]
- 29.Schrama M, Veen GC, Bakker EL, Ruifrok JL, Bakker JP, Olff H. 2013. An integrated perspective to explain nitrogen mineralization in grazed ecosystems. Perspect. Plant Ecol. Evol. Syst. 15, 32–44. ( 10.1016/j.ppees.2012.12.001) [DOI] [Google Scholar]
- 30.Du Toit JT, Olff H. 2014. Generalities in grazing and browsing ecology: using across-guild comparisons to control contingencies. Oecologia 174, 1075–1083. ( 10.1007/s00442-013-2864-8) [DOI] [PubMed] [Google Scholar]
- 31.Wolf A, Doughty CE, Malhi Y. 2013. Lateral diffusion of nutrients by mammalian herbivores in terrestrial ecosystems. PLoS ONE 8, e71352 ( 10.1371/journal.pone.0071352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sitters J, Bakker ES, Veldhuis MP, Veen GF, Olde Venterink H, Vanni MJ. 2017. The stoichiometry of nutrient release by terrestrial herbivores and its ecosystem consequences. Front. Earth Sci. 5, 32 ( 10.3389/feart.2017.00032) [DOI] [Google Scholar]
- 33.Sitters J, Maechler MJ, Edwards PJ, Suter W, Olde Venterink H. 2014. Interactions between C : N : P stoichiometry and soil macrofauna control dung decomposition of savanna herbivores. Funct. Ecol. 28, 776–786. ( 10.1111/1365-2435.12213) [DOI] [Google Scholar]
- 34.Goll DS, Brovkin V, Parida BR, Reick CH, Kattge J, Reich PB, van Bodegom PM, Niinemets Ü. 2012. Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosci. Discuss. 9, 3173–3232. ( 10.5194/bgd-9-3173-2012) [DOI] [Google Scholar]
- 35.Landman M, Schoeman DS, Hall-Martin AJ, Kerley GIH. 2014. Long-term monitoring reveals differing impacts of elephants on elements of a canopy shrub community. Ecol. Appl. 24, 2002–2012. ( 10.1890/14-0080.1) [DOI] [PubMed] [Google Scholar]
- 36.Stevens N, Erasmus BFN, Archibald S, Bond WJ. 2016. Woody encroachment over 70 years in South African savannahs: overgrazing, global change or extinction aftershock? Phil. Trans. R. Soc. B 371, 20150437 ( 10.1098/rstb.2015.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cromsigt JPGM, te Beest M. 2014. Restoration of a megaherbivore: landscape-level impacts of white rhinoceros in Kruger National Park, South Africa. J. Ecol. 102, 566–575. ( 10.1111/1365-2745.12218) [DOI] [Google Scholar]
- 38.van der Waal C, et al. 2011. Large herbivores may alter vegetation structure of semi-arid savannas through soil nutrient mediation. Oecologia 165, 1095–1107. ( 10.1007/s00442-010-1899-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asner GP, Elmore AJ, Olander LP, Martin RE, Harris AT. 2004. Grazing systems, ecosystem responses, and global change. Annu. Rev. Environ. Resour. 29, 261–299. ( 10.1146/annurev.energy.29.062403.102142) [DOI] [Google Scholar]
- 40.Prins HHT, van der Jeugd HP. 1993. Herbivore population crashes and woodland structure in East Africa. J. Ecol. 81, 305–314. ( 10.2307/2261500) [DOI] [Google Scholar]
- 41.Plas F, Howison RA, Mpanza N, Cromsigt JPGM, Olff H. 2016. Different-sized grazers have distinctive effects on plant functional composition of an African savannah. J. Ecol. 104, 864–875. ( 10.1111/1365-2745.12549) [DOI] [Google Scholar]
- 42.Guimarães PR Jr, Galetti M, Jordano P. 2008. Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS ONE 3, e1745 ( 10.1371/journal.pone.0001745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janzen DH, Martin PS. 1982. Neotropical anachronisms: the fruits the gomphotheres ate. Science 215, 19–27. ( 10.1126/science.215.4528.19) [DOI] [PubMed] [Google Scholar]
- 44.Pires MM, Guimarães PR, Galetti M, Jordano P. 2018. Pleistocene megafaunal extinctions and the functional loss of long-distance seed-dispersal services. Ecography 41, 153–163. ( 10.1111/ecog.03163) [DOI] [Google Scholar]
- 45.Culot L, Bello C, Batista JL, Couto HT, Galetti M. 2017. Synergistic effects of seed disperser and predator loss on recruitment success and long-term consequences for carbon stocks in tropical rainforests. Sci. Rep. 7, 7662 ( 10.1038/s41598-017-08222-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bello C, Galetti M, Pizo MA, Magnago LFS, Rocha MF, Lima RA, Peres CA, Ovaskainen O, Jordano P. 2015. Defaunation affects carbon storage in tropical forests. Sci. Adv. 1, e1501105 ( 10.1126/sciadv.1501105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poulsen JR, et al. 2017. Poaching empties critical Central African wilderness of forest elephants. Curr. Biol. 27, R134–R135. ( 10.1016/j.cub.2017.01.023) [DOI] [PubMed] [Google Scholar]
- 48.Sampson C, et al. 2018. New elephant crisis in Asia—early warning signs from Myanmar. PLoS ONE 13, e0194113 ( 10.1371/journal.pone.0194113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fragoso JM, Huffman JM. 2000. Seed-dispersal and seedling recruitment patterns by the last Neotropical megafaunal element in Amazonia, the tapir. J. Trop. Ecol. 16, 369–385. ( 10.1017/S0266467400001462) [DOI] [Google Scholar]
- 50.Blake S, Deem SL, Mossimbo E, Maisels F, Walsh P. 2009. Forest elephants: tree planters of the Congo. Biotropica 41, 459–468. ( 10.1111/j.1744-7429.2009.00512.x) [DOI] [Google Scholar]
- 51.Osuri AM, et al. 2016. Contrasting effects of defaunation on aboveground carbon storage across the global tropics. Nat. Commun. 7, 11351 ( 10.1038/ncomms11351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sobral M, Silvius KM, Overman H, Oliveira LF, Raab TK, Fragoso JM. 2017. Mammal diversity influences the carbon cycle through trophic interactions in the Amazon. Nat. Ecol. Evol. 1, 1670–1676. [DOI] [PubMed] [Google Scholar]
- 53.Campos-Arceiz A, Traeholt C, Jaffar R, Santamaria L, Corlett RT. 2012. Asian tapirs are no elephants when it comes to seed dispersal. Biotropica 44, 220–227. ( 10.1111/j.1744-7429.2011.00784.x) [DOI] [Google Scholar]
- 54.Landman M, Gaylard A, Mendela T, Kerley GIH. 2014. Impact of elephant on two woody trees, Boscia oleoides and Pappea capensis, in an arid thicket-Nama Karoo mosaic, Addo Elephant National Park. Koedoe 56, a1231 ( 10.4102/koedoe.v56i1.1231) [DOI] [Google Scholar]
- 55.Olofsson J, Stark S, Oksanen L. 2004. Reindeer influence on ecosystem processes in the tundra. Oikos 105, 386–396. ( 10.1111/j.0030-1299.2004.13048.x) [DOI] [Google Scholar]
- 56.Elmendorf SC, et al. 2012. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Change 2, 453–457. ( 10.1038/nclimate1465) [DOI] [Google Scholar]
- 57.Chapin FS, et al. 2005. Role of land-surface changes in Arctic summer warming. Science 310, 657–660. ( 10.1126/science.1117368) [DOI] [PubMed] [Google Scholar]
- 58.de Wit HA, Bryn A, Hofgaard A, Karstensen J, Kvalevåg MM, Peters GP. 2014. Climate warming feedback from mountain birch forest expansion: reduced albedo dominates carbon uptake. Glob. Change Biol. 20, 2344–2355. ( 10.1111/gcb.12483) [DOI] [PubMed] [Google Scholar]
- 59.Olofsson J, Oksanen L, Callaghan T, Hulme PE, Oksanen T, Suominen O. 2009. Herbivores inhibit climate-driven shrub expansion on the tundra. Glob. Change Biol. 15, 2681–2693. ( 10.1111/j.1365-2486.2009.01935.x) [DOI] [Google Scholar]
- 60.Cohen J, Pulliainen J, Ménard CB, Johansen B, Oksanen L, Luojus K, Ikonen J. 2013. Effect of reindeer grazing on snowmelt, albedo and energy balance based on satellite data analyses. Remote Sens. Environ. 135, 107–117. ( 10.1016/j.rse.2013.03.029) [DOI] [Google Scholar]
- 61.te Beest M, Sitters J, Ménard CB, Olofsson J. 2016. Reindeer grazing increases summer albedo by reducing shrub abundance in Arctic tundra. Environ. Res. Lett. 11, 125013 ( 10.1088/1748-9326/aa5128) [DOI] [Google Scholar]
- 62.Zimov NS, Zimov SA, Zimova AE, Zimova GM, Chuprynin VI, Chapin FS. 2009. Carbon storage in permafrost and soils of the mammoth tundra-steppe biome: role in the global carbon budget. Geophys. Res. Lett. 36, L02502 ( 10.1029/2008GL036332) [DOI] [Google Scholar]
- 63.Zimov SA. 2005. Pleistocene park: return of the mammoth's ecosystem. Science 308, 796–798. ( 10.1126/science.1113442) [DOI] [PubMed] [Google Scholar]
- 64.Jackson RB, Banner JL, Jobbágy EG, Pockman WT, Wall DH. 2002. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418, 623–626. ( 10.1038/nature00910) [DOI] [PubMed] [Google Scholar]
- 65.Cumming DH, Cumming GS. 2003. Ungulate community structure and ecological processes: body size, hoof area and trampling in African savannas. Oecologia 134, 560–568. ( 10.1007/s00442-002-1149-4) [DOI] [PubMed] [Google Scholar]
- 66.Chaplot V, Dlamini P, Chivenge P. 2016. Potential of grassland rehabilitation through high density-short duration grazing to sequester atmospheric carbon. Geoderma 271, 10–17. ( 10.1016/j.geoderma.2016.02.010) [DOI] [Google Scholar]
- 67.Savory A, Parsons SD. 1980. The Savory grazing method. Rangelands 2, 234–237. [Google Scholar]
- 68.Smith FA, Smith REE, Lyons SK, Payne JL. 2018. Body size downgrading of mammals over the late Quaternary. Science 360, 310–313. ( 10.1126/science.aao5987) [DOI] [PubMed] [Google Scholar]
- 69.Haas TC, Ferreira SM. 2016. Conservation risks: when will rhinos be extinct? IEEE Trans. Cybern. 46, 1721–1734. ( 10.1109/TCYB.2015.2470520) [DOI] [PubMed] [Google Scholar]
- 70.IUCN. 2017. The IUCN Red List of Threatened Species. Version 2017-3 http://www.iucnredlist.org Downloaded on 05 May 2018.
- 71.Pacala S, Socolow R. 2004. Stabilization wedges: solving the climate problem for the next 50 years with current technologies. Science 305, 968–972. ( 10.1126/science.1100103) [DOI] [PubMed] [Google Scholar]
- 72.Brancalion PH, Bello C, Chazdon RL, Galetti M, Jordano P, Lima RA, Medina A, Pizo M Reid JL. 2018. Maximizing biodiversity conservation and carbon stocking in restored tropical forests. Conserv. Lett. 11, e12454 ( 10.1111/conl.12454) [DOI] [Google Scholar]
- 73.Holdo RM, Sinclair AR, Dobson AP, Metzger KL, Bolker BM, Ritchie ME, Holt RD. 2009. A disease-mediated trophic cascade in the Serengeti and its implications for ecosystem C. PLoS Biol. 7, e1000210 ( 10.1371/journal.pbio.1000210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmitz OJ, et al. 2014. Animating the carbon cycle. Ecosystems 17, 344–359. ( 10.1007/s10021-013-9715-7) [DOI] [Google Scholar]
- 75.Lindsey PA, Nyirenda VR, Barnes JI, Becker MS, McRobb R, Tambling CJ, Taylor WA, Watson FG, t'Sas-Rolfes M. 2014. Underperformance of African protected area networks and the case for new conservation models: insights from Zambia. PLoS ONE 9, e94109 ( 10.1371/journal.pone.0094109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Craigie ID, Baillie JE, Balmford A, Carbone C, Collen B, Green RE, Hutton JM. 2010. Large mammal population declines in Africa's protected areas. Biol. Conserv. 143, 2221–2228. ( 10.1016/j.biocon.2010.06.007) [DOI] [Google Scholar]
- 77.Robson AS, Trimble MJ, Purdon A, Young-Overton KD, Pimm SL, Van Aarde RJ. 2017. Savanna elephant numbers are only a quarter of their expected values. PLoS ONE 12, e0175942 ( 10.1371/journal.pone.0175942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mallon DP, Zhigang J. 2009. Grazers on the plains: challenges and prospects for large herbivores in Central Asia. J. Appl. Ecol. 46, 516–519. ( 10.1111/j.1365-2664.2009.01654.x) [DOI] [Google Scholar]
- 79.Boshoff AF, Landman M, Kerley GIH. 2016. Filling the gaps on the maps: historical distribution patterns of some larger mammals in part of southern Africa. Trans. R. Soc. S. Afr. 71, 23–87. ( 10.1080/0035919X.2015.1084066) [DOI] [Google Scholar]
- 80.Boshoff AF, Kerley GIH. 2015. Lost herds of the Highveld: evidence from the written historical record. Afr J Wildl. Res. 45, 287–300. ( 10.3957/056.045.0287) [DOI] [Google Scholar]
- 81.Du Toit JG. 2007. Report: Role of the Private Sector in the Wildlife Industry. Tshwane, Wildlife Ranching SA, Du Toit Wilddienste. 87 pp.
- 82.Taylor WA, Lindsey PA, Davies-Mostert H.. 2016. An assessment of the economic, social and conservation value of the wildlife ranching industry and its potential to support the green economy in South Africa. Johannesburg, South Africa: The Endangered Wildlife Trust. [Google Scholar]
- 83.Kerley GIH, Knight MH, De Kock M. 1995. Desertification of subtropical thicket in the Eastern Cape, South Africa: are there alternatives? Environ. Monit. Assess. 37, 211–230. ( 10.1007/BF00546890) [DOI] [PubMed] [Google Scholar]
- 84.Carruthers J. 2008. Wilding the farm or farming the wild? The evolution of scientific game ranching in South Africa from the 1960s to the present. Trans. R. Soc. S. Afr. 63, 160–181. ( 10.1080/00359190809519207) [DOI] [Google Scholar]
- 85.Landman M, Kerley GIH. 2018. Final Report: Survey of small elephant populations in South Africa. Port Elizabeth: Centre for African Conservation Ecology, Nelson Mandela University.
- 86.Becken S, Mackey B. 2017. What role for offsetting aviation greenhouse gas emissions in a deep-cut carbon world? J. Air Transp. Manag. 63, 71–83. ( 10.1016/j.jairtraman.2017.05.009) [DOI] [Google Scholar]
- 87.Mackey B, Prentice IC, Steffen W, House JI, Lindenmayer D, Keith H, Berry S. 2013. Untangling the confusion around land carbon science and climate change mitigation policy. Nat. Clim. Change 3, 552–557. ( 10.1038/nclimate1804) [DOI] [Google Scholar]
- 88.Steffen W, et al. 2018. Trajectories of the Earth System in the Anthropocene. Proc. Natl Acad. Sci. USA, 201810141 ( 10.1073/pnas.1810141115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aengenheyster M, Feng QY, Ploeg FVD, Dijkstra HA. 2018. The point of no return for climate action: effects of climate uncertainty and risk tolerance. Earth Syst. Dyn. 9, 1085–1095. ( 10.5194/esd-9-1085-2018) [DOI] [Google Scholar]
- 90.Lasanta T, Arnáez J, Pascual N, Ruiz-Flaño P, Errea MP, Lana-Renault N. 2017. Space–time process and drivers of land abandonment in Europe. Catena 149, 810–823. ( 10.1016/j.catena.2016.02.024) [DOI] [Google Scholar]
- 91.Schierhorn F, Müller D, Beringer T, Prishchepov AV, Kuemmerle T, Balmann A. 2013. Post-Soviet cropland abandonment and carbon sequestration in European Russia, Ukraine, and Belarus. Global Biogeochem. Cycles 27, 1175–1185. ( 10.1002/2013GB004654) [DOI] [Google Scholar]
- 92.Navarro LM, Pereira HM. 2012. Rewilding abandoned landscapes in Europe. Ecosystems 15, 900–912. ( 10.1007/s10021-012-9558-7) [DOI] [Google Scholar]
- 93.Ripple WJ, Smith P, Haberl H, Montzka SA, McAlpine C, Boucher DH. 2014. Ruminants, climate change and climate policy. Nat. Clim. Change 4, 2–5. ( 10.1038/nclimate2081) [DOI] [Google Scholar]
- 94.Venter ZS, Hawkins HJ, Cramer MD. 2017. Implications of historical interactions between herbivory and fire for rangeland management in African savannas. Ecosphere 8, e01946 ( 10.1002/ecs2.1946) [DOI] [Google Scholar]
- 95.Wilson GR, Edwards MJ. 2008. Native wildlife on rangelands to minimize methane and produce lower-emission meat: kangaroos versus livestock. Conserv. Lett. 1, 119–128. ( 10.1111/j.1755-263X.2008.00023.x) [DOI] [Google Scholar]
- 96.Cloete PC, Taljaard PR, Grove B. 2007. A comparative economic case study of switching from cattle farming to game ranching in the Northern Cape Province. S Afr. J. Wildl. Res. 37, 71–78. ( 10.3957/0379-4369-37.1.71) [DOI] [Google Scholar]
- 97.Hoffman LC, Wiklund E. 2006. Game and venison—meat for the modern consumer. Meat Sci. 74, 197–208. ( 10.1016/j.meatsci.2006.04.005) [DOI] [PubMed] [Google Scholar]
- 98.Deinet S, Ieronymidou C, McRae L, Burfield IJ, Foppen RP, Collen B, Boehm M. 2013. Wildlife comeback in Europe. The recovery of selected mammal and bird species. Zoological Society of London, UK.
- 99.Côté SD, Rooney TP, Tremblay JP, Dussault C, Waller DM. 2004. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 35, 113–147. ( 10.1146/annurev.ecolsys.35.021103.105725) [DOI] [Google Scholar]
- 100.Wilmers CC, Schmitz OJ. 2016. Effects of gray wolf-induced trophic cascades on ecosystem carbon cycling. Ecosphere 7, e01501 ( 10.1002/ecs2.1501) [DOI] [Google Scholar]
- 101.Veldman JW, et al. 2015. Tyranny of trees in grassy biomes. Science 347, 484–485. ( 10.1126/science.347.6221.484-c) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.