Abstract
Background
The WHO estimates that a considerable number of people in Sub-Saharan Africa (SSA) rely on traditional, complementary and alternative medicine (TCAM) to meet their primary healthcare needs, yet there remains a dearth of research evidence on the overall picture of TCAM utilisation in the region.
Methods
We conducted a literature search of original articles examining TCAM use in SSA between 1 January 2006 and 28 February 2017, employing Medline, Cumulative Index to Nursing and Allied Health Literature, Allied and Complementary Medicine Database, Scopus, ProQuest, PubMed, Embase and African Journals Online databases. A critical appraisal of relevant articles reporting a quantitative or mixed-method design was undertaken.
Results
Despite the heterogeneity and general low quality of the identified literature, the review highlights a relatively high use of TCAM alone or in combination with orthodox medicine, in both general population and in specific health conditions in SSA. TCAM users compared with non-TCAM users are more likely to be of low socioeconomic and educational status, while there were inconsistencies in age, sex, spatial location and religious affiliation between TCAM users and non-TCAM users. Most TCAM users (55.8%–100%) in SSA fail to disclose TCAM use to their healthcare providers, with the main reasons for non-disclosure being fear of receiving improper care, healthcare providers’ negative attitude and a lack of enquiry about TCAM use from healthcare providers.
Conclusion
TCAM use in SSA is significant, although most studies emerge from a few countries. Factors associated with TCAM use in SSA are similar to those observed in other regions, but further research may be required to further elucidate challenges and opportunities related to TCAM use specific to SSA.
Keywords: complementary therapies, traditional medicine, Sub-Saharan Africa, systematic review, Integrative Medicine, Health System
Key questions.
What is already known?
There remains a dearth of research evidence in Sub-Saharan Africa (SSA) on the drivers and facilitators of traditional, complementary and alternative medicine (TCAM) use, factors associated with TCAM use, and the impact of TCAM use on broader healthcare.
What are the new findings?
Studies suggest a high use of TCAM (particularly TCAM products) in SSA, although most studies are limited to few countries, and there is a significant heterogeneity and low quality of some of the current scholarship.
TCAM is used due to its perceived low cost, alignment of TCAM with sociocultural, religious and spiritual values, and dissatisfaction with conventional healthcare.
Non-disclosure of TCAM use to healthcare providers is common among TCAM users in SSA, primarily due to the fear of receiving improper care from hospitals, negative attitude of healthcare providers towards TCAM and a lack of enquiry about TCAM use from healthcare providers.
What do the new findings imply?
Widespread TCAM use in SSA necessitates health departments and governments across the region to consider and familiarise themselves with the current role of TCAM and its future possibilities within the wider healthcare system.
Introduction
Traditional, complementary and alternative medicine (TCAM) refers to a set of healthcare practices (indigenous or imported) that are delivered outside of the mainstream healthcare system.1 In the African setting it may encompass local herbal medicines or products, indigenous healthcare practices (traditional bone setting), as well as imported complementary and alternative medicine products and practices (eg, acupuncture or chiropractic). Sub-Saharan Africa is one region of the world in which TCAM has long been held to be widespread, with a considerable number of its population relying on it to maintain their health or prevent and treat communicable and non-communicable diseases.2 3 The economic influence of TCAM is extensive, contributing at least R2.9 billion (US$2.2 million) to the South African economy alone.4
The increasing uptake of TCAM services across the continent in recent decades has attracted the attention of policy makers, researchers and healthcare professionals. In the past 20 years, the WHO regional office for Africa spearheaded the implementation of a regional strategy endorsed by African Heads of State in Lusaka, Zambia5 to promote the role of TCAM in health systems in the African region. The gains experienced since the adoption of the regional plan include policy formation in 36 countries and research promotion, including the establishment of TCAM research centres in some countries like Nigeria, Ghana and South Africa. The regional plan has also promoted the inclusion of TCAM courses into the curricula of healthcare training institutions in countries across the continent. For instance, such plan has seen the inclusion of TCAM courses in some South African6 and Ghanian7 universities. It has also promoted the training of TCAM practitioners and the local production and cultivation of medicinal plants, as well as the establishment of intellectual property rights for traditional medicine knowledge in few nations.5 Despite such progress, African countries continue to grapple with an absence of TCAM policy or its implementation, inadequate TCAM research infrastructure and insufficient regulation of TCAM products and practices.5 8 For instance, by 2005, only 32% and 27% of the African countries who responded to the WHO global survey had a national policy and law or regulation on TCAM.9
A steady rise in the prevalence of chronic non-communicable diseases is significantly contributing to Africa’s disease burden, and is adding burden to healthcare systems already strained due to the high incidence of infectious diseases.10 With high TCAM use for chronic health conditions reported outside of Africa,11 it is postulated that TCAM will play an integral role in the health and well-being of people suffering from chronic diseases in Africa as well.12 13 TCAM’s role in the provision of primary healthcare is recognised in some Sub-Saharan Africa countries’ health policy documents within the context of limited access to essential health services, especially among the rural poor.14 15
Considering the high utilisation of TCAM across Sub-Saharan Africa, it is necessary for policy decision makers, researchers and health professionals to recognise TCAM healthcare practices as integral to the health-seeking of populations and develop an effective response that safeguards their health and well-being. A proper policy and practice response to increasing TCAM use requires an indepth insight into the nature of TCAM use, including the profile of TCAM users as well as the drivers and barriers that facilitate and limit the use of TCAM. In direct response, this paper reports findings from the first comprehensive critical review of the prevalence of TCAM use alone and in combination with conventional medicine, sociodemographic characteristics of TCAM users, motivators of and barriers to TCAM use, safety and cost associated with TCAM use, as well as details around non-disclosure of TCAM use to health providers.
Methodology
Research design
The systematic review analyses the contemporary scholarship using an established approach developed for a number of health research topics.16–18
Search strategy
Peer-reviewed articles reporting on TCAM use in Sub-Saharan Africa were searched using the following databases: Cumulative Index to Nursing and Allied Health Literature, Allied and Complementary Medicine Database, Scopus, ProQuest, Medline, PubMed, African Journals Online, Embase and Google Scholar. Hand searching in the bibliography of relevant articles was also employed to help ensure the capture of all relevant peer-reviewed literature. Table 1 shows a summary of the search strategy employed. The definition of TCAM in our review was based on the definitions of traditional medicine and complementary medicine put forward by WHO.1 It encompasses local herbal medicines or products, indigenous healthcare practices (traditional bone setting), as well as imported complementary and alternative medicine products and practices (such as Chinese medicine and chiropractic). Vitamins and mineral supplements were not considered as TCAM products in our study, as these are usually used for general health prevention rather than for specific health purposes.
Table 1.
Search strategy
| Databases | Cumulative Index to Nursing and Allied Health Literature |
| Allied and Complementary Medicine Database | |
| Embase | |
| PubMed | |
| Medline | |
| Scopus | |
| ProQuest | |
| African Journals Online | |
| Other sources searched | Google Scholar, bibliographies of searched articles |
| Key searched terms | Traditional medicine OR traditional therapy OR African traditional medicine OR traditional healer OR traditional medicine practitioner OR traditional medicine healer OR traditional birth attendant OR bonesetter OR faith healer OR spiritualist OR Complementary therapies OR Complementary and alternative medicine (CAM) OR Herbal medicine OR herbal therapy OR medicinal herbs OR herbal extract OR herbal product OR herbal supplements OR herbal remedies OR home remedies OR medicinal plants OR herbalist OR medical herbalist, folklore medicine OR folklore therapy OR indigenous therapy OR indigenous medicine OR indigenous health AND Africa OR sub-Saharan Africa, Western Africa(including all countries in that region) OR Central Africa(including all countries in that region) OR eastern Africa(including all countries in that region) OR Southern Africa(including all countries in that region) AND use OR prevalence OR Pattern OR utilization OR attitude OR knowledge OR practice OR perception OR health seeking behaviour OR Health care utilization AND Adverse Drug Event OR Adverse Drug Reaction OR Drug Side Effects OR Drug Toxicity AND Cost OR Expenditure OR Income |
| Search limit | |
| Duration | 1 January 2006 and 28 February 2017 |
| Language | English |
| Population | Humans of all age groups |
| Location | Sub-Saharan Africa (SSA) |
| Types of study | All studies that report empirical findings in all population groups for the treatment and prevention of health conditions, in SSA using traditional, complementary and alternative medicine products and/or practitioners. |
| Type of publication | Peer-reviewed research articles. |
| Exclusion criteria | Review articles including systematic reviews and meta-analysis, editorial, commentaries, letter to the editor, clinical case studies, studies conducted outside SSA, clinical studies using randomised control trial design, and articles that failed to use systematic research design and reporting procedures. |
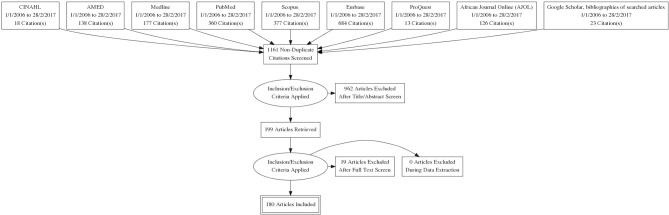
The results from the databases and hand searches were imported into EndNote V.X8. Duplicates were removed, and the remaining articles were screened based on the title, abstract and full text. Figure 1 gives a detailed algorithm of how papers were excluded and included.
Figure 1.
PRISMA flow diagram of included and excluded articles. AMED, Allied and Complementary Medicine Database; CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Search outcomes
We initially identified 1916 articles from the database search and from other sources. These papers were further screened using our inclusion criteria. One hundred and eighty papers met the inclusion criteria and were included in the critical review. A summary of the search process and categorical analysis of the reviewed studies are shown in figure 1 and online supplementary file 1, respectively.
bmjgh-2018-000895supp001.pdf (517.9KB, pdf)
Quality appraisal system
We employed an analytical tool adapted from previous critical reviews on complementary and alternative medicine use17 19 to evaluate the quality of the papers that met the review criteria. The tool appraised study methodology, participant characteristics and TCAM use, with 1 point given to each aspect under the three domains (methodology, reporting of participant characteristics and reporting TCAM use). A maximum potential score of 12 was tallied if all aspects of the three domains were met. Two authors separately analysed and allocated scores. Differences in the final score were resolved through discussion among the four authors, with the most senior author having to make the final decision. Only articles using mixed and quantitative designs were considered as they formed the greater part of the reviewed papers. For mixed-method studies, only the quantitative study component was appraised. Table 2 and the online supplementary file 2 provide the details of the quality appraisal tool and the summated score of each paper, respectively. Articles with summated scores ranging from 9 to 12, 6–8 and 0–5 were considered of good, fair and poor quality, respectively.
Table 2.
Quality appraisal scoring system
| Dimensions of quality appraisal | Codes | Points awarded |
| Methodology | ||
| Representative sampling strategy | A | 1 |
| Sample size ≥500 | B | 1 |
| Response rate >75% | C | 1 |
| Low recall bias on TCAM use within the past 12 months or less | D | 1 |
| Reporting participants’ characteristics | ||
| Age | E | 1 |
| Gender | F | 1 |
| Socioeconomic status (income or education) | G | 1 |
| Ethnicity/tribe | H | 1 |
| Location (urban or rural or district or region) | I | 1 |
| Reporting TCAM use | ||
| Definition of TCAM to respondents | J | 1 |
| Assessed use of TCAM | K | 1 |
| Type of TCAM | L | 1 |
| Total | 12 |
TCAM, traditional, complementary and alternative medicine.
bmjgh-2018-000895supp002.pdf (328.5KB, pdf)
Results
A total of 180 articles met the criteria for review. The included articles employed mixed-method (n=14), qualitative (n=12) and quantitative (n=154) research designs. Based on our search strategy, we were able to identify studies from 25 out of the total 48 countries which constitute Sub-Saharan Africa. Nearly three-quarters (72.8%) of included papers reported research conducted in just four countries: Nigeria (n=72, 40.0%), South Africa (n=26, 14.4%), Ghana (n=20, 11.1%) and Uganda (n=13, 7.2%). A summary of the included articles is shown in online supplementary file 1. There were very few articles with high methodological quality. Of the 165 articles that met the requirement for critical appraisal, 2 articles from South Africa20 21 had a total score of 11, and 7 papers from different countries had a score of 10.22–28 Less than a quarter (n=30, 18.2%) and close to two-thirds (n=106, 64.2%) of the articles appraised had total scores ranging from 9 to 12 and from 6 to 8, respectively. Methodological flaws were discovered in the selected articles, with only 8 (4.8%) of studies employing a nationally representative sampling strategy. Two-thirds (n=110, 66.7%) of the identified articles reported a sample size less than 500. Also, the tendency for recall bias based on whether TCAM was used within the past 12 months or less was identified in almost half (n=82, 49.7%) of the papers appraised. With regard to the reporting of TCAM use, close to two-thirds (66.1%) of the articles reviewed failed to provide a definition of TCAM. Details of the results of the critical appraisal scoring system are shown in online supplementary file 2. In general, the reviewed articles reported eight major themes: types of TCAM used, prevalence of use of TCAM, prevalence of concurrent use of TCAM and allopathic medicine, and sociodemographic profile of TCAM users, drivers and barriers to TCAM use, non-disclosure of TCAM use to healthcare providers, TCAM costs and reported adverse effects of TCAM use. In reporting the findings of our review, the prevalence of TCAM and the sociodemographic profile of TCAM users were categorised into general population studies (including both adult male and adult female participants and not limited to examining any single specific disease or condition) and subhealth or disease-specific populations. The categorisation was done to highlight the TCAM utilisation in the general population and in diseases or conditions and specific populations that are considered a public health or clinical issue in Sub-Saharan Africa. Disease conditions or populations with single publication were grouped as others.
Types of TCAM use
Based on the literature reviewed, biological-based therapies such as herbal therapy are the most common TCAM used in Sub-Saharan Africa, followed by faith-based healing methods (prayer/spirituality) and mind-body therapies (massage, traditional bone setting relaxation, mediation and yoga).29–53 A few studies examining TCAM use among university staff, patients with HIV/AIDS and patients with cancer reported homeopathy,54 prayer/spirituality21 55 and massage56 as the most common TCAM modalities used, respectively.
Prevalence of TCAM use in Sub-Saharan Africa
One hundred and twenty-six of the reviewed articles reported the prevalence of TCAM product (self-care and over-the-counter use) and practitioner use. Papers were divided into two main categories: those reporting TCAM use in the general population (including both adult male and adult female participants and not limited to examining any single specific disease or condition) and TCAM use in subpopulations (such as a specific clinical population). For reporting TCAM prevalence, articles were categorised as reporting on large sample studies (n≥500) and small sample studies (n<500). In general, the current literature suggests a varied prevalence but substantial use of TCAM in both general and subhealth populations (despite methodological limitations in many of the articles reviewed, including no formal or standardised definition of timeframe of use).
Prevalence of TCAM use in the general population
Twenty-six articles reported TCAM product use in the general population,12 44 45 48 49 57–77 reporting substantial prevalence rates ranging from 4.6% (urban settlement in Ethiopia)65 to 94% (semiurban settlements in Nigeria and Ethiopia),49 72 with an estimated average of 58.2%. At least half (n=23) of the study population in majority of the studies reported using TCAM products. Similar utilisation rates were observed among large sample size (≥500) studies44 45 57–60 65 68 76 and small sample size (<500) studies.12 48 49 61–64 66 67 69–75
A varied prevalence was also observed among the 10 articles20 27 28 58 63 78–81 that reported on TCAM practitioner utilisation (1.2%–67% (mean, 28.8%)). Among studies with large samples,20 27 28 58 79 a lower use of TCAM practitioner services was observed (1.2%–44.1% (average, 12.6%)) compared with studies63 78 80 81 with smaller samples (37.5%–67% (mean, 53.0%)).
Prevalence of TCAM use in health subpopulations
Pregnancy, childbirth, abortion and infertility
Eighteen studies reported the prevalence rates of TCAM use during pregnancy,24 82–95 childbirth94–96 and for pregnancy termination.97 Between 12% and 90.3% (mean, 48.4%) of pregnant women were reported to use a TCAM product during pregnancy, a rate consistent across studies drawn from large sample sizes84 85 93–95 (25.5%–67.5% (average, 45.3%)) and from small sample sizes24 83 86–92 (12%–90.3% (average, 50.1%)). There is limited literature concerning TCAM practitioner service utilisation during pregnancy, with only one Zambian82 and Nigerian98 study reporting the proportion of women seeking the service of a traditional medicine practitioner during their pregnancy (Zambia: 21%; Nigerian: 44.6%). A study of Ghanaian women94 (n=611) reported 11.7% used TCAM products during childbirth, while two Nigerian studies reporting large sample sizes identified 24.1% and 42.5% of postpartum women used the services of TCAM practitioners during childbirth, respectively.95 96 Close to a quarter (22%) of urban Tanzanian women seek help from a traditional medicine practitioner for pregnancy termination compared with 16.9% of their rural counterparts.97 There is also a limited literature with regards TCAM use for infertility, with only one Ugandan study reporting high use (76.2%) of herbal medicine among women seeking infertility care99 and a Nigerian study reporting high use (69%) of TCAM practitioner service among infertile couples.100 There is also a dearth of research evidence of TCAM use among menopausal women, with only one Nigerian study reporting a lower use (3.8%) TCAM practitioner service among menopausal women.101
Sexual health conditions
Three papers, of which two were drawn from large sample sizes (≥500), reported Zambian (68%) women used TCAM products to dry up and constrict the vaginal passage prior to sexual intercourse,102 and Ghanaian (56.3%) women used TCAM to manage gynaecological conditions.94 The third study (n=224) reported 54.9% of male and female Ugandans presenting with various types of sexually transmitted infections used TCAM.103
Hypertension, diabetes, cancer and asthma
Seven articles reported TCAM product utilisation rates among patients with hypertension32 33 39 46 104–106 and indicate a rate of between 19.5% and 67.8% (mean, 27.1%). Only one of the seven studies was drawn from a large sample of patients with hypertension (n=500) and the reported prevalence in this case was 24%.106
TCAM product use by patients with diabetes varied across the four identified studies drawn from smaller samples, with a higher rate reported in Tanzania (77.1%)107 compared with Nigeria (46%),43 Guinea (33%)108 and Kenya (12.4%).109 With regard to cancer, the available literature reports a high use of TCAM among patients with cancer in Sub-Saharan Africa (Nigeria: 65%34; Ethiopia: 79%110; Ghana: 73.5%56), although one Nigerian study did report a lower rate of use specifically for TCAM services.111 A Prospective Urban and Rural Epidemiological (PURE) study in South Africa reported a higher (61%) TCAM product use among general adult population with non-communicable diseases.12 With regard to TCAM use among patients with asthma, the reviewed literature was drawn from smaller sample size studies conducted in Nigeria, and they suggested that slightly over half of adult (50.5%)29 and a quarter of paediatric (25%)47 patients were TCAM product users.
HIV/AIDS
Twenty-four papers reported TCAM product 112 and practitioner113–115 usage among patients with HIV/AIDS. We observed a similar average prevalence of TCAM product (1.8%–96.8% (mean, 45.8%)) and practitioner use (17.6%–62% (mean, 45.0%)), respectively. Only one product-based59 and one practitioner-based113 studies were conducted using a large sample of patients with HIV/AIDS, and both reported utilisation rates of 1.8% and 62%, respectively.
Malaria and febrile illness
A total of eight papers reported TCAM use for malaria and febrile illness, with close to a quarter of children and at least half of adults reported using TCAM.22 116–122 Two studies reporting large sample sizes in Sierra Leone reported 22%121 and 24.7%22 TCAM products use among children, while the rates were higher among adult populations in Sierra Leone (55%)120 and Ghana (50.3%).119 An increased rate of TCAM use for uncomplicated malaria was recorded in Mali117 between 2003 (24%) and 2013 (58%). Another large population study in two rural districts in Mali documented 27% of individuals with uncomplicated malaria used TCAM alone for management of their health compared with 50% for complicated cases.116 On the other hand, a lower proportion (18.2%) of TCAM products was reported among 33 children with complicated malaria122 The only study reporting TCAM practitioner use for malaria was conducted in Nigeria and reported a prevalence of 49.7%.118
Mental and neurological disorders (epilepsy and mental health disorders)
A total of 11 articles reporting small sample sizes (<500) identified rates of TCAM use among patients with epilepsy40 47 123 and mental health conditions.23 124–131 Among children with epilepsy, Lagunju40 reported a high use (56.6%) of TCAM products compared with 38% reported by Oshikoya et al.47 On the other hand, Nwani et al 123 reported a high utilisation of TCAM practitioner service (65.5%) among adult patients with epilepsy. Seventy-six per cent of Nigerian patients with schizophrenia129 and 11.5% of patients with psychosis in South Africa131 used TCAM products. Among psychiatric patients in general, a higher proportion in Nigeria (73.5%)125 and Ethiopia (50.3%)23 used the service of a TCAM practitioner compared with their counterparts (in Ghana (23.3%)127 and Malawi (22.7%)128). Greater than two-thirds (69%–76%) of patients with schizophrenia in Nigeria124 129 seek care from a TCAM practitioner, whereas a lower proportion of patients with psychosis (11.5%–38.5%) in South Africa126 131 visit a TCAM practitioner. On the other hand, a Ugandan study stated that more than 80% of patients with psychosis simultaneously access both orthodox and traditional medicine systems.132 In Sudan, more than one-third (41%) of patients with mental disorders had sought the service of a TCAM practitioner prior to accessing conventional care.130
Musculoskeletal conditions
A total of seven (six Nigerian, one Ghanaian) studies drawn from small samples reported TCAM product and practitioner use for musculoskeletal conditions. Two studies reported 96.8% of peasant farmers41 and 40.2% of occupational drivers133 as using TCAM products for the management of musculoskeletal pain. Another study reported 47.2% of TCAM product use among patients with osteoarthritis.42 On the other hand, 52.3%, 31.6% and 21% of patients with bone fracture from north central134 135 and middle belt136 regions of Nigeria patronises traditional bonesetters (TBS), respectively. In the Ghanaian study, 29 of the 46 patients interviewed had received treatment from a TBS.137
Diarrhoea
Four studies were identified that reported TCAM product and practitioner use among individuals experiencing diarrhoea.96 121 138 139 A general population study from Kenya139 and a paediatric-based survey in Sierra Leone121 reported 97.45% and 31% of TCAM product use to manage diarrhoea, respectively. In addition to the Kenyan study, similar studies from Mali (57%)138 and Nigeria (11.3%)96 reported rates of TCAM use for diarrhoea management.
Eye diseases
Seven studies,38 140–144 five of which were conducted in Nigeria, reported that the TCAM use for ophthalmic conditions ranged from 1.6% to 83.2% (average, 28.2%). No study presented TCAM practitioner use for the management of ophthalmic conditions.
Surgical care
Two studies from Nigeria and South Africa reported studies on TCAM product use among surgical patients. In the Nigerian study, 40% of 60 patients surveyed used herbal medicine during their preoperative period,145 while in the South African study 7% of 495 surgical patients had used TCAM in the preceding 6 weeks.146
Others (infantile colic, tuberculosis, oral health and mycetoma)
A study in Nigeria reported that 32.8% and 3.1% use TCAM product and visited a traditional birth attendant to manage infantile colic, respectively.147 Also, studies conducted in Nigeria reported that 10% and 19.8% of patients suffering from tuberculosis148 and toothache149 had consulted with a traditional medicine practitioner, respectively. In addition, another Nigerian study reported that 31.9% of mothers with sick children visiting a private primary healthcare clinic used crude oil as traditional medication.150 In Sudan, 42.4% of patients with mycetoma reported using herbal medicines,151 while 57% of deceased patients who were terminally ill in Ethiopia visited a traditional and spiritual healer.152
General inpatients and outpatients
Five studies (three Nigerian, one South African and one Ugandan) assessed TCAM use among inpatients and outpatients. The current review indicates a higher TCAM utilisation rate among outpatients compared with inpatients. For instance, a Nigerian study among inpatient and outpatients reported that 72% of the 200 outpatients are currently using TCAM products, whereas 18.5% of the 65 inpatients are reportedly using TCAM products.153 Similar findings among outpatients were reported in similar studies in Nigeria (89.9%)30 and South Africa (60.9%)154 and among inpatients in Nigeria (14.2%)155 and Uganda(18.3%).156
Student population, healthcare professionals and academic staff
The reviewed literature indicates that 38.4% of high school students in Nigeria used a TCAM product157 compared with 81.8% and 75.1% TCAM product utilisation rates that were reported among Nigerian university students31 and university students and staff,158 respectively. A similar high utilisation rate was reported among university students in Ghana (89.1%).35 This rate was lower among medical students in both countries (Nigeria: 28%159; Ghana: 56.7%160) and among paramedical students in Nigeria (53.9%).161 In Sierra Leone, 59.1% and 55.6% of graduating medical and nursing students37 as well as all pharmacy students36 used TCAM products.
Among healthcare professionals, TCAM use was reported among medical doctors in Nigeria (20.7%)162 and professionals providing HIV/AIDS care in Durban, South Africa (23.5%).163 One study drawn from a large sample reported that 50.3% of the academic and administrative staff in a South African university54 used TCAM.
Prevalence of concurrent use of TCAM and allopathic medicines
Twenty-six papers were identified that reported on the concurrent use of TCAM products and conventional medicines within the general population44 64 164 and subhealth population.21 35 53 55 66 89 91 105 106 109 112 117 153 165–174 The prevalence of concomitant use ranged from 4.3% to 69.4% (mean, 30.5%). There is high prevalence of concurrent use with conventional medicine in the general population (mean, 54.9% (40%–63.7%)). The prevalence of co-use of TCAM and allopathic medicines among patients with HIV/AIDS had a mean of 20.3% (4.3%–47.9%).21 53 55 112 166–170 173 174 With regard to patients with hypertension, the utilisation rate was higher in a study conducted in Nigeria (47.5%)106 than in Uganda (14.3%),105 but a relatively lower utilisation rate (7%) was reported among patients with diabetes in Kenya.109 Among pregnant women, the prevalence of concurrent use was lower in Kenya (20%)89 than Uganda (64.1%)91 and Ghana (45%).172 Among the general outpatients, two studies in Nigeria reported a varied prevalence of 21%153 and 69.4%.165
Sociodemographic profile of TCAM users in the general population
The sociodemographic characteristics of users of TCAM were identified in a number of articles. TCAM users were reported in many studies to be more common in individuals with a lower socioeconomic status20 44 45 62 65 66 71 79 175 176 and who are unemployed and unskilled20 69 74 when compared with non-users. With regard to the link between age and TCAM use, the relevant papers reported variability based on where the study was conducted. Generally, studies conducted in urban or semiurban settings reported TCAM users to be younger (20–50 years),20 58 61 71 74 whereas those conducted in a rural setting reported TCAM users to more likely be older (>55 years).62 177 An inconsistent pattern was observed from the available literature with respect to educational status of TCAM users. While four included studies reported TCAM users to have little or no formal education,20 62 71 177 two other studies provided a contrasting view.61 74 Generally, TCAM users compared with non-TCAM users in the general population were more commonly reported to be married44 45 57 than not married.20 Two studies reported on the link between TCAM use and spatial location of respondents. A national household survey in South Africa identified rural residents as more likely to visit a TCAM practitioner than their urban counterparts,20 while a study among 324 residents of the Ashanti Region of Ghana did not find any significant difference between TCAM users residing in both locations.50 The available literature reports an equivocal relationship between TCAM use and gender. On one hand, two studies from Ethiopia and Nigeria identified women more than men as likely TCAM users,44 65 while another Nigerian study reported men as likely users of TCAM.45 With respect to religion, a community-based study in Enugu, Nigeria reported TCAM users were likely to be Christians than other religions,74 whereas another Nigerian study conducted in Imo State did not observe any significant difference.68 Meanwhile, two Nigerian studies reported no significant correlation between the sociodemographic characteristics of the respondents and TCAM use.60 72
In summary, TCAM users compared with non-TCAM users in the general population across Sub-Saharan Africa are more likely to be of low socioeconomic status, while there were inconsistencies in age, sex, educational status, spatial location and religious affiliation among TCAM users
Sociodemographic profile of TCAM users in health subpopulations
TCAM users in pregnancy, childbirth and abortion
The reviewed literature shows pregnant women who use TCAM were more likely to be of low socioeconomic status84 88 92 93 98 and less educated84 86 88 89 92 98 compared with non-users, although one study reported higher education completion as a predictor of TCAM use.84 TCAM users were also found to be younger (<30 years)84 98 and married84 98 compared with non-TCAM users.24 Meanwhile, three studies from Zambia,82 Mali90 and Ethiopia83 reported no sociodemographic difference between TCAM users and non-users. With regard to TCAM use during childbirth, two studies drawn from a large sample of Nigerian women show TCAM users are likely to be women who are less educated, from low socioeconomic background, Muslim and primiparous.95 96 With respect to pregnancy termination, a Tanzanian study indicates that women with primary education from both rural and urban settings were more likely to use herbs to induce abortion compared with those with at least a high school education.97
TCAM users among patients with HIV/AIDS
Patients with HIV/AIDS who identified as a TCAM user in a number of studies across Sub-Saharan Africa were more likely to be female,21 168 178–180 not married,180 181 of low socioeconomic status,21 180 younger (<39 years),25 179 180 unemployed,180 181 educated,21 25 from a rural area21 53 and of Christian religious denomination.180
TCAM users among patients with hypertension and diabetes
A review of the literature indicates that patients with hypertension who are male,33 39 46 of low income level,33 39 less educated,33 older106 and reside in rural area33 are more likely to be TCAM users. A Ugandan study did not find any significant association between the sociodemographic profile of patients with hypertension and TCAM use.105 A study undertaken in South Africa in 2010 found that TCAM users with hypertension compared with non-hypertensive TCAM users were more likely to be older, without a partner and unemployed.104 With regard to patients with diabetes, two studies from Kenya109 and Nigeria43 show that patients with diabetes who are older and have had at least a formal education were more likely to be TCAM users.
TCAM users among patients with cancer
A Ghanaian study reported that patients with cancer who were female were more likely users of TCAM,56 whereas a similar study in Ethiopia reported that patients who have attained at least secondary education, had monthly income of more than US$125, presented with comorbidity and at the advanced stage of their disease were likely users of TCAM.110 However, there were no statistically significant differences between TCAM users and non-users with regard to the type of cancer. Meanwhile, a Nigerian study did not find any significant difference between users and non-users of TCAM.34
TCAM users among patients with eye diseases
Patients with eye problems who are older (≥50 years),140 142 from a rural settlement,141 143 married141 and uneducated142 are likely to be TCAM users in Sub-Saharan Africa. However, another study from Nigeria identified younger age (<50 years) as a determinant of TCAM use for eye diseases.141 Also, a similar study from Zimbabwe reported traditional medicine users for eye conditions are likely to belong to the Apostolic sect, reside in a periurban area and unemployed.38
TCAM users among surgical patients
The two identified studies from Nigeria and South Africa conducted among presurgical patients found no statistically significant differences exist between TCAM users and non-users.145 146
TCAM users among patients with malaria and febrile illness
Two Sierra Leonean studies reported that TCAM use was associated with being male,120 Muslim121 and living in a rural area.120 In a study conducted among female residents in Nigeria, TCAM users were more likely to be older (≥50 years), and less educated, unemployed or had a blue-collar job.118
TCAM users presenting with sexual health conditions
A Ugandan study among patients with sexually transmitted infection reported that TCAM use was common among those who were married and educated.103 Ghanaian patients visiting gynaecological units and using TCAM were reported to be less educated and unskilled compared with non-users.94 A Zambian study examining the use of TCAM to achieve vaginal dryness prior to sexual intercourse revealed that the practice was common among married women and those who grew up in rural areas.102
TCAM users seeking infertility care
The two identified studies from Nigeria and Uganda indicated that TCAM users were likely to have attained at least secondary education,99 100 of low socioeconomic class,100 married, never conceived and older than 30 years.99 The Ugandan study also reported herbal medicine use was higher among women with less than 3 years of infertility.99
TCAM users with musculoskeletal diseases
With regard to musculoskeletal diseases, only one Nigerian study among patients with osteoarthritis was identified, and it reported no significant difference in respondent demographics between TCAM users and non-users.42 On the other hand, another Nigerian study that assessed the utilisation of services rendered by TBS reported that being young, male, married, having a skilled occupation and of low economic status were associated with visiting a TBS.134
TCAM users with mental illness and neurological disorders
Two Nigerian studies40 129 reported the characteristics of TCAM users with mental health disorders. Caregivers of children with epilepsy who came from low socioeconomic background and had lower levels of education were more likely to use TCAM.40 Patients with schizophrenia who are older (>40 years), less educated, reside in a rural setting and practise African traditional religion were more likely to use the services of a traditional healer.129 This same study further reported that patients with schizophrenia who are Christians were likely to visit a psychiatric hospital and faith healer compared with their counterparts practising African traditional religion.129 Another study from Sudan reported that mental health patients who visit traditional healers were men, with an average age of 31 years, illiterate or with only a primary education, and unemployed.130
TCAM users among students, healthcare professionals, academic staff and general outpatient population
A Ghanaian study observed that TCAM users were more likely to be Christians enrolled in non-science-related programmes at the university.35 In Sierra Leone, healthcare students’ gender, age and year of study were not associated with TCAM use,36 37 although being a Christian was associated with the use of spirituality/prayer among pharmacy students.36 With regard to the general outpatient population, a study in Nigeria reported that women who were older, less educated and whose occupation was fishing were more likely to use crude oil as traditional medication.150
Drivers of TCAM use
A handful of papers included in our review identified a number of pull and push factors promoting TCAM use. Key pull factors reported in the literature include relative low cost and flexibility of payment of TCAM products and services,29–31 42 45 49 60 68 72–76 78 86 88 91 98 106 108 110 118 134 139 150 161 181–190accessibility,22 30 31 49 57 60 72 73 76 88 98 108 118 134 175 183 184 186 187 190 191 and the perception of TCAM being natural and therefore safe as well as effective compared with conventional healthcare.20 29–32 42 45 48 49 57 73 74 76 78 85 86 88 91 106 108 118 134 139 141 150 157 175 183 187 188 192 Patients participating in the identified studies were also positively attracted to TCAM for other reasons such as alignment with a patient’s sociocultural, religious and spiritual values with regard to health and disease12 32 33 42 68 72 73 81 91 106 110 118 149 181 183 187 188 190 192 193 and the sense of patient autonomy of their health.183 Other pull factors of TCAM use identified from the literature are patients’ trust and confidence in their traditional medicine practitioner to share their personal secrets and the perceived privacy they enjoyed with their traditional medicine practitioner.187 194 Perceived psychosocial care and support provided by TCAM providers compared with orthodox healthcare providers have also been reported as a pull factor.98 110 183 In addition, recommendation by respected and trusted peers such as TCAM providers, elders, relatives and friends has also been reported as a factor that drives patients into using TCAM.12 54 111 134 135 161 186 Aggressive advertisement of TCAM products and services is another pull factor.54 73 76 186
The push factors mainly centred on dissatisfaction with conventional healthcare, and this includes long distance to health facilities,187 unavailability of drugs,22 91 186 187 difficulty and inequity in accessing care,22 91 106 186 negative attitude of healthcare providers,106 134 187 long waiting time, lengthy procedures and fear of being diagnosed of a serious disease.134 192
Barriers to TCAM use
A number of population and subpopulation studies reported factors limiting the use of TCAM in Sub-Saharan Africa. Studies conducted among Nigerian31 and Sierra Leonean36 undergraduate students and Nigerian medical doctors162 cited an absence of conclusive scientific evidence that supports TCAM practice as a common barrier to the use of TCAM. A similar finding was reported in a population-based study in Ethiopia.49 Also, lack of patient belief in the safety and efficacy of TCAM was identified as a barrier to TCAM use in studies conducted among patients with hypertension,33 pregnant women88 and patients with various health conditions,192 as well as in the general population.49 In addition, two population-based studies48 189 and a subhealth study192 reported that respondents were reluctant to use traditional medical care due to the perceived demonic nature of TCAM. Further, four population-based studies from Ghana183 184 189 and Tanzania73 and subpopulation studies from Nigeria31 and Ghana192 cited perceived lack of an appropriate dose for TCAM products and unhygienic practice in product preparation,31 73 183 184 189 as well as the unregulated TCAM practitioner practice,183 192 as deterrent to using TCAM. Other barriers to TCAM use were an absence of health financing for traditional health care183 and a perceived lack of education and training among TCAM practitioners.36 183 189
Non-disclosure of TCAM use to healthcare providers
Twenty-five subhealth population studies reported on patients’ non-disclosure of TCAM use to their conventional healthcare providers.21 26 33 34 39 40 43 51 52 56 66 82 88 89 91 106 110–112 145 153 169 194–196 The non-disclosure rate of TCAM use to healthcare providers ranged from 55.8% to 100%, with an average of 83.0%. With regard to reasons for non-disclosure, four studies conducted among pregnant women in Uganda91 and Zambia,82 patients with hypertension in Ghana39 and patients with HIV/AIDS in South Africa195 cited fear of receiving improper care as a reason for not disclosing their TCAM use status to their healthcare provider. Another reason for non-disclosure of TCAM use was the conventional medicine provider’s negative attitude with perceived lack of support and understanding that lead to mistrust and stigma from conventional providers. Such reasons were put forward by patients with HIV/AIDS in South Africa,194 Uganda196 and Ghana,51 as well as patients with cancer in Nigeria111 and pregnant women in Uganda.91 The perception among Ethiopian patients with cancer110 and Ugandan patients with HIV/AIDS196 that their conventional healthcare providers lack knowledge about TCAM was another reason for not divulging their TCAM use status. Healthcare providers’ lack of enquiry about TCAM use was also cited among patients with cancer,34 epilepsy40 and diabetes43 in Nigeria. A study in Uganda reported that two-thirds of patients were open for discussion on TCAM use if initiated by their healthcare provider, and majority of them were also willing to adhere to their advice on TCAM use.196
Cost of TCAM use
We identified 15 papers that reported on the cost of TCAM use.20 21 25 27 47 55 71 111 112 117 130 146 167 197 198 The current literature shows a conflicting picture among studies that compared cost incurred between TCAM and conventional care. Some studies suggest that there is relatively low cost involved in using TCAM therapy compared with conventional care, although there is variation on how cost was measured in the two groups. For example, a Ghanaian national survey revealed that the average total household cost in the last 12 months for conventional care was slightly higher (US$33.43) than costs incurred in seeking TCAM treatment (US$30.33).27 Also, a study conducted among patients with cancer in Nigeria indicates cost of TCAM ranged from no cost to US$31.25, compared with the minimum cost of US$250 for conventional care.111 In Mali, half of conventional malaria treatment costs ranged from no cost to US$116, whereas TCAM treatment ranged from no cost for three-quarters of patients to US$100.117 In other studies, the cost of TCAM was higher than conventional care. For example, a Cameroonian study reported that the cost of TCAM treatment per day (US$1.5) was higher than conventional treatment (US$0.77).197 Although cost was not stated, Sorketti and colleagues130 in Sudan reported that more than three-quarters of psychiatric patients reported that the cost of treatment in traditional health centres was not less than conventional psychiatric service. Although no comparison was made with regard to conventional care, cost for TCAM services was relatively high in certain cases. For instance, in Tanzania, the maximum cost of TCAM treatment for epilepsy was US$100.119 A relatively high cost of TCAM practitioner services was also reported among the general population in South Africa, where the median cost for the last visit to a traditional healer was calculated at US$21. TCAM costs can take up a significant part of the household healthcare budget. Sixty-four per cent of households in a South African study spent more than 10% of their monthly spending,20 whereas majority (92.2%) of patients with HIV/AIDS who are TCAM users in Nigeria spent less than 20% of their monthly income on herbal treatment.112 Among surgical patients in South Africa, close to one-third (30%) paid less than US$60 for TCAM services.146
Findings from studies that assessed the cost per month of TCAM use alone reported an average of US$47.5 (US$12.60–US$96.88).21 25 47 55 167 In comparison between self-treatment and TCAM practitioner use, the average financial cost in Burkina Faso for TCAM self-care (US$2.85) was almost half of that for practitioner use (US$4.77).71
Safety of TCAM (self-reported or observed adverse effects)
Twenty-four papers reported on the perceived adverse effects due to TCAM or in combination with allopathic medicines.33–35 44 47 52 55 59 66 69 76 84–86 88 106 110 145 146 153 156 199 200 Fifteen of the studies33 34 44 47 69 76 84–86 88 110 145 146 153 199 200 reported on the prevalence of respondents’ perceived side effects due to TCAM products use (4%–53.3% (mean, 19.1%)), whereas four35 66 106 153 reported on respondents’ perceived side effects due to concomitant use of TCAM products and conventional medicines (2%–56.5% (mean, 23.5%)). One Nigerian study153 among outpatients reported a relatively high prevalence of TCAM adverse effects when used alone (9%) than when used in combination with conventional medicine (2%). Gastrointestinal disturbances (nausea, vomiting, diarrhoea and abdominal pain) followed by dizziness, headache and malaise were the most common types of adverse effects reported in 1134 44 52 55 76 84–86 145 146 153 201 of the 1234 44 52 55 59 76 84–86 145 146 153 201 studies that reported on the types of respondents’ perceived side effects. A Ugandan study among patients with HIV/AIDS and non-HIV/AIDS individuals reported that TCAM use was independently associated with the occurrence of liver fibrosis,59 while another among general inpatients indicated that 10 adverse effects were associated with the use of herbal products prior to admission.156
Discussion
This paper reports the first ever systematic review of the available research evidence on the contemporary use of TCAM in Sub-Saharan Africa. While there are assumptions about high use of TCAM (eg, 80%) across the continent that circulate in the literature, there has been no systematic review to date to substantiate that claim. Our review attempts to provide an estimate of TCAM (product and practitioners) prevalence both in the general population and health subpopulations based on the review of the current literature. The reviewed studies report varied TCAM (product and practitioners) use prevalence rates within and across countries in line with previous reviews.202 203 Such variability is possibly attributed to differences in TCAM definition and sample size in different studies and the high tendency for recall bias due to variations in timeframes over which TCAM use was evaluated. For instance, two large Nigerian studies report different prevalence rates of TCAM use during childbirth due to differences in study location, method of sampling and the way TCAM use was assessed.95 96 Despite an apparent conflict between variability in reported TCAM utilisation rates across studies, and the high sample size and response rate of many of these studies, our review highlights a relatively high use of TCAM both in the general population and health subpopulations, which resonate with findings of previous studies conducted in developed nations204 205 and estimates by the WHO.1 The push and pull factors of TCAM use identified in our review also resonate with the drivers of TCAM use reported outside of Africa,206 and appear to be important drivers underpinning the high use of TCAM across the continent. As in countries outside of Africa, the high use of TCAM also underscores the substantial role of TCAM as a source of basic healthcare to populations across Africa. It is imperative for health departments and governments across Sub-Saharan Africa to at least consider and inform themselves regarding TCAM, its current role and future possibilities in wider healthcare systems. This can be in the form of TCAM policy design and implementation, promoting TCAM training, research and development, as well as encouraging consideration of the integration of TCAM into mainstream healthcare system.1 It is worth noting that as a means of advancing TCAM use, some institutions have integrated TCAM into medical education curriculum within and outside of Africa.6 207
Our review also identified a variation in patient non-disclosure rates across studies which resonates with findings from research outside Africa.208 209 The lowest estimate identified in our review is sufficiently large to warrant concern over communication between patients who are TCAM users and their healthcare providers. Insufficient disclosure of TCAM use also highlights the need to have a clear insight into the barriers to disclosure. The available literature outside of Africa indicates that individual and/or contextual factors may influence patients’ decision not to disclose his/her TCAM use status.208 210 However, the reasons for non-disclosure identified in our review are informed by differences in beliefs and attitudes regarding conventional medicine and TCAM between patients and healthcare providers. In order to improve patient–provider communication about TCAM at the health service delivery level, it is important for healthcare professionals to be aware that their patients are likely users of TCAM and to encourage and facilitate an open dialogue about TCAM use as routine in their interaction with patients. Such communication regarding TCAM should imbibe the culture of shared decision making211 about therapeutic options for patients since it promotes patient satisfaction and active patient involvement in their care.212 Also, TCAM policy and practice guidelines should incorporate culturally sensitive patient education about the usefulness of patient disclosure of TCAM use to their conventional healthcare providers. In addition, to leverage communication about TCAM use with patients, conventional healthcare providers are required to be knowledgeable about commonly used TCAM products and practices to better advise their patients and the public on their risks and benefits. The current literature, although scanty, indicates a deficiency in knowledge about TCAM products and practices among healthcare providers in Sub-Saharan Africa213 214 and outside of Africa.215 216 As it has been suggested in studies outside of Africa,217 218 there is a need for the inclusion of common TCAM modalities into the existing curricula of healthcare training institutions and continuous professional education programme of the various healthcare cadres, as well as making reputable TCAM pharmacopoeias available in health facilities across Africa.
The reasons for the high TCAM patronage across the continent were also examined in our review, and they appear to resonate with the push and pull factors reported in studies conducted outside of Africa.219 220 Possible underlying structural factors that help explain the drivers of TCAM use include the fact that Sub-Saharan Africa is host to the largest population of people who are economically disadvantaged,221 and access to conventional care is limited due to cost and distance.222 Therefore, TCAM offers an affordable and easily accessible healthcare option. Also, because TCAM is publicly available and allows active patient involvement in health decision making, it offers users greater freedom and ownership in terms of their healthcare choices compared with conventional care in which health decisions are generally controlled by the healthcare professional.223
Our review also indicates that TCAM was used concurrently with conventional medicine, indicating that TCAM is mostly used as a complementary therapy rather than an alternative to conventional care. This may be linked to a growing paradigm shift among patients towards a holistic attitude in health that aligns with the philosophy of TCAM and acknowledges the insufficiencies of biomedical care.224 Given the complex interplay of factors influencing the pluralistic nature of health service utilisation in Sub-Saharan Africa,225 further research exploring the factors influencing the decision to use TCAM is required. The concurrent use of TCAM and allopathic medicines is known to also potentially undermine patient safety and health outcome due to herb–drug interactions leading to serious adverse effect and therapeutic failure of conventional medications.226–228 It is imperative that clinical studies be conducted to provide evidence of clinical interactions between conventional medicine and commonly used TCAM modalities in Sub-Saharan Africa. Such information is of value to healthcare providers when interacting with their patients as it will help provide an opportunity for well-informed therapeutic choices to be made that will contribute to maximising patient health outcomes.
Our review indicates a relatively low prevalence of self-reported TCAM adverse effects when used alone or in combination with allopathic medicines compared with what has been reported outside of Africa. Although not verified, it shows that TCAM like any conventional medicine is not free of adverse effects.229 The relatively low prevalence may be due to the fact that patient non-disclosure rate across the Sub-Saharan Africa is high and the fact that TCAM is often considered natural and therefore safe. TCAM adverse effects can be due to inherent toxicity or due to quality issues such as poor quality of TCAM products, incorrect or misidentified, or adulterated or contaminated TCAM products. Despite the majority of side effects being reported were gastrointestinal disturbances in our review, serious adverse effects such as liver and kidney toxicities have been cited in the literature.230 231 This has led the WHO to develop guidelines for the safety monitoring of herbal medicine product within the existing WHO pharmacovigilance framework.232 However, TCAM product safety regulation across Africa is still a challenge as many countries across Africa lack adequate regulatory framework to ensure the safety and quality of TCAM.233 Such challenge is compounded by the fact that there are few preclinical and postmarket TCAM safety and quality data as in addition to confusing nomenclature over plant species, varied cultural differences and traditional practices across Africa.234 Despite its limitations, the use of pharmacovigilance principles as part of the overall regulatory framework for TCAM in Africa is urgently needed. The incorporation of pharmacovigilance questions into ethnobotanical or ethnopharmacological studies is worth experimenting.235 Thus, the systematic collection and analysis of TCAM safety data is crucial in order to protect patients and the public at large.
Our review also identifies, among the general population and some health subpopulations, a relatively high level of self-directed TCAM use compared with the levels of practitioner-directed TCAM use. This may be explained by the significant amount of studies that focused on TCAM product use compared with TCAM practitioner use, or that the decision to use TCAM is more often influenced by family, friends and neighbours than traditional medicine practitioners.12 111 National representative studies that specifically look at TCAM practitioner utilisation both in general populations and among health subpopulations will provide further insight into the extent of TCAM practitioners’ contribution to the health delivery system.
In the general population in particular, the available literature indicates that TCAM users are likely to be of low socioeconomic class who are unemployed and unskilled. This suggests that low socioeconomic status in the society influences the decision to use TCAM given the fact that TCAM is a low-cost healthcare option compared with biomedicine. With particular reference to educational status, the result of the majority of the relevant studies reviewed contrasted with studies from high-income countries that show that TCAM users are more likely to have attained a high level of education than non-users,206 236 237 but in line with current scholarship in other low and middle-income nations.238 The low levels of health literacy239 and low access to evidence-based health information across most populations in Sub-Saharan Africa240 may explain such correlation. Our review reports conflicting findings on the relationship between TCAM utilisation and age, gender, religion and spatial location. This is in contrast with reviews that focused on studies outside of Africa, particularly with respect to gender in which women were more likely TCAM users compared with men.206 236 241 Variations in study design, sample size and data analysis may also explain the differences in TCAM user profile across studies, principally where statistical methods employed did not allow for the impact of confounders to be measured,242 thereby affecting the validity of the findings. Well-designed nationwide, or multinational, studies in which data are appropriately analysed are required to understand how sociodemographic factors affect TCAM use in and across Sub-Saharan Africa.
Our review highlights that there has been a disproportionate research focus on TCAM use among HIV/AIDS and obstetric patients in Sub-Saharan Africa. This pattern suggests the current TCAM research priorities in Sub-Saharan Africa and underscores the historic public health policy and research attention dedicated to infectious diseases and maternal child health in Sub-Saharan Africa.243 Areas that have received less attention in the TCAM research agenda in Sub-Saharan Africa include cancer, diabetes, musculoskeletal conditions and surgery. Given the steady rise in non-communicable diseases in the region10 and the high rate of TCAM use by individuals with these conditions,11 12 greater policy attention and research focus are needed to understand TCAM role in non-communicable diseases prevention and treatment in Sub-Saharan Africa. Specifically, well-designed, large-scale studies that look at how TCAM interfaces or interacts with conventional care for non-communicable disease conditions are urgently needed. Also, for both communicable and non-communicable health conditions, the interprofessional dynamics and provider–patient communication with regard to TCAM use need to be further explored.
Review limitations and future research directions
Our review highlights key research gaps that need to be addressed that currently challenge attempts to provide a comprehensive overview of TCAM use across Sub-Saharan Africa. First, most of the studies in our review were from Nigeria, South Africa and Ghana. Additional data are required from other Sub-Saharan Africa countries where little research has been conducted to know whether findings from less researched countries are in line or in contrast to the current evidence synthesised in our review. Second, of those articles appraised for quality, the majority had critical methodological flaws, such as employing a non-representative sample strategy, reporting a small sample size, incorporating a substantial risk of recall bias and failing to provide a definition of TCAM in their studies, all of which undermine the integrity of research findings and challenge the ability to draw definitive conclusions and conduct proper comparisons across different studies. Similar methodological limitations have been reported elsewhere.236 238 It is of utmost importance that the quality of empirical research into TCAM use in Sub-Saharan Africa is improved by using standardised methodology that follows good research design and practice.244 Third, our review identified the low cost of TCAM as a key driver of TCAM use, and low socioeconomic status was identified as a possible predictor of TCAM use in our review. However, only few studies in our review compared patient-reported cost of TCAM care and conventional healthcare and it shows a conflicting picture. Thus, there is a dearth of research evidence on the actual cost incurred by patients when seeking TCAM care. An economic analysis of TCAM use relative to conventional healthcare in general and subhealth populations is a worthy focus within the future TCAM research agenda in Sub-Saharan Africa. Fourth, the majority of the papers reviewed in our study employed quantitative survey design and analysis, providing a useful snapshot of TCAM utilisation patterns. Such research design, although useful, provides limited information regarding the decision-making process around TCAM use and patient–provider communication about TCAM use. Quantitative survey design also failed to provide an indepth insight with regard to the interface between the lived experience of TCAM use and the user’s religion and culture. As such, it is important that qualitative studies that focused on TCAM use and practice are conducted to help provide cultural and social insights that are useful to healthcare providers, policy makers and patients. Fifth, only studies published in English were considered in our review. We decided to include only English papers because none of us are proficient in French and other languages. Given that the full text of each included article is thoroughly read and understood before data are extracted, it will be unwise to rely on the English abstract of papers written in other languages given our limitation in other languages other than English. It is possible that an inclusion of studies published in other languages would have influenced our results. Finally, there are new publications beyond our search duration which if included may have influenced our findings. It is good for readers to take this and other aforementioned limitations when interpreting our findings.
Conclusion
TCAM use appears widespread across many countries in Sub-Saharan Africa, although most studies emerge from just a few countries including Nigeria, South Africa, Ghana and Uganda. Stakeholders involved in the healthcare sector in these countries should be mindful of this critical role of TCAM in healthcare service delivery across these countries. Further research in Sub-Saharan Africa should address a number of gaps identified in the current scholarship in order to help inform policy design and practice, as well as contribute to providing safe, efficient and harmonised healthcare for all in Sub-Saharan Africa.
Acknowledgments
Successful completion of this manuscript was made possible through participation in the Twelve Weeks to Publication Program funded by Faculty of Health, University of Technology Sydney and with the active support and contributions made by the facilitators and other program participants.
Footnotes
Handling editor: Dr Stephanie M Topp
Contributors: PBJ and JW conceived of the study. All authors contributed to the study design. PBJ did the database search and data extraction, while JW, AS and JA supervised the process. PBJ wrote the first draft of the manuscript, while JW, AS and JA contributed to the intellectual content and reviewed the subsequent and final drafts of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data available.
References
- 1. World Health Organization WHO traditional medicine strategy 2014-2023. Geneva, 2014. [Google Scholar]
- 2. World Health Organization Promoting the role of traditional medicine in health systems: a strategy for the african region, 2000. [Google Scholar]
- 3. Bannerman RH. The role of traditional medicine in primary health care : Traditional medicine and health care coverage. Geneva: WHO, 1983: 318–27. [Google Scholar]
- 4. Mander M, Ntuli L, Diederichs N. Economics of the traditional medicine trade in South Africa: health care delivery. S Afr Health Rev 2007:189–96. [Google Scholar]
- 5. Kasilo OM, Trapsida J-M, Mwikisa Ngenda C. An overview of the traditional medicine situation in the African region. African Health Monitor 2010:7–15. [Google Scholar]
- 6. Chitindingu E, George G, Gow J. A review of the integration of traditional, complementary and alternative medicine into the curriculum of South African medical schools. BMC Med Educ 2014;14:40 10.1186/1472-6920-14-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. KNUST Programmes of Study, College of Health Sciences. 2018. Available from: https://www.knust.edu.gh/admissions/prospective/ugprogrammes [Accessed 7 June 2018].
- 8. Abdullahi AA. Trends and challenges of traditional medicine in Africa. Afr J Tradit Complement Altern Med 2011;8(5 Suppl):115-23 10.4314/ajtcam.v8i5S.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO National policy on traditional medicine and regulation of herbal medicines: report of a WHO global survey. Geneva: World Health Organization, 2005. [Google Scholar]
- 10. Dalal S, Beunza JJ, Volmink J, et al. Non-communicable diseases in sub-Saharan Africa: what we know now. Int J Epidemiol 2011;40:885–901. 10.1093/ije/dyr050 [DOI] [PubMed] [Google Scholar]
- 11. Saydah SH, Eberhardt MS. Use of complementary and alternative medicine among adults with chronic diseases: United States 2002. J Altern Complement Med 2006;12:805–12. 10.1089/acm.2006.12.805 [DOI] [PubMed] [Google Scholar]
- 12. Hughes GD, Aboyade OM, Beauclair R, et al. Characterizing herbal medicine use for noncommunicable diseases in urban South Africa. Evidence-Based Complementary and Alternative Medicine 2015;2015:1–10. 10.1155/2015/736074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ayele AA, Tegegn HG, Haile KT, et al. Complementary and alternative medicine use among elderly patients living with chronic diseases in a teaching hospital in Ethiopia. Complement Ther Med 2017;35:115–9. 10.1016/j.ctim.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 14. Sambo L. Health systems and primary health care in the African region In: African Health Monitor. 14, 2011: 2–3. [Google Scholar]
- 15. Campbell-Hall V, Petersen I, Bhana A, et al. Collaboration between traditional practitioners and primary health care staff in South Africa: developing a workable partnership for community mental health services. Transcult Psychiatry 2010;47:610–28. 10.1177/1363461510383459 [DOI] [PubMed] [Google Scholar]
- 16. Adams J, Lui CW, Sibbritt D, et al. Attitudes and referral practices of maternity care professionals with regard to complementary and alternative medicine: an integrative review. J Adv Nurs 2011;67:472–83. 10.1111/j.1365-2648.2010.05510.x [DOI] [PubMed] [Google Scholar]
- 17. Adams J, Barbery G, Lui CW. Complementary and alternative medicine use for headache and migraine: a critical review of the literature. Headache 2013;53:459–73. 10.1111/j.1526-4610.2012.02271.x [DOI] [PubMed] [Google Scholar]
- 18. Peng W, Adams J, Sibbritt DW, et al. Critical review of complementary and alternative medicine use in menopause: focus on prevalence, motivation, decision-making, and communication. Menopause 2014;21:536–48. 10.1097/GME.0b013e3182a46a3e [DOI] [PubMed] [Google Scholar]
- 19. Fejer R, Kyvik KO, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J 2006;15:834–48. 10.1007/s00586-004-0864-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nxumalo N, Alaba O, Harris B, et al. Utilization of traditional healers in South Africa and costs to patients: findings from a national household survey. J Public Health Policy 2011;32 Suppl 1:S124–S136. 10.1057/jphp.2011.26 [DOI] [PubMed] [Google Scholar]
- 21. Peltzer K, Preez NF-du, Ramlagan S, et al. Use of traditional complementary and alternative medicine for HIV patients in KwaZulu-Natal, South Africa. BMC Public Health 2008;8:255 10.1186/1471-2458-8-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diaz T, George AS, Rao SR, et al. Healthcare seeking for diarrhoea, malaria and pneumonia among children in four poor rural districts in Sierra Leone in the context of free health care: results of a cross-sectional survey. BMC Public Health 2013;13:157 10.1186/1471-2458-13-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Girma E, Tesfaye M. Patterns of treatment seeking behavior for mental illnesses in Southwest Ethiopia: a hospital based study. BMC Psychiatry 2011;11:138 10.1186/1471-244X-11-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mureyi DD, Monera TG, Maponga CC. Prevalence and patterns of prenatal use of traditional medicine among women at selected harare clinics: a cross-sectional study. BMC Complement Altern Med 2012;12:164 10.1186/1472-6882-12-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peltzer K, Friend-du Preez N, Ramlagan S, et al. Traditional complementary and alternative medicine and antiretroviral treatment adherence among HIV patients in KwaZulu-Natal, South Africa. Afr J Tradit Complement Altern Med 2010;7 10.4314/ajtcam.v7i2.50871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peltzer K, Preez NF, Ramlagan S, et al. Antiretrovirals and the use of traditional, complementary and alternative medicine by HIV patients in Kwazulu-Natal, South Africa: a longitudinal study. Afr J Tradit Complement Altern Med 2011;8:337–45. 10.4314/ajtcam.v8i4.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oyebode O, Kandala NB, Chilton PJ, et al. Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Policy Plan 2016;31:984–91. 10.1093/heapol/czw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peltzer K, Pengpid S. Prevalence and determinants of traditional, complementary and alternative medicine provider use among adults from 32 countries. Chin J Integr Med 2018;24:584–90. 10.1007/s11655-016-2748-y [DOI] [PubMed] [Google Scholar]
- 29. Adeyeye O, Onadeko B, Ogunleye O. The use of complementary and alternative medicine by asthma patients receiving care in an urban tertiary centre in Nigeria. Int J Biol Med Res 2011;(4):1026-–30. [Google Scholar]
- 30. Adinma E, Azuike E, Okafor-Udah C. Pattern and practice of complimentary and alternative medication amongst patients in a tertiary hospital in Nigeria. Eur J Prev Med 2015;3:44–8. [Google Scholar]
- 31. Ahwinahwi U, Chukwudi K. Perception and Use of Complementary and Alternative Medicine (CAM) among undergraduate students in a Nigerian University. J Appl Pharm Sci 2016;6:096–101. 10.7324/JAPS.2016.60617 [DOI] [Google Scholar]
- 32. Amira OC, Okubadejo NU. Frequency of complementary and alternative medicine utilization in hypertensive patients attending an urban tertiary care centre in Nigeria. BMC Complement Altern Med 2007;7:30 10.1186/1472-6882-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Asfaw Erku D, Basazn Mekuria A, Erku DA, Mekuria AB. Prevalence and correlates of complementary and alternative medicine use among hypertensive patients in Gondar Town, Ethiopia. Evid Based Complement Alternat Med 2016;2016:1–7. 10.1155/2016/6987636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ezeome ER, Anarado AN. Use of complementary and alternative medicine by cancer patients at the University of Nigeria Teaching Hospital, Enugu, Nigeria. BMC Complement Altern Med 2007;7:28 10.1186/1472-6882-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gyasi RM, Agyemang-Duah W, Mensah CM, et al. Unconventional medical practices among Ghanaian students: a university-based survey. J Tradit Complement Med 2017;7:126–32. 10.1016/j.jtcme.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. James PB, Bah AJ, Awareness BAJ. Awareness, use, attitude and perceived need for Complementary and Alternative Medicine (CAM) education among undergraduate pharmacy students in Sierra Leone: a descriptive cross-sectional survey. BMC Complement Altern Med 2014;14:438 10.1186/1472-6882-14-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. James PB, Bah AJ, Kondorvoh IM. Exploring self-use, attitude and interest to study complementary and alternative medicine (CAM) among final year undergraduate medical, pharmacy and nursing students in Sierra Leone: a comparative study. BMC Complement Altern Med 2016;16:121 10.1186/s12906-016-1102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jaya Y, Masanganise R. The prevalence, types and effects of traditional eye medicine use among newly presenting patients at Sekuru Kaguvi Hospital Eye Unit in Harare, Zimbabwe. Cent Afr J Med 2014;60(5-8):36–44. [PubMed] [Google Scholar]
- 39. Kretchy IA, Owusu-Daaku F, Danquah S. Patterns and determinants of the use of complementary and alternative medicine: a cross-sectional study of hypertensive patients in Ghana. BMC Complement Altern Med 2014;14:44 10.1186/1472-6882-14-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lagunju IA. Complementary and alternative medicines use in children with epilepsy in Ibadan, Nigeria. Afr J Med Med Sci 2013;42:15–23. [PubMed] [Google Scholar]
- 41. Mbada CE, Adeyemi TL, Adedoyin RA, et al. Prevalence and modes of complementary and alternative medicine use among peasant farmers with musculoskeletal pain in a rural community in South-Western Nigeria. BMC Complement Altern Med 2015;15:164 10.1186/s12906-015-0695-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Obalum DC, Ogo CN. Usage of Complementary and Alternative Medicine (CAM) among osteoarthritis patients attending an urban multi-specialist hospital in Lagos, Nigeria. Niger Postgrad Med J 2011;18:44–7. [PubMed] [Google Scholar]
- 43. Ogbera AO, Dada O, Adeyeye F, et al. Complementary and alternative medicine use in diabetes mellitus. West Afr J Med 2010;29:158–62. [DOI] [PubMed] [Google Scholar]
- 44. Okoronkwo I, Onyia-pat J-lovena, Okpala P, et al. Patterns of complementary and alternative medicine use, perceived benefits, and adverse effects among adult users in Enugu Urban, Southeast Nigeria. Evid Based Complementary Altern Med 2014;2014:1–6. 10.1155/2014/239372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Onyiapat JL, Okoronkwo IL, Ogbonnaya NP. Complementary and alternative medicine use among adults in Enugu, Nigeria. BMC Complement Altern Med 2011;11:19 10.1186/1472-6882-11-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Osamor PE, Owumi BE. Complementary and alternative medicine in the management of hypertension in an urban Nigerian community. BMC Complement Altern Med 2010;10:36 10.1186/1472-6882-10-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oshikoya KA, Senbanjo IO, Njokanma OF, et al. Use of complementary and alternative medicines for children with chronic health conditions in Lagos, Nigeria. BMC Complement Altern Med 2008;8:66 10.1186/1472-6882-8-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Galabuzi C, Agea J, Fungo B. Traditional medicine as an alternative form of health care system: a pre liminary case study of Nangabo sub-county, central Uganda. Afr J Tradit Complement Altern Med 2010;7:11–16. 10.4314/ajtcam.v7i1.57224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gari A, Yarlagadda R, Wolde-Mariam M. Knowledge, attitude, practice, and management of traditional medicine among people of Burka Jato Kebele, West Ethiopia. J Pharm Bioallied Sci 2015;7:136 10.4103/0975-7406.148782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gyasi RM, Asante F, Segbefia AY, et al. Does spatial location matter? Traditional therapy utilisation among the general population in a Ghanaian rural and urban setting. Complement Ther Med 2015;23:439–50. 10.1016/j.ctim.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 51. Gyasi RM, Tagoe-Darko E, Mensah CM. Use of traditional medicine by HIV/AIDS patients in Kumasi Metropolis, Ghana: a cross-sectional survey. Am Int J Contemp Res 2013;4:117–29. [Google Scholar]
- 52. Suroowan S, Mahomoodally F. Complementary and alternative medicine use among Mauritian women. Complement Ther Clin Pract 2013;19:36–43. 10.1016/j.ctcp.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 53. Ekwunife OI, Oreh C, Ubaka CM. Concurrent use of complementary and alternative medicine with antiretroviral therapy reduces adherence to HIV medications. Int J Pharm Pract 2012;20:340–3. 10.1111/j.2042-7174.2012.00204.x [DOI] [PubMed] [Google Scholar]
- 54. van Staden AM, Joubert GB. Interest in and willingness to use complementary, alternative and traditional medicine among academic and administrative university staff in Bloemfontein, South Africa. Afr J Tradit Complement Altern Med 2014;11:61–6. 10.4314/ajtcam.v11i5.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oshikoya KA, Oreagba IA, Ogunleye OO, et al. Use of complementary medicines among HIV-infected children in Lagos, Nigeria. Complement Ther Clin Pract 2014;20:118–24. 10.1016/j.ctcp.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 56. Yarney J, Donkor A, Opoku SY, et al. Characteristics of users and implications for the use of complementary and alternative medicine in Ghanaian cancer patients undergoing radiotherapy and chemotherapy: a cross- sectional study. BMC Complement Altern Med 2013;13:16 10.1186/1472-6882-13-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Abodunrin OL, Omojasola T, Rojugbokan OO. Utilization of alternative medical services by people of a north central city of Nigeria. East Afr J Public Health 2011;8:82–7. [PubMed] [Google Scholar]
- 58. Allabi AC, Busia K, Ekanmian V, et al. The use of medicinal plants in self-care in the Agonlin region of Benin. J Ethnopharmacol 2011;133:234–43. 10.1016/j.jep.2010.09.028 [DOI] [PubMed] [Google Scholar]
- 59. Auerbach BJ, Reynolds SJ, Lamorde M, et al. Traditional herbal medicine use associated with liver fibrosis in rural Rakai, Uganda. PLoS One 2012;7:e41737 10.1371/journal.pone.0041737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bamidele JO, Adebimpe WO, Oladele EA. Knowledge, attitude and use of alternative medical therapy amongst urban residents of Osun State, southwestern Nigeria. Afr J Tradit Complement Altern Med 2009;6:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Banwat ME, A. EA, I. AI, et al. Alternative medicine use among workers in an urban setting in north-central Nigeria. Int J Biomed Res 2015;6:268–73. 10.7439/ijbr.v6i4.1919 [DOI] [Google Scholar]
- 62. Chintamunnee V, Mahomoodally MF. Herbal medicine commonly used against non-communicable diseases in the tropical island of Mauritius. J Herb Med 2012;2:113–25. 10.1016/j.hermed.2012.06.001 [DOI] [Google Scholar]
- 63. De Jager GF, Prinsloo EAM, Joubert G. Use of traditional medicine versus use of the community-based primary health care clinic by the San community at Platfontein. South African Family Practice 2010;52:542–7. 10.1080/20786204.2010.10874045 [DOI] [Google Scholar]
- 64. Duru CB, Diwe KC, Uwakwe KA. Combined orthodox and traditional medicine use among hous eholds in Orlu, Imo State, Nigeria: prevalence and determinants. World Journal of Preventive Medicine 2016;4:5–11. [Google Scholar]
- 65. Flatie T, Gedif T, Asres K, et al. Ethnomedical survey of Berta ethnic group Assosa Zone, Benishangul-Gumuz regional state, mid-west Ethiopia. J Ethnobiol Ethnomed 2009;5:14 10.1186/1746-4269-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gyasi RM, Siaw LP, Mensah CM. Prevalence and pattern of traditional medical therapy utilisation in Kumasi Metropolis and Sekyere South District, Ghana. J Ethnopharmacol 2015;161:138–46. 10.1016/j.jep.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 67. Mee P, Wagner RG, Gómez-Olivé FX, et al. Changing use of traditional healthcare amongst those dying of HIV related disease and TB in rural South Africa from 2003 - 2011: a retrospective cohort study. BMC Complement Altern Med 2014;14:504 10.1186/1472-6882-14-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Opara EO KK. Factors affecting utilization of herbal medicine as livelihood alternatives among residents of imo state: the role of social work professionals. J Humanit Soc Sci 2016;21:66–78. [Google Scholar]
- 69. Oreagba IA, Oshikoya KA, Amachree M. Herbal medicine use among urban residents in Lagos, Nigeria. BMC Complement Altern Med 2011;11:117 10.1186/1472-6882-11-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Osemene KP, Elujoba AA, Ilori MO. A comparative assessment of herbal and orthodox medicines in Nigeria. Niger J Nat Prod Med 2013;17:77–81. 10.4314/njnpm.v17i1.7 [DOI] [Google Scholar]
- 71. Pouliot M. Relying on nature's pharmacy in rural Burkina Faso: empirical evidence of the determinants of traditional medicine consumption. Soc Sci Med 2011;73:1498–507. 10.1016/j.socscimed.2011.08.037 [DOI] [PubMed] [Google Scholar]
- 72. Sarki ZM DM. Socio-demographic factors and utilization of traditional medicine in Kazaure Town, Jigawa State, Nigeria. Int Journal of Emerging Knowledge 2015;3:9–20. [Google Scholar]
- 73. Stanifer JW, Patel UD, Karia F, et al. The determinants of traditional medicine use in northern Tanzania: a mixed-methods study. PLoS One 2015;10:e0122638 10.1371/journal.pone.0122638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Usifoh S, Udezi A. Social and economic factors influencing the patronage and use of complementary and alternative medicine in Enugu. Journal of Pharmacy & Bioresources 2013;10:17–24. [Google Scholar]
- 75. Wassie SM, Aragie LL, Taye BW, et al. Knowledge, attitude, and utilization of traditional medicine among the communities of merawi town, northwest ethiopia: A cross-sectional study. Evid Based Complement Alternat Med 2015;2015:1–7. 10.1155/2015/138073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jimoh AO, Sani Z, Abubakar K. Safety concerns and determinants of complementary and alternative medicine use in a sub- Concerns and Determinants of Complementary and Alternative Medicine Use in a Sub-urban area of Sokoto, North Western Nigeria. Journal of Medical Sciences 2013;13:737–42. 10.3923/jms.2013.737.742 [DOI] [Google Scholar]
- 77. Awad AI, Eltayeb IB, Capps PA. Self-medication practices in Khartoum State, Sudan. Eur J Clin Pharmacol 2006;62:317–24. 10.1007/s00228-006-0107-1 [DOI] [PubMed] [Google Scholar]
- 78. Birhan W, Giday M, Teklehaymanot T. The contribution of traditional healers' clinics to public health care system in Addis Ababa, Ethiopia: a cross-sectional study. J Ethnobiol Ethnomed 2011;7:39 10.1186/1746-4269-7-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kruk ME, Rockers PC, Varpilah ST, et al. Which doctor?: determinants of utilization of formal and informal health care in postconflict liberia. Med Care 2011;49:585–91. 10.1097/MLR.0b013e31820f0dd4 [DOI] [PubMed] [Google Scholar]
- 80. Mathibela MK, Egan BA, Du Plessis HJ, et al. Socio-cultural profile of Bapedi traditional healers as indigenous knowledge custodians and conservation partners in the Blouberg area, Limpopo Province, South Africa. J Ethnobiol Ethnomed 2015;11:49 10.1186/s13002-015-0025-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mbereko A, Mahlatini P. Understanding contributions of traditional healers to the prevention, care and support in the fight against HIV and AIDS Pandemic in Kariba, Zimbabwe. Int J Sociol Anthropol 2014;6:136. [Google Scholar]
- 82. Banda Y, Chapman V, Goldenberg RL, et al. Use of traditional medicine among pregnant women in Lusaka, Zambia. J Altern Complement Med 2007;13:123–8. 10.1089/acm.2006.6225 [DOI] [PubMed] [Google Scholar]
- 83. Bayisa B, Tatiparthi R, Mulisa E. Use of herbal medicine among pregnant women on antenatal care at nekemte hospital, Western ethiopia. Jundishapur J Nat Pharm Prod 2014;9:e17368 doi:10.17795/jjnpp-17368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Duru CB, Uwakwe KA, Chinomnso NC. Socio-demographic determinants of herbal medicine use in pregnancy among nigerian women attending clinics in a tertiary hospital in Imo State, South-East, Nigeria. American Journal of Medicine Studies 2016;4:1–10. [Google Scholar]
- 85. Fakeye TO, Adisa R, Musa IE. Attitude and use of herbal medicines among pregnant women in Nigeria. BMC Complement Altern Med 2009;9:53 10.1186/1472-6882-9-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Laelago T, Yohannes T, Lemango F. Prevalence of herbal medicine use and associated factors among pregnant women attending antenatal care at public health facilities in Hossana Town, Southern Ethiopia: facility based cross sectional study. Arch Public Health 2016;74:7 10.1186/s13690-016-0118-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Malan DF, Neuba DF. Traditional practices and medicinal plants use during pregnancy by Anyi-Ndenye women (Eastern Côte d'Ivoire). Afr J Reprod Health 2011;15:85–93. [PubMed] [Google Scholar]
- 88. Mekuria AB, Erku DA, Gebresillassie BM, et al. Prevalence and associated factors of herbal medicine use among pregnant women on antenatal care follow-up at University of Gondar referral and teaching hospital, Ethiopia: a cross-sectional study. BMC Complement Altern Med 2017;17:86 10.1186/s12906-017-1608-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mothupi MC. Use of herbal medicine during pregnancy among women with access to public healthcare in Nairobi, Kenya: a cross-sectional survey. BMC Complement Altern Med 2014;14:432 10.1186/1472-6882-14-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nergard CS, Ho TP, Diallo D, et al. Attitudes and use of medicinal plants during pregnancy among women at health care centers in three regions of Mali, West-Africa. J Ethnobiol Ethnomed 2015;11:73 10.1186/s13002-015-0057-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nyeko R, Tumwesigye NM, Halage AA. Prevalence and factors associated with use of herbal medicines during pregnancy among women attending postnatal clinics in Gulu district, Northern Uganda. BMC Pregnancy Childbirth 2016;16:296 10.1186/s12884-016-1095-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ologe MO, Aboyeji AP, Ijaiya MA, et al. Herbal use among pregnant mothers in Ilorin, Kwra State, Nigeria. J Obstet Gynaecol 2008;28:720–1. 10.1080/01443610802461912 [DOI] [PubMed] [Google Scholar]
- 93. Tamuno I, Omole-Ohonsi A, Fadare J. Use of herbal medicine among pregnant women attending a tertiary hospital in northern Nigeria.. The Internet Journal of Gynecology and Obstetrics 2011;15. [Google Scholar]
- 94. Addo V. Herbal medicines: socio-demographic characteristics and pattern of use by patients in a tertiary obstetrics and gynaecology unit. J Sci Tech 2007;27:149–55. [Google Scholar]
- 95. Olusanya BO, Inem VA, Abosede OA. Infants delivered in maternity homes run by traditional birth attendants in urban Nigeria: a community-based study. Health Care Women Int 2011;32:474–91. 10.1080/07399332.2011.565531 [DOI] [PubMed] [Google Scholar]
- 96. Sarmiento I, Zuluaga G, Andersson N. Traditional medicine used in childbirth and for childhood diarrhoea in Nigeria's Cross River State: interviews with traditional practitioners and a statewide cross-sectional study. BMJ Open 2016;6:e010417 10.1136/bmjopen-2015-010417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rasch V, Kipingili R. Unsafe abortion in urban and rural Tanzania: method, provider and consequences. Trop Med Int Health 2009;14:1128–33. 10.1111/j.1365-3156.2009.02327.x [DOI] [PubMed] [Google Scholar]
- 98. Ebuehi OM, Akintujoye I. Perception and utilization of traditional birth attendants by pregnant women attending primary health care clinics in a rural Local Government Area in Ogun State, Nigeria. Int J Womens Health 2012;4:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kaadaaga HF, Ajeani J, Ononge S, et al. Prevalence and factors associated with use of herbal medicine among women attending an infertility clinic in Uganda. BMC Complement Altern Med 2014;14:27 10.1186/1472-6882-14-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ola TM, Aladekomo FO, Oludare BA. Determinants of the choice of treatment outlets for infertility in Southwest Nigeria. Rawal Medical Journal 2008;33:193–6. [Google Scholar]
- 101. Dienye PO, Judah F, Ndukwu G. Frequency of symptoms and health seeking behaviours of menopausal women in an out-patient clinic in Port Harcourt, Nigeria. Glob J Health Sci 2013;5:39–47. 10.5539/gjhs.v5n4p39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mbikusita-Lewanika M, Stephen H, Thomas J. The prevalence of the use of 'dry sex' traditional medicines, among Zambian women, and the profile of the users. Psychol Health Med 2009;14:227–38. 10.1080/13548500802270364 [DOI] [PubMed] [Google Scholar]
- 103. Nuwaha F, Muganzi E. Predictors of use of traditional medicine by patients with sexually transmitted infections in southwest Uganda. J Altern Complement Med 2008;14:733–9. 10.1089/acm.2007.7160 [DOI] [PubMed] [Google Scholar]
- 104. Hughes GD, Aboyade OM, Clark BL, et al. The prevalence of traditional herbal medicine use among hypertensives living in South African communities. BMC Complement Altern Med 2013;13:38 10.1186/1472-6882-13-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nuwaha F, Musinguzi G. Use of alternative medicine for hypertension in Buikwe and Mukono districts of Uganda: a cross sectional study. BMC Complement Altern Med 2013;13:301 10.1186/1472-6882-13-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Olisa NS, Oyelola FT. Evaluation of use of herbal medicines among ambulatory hypertensive patients attending a secondary health care facility in Nigeria. Int J Pharm Pract 2009;17:101–5. 10.1211/ijpp.17.02.0005 [DOI] [PubMed] [Google Scholar]
- 107. Lunyera J, Wang D, Maro V, et al. Traditional medicine practices among community members with diabetes mellitus in Northern Tanzania: an ethnomedical survey. BMC Complement Altern Med 2016;16:282 10.1186/s12906-016-1262-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Baldé NM, Youla A, Baldé MD, et al. Herbal medicine and treatment of diabetes in Africa: an example from Guinea. Diabetes Metab 2006;32:171–5. 10.1016/S1262-3636(07)70265-3 [DOI] [PubMed] [Google Scholar]
- 109. Mwangi J, Gitonga L. Perceptions and use of herbal remedies among patients with diabetes mellitus in Murang’a North District, Kenya. Open Journal of Clinical Diagnostics 2014;04:152–72. 10.4236/ojcd.2014.43024 [DOI] [Google Scholar]
- 110. Erku DA. Complementary and alternative medicine use and its association with quality of life among cancer patients receiving chemotherapy in Ethiopia: a cross-sectional study. Evid Based Complement Alternat Med 2016;2016:8:1–8. 10.1155/2016/2809875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Asuzu C, Elumelu‐Kupoluyi T, Asuzu M. A pilot study of cancer patients' use of traditional healers in the Radiotherapy Department, University College Hospital, Ibadan, Nigeria. Psycho-Oncol 2015. [DOI] [PubMed] [Google Scholar]
- 112. Onifade AA, Ajeigbe KO, Omotosho IO, et al. Attitude of HIV patients to herbal remedy for HIV infection in Nigeria. Niger J Physiol Sci 2013;28:109–12. [PubMed] [Google Scholar]
- 113. Audet CM, Blevins M, Rosenberg C, et al. Symptomatic HIV-positive persons in rural Mozambique who first consult a traditional healer have delays in HIV testing: a cross-sectional study. J Acquir Immune Defic Syndr 2014;66:e80–e86. 10.1097/QAI.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Horwitz RH, Tsai AC, Maling S, et al. No association found between traditional healer use and delayed antiretroviral initiation in rural Uganda. AIDS Behav 2013;17:260–5. 10.1007/s10461-011-0132-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Thielman NM, Ostermann J, Whetten K, et al. Reduced adherence to antiretroviral therapy among HIV-infected Tanzanians seeking cure from the Loliondo healer. J Acquir Immune Defic Syndr 2014;65:e104–e109. 10.1097/01.qai.0000437619.23031.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Diallo D, Graz B, Falquet J, et al. Malaria treatment in remote areas of Mali: use of modern and traditional medicines, patient outcome. Trans R Soc Trop Med Hyg 2006;100:515–20. 10.1016/j.trstmh.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 117. Graz B, Willcox M, Berthé D, et al. Home treatments alone or mixed with modern treatments for malaria in Finkolo AC, South Mali: reported use, outcomes and changes over 10 years. Trans R Soc Trop Med Hyg 2015;109:209–13. 10.1093/trstmh/tru181 [DOI] [PubMed] [Google Scholar]
- 118. Jombo GTA, Mbaawuaga EM, Denen AP, et al. Utilization of traditional healers for treatment of malaria among female residents in Makurdi city and its environs. Asian Pac J Trop Biomed 2010;3:563–6. 10.1016/S1995-7645(10)60136-8 [DOI] [Google Scholar]
- 119. Mensah CM, Gyasi RM. Use of herbal medicine in the management of malaria in the urban-periphery, Ghana. J Biol Agric Healthc 2012;2:113–22. [Google Scholar]
- 120. Ranasinghe S, Ansumana R, Lamin JM, et al. Herbs and herbal combinations used to treat suspected malaria in Bo, Sierra Leone. J Ethnopharmacol 2015;166:200–4. 10.1016/j.jep.2015.03.028 [DOI] [PubMed] [Google Scholar]
- 121. Bakshi SS, McMahon S, George A, et al. The role of traditional treatment on health care seeking by caregivers for sick children in Sierra Leone: results of a baseline survey. Acta Trop 2013;127:46–52. 10.1016/j.actatropica.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 122. Eseigbe EE, Anyiam JO, Ogunrinde GO. Health care seeking behavior among caregivers of sick children who had cerebral malaria in Northwestern Nigeria. Malar Res Treat 2012;2012:4 10.1155/2012/954975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nwani PO, Arinzechi EO, Asomugha AL, et al. Illness concept among people with epilepsy and their caregivers and preferred treatment methods in a suburban community in Southeast Nigeria. West Afr J Med 2013;32:26–30. [PubMed] [Google Scholar]
- 124. Adeosun II AAA, Adewumi TA, Jeje OO. The pathways to the first contact with mental health services among patients with schizophrenia in Lagos, Nigeria. Schizophr Res Treatment 2013;2013:769161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Aghukwa CN. Care seeking and beliefs about the cause of mental illness among Nigerian psychiatric patients and their families. Psychiatr Serv 2012;63:616–8. 10.1176/appi.ps.201000343 [DOI] [PubMed] [Google Scholar]
- 126. Burns JK, Jhazbhay K, Kidd M, et al. Causal attributions, pathway to care and clinical features of first-episode psychosis: a South African perspective. Int J Soc Psychiatry 2011;57:538–45. 10.1177/0020764010390199 [DOI] [PubMed] [Google Scholar]
- 127. Ibrahim A, Hor S, Bahar OS, et al. Pathways to psychiatric care for mental disorders: a retrospective study of patients seeking mental health services at a public psychiatric facility in Ghana. Int J Ment Health Syst 2016;10:63 10.1186/s13033-016-0095-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kauye F, Udedi M, Mafuta C. Pathway to care for psychiatric patients in a developing country: Malawi. Int J Soc Psychiatry 2015;61:121–8. 10.1177/0020764014537235 [DOI] [PubMed] [Google Scholar]
- 129. Odinka PC, Oche M, Ndukuba AC, et al. The socio-demographic characteristics and patterns of help-seeking among patients with schizophrenia in south-east Nigeria. J Health Care Poor Underserved 2014;25:180–91. 10.1353/hpu.2014.0055 [DOI] [PubMed] [Google Scholar]
- 130. Sorketti EA, Zainal NZ, Habil MH. The characteristics of people with mental illness who are under treatment in traditional healer centres in Sudan. Int J Soc Psychiatry 2012;58:204–16. 10.1177/0020764010390439 [DOI] [PubMed] [Google Scholar]
- 131. Tomita A, Burns JK, King H, et al. Duration of untreated psychosis and the pathway to care in KwaZulu-Natal, South Africa. J Nerv Ment Dis 2015;203:222–5. 10.1097/NMD.0000000000000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Abbo C. Profiles and outcome of traditional healing practices for severe mental illnesses in two districts of Eastern Uganda. Glob Health Action 2009;4(1-15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Akinpelu A, Oyewole O, Odole A. Prevalence of musculoskeletal pain and health seeking behaviour among occupational drivers in Ibadan, Nigeria. Afr J Biomed Res 2011;14:89–94. [Google Scholar]
- 134. Aderibigbe SA, Agaja SR, Bamidele JO. Determinants of utilization of traditional bone setters in Ilorin, north central Nigeria. J Prev Med Hyg 2013;54:35–40. [PMC free article] [PubMed] [Google Scholar]
- 135. Onyemaechi NO, Lasebikan OA, Elachi IC, et al. Patronage of traditional bonesetters in Makurdi, north-central Nigeria. Patient Prefer Adherence 2015;9:275–9. 10.2147/PPA.S76877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nwadiaro H, Ozoilo K, Nwadiaro P, et al. Determinants of patronage of traditional bone setters in the middle belt of Nigeria. Journal of the National Association of Resident Doctors of Nigeria 2008;17:356–9. [DOI] [PubMed] [Google Scholar]
- 137. Aries MJ, Joosten H, Wegdam HH, et al. Fracture treatment by bonesetters in central Ghana: patients explain their choices and experiences. Trop Med Int Health 2007;12:564–74. [DOI] [PubMed] [Google Scholar]
- 138. Farag TH, Kotloff KL, Levine MM, et al. Seeking care for pediatric diarrheal illness from traditional healers in Bamako, Mali. Am J Trop Med Hyg 2013;89(1 Suppl):12–753. 10.4269/ajtmh.12-0753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Njoroge GN, Kibunga JW. Herbal medicine acceptance, sources and utilization for diarrhoea management in a cosmopolitan urban area (Thika, Kenya). African Journal of Ecology 2007;45(s1):65–70. 10.1111/j.1365-2028.2007.00740.x [DOI] [Google Scholar]
- 140. Achigbu E, Achigbu K. Traditional medication use among out-patients attending the eye clinic of a secondary health facility in Owerri, South-East Nigeria. Orient Journal of Medicine 2014;26(3-4):107–13. [Google Scholar]
- 141. Eze BI, Chuka-Okosa CM, Uche JN. Traditional eye medicine use by newly presenting ophthalmic patients to a teaching hospital in south-eastern Nigeria: socio-demographic and clinical correlates. BMC Complement Altern Med 2009;9:40 10.1186/1472-6882-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nwosu SN, Obidiozor JU. Incidence and risk factors for traditional eye medicine use among patients at a tertiary eye hospital in Nigeria. Niger J Clin Pract 2011;14:405–7. 10.4103/1119-3077.91744 [DOI] [PubMed] [Google Scholar]
- 143. Ukponmwan CU, Momoh N. Incidence and complications of traditional eye medications in Nigeria in a teaching hospital. Middle East Afr J Ophthalmol 2010;17:315 10.4103/0974-9233.71596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ajite KO, Fadamiro OC. Prevalence of harmful/traditional medication use in traumatic eye injury. Glob J Health Sci 2013;5:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Onyeka TC, Ezike HA, Nwoke OM, et al. Herbal medicine: a survey of use in Nigerian presurgical patients booked for ambulatory anaesthesia. BMC Complement Altern Med 2012;12:130 10.1186/1472-6882-12-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Nethathe G, Matamba T, Malumalu J, et al. Traditional medicine use in surgical patients in a South African tertiary hospital. South Afr J Anaesth Analg 2016;22:89–92. [Google Scholar]
- 147. Oshikoya KA, Senbanjo IO, Njokanma OF. Self-medication for infants with colic in Lagos, Nigeria. BMC Pediatr 2009;9:9 10.1186/1471-2431-9-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ukwaja KN, Alobu I, Nweke CO, et al. Healthcare-seeking behavior, treatment delays and its determinants among pulmonary tuberculosis patients in rural Nigeria: a cross-sectional study. BMC Health Serv Res 2013;13:25 10.1186/1472-6963-13-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lawal FB, Taiwo JO, Oke GA. Factors influencing awareness and attendance of traditional oral health care practices by residents of a peri-urban community in Ibadan, Nigeria. Afr Health Sci 2015;15:233–9. 10.4314/ahs.v15i1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Dienye P, Akani A, Itimi K. Uses of crude oil as traditional medicine: a survey of mothers in a rural clinic in South-south Nigeria. Rural Remote Health 20121858:12. [PubMed] [Google Scholar]
- 151. Ezaldeen EA, Fahal AH, Osman A. Mycetoma herbal treatment: the mycetoma research centre, Sudan experience. Plos Neglect Trop Dis 2013;7:e2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Reniers G, Tesfai R. Health services utilization during terminal illness in Addis Ababa, Ethiopia. Health Policy Plan 2009;24:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Fakeye TO, Tijani A, Adebisi O. A survey of the use of herbs among patients attending secondary-level health care facilities in Southwestern Nigeria. J Herb Pharmacother 2008;7(3-4):213–27. 10.1080/15228940802152901 [DOI] [PubMed] [Google Scholar]
- 154. Marais A, Steenkamp V, Du Plooy WJ. Conditions frequently self-treated with herbal remedies by patients visiting a tertiary hospital in Gauteng, South Africa. S Afr Fam Pract 2015;57:8–11. [Google Scholar]
- 155. Fakeye TO, Adisa R, Olatunji E. Self medication among hospitalized patients in selected secondary health facilities in South Western Nigeria. J Pharm Pract 2010;8:233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kiguba R, Ononge S, Karamagi C, et al. Herbal medicine use and linked suspected adverse drug reactions in a prospective cohort of Ugandan inpatients. BMC Complement Altern Med 2016;16:145 10.1186/s12906-016-1125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Alade GO, Okpako E, Ajibesin KK, et al. Indigenous knowledge of herbal medicines among adolescents in Amassoma, Bayelsa State, Nigeria. Glob J Health Sci 2016;8:217 10.5539/gjhs.v8n1p217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nworu CS, Udeogaranya PO, Okafor CK, et al. Perception, usage and knowledge of herbal medicines by students and academic staff of University of Nigeria: A survey. European Journal of Integrative Medicine 2015;7:218–27. 10.1016/j.eujim.2015.01.005 [DOI] [Google Scholar]
- 159. Enwere OO. Herbs in orthodox practice: a view by medical students. Afr J Tradit Complement Altern Med 2009;6:203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ameade EP, Amalba A, Helegbe GK, et al. Medical students' knowledge and attitude towards complementary and alternative medicine - A survey in Ghana. J Tradit Complement Med 2016;6:230–6. 10.1016/j.jtcme.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Adomi PO. Herbal medicine usage by paramedical students of Delta State University Abraka, Nigeria. Australian Journal of Herbal Medicine 2014;26:114–8. [Google Scholar]
- 162. Awodele O, Agbaje EO, Abiola OO, et al. Doctors’ attitudes towards the use of herbal medicine in Lagos, Nigeria. Journal of Herbal Medicine 2012;2:16–22. 10.1016/j.hermed.2012.02.002 [DOI] [Google Scholar]
- 163. Mbutho NP, Gqaleni N, Korporaal CM. Traditional complementary and alternative medicine: knowledge, attitudes and practices of health care workers in HIV and AIDS clinics in Durban hospitals. Afr J Tradit Complement Altern Med 2012;9(3 Suppl):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Chingwaru W, Prevalence VJ. perceptions and factors influencing the use of traditional and complementary medicine (T&CM) in Zimbabwe’s adult population: The case of Bindura District. Eur J Integr Med 2016;8:484–93. [Google Scholar]
- 165. ADIBE M. Prevalence of concurrent use of herbal and synthetic medicines among outpatients in a mission hospital in Nigeria. Int J Drug Dev Res 2009. [Google Scholar]
- 166. Awodele O, Olayemi SO, Adeyemo TA, et al. Use of complementary medicine amongst patients on antiretroviral drugs in an HIV treatment centre in Lagos, Nigeria. Curr Drug Saf 2012;7:120–5. 10.2174/157488612802715627 [DOI] [PubMed] [Google Scholar]
- 167. Babb DA, Pemba L, Seatlanyane P, et al. Use of traditional medicine by HIV-infected individuals in South Africa in the era of antiretroviral therapy. Psychol Health Med 2007;12:314–20. 10.1080/13548500600621511 [DOI] [PubMed] [Google Scholar]
- 168. Langlois-Klassen D, Kipp W, Jhangri GS, et al. Use of traditional herbal medicine by AIDS patients in Kabarole District, western Uganda. Am J Trop Med Hyg 2007;77:757–63. 10.4269/ajtmh.2007.77.757 [DOI] [PubMed] [Google Scholar]
- 169. Lubinga SJ, Kintu A, Atuhaire J, et al. Concomitant herbal medicine and Antiretroviral Therapy (ART) use among HIV patients in Western Uganda: a cross-sectional analysis of magnitude and patterns of use, associated factors and impact on ART adherence. AIDS Care 2012;24:1375–83. 10.1080/09540121.2011.648600 [DOI] [PubMed] [Google Scholar]
- 170. Malangu N. Self-reported use of traditional, complementary and over-the-counter medicines by HIV-infected patients on antiretroviral therapy in Pretoria, South Africa. Afr J Tradit Complement Altern Med 2007;4:273–8. 10.4314/ajtcam.v4i3.31219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Stanifer JW, Lunyera J, Boyd D, et al. Traditional medicine practices among community members with chronic kidney disease in northern Tanzania: an ethnomedical survey. BMC Nephrol 2015;16:170 10.1186/s12882-015-0161-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Aryeetey R, Aikins M, Dako-Gyeke P, et al. Pathways utilized for antenatal health seeking among women in the Ga East District, Ghana. Ghana Med J 2015;49:44–9. 10.4314/gmj.v49i1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Mncengeli S, Manimbulu NM, Panjasaram N. Concurrent use of Antiretroviral and African traditional medicines amongst people living with HIV/AIDS (PLWA) in the eThekwini Metropolitan area of KwaZulu Natal. Afr Health Sci 2016;16:1118–30. 10.4314/ahs.v16i4.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Tamuno I. Traditional medicine for HIV infected patients in antiretroviral therapy in a tertiary hospital in Kano, Northwest Nigeria. Asian Pac J Trop Med 2011;4:152–5. 10.1016/S1995-7645(11)60058-8 [DOI] [PubMed] [Google Scholar]
- 175. Gyasi RM, Mensah CM, Siaw LP. Predictors of traditional medicines utilisation in the Ghanaian health care practice: interrogating the Ashanti situation. J Community Health 2015;40:314–25. 10.1007/s10900-014-9937-4 [DOI] [PubMed] [Google Scholar]
- 176. Sato A. Does socio-economic status explain use of modern and traditional health care services? Soc Sci Med 2012;75:1450–9. 10.1016/j.socscimed.2012.05.032 [DOI] [PubMed] [Google Scholar]
- 177. Ladele AA, Bisi-Amosun OO. Level of utilization of traditional and orthodox medicines by rural dwellers in Ile-Ogbo Community of Osun State, Nigeria. Journal of Agricultural Extension 2014;18:155–68. 10.4314/jae.v18i1.14 [DOI] [Google Scholar]
- 178. Monera TG, Maponga CC. Prevalence and patterns of Moringa oleifera use among HIV positive patients in Zimbabwe: a cross-sectional survey. Journal of public Health in Africa 2012;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Namuddu B, Kalyango JN, Karamagi C, et al. Prevalence and factors associated with traditional herbal medicine use among patients on highly active antiretroviral therapy in Uganda. BMC Public Health 2011;11:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Nlooto M, Naidoo P, Traditional NP. Traditional, complementary and alternative medicine use by HIV patients a decade after public sector antiretroviral therapy roll out in South Africa: a cross sectional study. BMC Complement Altern Med 2016;16:128 10.1186/s12906-016-1101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Otang WM, Grierson DS, Prevalence NRN. perceived benefits and effectiveness of herbal medicine in the management of symptoms of opportunistic fungal infections in HIV/AIDS patients in the Eastern Cape, South Africa. Afr J Biotechnol 2011;10:19458–63. [Google Scholar]
- 182. Akeju DO, Oladapo OT, Vidler M, et al. Determinants of health care seeking behaviour during pregnancy in Ogun State, Nigeria. Reprod Health 2016;13 Suppl 1:32 10.1186/s12978-016-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Gyasi RM, Asante F, Yeboah JY, et al. Pulled in or pushed out? Understanding the complexities of motivation for alternative therapies use in Ghana. Int J Qual Stud Health Well-being 2016;11:29667 10.3402/qhw.v11.29667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Gyasi RM, Mensah CM, Adjei PO-W, et al. Public perceptions of the role of traditional medicine in the health care delivery system in Ghana. Glob J Health Sci 2011;3:40–9. 10.5539/gjhs.v3n2p40 [DOI] [Google Scholar]
- 185. Kaingu CK, Oduma JA, Kanui TI. Practices of traditional birth attendants in Machakos District, kenya. J Ethnopharmacol 2011;137:495–502. 10.1016/j.jep.2011.05.044 [DOI] [PubMed] [Google Scholar]
- 186. Rutebemberwa E, Lubega M, Katureebe SK, et al. Use of traditional medicine for the treatment of diabetes in Eastern Uganda: a qualitative exploration of reasons for choice. BMC Int Health Hum Rights 2013;13:1 10.1186/1472-698X-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Mwaka AD, Okello ES, Orach CG. Barriers to biomedical care and use of traditional medicines for treatment of cervical cancer: an exploratory qualitative study in northern Uganda. Eur J Cancer Care 2015;24:503–13. 10.1111/ecc.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Okafor IP, Sekoni AO, Ezeiru SS, et al. Orthodox versus unorthodox care: a qualitative study on where rural women seek healthcare during pregnancy and childbirth in Southwest, Nigeria. Malawi Med J 2014;26:45–9. [PMC free article] [PubMed] [Google Scholar]
- 189. Tabi MM, Powell M, Hodnicki D. Use of traditional healers and modern medicine in Ghana. Int Nurs Rev 2006;53:52–8. 10.1111/j.1466-7657.2006.00444.x [DOI] [PubMed] [Google Scholar]
- 190. Scott K, McMahon S, Yumkella F, et al. Navigating multiple options and social relationships in plural health systems: a qualitative study exploring healthcare seeking for sick children in Sierra Leone. Health Policy Plan 2014;29:292–301. 10.1093/heapol/czt016 [DOI] [PubMed] [Google Scholar]
- 191. Gyasi RM. Relationship between health insurance status and the pattern of traditional medicine utilisation in Ghana. Evid Based Complement Alternat Med 2015;2015:1–10. 10.1155/2015/717926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Aziato L, Antwi HO. Facilitators and barriers of herbal medicine use in Accra, Ghana: an inductive exploratory study. BMC Complement Altern Med 2016;16:142 10.1186/s12906-016-1124-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Appelbaum Belisle H, Hennink M, Ordóñez CE, et al. Concurrent use of traditional medicine and ART: perspectives of patients, providers and traditional healers in Durban, South Africa. Glob Public Health 2015;10:71–87. 10.1080/17441692.2014.967709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Puoane TR, Hughes GD, Uwimana J, et al. Why HIV positive patients on antiretroviral treatment and/or cotrimoxazole prophylaxis use traditional medicine: perceptions of health workers, traditional healers and patients: a study in two provinces of South Africa. Afr J Tradit Complement Altern Med 2012;9:495–502. 10.4314/ajtcam.v9i4.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Hughes GD, Puoane TR, Clark BL, et al. Prevalence and predictors of traditional medicine utilization among persons living with AIDS (PLWA) on antiretroviral (ARV) and prophylaxis treatment in both rural and urban areas in South Africa. Afr J Tradit Complement Altern Med 2012;9:470–84. 10.4314/ajtcam.v9i4.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Langlois-Klassen D, Kipp W, Rubaale T. Who's talking? Communication between health providers and HIV-infected adults related to herbal medicine for AIDS treatment in western Uganda. Soc Sci Med 2008;67:165–76. [DOI] [PubMed] [Google Scholar]
- 197. Labhardt ND, Aboa SM, Manga E. Bridging the gap: how traditional healers interact with their patients. A comparative study in Cameroon. Trop Med Int Health 2010; 15;(9):1099–108. [DOI] [PubMed] [Google Scholar]
- 198. Winkler AS, Mayer M, Ombay M. Attitudes towards African traditional medicine and Christian spiritual healing regarding treatment of epilepsy in a rural community of northern Tanzania. Afr J Tradit Complement Altern Med 2009;7:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Moshabela M, Pronyk P, Williams N, et al. Patterns and implications of medical pluralism among HIV/AIDS patients in rural South Africa. AIDS Behav 2011;15:842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Awodele O, Amagon K, Usman S, et al. Safety of herbal medicines use: case study of ikorodu residents in Lagos, Nigeria. Curr Drug Saf 2013;9:138–44. 10.2174/1574886308666131229104104 [DOI] [PubMed] [Google Scholar]
- 201. Tchacondo T, Karou SD, Batawila K, et al. Herbal remedies and their adverse effects in Tem tribe traditional medicine in Togo. Afr J Tradit Complement Altern Med 2011;8:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Ernst E. Prevalence surveys: to be taken with a pinch of salt. Complement Ther Clin Pract 2006;12:272–5. 10.1016/j.ctcp.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 203. Hall HG, Griffiths DL, McKenna LG. The use of complementary and alternative medicine by pregnant women: a literature review. Midwifery 2011;27:817–24. 10.1016/j.midw.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 204. Clarke TC, Black LI, Stussman BJ. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report 2015;79:1. [PMC free article] [PubMed] [Google Scholar]
- 205. Xue CC, Zhang AL, Lin V, et al. Complementary and alternative medicine use in Australia: a national population-based survey. J Altern Complement Med 2007;13:643–50. 10.1089/acm.2006.6355 [DOI] [PubMed] [Google Scholar]
- 206. Reid R, Steel A, Wardle J, et al. Complementary medicine use by the Australian population: a critical mixed studies systematic review of utilisation, perceptions and factors associated with use. BMC Complement Altern Med 2016;16:176 10.1186/s12906-016-1143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Quartey NK, Ma PH, Chung VC, et al. Complementary and alternative medicine education for medical profession: systematic review. Evid Based Complement Alternat Med 2012;2012:13 10.1155/2012/656812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Davis EL, Oh B, Butow PN, et al. Cancer patient disclosure and patient-doctor communication of complementary and alternative medicine use: a systematic review. Oncologist 2012;17:1475–81. 10.1634/theoncologist.2012-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Robinson A, McGrail MR. Disclosure of CAM use to medical practitioners: a review of qualitative and quantitative studies. Complement Ther Med 2004;12(2-3):90–8. 10.1016/j.ctim.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 210. Thomson P, Jones J, Evans JM, et al. Factors influencing the use of complementary and alternative medicine and whether patients inform their primary care physician. Complement Ther Med 2012;20(1-2):45–53. 10.1016/j.ctim.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 211. Salzburg Global Seminar Salzburg statement on shared decision making. BMJ 2011;342:d1745 10.1136/bmj.d1745 [DOI] [PubMed] [Google Scholar]
- 212. Oh B, Butow P, Mullan B, et al. Patient-doctor communication: use of complementary and alternative medicine by adult patients with cancer. J Soc Integr Oncol 2010;8:56-64. [PubMed] [Google Scholar]
- 213. Asmelashe Gelayee D, Binega Mekonnen G, Asrade Atnafe S, et al. Herbal medicines: personal use, knowledge, attitude, dispensing practice, and the barriers among community pharmacists in Gondar, Northwest Ethiopia. Evid Based Complement Alternat Med 2017;2017:6480142:1–6. 10.1155/2017/6480142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Fakeye TO, Onyemadu O. Evaluation of knowledge base of hospital pharmacists and physicians on herbal medicines in Southwestern Nigeria. Pharm Pract 2008;6:88 10.4321/S1886-36552008000200005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Suchard JR, Suchard MA, Steinfeldt JL. Physician knowledge of herbal toxicities and adverse herb-drug interactions. Eur J Emerg Med 2004;11:193–7. 10.1097/01.mej.0000134721.72356.f7 [DOI] [PubMed] [Google Scholar]
- 216. Wahner-Roedler DL, Lee MC, Chon TY, et al. Physicians' attitudes toward complementary and alternative medicine and their knowledge of specific therapies: 8-year follow-up at an academic medical center. Complement Ther Clin Pract 2014;20:54–60. 10.1016/j.ctcp.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 217. Kemper KJ, Gardiner P, Gobble J, et al. Expertise about herbs and dietary supplements among diverse health professionals. BMC Complement Altern Med 2006;6:15 10.1186/1472-6882-6-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Clement YN, Williams AF, Khan K, et al. A gap between acceptance and knowledge of herbal remedies by physicians: the need for educational intervention. BMC Complement Altern Med 2005;5:20 10.1186/1472-6882-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Sibbritt D, Adams J, Murthy V. The prevalence and determinants of Chinese medicine use by Australian women: analysis of a cohort of 10,287 women aged 56-61 years. Am J Chin Med 2013;41:281–91. 10.1142/S0192415X13500201 [DOI] [PubMed] [Google Scholar]
- 220. Magin PJ, Adams J, Heading GS, et al. Complementary and alternative medicine therapies in acne, psoriasis, and atopic eczema: results of a qualitative study of patients' experiences and perceptions. J Altern Complement Med 2006;12:451–7. 10.1089/acm.2006.12.451 [DOI] [PubMed] [Google Scholar]
- 221. Cruz M, Foster J, Quillin B. Ending extreme poverty and sharing prosperity: Progress and policies. Policy Research Note PRN/15/03, World Bank Group 2015. [Google Scholar]
- 222. Peters DH, Garg A, Bloom G, et al. Poverty and access to health care in developing countries. Ann N Y Acad Sci 2008;1136:161–71. 10.1196/annals.1425.011 [DOI] [PubMed] [Google Scholar]
- 223. Bishop FL, Yardley L, Lewith GT. A systematic review of beliefs involved in the use of complementary and alternative medicine. J Health Psychol 2007;12:851–67. 10.1177/1359105307082447 [DOI] [PubMed] [Google Scholar]
- 224. Stratton TD, McGivern-Snofsky JL. Toward a sociological understanding of complementary and alternative medicine use. J Altern Complement Med 2008;14:777–83. 10.1089/acm.2007.7006 [DOI] [PubMed] [Google Scholar]
- 225. Olsen WC, Sargent C. African Medical Pluralism. Indianapolis: Indiana University Press, 2017. [Google Scholar]
- 226. Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 2009;69:1777–98. 10.2165/11317010-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 227. Kamsu-Foguem B, Foguem C. Adverse drug reactions in some African herbal medicine: literature review and stakeholders' interview. Integr Med Res 2014;3:126–32. 10.1016/j.imr.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Luyckx VA, Naicker S. Acute kidney injury associated with the use of traditional medicines. Nat Clin Pract Nephrol 2008;4:664–71. 10.1038/ncpneph0970 [DOI] [PubMed] [Google Scholar]
- 229. Delgoda R, Ellington C, Barrett S, et al. The practice of polypharmacy involving herbal and prescription medicines in the treatment of diabetes mellitus, hypertension and gastrointestinal disorders in Jamaica. West Indian Med J 2004;53:400–5. [PubMed] [Google Scholar]
- 230. Nortier JL, Vanherweghem JL. For patients taking herbal therapy--lessons from aristolochic acid nephropathy. Nephrol Dial Transplant 2007;22:1512–7. 10.1093/ndt/gfm167 [DOI] [PubMed] [Google Scholar]
- 231. Oshikoya KA, Njokanma OF, Chukwura HA, et al. Adverse drug reactions in Nigerian children. Paediatr Perinat Drug Ther 2007;8:81–8. 10.1185/146300907X199858 [DOI] [Google Scholar]
- 232. WHO WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. 2004. Geneva: World Health Organization vii, 2015. [Google Scholar]
- 233. Kasilo OM, Trapsida J-M, régional pour l'Afrique B. Regulation of traditional medicine in the WHO African region. African Health Monitor 2010:25–31. [Google Scholar]
- 234. Adewunmi CO, Ojewole JAO. Safety of traditional medicines, complementary and alternative medicines in Africa. Afr J Tradit Complement Altern Med 2006;1:1–3. [Google Scholar]
- 235. Rodrigues E, Barnes J. Pharmacovigilance of herbal medicines. Drug Saf 2013;36:1–12. 10.1007/s40264-012-0005-7 [DOI] [PubMed] [Google Scholar]
- 236. Seo HJ, Baek SM, Kim SG, et al. Prevalence of complementary and alternative medicine use in a community-based population in South Korea: a systematic review. Complement Ther Med 2013;21:260–71. 10.1016/j.ctim.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 237. Ernst E. Prevalence of use of complementary/alternative medicine: a systematic review. Bull World Health Organ 2000;78:258–66. [PMC free article] [PubMed] [Google Scholar]
- 238. Suswardany DL, Sibbritt DW, Supardi S, et al. A critical review of traditional medicine and traditional healer use for malaria and among people in malaria-endemic areas: contemporary research in low to middle-income Asia-Pacific countries. Malar J 2015;14:98 10.1186/s12936-015-0593-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Thompson J, Havenga Y, Naude S. The health literacy needs of women living with HIV/AIDS. Health SA Gesondheid 2015;20:11–21. 10.1016/j.hsag.2015.03.001 [DOI] [Google Scholar]
- 240. Forland F, Rohwer AC, Klatser P, et al. Strengthening evidence-based healthcare in Africa. Evid Based Med 2013;18:204–6. 10.1136/eb-2012-101143 [DOI] [PubMed] [Google Scholar]
- 241. Frass M, Strassl RP, Friehs H, et al. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J 2012;12:45–56. [PMC free article] [PubMed] [Google Scholar]
- 242. Greenland S, Morgenstern H. Confounding in health research. Annu Rev Public Health 2001;22:189–212. 10.1146/annurev.publhealth.22.1.189 [DOI] [PubMed] [Google Scholar]
- 243. World Health Organization Regional Office for Africa The health of the people: the African regional health report. World Health Organization, 2006. [Google Scholar]
- 244. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2018-000895supp001.pdf (517.9KB, pdf)
bmjgh-2018-000895supp002.pdf (328.5KB, pdf)