Summary
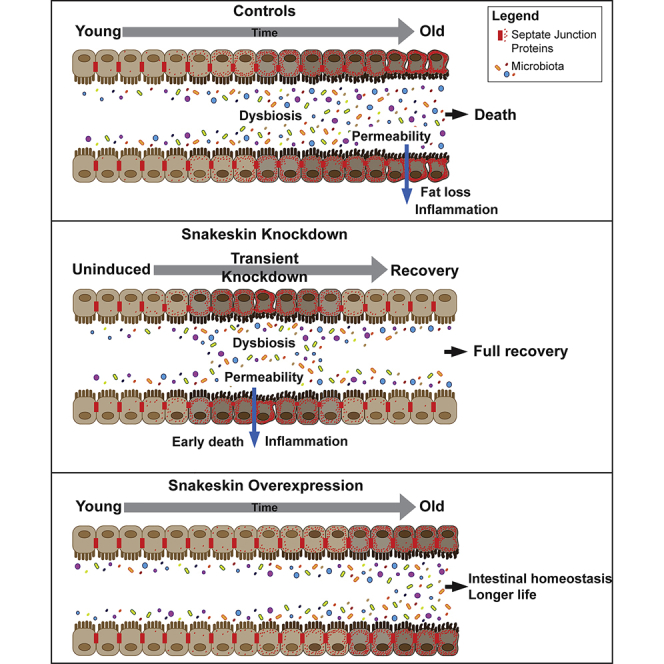
Intestinal barrier dysfunction is an evolutionarily conserved hallmark of aging, which has been linked to microbial dysbiosis, altered expression of occluding junction proteins, and impending mortality. However, the interplay between intestinal junction proteins, age-onset dysbiosis, and lifespan determination remains unclear. Here, we show that altered expression of Snakeskin (Ssk), a septate junction-specific protein, can modulate intestinal homeostasis, microbial dynamics, immune activity, and lifespan in Drosophila. Loss of Ssk leads to rapid and reversible intestinal barrier dysfunction, altered gut morphology, dysbiosis, and dramatically reduced lifespan. Remarkably, restoration of Ssk expression in flies showing intestinal barrier dysfunction rescues each of these phenotypes previously linked to aging. Intestinal up-regulation of Ssk protects against microbial translocation following oral infection with pathogenic bacteria. Furthermore, intestinal up-regulation of Ssk improves intestinal barrier function during aging, limits dysbiosis, and extends lifespan. Our findings indicate that intestinal occluding junctions may represent prolongevity targets in mammals.
Subject Areas: Molecular Mechanism of Behavior, Microbiome, Functional Aspects of Cell Biology, Model Organism
Graphical Abstract
Highlights
-
•
Loss of Ssk leads to intestinal barrier dysfunction, dysbiosis, and early-onset death
-
•
Restoration of Ssk reverses each of these age-associated phenotypes
-
•
Up-regulation of Ssk prevents bacterial translocation upon pathogenic infection
-
•
Ssk up-regulation improves barrier integrity, limits dysbiosis, and extends lifespan
Molecular Mechanism of Behavior; Microbiome; Functional Aspects of Cell Biology; Model Organism
Introduction
The intestinal epithelium acts as a selectively permeable barrier that permits the absorption of nutrients, ions, and water, while maintaining an effective defense against intraluminal toxins, antigens, and enteric microorganisms. In recent years, studies in diverse organisms, including worms (Dambroise et al., 2016, Gelino et al., 2016), flies (Dambroise et al., 2016, Rera et al., 2011, Rera et al., 2012), fish (Dambroise et al., 2016), rodents (Thevaranjan et al., 2017), and primates (Mitchell et al., 2017, Tran and Greenwood-Van Meerveld, 2013), have shown that intestinal barrier dysfunction is a pathophysiological hallmark of aging (Hu and Jasper, 2017). Loss of intestinal barrier function, in aged flies, is linked to organismal health decline, including loss of motor activity, systemic metabolic defects, and impending mortality (Clark et al., 2015, Rera et al., 2012). In addition, numerous gastrointestinal and systemic diseases, including inflammatory bowel diseases (IBDs), diabetes, multiple sclerosis, heart disease, autism, and Parkinson disease, have been associated with barrier dysfunction of the intestine (Choi et al., 2017, Farhadi et al., 2003, Hu and Jasper, 2017). Together, these findings indicate that identification of molecular targets for therapeutic intervention to improve or restore intestinal barrier function holds promise toward the goal of prolonging both health span and lifespan.
Occluding junctions play critical roles in epithelial barrier function, restricting the free diffusion of solutes between cells, as well as in the regulation of paracellular transport. In vertebrates, the occluding junctions are called tight junctions and their functional roles are well characterized (Buckley and Turner, 2018). A functionally analogous structure, called the septate junction (SJ), exists in invertebrates. In arthropods, there exist two types of SJs with morphological differences: pleated SJs, which are found in ectodermally derived epithelial cells and glial cells, and the smooth SJs (sSJs), which are found in endodermally derived epithelia, such as the midgut (Tepass and Hartenstein, 1994). Patients with IBDs can display an increase in gut permeability, or “leaky gut,” and changes in occluding junction expression (Odenwald and Turner, 2017). Although it is unclear what role occluding junction modulation plays in the initiation or perpetuation of IBDs, it has been proposed that the cellular machinery that maintains the intestinal barrier may represent a therapeutic target in both intestinal and systemic diseases (Odenwald and Turner, 2017).
Previous work in Drosophila has identified a number of age-related intestinal changes, including intestinal stem cell (ISC) deregulation (Biteau et al., 2008, Choi et al., 2008, Park et al., 2009), which are associated with a decline in intestinal function (Jasper, 2015). Age-related alterations in intestinal epithelial junction expression and localization have also been observed in both flies (Clark et al., 2015, Resnik-Docampo et al., 2017) and mammals (Meier and Sturm, 2009, Ren et al., 2014, Tran and Greenwood-Van Meerveld, 2013), yet the causal relationships between changes in occluding junction function, intestinal homeostasis, and organismal aging are only beginning to be understood. Recent work in Drosophila has shown that acute depletion of the tricellular junction (TCJ) protein, Gliotactin (Gli), in young flies leads to an increase in ISC proliferation and early-onset intestinal barrier dysfunction (Resnik-Docampo et al., 2017), indicating that altered TCJ function is sufficient to induce changes in ISC behavior previously observed in aged animals. Furthermore, age-related alterations in the composition and load of the intestinal microbiota have been shown to influence intestinal function and lifespan (Clark et al., 2015, Clark and Walker, 2018, Thevaranjan et al., 2017). Indeed, age-onset microbial dysbiosis is tightly linked to intestinal barrier dysfunction in both flies and mice (Clark et al., 2015, Thevaranjan et al., 2017). Critically, however, the question of whether manipulating intestinal occluding junction expression can delay age-onset dysbiosis and/or positively affect lifespan has not been addressed in any organism.
In this study, we show that Snakeskin (Ssk), an sSJ-specific protein (Furuse and Izumi, 2017, Yanagihashi et al., 2012), plays an important role in controlling the density and composition of the gut microbiota and that up-regulation of Ssk during aging can prolong Drosophila lifespan. More specifically, loss of intestinal Ssk in adults leads to rapid-onset intestinal barrier dysfunction, changes in gut morphology, altered expression of antimicrobial peptides (AMPs), and microbial dysbiosis. Critically, we show that these phenotypes, including intestinal barrier dysfunction and dysbiosis, can be reversed upon restored Ssk expression. Consistent with a critical role for intestinal junction proteins in organismal viability, loss of intestinal Ssk in adult animals leads to the rapid depletion of metabolic stores and rapid death. Importantly, restoring Ssk expression in flies showing intestinal barrier dysfunction prevents early-onset mortality. Moreover, intestinal up-regulation of Ssk in normal flies protects against microbial translocation, limits age-onset dysbiosis, and prolongs lifespan. Our findings support the idea that occluding junction modulation could prove an effective therapeutic approach to prolong both intestinal and organismal health during aging in other species, including mammals.
Results
Alterations in Septate Junction Proteins Occur before the Smurf Phenotype
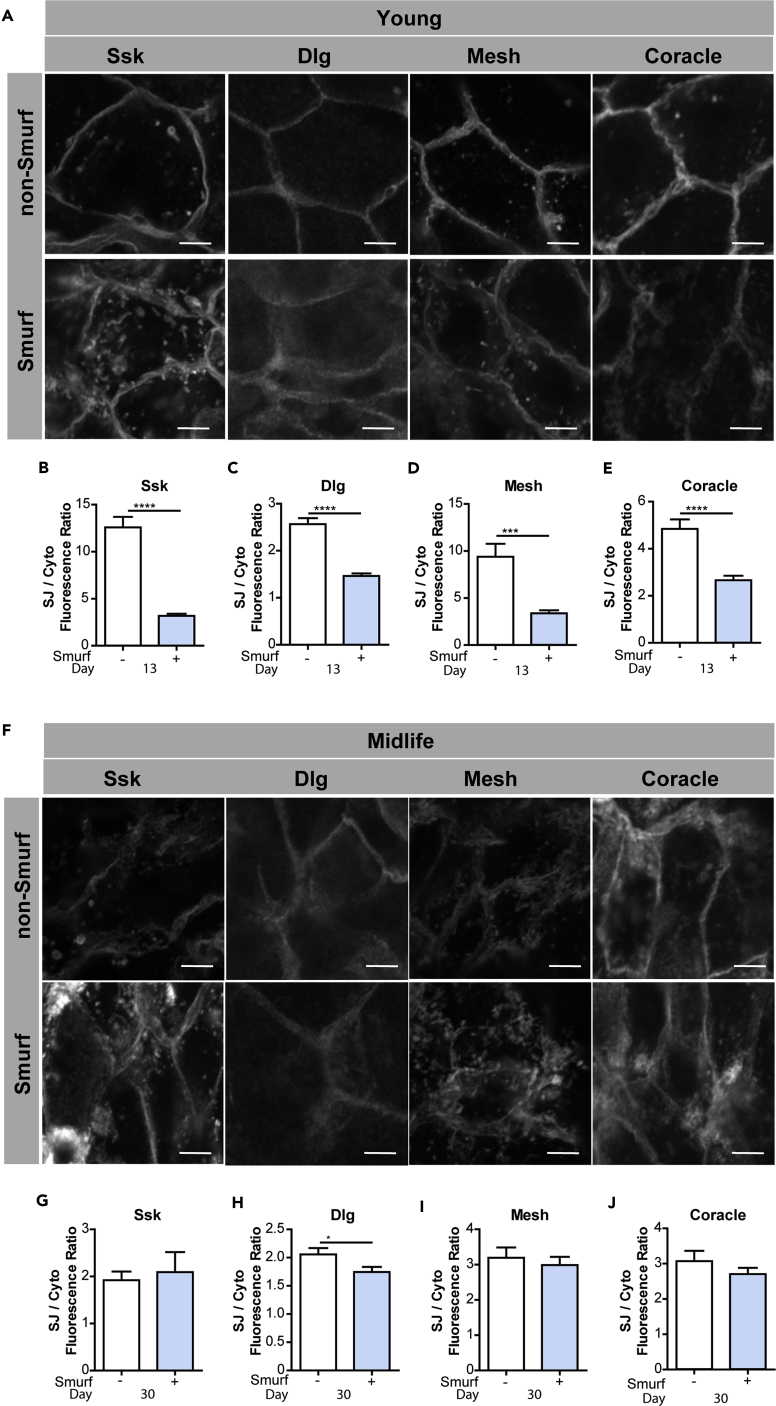
Loss of intestinal barrier function can be detected in living flies, via the Smurf assay, due to the leakage of a non-absorbable blue dye outside of the gut post-feeding (Rera et al., 2011, Rera et al., 2012). Previous work showed that flies exhibiting age-onset intestinal barrier dysfunction, or “Smurfs,” display decreased expression of SJ and adherens junction (AJ) genes and proteins, relative to age-matched controls (Clark et al., 2015). Here, we utilized confocal immunofluorescence microscopy and quantitative PCR (qPCR) to further investigate the temporal dynamics between age-onset intestinal barrier dysfunction and alterations in junction protein localization or transcript expression. In young flies, aged 13 days, significant changes in the SJ proteins Discs large (Dlg), Coracle (Cora), Ssk, and Mesh were observed in the midgut of Smurf flies, and these SJ proteins appeared to accumulate in the cytoplasm (Figures 1A–1E). More specifically, in guts from Smurf flies, we observed an increase in junction proteins localized in the cytoplasm, relative to the plasma membrane, than in the non-Smurf flies, revealing that, in young flies, mislocalization of junction proteins is correlated with barrier dysfunction. In midlife, at age 30 days, we observed a mislocalization of SJ proteins occurring in both the non-Smurf and Smurf flies when compared with young non-Smurf flies (Figures 1F–1J). These data reveal that alterations in SJ proteins occur before the onset of the Smurf phenotype. Older flies, aged 45 days, also showed no significant difference between Smurf and non-Smurf flies and exhibited severe mislocalization of SJ proteins (data not shown). Consistent with previous work (Resnik-Docampo et al., 2017), we failed to observe a decrease in transcript levels of SJ and AJ genes, in non-Smurfs during aging, indicating that downregulation of junction transcripts is not a primary mechanism underlying age-onset barrier dysfunction (Figures S1A–S1I). However, upon loss of intestinal barrier function, there is a decrease in mRNAs of genes encoding junction proteins (Clark et al., 2015).
Figure 1.
Alterations in SJs in Posterior Midguts of Smurf and Non-Smurf Flies
(A–E) SJ protein localization in ECs in Smurf or Non-Smurf midguts in 13-day-old w1118 female flies. Representative images (A) and SJ/cytoplasm fluorescence ratios (B–E) for Ssk, Dlg, Mesh, and Coracle. SJ protein mislocalization is observed in Smurf ECs, represented by an increase in cytoplasmic localization. n ≥ 14 midguts per condition; n = 10 ECs were observed per midgut; scale bar, 5 μm.
(F–J) SJ protein localization in ECs in Smurf or non-Smurf midguts in 30-day-old w1118 female flies. Representative images (F) and SJ/cytoplasm fluorescence ratios (G–J) for Ssk, Dlg, Mesh, and Coracle. SJ protein mislocalization is observed in both Smurf and non-Smurf ECs, represented by an increase in cytoplasmic localization. n ≥ 14 midguts per condition; n = 10 ECs were observed per midgut; scale bar, 5 μm.
Samples were dissected and stained in parallel under the same conditions; pictures taken at same laser intensity. Data analyzed utilizing a two-tailed unpaired Student's t test, and the error bars are the SEM range of those averages. *p < 0.05; ***p < 0.001; ****p < 0.0001 represent a statistically significant difference. See also Figure S1.
Adult-Onset Loss of Ssk Leads to Reversible Intestinal Barrier Dysfunction
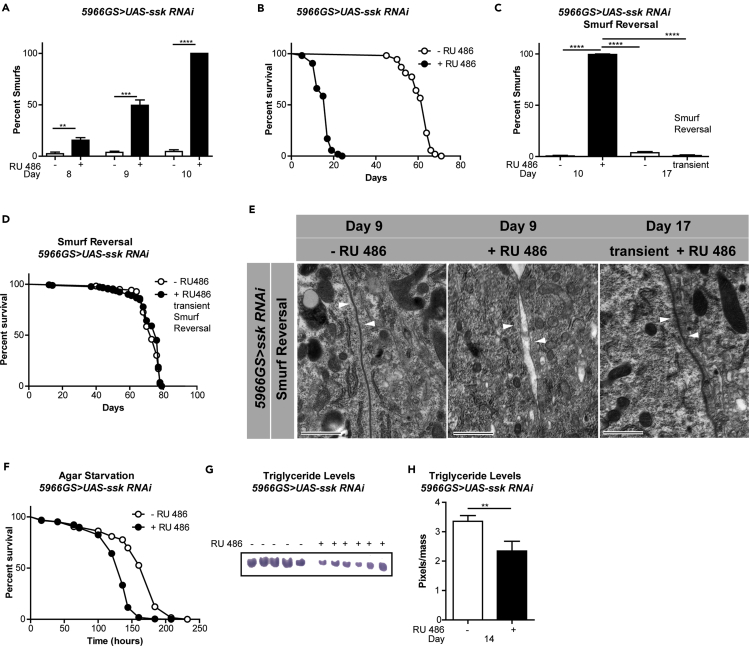
Loss of Ssk results in defective SJ formation and is embryonically lethal in Drosophila (Yanagihashi et al., 2012). In addition, it has been shown that RNAi-mediated depletion of Ssk in developing larvae impairs intestinal barrier function (Yanagihashi et al., 2012). Here, we set out to determine whether Ssk regulates intestinal barrier function in the adult gut. To do so, we utilized an RU486-inducible GeneSwitch driver that is expressed in gut enteroblasts (EBs) and post-mitotic enterocytes (ECs), 5966GS (Mathur et al., 2010), crossed to a UAS-ssk RNAi line (Yanagihashi et al., 2012). Initially, we confirmed an RU486-dependent reduction in ssk expression in the adult intestine (Figure S2A). Upon adult onset, where flies were fed RU486 starting at the age of 3 days, intestine-specific ssk knockdown led to a rapid and pronounced decrease in intestinal barrier integrity (Figure 2A). Indeed, upon 7 days of ssk knockdown, 100% of female flies displayed the Smurf phenotype. Furthermore, adult-onset knockdown of ssk conferred early-onset mortality (Figure 2B). This observation is consistent with previous results showing that the Smurf phenotype is a harbinger of death in aging flies (Rera et al., 2012). Early-onset mortality also occurs when knockdown of ssk is initiated in midlife (day 35 or day 45, Figure S2B). In addition, adult-onset loss of intestinal Ssk leads to intestinal barrier dysfunction and early death in male flies (Figures S2C and S2D).
Figure 2.
Loss of Intestinal Ssk Leads to Rapid and Reversible Intestinal Barrier Dysfunction and Early-Onset Mortality
(A) Intestinal integrity of 5966GS > UAS-ssk RNAi females with or without RU486-mediated transgene induction from day 3 onward. Percentage Smurfs were assessed at 8, 9, and 10 days of age. One-way ANOVA/Bonferroni's multiple comparisons test; n > 179 flies/condition.
(B) Survival curves of 5966GS > UAS-ssk RNAi females with or without RU486-mediated transgene induction from day 3 onward. p < .0001, log rank test; n > 107 flies/condition. Median lifespan 17 days and 64 days, respectively. Maximum lifespan 24 days and 71 days, respectively.
(C) Reversal of intestinal integrity of 5966GS > UAS-ssk RNAi females with or without RU486-mediated transgene induction from day 3 until day 10, when all the induced flies become Smurfs, then moved to RU486− food for 7 days and assayed again for barrier integrity on day 17. n > 222/condition; p < 0.0001, one-way ANOVA/Bonferroni's multiple comparisons test.
(D) Survival curves of 5966GS > UAS-ssk RNAi females with or without RU486-mediated transgene induction from day 3 to day 10, then without RU486-mediated transgene induction from day 10 onward. No statistical difference, log rank test; n > 182 flies/condition. Median lifespan 76 and 77 days, respectively.
(E) Electron micrographs of histological sections from dissected midguts of 5966GS > UAS-ssk RNAi females with or without RU486-mediated transgene induction from day 3 to day 9 or transiently from day 3 until day 10, when all the induced flies become Smurfs, then moved to RU486− food for 7 days and imaged at day 17. Arrows point to SJ; scale bar, 800 nm.
(F) Survival curves without food of 5966GS > UAS-ssk RNAi females at 9 days of age with or without RU486-mediated transgene induction from day 3 to day 9, then placed on agar until death. p < 0.0001; log rank test; n > 188 flies.
(G and H) Thin-layer chromatography image (G) and quantification (H) of whole-body lipid stores of 5966GS > UAS-ssk RNAi females at 14 days of age with or without RU486-mediated transgene induction from day 3 to day 14. n = 5 and n = 6 biological replicates with five flies per replicate; p < 0.01; two-tailed unpaired Student's t test.
**p < 0.01; ***p < 0.001; ****p < 0.0001 represent a statistically significant difference. Error bars on bar graphs depict mean ± SEM. See also Figure S2.
To better understand the role of ssk expression in barrier integrity in the adult intestine, we examined whether restoring ssk expression could rescue intestinal barrier function in Smurf flies. To do so, we examined the impact of transient knockdown of Ssk in the adult intestine. Following 7 days of RU486-mediated Ssk knockdown, after which time 100% of the flies displayed the Smurf phenotype, flies were allowed to recover for 1 week on food lacking RU486. Remarkably, we observed that upon restoration of ssk mRNA levels (Figure S3J), the Smurf phenotype is fully reversed, with the formerly Smurf flies now becoming non-Smurf ones (Figure 2C). Restoring intestinal barrier function in Smurf flies also prevented early-onset mortality (Figure 2D). Using an independently generated UAS-ssk RNAi line (VDRC, 11906GD, labeled UAS-ssk RNAi (III)), we confirmed that adult-onset intestinal knockdown of ssk led to reversible intestinal barrier dysfunction and early-onset mortality (Figures S2E–S2G). Feeding RU486 to control flies did not alter the intestinal barrier function or lifespan (Figures S2H and S2I). Consistent with the Smurf phenotype, electron microscopy revealed distinct gaps in SJs between adjacent ECs in midguts following intestinal knockdown of ssk (Figure 2E). This is completely reversed upon restoration of ssk mRNA levels, with the SJ becoming tight and no longer containing gaps between adjacent cells (Figure 2E).
Age-onset intestinal barrier dysfunction is linked to systemic metabolic defects, including reduced metabolic stores and impaired insulin/insulin growth factor signaling (Rera et al., 2012). Hence, we set out to determine whether acute loss of intestinal Ssk recapitulates these systemic metabolic phenotypes, previously linked to aging. Short-term, intestine-specific RNAi of ssk reduced survival on an agar-only diet (Figure 2F). This sensitivity to starvation was linked to reduced triglyceride stores (Figures 2G and 2H). One of the hallmarks of aging is the metabolic syndrome, also known as the insulin resistance syndrome, a clinical condition comprising physiological and biochemical abnormalities predisposing individuals to a host of age-onset diseases, including type 2 diabetes and cardiovascular disease (Lusis et al., 2008). In flies, aging and age-onset intestinal barrier dysfunction have been linked to Drosophila FOXO (dFOXO) activation (Guo et al., 2014, Morris et al., 2012, Rera et al., 2012), which is normally induced when IIS is repressed (Teleman, 2009). To gain insight into the impact of Ssk knockdown on dFOXO activation, we assayed the expression levels of two dFOXO target genes—Insulin-like receptor (InR) and Ecdysone-inducible gene L2 (ImpL2). Both InR and ImpL2 mRNA levels were significantly elevated in the intestine, following 6 days of ssk knockdown, and occurred before detectable barrier dysfunction (Figures S2J and S2K). In addition, InR and ImpL2 mRNA levels returned to normal 1 week after transient ssk knockdown (Figures S2L and S2M), indicating that restoring intestinal Ssk rescues dFOXO activation. Feeding RU486 to control flies did not alter FOXO activation (Figures S2N and S2O), neither was a midlife elevation in FOXO targets observed in non-Smurfs (Figures S2P and S2Q).
Ssk Knockdown Results in Mislocalization of Septate Junction Proteins and Altered Gut Morphology
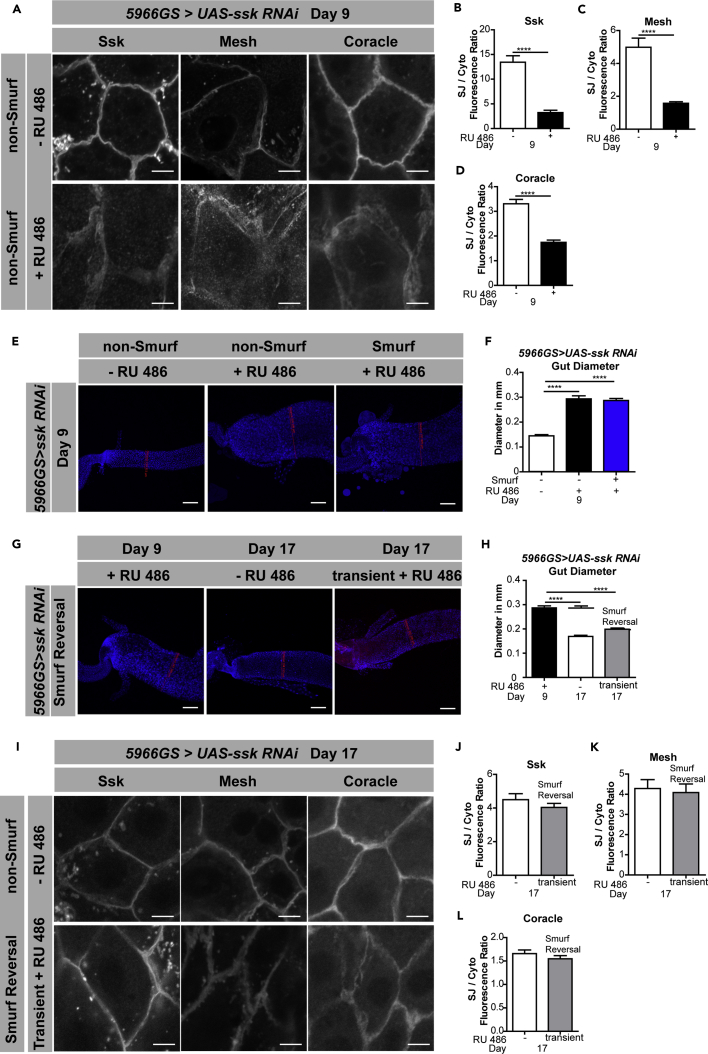
Ssk forms a protein complex with Mesh and Tetraspanin 2A (Tsp2A), and each of these three components are necessary for SJ formation during development (Furuse and Izumi, 2017, Izumi et al., 2012). We set out to investigate the role of Ssk in the maintenance of the SJ in the adult midgut. Immunofluorescent images of SJ components revealed mislocalization of junction proteins following 6 days of Ssk knockdown (Figures 3A–3D and S3A). In addition to Ssk mislocalization, there was a mislocalization of Mesh and Cora, reinforcing the idea that Ssk is required for proper localization of other SJ components or maintenance of the SJ in the adult fly, similar to its role in larvae (Furuse and Izumi, 2017, Izumi et al., 2012). In addition to changes in protein localization, there was also a reduction in the transcripts of both SJ and AJ components upon Ssk knockdown (Figure S3B). These changes in gene expression occurred before the Smurf phenotype (Figures S3C–S3H). Interestingly, after 11 days of Ssk knockdown, when the flies are close to death, we observed an increase in junction gene mRNA levels (Figure S3I), consistent with findings in flies with acute loss of the TCJ protein Gli (Resnik-Docampo et al., 2017). In addition to changes in junction protein localization and gene expression, there was also a striking effect on gut morphology. Specifically, the posterior midgut appeared shorter and wider, with a diameter almost twice that of control guts (Figures 3E and 3F).
Figure 3.
Loss of Intestinal Ssk Leads to Altered Gut Morphology and SJs
(A–D) SJ protein localization in ECs in 9-day-old 5966GS > UAS-ssk RNAi female flies with RU486-mediated transgene induction from day 3 to day 9. Representative images (A) and SJ/cytoplasm fluorescence ratios for (B) Ssk, (C) Mesh, and (D) Coracle. SJ protein mislocalization is observed in the presence of RU486 ECs, represented by an increase in cytoplasmic localization. Two-tailed unpaired Student's t test. n > 14 midguts per condition; n = 10 ECs were observed per midgut; scale bar, 5 μm.
(E and F) Representative images (E) and quantification (F) of posterior midgut diameters from dissected intestines of 5966GS > UAS-ssk RNAi female flies on day 9 with or without RU486-mediated transgene induction from day 3 to day 9. The red line shows the location measured. A significant increase in the midgut diameter is observed in Ssk knockdown flies in both Smurfs and non-Smurfs. n > 14 midguts per condition; scale bar, 50 μm.
(G and H) Representative images (G) and quantification (H) of posterior midgut diameters from dissected intestines of 5966GS > UAS-ssk RNAi female flies on day 9 with RU486-mediated transgene induction from day 3 to day 9, or on day 17, with and without RU486-mediated transgene induction from day 3 to day 10 when all induced flies become Smurfs, then removed from RU486-mediated induction for 7 more days until day 17, where flies were assayed with blue dye, to now be non-Smurfs. The red line shows the location measured. Transiently induced midgut diameter has returned to control levels. n > 14 midguts per condition; scale bar, 50 μm.
(I–L) SJ protein localization in ECs in midguts of 17-day-old 5966GS > UAS-ssk RNAi female flies with and without RU486-mediated transgene induction from day 3 to day 10 when all induced flies become Smurfs, then removed from RU486-mediated induction for 7 more days until day 17, where flies were assayed with blue dye, to now be non-Smurfs. Representative images (I) and SJ/cytoplasm fluorescence ratios for (J) Ssk, (K) Mesh and (L) Coracle. There was no significant difference in SJ protein localization, two-tailed unpaired t test. n > 14 midguts per condition; n = 10 ECs were observed per midgut; scale bar, 5 μm.
Samples were dissected and stained in parallel under same conditions, pictures taken at same laser intensity. Data analyzed with one-way ANOVA/Tukey's multiple comparisons test, unless otherwise stated, and the error bars are the SEM range of those averages. ****p < 0.0001 represent a statistically significant difference. See also Figure S3.
Remarkably, these alterations in gut morphology, occluding junction localization, and mRNA levels, that occurred following ssk knockdown, were also completely reversible. After inducing transient ssk knockdown for 7 days, until all the flies became Smurfs, followed by recovery of the Smurf flies on food lacking RU486 for 7 days, the gut morphology returned to control diameters (Figures 3G and 3H), SJ protein localization appeared normal (Figures 3I–3L), and junction gene mRNA levels returned to control levels (Figure S3J). Feeding RU486 to control flies did not alter mRNA levels of junction components, protein localization, or gut morphology in control flies (Figures S3K–S3Q). These findings illustrate that reducing the expression of Ssk causes the mislocalization of other SJ components, changes in gut morphology, and alterations in junction gene mRNA levels and that restoring Ssk expression reverses these changes.
Loss of Ssk Leads to Intestinal Stem Cell Overproliferation
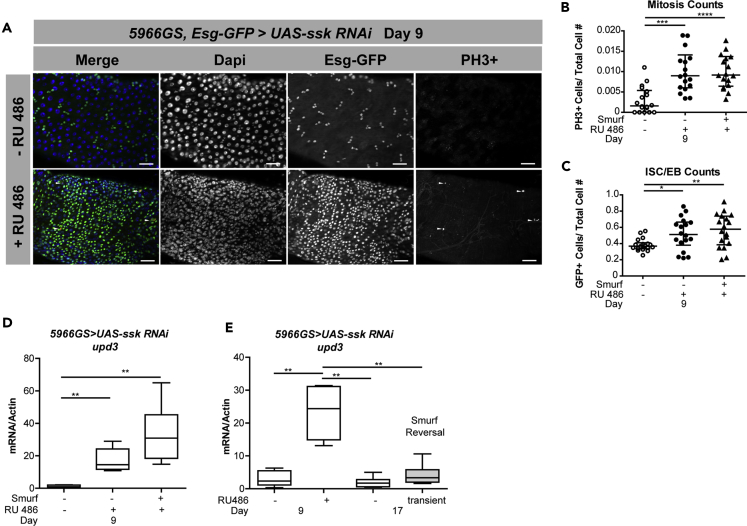
The Drosophila midgut epithelium is composed of ISCs that self-renew to maintain the ISC population and generate daughter cells termed EBs that differentiate to produce the secretory enteroendocrine cells and absorptive ECs (Micchelli and Perrimon, 2006, Ohlstein and Spradling, 2006). During aging, and in response to intestinal damage or pathogenic infection, an increase in ISC proliferation is observed, which is accompanied by a block in terminal differentiation, whereby differentiating cells exhibit both ISC/EB and EC markers (Biteau et al., 2008, Buchon et al., 2009, Choi et al., 2008, Jiang et al., 2009, Park et al., 2009). As previous work had indicated that depletion of SJ components from ECs was sufficient to trigger ISC proliferation in young flies, similar to what was observed during aging (Resnik-Docampo et al., 2017), we wanted to assess stem cell proliferation upon adult-onset depletion of Ssk from intestinal ECs. A pronounced increase in ISC proliferation and a decrease in terminal differentiation of progenitor cells was observed before Smurf detection (Figures 4A–4C), implying that the perturbation of SJs in the midgut of 9-day-old flies is sufficient to stimulate an increase in stem cell proliferation.
Figure 4.
Loss of Intestinal Ssk Leads to Overproliferation of Intestinal Stem Cells
(A–C) Representative images (A) and quantification showing mitosis counts (B) and changes in ISC/EB number (C) of posterior midguts from dissected intestines of 5966GS, esg:GFP > UAS-ssk RNAi female flies on day 9 with RU486-mediated transgene induction from day 3 to day 9. Ssk knockdown causes an increase in esg+ISC/EBs (marked by esg:GFP, green) and ISC proliferation (marked by arrowheads PH3, red) compared with controls. Scale bar, 50 μm. (B) n = 18 midguts/condition. Each data point is an average proportion calculated from four independent images per midgut, and the error bars represent the mean ± SEM of those averages; one-way ANOVA/Tukey's multiple comparisons test. ****p < 0.0001; ***p < 0.001. (C) n = 17 midguts/condition. Each data point is an average proportion calculated from four independent images per midgut, and the error bars represent the median with interquartile range of those averages. Kruskal-Wallis/Dunn multiple comparisons test. **p < 0.01; *p < 0.05.
(D) upd3, unpaired3, gene expression assayed by qPCR from dissected intestines in Smurf and non-Smurf 5966GS > UAS-ssk RNAi female flies on day 9 with or without RU486-mediated transgene induction from day 3 to day 9. n = 6 replicates of five intestines.
(E) upd3 gene expression assayed by qPCR from dissected intestines in 5966GS > UAS-ssk RNAi female flies at 9 days of age with or without RU486-mediated transgene induction from day 3 until day 10, when all the induced flies become Smurfs, and then moved to RU486 food for 7 days and assayed on day 17. n = 6 replicates of five intestines.
Boxplots display the first and third quartile, with the horizontal bar at the median and whiskers showing the most extreme data point, which is no more than 1.5 times the interquartile range from the box. Wilcoxon test unless otherwise stated. See also Figure S4.
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway has been reported to stimulate ISC proliferation in response to damage, infection, or stress (Jiang et al., 2009, Resende and Jones, 2012). Hence, we examined the mRNA levels of unpaired 3 (upd3), one of the cytokines that activates the JAK/STAT pathway in flies. Adult-onset depletion of Ssk resulted in elevations in upd3 mRNA levels before Smurf detection (Figure 4D). Feeding RU486 to control flies did not affect upd3 expression (Figure S4A). Moreover, when RU486 is removed, thereby eliminating Ssk knockdown, and the flies are allowed to recover for a week, upd3 levels return to normal (Figure 4E). Interestingly, we observed a significant increase in upd3 mRNA levels in non-Smurf flies in midlife (day 30; Figure S4B), corresponding to the mislocalization and altered expression of SJ-related proteins (Figure 1F), which is similar to the SJ protein modulation observed in aged flies (Resnik-Docampo et al., 2017). This suggests a correlation between junctional protein mislocalization, activation of the JAK/STAT pathway, and initiation of stem cell overproliferation before detectable intestinal barrier failure.
Loss of Intestinal Ssk Leads to Reversible Microbial Dysbiosis
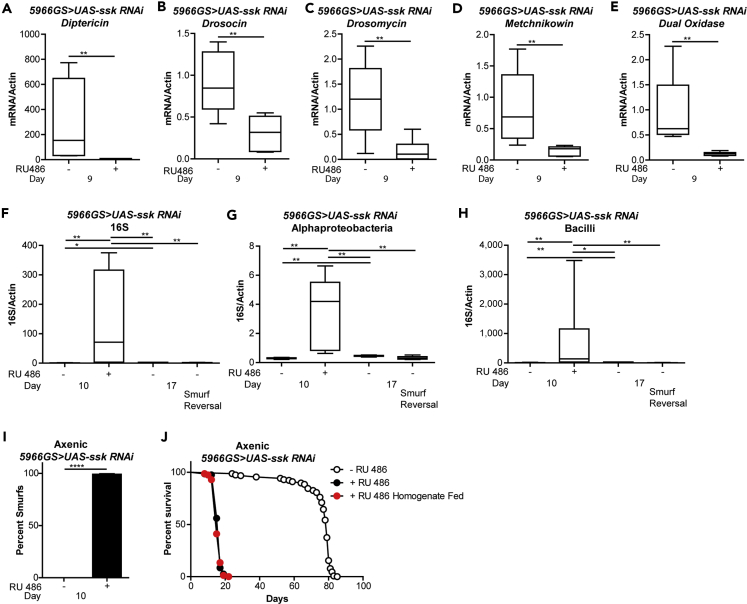
In both Drosophila and mice, age-onset intestinal barrier dysfunction is linked to microbial dysbiosis (Clark et al., 2015, Thevaranjan et al., 2017). Control of the commensal microbiota and innate immune responses to pathogenic bacteria are achieved primarily by two pathways acting in Drosophila gut ECs: expression and activation of dual oxidase (Duox), which initiates an oxidative burst response to produce high levels of reactive oxygen species (Ha et al., 2005, Ha et al., 2009a, Ha et al., 2009b), and activation of the immune deficiency (IMD/Relish) pathway, which activates the nuclear factor-κB-like transcription factor Relish and induces the expression of AMPs (Leulier and Royet, 2009). Previous studies have reported a large increase in AMP expression in flies showing age-onset intestinal barrier dysfunction (Clark et al., 2015, Rera et al., 2012). Therefore, we wanted to examine the relationship between immunity gene expression and intestinal Ssk expression. Short-term intestinal Ssk knockdown, for 6 days, led to a decrease in AMP expression (Figures 5A–5D), as well as decreased expression of Duox (Figure 5E). This reduction in immunity gene mRNA levels occurred in non-Smurf as well as Smurf flies (Figure S5A), revealing that this occurs before detectable barrier dysfunction. Feeding RU486 to control flies did not affect immunity gene expression in control flies (Figures S5B and S5C). Knockdown of Ssk for 11 days led to the activation of an immune response, with flies now exhibiting elevated AMPs and Duox levels (Figures S5D and S5E), which is consistent with previous data in aged Smurf flies (Clark et al., 2015, Rera et al., 2012).
Figure 5.
Loss of Intestinal Ssk Leads to Reversible Microbial Dysbiosis
(A–E) Immunity gene expression assayed by qPCR from dissected intestines of 5966GS > UAS-ssk RNAi female flies on day 9 with or without RU486-mediated transgene induction from day 3 to day 9. Diptericin (A), Drosocin (B), Drosomycin (C), Metchnikowin (D), and Dual Oxidase (E). n = 6 replicates of five intestines per replicate.
(F) Reversal of bacterial levels assayed by qPCR of 16S with universal primers in surface-sterilized Smurf and non-Smurf 5966GS > UAS-ssk RNAi female flies at 9 days of age with or without RU486-mediated transgene induction from day 3 to day 10, when all induced flies become Smurfs, then removed from RU486-mediated induction for 7 more days until day 17, where flies were assayed to now be non-Smurfs. n = 6 replicates of five whole flies per replicate.
(G and H) Reversal of bacterial levels assayed by taxon-specific qPCR of the Alphaproteobacteria or Bacilli 16S rRNA gene in 5966GS > UAS-ssk RNAi female flies at 9 days of age with or without RU486-mediated transgene induction from day 3 to day 10, when all induced flies become Smurfs, then removed from RU486-mediated induction for 7 more days until day 17, where flies were assayed to now be non-Smurfs. n = 6 replicates of 5 whole flies per replicate.
(I) Intestinal integrity of axenic 5966GS > UAS-ssk RNAi female flies at 10 days of age with or without RU486-mediated transgene induction from day 3 to day 10. p < 0.0001, two-tailed unpaired Student's t test; n > 155 flies/condition.
(J) Survival curves of 5966GS > UAS-ssk RNAi females conventionally reared, axenically reared, and axenically treated and exposed to fly homogenate as embryos, with or without RU486-mediated transgene induction from day 3 onward. p < .0001, log rank test; n > 155 flies/condition. Boxplots display the first and third quartiles, with the horizontal bar at the median and whiskers showing the most extreme data point, which is no more than 1.5 times the interquartile range from the box. Error bars on bar graphs depict mean ± SEM.
*p < 0.05; **p < 0.01; ****p < 0.0001; Wilcoxon test unless otherwise stated. See also Figure S5.
To determine whether altered immune gene expression was linked to changes in gut bacteria, we utilized qPCR with universal primers to the bacterial 16S rRNA gene to characterize alterations in microbiota dynamics in response to loss of intestinal Ssk. Short-term intestinal Ssk knockdown, for 7 days, led to a significant elevation in bacterial loads (Figure 5F), with these changes occurring before Smurf detection (Figures S5F and S5G). Next, we utilized primers to the 16S rRNA gene that are specific to the classes Bacilli, Gammaproteobacteria, and Alphaproteobacteria (Clark et al., 2015). Upon knockdown of intestinal Ssk, there was an increase in Alphaproteobacteria and Bacilli (Figures S5H), with these changes also occurring before Smurf detection (Figures S5I). There was no significant change in Gammaproteobacteria (Figure S5J). Elevations in microbial content and dysbiosis were also observed upon knockdown of Ssk in aged flies (75 days old), implying that even when there are higher levels of commensal bacteria in aged flies, perturbing SJs can still have a significant impact on microbiota dynamics (Figures S5K and S5L). Strikingly, restoring Ssk in the adult intestine by removing RU486 led to restoration of commensal homeostasis after 7 days (Figures 5F–5H and S5M), which was maintained throughout the lifespan of the fly (Figures S5N and S5O). Together, these data support the idea that loss of Ssk, alone, can cause dysbiosis, which is reversible upon resumption of Ssk expression. Restoring Ssk expression for 7 days, which promotes commensal homeostasis, was associated with increased expression of certain AMPs, including a significant increase in Diptericin (Figures S5P and S5Q).
Loss of commensal control during aging, including a large increase in microbial load, impairs intestinal function and drives mortality in aged flies showing intestinal barrier dysfunction (Clark et al., 2015). Hence, we set out to examine whether dysbiosis in Ssk knockdown flies contributes to intestinal barrier dysfunction and/or limits lifespan. When flies were maintained under axenic conditions (Figure S5R) or fed antibiotics, intestinal knockdown of Ssk conferred rapid intestinal barrier dysfunction and mortality at a similar rate as in conventionally raised flies (Figures 5I, 5J, and S5S). In addition, under axenic conditions, intestinal Ssk knockdown still led to an increase in dFOXO targets, mislocalization of SJ components (data not shown), altered gut morphology, and an increase of upd3, with no significant alterations in AMP or Duox transcripts (Figures S5T–S5Y). Together, these findings indicate that loss of intestinal Ssk does not affect intestinal barrier function or lifespan via altered microbial dynamics or an altered immune response. This suggests that altering SJ integrity, by knocking down one critical component, is sufficient to lead to hallmarks of the aged gut and a greatly diminished lifespan, even in the absence of bacteria.
Up-Regulation of Ssk Prevents Bacterial Translocation upon Pathogenic Infection
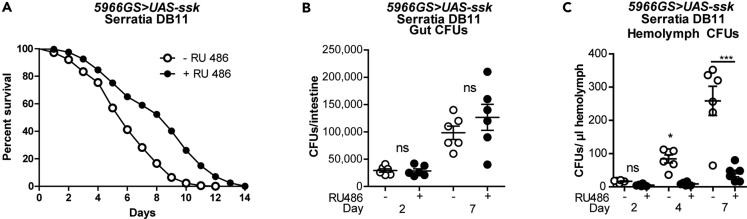
Serratia marcescens is an entomopathogenic bacterium that opportunistically infects a wide range of hosts, including humans. If ingested by Drosophila, S. marcescens can traverse the intestinal epithelium into the body cavity, leading to death within 6 days (Nehme et al., 2007). Here, we set out to determine whether manipulating Ssk expression can protect against S. marcescens-induced pathogenicity. To do so, we have used S. marcescens Db11 (Nehme et al., 2007). First, using a UAS-ssk transgene (Yanagihashi et al., 2012), we confirmed an RU486-dependent induction of ssk in the intestine (Figure S6A). Intestinal up-regulation of Ssk for 20 days resulted in a significant increase in survival following oral infection of Db11 (Figure 6A). Importantly, we observed no difference in bacterial loads in dissected guts following oral infection of Db11 (Figure 6B). However, intestinal up-regulation of Ssk led to a highly significant reduction in bacterial load in the hemolymph, which is the circulatory system of the fly (Figure 6C). These findings indicate that the up-regulation of Ssk can improve intestinal barrier integrity under these conditions to limit bacterial translocation. Feeding RU486 to control flies did not alter the lifespan or bacterial levels in the gut or hemolymph following oral infection of Db11 (Figures S6B–S6D).
Figure 6.
Intestinal Ssk Reduces Bacterial Translocation upon Oral Infection with Pathogenic Bacteria
(A) Survival curves of 5966GS > UAS-ssk females with or without RU486-mediated transgene induction from day 3 until day 20; then Db11 bacteria were fed to the flies and survival plotted. p < .0001, log rank test; n > 216 flies/condition.
(B) S. marcescens Db11 colony-forming units (CFUs) from dissected midguts of 5966GS > UAS-ssk females with or without RU486-mediated transgene induction from day 3 until day 20. There is no significant difference; one-way ANOVA/Tukey's multiple comparisons test. Error bars depict mean ± SEM.
(C) S. marcescens Db11 CFUs from the hemolymph of 5966GS > UAS-ssk females with or without RU486-mediated transgene induction from day 3 until day 20. There is significantly less bacteria in the hemolymph when Ssk is up-regulated. ***p < .0001; one-way ANOVA/Tukey's multiple comparisons test. Error bars depict mean ± SEM.
See also Figure S6.
Up-Regulation of Ssk Improves Barrier Integrity, Limits Dysbiosis during Aging, and Extends Lifespan
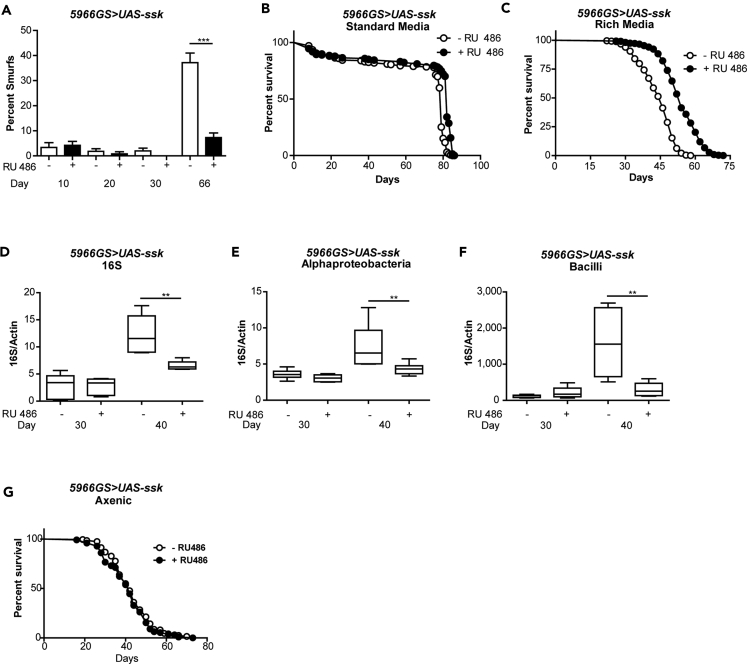
Next, we set out to determine whether Ssk could improve intestinal homeostasis during aging and/or promote longevity. We overexpressed Ssk in wild-type flies and conducted a series of physiological assays to examine the impact of Ssk on aging and lifespan determination. Remarkably, we observed a delay in the onset of intestinal barrier dysfunction during aging upon intestinal Ssk up-regulation (Figure 7A). Moreover, intestinal up-regulation of Ssk resulted in a modest extension of lifespan when compared with isogenic controls (Figure 7B). Interestingly, on a diet containing yeast extract, which is more nutritionally dense than whole yeast (Bass et al., 2007), intestinal up-regulation of Ssk resulted in a more pronounced extension of lifespan (Figure 7C). This may be because as the concentration of yeast is increased, the bacterial load in control flies is elevated with a concomitant decrease in lifespan (Figures S7A and S7B). Up-regulating intestinal Ssk during aging also delayed the age-related increase in bacterial load and reduced the levels of both Alphaproteobacteria and Bacilli in aged animals (Figures 7D–7F and S7C). Importantly, the lifespan extension mediated by intestinal Ssk is eliminated when flies are either treated with antibiotics or raised under axenic conditions (Figures 7G and S7D). Together, our findings are consistent with a model in which up-regulating intestinal Ssk prolongs lifespan via improved commensal homeostasis.
Figure 7.
Up-Regulation of Ssk Improves Intestinal Integrity during Aging and Prolongs Lifespan
(A) Intestinal integrity of 5966GS > UAS-ssk females with or without RU486-mediated transgene induction from day 3 onward on standard media. Percentage Smurfs were assessed with blue dye at 10, 20, 30, and 66 days of age. ***p < .0001, one-way ANOVA/Bonferroni's multiple comparisons test; n > 174 flies/condition.
(B) Survival curves of 5966GS > UAS-ssk females with or without RU486-mediated transgene induction from day 3 onward on standard media. p < .0001, log rank test; n > 159 flies/condition. Median lifespan 79 and 82 days, respectively.
(C) Survival curves of 5966GS > UAS-ssk females with or without RU486-mediated transgene induction from day 3 onward on rich media. p < .0001, log rank test; n > 288 flies/condition. Median lifespan 48 days and 54 days, respectively.
(D) Bacterial levels assayed by qPCR of 16S with universal primers in 5966GS > UAS-ssk female flies at 30 and 40 days of age with or without RU486-mediated transgene induction from day 3 onward on rich media, n = 6 replicates of 5 flies.
(E and F) Bacterial levels assayed by taxon-specific qPCR of the Alphaproteobacteria or Bacilli 16S rRNA gene in 5966GS > UAS-ssk female flies at 30 and 40 days of age with or without RU486-mediated transgene induction from day 3 onward on rich media, n = 6 replicates of 5 flies.
(G) Survival curves of axenic 5966GS > UAS-ssk females with or without RU486-mediated transgene induction from day 3 onward on rich media. There was no significant difference, log rank test; n > 197 flies/condition. Median lifespan 42 days.
Boxplots display the first and third quartile, with the horizontal bar at the median and whiskers showing the most extreme data point, which is no more than 1.5 times the interquartile range from the box. Error bars on bar graphs depict mean ± SEM. **p < 0.01; ***p < 0.001; Wilcoxon test unless otherwise stated. See also Figure S7.
Discussion
Aging is characterized by progressive health decline leading to mortality, yet the underlying pathophysiology remains elusive. There is an emerging understanding that maintaining intestinal barrier function during aging is critical to organismal health and longevity (Cesar Machado and da Silva, 2016, Choi et al., 2017, Clark et al., 2015, Clark and Walker, 2018, Hu and Jasper, 2017, Rera et al., 2013, Rera et al., 2012, Thevaranjan et al., 2017). At the same time, an age-related remodeling of epithelial junctions has been implicated in loss of barrier function in aged animals (Clark et al., 2015, Meier and Sturm, 2009, Ren et al., 2014, Resnik-Docampo et al., 2017, Tran and Greenwood-Van Meerveld, 2013). These findings suggest that strategies to maintain epithelial junctions during aging may prove effective toward the goal of prolonging healthy lifespan. In this study, we have used the fruit fly, Drosophila, as a model to study the impact of altered expression of the SJ-specific protein Ssk on intestinal barrier function, commensal homeostasis, and lifespan. We show that Ssk is required to maintain barrier function and commensal homeostasis in the adult intestine. Indeed, loss of intestinal Ssk leads to rapid-onset microbial dysbiosis, immune gene modulation, barrier dysfunction, and mortality, although mortality was not due to dysbiosis. Furthermore, restoring Ssk led to resumption of barrier integrity and reversed these phenotypes, previously linked to aging. Remarkably, up-regulating Ssk in the adult intestine protected against oral infection with pathogenic bacteria. Improved survival under these conditions is linked to a reduction in bacterial translocation, consistent with improved intestinal barrier function. Finally, intestinal overexpression of Ssk during aging delayed the onset of dysbiosis and intestinal barrier dysfunction and prolonged lifespan. The prolongevity effects of Ssk were eliminated when flies were maintained axenically, consistent with a key role for Ssk-mediated alterations in microbiota dynamics for its effects on longevity.
Previous work indicated that dysbiosis precedes and predicts intestinal barrier dysfunction in aged flies (Clark et al., 2015). Here, we show that there is a mislocalization of SJs during midlife, before the detection of intestinal barrier failure. Hence, it is possible that alterations in SJs occur either before or concurrent with changes in microbial dynamics. It has previously been reported that age-related changes in SJ levels and localization were particularly noticeable at TCJs (Resnik-Docampo et al., 2017). Depletion of the Drosophila TCJ protein Gli led to ISC overproliferation and disruption of the intestinal barrier. Interestingly, however, acute loss of Gli does not lead to dysbiosis (Resnik-Docampo et al., 2017, Resnik-Docampo et al., 2018). This may be because TCJs contribute to only a small percentage of the barrier in the fly intestine, when compared with the bicellular junctions.
Ssk forms a complex with Mesh, another sSJ-specific protein, and these proteins are mutually interdependent for their localization (Furuse and Izumi, 2017, Izumi et al., 2012). Interestingly, it was recently reported that Mesh regulates Duox expression to modulate the load and composition of the gut microbiota (Xiao et al., 2017). Consistent with this model, we observe a significant inhibition of Duox expression upon intestinal Ssk knockdown. At the same time, however, we observe reduced expression of several AMPs in Ssk knockdown flies. Furthermore, we observe elevated AMP and Duox expression after longer periods of Ssk knockdown, which is consistent with previously observed AMP elevations observed in Smurf flies and in aged non-Smurf flies (Clark et al., 2015). These data demonstrate that knocking down one SJ protein that disturbs intestinal junction integrity leads to an initial repression of AMPs that coincides with rapid elevations in microbial load, followed by an increase in immune activation, before death. These data provide a possible mechanism for the initial increase in microbial content observed before detectable barrier permeability, yet recapitulate the later elevation in immune gene expression observed in aged flies. Further studies are required to determine the mechanistic relationships between SJs, immune homeostasis, and microbiota dynamics during aging.
In humans, compromised intestinal barrier function has been linked to a number of intestinal and systemic diseases (Choi et al., 2017). Although the existing clinical data have not established a causal role, the idea of targeting and restoring the intestinal epithelial barrier has been proposed as a potential therapeutic approach (Odenwald and Turner, 2017). A better understanding of the mechanisms underlying barrier regulation may aid this goal. At present, therapeutic approaches to treat intestinal barrier dysfunction have largely focused on reducing inflammation. Indeed, anti-tumor necrosis factor (TNF) therapy has been used to successfully treat intestinal barrier dysfunction in the context of Crohn disease (Noth et al., 2012, Suenaert et al., 2002). Consistent with this, TNF-deficient mice display improved intestinal barrier dysfunction during aging (Thevaranjan et al., 2017). Interestingly, anti-TNF therapy can reverse age-onset microbiota changes in mice (Thevaranjan et al., 2017). These data reveal that the composition of the microbiota can be altered by the inflammatory status of the host. Taken together, these studies support a model whereby an age-related increase in microbial translocation induces an inflammatory response, which contributes to dysbiosis. In this feedforward model, dysbiosis contributes to intestinal barrier dysfunction and increased microbial translocation (Thevaranjan et al., 2017). Previous work, in Drosophila, has shown that intestinal immune activation leads to intestinal barrier dysfunction and early-onset mortality (Clark et al., 2015). Our current study adds to this model by showing that occluding junction modulation can, by itself, induce dysbiosis and mortality. In fact, under axenic conditions, and in the absence of an inflammatory response, occluding junction modulation, alone, is sufficient to induce certain markers of aging and mortality. This is consistent with an important role for SJs in the feedforward model. Given our findings, it will be important to determine whether interventions that maintain the intestinal epithelial barrier in aging mammals can prolong intestinal and/or organismal health during aging.
Limitations of Study
Although Drosophila melanogaster is a well-studied and highly tractable model organism of proven utility in understanding mechanisms of aging, development, and disease, the relevance of molecular mechanisms uncovered in this work to human aging and disease remains to be demonstrated experimentally.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank M. Furuse and the VDRC for fly stocks. In addition, stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. We thank Chunni Zhu and the UCLA BRI Microscopic Techniques and Electron Microscopy Core Facility for help with electron microscopy. This work was supported by the NIH grants AG040288 (D.L.J. and D.W.W.), AG028092 and DK105442 (D.L.J.), AG049157 and AG037514 (D.W.W.), and GM105775 and AG045842 (M.S.-H.). M.U. is a Charles H. Revson Senior Fellow and supported by the NIH training grant 5T32DK007328. This research was conducted while D.W.W. was a Julie Martin Mid-Career Awardee in Aging Research supported by The Ellison Medical Foundation and AFAR.
Author Contributions
A.M.S., M.R-D. and M.U. designed, performed, and analyzed experiments. R.I.C., M.S-H., D.L.J., and D.W.W designed and analyzed experiments. A.M.S. and D.W.W wrote the paper with input and comments from all authors.
Declaration of Interests
The authors declare no competing interests.
Published: November 30, 2018
Footnotes
Supplemental Information includes Transparent Methods and seven figures and can be found with this article online at https://doi.org/10.1016/j.isci.2018.10.022.
Supplemental Information
References
- Bass T.M., Grandison R.C., Wong R., Martinez P., Partridge L., Piper M.D. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B., Hochmuth C.E., Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Broderick N.A., Chakrabarti S., Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley A., Turner J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 2018;10:1–16. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesar Machado M.C., da Silva F.P. Intestinal barrier dysfunction in human pathology and aging. Curr. Pharm. Des. 2016;22:4645–4650. doi: 10.2174/1381612822666160510125331. [DOI] [PubMed] [Google Scholar]
- Choi N.-H., Kim J.-G., Yang D.-J., Kim Y.-S., Yoo M.-A. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W., Yeruva S., Turner J.R. Contributions of intestinal epithelial barriers to health and disease. Exp. Cell Res. 2017;358:71–77. doi: 10.1016/j.yexcr.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.I., Salazar A., Yamada R., Fitz-Gibbon S., Morselli M., Alcaraz J., Rana A., Rera M., Pellegrini M., Ja W.W. Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep. 2015;12:1656–1667. doi: 10.1016/j.celrep.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.I., Walker D.W. Role of gut microbiota in aging-related health decline: insights from invertebrate models. Cell. Mol. Life Sci. 2018;75:93–101. doi: 10.1007/s00018-017-2671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambroise E., Monnier L., Ruisheng L., Aguilaniu H., Joly J.S., Tricoire H., Rera M. Two phases of aging separated by the Smurf transition as a public path to death. Sci. Rep. 2016;6:23523. doi: 10.1038/srep23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi A., Banan A., Fields J., Keshavarzian A. Intestinal barrier: an interface between health and disease. J. Gastroenterol. Hepatol. 2003;18:479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- Furuse M., Izumi Y. Molecular dissection of smooth septate junctions: understanding their roles in arthropod physiology. Ann. N. Y Acad. Sci. 2017;1397:17–24. doi: 10.1111/nyas.13366. [DOI] [PubMed] [Google Scholar]
- Gelino S., Chang J.T., Kumsta C., She X., Davis A., Nguyen C., Panowski S., Hansen M. Intestinal autophagy improves healthspan and longevity in C. elegans during dietary restriction. PLoS Genet. 2016;12:e1006135. doi: 10.1371/journal.pgen.1006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Karpac J., Tran S.L., Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell. 2014;156:109–122. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha E.M., Lee K.A., Park S.H., Kim S.H., Nam H.J., Lee H.Y., Kang D., Lee W.J. Regulation of DUOX by the galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev. Cell. 2009;16:386–397. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Ha E.M., Lee K.A., Seo Y.Y., Kim S.H., Lim J.H., Oh B.H., Kim J., Lee W.J. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nat. Immunol. 2009;10:949–957. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- Ha E.M., Oh C.T., Bae Y.S., Lee W.J. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Hu D.J., Jasper H. Epithelia: understanding the cell biology of intestinal barrier dysfunction. Curr. Biol. 2017;27:R185–R187. doi: 10.1016/j.cub.2017.01.035. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Yanagihashi Y., Furuse M. A novel protein complex, Mesh-Ssk, is required for septate junction formation in the Drosophila midgut. J. Cell Sci. 2012;125:4923–4933. doi: 10.1242/jcs.112243. [DOI] [PubMed] [Google Scholar]
- Jasper H. Exploring the physiology and pathology of aging in the intestine of Drosophila melanogaster. Invertebr. Reprod. Dev. 2015;59:51–58. doi: 10.1080/07924259.2014.963713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Patel P.H., Kohlmaier A., Grenley M.O., McEwen D.G., Edgar B.A. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulier F., Royet J. Maintaining immune homeostasis in fly gut. Nat. Immunol. 2009;10:936–938. doi: 10.1038/ni0909-936. [DOI] [PubMed] [Google Scholar]
- Lusis A.J., Attie A.D., Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat. Rev. Genet. 2008;9:819–830. doi: 10.1038/nrg2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur D., Bost A., Driver I., Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J., Sturm A. The intestinal epithelial barrier: does it become impaired with age? Dig. Dis. 2009;27:240–245. doi: 10.1159/000228556. [DOI] [PubMed] [Google Scholar]
- Micchelli C.A., Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Mitchell E.L., Davis A.T., Brass K., Dendinger M., Barner R., Gharaibeh R., Fodor A.A., Kavanagh K. Reduced Intestinal motility, mucosal barrier function, and inflammation in aged monkeys. J. Nutr. Health Aging. 2017;21:354–361. doi: 10.1007/s12603-016-0725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.N., Coogan C., Chamseddin K., Fernandez-Kim S.O., Kolli S., Keller J.N., Bauer J.H. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim. Biophys. Acta. 2012;1822:1230–1237. doi: 10.1016/j.bbadis.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme N.T., Liegeois S., Kele B., Giammarinaro P., Pradel E., Hoffmann J.A., Ewbank J.J., Ferrandon D. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 2007;3:e173. doi: 10.1371/journal.ppat.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noth R., Stuber E., Hasler R., Nikolaus S., Kuhbacher T., Hampe J., Bewig B., Schreiber S., Arlt A. Anti-TNF-alpha antibodies improve intestinal barrier function in Crohn's disease. J. Crohns Colitis. 2012;6:464–469. doi: 10.1016/j.crohns.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Park J.-S., Kim Y.-S., Yoo M.-A. The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging. 2009;1:637–651. doi: 10.18632/aging.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W.Y., Wu K.F., Li X., Luo M., Liu H.C., Zhang S.C., Hu Y. Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin. Exp. Res. 2014;26:183–191. doi: 10.1007/s40520-013-0148-0. [DOI] [PubMed] [Google Scholar]
- Rera M., Azizi M.J., Walker D.W. Organ-specific mediation of lifespan extension: more than a gut feeling? Ageing Res. Rev. 2013;12:436–444. doi: 10.1016/j.arr.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M., Bahadorani S., Cho J., Koehler C.L., Ulgherait M., Hur J.H., Ansari W.S., Lo T., Jr., Jones D.L., Walker D.W. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M., Clark R.I., Walker D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. U S A. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende L.P., Jones D.L. Local signaling within stem cell niches: insights from Drosophila. Curr. Opin. Cell Biol. 2012;24:225–231. doi: 10.1016/j.ceb.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik-Docampo M., Koehler C.L., Clark R.I., Schinaman J.M., Sauer V., Wong D.M., Lewis S., D'Alterio C., Walker D.W., Jones D.L. Tricellular junctions regulate intestinal stem cell behaviour to maintain homeostasis. Nat. Cell Biol. 2017;19:52–59. doi: 10.1038/ncb3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik-Docampo M., Sauer V., Schinaman J.M., Clark R.I., Walker D.W., Jones D.L. Keeping it tight: the relationship between bacterial dysbiosis, septate junctions, and the intestinal barrier in Drosophila. Fly (Austin) 2018;12:1–7. doi: 10.1080/19336934.2018.1441651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaert P., Bulteel V., Lemmens L., Noman M., Geypens B., Van Assche G., Geboes K., Ceuppens J.L., Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am. J. Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- Teleman A.A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2009;425:13–26. doi: 10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- Tepass U., Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev. Biol. 1994;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Thevaranjan N., Puchta A., Schulz C., Naidoo A., Szamosi J.C., Verschoor C.P., Loukov D., Schenck L.P., Jury J., Foley K.P. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–466.e4. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L., Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:1045–1056. doi: 10.1093/gerona/glt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Yang L., Pang X., Zhang R., Zhu Y., Wang P., Gao G., Cheng G. A Mesh-Duox pathway regulates homeostasis in the insect gut. Nat. Microbiol. 2017;2:17020. doi: 10.1038/nmicrobiol.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihashi Y., Usui T., Izumi Y., Yonemura S., Sumida M., Tsukita S., Uemura T., Furuse M. Snakeskin, a membrane protein associated with smooth septate junctions, is required for intestinal barrier function in Drosophila. J. Cell Sci. 2012;125:1980–1990. doi: 10.1242/jcs.096800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.