Abstract
In contrast to that of the Pleistocene epoch, between approximately 2.6 million and 10 000 years before present, the extant community of large herbivores in Arctic tundra is species-poor predominantly due to human extinctions. We here discuss how this species-poor herbivore guild influences tundra ecosystems, especially in relation to the rapidly changing climate. We show that present herbivore assemblages have large effects on tundra ecosystem composition and function and suggest that the effect on thermophilic species expected to invade the tundra in a warmer climate is especially strong, and that herbivores slow ecosystem responses to climate change. We focus on the ability of herbivores to drive transitions between different vegetation states. One such transition is between tundra and forest. A second vegetation transition discussed is between grasslands and moss- and shrub-dominated tundra. Contemporary studies show that herbivores can drive such state shifts and that a more diverse herbivore assemblage would have even higher potential to do so. We conclude that even though many large herbivores, and especially the megaherbivores, are extinct, there is a potential to reintroduce large herbivores in many arctic locations, and that doing so would potentially reduce some of the unwanted effects of a warmer climate.
This article is part of the theme issue ‘Trophic rewilding: consequences for ecosystems under global change’.
Keywords: herbivory, climate change, state shifts, treeline shifts, mammoth steppe, rewilding
1. The Arctic herbivore species assemblage
Herbivores are known to be important components of Arctic ecosystems, and many species like the large reindeer and caribou herds, and high densities of voles and lemmings during peak years, are well known to exert large effects on the ecosystems when they feed on the plants and serve as food for predators [1,2]. Despite their importance, the diversity of herbivores is low in the Arctic. About 50 species of mammalian herbivores are found in the Arctic as a whole, and most regions have a local species richness of mammalian herbivores below 10 [3]. Most of these herbivores are small- or medium-sized rodents, and only six large mammalian herbivores occur in the Arctic [3]. Four of these six herbivores have narrow distributional ranges and are only found in marginally Arctic habitats (moose, Dall's sheep, Siberian bighorn sheep and American bison). This means that the only two large herbivores with wide distributional ranges are caribou/reindeer (Rangifer tarandus; hereafter ‘caribou’, unless used in reference to the domestic or semi-domestic sub-species) [4,5] and muskoxen (Ovibos moschatus) [6]. The species richness of large herbivores in the Arctic thus varies between 0 and 2 species in most Arctic regions. The most widespread of the two herbivores, caribou, are also domesticated in many of these regions. We will here explore how this low species richness of large herbivores influences the vulnerability of arctic ecosystems to climate change, especially warming. We also discuss implications of the fact that only domesticated herbivores exist in many parts of the Arctic.
In contrast to current conditions, during the Pleistocene, most of the Arctic was covered by a vast, productive graminoid- and forb-rich biome commonly referred to as the mammoth steppe, grazed by large herds of a diverse assemblage of large herbivores, including woolly mammoth, woolly rhino, steppe wisent, wild horse, wapiti (elk), wild ass, snow sheep, camel, Saiga antelope, helmeted muskox, muskox and caribou [7,8]. Between 10 000 and 50 000 years before present, almost all of these megaherbivores were extinct in the Arctic, as well as the rest of the world outside Africa [9]. The relative influence of humans and rapid climatic warming during the Pleistocene–Holocene transition in this mass extinction has been widely debated. The extinction of large- and slow-breeding animals strongly implicates human hunting in such extinctions, although it is plausible that rapid climate changes during the same period also contributed [9–11]. For at least two formerly abundant Pleistocene large herbivores, the steppe bison and woolly mammoth, paleoecological evidence and bioclimatic envelope modelling suggest that human expansion into the far north probably coincided with rapid warming in the demise of such species [12,13]. It is thus clear that human hunting has at least contributed to the large-scale extinction of large herbivores, and contributed to large vegetation changes and shaping the presently species-poor tundra.
Another way humans have influenced the Arctic herbivore assemblage is through the domestication of reindeer [4,14]. Domesticated reindeer are today found throughout the Eurasian Arctic [4] and are the only large herbivore present in many areas today. On the other hand, in large parts of the Arctic, domesticated reindeer still migrate according to their traditional migration routes, and there is no empirical evidence that effects of reindeer on tundra vegetation differ substantially from effects of caribou [15].
We will in this paper present different perspectives on how extant large herbivores in the Arctic influence these ecosystems. We will discuss how large herbivores are likely to interact with climate to shape future ecosystems, and if their low richness reduces their ability to do so. Finally, we will consider paleo-ecological state shifts in a discussion of the role of herbivores in the state shifts occurring in the Arctic today, especially the expansion of trees and shrubs, and the disappearance of the mammoth steppe at the end of the Pleistocene. The conceptual models presented above serve as the basis for understanding how reintroduction of large herbivores in a rewilding context will influence the structure and function of arctic ecosystems. Finally, we briefly review the potential to reintroduce large herbivores in the Arctic and present conclusions about the potential consequences of rewilding the arctic herbivore assembly.
2. Effects of large herbivores on tundra vegetation
Throughout the Arctic, large herbivores are well known to influence plant community structure [15–17], and ecosystem processes and functions [18–21]. It is, however, difficult to quantify the magnitude of these effects. In areas where natural experiments allow long-term and large-scale comparisons, the effects can be considerable. On islands where reindeer have been introduced, dramatic changes in the vegetation have been observed, especially dramatic declines of lichens and deciduous shrubs [22,23]. Reindeer also have large effects on tundra vegetation in studies using reindeer management fences that have separated different grazing regimes for decades. The dramatic decline of lichens and deciduous shrubs is also the most prominent effect here [24,25], but strong effects of reindeer on tree recruitment and densities of larger trees [26] as well as dramatic vegetation shifts to graminoid-dominated vegetation [25,27] have been observed.
In contrast to the large effects recorded in these long-term natural experiments, the vegetation responses to excluding large mammalian herbivores are often much smaller. In the most recent meta-analyses of reindeer exclosure studies, it was concluded that effects of reindeer on lichens were negative, while the effects on forbs, graminoids, woody species and bryophytes were weak or non-significant [15]. Although no similar meta-analyses exist for muskoxen, the results would probably be at least comparable to the effects of reindeer. For instance, in Western Greenland where caribou and muskoxen coexist, a multi-annual field experiment revealed that the most prominent effects on tundra plant community composition, species richness and community dynamics were more strongly related to muskox exclusion than to caribou exclusion [28,29]. However, in that location, caribou density is far lower than is typical of densities maintained through reindeer herding. In high arctic Greenland, where muskoxen are the sole species of large herbivore, they have been estimated to remove only 0.04–0.17% of aboveground plant biomass during the summer months [30]. One reason for the partly contrasting results in these different types of studies could be that the response of Arctic vegetation is slow, and that the response of vegetation in the exclosures after a decade or two is still only transient, while the responses in the natural experiments, where the different grazing regimes have been prevailing for several decades or centuries, are closer to the equilibrium state. Another explanation could be that increasing and decreasing herbivore densities have very different effects on the vegetation [31]. When herbivores are excluded, plants that cannot tolerate grazing will not be present to begin with. It will thus be the plants that are present to begin with and thrive best in the absence of herbivores that will increase in abundance. If no species are present in the grazed system with the potential to grow tall or accumulate biomass, these species have to colonize the area before dramatic changes can be expected [32,33]. The ungrazed vegetation might be difficult to invade, because the vegetation gets denser and gaps suitable for regeneration from seeds are decreasing [34–36]. Increased herbivore densities in areas with historically low herbivore densities can, on the other hand, rapidly change the vegetation because grazing intolerant plants will quickly be eradicated, and grazing tolerant species can colonize in the gaps [32].
3. Herbivores constrain vegetation responses to climate warming
In a warmer climate, arctic tundra plants may be expected to grow earlier and faster, and increase in abundance [37,38]. This has been recorded as an increase in shrub abundance [39,40] and greening of the Arctic recorded from satellite-derived vegetation indices [41] in most of the Arctic. As herbivores are well known to reduce vegetation density of shrubs in particular [42], a higher density of herbivores will be expected to at least partly counteract these responses to a changing climate [28,29]. Concomitantly, any constraint imposed by large herbivores on shrub expansion in response to warming in the Arctic may also constrain the carbon uptake response of shrubs to warming. For instance, in addition to constraining the positive abundance response of dwarf birch and grey willow to experimental warming at a low arctic site in Greenland [28], large herbivores are estimated to reduce the carbon uptake response to warming of the vegetation in the same area by a factor of four [18]. A special aspect of herbivory in the Arctic is that the effect of herbivory appears to be especially strong on species that are expected to be favoured by warming. In a warming Arctic, we expect thermophilic species to invade and increase in abundance [37,38,42]. To grow fast and to be able to compete for light in denser vegetation, these species are typically taller and have higher N concentrations, shoot : root ratio and specific leaf area [33,34]. The same traits will also make the plants more susceptible to herbivory [43]. Experiments have shown that thermophilic species invading the tundra are indeed more sensitive to herbivory than the local plant species [44,45]. Reindeer and caribou are thus expected to constrain the vegetation changes that are projected to happen in a warmer climate [44,45]. A more diverse herbivore assembly would probably be even more effective in constraining projected vegetation changes, and rewilding of the herbivore guild might thus be a tool to preserve arctic vegetation in a warmer future.
4. Herbivores and woody vegetation
The ecotone between areas covered by erect woody vegetation (trees and tall shrubs) and open tundra heath or grasslands is one of the most obvious vegetation boundaries in northern ecosystems and often used to define the extent of the tundra. The distribution of trees and shrubs are, to a large extent, controlled by climatic conditions. High altitude treelines, defined as the highest occurrence of groups of trees greater than 3 m, are globally associated with a seasonal mean ground temperature of 6.7°C [46]. Moreover, paleoecological records [47] and contemporary studies [47] show clearly that treelines have moved to higher or lower altitudes in response to a changing climate.
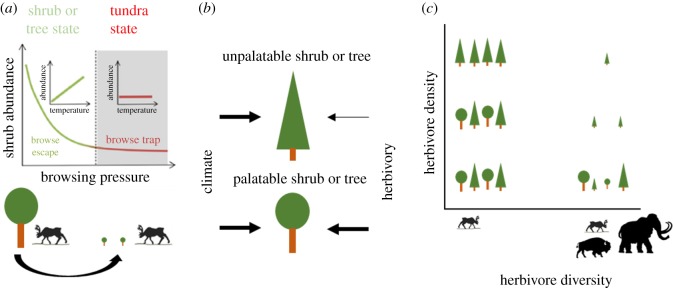
Although the observed treeline changes are linked to increases in temperature, they also reflect changes in land use and herbivore pressure, at least at regional scales and decennial time scales. At treelines across different geographical regions, studies have demonstrated that herbivory can exacerbate or constrain climate-driven distributional shifts in tall shrub and tree species [26,43,48–54]. The large mammalian herbivores present in the Arctic today do not damage large trees that have been able to grow above browsing height. However, a recent study demonstrated that where reindeer are present in high enough densities, they can prevent small shrub ramets from becoming tall and abundant. Where reindeer densities were above a threshold of approximately 5 animals km−2, shrubs were kept in a browse trap, and shrubs in grasslands were at low height and low abundance. At reindeer densities below this threshold, shrubs were taller and more abundant indicating reindeer were no longer in control of the grassland state [55] (figure 1b). Since reindeer can severely hamper the regeneration of mountain birch stands by limiting regeneration from basal shoots and re-establishment of individual trees from saplings [26,57], similar threshold densities are expected for tree regeneration and treeline expansion. Although reindeer do not damage large trees, to understand their potential to cause transitions between vegetation states dominated by erect woody vegetation and open tundra, it also must be considered that recurrent disturbances like insect outbreaks [58], fires [59] and avalanches [60] occasionally wipe out the larger trees in treeline forests. If reindeer prevent trees from regenerating, these areas can be deforested.
Figure 1.
A conceptual framework for how herbivores influence vegetation (trees and shrubs). (a) Reindeer browse palatable shrubs that are within their physical range, and shrubs and tree density can thus be kept low by herbivores independent of climatic conditions if herbivore density is high enough (modified from Bråthen et al. [55]). Although reindeer do not browse large trees, the wood encroachment could still be reversible following disturbances like insect outbreaks, fire and avalanches. (b) The effect of extant herbivores is expected to be stronger on palatable plants than on unpalatable plants (modified from Christie et al. [19]). (c) Which plants that are palatable or not depends on the herbivore present. With a diverse assemblage of herbivores including megaherbivores present, few species will be unpalatable to all herbivores, and trees cannot grow out of the browsing zone (modified from Bakker et al. [56]).
The capacity of herbivores to regulate tree and shrub abundance has been proposed to be linked to the palatability of the trees and shrubs present [19,61]. Especially willow and birch species at Scandinavian treelines are highly palatable to herbivores, and this could be one reason that reindeer, albeit a comparatively poor browser, are able to keep these tundra ecosystems open [26,54,62]. Globally, most of the Arctic and alpine treelines are formed by evergreen conifers that are relatively unpalatable, at least for reindeer [63]. The strong effect of reindeer at treelines might thus be specific to Scandinavia, or other sites where palatable deciduous shrubs form the treeline. However, because extant reindeer densities also reduce the establishment of less palatable species like Siberian larch, Scotts Pine and Norwegian spruce [57], reindeer might still be able to preserve open tundra in areas with less palatable tree species, but they will be far less efficient in doing so.
The palatability of trees and shrubs is not only a function of plant chemistry, but also a function of the herbivore assembly [63]. In Arctic ecosystems dominated by only reindeer, or reindeer and muskoxen, many plants might be unpalatable and the potential for present herbivores to reach densities where they can keep the trees in a browsing trap might be low. However, with a more species-rich herbivore assemblage, consisting of a range of herbivores with different feeding preferences, fewer species would be unpalatable to the whole herbivore guild. Moose, for example, feed preferentially on Scott's pine [64], even though it is avoided by reindeer [65]. Moreover, if the herbivore guild also contains megahebivores that could browse higher up in trees and even damage large trees, trees can no longer grow out of the browsing zone. African elephants have, for example, a strong effect on woody plants due to their physical strength and height, causing damage by feeding high up in tree crowns, but also by pushing them over [66], and mammoth and mastodonts are expected to have done the same. As stated above, during the Pleistocene, the herbivore assemblage was much more diverse than today, and included both megaherbivores, grazers and more specialized browsers. The ability of such a diverse herbivore assemblage to drive state transitions between forest and open tundra is expected to have been much stronger than what is observed from the depauperate herbivore assemblages of today. We could thus expect present species-poor herbivore assemblages to, in most cases, only reduce the abundance of palatable trees, but only have small effects on the position of the actual treeline in systems where unpalatable species are present. However, if more specialist browsers and megaherbivores were present, tree species unpalatable to the herbivores present today could be palatable for another herbivore species and thus also be expected to be trapped in browsing traps [56] (figure 1c). Rewilding the herbivore guild by introducing more herbivore species would thus have a potential to slow down the rising treeline and the increase of woody plants currently occurring in the Arctic in response to a warmer climate.
5. The mammoth steppe
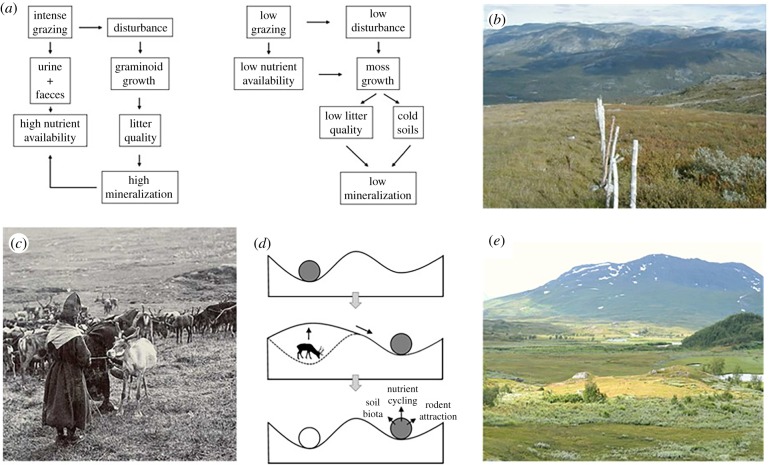
To understand how the tundra would be influenced by a diverse herbivore assembly, we have to look back to the Late Pleistocene and its diverse herbivore assembly. One of the most controversial hypotheses about the influence of large herbivores on arctic tundra vegetation is that human extinction of megaherbivores, rather than climate, drove the disappearance of the so-called mammoth steppe vegetation [7]. The importance of understanding this process today is accentuated by the fact that it also suggests that soil carbon storage decreased by more than 1000 Gt during this vegetation shift [67], and that the expected massive loss of soil carbon in the face of a warming Arctic could be inhibited by restoring megaherbivores that would reduce permafrost thawing [68]. During the Late Pleistocene, the mammoth steppe was probably the Earth's most extensive biome [69], and a diverse set of megaherbivores existing at high densities grazed these extensive graminoid-forb plains, much like the African savannah today. The densities of large herbivores inhabiting the mammoth steppe in Alaska and northeastern Siberia have been estimated to have been as high as 105 kg ha−1, [68], which is more than an order of magnitude greater than present herbivore densities. For a comparison, domesticated reindeer in Scandinavia today rarely exceed 3 kg ha−1. Zimov et al. [7,67] proposed that this high density of herbivores resulted in the graminoid dominance of the mammoth steppe, which in turn contributed to a high nutrient availability both by providing high quality litter that decomposed quickly and by maintaining a high rate of transpiration which resulted in dry oxygenated soils, further contributing to a high nutrient mineralization rate and nutrient availability. So, when megaherbivores were driven extinct, presumably by human hunting or its interaction with climatic warming, the system shifted to one dominated by chemically defended dwarf birch and moss, with wetter and colder soils and low litter quality, resulting in reduced nutrient availability [67] (figure 2a).
Figure 2.
A conceptual framework for how herbivores drive vegetation state shifts between moss and shrub tundra and grassland. (a) During the Late Pleistocene, the extinction of megaherbivores has been proposed to cause a vegetation state shift from productive grasslands with a high nutrient cycling to lowly productive tundra with a slow nutrient cycling (modified from Zimov et al. [67]). (b) Reindeer are today causing reversed vegetation transitions close to management fences where they are found in high densities, and the effects on nutrient cycling follow the conceptual figure presented in (a). (c and e) Historical reindeer herding also caused similar shifts in tundra vegetation centuries ago, and the productive grassland state is still maintained a century after active use ceased. (d) This can be illustrated by a ball representing an ecosystem that can be pushed by a herbivore into different points of attractions and remain in these different states even after the herbivores are removed (adapted from Egelkraut et al. [35]).
One way to evaluate this hypothesis is to investigate the timing of the megaherbivore extinction, vegetation shifts and human colonization. This work has already been reviewed in detail [69,70], and the best data available today seem to indicate that megaherbivores declined first after human population had increased, and the mammoth steppe changed from grasslands to heathlands at least partly as a consequence of megaherbivore extinctions. For example, analysis of megafaunal herbivore abundance from dung spore abundance may suggest that megaherbivore abundance, including mammoth, declines well before final extinction and major floral reorganization [71]. It is thus plausible that human extinction of megaherbivores contributed to the vegetation shift characteristic of the Pleistocene–Holocene transition at high northern latitudes.
A complementary way to evaluate this hypothesis is to investigate whether contemporary studies support the notion that herbivores can indeed drive such state transitions (figure 2b–e). The high density and species-rich large herbivore communities of the African savannahs are indeed today contributing to maintaining the productive graminoid-dominated savannahs by promoting nutrient cycling and primary production [72,73], in a corresponding way to how Zimov et al. [7] proposed that megaherbivores shaped the mammoth steppe during the Pleistocene. The critical question is, however, whether herbivores could drive such vegetation shifts in the climatic conditions found in the tundra today. Early work [7] and more recent reviews [74] indicate that herbivores can drive vegetation shifts from moss- and shrub-dominated tundra to graminoid-dominated vegetation. Since then, numerous studies have added support for the notion that herbivores can indeed drive these types of regime shifts in arctic tundra. Numerous studies have shown that reindeer can drive vegetation shifts from moss- or shrub-dominated tundra to graminoid-dominated vegetation both when concentrated by fences [25,27] and elsewhere [75–78]. A striking example that herbivores can cause graminoid expansion at large spatial scales is the introduction of caribou to herbivore-free islands off the coast of Alaska. Fifty years after introducing the caribou, graminoid biomass had more than doubled across the islands [23]. Moreover, studies using historical Sami reindeer herding sites (historical milking grounds) show that when these grasslands are created, they can be stable for centuries [35]. Modern ecological studies question if we should refer to the mammoth steppe as grasslands, because ancient DNA metabarcoding suggests that it was rather dominated by forbs until the appearance of moist tundra dominated by woody plants, and that both forbs and graminoids were important parts of the megaherbivores diet [8]. This does not contradict contemporary data, because the grazing-induced grasslands close to management fences [25] (figure 2b) or historical milking grounds [35] (figure 2c–e) both have high densities and richness of forbs.
Another aspect of this hypothesis that can be tested by contemporary data is whether herbivores support a higher primary production via increased summer soil temperatures and increased nutrient cycling (figure 2a). Studies of Svalbard reindeer [77,79,80], as well as domesticated reindeer in Fennoscandia, clearly show that reindeer grazing increases summer soil temperatures by reducing the insulating capacity of the moss- or dwarf shrub layer [27,81,82], litter quality [83], nitrogen mineralization rates in the soil and primary production [27,81]. As well, graminoid density and tissue nitrogen concentration are greater on intensely grazed swards on caribou summer ranges in Alaska than on lightly grazed swards [84]. Contemporary studies also indicate that urine and faeces are important nutrient sources for tundra plants, and that the nutrients are often taken up by plants before it reaches the organic soil horizon [85,86]. Caribou and reindeer can thus, at least at a local scale, drive state shifts similar to the one that occurred in the Late Pleistocene. Moreover, a more diverse herbivore guild including large megaherbivores would probably have even greater potential to drive vegetation state shifts (figure 2c).
One crucial unknown, however, is how this herbaceous vegetation state could support the high density of herbivores. Although individual studies have reported that herbivores can cause a 10-fold increase in productivity (cited in [7]), only modest increases in productivity have been recorded in most studies [20,27,35,87,88]. So, even though contemporary data suggest that the diverse herbivore guild grazing the mammoth steppe could drive these kinds of vegetation shifts, it remains an open question whether the present tundra vegetation could be shifted to an herbaceous mammoth steppe under current climate if a diverse assembly of herbivores were introduced.
6. Implications for rewilding of tundra ecosystems
The Arctic tundra is one of the biomes on Earth that is least influenced by humans. Nonetheless, human exploitation has resulted in a species-poor assemblage of large herbivores that probably has had large effects on the structure and function of Arctic ecosystems. Part of this process is irreversible using methods available today, because most species of megaherbivores that once inhabited the tundra have been extinct for millennia, including woolly mammoth, woolly rhino and mastodon. Even though the potential to clone these species has been seriously discussed [89], current rewilding efforts must rely on the species that are extant.
Since the current Arctic large herbivore assembly is so species-poor, the potential to increase the diversity is substantial. Both reindeer [22,23] and muskoxen [90] have been introduced to many places in the Arctic during the last century. Some of these introductions have been successful and resulted in large stable populations like muskoxen on eastern Greenland or caribou on parts of Svalbard. However, in other sites, introduced populations have crashed [22], and introductions have failed. It does not, to our knowledge, exist any systematic evaluations of the introduction of reindeer and muskoxen in the Arctic, so presently the eventual success of such introductions must be based on our knowledge of the basic requirements of these animals. It is difficult to identify any additional herbivores that are suitable for rewilding the Arctic herbivore assembly in general. Most of the Arctic specialists, including megaherbivores, are extinct and the remaining large herbivores occasionally found in the Arctic are predominantly boreal species, and many of them have restricted distribution. Introducing these species to new places will surely involve ethical concerns of spreading invasive species [91]. However, in most of the Arctic, there are locally large herbivores present that could be used in rewilding efforts. In Scandinavia and parts of Russia, moose can be found in the open tundra predominantly along streams, and American bison is present in and close to the tundra in many places in North America. Both these species could be suitable targets if there is a wish to increase the diversity of large herbivores in the Arctic. There are numerous other extant herbivores that could be suitable in this context as well. Horses, ass and camels are still common in a global context, but probably not genetically similar to the ones historically grazing on the tundra. Large-scale introductions of these herbivores would require biological and ethical considerations outside the scope of this paper. Identifying potential herbivores invading the Arctic in the future and species suitable for introduction programmes is an important future research task.
The most ambitious rewilding effort in the Arctic is the Pleistocene Park founded by Sergei Zimov. The Pleistocene Park is a nature reserve on the Kolyma River south of Chersky in the Sakha Republic, Russia, where large herbivores are reintroduced to restore the Pleistocene mammoth steppe and save the large amount of permafrost stored in the yeodoma permafrost soils. In an area of about 1600 ha, herbivores including horses, moose, reindeer, muskox, wapiti and bison have been reintroduced. The exact success of all these introductions are not evaluated scientifically, but at least it is reported on their webpage (http://www.pleistocenepark.ru/en) that all these herbivores survive in the park. Although such ambitious rewilding efforts are clearly controversial, and surely going to be restricted to isolated efforts, the potential gains in protecting the carbon stored in the permafrost [68] and increasing the albedo [82] might potentially motivate extreme action.
A remaining question is also how certain we can be that a rewilding of the herbivore assembly will provide the ecosystem services that we have discussed earlier in this paper. There is solid evidence that herbivores will reduce the abundance of shrubs and that higher densities and diversity of herbivores will be more efficient in doing so. There is also solid evidence that this reduced shrub abundance will result in a higher albedo [82]. The effects on the carbon storage are, however, uncertain. As stated above, it has been proposed that the high herbivore densities preserved the permafrost and the carbon stored in it at the Late Pleistocene by reducing the insulation of the soil especially during winter [68]. Although this is plausible, several contemporary studies have recorded warmer soils during summer in areas grazed by reindeer [82,83]. Reindeer also reduce the gross primary production in the short term by reducing the abundance of shrubs [18], and a neutral effect of reindeer on carbon storage have been recorded in several studies [27,35]. Moreover, permafrost collapsed after shrub removal experiment and turned the tundra into a carbon and methane source [92]. More research is needed to understand how a rewilding of the herbivore assembly would influence soil carbon storage in the Arctic.
Data accessibility
This article has no additional data.
Authors' contribution
J.O. wrote the manuscript with substantial contributions from E.P.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Swedish Research Council grant no. 2017-04515 to J.O.
References
- 1.Ims RA, Fuglei E. 2005. Trophic interactions in tundra ecosystems and the impact of climate change. Bioscience 55, 311–322. ( 10.1641/0006-3568(2005)055%5B0311:TICITE%5D2.0.CO;2) [DOI] [Google Scholar]
- 2.Post E, et al. 2009. Ecological dynamics across the Arctic associated with recent climate change. Science 325, 1355–1358. ( 10.1126/science.1173113) [DOI] [PubMed] [Google Scholar]
- 3.Barrio IC, et al. 2016. Biotic interactions mediate patterns of herbivore diversity in the Arctic. Global Ecol. Biogeogr. 25, 1108–1118. ( 10.1111/geb.12470) [DOI] [Google Scholar]
- 4.Uboni A, Horstkotte T, Kaarlejärvi E, Seveque A, Stammler F, Olofsson J, Forbes BC, Moen J. 2016. Long-term trends and role of climate in the population dynamics for Eurasian reindeer. PLoS ONE 11, e0158359 ( 10.1371/journal.pone.0158359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallory CD, Boyce MS. 2018. Observed and predicted effects of climate change on Arctic caribou and reindeer. Environ. Rev. 26, 13–26. ( 10.1139/er-2017-0032) [DOI] [Google Scholar]
- 6.Gunn A, Forchhammer MC. 2008. Ovibos moschatus. In: IUCN 2018. The IUCN Red List of Threatened Species. Version 2018-1. (http://www.iucnredlist.org) [Online; downloaded on 03 June 2018.].
- 7.Zimov SA, Chuprynin VI, Oreshko AP, Chapin FS, Reynolds JF, Chapin MC. 1995. Steppe-tundra transition—a herbivore-driven biome shift at the end of the pleistocene. Am. Nat. 146, 765–794. ( 10.1086/285824) [DOI] [Google Scholar]
- 8.Willerslev E, et al. 2014. Fifty thousand years of Arctic vegetation and megafaunal diet. Nature 506, 47–52. ( 10.1038/nature12921) [DOI] [PubMed] [Google Scholar]
- 9.Koch PL, Barnosky AD. 2006. Late Quaternary extinctions: state of the debate. Annu. Rev. Ecol., Evol. Syst. 37, 215–250. ( 10.1146/annurev.ecolsys.34.011802.132415) [DOI] [Google Scholar]
- 10.Carrasco MA, Barnosky AD, Graham RW. 2009. Quantifying the extent of North American mammal extinction relative to the pre-Anthropogenic baseline. PLoS ONE 4, e8331 ( 10.1371/journal.pone.0008331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheremetev IS, Rozenfeld SB, Sipko TP, Gruzdev AR. 2014. Extinction of large herbivore mammals: niche characteristics of musk ox Ovibos moschatus and reindeer Rangifer tarandus coexisting in isolation. Zh. Obshch. Biol. 75, 62–73. [PubMed] [Google Scholar]
- 12.Shapiro B, et al. 2004. Rise and fall of the Beringian steppe bison. Science 306, 1561–1565. ( 10.1126/science.1101074) [DOI] [PubMed] [Google Scholar]
- 13.Nogués-Bravo D, Rodiguez J, Hortal J, Batra P, Araujo MB. 2008. Climate change, humans, and the extinction of the woolly mammoth. PLoS Biol. 6, 685–692. ( 10.1371/journal.pbio.0060079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjørnstad G, Flagstad Ø, Hufthammer AK, Røed KH. 2012. Ancient DNA reveals a major genetic change during the transition from hunting economy to reindeer husbandry in northern Scandinavia. J. Archaeol. Sci. 39, 102–108. ( 10.1016/j.jas.2011.09.006) [DOI] [Google Scholar]
- 15.Bernes C, Bråthen KA, Forbes BC, Speed JDM, Moen J. 2015. What are the impacts of reindeer/caribou (Rangifer tarandus L.) on arctic and alpine vegetation? A systematic review. Environ. Evidence 4, 4 ( 10.1186/s13750-014-0030-3) [DOI] [Google Scholar]
- 16.Jefferies RL, Klein DR, Shaver GR. 1994. Vertebrate herbivores and northern plant communities—reciprocal influences and responses. Oikos 71, 193–206. ( 10.2307/3546267) [DOI] [Google Scholar]
- 17.Suominen O, Olofsson J. 2000. Impacts of semi-domesticated reindeer on structure of tundra and forest communities: a review. Ann. Zool. Fennici 37, 233–249. [Google Scholar]
- 18.Cahoon SMP, Sullivan PF, Post E, Welker JM. 2012. Large herbivores limit CO2 uptake and suppress carbon cycle responses to warming in West Greenland. Glob. Chang. Biol. 18, 469–479. ( 10.1111/j.1365-2486.2011.02528.x) [DOI] [Google Scholar]
- 19.Christie KS, Bryant JP, Gough L, Ravolainen VT, Ruess RW, Tape KD. 2015. The role of vertebrate herbivores in regulating shrub expansion in the Arctic: a synthesis. BioScience 65, 1123–1133. [Google Scholar]
- 20.Väisänen M, Ylänne H, Kaarlejärvi E, Sjögersten S, Olofsson J, Crout N, Stark S. 2014. Consequences of warming on tundra carbon balance determined by reindeer grazing history. Nat. Clim. Change 4, 384–388. ( 10.1038/nclimate2147) [DOI] [Google Scholar]
- 21.Vowles T, Lindwall F, Ekblad A, Bahram M, Furneaux BR, Ryberg M, Björk RG. 2018. Complex effects of mammalian grazing on extrametrical mycelial biomass in the Scandes forest-tundra ecotone. Ecol. Evol. 8, 1019–1030. ( 10.1002/ece3.3657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein DR. 1968. Introduction, increase, and crash of reindeer on St. Matthew Island. J. Wildl. Manage. 32, 350–367. ( 10.2307/3798981) [DOI] [Google Scholar]
- 23.Ricca MA, Miles AK, van Vuren DH, Eviner VT. 2015. Impacts of introduced Rangifer on ecosystem processes of maritime tundra on subarctic islands. Ecosphere 7, e01219 ( 10.1002/ecs2.1219) [DOI] [Google Scholar]
- 24.Kitti H, Forbes B, Oksanen L. 2009. Long- and short-term effects of reindeer grazing on tundra wetland vegetation. Polar Biol. 32, 253–361. ( 10.1007/s00300-008-0526-9) [DOI] [Google Scholar]
- 25.Olofsson J, Kitti H, Rautiainen P, Stark S, Oksanen L. 2001. Effects of summer grazing by reindeer on composition of vegetation, productivity and nitrogen cycling. Ecography 24, 13–24. ( 10.1034/j.1600-0587.2001.240103.x) [DOI] [Google Scholar]
- 26.Biuw M, et al. 2014. Long-term impacts of contrasting management of large ungulates in the Arctic tundra-forest ecotone: ecosystem structure and climate feedback. Ecosystems 17, 890–905. ( 10.1007/s10021-014-9767-3) [DOI] [Google Scholar]
- 27.Olofsson J, Stark S, Oksanen L. 2004. Reindeer influence on ecosystem processes in the tundra. Oikos 105, 386–396. ( 10.1111/j.0030-1299.2004.13048.x) [DOI] [Google Scholar]
- 28.Post E, Pedersen C. 2008. Opposing plant community responses to warming with and without herbivores. Proc. Natl Acad. Sci. USA 34, 12 353–12 358. ( 10.1073/pnas.0802421105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Post E. 2013. Erosion of community diversity and stability by herbivore removal under warming. Proc. R. Soc. B 280, 20122722 ( 10.1098/rspb.2012.2722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosbacher JB, Kristensen DK, Michelsen A, Stelvig M, Schmidt NM. 2016. Quantifying muskox plant biomass removal and spatial relocation of nitrogen in a high arctic tundra ecosystem. Arct. Antarct. Alp Res. 48, 229–240. ( 10.1657/AAAR0015-034) [DOI] [Google Scholar]
- 31.Olofsson J. 2006. Short- and long-term effects of changes in reindeer grazing pressure on tundra heath vegetation. J. Ecol. 94, 431–440. ( 10.1111/j.1365-2745.2006.01100.x) [DOI] [Google Scholar]
- 32.Olofsson J, te Beest M, Ericson L. 2013. Complex biotic interactions drive long-term vegetation dynamics in a subarctic ecosystem. Phil. Trans. R. Soc. B 368, 20120486 ( 10.1098/rstb.2012.0486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskelinen A, Kaarlejärvi E, Olofsson J. 2017. Herbivory and nutrient limitation protect warming tundra from lowland species' invasion and diversity loss. Glob. Change Biol. 23, 245–255. ( 10.1111/gcb.13397) [DOI] [PubMed] [Google Scholar]
- 34.Kaarlejärvi E, Eskelinen A, Olofsson J. 2017. Herbivores rescue diversity in warming tundra by modulating trait-dependent species losses and gains. Nat. Commun. 8, 419 ( 10.1038/s41467-017-00554-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egelkraut D, Aronsson KÅ, Allard A, Åkerholm M, Stark S, Olofsson J. 2018. Multiple feedbacks contribute to a centennial legacy of reindeer on tundra vegetation. Ecosystems. In press ( 10.1007/s10021-018-0239-z) [DOI] [Google Scholar]
- 36.Vowles T, Lovehav C, Molau U, Björk RG. 2017. Contrasting impacts of reindeer grazing in two tundra grasslands. Environ. Res. Lett. 12, 034018 ( 10.1088/1748-9326/aa62af) [DOI] [Google Scholar]
- 37.Elmendorf SC, et al. 2012. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Change 2, 453–457. ( 10.1038/nclimate1465) [DOI] [Google Scholar]
- 38.Elmendorf SC, et al. 2012. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol. Lett. 15, 164–175. ( 10.1111/j.1461-0248.2011.01716.x) [DOI] [PubMed] [Google Scholar]
- 39.Myers-Smith IH, et al. 2011. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Envir. Res. Lett. 6, 045509 ( 10.1088/1748-9326/6/4/045509) [DOI] [Google Scholar]
- 40.Myers-Smith IH, et al. 2015. Climate sensitivity of shrub growth across the tundra biome. Nat. Clim. Change 5, 887–892. ( 10.1038/nclimate2697) [DOI] [Google Scholar]
- 41.Zhu Z, et al. 2016. Greening of the earth and its drivers. Nat. Clim. Change 6, 791–795. ( 10.1038/nclimate3004) [DOI] [Google Scholar]
- 42.Gottfried M, et al. 2012. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2, 111–115. ( 10.1038/nclimate1329) [DOI] [Google Scholar]
- 43.Olofsson J, Oksanen L, Callaghan T, Hulme PE, Oksanen T, Suominen O. 2009. Herbivores inhibit climate-driven shrub expansion on the tundra. Global Change Biol. 15, 2681–2693. ( 10.1111/j.1365-2486.2009.01935.x) [DOI] [Google Scholar]
- 44.Kaarlejärvi E, Eskelinen A, Olofsson J. 2013. Herbivory prevents positive responses of lowland plants to warmer and more fertile conditions at high altitudes. Funct. Ecol. 27, 1244–1253. ( 10.1111/1365-2435.12113) [DOI] [Google Scholar]
- 45.Kaarlejärvi E, Olofsson J. 2014. Concurrent biotic interactions influence plant performance at their altitudinal distribution margins. Oikos 123, 943–952. ( 10.1111/oik.01261) [DOI] [Google Scholar]
- 46.Körner C, Paulsen J. 2004. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 31, 713–732. ( 10.1111/j.1365-2699.2003.01043.x) [DOI] [Google Scholar]
- 47.Kullman L. 2013. Ecological tree line history and paleoclimate: review of megafossil evidence from the Swedish Scandes. Boreas 42, 555–567. ( 10.1111/bor.12003) [DOI] [Google Scholar]
- 48.den Herder M, Virtanen R, Roininen H. 2008. Reindeer herbivory reduces willow growth and grouse forage in a forest-tundra ecotone. Basic Appl. Ecol. 9, 324–331. ( 10.1016/j.baae.2007.03.005) [DOI] [Google Scholar]
- 49.Munier A, Hermanutz L, Jacobs J, Lewis K. 2010. The interacting effects of temperature, ground disturbance, and herbivory on seedling establishment: implications for treeline advance with climate warming. Plant Ecol. 210, 19–30. ( 10.1007/s11258-010-9724-y) [DOI] [Google Scholar]
- 50.Speed JDM, Austrheim G, Hester AJ, Mysterud A. 2011. Browsing interacts with climate to determine tree-ring increment. Funct. Ecol. 25, 1018–1023. ( 10.1111/j.1365-2435.2011.01877.x) [DOI] [Google Scholar]
- 51.Speed JDM, Austrheim G, Hester AJ, Mysterud A. 2010. Experimental evidence for herbivore limitation of the treeline. Ecology 91, 3414–3420. ( 10.1890/09-2300.1) [DOI] [PubMed] [Google Scholar]
- 52.Speed JDM, Austrheim G, Hester AJ, Mysterud A. 2011. Growth limitation of mountain birch caused by sheep browsing at the altitudinal treeline. Forest Ecol. Manag. 261, 1344–1352. ( 10.1016/j.foreco.2011.01.017) [DOI] [Google Scholar]
- 53.Cairns DM, Lafon C, Moen J, Young A. 2007. Influences of animal activity on treeline position and pattern: implications for treeline responses to climate change. Phys. Geogr. 28, 419–433. ( 10.2747/0272-3646.28.5.419) [DOI] [Google Scholar]
- 54.Cairns DM, Moen J. 2004. Herbivory influences tree lines. J. Ecol. 92, 1019–1024. ( 10.1111/j.1365-2745.2004.00945.x) [DOI] [Google Scholar]
- 55.Bråthen KA, Ravolainen VT, Stien A, Tveraa T, Ims RA. 2017. Rangifer management controls a climate-sensitive tundra state transition. Ecol. Appl. 27, 2416–2427. ( 10.1002/eap.1618) [DOI] [PubMed] [Google Scholar]
- 56.Bakker ES, Gill JL, Johnson CN, Vera FWM, Sandom CJ, Asner GP, Svenning JC. 2015. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl Acad. Sci. USA 113, 847–855. ( 10.1073/pnas.1502545112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bougnounou F, Hulme PE, Oksanen L, Suominen O, Olofsson J. 2018. Role of climate and herbivory on native and alien conifer seedling recruitment at and above the Fennoscandian treeline. J. Veg. Sci. 29, 573–584. ( 10.1111/jvs.12637) [DOI] [Google Scholar]
- 58.Jepsen JU, Biuw M, Ims RA, Kapari L, Schott T, Vingstad OPL, Hagen SB. 2013. Ecosystem impacts of range expanding forest defoliator at the forest-tundra ecotone. Ecosystems 16, 561–575. ( 10.1007/s10021-012-9629-9) [DOI] [Google Scholar]
- 59.Cansler CA, McKenzie D, Halpern CB. 2018. Fire enhances the complexity of forest structure in alpine treeline ecotones. Ecosphere 9, e02091 ( 10.1002/ecs2.2091) [DOI] [Google Scholar]
- 60.Walsh SJ, Butler DR, Allen TR, Malanson GP. 1994. Influence of snow patterns and snow avalanches on the alpine treeline ecotone. J. Veg. Sci. 5, 657–672. ( 10.2307/3235881) [DOI] [Google Scholar]
- 61.Bryant JP, Joly K, Chapin FS, DeAngelis DL, Kielland K. 2014. Can antibrowsing defense regulate the spread of woody vegetation in arctic tundra? Ecography 37, 204–211. ( 10.1111/j.1600-0587.2013.00436.x) [DOI] [Google Scholar]
- 62.Sankey TT, Montagne C, Graumlich L, Lawrence R, Nielsen J. 2006. Lower forest-grassland ecotones and 20th century livestock herbivory effects in northern Mongolia. Forest Ecol. Manage. 233, 36–44. ( 10.1016/j.foreco.2006.05.070) [DOI] [Google Scholar]
- 63.Niemelä P, Chapin FS, Danell K, Bryant JP. 2001. Herbivory-mediated responses of selected boreal forests to climatic change. Clim. Change 48, 427–440. ( 10.1023/A:1010787714349) [DOI] [Google Scholar]
- 64.Milligan HT, Koricheva J. 2013. Effects of tree species richness and composition on moose winter browsing damage and foraging selectivity: an experimental study. J. Animal Ecol. 82, 739–748. ( 10.1111/1365-2656.12049) [DOI] [PubMed] [Google Scholar]
- 65.Roturier S, Bergsten U. 2006. Influence of soil scarification on reindeer foraging and damage to planted Pinus sylvestris seedlings. Scand. J. Forest Res. 21, 209–220. ( 10.1080/02827580600759441) [DOI] [Google Scholar]
- 66.Asner GP, Levick SR. 2012. Landscape-scale effects of herbivores on treefall in African savannas. Ecol. Lett. 15, 1211–1217. ( 10.1111/j.1461-0248.2012.01842.x) [DOI] [PubMed] [Google Scholar]
- 67.Zimov SA, Zimov NS, Tikhonov AN, Chapin FS. 2012. Mammoth steppe: a high-productivity phenomenon. Quaternary Sci. Rev. 57, 26–45. ( 10.1016/j.quascirev.2012.10.005) [DOI] [Google Scholar]
- 68.Zimov NS, Zimov SA, Zimova AE, Zimova GM, Chuprynin VI, Chapin FS. 2009. Carbon storage in permafrost and soils of the mammoth tundra-steppe biome: role in the global carbon budget. Geophys. Res. Lett. 36, L02502 ( 10.1029/2008GL036332) [DOI] [Google Scholar]
- 69.Guthrie RD. 1990. Frozen fauna of the mammoth steppe. Chicago: The University of Chicago Press. [Google Scholar]
- 70.Guthrie RD. 2006. New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature 441, 207–209. ( 10.1038/nature04604) [DOI] [PubMed] [Google Scholar]
- 71.Robinson GS, Burney LP, Burney DA. 2005. Landscape paleoecology and megafaunal extinction in southeastern New York State. Ecol. Monogr. 75, 295–315. ( 10.1890/03-4064) [DOI] [Google Scholar]
- 72.McNaughton SJ. 1984. Grazing lawns—animals in herds, plant form, and coevolution. Am. Nat. 124, 863–886. ( 10.1086/284321) [DOI] [Google Scholar]
- 73.McNaughton SJ, Banyikwa FF, McNaughton MM. 1997. Promotion of the cycling of diet-enhancing nutrients by African grazers. Science 278, 1798–1800. ( 10.1126/science.278.5344.1798) [DOI] [PubMed] [Google Scholar]
- 74.van der Wal R. 2006. Do herbivores cause habitat degradation or vegetation state transition? Evidence from the tundra. Oikos 114, 177–186. ( 10.1111/j.2006.0030-1299.14264.x) [DOI] [Google Scholar]
- 75.Thing H. 1984. Feeding ecology of the West Greenland caribou (Caribou tarandus groenlandicus) in Sisimiut-Kangerlussuaq region. Danish Rev. Game Biol. 12, 1–55. [Google Scholar]
- 76.Manseau M, Huot J, Crête M. 1996. Effects of summer grazing by caribou on composition and productivity of vegetation: community and landscape level. J. Ecol. 84, 503–513. ( 10.2307/2261473) [DOI] [Google Scholar]
- 77.van der Wal R, Brooker RW. 2004. Mosses mediate grazer impacts on grass abundance in arctic ecosystems. Funct. Ecol. 18, 77–86. ( 10.1111/j.1365-2435.2004.00820.x) [DOI] [Google Scholar]
- 78.Forbes BC, Fauria MM, Zetterberg P. 2010. Russian Arctic warming and greening are closely tracked by tundra shrub willows. Global Change Biol. 16, 1542–1554. ( 10.1111/j.1365-2486.2009.02047.x) [DOI] [Google Scholar]
- 79.van der Wal R, van Lieshout SMJ, Loonen MJJE. 2001. Herbivore impact on moss depth, soil temperature and arctic plant growth. Polar Biol. 24, 29–32. ( 10.1007/s003000000170) [DOI] [Google Scholar]
- 80.van der Wal R, Bardgett RD, Harrison KA, Stien A. 2004. Vertebrate herbivores and ecosystem control: cascading effects of faeces on tundra ecosystems. Ecography 27, 242–252. ( 10.1111/j.0906-7590.2004.03688.x) [DOI] [Google Scholar]
- 81.Stark S, Väisänen M. 2014. Insensitivity of soil microbial activity to temporal variation in soil N in subarctic tundra: evidence from responses to large migratory grazers. Ecosystems 17, 906–917. ( 10.1007/s10021-014-9768-2) [DOI] [Google Scholar]
- 82.te Beest M, Sitters J, Menard CB, Olofsson J. 2018. Reindeer grazing increases summer albedo by reducing shrub abundance in Arctic tundra. Environ. Res. Lett., 11, 125013 ( 10.1088/1748-9326/aa5128) [DOI] [Google Scholar]
- 83.Olofsson J, Oksanen L. 2002. Role of litter decomposition for the increased primary production in areas heavily grazed by reindeer: a litterbag experiment. Oikos 96, 507–515. ( 10.1034/j.1600-0706.2002.960312.x) [DOI] [Google Scholar]
- 84.Post ES, Klein DR. 1996. Relationships between graminoid growth form and levels of grazing by caribou (Rangifer tarandus) in Alaska. Oecologia 107, 364–372. ( 10.1007/BF00328453) [DOI] [PubMed] [Google Scholar]
- 85.Barthelemy H, Stark S, Olofsson J. 2015. Strong responses of subarctic plant communities to long-term reindeer feces manipulation. Ecosystems 18, 740–751. ( 10.1007/s10021-015-9856-y) [DOI] [Google Scholar]
- 86.Barthelemy H, Stark S, Michelsen A, Olofsson J. 2018. Urine is an important source for plants irrespectively of vegetation composition in an Arctic tundra: insights from a N-15 enriched urea tracer experiment. J. Ecol. 106, 367–378. ( 10.1111/1365-2745.12820) [DOI] [Google Scholar]
- 87.Barthelemy H, Stark S, Kytöviita MM, Olofsson J. 2017. Grazing decrease N partitioning among coexisting plant species. Funct. Ecol. 31, 2051–2060. ( 10.1111/1365-2435.12917) [DOI] [Google Scholar]
- 88.Olofsson J. 2009. Effects of simulated reindeer grazing, trampling, and waste products on nitrogen mineralization and primary production. Arct. Antarct. Alp. Res. 41, 330–338. ( 10.1657/1938-4246-41.3.330) [DOI] [Google Scholar]
- 89.Shapiro B. 2016. How to clone a mammoth: the science of de-extinction. Princeton, NJ: Princeton University Press. [Google Scholar]
- 90.Lehenaff D, Crête M. 1989. Introduction of muskoxen in northern Quebec—the demographic explosion of a colonizing herbivore. Can. J. Zool. 67, 1102–1105. ( 10.1139/z89-156) [DOI] [Google Scholar]
- 91.Pauchard A, et al. 2016. Non-native and native organisms moving into high elevation and high latitude ecosystems in an era of climate change: new challenges for ecology and conservation. Biol. Inv. 18, 345–353. ( 10.1007/s10530-015-1025-x) [DOI] [Google Scholar]
- 92.Nauta AL, et al. 2015. Permafrost collapse after shrub removal shifts tundra ecosystems to a methane source. Nat. Climate Change 5, 67–70. ( 10.1038/nclimate2446) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.