Abstract
The characteristics of the muscles of the thoracic limb were evaluated in 22 specimens of Lycalopex gymnocercus. Descriptive and comparative analyses showed similarity with other canids in terms of topography and tendon insertions. Differences with the domestic dog were observed in the pectoralis profundus, triceps brachii and interflexorii muscles. Intraspecific variations were observed in the rhomboideus capitis, serratus ventralis cervicis, extensor carpi radialis, extensor digiti I and II, lumbricales, flexor digiti I brevis, abductor digiti I brevis, and flexor digiti V muscles. The analyses of muscle architecture carried out in nine specimens showed that there was no difference in muscle percentage mass in the thoracic limb of males and females, but a young specimen showed significant lower percentage mass. The triceps brachii caput longus muscle showed the greatest mass, the subscapularis muscle showed the greatest physiological cross‐sectional area value, and the extrinsic muscles, in general, presented the longest fascicles and higher architectural indexes. Muscle architecture data were compatible with those of a thoracic limb adapted to fast cursorial locomotion that prioritizes movements in a sagittal plane instead of rotation or adduction/abduction. There was a high association between functional percentage mass of the muscles in the thoracic limb and phylogeny in the Carnivora order. It may be inferred that carnivoran muscle mass is largely determined by phylogeny.
Keywords: Azara's fox, forelimb, muscle architecture, wild carnivorans
Introduction
Lycalopex gymnocercus (G. Fisher, 1814), known as the Pampas fox, Azara's fox or Azara's zorro, is a medium‐sized South American fox (3–8 kg) that prefers open habitats such as the Pampas planes (Luengos Vidal et al. 2012). It is found in eastern Bolivia, western and central Paraguay, Uruguay, northern and central Argentina, and southern Brazil (Lucherini & Luengos Vidal, 2008). It is an omnivorous animal that preys on hares, armadillos, opossums, small rodents, lizards, fish, birds, insects, besides eating fruits (Queirolo et al. 2013). In view of the omnivorous habit, it can be speculated that the functional demand of the thoracic limbs may result in little versatility and adaptations for walking in open areas. There are more free‐living males than females and, although these animals are lone hunters, couples may be observed from the moment of mating to the time offspring leave the den (Lucherini & Luengos Vidal, 2008; Queirolo et al. 2013). They live up to 14 years in captivity, but only a few years in the wild (Crespo, 1971). The genus Lycalopex includes at least three other species of foxes that evolved and spread throughout South America (Tchaicka et al. 2016).
Detailed studies of the anatomy of the L. gymnocercus include the description of its encephalic vascularization (Depedrini & Campos, 2003, 2007), the topography of its lumbar intumescence and medullary conus (Souza Junior et al. 2014), and lumbosacral (Lorenzão et al. 2016), its brachial plexus formation (Souza Junior et al. 2016), and many aspects of its thoracic limb osteology (Souza Junior et al. 2018).
The thoracic limb is involved in different activities, such as cursorial locomotion, weight support and prey capture, as well as climbing, swimming, digging and mating behavior. This dynamism in the morphology of the thoracic limb reflects ecological variations, such as prey size and type, habitat preference, and the ability to perform some movements (Ewer, 1973; Meachen‐Samuels & Van Valkenburgh, 2009; Fabre et al. 2013, 2015; Meloro et al. 2013). Together with craniodental data, the analysis of the thoracic limb may aid in extrapolating hunting behavior of extinct species (Iwaniuk et al. 1999; Andersson & Werdelin, 2003).
In spite of the availability of anatomical descriptions of thoracic limb myology in several species of carnivorans (Macalister, 1870; Windle, 1888; Windle & Parsons, 1897; Barone, 1967; Leach, 1977; Spoor & Badoux, 1986a; Feeney, 1999; Fisher et al. 2009; Santos et al. 2010; Julik et al. 2012; Ercoli et al. 2015; Pereira et al. 2016; Viranta et al. 2016), determination and analysis of quantitative architectural parameters are still scarce in both domestic (Shahar & Milgram, 2005; Williams et al. 2008) and wild carnivorans (Hudson et al. 2011; Moore et al. 2013; Cuff et al. 2016). Despite the functional importance and correlation with ecological aspects, there are few data on muscle architecture of the thoracic limb in wild canids.
Architectural data are properties that reveal the function of skeletal muscles, and the understanding of these data has great practical importance (Lieber & Fridén, 2000; Ward et al. 2009). The measurements that are required to assess muscle architecture include: muscle mass, muscle length, length of the fibers (or fascicles), and pennation angle (angle of the fiber relative to the force‐generating axis). Based on these data, physiological cross‐sectional area (PCSA) and architectural index (AI) may be calculated (Lieber & Fridén, 2000).
The PCSA of a muscle corresponds to the relationship between the volume of the muscle and the length of its fascicles, and represents the best architectural data to compare the force‐generating capacity between different muscles (Lieber & Fridén, 2000; Shahar & Milgram, 2005). On the other hand, the AI is the ratio of fiber length to total muscle length, and it can reflect the number of sarcomeres in series in a muscle. It is proportional to the potential velocity of muscle contraction (Shahar & Milgram, 2005).
Although some metabolic parameters, such as distribution of fiber types, may substantially influence contractile properties, architectural data are the best predictors of the muscle function (Ward et al. 2009). Imaging methods, such as magnetic resonance, computed tomography and ultrasound, as well as muscle biopsy, are not able to determine actual architectural data, as they do not take into account variations in fiber length and orientation throughout the length of the muscle (Lieber & Fridén, 2000). However, the analysis of cadaver specimens continues to be a viable method to gather architectural data in animals since the first studies carried out with pelvic limbs of domestic felids by Sacks & Roy (1982).
The objective of the present study was to analyze the morphofunctional characteristics of thoracic limb muscles of L. gymnocercus in an anatomical and quantitative context, and compare them with descriptive and architectural data available for other carnivorans.
Materials and methods
Sampling
This study was based on 22 cadavers (21 adults and one puppy) of L. gymnocercus (Table 1) that were found on highways in the southwestern part of the state of Rio Grande do Sul, Brazil. The Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) approved the study (SISBIO authorization number 33667). The right thoracic limb of a male specimen of Cerdocyon thous (crab‐eating‐fox) was also dissected for muscle mass comparison.
Table 1.
Specimens of Lycalopex gymnocercus analysed in this study
| Register number | Sex | Reason | Body mass (kg) | Preservation | Site (city) |
|---|---|---|---|---|---|
| 5134 | F | Check variations | – | Formaldehyde | Uruguaiana |
| 5261 | M | Check variations | – | Formaldehyde | São Francisco de Assis |
| 5269 | M | Check variations | – | Formaldehyde | São Gabriel |
| 5274 | M | Anatomical description and check variations | – | Formaldehyde | Uruguaiana |
| 5603 | M | Check variations | – | Formaldehyde | Barra do Quaraí |
| 8414 | M | Anatomical description and check variations | – | Formaldehyde | Uruguaiana |
| 8433 | F | Anatomical description and check variations | – | Formaldehyde | Dilermando de Aguiar |
| 8434 | F | Check variations | – | Formaldehyde | Uruguaiana |
| 8501 | M | Check variations | – | Formaldehyde | Alegrete |
| 8519 | F | Anatomical description and check variations | – | Formaldehyde | Uruguaiana |
| 8532 | F | Check variations | – | Formaldehyde | Uruguaiana |
| 8533 | F | Check variations | – | Formaldehyde | São Gabriel |
| 8576 | F | Representation of muscle attachments | – | Dry bones | Uruguaiana |
| 8582 | F | Muscle architecture | 5.7 | Freezing (−20 °C) | Uruguaiana |
| 8583 | M | Muscle architecture | 5.5 | Freezing (−20 °C) | Itaqui |
| 8584 | M | Muscle architecture | 5.9 | Freezing (−20 °C) | São Gabriel |
| 8585 | F | Muscle architecture and check variations | 4.9 | Freezing (−20 °C) | Alegrete |
| 8586 | F | Muscle architecture and check variations | 5.1 | Freezing (−20 °C) | Uruguaiana |
| 8587 | M | Muscle architecture | 4.4 | Freezing (−20 °C) | Uruguaiana |
| 8588 | M | Muscle architecture | 5.4 | Freezing (−20 °C) | Santiago |
| 8589 | M | Muscle architecture and check variations | 2.2 | Freezing (−20 °C) | Uruguaiana |
| 8590 | M | Muscle architecture and check variations | 6.3 | Freezing (−20 °C) | Vila Nova do Sul |
Anatomical description
For the identification of the muscles, the right thoracic limbs of four animals (two males, 5274 and 8414; and two females, 8433 and 8519) were dissected. Cadavers were preserved in 10% formaldehyde. Dissections consisted of removal of the skin and superficial fascia, followed by removal of the remainders of connective tissue, and identification of the muscles and their respective bone attachments. After that, each muscle was removed and the attachment points were precisely marked with permanent markers of different colors on the bones of the right thoracic limb of a female specimen (8576). These bones were macerated and cleaned beforehand. Before bones were marked, they were photographed with an 18‐MP Canon® camera model EOS Rebel T3i. The photographs in .JPG format were edited in Adobe Illustrator CC® software for the contour and bone accidents to be reliably reproduced in schematic drawings. The painted areas in the bones of specimen 8576 were reproduced in schemes that enabled a reliable representation of the muscle insertion points. When there were variations between the muscles in these four specimens, an additional 12 specimens were utilized to check for frequencies of variations. The muscles and bone structures were named according to the ICVGAN (2017).
Muscle architecture
The right thoracic limbs of nine dead L. gymnocercus frozen at −20 °C immediately after collection were analyzed for muscle architecture data. Initially, the specimens were thawed in a chamber at mean temperature equal to 2 °C for about 48 h, and body mass was assessed after complete thawing using an electronic digital scale with 0.1 kg resolution (Kruuse®). After that, the skin and superficial fascia of the cervical and thoracic regions and of the right thoracic limb were removed, exposing the musculature.
Muscles were dissected for individualization, and extrinsic muscles were released from their origins; vessels and nerves of the axillary region were transected to release the thoracic limb from the rest of the body. Each muscle was carefully removed from its bone attachment, and tendons were excised. A scale with 0.01 g resolution (Marte®) was used to assess the mass of the muscle belly. The length of the muscle belly was measured on a flat surface with a tape measure (1 mm scale), and the pennation angle was estimated with a protractor (2 ° resolution). Only the small muscles that both originated and inserted on the bones of the hand did not have their architectural data determined, given the minuscule size of their fascicles, and the absence of comparative data for other carnivorans.
After muscles were removed, weighed and measured, they were immersed in 10% formaldehyde for 48 h. Then, they were washed in saline solution and transferred to a container with 20% sulfuric acid for 7–10 days. The acid enabled the separation of muscle fascicles for the measurements, as reported by Sacks & Roy (1982), Delp et al. (2001), Shahar & Milgram (2005) and Perry et al. (2014). Then, the lengths of five fascicles of different regions of each muscle were measured with a flexible tape (readability of ± 1 mm), and arithmetic means were calculated. Payne et al. (2006) and Williams et al. (2008) defined a fascicle as a bundle of individual fibers that was large enough to be seen by the naked eye.
The PCSA of each muscle was estimated with the following equation:
where m is the mass of the muscle belly in grams, α is the pennation angle, p is muscle density, which is considered to be 1.06 g cm−3 (Mendez & Keys, 1960), and l is the arithmetic mean of the length of the muscle fascicles.
The AI for each muscle was calculated as:
where L is the length of the muscle belly.
Comparisons of the architectural data between specimens of L. gymnocercus of different sizes and ages, and with other species in the Carnivora order available in the literature were enabled by the concept of geometric similarity for data standardization. This concept, explained by Alexander (2006) and adopted by several authors (Payne et al. 2006; Sharir et al. 2006; Michilsens et al. 2009; Moore et al. 2013; Rose et al. 2013; Webster et al. 2014; Cuff et al. 2016), determines that the mass of a structure is directly scaled with the body mass of an individual, the length with body mass1/3, and the areas with body mass2/3. Therefore, the mass of the muscles was calculated in relation to the body mass, the length of the fascicles with body mass1/3, and PCSA with body mass2/3.
After scaling, the architectural data of each muscle were compared by Student's t‐test for independent samples between males (n = 5) and females (n = 3). The t‐test was also used to compare the masses of the muscles of the young male specimen (8589) (n = 1) and the adult ones (n = 8). In both comparisons, P < 0.05 was adopted as the significance level.
For the comparative analysis of muscle masses, intrinsic muscles were classified into one of 10 functional groups. To do this, the main action of the muscle was considered the movement with major mechanical advantage, usually exerted on its distal insertion tendon. Thus, a muscle such as biceps brachii that acts distally in the flexion of the elbow and proximally aiding in shoulder extension was only placed in the elbow flexor group.
The following functional groups were determined: shoulder extensors (supraspinatus and coracobrachialis), shoulder flexors (infraspinatus, deltoideus, teres major and teres minor), elbow extensors (anconeus, triceps brachii and tensor fasciae antebrachi), elbow flexors (biceps brachii and brachialis), extensors of the carpus (extensor carpi radialis), flexors of the carpus (flexor carpi radialis, flexor carpi ulnaris and ulnaris lateralis), digit extensors (extensor digitorum communis, extensor digitorum lateralis and abductor pollicis longus), digit flexors (flexor digitorum superficialis and flexor digitorum profundus), supinator (supinator and brachiorradialis) and pronator muscles (pronator teres and pronator quadratus). Although the subscapularis muscle may aid both shoulder extension and flexion, it was considered that its main function was medial stabilization of the shoulder and aiding the pectorales superficialis muscle in the adduction of the limb (Evans & DeLahunta, 2013). Therefore, the subscapularis muscle was not included in the functional groups listed above.
The mass of the muscles in each functional group was summed, and the percentage of each group in the total mass of intrinsic muscles of the limb was calculated. This percentage calculation was performed for L. gymncercus (n = 8) and for the C. thous (n = 1) specimen in the present study; it was also calculated for other species in the Carnivora order based on data in the literature: the canids Vulpes vulpes (n = 5), Urocyon cinereoargenteus (n = 4), Canis latrans (n = 1) analyzed by Feeney (1999); mongrel domestic dogs (n = 4), as reported by Shahar & Milgram (2005), and Greyhound domestic dogs (n = 7), evaluated by Williams et al. (2008); the mustelids Aonyx cinerea, analyzed by Macalister (1870), Martes pennanti (n = 4), by Feeney (1999), Taxidea taxus (n = 6), by Moore et al. (2013), and Galictis cuja (n = 2), by Ercoli et al. (2015); the procyonid Procyon lotor (n = 2), by Feeney (1999); the hyaenid Hyaena hyaena (n = 1), by Spoor & Badoux (1986a); the felids Acinonyx jubatus (n = 8) by Hudson et al. (2011), Leopardus pardalis (n = 1) by Julik et al. (2012), Lynx lynx (n = 4) by Viranta et al. (2016), Felis nigripes (n = 1), Felis silvestres (n = 1), Caracal caracal (n = 1), Panthera uncia (n = 1), Panthera onca (n = 1), Panthera tigris (n = 1) and Panthera leo (n = 1) by Cuff et al. (2016).
Data on the percentage mass of each functional group for each species were recorded in a spreadsheet. Based on the original percentage values of each species, a cluster analysis was carried out using Ward's minimum variance method, and Euclidean distances were calculated to plot a dendrogram. Last, variance analysis (one‐way anova) complemented by Tukey test was used to compare the percentage mass of each functional group in the three groups of species cited above, considering P < 0.05. All analyses were carried out in BioEstat 5.3® software.
Results
Descriptive aspects
The muscles identified in the dissection of the L. gymnocercus specimens were divided into extrinsic (tendon of origin outside the thoracic limb, and insertions on the bones of the limb) and intrinsic muscles (origin and insertion on the thoracic limb). Descriptive data on extrinsic muscles are summarized in Table 2, and on intrinsic muscles in Table 3. The precise points of bone attachments of the muscles of the thoracic limb are shown in Figs 1, 2, 3, 4.
Table 2.
Origin, insertion and action of thoracic limb extrinsic muscles of Lycalopex gymnocercus
| Muscle | Abbrev. | Origin | Insertion | Main action |
|---|---|---|---|---|
| Cleidocervicalis | CLC | Mid‐dorsal fibrous raphe of the cranial end of the neck (at the level of C1–C4) | Intersectio clavicularis | Protract the limb |
| Cleidomastoideus | CLM | Mastoid process of temporal bone | Intersectio clavicularis | Protract the limb |
| Cleidobrachialis | CLB | Intersectio clavicularis | Distal third of the cranial surface of the humerus | Protract the limb |
| Latissimus dorsi | LTD | Muscle attachment from T5 to T8, and in thoracolumbar fascia from T9 to L3 | Tuberositas teres major | Retract the limb and flex the shoulder joint |
| Omotransversarius | OMT | Ventral aspect of wing of atlas | Acromion and supra‐hamate process | Protract the limb |
| Pectoralis descendens | PCD | First sternebrae | From the crista tuberculi majoris to the middle third of humeral diaphysis | Adduct the limb; stability |
| Pectoralis transversus | PCT | First three sternebrae | From the middle to the distal third of the humeral diaphysis | Adduct the limb; stability |
| Pectoralis profundus | PCP | From all sternebrae and deep fascia over xiphoid and cranial abdominal regions | Medial face of the tuberculum majus | Adduct the limb, retract the limb caudally, flex the shoulder joint |
| Rhomboideus capitis | RHCa | Nuchal crest (inconstant) | Fusion to the middle third of the rhomboideus cervicis | Elevate the limb |
| Rhomboideus cervicis | RHC | Median raphe from C2 to T3 | Dorsal margin and angulus cranialis of the scapula | Elevate and protract the limb |
| Rhomboideus thoracis | RHT | Spinous process of T4 and T5 | Dorsal margin and angulus caudalis of the scapula | Elevate and retract the limb |
| Serratus ventralis cervicis | SVC | Transverse processes of C4 to C7 | Facies serrata | Stability of the limb in relation to the trunk; protract the limb |
| Serratus ventralis thoracis | SVT | Medium third of the first 8 or 9 first ribs | Facies serrata | Stability of the limb in relation to the trunk; retract the limb |
| Trapezius pars cervicalis | TPC | Median raphe from C4 to C7 | Spina scapulae | Elevate, protract and abduct the limb |
| Trapezius pars thoracica | TPT | Median raphe from T1 to T9 | Spina scapulae | Elevate, retract and abduct the limb |
Table 3.
Origin, insertion and action of thoracic limb intrinsic musculature of Lycalopex gymnocercus
| Muscle | Abbrev. | Origin | Insertion | Main action |
|---|---|---|---|---|
| Coracobrachialis | CRB | Coracoid process of scapula | Distally on lesser tubercle of the humerus | Extension of shoulder |
| Deltoideus pars scapularis | DLS | Caudal surface of scapular spine | Tuberositas deltoidea | Flexion of shoulder |
| Deltoideus pars acromialis | DLA | Acromion | Tuberositas deltoidea and distally to medium third of humerus | Flexion of shoulder |
| Infraspinatus | INS | Infraspinous fossa | Caudodistally on tuberculum majus | Flexion of shoulder |
| Subscapularis | SBS | Medial surface of the scapula | Proximal margin of tuberculum minus | Adduction of shoulder |
| Supraspinatus | SPS | Supraspinous fossa | Tuberculum majus | Extension of shoulder |
| Teres major | TMJ | Caudal angle and dorsal third of the scapula | Teres major tuberosity | Flexion of shoulder |
| Teres minor | TMI | Ventral third of caudal margin of the scapula | Distally to tuberculum majus | Flexion of shoulder |
| Biceps brachii | BBR | Supraglenoid tuberosity | Proximal third of radius and distally to medial coronoid process of ulna | Flexion of elbow and extension of shoulder |
| Brachialis | BRC | Caudolaterally in proximal third of humerus | Distally to medial coronoid process of ulna | Flexion of elbow |
| Triceps brachii caput longum | TBLo | Caudal margin of scapula | Proximal tip and caudal surface of olecranon tuber | Extension of elbow and flexion of shoulder |
| Triceps brachii caput laterale | TBLa | Tricipital line of humerus | Lateral elevation of olecranon tuber | Extension of elbow |
| Triceps brachii caput mediale | TBM | Proximally to tuberositas teres major on the proximal humeral medial surface | Medial elevation of olecranon tuber | Extension of elbow |
| Triceps brachii caput accessorium | TBA | Proximal caudal part of the neck of the humerus | Medial elevation of olecranon tuber | Extension of elbow |
| Anconeus | ANC | Lateral epicondilar crest and olecrani fossae | Lateral surface of olecranon | Extension of elbow |
| Tensor fasciae antebrachii | TFA | From aponeurosis with latissimus dorsi in the axillary region | Antebrachial fascia | Extension of elbow and tensioning of antebrachial fascia |
| Brachioradialis | BRR | Lateral supracondylar crest of humerus | Medial styloid process of humerus | Supination |
| Extensor carpi radialis | ECR | Lateral supracondylar crest of humerus | Tuberosity of metacarpals II and III | Extension of carpal joint |
| Extensor digitorum comunis | EDC | Lateral epicondyle of humerus | Processus extensorius of distal phalanx of digits II–V | Extension of four main digits |
| Extensor digitorum lateralis | EDL | Lateral epicondyle of humerus | Processus extensorius of distal phalanx of digits (III) IV–V | Extension of two or three lateral digits |
| Ulnaris lateralis | UNL | Lateral epicondyle of humerus | Laterally on the base of metacarpal V | Flexion of carpal joint |
| Supinator | SUP | Lateral epicondyle of humerus | Cranial and medial surfaces of proximal radius | Supination |
| Extensor digiti I and II | EDI‐EDII | Lateral distal half of ulna | Head of the metacarpal I and tendon of extensor digitorum communis to digit II | Extension of digits I and II |
| Abductor digiti I longus | ABIL | Lateral surface of radius and ulna | Base of metacarpal I | Extension and abduction of digit I |
| Pronator teres | PRT | Medial epicondyle of humerus | Middle third of the cranial surface of radial diaphysis | Pronation |
| Flexor carpi radialis | FCR | Medial epicondyle of humerus | Palmar surface of base of metacarpals II and III | Flexion of carpal joint |
| Flexor digitorum superficialis | FDS | Medial epicondyle of humerus | Palmar surface of base of middle phalanx | Flexion of digits II–V |
| Flexor carpi ulnaris caput humerale | FCUH | Medial epicondyle of humerus | Accessory carpal bone | Flexion of carpus |
| Flexor carpi ulnaris caput ulnare | FCUU | Caudal margin of proximal third of ulna | Accessory carpal bone | Flexion of carpus |
| Flexor digitorum profundus caput humerale | FDPH | Medial epicondyle of humerus | Flexor tubercule of the distal phalanx of the digits I–V | Flexion of digits I–V |
| Flexor digitorum profundus caput radiale | FDPR | Proximal second quarter of craniomedial surface of radius | Flexor tubercle of distal phalanx of the digits I–V | Flexion of digits I–V |
| Flexor digitorum profundus caput ulnare | FDPU | Caudal surface of ulna, distally from olecranon to medium third of ulna | Flexor tubercle of distal phalanx of digits I–V | Flexion of digits I–V |
| Pronator quadratus | PRQ | Medial surface of body of ulna | Medial surface of body of radius | Pronation |
| Interflexorius | IFL | From humeral head of m. flexor digitorum profundus | Fusion with tendons of m. flexor digitorum superficialis to digits II and III | Flexion of digits II and III |
| Flexor digitorum brevis | FDB | From tendon of m. flexor digitorum superficialis to digit V | Proximal phalanx of digit V | Flexion of digit V |
| Lumbricales | LMB | Aponeurosis of tendons of m. flexor digitorum profundus | Proximal phalanx of digits III, IV and V | Flexion of digit III–V |
| Interosseous I, II, III and IV | INT | Basis of metacarpals II‐V | Proximal sesamoids and proximal phalanx of digits II–V | Flexion of digits II–V |
| Abductor digiti I brevis | ABIB | Flexor retinaculum | Fusion with the tendon of abductor digiti I longus | Abduction of digit I |
| Flexor digiti I brevis | FDB | Radiate carpal ligament | Proximal sesamoid of digit I | Flexion of digit I |
| Adductor digiti I | ADI | Flexor retinaculum | Proximal phalanx of digit I | Adduction of digit I |
| Abductor digiti V | ABV | Accessory carpal bone | Proximal phalanx of digit V | Abduction of digit V |
| Flexor digiti V | FDV | From ligament of accessory carpal bone to metacarpal IV | Fusion with tendon of m. abductor digiti V | Flexion of digit V |
| Adductor digiti V | ADV | Radiate carpal ligament | Medial surface of metacarpal V | Adduction of digit V |
| Adductor digit II | ADII | Radiate carpal ligament | Axial surface of base of proximal phalanx of digit II | Adduction of digit II |
Figure 1.
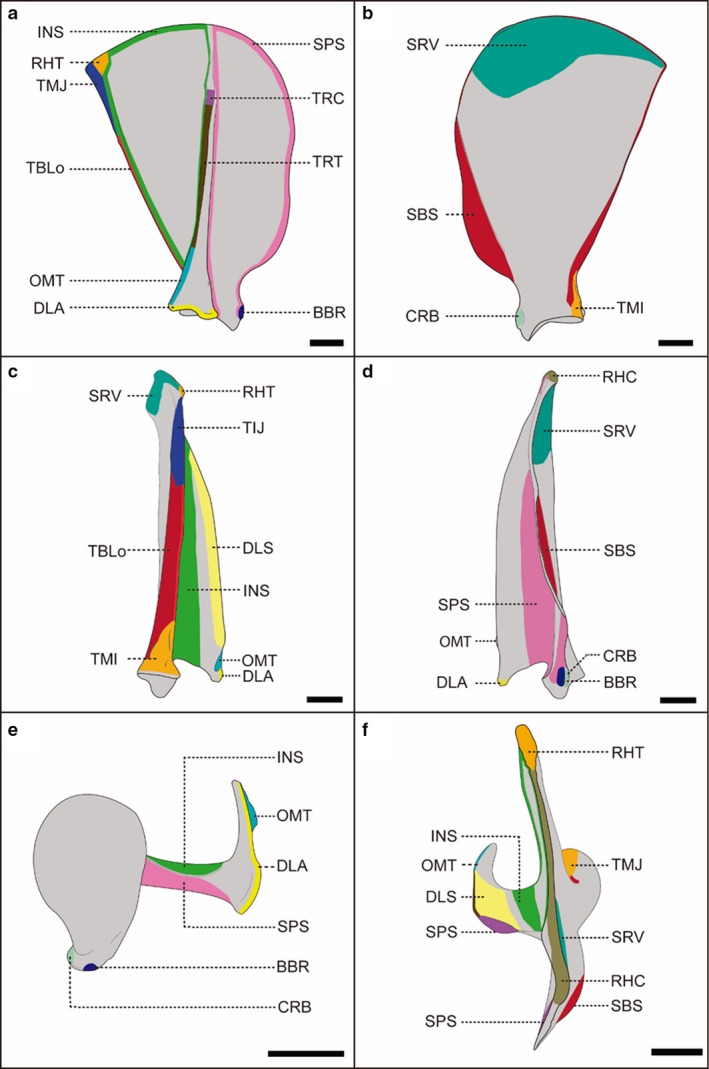
Schematic representation of the scapula of a female, adult specimen of Lycalopex gymnocercus (8576) illustrating muscle insertions. Lateral (a), medial (b), caudal (c), cranial (d), ventral (e) and dorsal (f) views. Intrinsic muscles: BBR, biceps brachii; CRB, coracobrachialis; DLA, deltoideus pars acromialis; DLS, deltoideus pars scapularis; INF, infraspinatus; TMI, teres minor; TMJ, teres major; SBS, subscapularis; SPS, supraspinatus; TBLo, triceps brachii caput longum. Extrinsic muscles: OMT, omotransversarius; RHC, rhomboideus cervicis; RHT, rhomboideus thoracis; SV, serratus ventralis; TPC, trapezius pars cervicalis; TPT, trapezius pars thoracica. Scale bar: 10 mm.
Figure 2.
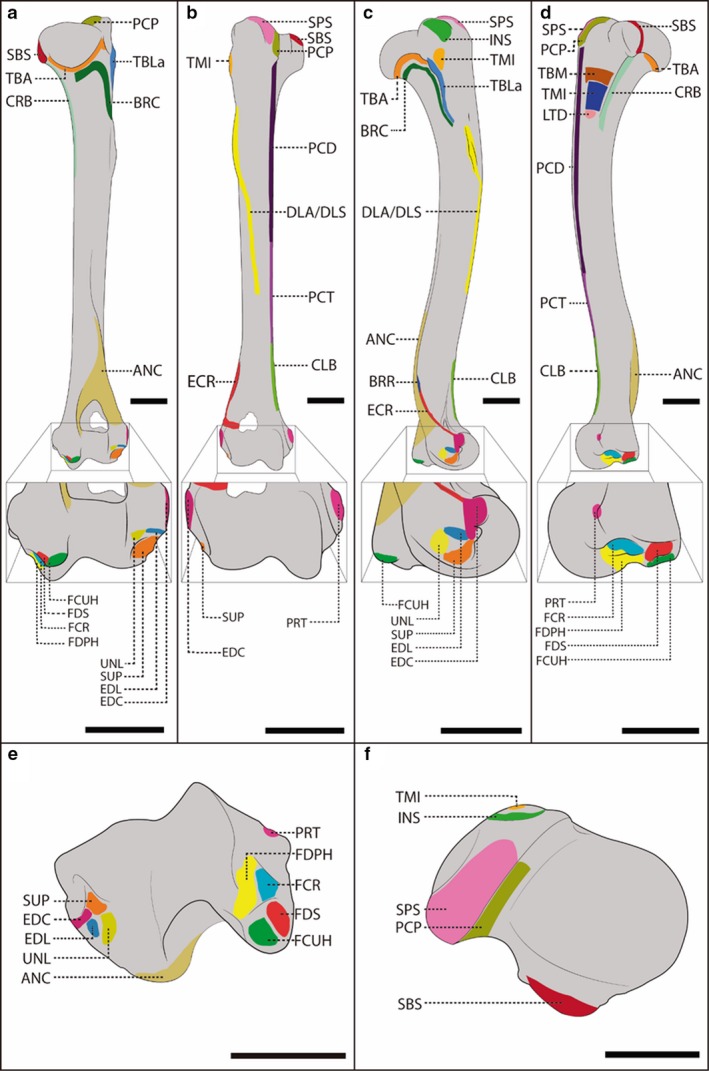
Schematic representation of the humerus of a female, adult specimen of Lycalopex gymnocercus (8576) illustrating muscle insertions. Caudal (a), cranial (b), lateral (c), medial (d), distal (e) and proximal (f) views, with details on the areas of muscle insertion of the extrinsic muscles: CLB, cleidobrachialis; LTD, latissimus dorsi; PCP, pectoralis profundus; PCD, pectoralis descendens; PCT, pectoralis transversus; and of the intrinsic musles: ANC, anconeus; BRC, brachialis; BRR, brachioradialis; CRB, coracobrachialis; DLA/DLS, deltoideus p. acromialis/deltoideus p. scapularis; EDC, extensor digitorum comunis; EDL, extensor digitorum lateralis; ECR, extensor carpi radialis; FDPH, flexor digitorum profundus caput humerale; FDS, flexor digitorum superficialis; FCR, flexor carpi radialis; FCUH, flexor carpi ulnaris caput humerale; INS, infraspinatus; PRT, pronator teres; TMJ, teres major; TMI, teres minor; SBS, subscapularis; SUP, supinator; SPS, supraspinatus; TBLa, triceps brachii caput lateralis; TBA, triceps brachii caput accessorium; TBM, triceps brachii caput medialis; UNL, ulnaris lateralis. Scale bar: 10 mm.
Figure 3.
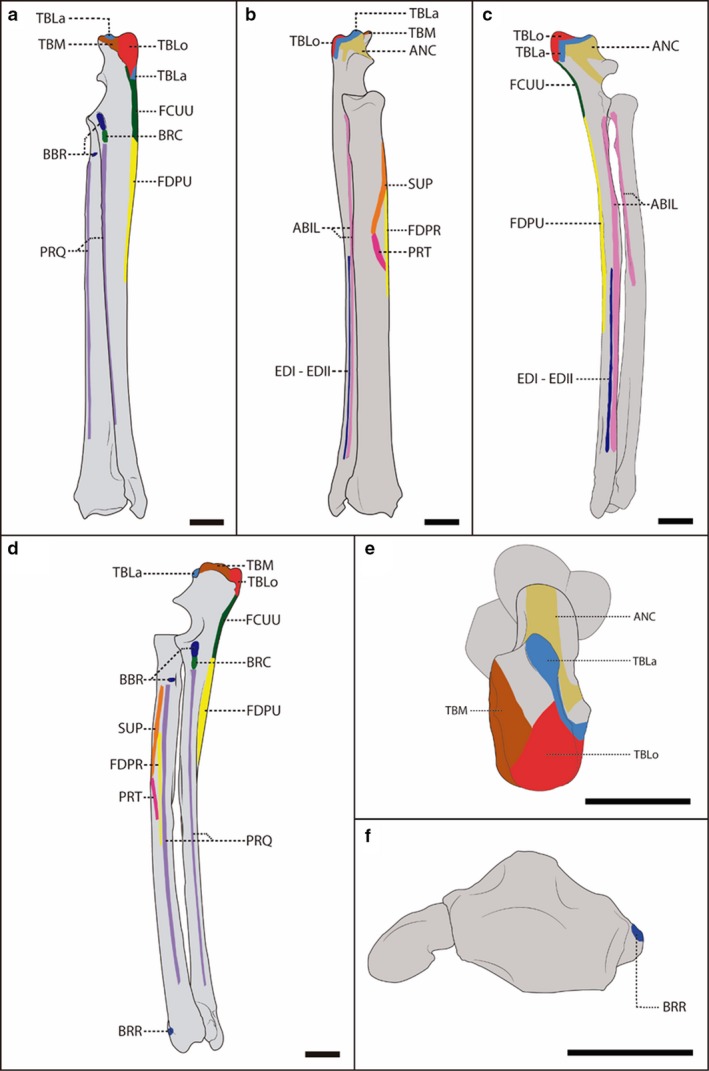
Schematic representation of the radius and ulna of a female, adult specimen of Lycalopex gymnocercus (8576) illustrating muscle insertions. Caudal (a), cranial (b), lateral (c), medial (d), proximal (e) and distal (f) views, with details on the areas of muscle insertion of the intrinsic muscles: ABIL, abductor digiti I longus; ANC, anconeus; BBR, biceps brachii; BRC, brachialis; BRR, brachioradialis; TBLa, triceps brachii caput lateralis; TBLo, triceps brachii caput longum; TBM, triceps brachii caput medialis; FCUU, flexor carpi ulnaris caput ulnare; FDPR, flexor digitorum profundus caput radiale; FDPU, flexor digitorum profundus caput ulnare; EDI‐EDII, extensor digiti I and II; PRQ, pronator quadrates; PRT, pronator teres; SUP, supinator. Scale bar: 10 mm.
Figure 4.
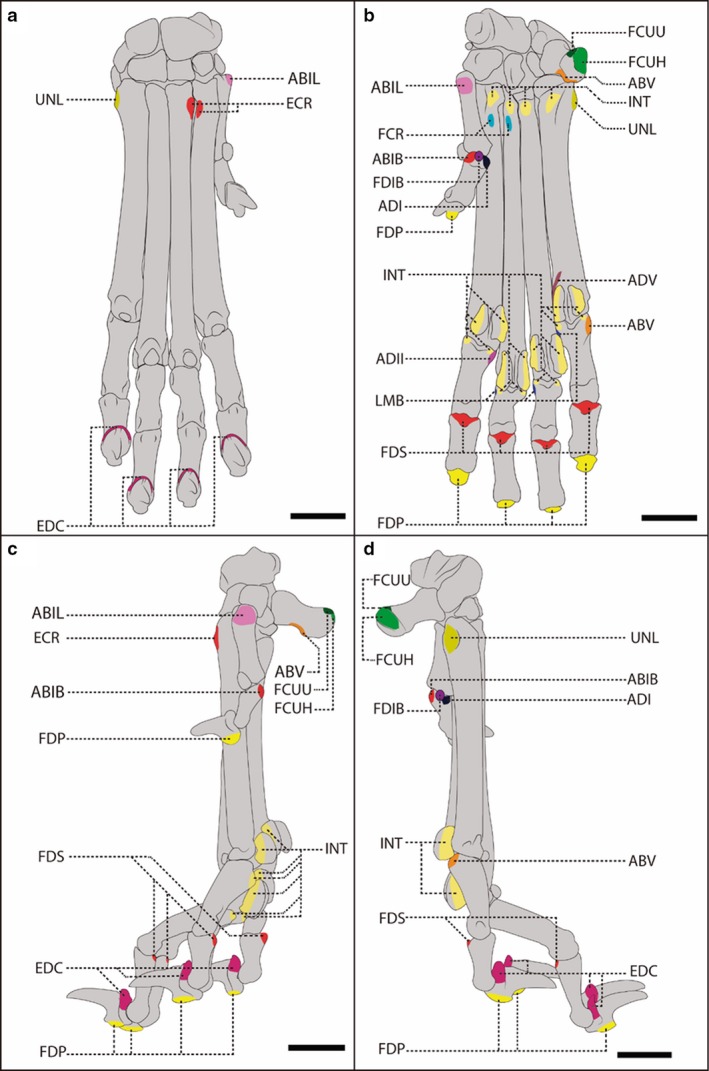
Schematic representation of the carpal, metacarpal, phalangeal and sesamoid bones of a female, adult specimen of Lycalopex gymnocercus (8576) illustrating muscle insertions. Dorsal (a), palmar (b), medial (c) and lateral (d) views, with details on the areas of muscle insertion of the intrinsic muscles: ABIB, abductor digiti I brevis; ABIL, abductor digiti I longus; ABV, abductor digiti V; ADI, adductor digiti I; ADII, adductor digiti I II; ADV, adductor digiti V; EDC, extensor digitorum comunis, extensor digitorum lateralis, and extensor digiti I and II ; FDP, flexor digitorum profundus; FDS, flexor digitorum superficialis; FCR, flexor carpi radialis; INT, interosseous; LMB, lumbricales; UNL, ulnaris lateralis; FCUH, flexor carpi ulnaris caput humerale; FCUU, flexor carpi ulnaris caput ulnare. Scale bar: 10 mm.
Intraspecific variations were identified in the muscles rhomboideus capitis, serratus ventralis cervicis, extensor carpi radialis, extensor digiti I and II, lumbricales, flexor digiti I brevis, abductor digiti I brevis, and flexor digiti V.
The rhomboideus capitis muscle was inconstant in L. gymnocercus (Fig. 5). Between the 21 specimens dissected, it was absent in 11 individuals (52.4%), bilaterally present in seven (33.3%), and unilaterally present in three individuals (14.3%). Bilateral occurrence was more frequent in males (five of 13 individuals, 38.4%) than in females (two in eight specimens, 25%). In the three individuals that showed the muscle rhomboideus capitis unilaterally and in two that showed it bilaterally, the muscle was only a thin muscle strip. In two male specimens in which the muscle was absent, a thin muscle strip was observed bound to the cranial margin of the muscle serratus ventralis cervicis, which was more visible and dettached as it approached the nuchal crest.
Figure 5.
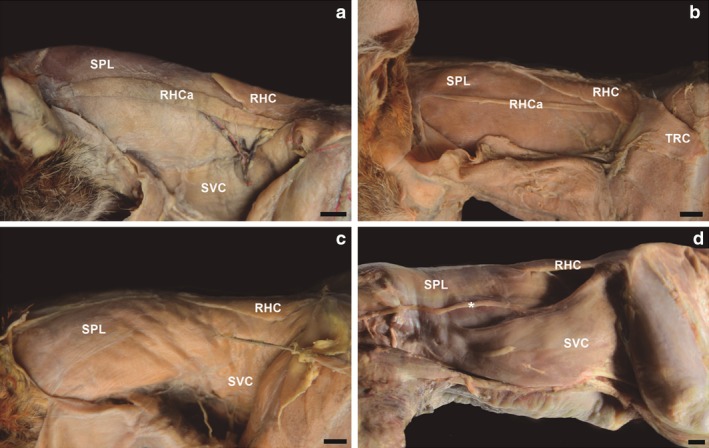
Photomacrographs of the muscles in the lateral cervical region of four adult specimens of Lycalopex gymnocercus. The most common presentation was a well‐developed m. rhomboideus capitis (a). However, variations with little developed (b) or absent (c) m. rhomboideus capitis were also observed. Another variation was a thin muscle strip (*) apparent in m. serratus ventralis cervicis in specimens that did not show m. rhomboideus capitis. SVC, m. serratus ventralis cervicis; RHC, rhomboideus cervicis; RHCa, m. rhomboideus capitis; SPL, m. splenius; TRC, m. trapezius pars cervicalis. Scale bar: 10 mm.
In one L. gymnocercus female specimen (8433), the muscle extensor carpi radialis showed three insertion tendons on both sides (Fig. 6). In this case, the tendon of the extensor carpi radialis brevis muscle was separated, with the two parts axially and abaxially inserted on the base of metacarpal III. In another male specimen (8533), the tendon of the extensor carpi radialis longus muscle discreetly bifurcated near the insertion on metacarpal II.
Figure 6.
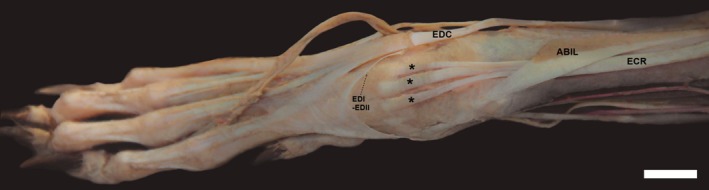
Photomacrographs of the dorsal region of the right hand of a female specimen (8433) of Lycalopex gymnocercus evidencing three possible tendon insertions (*) of: ECR, m. extensor carpi radialis; EDC, tendon of m. extensor digitorum communis; ABIL, m. abdutcor digiti I longus; and EDI‐EDII, tendon of m. extensor digiti I and II insertion (arrow). Scale bar: 10 mm.
The muscle extensor digiti I and II of L. gymnocercus presented variations in insertion. In most cases, the insertion tendon was dorsally divided near the base of metacarpal III. The medial division was a delicate tendon to metacarpal I, and the lateral division followed a distal path to join the tendon of the muscle extensor digitorum communis to digit II, near the medial aspect of the metacarpophalangeal joint. In the right antimere of a male specimen (5274), a thin strip was observed also to digit III.
Three lumbricales muscles were observed in 19 (90.5%) of the specimens. However, there were only two lumbricales muscles in two females (8585 and 8586, 9.5%). In these two specimens, the lumbrical muscle that inserts on the fifth digit was lacking. Among the specific muscles of digit I (thumb), one female specimen (8433) lacked the flexor digiti I brevis, and one male (8590) lacked the abductor digiti I brevis. In two specimens (8586 and 8589), the flexor digiti V was absent. The flexor digitorum brevis was not present in six specimens (28.6%).
Muscle architecture
The mean mass of the muscles of the thoracic limb in adult specimens (n = 8) of L. gymnocercus was 345.51 ± 58.31 g, corresponding to 6.37 ± 0.62% of the body mass of the individuals. In females (n = 3), the mean mass was 329.17 ± 63.08 g, and in males (n = 5) it was 355.31 ± 60.34 g, corresponding to 6.25 ± 0.68% and 6.45 ± 0.65% of the body mass, respectively. The percentage was not influenced by sex (P = 0.70). The young specimen weighed 2.2 kg, and the muscles of its right thoracic limb weighed 120.95 g, which represented only 5.49% of its body mass, demonstrating that the young specimen had proportionally less muscle mass in the limb compared with the average adult, with a significant difference (P = 0.04). Therefore, the results presented and discussed here for L. gymnocercus discounted the data of the young specimen; only data on the eight adult individuals were used. However, the percentage mass distribution of each functional group was identical between the young and adult individuals (P = 1.00).
The triceps brachii caput longus muscle was the muscle that showed the greatest mean mass (39.66 ± 8.61 g), and the supinator had the smallest (0.49 ± 0.08 g; Table 4). The latissimus dorsi muscle presented the longest fascicles, the cleidomastoideus muscle showed the greatest AI, and the subscapularis muscle had the greatest PCSA value (Fig. 7). These data did not include the small muscles that both originated and inserted on the bones of the hand.
Table 4.
Arithmetic means and respective standard deviations of mass (M), mean fascicle length (Mfasc), physiological cross‐sectional area (PCSA) and architectural indexes (AI) of the muscles of the thoracic limb in adult specimens of Lycalopex gymnocercus (n = 8), according to sex
| Muscle | All individuals (n = 8) | Males (n = 5) | Females (n = 3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M (g) | Mfasc (cm) | PCSA (cm2) | AI | M (g) | Mfasc (cm) | PCSA (cm2) | AI | M (g) | Mfasc (cm) | PCSA (cm2) | AI | |
| ABIL | 1.00 ± 0.13 | 0.71 ± 0.19 | 1.38 ± 0.45 | 0.08 ± 0.03 | 0.97 ± 0.09 | 0.73 ± 0.24 | 1.33 ± 0.51 | 0.08 ± 0.03 | 1.04 ± 0.18 | 0.68 ± 0.11 | 1.46 ± 0.43 | 0.07 ± 0.01 |
| ANC | 1.65 ± 0.47 | 1.67 ± 0.52 | 0.91 ± 0.35 | 0.34 ± 0.14 | 1.89 ± 0.31 | 1.68 ± 0.61 | 1.04 ± 0.39 | 0.30 ± 0.16 | 1.25 ± 0.45 | 1.64 ± 0.45 | 0.70 ± 0.13 | 0.40 ± 0.12 |
| BBR | 7.15 ± 1.36 | 1.55 ± 0.42 | 4.45 ± 1.40 | 0.16 ± 0.04 | 7.42 ± 1.41 | 1.46 ± 0.52 | 4.98 ± 1.49 | 0.15 ± 0.05 | 6.71 ± 1.42 | 1.71 ± 0.08 | 3.57 ± 0.73 | 0.18 ± 0.02 |
| BRC | 4.29 ± 0.74 | 3.49 ± 0.77 | 1.17 ± 0.32 | 0.33 ± 0.07 | 4.48 ± 0.56 | 3.59 ± 0.90 | 1.20 ± 0.41 | 0.34 ± 0.08 | 3.96 ± 1.03 | 3.31 ± 0.63 | 1.11 ± 0.16 | 0.31 ± 0.04 |
| CLB | 5.35 ± 1.62 | 6.83 ± 0.74 | 0.74 ± 0.21 | 0.64 ± 0.08 | 5.38 ± 1.41 | 6.96 ± 0.63 | 0.74 ± 0.20 | 0.67 ± 0.09 | 5.30 ± 2.27 | 6.62 ± 1.02 | 0.75 ± 0.28 | 0.59 ± 0.02 |
| CLCe | 6.51 ± 2.18 | 12.11 ± 1.63 | 0.51 ± 0.19 | 0.76 ± 0.11 | 6.30 ± 2.32 | 12.83 ± 1.63 | 0.46 ± 0.14 | 0.78 ± 0.12 | 6.84 ± 2.38 | 10.92 ± 0.76 | 0.60 ± 0.25 | 0.73 ± 0.10 |
| CLM | 6.46 ± 1.55 | 12.80 ± 1.57 | 0.49 ± 0.15 | 0.79 ± 0.05 | 6.63 ± 1.35 | 13.26 ± 1.86 | 0.48 ± 0.14 | 0.81 ± 0.06 | 6.16 ± 2.13 | 12.03 ± 0.52 | 0.49 ± 0.19 | 0.77 ± 0.01 |
| CRB | 0.78 ± 0.19 | 0.90 ± 0.23 | 0.87 ± 0.33 | 0.24 ± 0.10 | 0.82 ± 0.22 | 0.94 ± 0.24 | 0.88 ± 0.42 | 0.24 ± 0.11 | 0.72 ± 0.11 | 0.82 ± 0.25 | 0.85 ± 0.17 | 0.23 ± 0.10 |
| DLA | 3.20 ± 0.81 | 1.41 ± 0.27 | 2.11 ± 0.34 | 0.27 ± 0.05 | 3.27 ± 0.71 | 1.40 ± 0.29 | 2.16 ± 0.32 | 0.26 ± 0.05 | 3.07 ± 1.11 | 1.41 ± 0.31 | 2.01 ± 0.42 | 0.28 ± 0.05 |
| DLS | 4.82 ± 1.24 | 3.57 ± 0.76 | 1.30 ± 0.40 | 0.45 ± 0.11 | 4.84 ± 1.31 | 3.44 ± 0.82 | 1.35 ± 0.40 | 0.41 ± 0.09 | 4.79 ± 1.39 | 3.77 ± 0.75 | 1.23 ± 0.48 | 0.51 ± 0.14 |
| ECR | 5.33 ± 1.06 | 2.33 ± 0.43 | 2.15 ± 0.50 | 0.26 ± 0.06 | 5.66 ± 0.88 | 2.18 ± 0.36 | 2.41 ± 0.42 | 0.24 ± 0.06 | 4.79 ± 1.28 | 2.58 ± 0.49 | 1.71 ± 0.23 | 0.28 ± 0.08 |
| EDC | 1.98 ± 0.37 | 1.39 ± 0.37 | 1.38 ± 0.33 | 0.18 ± 0.05 | 2.05 ± 0.36 | 1.46 ± 0.28 | 1.35 ± 0.37 | 0.18 ± 0.03 | 1.87 ± 0.42 | 1.28 ± 0.53 | 1.43 ± 0.30 | 0.18 ± 0.08 |
| EDL | 0.92 ± 0.28 | 1.22 ± 0.21 | 0.75 ± 0.34 | 0.16 ± 0.05 | 1.04 ± 0.26 | 1.14 ± 0.20 | 0.89 ± 0.34 | 0.14 ± 0.03 | 0.71 ± 0.16 | 1.35 ± 0.17 | 0.51 ± 0.18 | 0.19 ± 0.07 |
| FCR | 1.19 ± 0.25 | 1.12 ± 0.20 | 1.00 ± 0.20 | 0.19 ± 0.04 | 1.18 ± 0.32 | 1.08 ± 0.15 | 1.01 ± 0.23 | 0.17 ± 0.01 | 1.21 ± 0.11 | 1.17 ± 0.30 | 0.99 ± 0.17 | 0.21 ± 0.07 |
| FCUH | 2.70 ± 0.41 | 1.03 ± 0.23 | 2.54 ± 0.57 | 0.09 ± 0.02 | 2.90 ± 0.32 | 0.95 ± 0.17 | 2.88 ± 0.24 | 0.08 ± 0.01 | 2.36 ± 0.31 | 1.16 ± 0.29 | 1.97 ± 0.52 | 0.11 ± 0.03 |
| FCUU | 0.89 ± 0.20 | 0.93 ± 0.25 | 0.91 ± 0.24 | 0.14 ± 0.03 | 0.89 ± 0.25 | 0.86 ± 0.25 | 0.98 ± 0.28 | 0.13 ± 0.04 | 0.89 ± 0.08 | 1.06 ± 0.23 | 0.80 ± 0.14 | 0.16 ± 0.03 |
| FDPH | 8.73 ± 1.08 | 1.44 ± 0.31 | 5.65 ± 1.60 | 0.12 ± 0.04 | 8.76 ± 1.34 | 1.24 ± 0.13 | 6.30 ± 1.70 | 0.10 ± 0.01 | 8.69 ± 0.71 | 1.77 ± 0.22 | 4.56 ± 0.65 | 0.16 ± 0.03 |
| FDPR | 0.50 ± 0.19 | 1.26 ± 0.44 | 0.41 ± 0.21 | 0.19 ± 0.08 | 0.55 ± 0.23 | 1.04 ± 0.28 | 0.51 ± 0.21 | 0.15 ± 0.05 | 0.43 ± 0.11 | 1.61 ± 0.47 | 0.25 ± 0.04 | 0.24 ± 0.09 |
| FDPU | 0.90 ± 0.26 | 0.80 ± 0.18 | 1.07 ± 0.26 | 0.09 ± 0.02 | 0.96 ± 0.31 | 0.80 ± 0.20 | 1.13 ± 0.32 | 0.08 ± 0.03 | 0.81 ± 0.12 | 0.80 ± 0.16 | 0.95 ± 0.07 | 0.09 ± 0.01 |
| FDS | 2.65 ± 0.49 | 1.30 ± 0.60 | 2.32 ± 1.14 | 0.12 ± 0.07 | 2.80 ± 0.58 | 1.06 ± 0.51 | 2.84 ± 1.09 | 0.09 ± 0.04 | 2.40 ± 0.10 | 1.70 ± 0.60 | 1.45 ± 0.60 | 0.17 ± 0.08 |
| INS | 17.70 ± 2.88 | 2.30 ± 0.64 | 7.17 ± 1.56 | 0.25 ± 0.08 | 18.23 ± 3.04 | 2.12 ± 0.36 | 7.76 ± 1.34 | 0.26 ± 0.04 | 16.82 ± 2.95 | 2.60 ± 0.99 | 6.17 ± 1.58 | 0.30 ± 0.11 |
| LTD | 34.27 ± 5.97 | 14.89 ± 1.37 | 2.08 ± 0.51 | 0.63 ± 0.06 | 33.85 ± 6.67 | 14.89 ± 1.08 | 2.04 ± 0.49 | 0.63 ± 0.07 | 34.96 ± 5.89 | 14.89 ± 2.06 | 2.15 ± 0.63 | 0.61 ± 0.06 |
| OMT | 5.75 ± 1.19 | 13.97 ± 1.97 | 0.40 ± 0.10 | 0.76 ± 0.11 | 5.80 ± 1.33 | 15.02 ± 1.61 | 0.37 ± 0.12 | 0.80 ± 0.12 | 5.66 ± 1.20 | 12.21 ± 0.99 | 0.43 ± 0.08 | 0.69 ± 0.04 |
| PCD | 3.43 ± 0.77 | 6.60 ± 1.25 | 0.51 ± 0.18 | 0.70 ± 0.10 | 3.46 ± 0.86 | 6.56 ± 1.55 | 0.53 ± 0.20 | 0.70 ± 0.11 | 3.38 ± 0.76 | 6.67 ± 0.78 | 0.49 ± 0.16 | 0.69 ± 0.12 |
| PCP | 36.43 ± 7.85 | 10.71 ± 1.00 | 3.21 ± 0.76 | 0.51 ± 0.07 | 36.45 ± 7.97 | 10.81 ± 1.14 | 3.17 ± 0.75 | 0.49 ± 0.08 | 36.39 ± 9.41 | 10.55 ± 0.92 | 3.28 ± 0.96 | 0.53 ± 0.06 |
| PCT | 10.26 ± 2.45 | 5.74 ± 0.73 | 1.69 ± 0.35 | 0.74 ± 0.10 | 10.44 ± 2.51 | 5.79 ± 0.93 | 1.70 ± 0.30 | 0.77 ± 0.10 | 9.95 ± 2.85 | 5.64 ± 0.36 | 1.67 ± 0.51 | 0.68 ± 0.09 |
| PRQ | 0.90 ± 0.27 | 0.50 ± 0.05 | 1.70 ± 0.43 | 0.05 ± 0.00 | 0.90 ± 0.30 | 0.52 ± 0.04 | 1.64 ± 0.50 | 0.05 ± 0.00 | 0.90 ± 0.26 | 0.47 ± 0.06 | 1.79 ± 0.34 | 0.05 ± 0.01 |
| PRT | 0.97 ± 0.29 | 0.85 ± 0.32 | 1.16 ± 0.42 | 0.16 ± 0.06 | 1.08 ± 0.29 | 0.85 ± 0.36 | 1.29 ± 0.43 | 0.16 ± 0.06 | 0.79 ± 0.22 | 0.84 ± 0.32 | 0.95 ± 0.35 | 0.14 ± 0.07 |
| RHC | 3.51 ± 1.00 | 5.13 ± 1.09 | 0.63 ± 0.11 | 0.45 ± 0.07 | 3.90 ± 1.11 | 5.40 ± 1.16 | 0.66 ± 0.11 | 0.48 ± 0.05 | 2.85 ± 0.07 | 4.69 ± 1.00 | 0.58 ± 0.10 | 0.39 ± 0.07 |
| RHT | 6.91 ± 1.07 | 3.20 ± 0.92 | 2.14 ± 0.54 | 0.54 ± 0.12 | 7.12 ± 1.17 | 3.35 ± 1.08 | 2.13 ± 0.66 | 0.56 ± 0.11 | 6.56 ± 0.99 | 2.93 ± 0.68 | 2.16 ± 0.36 | 0.50 ± 0.16 |
| SBS | 13.80 ± 2.37 | 1.04 ± 0.25 | 12.61 ± 2.17 | 0.14 ± 0.03 | 14.45 ± 2.31 | 1.05 ± 0.33 | 13.27 ± 2.44 | 0.13 ± 0.03 | 12.70 ± 2.47 | 1.01 ± 0.08 | 11.50 ± 1.29 | 0.14 ± 0.02 |
| SPS | 23.30 ± 4.91 | 3.15 ± 0.58 | 6.69 ± 1.24 | 0.32 ± 0.06 | 24.93 ± 3.26 | 3.18 ± 0.65 | 7.17 ± 1.27 | 0.31 ± 0.07 | 20.60 ± 6.74 | 3.09 ± 0.58 | 5.90 ± 0.80 | 0.32 ± 0.03 |
| SUP | 0.50 ± 0.08 | 1.10 ± 0.52 | 0.48 ± 0.17 | 0.33 ± 0.13 | 0.51 ± 0.09 | 1.07 ± 0.63 | 0.52 ± 0.18 | 0.30 ± 0.15 | 0.47 ± 0.07 | 1.14 ± 0.35 | 0.41 ± 0.14 | 0.36 ± 0.11 |
| SVC | 16.47 ± 2.17 | 7.21 ± 0.90 | 2.04 ± 0.37 | 0.56 ± 0.09 | 17.01 ± 2.38 | 7.35 ± 1.05 | 2.09 ± 0.48 | 0.59 ± 0.09 | 15.58 ± 1.82 | 6.99 ± 0.77 | 1.95 ± 0.04 | 0.51 ± 0.05 |
| SVT | 17.50 ± 4.00 | 2.79 ± 0.70 | 5.47 ± 2.18 | 0.37 ± 0.11 | 18.42 ± 4.64 | 2.66 ± 0.85 | 5.94 ± 2.74 | 0.38 ± 0.12 | 15.98 ± 2.71 | 3.02 ± 0.35 | 4.70 ± 0.36 | 0.36 ± 0.09 |
| TBA | 5.48 ± 1.27 | 4.17 ± 0.79 | 1.23 ± 0.17 | 0.45 ± 0.09 | 5.63 ± 1.41 | 4.29 ± 0.94 | 1.23 ± 0.17 | 0.46 ± 0.11 | 5.23 ± 1.23 | 3.96 ± 0.57 | 1.23 ± 0.20 | 0.43 ± 0.06 |
| TBLa | 13.80 ± 3.16 | 4.17 ± 0.59 | 3.02 ± 0.43 | 0.43 ± 0.07 | 14.33 ± 3.72 | 4.36 ± 0.67 | 2.96 ± 0.35 | 0.45 ± 0.07 | 12.92 ± 2.31 | 3.86 ± 0.23 | 3.12 ± 0.61 | 0.40 ± 0.05 |
| TBLo | 39.66 ± 8.61 | 3.30 ± 0.52 | 10.99 ± 1.88 | 0.30 ± 0.04 | 40.82 ± 9.82 | 3.44 ± 0.54 | 10.88 ± 2.27 | 0.31 ± 0.04 | 37.72 ± 7.60 | 3.05 ± 0.47 | 11.16 ± 1.39 | 0.28 ± 0.03 |
| TBM | 6.76 ± 1.40 | 3.40 ± 0.53 | 1.89 ± 0.54 | 0.38 ± 0.07 | 7.02 ± 1.46 | 3.33 ± 0.46 | 2.02 ± 0.67 | 0.35 ± 0.07 | 6.32 ± 1.46 | 3.51 ± 0.73 | 1.66 ± 0.09 | 0.41 ± 0.08 |
| TFA | 1.46 ± 0.38 | 5.50 ± 1.17 | 0.26 ± 0.06 | 0.64 ± 0.10 | 1.57 ± 0.36 | 5.32 ± 1.48 | 0.29 ± 0.04 | 0.61 ± 0.09 | 1.27 ± 0.42 | 5.81 ± 0.37 | 0.21 ± 0.06 | 0.68 ± 0.14 |
| TMJ | 8.15 ± 1.35 | 5.16 ± 0.89 | 1.55 ± 0.46 | 0.54 ± 0.10 | 8.46 ± 1.12 | 5.10 ± 0.94 | 1.60 ± 0.39 | 0.52 ± 0.10 | 7.62 ± 1.79 | 5.27 ± 0.98 | 1.45 ± 0.65 | 0.56 ± 0.12 |
| TMI | 0.73 ± 0.12 | 1.14 ± 0.22 | 0.62 ± 0.13 | 0.41 ± 0.14 | 0.77 ± 0.12 | 1.07 ± 0.23 | 0.69 ± 0.09 | 0.36 ± 0.09 | 0.65 ± 0.10 | 1.26 ± 0.19 | 0.50 ± 0.11 | 0.47 ± 0.20 |
| TRC | 4.20 ± 1.24 | 5.55 ± 1.10 | 0.68 ± 0.14 | 0.49 ± 0.08 | 4.35 ± 1.42 | 5.70 ± 1.23 | 0.67 ± 0.18 | 0.51 ± 0.09 | 3.94 ± 1.12 | 5.28 ± 1.00 | 0.68 ± 0.07 | 0.47 ± 0.07 |
| TRT | 4.74 ± 0.95 | 3.84 ± 0.54 | 0.91 ± 0.23 | 0.46 ± 0.07 | 4.95 ± 1.13 | 3.78 ± 0.69 | 0.90 ± 0.30 | 0.46 ± 0.08 | 4.38 ± 0.53 | 3.93 ± 0.22 | 0.94 ± 0.07 | 0.46 ± 0.04 |
| UNL | 1.86 ± 0.29 | 0.68 ± 0.23 | 2.79 ± 0.95 | 0.07 ± 0.02 | 2.07 ± 0.09 | 0.65 ± 0.21 | 3.18 ± 0.90 | 0.07 ± 0.02 | 1.52 ± 0.12 | 0.73 ± 0.29 | 2.15 ± 0.74 | 0.08 ± 0.03 |
Figure 7.
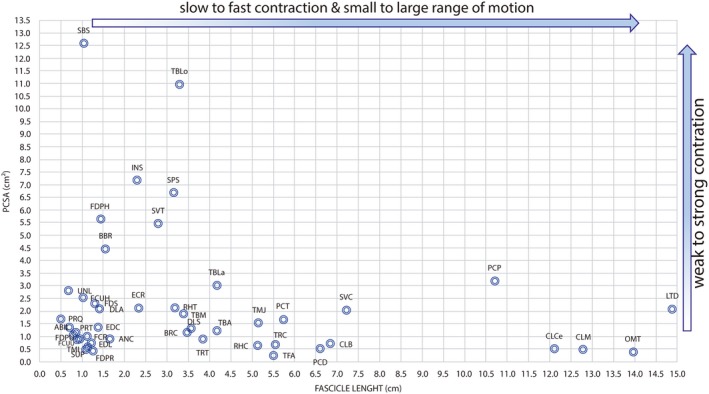
Scatter graph showing fiber length and physiological cross‐sectional area (PCSA) of muscles in the human lower limb. Fiber length is proportional to muscle excursion, and PCSA is proportional to maximum muscle force. Thus, this graph can be used to compare relative force and excursion of muscles within the thoracic limb of adult specimens of Lycalopex gymnocercus (n = 8).
Mean values for architectural data that were scaled in relation to body mass of the specimens were used in the comparison between the sexes (Table 5). Considering P < 0.05 as significant, the muscles anconeus, extensor digitorum lateralis and ulnar lateralis showed masses that were significantly greater in males; the humeral head of the flexor digitorum profundus muscle presented fascicles that were, on average, shorter in males, and the omotransversarius muscles was shorter in females; the PCSA values of the muscles flexor carpi radialis, flexor carpi ulnaris caput humeralis and tensor fasciae antebrachi were larger in males.
Table 5.
Arithmetic means and respective standard deviations of architectural data normalized by geometric similarity of thoracic limb muscles of Lycalopex gymnocercus adult specimens (n = 8), according to sex
| Muscle | All individuals (n = 8) | Males (n = 5) | Females (n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M | Mfasc | PCSA | M | Mfasc | PCSA | M | Mfasc | PCSA | |
| ABIL | 0.19 ± 0.03 | 0.41 ± 0.11 | 0.45 ± 0.14 | 0.18 ± 0.02 | 0.42 ± 0.14 | 0.43 ± 0.14 | 0.20 ± 0.03 | 0.39 ± 0.06 | 0.49 ± 0.15 |
| ANC | 0.30 ± 0.07 | 0.95 ± 0.28 | 0.30 ± 0.11 | 0.34 ± 0.04* | 0.96 ± 0.34 | 0.34 ± 0.12 | 0.24 ± 0.07* | 0.95 ± 0.24 | 0.23 ± 0.04 |
| BBR | 1.32 ± 0.14 | 0.89 ± 0.24 | 1.46 ± 0.41 | 1.34 ± 0.14 | 0.83 ± 0.29 | 1.62 ± 0.44 | 1.27 ± 0.17 | 0.99 ± 0.04 | 1.19 ± 0.18 |
| BRC | 0.79 ± 0.09 | 2.00 ± 0.42 | 0.38 ± 0.10 | 0.82 ± 0.07 | 2.05 ± 0.50 | 0.39 ± 0.13 | 0.75 ± 0.13 | 1.91 ± 0.32 | 0.37 ± 0.05 |
| CLB | 0.99 ± 0.26 | 3.92 ± 0.44 | 0.24 ± 0.06 | 0.98 ± 0.23 | 3.98 ± 0.42 | 0.24 ± 0.06 | 0.99 ± 0.35 | 3.83 ± 0.56 | 0.25 ± 0.08 |
| CLCe | 1.20 ± 0.37 | 6.95 ± 0.82 | 0.17 ± 0.06 | 1.15 ± 0.41 | 7.31 ± 0.75 | 0.15 ± 0.04 | 1.29 ± 0.34 | 6.34 ± 0.57 | 0.20 ± 0.07 |
| CLM | 1.19 ± 0.24 | 7.34 ± 0.74 | 0.16 ± 0.05 | 1.21 ± 0.23 | 7.55 ± 0.83 | 0.16 ± 0.05 | 1.16 ± 0.31 | 6.98 ± 0.48 | 0.16 ± 0.05 |
| CRB | 0.15 ± 0.03 | 0.52 ± 0.14 | 0.29 ± 0.11 | 0.15 ± 0.04 | 0.54 ± 0.16 | 0.29 ± 0.13 | 0.14 ± 0.02 | 0.47 ± 0.13 | 0.29 ± 0.07 |
| DLA | 0.59 ± 0.12 | 0.81 ± 0.14 | 0.69 ± 0.11 | 0.60 ± 0.11 | 0.80 ± 0.15 | 0.71 ± 0.12 | 0.58 ± 0.16 | 0.82 ± 0.16 | 0.67 ± 0.11 |
| DLS | 0.89 ± 0.18 | 2.05 ± 0.44 | 0.43 ± 0.11 | 0.87 ± 0.19 | 1.96 ± 0.45 | 0.44 ± 0.11 | 0.91 ± 0.20 | 2.19 ± 0.47 | 0.41 ± 0.15 |
| ECR | 0.98 ± 0.13 | 1.34 ± 0.23 | 0.70 ± 0.15 | 1.03 ± 0.09 | 1.24 ± 0.19 | 0.78 ± 0.12* | 0.91 ± 0.17 | 1.49 ± 0.24 | 0.57 ± 0.06* |
| EDC | 0.37 ± 0.05 | 0.80 ± 0.20 | 0.46 ± 0.11 | 0.37 ± 0.06 | 0.83 ± 0.16 | 0.44 ± 0.11 | 0.35 ± 0.05 | 0.74 ± 0.29 | 0.48 ± 0.12 |
| EDL | 0.17 ± 0.04 | 0.71 ± 0.14 | 0.24 ± 0.10 | 0.19 ± 0.03* | 0.66 ± 0.14 | 0.28 ± 0.09 | 0.14 ± 0.02* | 0.79 ± 0.12 | 0.17 ± 0.05 |
| FCR | 0.22 ± 0.04 | 0.64 ± 0.11 | 0.33 ± 0.07 | 0.22 ± 0.06 | 0.62 ± 0.09 | 0.33 ± 0.07 | 0.23 ± 0.01 | 0.68 ± 0.16 | 0.33 ± 0.07 |
| FCUH | 0.50 ± 0.07 | 0.59 ± 0.13 | 0.84 ± 0.20 | 0.53 ± 0.04 | 0.54 ± 0.09 | 0.94 ± 0.13* | 0.46 ± 0.09 | 0.67 ± 0.18 | 0.66 ± 0.19* |
| FCUU | 0.16 ± 0.03 | 0.53 ± 0.13 | 0.30 ± 0.07 | 0.16 ± 0.04 | 0.49 ± 0.13 | 0.32 ± 0.08 | 0.17 ± 0.01 | 0.61 ± 0.11 | 0.27 ± 0.06 |
| FDPH | 1.62 ± 0.17 | 0.83 ± 0.19 | 1.85 ± 0.47 | 1.60 ± 0.22 | 0.71 ± 0.09* | 2.04 ± 0.49 | 1.66 ± 0.02 | 1.02 ± 0.12* | 1.53 ± 0.23 |
| FDPR | 0.09 ± 0.03 | 0.72 ± 0.26 | 0.13 ± 0.06 | 0.10 ± 0.03 | 0.60 ± 0.16 | 0.16 ± 0.06 | 0.08 ± 0.02 | 0.94 ± 0.28 | 0.08 ± 0.01 |
| FDPU | 0.17 ± 0.04 | 0.46 ± 0.09 | 0.35 ± 0.08 | 0.17 ± 0.04 | 0.45 ± 0.10 | 0.37 ± 0.10 | 0.16 ± 0.03 | 0.46 ± 0.09 | 0.32 ± 0.04 |
| FDS | 0.49 ± 0.07 | 0.75 ± 0.35 | 0.76 ± 0.38 | 0.51 ± 0.09 | 0.60 ± 0.29 | 0.93 ± 0.37 | 0.46 ± 0.04 | 0.99 ± 0.36 | 0.49 ± 0.21 |
| INS | 3.29 ± 0.50 | 1.33 ± 0.39 | 2.34 ± 0.41 | 3.33 ± 0.49 | 1.21 ± 0.22 | 2.52 ± 0.32 | 3.23 ± 0.63 | 1.51 ± 0.61 | 2.06 ± 0.42 |
| LTD | 6.34 ± 0.81 | 8.56 ± 0.92 | 0.68 ± 0.14 | 6.15 ± 0.92 | 8.51 ± 0.74 | 0.66 ± 0.14 | 6.65 ± 0.59 | 8.65 ± 1.35 | 0.72 ± 0.17 |
| OMT | 1.06 ± 0.19 | 8.01 ± 1.04 | 0.13 ± 0.03 | 1.06 ± 0.22 | 8.57 ± 0.81* | 0.12 ± 0.04 | 1.08 ± 0.18 | 7.08 ± 0.62* | 0.15 ± 0.02 |
| PCD | 0.63 ± 0.12 | 3.78 ± 0.68 | 0.17 ± 0.06 | 0.63 ± 0.15 | 3.73 ± 0.81 | 0.17 ± 0.07 | 0.64 ± 0.09 | 3.87 ± 0.52 | 0.16 ± 0.04 |
| PCP | 6.70 ± 0.97 | 6.16 ± 0.65 | 1.05 ± 0.21 | 6.58 ± 0.90 | 6.18 ± 0.73 | 1.02 ± 0.20 | 6.90 ± 1.24 | 6.12 ± 0.64 | 1.09 ± 0.26 |
| PCT | 1.89 ± 0.36 | 3.29 ± 0.39 | 0.55 ± 0.10 | 1.90 ± 0.40 | 3.30 ± 0.48 | 0.55 ± 0.09 | 1.88 ± 0.38 | 3.27 ± 0.25 | 0.56 ± 0.14 |
| PRQ | 0.17 ± 0.05 | 0.29 ± 0.02 | 0.56 ± 0.15 | 0.17 ± 0.06 | 0.29 ± 0.02 | 0.54 ± 0.17 | 0.17 ± 0.05 | 0.27 ± 0.03 | 0.60 ± 0.13 |
| PRT | 0.18 ± 0.04 | 0.48 ± 0.18 | 0.38 ± 0.13 | 0.19 ± 0.04 | 0.48 ± 0.19 | 0.42 ± 0.14 | 0.15 ± 0.03 | 0.49 ± 0.19 | 0.32 ± 0.12 |
| RHC | 0.64 ± 0.14 | 2.94 ± 0.57 | 0.21 ± 0.03 | 0.70 ± 0.15 | 3.06 ± 0.57 | 0.21 ± 0.03 | 0.55 ± 0.05 | 2.73 ± 0.63 | 0.19 ± 0.03 |
| RHT | 1.28 ± 0.15 | 1.84 ± 0.53 | 0.70 ± 0.16 | 1.30 ± 0.16 | 1.92 ± 0.62 | 0.69 ± 0.20 | 1.25 ± 0.14 | 1.70 ± 0.41 | 0.72 ± 0.10 |
| SBS | 2.55 ± 0.29 | 0.59 ± 0.14 | 4.14 ± 0.61 | 2.63 ± 0.29 | 0.60 ± 0.18 | 4.32 ± 0.73 | 2.41 ± 0.27 | 0.59 ± 0.04 | 3.85 ± 0.23 |
| SPS | 4.30 ± 0.69 | 1.80 ± 0.29 | 2.21 ± 0.41 | 4.54 ± 0.39 | 1.81 ± 0.32 | 2.35 ± 0.47 | 3.89 ± 0.97 | 1.78 ± 0.29 | 1.97 ± 0.19 |
| SUP | 0.09 ± 0.01 | 0.63 ± 0.28 | 0.16 ± 0.05 | 0.09 ± 0.01 | 0.61 ± 0.35 | 0.17 ± 0.06 | 0.09 ± 0.01 | 0.66 ± 0.19 | 0.14 ± 0.05 |
| SVC | 3.05 ± 0.27 | 4.14 ± 0.53 | 0.67 ± 0.11 | 3.10 ± 0.33 | 4.20 ± 0.66 | 0.68 ± 0.15 | 2.97 ± 0.15 | 4.04 ± 0.31 | 0.66 ± 0.03 |
| SVT | 3.21 ± 0.47 | 1.60 ± 0.39 | 1.79 ± 0.67 | 3.32 ± 0.57 | 1.51 ± 0.47 | 1.92 ± 0.85 | 3.04 ± 0.28 | 1.75 ± 0.18 | 1.58 ± 0.13 |
| TBA | 1.01 ± 0.18 | 2.38 ± 0.39 | 0.41 ± 0.06 | 1.02 ± 0.20 | 2.44 ± 0.46 | 0.40 ± 0.08 | 0.99 ± 0.15 | 2.29 ± 0.31 | 0.41 ± 0.05 |
| TBLa | 2.54 ± 0.39 | 2.39 ± 0.30 | 0.99 ± 0.11 | 2.58 ± 0.45 | 2.48 ± 0.34 | 0.96 ± 0.07 | 2.46 ± 0.33 | 2.24 ± 0.19 | 1.04 ± 0.15 |
| TBLo | 7.27 ± 0.91 | 1.89 ± 0.27 | 3.60 ± 0.45 | 7.33 ± 1.02 | 1.96 ± 0.27 | 3.51 ± 0.52 | 7.16 ± 0.88 | 1.77 ± 0.25 | 3.74 ± 0.35 |
| TBM | 1.25 ± 0.19 | 1.95 ± 0.31 | 0.62 ± 0.17 | 1.27 ± 0.21 | 1.90 ± 0.30 | 0.65 ± 0.21 | 1.20 ± 0.19 | 2.03 ± 0.38 | 0.56 ± 0.02 |
| TFA | 0.27 ± 0.06 | 3.16 ± 0.66 | 0.08 ± 0.02 | 0.29 ± 0.06 | 3.04 ± 0.84 | 0.09 ± 0.01* | 0.24 ± 0.06 | 3.37 ± 0.14 | 0.07 ± 0.01* |
| TMJ | 1.51 ± 0.20 | 2.97 ± 0.52 | 0.51 ± 0.14 | 1.55 ± 0.20 | 2.91 ± 0.52 | 0.52 ± 0.13 | 1.44 ± 0.22 | 3.06 ± 0.63 | 0.48 ± 0.19 |
| TMI | 0.14 ± 0.03 | 0.65 ± 0.13 | 0.20 ± 0.05 | 0.14 ± 0.03 | 0.61 ± 0.12 | 0.23 ± 0.05 | 0.12 ± 0.01 | 0.73 ± 0.11 | 0.17 ± 0.03 |
| TRC | 0.78 ± 0.22 | 3.17 ± 0.56 | 0.22 ± 0.05 | 0.79 ± 0.25 | 3.24 ± 0.62 | 0.22 ± 0.06 | 0.75 ± 0.20 | 3.06 ± 0.56 | 0.23 ± 0.02 |
| TRT | 0.88 ± 0.15 | 2.20 ± 0.26 | 0.30 ± 0.08 | 0.90 ± 0.18 | 2.15 ± 0.31 | 0.30 ± 0.10 | 0.84 ± 0.08 | 2.28 ± 0.17 | 0.31 ± 0.01 |
| UNL | 0.35 ± 0.06 | 0.39 ± 0.13 | 0.92 ± 0.32 | 0.38 ± 0.05* | 0.37 ± 0.11 | 1.05 ± 0.33 | 0.29 ± 0.04* | 0.43 ± 0.18 | 0.71 ± 0.21 |
Values followed by ‘*’ show significant differences (P < 0.05) between sexes in Student's t‐test. M, mass; Mfasc, mean fascicle length; PCSA, physiological cross‐sectional area.
Comparative data between muscle percentage mass for each functional group in relation to the total muscle mass of the limb, considering only the intrinsic muscles and according to the species in the order Carnivora are shown in Table 6. In L. gymnocercus, the elbow extensor muscles formed the functional group with the greatest mass (40.47%). The mass percentage of some functional groups is greater in some groups of species, as evidenced by the analysis of variance (Table 7). For example, canids have significantly more mass in elbow extensors, whereas Musteloidea have greater mass in muscles involved in digit flexion and supination.
Table 6.
Muscle percentage mass distribution according to the functional group, in different species of the order Carnivora
| Species | Source | Family | n | ExS | FlS | ExE | FlE | ExC | FlC | ExD | FlD | Sup | Pron |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lycalopex gymnocercus | Present study | Canidae | 8 | 14.18 | 20.35 | 40.47 | 6.71 | 3.18 | 3.88 | 2.29 | 7.53 | 0.29 | 1.12 |
| Cerdocyon thous | Present study | Canidae | 1 | 15.48 | 20.30 | 37.64 | 7.43 | 3.18 | 4.30 | 2.12 | 7.88 | 0.37 | 1.30 |
| Canis familiaris (mongrel) | Shahar & Milgram (2005) | Canidae | 4 | 13.11 | 20.73 | 40.56 | 6.78 | 3.07 | 5.39 | 2.08 | 7.42 | 0.28 | 0.58 |
| Canis familiaris (Greyh.) | Williams et al. (2008) | Canidae | 7 | 14.38 | 21.57 | 44.56 | 5.80 | 2.49 | 3.37 | 2.62 | 4.85 | 0.00 | 0.37 |
| Canis latrans | Feeney (1999) | Canidae | 1 | 11.80 | 20.82 | 39.92 | 7.04 | 3.28 | 5.28 | 1.82 | 9.11 | 0.40 | 0.55 |
| Vulpes vulpes | Feeney (1999) | Canidae | 5 | 14.08 | 20.61 | 40.53 | 7.24 | 2.81 | 4.21 | 1.58 | 7.54 | 0.53 | 0.88 |
| Urocyon cinereoargenteus | Feeney (1999) | Canidae | 4 | 16.06 | 20.63 | 39.56 | 6.10 | 3.32 | 4.54 | 1.57 | 6.81 | 0.52 | 0.87 |
| Martes pennanti | Feeney (1999) | Mustelidae | 4 | 9.09 | 14.07 | 32.73 | 10.90 | 4.69 | 9.39 | 2.01 | 11.40 | 3.02 | 2.68 |
| Taxidea taxus | Moore et al. (2013) | Mustelidae | 6 | 7.23 | 12.06 | 36.04 | 3.59 | 2.29 | 6.30 | 5.82 | 20.18 | 3.27 | 3.23 |
| Aonyx cinerea | Macalister (1870) | Mustelidae | 1 | 9.10 | 16.24 | 34.58 | 6.24 | 4.94 | 7.31 | 3.64 | 9.88 | 4.94 | 3.13 |
| Procyon lotor | Feeney (1999) | Mustelidae | 2 | 10.45 | 20.89 | 30.21 | 12.51 | 1.56 | 6.22 | 2.85 | 9.59 | 3.11 | 2.59 |
| Galictis cuja | Ercoli et al. (2015) | Mustelidae | 2 | 12.86 | 11.32 | 38.04 | 7.68 | 3.51 | 7.57 | 3.80 | 10.17 | 2.62 | 2.44 |
| Lynx lynx | Viranta et al. (2016) | Felidae | 4 | 11.84 | 20.09 | 31.20 | 10.10 | 3.92 | 5.69 | 4.39 | 9.55 | 0.95 | 2.27 |
| Acinonyx jubatus | Hudson et al. (2011) | Felidae | 8 | 17.81 | 24.42 | 33.03 | 9.41 | 1.02 | 3.06 | 1.94 | 6.91 | 1.20 | 1.20 |
| Leopardus pardalis | Julik et al. (2012) | Felidae | 1 | 14.25 | 24.60 | 26.77 | 9.93 | 2.91 | 5.07 | 3.53 | 8.11 | 2.93 | 1.90 |
| Felis nigripes | Cuff et al. (2016) | Felidae | 1 | 15.37 | 25.40 | 29.54 | 8.96 | 1.92 | 5.14 | 3.92 | 6.61 | 0.99 | 2.15 |
| Felis silvestris | Cuff et al. (2016) | Felidae | 1 | 13.41 | 20.14 | 32.17 | 9.58 | 3.62 | 5.42 | 4.09 | 8.60 | 0.92 | 2.05 |
| Caracal caracal | Cuff et al. (2016) | Felidae | 1 | 16.35 | 21.33 | 31.17 | 8.62 | 3.04 | 5.76 | 2.88 | 7.57 | 0.91 | 2.37 |
| Panthera uncia | Cuff et al. (2016) | Felidae | 1 | 12.83 | 22.86 | 32.37 | 8.87 | 3.01 | 4.05 | 4.15 | 8.12 | 1.56 | 2.18 |
| Panthera onca | Cuff et al. (2016) | Felidae | 1 | 17.17 | 18.84 | 31.50 | 7.17 | 3.61 | 8.38 | 2.70 | 5.82 | 2.34 | 2.47 |
| Panthera tigris | Cuff et al. (2016) | Felidae | 1 | 14.08 | 21.00 | 32.16 | 10.24 | 1.83 | 4.07 | 3.47 | 7.18 | 2.26 | 3.73 |
| Panthera leo | Cuff et al. (2016) | Felidae | 1 | 11.49 | 20.54 | 31.98 | 8.22 | 3.20 | 7.21 | 5.99 | 5.79 | 3.24 | 2.34 |
| Hyaena hyaena | Spoor & Badoux (1986a) | Hyaenidae | 1 | 14.70 | 26.70 | 25.35 | 7.89 | 3.81 | 6.28 | 4.08 | 10.36 | 0.27 | 0.56 |
ExS, extensors of the shoulder joint; FlS, flexors of the shoulder joint; ExE, extensors of the elbow joint; FlE, flexors of the elbow joint; ExC, extensor of the carpal joint; FlC, flexors of the carpal joint; ExD, extensors of the phalangeal joints; FlD, flexors of the phalangeal joints; Sup, supinators; Pron, pronators.
Table 7.
Mean percentage mass of intrinsic muscles for each functional group of the thoracic limb of specimens in the order Carnivora, grouped by canids, musteloidea and feliformia
| Functional group | Canids (n = 7) | Musteloidea (n = 5) | Feliformia (n = 11) |
|---|---|---|---|
| ExS (%) | 14.2ª | 9.7b | 14.5ª |
| FlS (%) | 20.7ª | 14.9b | 22.4ª |
| ExE (%) | 40.5ª | 34.4b | 30.6c |
| FlE (%) | 6.7ª | 8.2ab | 9.0b |
| ExC (%) | 3.1ª | 3.4ª | 2.9ª |
| FlC (%) | 4.4ª | 7.4b | 5.5ac |
| ExD (%) | 2.0a | 3.6b | 3.7b |
| FlD (%) | 7.3ª | 12.2b | 7.7ª |
| Sup (%) | 0.3ª | 3.4b | 1.6c |
| Pron (%) | 0.8ª | 2.8b | 2.1b |
| Total (%) | 100 | 100 | 100 |
Values followed by different letters in the same row show statistically significant differences according to Tukey test (P < 0.05).
Cluster analysis with the minimum variance method yielded a dendrogram that demonstrated that muscle percentage mass distribution in the functional groups is clearly associated with the phylogenetic proximity between the species (Fig. 8).
Figure 8.
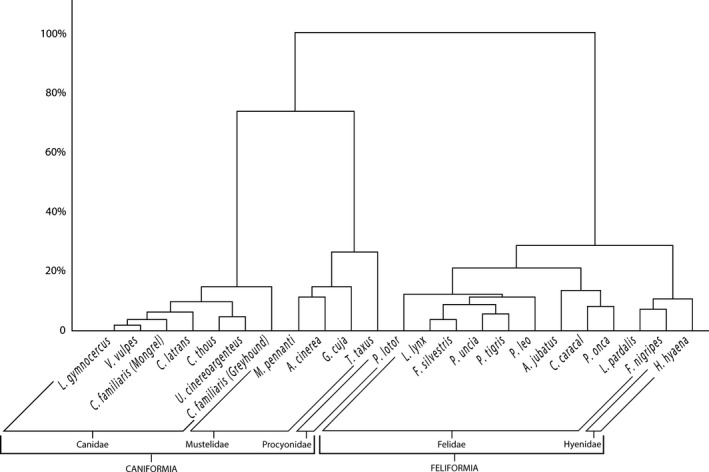
Dendrogram obtained from cluster analysis with Ward's minimum variance method and Euclidian distance based on the percentage mass of muscles according to the functional group in specimens of the Carnivora order.
Discussion
Descriptive and comparative aspects
The anatomical position of the muscles in the thoracic limb of L. gymnocercus is very similar to that described for domestic dogs in textbooks (Clair, 1986; Nickel et al. 1986; Evans & DeLahunta, 2013). Therefore, in the descriptive analysis, the present discussion focused on those characteristics that had comparative, phylogenetic and/or functional meaning for the order Carnivora. The intraspecific differences observed in the dissection procedures were emphasized. Muscles that were identical to those of domestic dogs or had little comparative importance were not discussed in detail.
The anatomical comparison of the muscles is a challenging task due to the variation in terminology. Older reports (Macalister, 1870; Windle, 1888; Windle & Parsons, 1897; Carlsson, 1905) employed a nomenclature that is very different from the current one. Even more recent studies that are rich in evolutionary and phylogenetical inferences preserve part of this nomenclature (Fisher et al. 2009; Julik et al. 2012; Ercoli et al. 2015). A large number of studies that have a more descriptive scope adopt the Nomina Anatomica Veterinaria that was current at that time (Barone, 1967; Leach, 1977; McClearn, 1985; Spoor & Badoux, 1986a; Feeney, 1999; Concha et al. 2004; Santos et al. 2010; Hudson et al. 2011; Moore et al. 2013; Carvalho & Souza Junior, 2014; García et al. 2015; Silva et al. 2015; Pereira et al. 2016; Viranta et al. 2016). Therefore, the present study adopted the nomenclature recommended by the ICVGAN (2017), and results were compared based on the interpretation of descriptive texts and illustrations in studies of different times and emphases.
In spite of the overall similarity in muscle anatomical position between L. gymnocercus and the domestic dog, some differences were observed: the muscle pectoralis profundus of L. gymnocercus showed three well‐defined parts (Fig. 9); the insertion tendon of the triceps brachii muscle (common to the four heads) was divided into two parts: a caudolateral one, containing the tendons for the long and lateral heads; and a medial one, including the tendons for the medial and accessory heads. The interflexorius muscle showed thin insertion tendons fused with those of the flexor digitorum superficialis muscle of digits II and III, although there are no obvious functional meanings for such differences.
Figure 9.
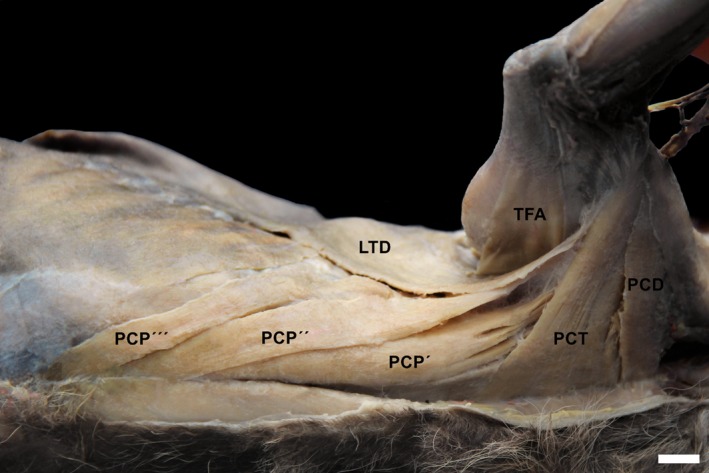
Photomacrograph of the muscles of the pectoral region of an adult specimen of Lycalopex gymnocercus evidencing the division of m. pectoralis profundus in three parts: carnial (PCP′), medial (PCP′′) and caudal (PCP′′′). PCD, m. pectoralis descendens; PCT, m. pectoralis transversus; TFA, m. tensor fasciae antebrachi; LTD, m. latissumus dorsi. Scale bar: 10 mm.
Intraspecific variations were found in several muscles of L. gymnocercus and are not new in Carnivora (Windle & Parsons, 1897; Julik et al. 2012; Ercoli et al. 2015). Fisher et al. (2009) identified a wide range of anatomical variations in Ailurus fulgens, and recommended the use of numerous samples to draw inferences on soft tissues.
Extrinsic muscles
The trapezius muscle of L. gymnocercus presented, invariably, a continuous fibrous band that was divided into two parts, a cervical and a thoracic one. In the domestic dog, this band is reported to be variable or, sometimes, absent (Sharir et al. 2006; Evans & DeLahunta, 2013). This band, called the fibrous interval by Windle & Parsons (1897), was recognized in Carnivora, such as P. lotor (Windle & Parsons, 1897), V. vulpes (Feeney, 1999) and G. cuja (Ercoli et al. 2015). Ercoli et al. (2015) emphasized the need to investigate this anatomical characteristic, as it may have phylogenetic importance in the Carnivora order.
The muscle pectoralis profundus showed three parts: a main, cranial one that originated from the manubrium to the penultimate sternebrae; an intermediate one that originated from the penultimate sternebrae to the xiphoid process; and a caudal, smaller one that originated from the xiphoid process to 1 cm caudal to it. In the domestic dog, only two parts are known: a main (deep) one, and a smaller one (superficial or abdominal; Evans & DeLahunta, 2013). Carlsson (1905) also illustrated three parts of the pectoralis profundus muscle in Otocyon megalotis. However, the intermediate part was larger. Spoor & Badoux (1986a) and Fisher et al. (2009) reported three parts for the pectoralis profundus muscle, although with a small, band‐shaped abdominal portion located deep to the caudal part in H. hyaena and A. fulgens, respectively. Ercoli et al. (2015) speculated that the body mass and strong arm effort were actually the main factors to be considered. The preponderance of a unique muscular mass observed in some large carnivorans could be related to a greater adduction force or propulsion at the cost of the precision of the movements. Considering the similar locomotor habits, the more complex pectorals muscles and smaller body mass of L. gymnocercus, in comparison with Canis, fit with this morpho‐functional relationship.
The majority of the specimens analyzed (52.4%) lacked the rhomboideus capitis muscle, whose function is to elevate the limb, cranially rotate the scapula and aid in the lateral movements of the neck during prey laceration. Among canids, it is described as a delicate structure in V. vulpes, C. latrans and U. cinereoargenteus (Feeney, 1999), and the domestic dog (Evans & DeLahunta, 2013). In L. gymnocercus, it seems to be disappearing with the loss of its main ancestral function and the acquisition of omnivore habits, agreeing with the criteria for a vestigial structure (Senter & Moch, 2015). In fact, it is well developed in species that have a hypercarnivore diet and carry out vigorous movements with the neck to lacerate the prey (Ercoli et al. 2015). Some mustelids, such as M. pennanti and G. cuja, develop a fourth rhomboid muscle, the rhomboideus profundus (Feeney, 1999; Ercoli et al. 2015).
The muscle serratus ventralis cervicis in L. gymnocercus originated in the transversal processes from C4 to C7, forming four clear divisions. This origin is similar to that reported by some specimens of Civettictis civetta, Genetta tigrina and Eira barbara by Windle & Parsons (1897). In the domestic dog, its origin can reach cranially the transverse processes of C4 or C3 (Nickel et al. 1986; Evans & DeLahunta, 2013) or even from C2 (Sharir et al. 2006) and yield five or six divisions. In Carnivora, such as A. fulgens, the origin is as cranial as the wing of the atlas (Fisher et al. 2009) and, in others, such as L. lynx, from C5 (Viranta et al. 2016). No reports were found in the literature on a thin strip originating in the serratus ventralis cervicis towards the nuchal crest observed in two specimens of L. gymnocercus that did not show the rhomboideus capitis muscle. This thin strip of the serratus ventralis cervicis muscle may be homologous to rhomboideus capitis.
Intrinsic muscles
A divergence in nomenclature was identified for the coracobrachialis and articularis humeri muscles. The ICVGAN (2017) and authors of textbooks in veterinary anatomy (Clair, 1986; Nickel et al. 1986; Liebich et al. 2016) report a coracobrachialis muscle that originates from a tendon on the coracoid process and inserts on the tuberositas teres major or distally to it. Lycalopex gymnocercus shows the coracobrachialis muscle that is described in all families of the Carnivora order, except for the viverrid Ginetta sp. (Windle & Parsons, 1897) and some mustelids (Fisher et al. 2009). Thus, the coracobrachialis muscle has been considered a plesiomorphic trait in the carnivorans (Ercoli et al. 2015).
The biceps brachii muscle of L. gymnocercus showed a single belly. Among Carnivora, only the families Ailuridae, Ursidae, Procyonidae and Viverridae include species that have an additional short head in the biceps brachii muscle (Windle, 1888; Windle & Parsons, 1897; Fisher et al. 2009). Besides, in L. gymnocercus this muscle inserted via two tendons, one on the radius and one on the ulna. In canids, the muscle can insert onto both bones (Evans & DeLahunta, 2013; Pereira et al. 2016) or solely on the ulna (Feeney, 1999). In felids and musteloids species, the muscle usually inserts exclusively on the radius (Barone, 1967; Nickel et al. 1986; Feeney, 1999; Concha et al. 2004; Julik et al. 2012; Ercoli et al. 2015). In the case of L. gymnocercus, the insertion of the biceps brachii muscle on the ulna reflects its main function as an elbow flexor, whereas the insertion on the radius enables some degree of supination.
The triceps brachii muscle presented four heads, as described for domestic carnivorans in general (Clair, 1986; Nickel et al. 1986), and for wild ones, such as H. hyaena (Spoor & Badoux, 1986a), P. lotor, V. vulpes, C. latrans and U. cinereoargenteus (Feeney, 1999), Nasua nasua (Santos et al. 2010) and Chrysocyon brachyurus (Pereira et al. 2016). The proximal subdivision of caput longum was considered to be an additional head by Windle & Parsons (1897), making it difficult to draw a comparison on the number of heads. Fisher et al. (2009) and Viranta et al. (2016) describe five heads in A. fulgens and L. lynx, respectively. In these studies, the authors subdivide caput mediale into two parts. According to Ercoli et al. (2015), the presence of five heads, including a caput angulare that originates in the caudal angle of the scapula, is a characteristic of mustelids and mephitids.
The insertion tendon that is common to the four heads of the muscle triceps brachii was subdivided into a part composed by the tendons of the long and lateral heads, and another, a medial one, composed by the tendons of the medial and accessory heads. This arrangement is similar to the one described in domestic cats but not in dogs (Nickel et al. 1986; Evans & DeLahunta, 2013).
The muscle brachioradialis was found bilaterally in 16 (76.2%) specimens, unilaterally in three (14.3%) and absent in two (9.5%) of the 21 individuals that were dissected. In one of the specimens, the insertion was on the middle third of the extensor carpii radialis. These results are similar to those previously reported on the fact that the brachioradialis muscle tends to be reduced or absent in canids and hyaenids (Spoor & Badoux, 1986a; Feeney, 1999), and well developed in the other families that need supination (Souza Junior et al. 2015).
In domestic carnivores, the extensor carpi radialis muscle is reported to be composed by two muscles: extensor carpi radialis longus (insertion on metacarpal II); and extensor carpi radialis brevis (insertion on metacarpal III; Nickel et al. 1986). According to Windle & Parsons (1897), the degree of separation or fusion of both muscles is an interesting comparative issue to analyze and is encountered in canids (Feeney, 1999). In L. gymnocercus the fusion of both muscles reflects a tendency of simplification in specialized cursorial species, as the limb is restricted to movements in the sagittal plane.
In one L. gymnocercus specimen, three insertion tendons were observed in the extensor carpi radialis muscle and, in a male, the insertion tendon of the long part was bifurcated near the insertion on metacarpal II. Additional variations in the insertion of this muscle have been reported in carnivorans (Windle & Parsons, 1897; Evans & DeLahunta, 2013) without any clear functional meaning.
In L. gymnocercus, the tendons of the extensorum digitorum lateralis muscle were more delicate than those of the muscle extensorum digitorum communis. This finding corroborates the report by Feeney (1999) that showed that this difference is clear in canids, whereas in M. pennanti and P. lotor, the tendons may be equally strong. The mass ratio of the extensorum digitorum communis and extensorum digitorum lateralis muscle was about 2 : 1 in L. gymnocercus, similar to the findings in the canids C. latrans, V. vulpes and U. cinereoargenteus; it is greater than in M. pennanti and P. lotor (Feeney, 1999). The extensor digitorum lateralis muscle may possibly be more developed in Musteloidea compared with canids, as the mustelids need more independent movements in each digit (Feeney, 1999).
The site of insertion of the supinator muscle on the radius of carnivorans is the characteristic that is most commonly analyzed in this muscle. In L. gymnocercus, it was inserted on the proximal third of the radius, reaching about 35% of the length of this bone, and confirming the findings of a previous report (Silva et al. 2015). This type of insertion was identical to the description by Feeney (1999) on other specialized cursorial canids, V. vulpes and C. latrans (34%), and by Silva et al. (2015) on C. thous (40%). In the canid U. cinereoargenteus, a tree climber, the muscle reaches almost half of the diaphysis of the radius (47%; Feeney, 1999). In the domestic dog, it was described as reaching only the proximal fourth of the radius (Nickel et al. 1986; Evans & DeLahunta, 2013). Therefore, the reach of this muscle may reflect a demand for external rotation of the hand in the different species. The demand is less in the domestic dog, intermediate in wild, specialized cursorial canids, and greater in the climber canid U. cinereoargenteus.
In non‐canid Carnivora, the supinator was described as little developed and covering one‐third of the diaphyses in H. hyaena (Windle & Parsons, 1897; Spoor & Badoux, 1986a) and A. jubatus (Hudson et al. 2011); about 40% of the diaphysis in P. lotor (Feeney, 1999); about 42% of the diaphysis in Procyon cancrivorus (Silva et al. 2015); the proximal half of the diaphysis in N. nasua (Santos et al. 2010), Meles sp. (Windle & Parsons, 1897), Puma concolor (Concha et al. 2004) and A. fulgens (Fisher et al. 2009); two‐thirds in P. leo (Barone, 1967), L. pardalis (Julik et al. 2012) and G. cuja (Ercoli et al. 2015); between two‐thirds and three‐quarters in Ursus americanus (Windle & Parsons, 1897); and three‐quarters in Mustela putorius, Lutra lutra (Windle & Parsons, 1897), M. pennanti (Feeney, 1999), T. taxus (Moore et al. 2013) and L. lynx (Viranta et al. 2016). Therefore, the supinator muscle is more developed in non‐canid Carnivora, especially in non‐cursorial or hand dexterous species. Among these, it is still more developed in those species that use the thoracic limb to swim and capture larger prey. Although A. jubatus feeds on larger animals, the muscle does not seem to be extremely expressive due to the prioritization of movements on the sagittal plane for high speed.
The extensor digiti I and II muscle of L. gymnocercus showed variations in its insertion. One male L. gymnocercus showed a delicate tendinous contribution to the insertion on digit III, a variation considered occasional in the domestic dog (Nickel et al. 1986; Evans & DeLahunta, 2013). In another female specimen, there were no defined insertion tendons, but an aponeurosis that was fused to the tendon of the extensor digitorum communis muscle. This variation was described in a C. thous specimen (García et al. 2015), and may reflect a joint action of both muscles as isolated extension movements of the fingers are not necessary.
In L. gymnocercus the pronator teres muscle covered 48% of the length of the radius, and was, therefore, located distally to the supinator muscle (Silva et al. 2015). In the other canid species, its covering length does not exceed half of the radius (Windle & Parsons, 1897; Feeney, 1999; Evans & DeLahunta, 2013; Silva et al. 2015).
Among non‐canids, the pronator teres muscle was observed up to the proximal third of the radius only in H. hyaena (Spoor & Badoux, 1986a). It may be observed that the muscle is shorter in canids, a little more developed in felids, viverrids and hyaenids, and very significant in ursids, procyonids and mustelids (Windle & Parsons, 1897; Barone, 1967; Feeney, 1999; Moore et al. 2013; Silva et al. 2015; Viranta et al. 2016). Therefore, more distally inserted pronator teres muscles can be associated with the need for hand rotation in carnivoran species. However, other anatomical characteristics, besides the point of insertion, need to be considered for the pronation movement to become more or less relevant. For example, in musteloids, the tendon of origin of the pronator teres muscle is located more proximally with respect to the center of rotation of the elbow than in the canids (Feeney, 1999), a feature that determines a more developed muscle also proximally. Although some hyaenids and canids species may present a more distal insertion of this muscle, the skeletal conformation of the forearm, with the less curved radius and narrower interosseous space, is imperative to prevent a relevant rotation movement of the hands.
The flexor digitorum superficialis muscle in L. gymnocercus was inserted in the middle phalanx of digits II–V, similar to the description in domestic dogs (Nickel et al. 1986; Evans & DeLahunta, 2013) and wild canids (Feeney, 1999). However, Windle & Parsons (1897), when referring to the flexor sublimis digitorum muscle, reported in one dog insertions only on digits II, III and IV. Some authors called it the palmaris longus muscle and used the term flexor digitorum superficialis muscle for the interflexorius muscle (Fisher et al. 2009; Julik et al. 2012; Ercoli et al. 2015). This difference in nomenclature makes it difficult for comparative aspects to be analyzed, as recognized by Ercoli et al. (2015). The ICVGAN (2017) does not mention the palmaris longus muscle; this nomenclature is adopted from human anatomy for a muscle that also originates in the medial epicondyle of the humerus but is inserted on the retinaculum and palmar aponeurosis (Martini et al. 2009). In non‐canids, the insertion of the flexor digitorum superficialis muscle occurs through a varied distribution of tendons to the middle phalanges of digits I–V, although the digits III and IV invariably receive tendons (Windle & Parsons, 1897; Barone, 1967; Leach, 1977; McClearn, 1985; Nickel et al. 1986; Spoor & Badoux, 1986a; Feeney, 1999; Concha et al. 2004; Fisher et al. 2009; Santos et al. 2010; Julik et al. 2012; Moore et al. 2013; Ercoli et al. 2015; Viranta et al. 2016). This should happen because these digits are longer and axially located in the hands.
The pronator quadratus muscle originated on the ulna and inserted on the radius, occupying the interosseous space throughout its extension. This reach of the muscle in L. gymnocercus is typical in canids (Feeney, 1999; Evans & DeLahunta, 2013) and hyaenids (Windle & Parsons, 1897; Spoor & Badoux, 1986a), species that have a narrower interosseous space and, therefore, perform a more subtle pronation. In the other families (felids, ursids, ailurids, procyonids, viverrids and mustelids), this muscle tends to be thicker, to fill a wider interosseous space, and to be placed from half or distal third of the forearm (Windle & Parsons, 1897; Barone, 1967; Leach, 1977; Feeney, 1999; Fisher et al. 2009; Julik et al. 2012; Moore et al. 2013; Ercoli et al. 2015; Viranta et al. 2016). This position closer to the hand, which needs to be internally rotated, gives some mechanical advantage to a more relevant pronation.
The flexor digitorum brevis muscle of L. gymnocercus was recognized as a very delicate fleshy muscle adhered to the palmar aspect of the tendon of the flexor digitorum superficialis muscle to digit V, exactly as described in the domestic dog (Nickel et al. 1986; Evans & DeLahunta, 2013), C. thous (Carvalho & Souza Junior, 2014), H. hyaena (Spoor & Badoux, 1986a) and A. fulgens (Fisher et al. 2009). Its presence was not always identified in the dissections, and it was recognized in 15 of the 21 (71.4%) specimens that were dissected, perhaps because the fleshy part could not have been seen in all the cases. Whenever present, its tendon inserted in the proximal phalanx of digit V. Despite its close relation with the superficial digital flexor muscle, it is considered a single muscle according to the ICVGAN (2017), as it can be entirely isolated from origin to insertion by dissection. Due to its small size and differences in nomenclature, the presence of this muscle is difficult to determine from the literature. In felids, it joins the tendon of the flexor digitorum superficialis muscle to digits IV and V and, occasionally, to digit III (Nickel et al. 1986; Julik et al. 2012). Ercoli et al. (2015) reported that the muscle was absent in G. cuja, and considered it absent in all mustelids.
In L. gymnocercus, three lumbricales muscle were found, except in two females that showed only two muscles. In the domestic dog, the most common pattern is three muscles (Nickel et al. 1986; Evans & DeLahunta, 2013), as described in other canids, such as V. vulpes, C. latrans, U. cinereoargenteus (Feeney, 1999) and C. thous (Carvalho & Souza Junior, 2014), one viverrid C. civetta, one hyaenid Proteles cristata, and the mustelids L. lutra, A. cinerea and M. putorius (Windle & Parsons, 1897). In H. hyaena, two muscles were also reported (Spoor & Badoux, 1986a). The species in the Carnivora order in which four lumbricales muscles are described are domestic felids (Nickel et al. 1986), as well as P. leo (Barone, 1967), L. pardalis (Julik et al. 2012), C. civetta, Genetta genetta, Herpestes edwardsi, Hyaena hyaena, Crocuta crocuta, Ursus maritimus, U. americanus, P. lotor, Nasua sp., Potos flavus, Meles meles, L. lutra (Windle & Parsons, 1897), M. pennanti (Feeney, 1999), G. cuja (Ercoli et al. 2015) and A. fulgens (Fisher et al. 2009). Based on these observations, it may be inferred that the lumbricales muscles tend to be more numerous in species that are more dependent on their hands.
Among specific muscles to digit I (thumb), L. gymnocercus presented the same ones found in domestic carnivorans (Nickel et al. 1986). In a female specimen (8433), only the adductor and abductor muscles were found, and the flexor digiti I brevis was not observed. In a male (8590), the abductor digiti I brevis muscle was absent. The intraspecific absence of this muscle was reported in a domestic dog by Windle & Parsons (1897).
Among the muscles that act upon digit V, the muscles abductor digiti V, adductor digiti V and flexor digiti V were found in L. gymnocercus. In two specimens, the flexor digiti V muscle was not found. These three muscles are present in all species in the order Carnivora that have been analyzed (Barone, 1967; Nickel et al. 1986; Fisher et al. 2009; Julik et al. 2012; Ercoli et al. 2015), except for H. hyaena, which does not have the flexor digiti V muscle (Spoor & Badoux, 1986a). Feeney (1999) emphasized that the abductor digiti V is stronger in M. pennanti and P. lotor than in the canids V. vulpes, C. latrans and U. cinereoargenteus. Among the canids, it is stronger in the later one, which might spread the fingers to climb trees.
Muscle architecture
If antimeric symmetry is assumed, it may be considered that muscles of the thoracic limbs represent 12.7% of the body mass in L. gymnocercus. This percentage was identical to that found in the present study in C. thous (12.7%), but smaller than that estimated by Williams et al. (2008) for Greyhound dogs (18.6%); Hudson et al. (2011) for A. jubatus (15.1%); Cuff et al. (2016) for F. nigripes (18.5%), L. pardalis (14.4%), P. tigris (16.6%), C. caracal (16.2%), P. onca (16.1%) and P. leo (14.3%). However, it was greater than that estimated by Cuff et al. (2016) for F. silvestris (7.1%) and P. uncia (11.4%).
Although the sum of the mass of thoracic limb muscles in male specimens of L. gymnocercus was, on average, greater than in females, this difference was not significant. The comparison between normalized architectural data (mass, mean fascicle length and PCSA) also showed few muscles with significant differences between adult males and females. This finding is in agreement with the observation that the architecture of a given muscle is extremely consistent among individuals of the same species (Lieber & Fridén, 2000). The normalized value of PCSA was greater in carpal flexors in males, maybe because their action is related to propulsion and prey capture. Male home ranges for L. gymnocercus are bigger than those of females (Maffei et al. 2007). Males with contiguous living areas have much smaller overlap areas than females with contiguous areas (Luengos Vidal et al. 2012). Because of this, it may be suggested that males have to move further to patrol their territories and keep other males out of their territories.
The comparison between the young specimen and the adults showed that the ratios between the mass of functional groups were similar. On the other hand, it was estimated that from 4 months old to the adult phase, there is a mass gain of 16% in the thoracic limb in relation to body mass. This growth may be justified by the demand for long‐distance foraging and prey capture when the offspring leaves the den and becomes independent (Lucherini & Luengos Vidal, 2008). However, this proposal should be considered as a preliminary result due to the minimal representation of the younger stages in the present study.
The long head of the triceps brachii muscle showed the greatest mass among all muscles (intrinsic and extrinsic) of the thoracic limb. This was also observed in mongrel dogs by Shahar & Milgram (2005), and in A. jubatus by Hudson et al. (2011). This muscle also shows the greatest mass among the intrinsic muscle in A. cinerea (Macalister, 1870), H. hyaena (Spoor & Badoux, 1986a), Greyhound dogs (Williams et al. 2008), G. cuja (Ercoli et al. 2015), and in several felids (Julik et al. 2012; Cuff et al. 2016). Compared with the other heads of the triceps brachii muscle, the long head seems to be more developed in canids and hyaenids than in other families (Spoor & Badoux, 1986a; Feeney, 1999; Moore et al. 2013). The triceps brachii muscle is the most important one in elbow extension during high‐speed locomotion (Julik et al. 2012). The muscle is voluminous, shows great mass and high PCSA, with low AI, near 0.30. These architectural characteristics reflect a muscle that is able to generate great force, which was demonstrated to be crucial to stabilize the elbow and shoulder joints on the ground during the support phase of walking, trotting and running (Goslow et al. 1981). Maintenance of the elbow extension in support phase seems to be essential to counteract the impact on the thoracic limb during maximum acceleration or running (Williams et al. 2008). Besides, the muscle is crucial in limb propulsion (Goslow et al. 1981).
Among the thoracic limb intrinsic muscles of L. gymnocercus, the greatest PCSA value was found in the subscapularis. Its great capacity to generate force is justified by its main function, which is medial stabilization of the glenohumeral joint, a spheroidal joint that does not have extracapsular ligaments (Evans & DeLahunta, 2013). Therefore, glenohumeral stability is ensured by the tendons of the muscles that have great PCSA, such as the subscapularis, infraspinatus, supraspinatus and biceps brachii. In addition, these muscles aid in restricting the movements of the shoulder articulation on the sagittal plane in specialized cursorial species.
The flexor digitorum profundus muscle, especially its humeral head, was the muscle in the antebrachial region that showed the greatest capacity to generate force. This finding is similar to that in mongrel (Shahar & Milgram, 2005), Greyhound dogs (Williams et al. 2008), and T. taxus (Moore et al. 2013), and different from the findings in A. jubatus (Hudson et al. 2011). Strikingly, in A. jubatus the flexor digitorum superficialis muscle was described as having the greatest PCSA value in this region. Especially with regard to the flexor muscles of the digits, some differences in nomenclature have been found for a long time among various studies. Therefore, this may result in inappropriate identification of some muscles by different authors, and a misleading result would occur in the comparisons of some non‐homologous muscles. Nevertheless, some comparative inferences about these muscles should be interpreted with caution. Spoor & Badoux (1986b) argued that the variety in the development of the long digital forelimb flexors in carnivorans represents the various intermediate stages between the morphology in man and in the dog, and proposed some changes in the nomenclature to better determine the correct homologies. However, in the present study it was preferred to adopt the official nomenclature for domestic mammals (ICVGAN 2017) because it has didactic advantages when comparing L. gymnocercus with domestic dog. Furthermore, the adoption of the ICVGAN (2017) favors the application of the anatomical knowledge to wild animal veterinary medicine procedures.
Among extrinsic muscles, the pectoralis profundus and latissimus dorsi showed the greatest mass and the highest AIs (0.51 and 0.63, respectively), similar to Greyhound dogs (Williams et al. 2008). This finding demonstrates that these muscles enable wide ranges of movement with fast contraction speed (Evans & DeLahunta, 2013). Both have an important role in limb retraction during change in gait, particularly for the pectoralis profundus muscle, which features great PCSA compared with the other extrinsic muscles, with decisive action in the propulsion of fast cursorial species, as it was explained in Greyhound dogs (Williams et al. 2008).
The greatest PCSA value among the extrinsic muscles was observed for the serratus ventralis thoracis muscle (5.47 cm2). Together with a low to moderate AI (0.37), its architectural data indicate a strong muscle that is able to support the limb connected to the trunk during the support phase. In fact, Carrier et al. (2006) determined that the serratus ventralis thoracis muscle is the main anti‐gravitational muscle in dogs, and its activity increased when mass was added to the trunk, and when the dogs ran downhill.
Although PCSA enables a very reasonable estimation on the force that the muscle is able to generate, few studies provide sufficient data for normalization and subsequent comparisons among species in the Carnivora order. The limitations in comparison are the lack of availability of data on extrinsic muscles (Moore et al. 2013), lack of knowledge on the body mass of the specimens (Shahar & Milgram, 2005; Hudson et al. 2011) or, less importantly, data that are restricted to a single specimen of each species (Cuff et al. 2016). In the present study, mean architectural data of L. gymnocercus were presented both as raw values and normalized in relation to the body mass of the specimens, in order to make future comparative inferences easier.
Another unfavorable aspect is that the determination of PCSA may be influenced by several methodological interferences pointed out by Lieber & Fridén (2000). For example, the pennation angle may show a wide variation between the superficial and the deeper part of the muscle, although the impact of the pennation angle in PCSA calculation occurs only in the few cases in which the angle is greater than 30 °.
If, on one hand, PCSA estimations are still scarce in Carnivora, determination of the mass of each muscle in the thoracic limb has been performed since the 19th century (Macalister, 1870) and is available in studies of several species in this order, either in percentage (Feeney, 1999; Ercoli et al. 2015) or in absolute values (Macalister, 1870; Shahar & Milgram, 2005; Williams et al. 2008; Hudson et al. 2011; Julik et al. 2012; Moore et al. 2013; Ercoli et al. 2015; Cuff et al. 2016). In a way, mass is one of the variables that interferes in PCSA values, that is, in the ability of the muscle to generate force.
Therefore, in this study, the percentage mass of 10 functional muscle groups was compared with the total mass of the intrinsic muscles (the body mass is not always informed in the available reports) in 22 species in the order Carnivora. These 10 functional groups were composed of muscles with both origin and insertion on the bones of the limb and acted specifically on the joints of the thoracic limb. This functional relevance, together with the limitation in data on extrinsic muscles for several species, determined the use of only intrinsic muscles in the calculations.
The significant discriminatory characteristics (P < 0.05) of canids, compared with specimens of the superfamily Musteloidea and suborder Feliformia, were: greater percentage mass in the group of elbow extensors and smaller in the group of elbow flexors, digit extensors, supinators and pronators. These findings are in accordance with the concept that cursorial, fast locomotion tends to concentrate muscles proximally and prioritize movements in the sagittal plane (Ewer, 1973; Feeney, 1999; Kardong, 2011). Based on this premise, canids concentrate a greater percentage of muscle mass proximally (82.1% of the mass of the intrinsic muscle acts on the shoulder and elbow) than the feliforms (76.5%) and musteoloid (67.2%) specimens. On the other hand, the percentage mass of the distal muscles (that act on the carpus and digits, and perform hand rotation) corresponds to 32.8% in Musteloidea, 23.5% in Feliformia specimens and only 17.9% in Canidae, which is due to the fact that mustelids and procyonids demand more force and manual ability to swim, capture prey in water and dig (Fabre et al. 2013).
The dendrogram (Fig. 8) generated from percentage of muscle mass of each species evidenced that the muscle mass of the functional groups reproduced phylogenetic proximity among more than 20 species, and was even superposed to the functional aspect. The smaller Euclidean distances among species involved C. latrans and Canis familiaris, and L. gymnocercus and V. vulpes, animals that are phylogenetically close, and perform similar movements. The greatest distance was observed between T. taxus and A. jubatus, two phylogenetically distant species, the former with fossorial habits and the second with fast cursorial habits (Hunter, 2011).
The dendrogram showed proximity between canids, and Greyhound dogs were the one placed further, possibly because of the artificial selection for fast locomotion. Canids were the most homogenous group of species in relation to the percentage mass of the muscles grouped by function. Mustelids also showed species grouping based on the similarity of muscle distribution. In spite of the differences in movement, the dendrogram showed mustelids near canids, which is in accordance with the phylogenetic proximity criterion, as both are part of the suborder Caniformia (Eizirik et al. 2010).
The members of the suborder Feliformia formed a third isolated group. Different from what was initially supposed, H. hyaena, a species whose limb use and external conformation are similar to canids, appeared close to felids. Again, grouping based on distribution of mass reflected phylogenetic proximity instead of superficial perception of limb conformation, as H. hyaena belongs to the suborder Feliformia (Eizirik et al. 2010).
Among the 22 species that were compared, the only case in which approximation based on the distribution of muscle percentage mass was different from phylogenetic expectations was for P. lotor. This species was grouped with felids, whereas in evolutionary terms, procyonids are closer to mustelids (Ewer, 1973). The explanation for this finding was that, compared with mustelids, P. lotor presented more mass in elbow and shoulder flexors and less in elbow and carpus extensors, making it closer to felids. In this specific case, the functional similarity, mainly in terms of climbing ability, places P. lotor closer to felids and further from most mustelids. Analysis of complete architectural data may aid in the understanding of the proximity of this species with felids.
Last, it may be concluded that L. gymnocercus has muscles in the thoracic limb adapted to fast cursorial locomotion that prioritize movement in the sagittal plane instead of elaborate manual movements. For example, the insertion of the cleidobrachialis muscle on the humerus, the single belly of biceps brachii, the fusion of the parts of the extensor carpi radialis muscle, the proximal insertion of the supinator muscle, the insertion of the flexor digitorum superficialis muscle on digits II–V, the complete separation of the heads in flexor carpi ulnaris and the use of the whole interosseous space by the pronator quadratus muscle are characteristics that are conserved in canids. Based on the comparison of multivariate analyses, it may be suggested that, at least in relation to the mass of the muscles in the thoracic limb, phylogeny imposes limitations to morphofunctional characteristics, even in species that are subjected to similar ecological pressures. Data for other carnivorans may more comprehensively validate these findings.
Author's contributions
PSJ conceived the study; PSJ and CBK collected the samples; PSJ, WVS, LMPRS, NCC and ECS performed the dissections and collected architectural data; ECS made photomacrographs and image editions; PSJ, CBK, MAF and ALQS analysed data and contributed in discussion section; ALQS supervised the study; PSJ drafted the study design and wrote the manuscript, which was co‐edited with all co‐authors.
References
- Alexander RM (2006) Consequences of size differences In: Principles of Animal Locomotion. (ed. Alexander RM.), pp. 53–67. Princeton: Princeton University Press. [Google Scholar]
- Andersson K, Werdelin L (2003) The evolution of cursorial carnivores in the tertiary: implications of elbow‐joint morphology. Proc Biol Sci 270, S163–S165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone R (1967) La Myologie Du Leon (Panthera leo). Mammalia 31, 459–514. [Google Scholar]
- Carlsson A (1905) Ist Otocyon caffer Die Ausgangsform Des Hundegeschlechts Oder Nicht? Zool Jahrb Abt Syst Geogr Biol Tier 22, 717–754. [Google Scholar]
- Carrier DR, Deban SM, Fischbein T (2006) Locomotor function of the pectoral girdle ‘muscular sling’ in Trotting Dogs. J Exp Biol 209, 2224–2237. [DOI] [PubMed] [Google Scholar]
- Carvalho NC, Souza Junior P (2014) Disposição Anatômica dos músculos digitais da mão em Cerdocyon thous (Linnaeus, 1766) In: Anais do XLI Congresso Brasileiro de Medicina Veterinária. SOVERGS: Gramado; Available at: http://sovergs.com.br/site/conbravet2014/artigos/trabalhos_1719.htm (Accessed: 06/02/2017) [Google Scholar]
- Clair LES (1986) Músculos do Carnívoro In: Anatomia dos Animais Domésticos, 5th edn (ed. Getty R.), pp. 1416–1444. Rio de Janeiro: Guanabara Koogan. [Google Scholar]
- Concha I, Adaro L, Borroni C, et al. (2004) Consideraciones anatómicas sobre la musculatura intrínseca del miembro torácico del Puma (Puma concolor). Int J Morphol 22, 121–125. [Google Scholar]
- Crespo JA (1971) Ecología del Zorro Gris Dusicion gymnocercus antiquus (Ameghino) en la provincia de La Pampa. Rev Mus Argent Ci Nat Ecol 5, 147–205. [Google Scholar]
- Cuff AR, Sparkes EL, Randau M, et al. (2016) The scaling of postcranial muscles in cats (Felidae) I: forelimb, cervical, and thoracic muscles. J Anat 229, 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp SL, Suryanarayanan S, Murray WM, et al. (2001) Architecture of the rectus abdominis, quadratus lumborum, and erector spinae. J Biomech 34, 371–375. [DOI] [PubMed] [Google Scholar]
- Depedrini JS, Campos RA (2003) Systematic study of the brain base arteries in the Pampas Fox (Dusicyon gymnocercus). Braz J Morphol Sci 20, 181–188. [Google Scholar]
- Depedrini JS, Campos RA (2007) Systematization, distribution and territory of the caudal cerebral artery on the surface of the brain in Pampas Foxes (Pseudalopex gymnocercus). Braz J Morphol Sci 24, 126–136. [Google Scholar]
- Eizirik E, Murphy WJ, Koepfli KP, et al. (2010) Pattern and timing of diversification of the mammalian order carnivora inferred from multiple nuclear gene sequences. Mol Phylogenet Evol 56, 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercoli MD, Álvarez A (2016) A novel series of forepaw muscles for mammals observed in the Patagonian weasel Lyncodon patagonicus . J Mammal 97, 1295–1303. [Google Scholar]
- Ercoli MD, Álvarez A, Stefanini MI, et al. (2015) Muscular anatomy of the forelimbs of the Lesser Grison (Galictis cuja) and a functional and phylogenetic overview of mustelidae and other Caniformia. J Mamm Evol 22, 57–91. [Google Scholar]
- Evans HE, DeLahunta A (2013) Miller's Anatomy of the Dog, 4th edn. St Louis: Saunders Elsevier. [Google Scholar]
- Ewer FG (1973) The Carnivores. Ithaca: Cornell University Press. [Google Scholar]
- Fabre AC, Cornette R, Slater G (2013) Getting a grip on the evolution of grasping in musteloid carnivorans: a three‐dimensional analysis of forelimb shape. J Evol Biol 26, 1521–1535. [DOI] [PubMed] [Google Scholar]
- Fabre AC, Cornette R, Goswami A (2015) Do constraints associated with the locomotor habitat drive the evolution of forelimb shape? A case study in musteloid carnivorans. J Anat 226, 596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney S (1999) Comparative osteology, myology and locomotor specializations of the fore and hind limbs of the North American Foxes Vulpes vulpes and Urocyon cinereoargenteus. Dissertation of Biology Department: University of Massachusets Amherst.
- Fisher RE, Adrian B, Barton M, et al. (2009) The phylogeny of the red panda (Ailurus fulgens): evidence from the forelimb. J Anat 215, 611–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García JFV, Pérez JSE, Buitrago CAS (2015) Descripción Anatómica del Músculo Extensor del I y II Dedo de la Mano del Zorro Perruno (Cerdocyon thous Linnaeus, 1766). Int J Morphol 33, 1455–1459. [Google Scholar]
- Goslow GE Jr, Seehermanf HJ, Taylor CR, et al. (1981) Electrical activity and relative length changes of dog limb muscles as a function of speed and gait. J Exp Biol 94, 15–42. [DOI] [PubMed] [Google Scholar]
- Hudson PE, Corr SA, Payne‐Davis RC, et al. (2011) Functional anatomy of the Cheetah (Acinonyx jubatus) forelimb. J Anat 218, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter L (2011) Carnivores of the World. Princeton: Princeton University Press. [Google Scholar]
- International Committee on Veterinary Gross Anatomy Nomenclature (ICVGAN) (2017) Nomina Anatomica Veterinaria, 5th edn. Hannover: Editorial Committee. [Google Scholar]
- Iwaniuk AN, Pellis SM, Whishaw IQ (1999) The relationship between forelimb morphology and behaviour in North American carnivores (Carnivora). Can J Zool 77, 1064–1074. [Google Scholar]
- Julik E, Zack S, Adrian B, et al. (2012) Functional anatomy of the forelimb muscles of the Ocelot (Leopardus pardalis). J Mamm Evol 19, 277–304. [Google Scholar]
- Kardong K (2011) Vertebrates: Comparative Anatomy, Function and Evolution, 6th edn. London: McGraw‐Hill Education. [Google Scholar]
- Leach D (1977) The forelimb musculature of marten (Martes americana turton) and fishes (Martes pennanti erxleben). Can J Zool 55, 31–41. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Fridén J (2000) Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 23, 1647–1666. [DOI] [PubMed] [Google Scholar]
- Liebich HG, Maierl J, König HE (2016) Membros Torácicos ou Anteriores (Membra Thoracica) In: Anatomia dos Animais Domésticos, 6th edn (eds König HE, Liebich HG.), pp. 151–222. Porto Alegre: Artmed. [Google Scholar]
- Lorenzão CJ, Zimpel AV, Novakoski E, et al. (2016) Comparison of the lumbosacral nerves formation in Pampas Fox (Pseudalopex gymnocercus) and Crab‐Eating‐Fox (Cerdocyon thous) in relationship to plexus models in dogs. Anat Rec 299, 361–369. [DOI] [PubMed] [Google Scholar]
- Lucherini M, Luengos Vidal EM (2008) Lycalopex gymnocercus (Carnivora: Canidae). Mamm Species 820, 1–9. [Google Scholar]
- Luengos Vidal EM, Sillero‐Zubiri C, Marino J, et al. (2012) Spatial organization of the Pampas Fox in a Grassland Relict of Central Argentina: a flexible system. J Zool 287, 133–141. [Google Scholar]
- Macalister A (1870) On the anatomy of Aonyx . Proc R Irish Acad Sci 1, 539–547. [Google Scholar]
- Maffei L, Paredes R, Segundo A, et al. (2007) Home range and activity of two sympatric fox species in the Bolivian Dry Chaco. Canid News 10.4 (online) http://www.canids.org/canidnews/10/ Sympatric_foxes_in_Bolivia.pdf.
- Martini FH, Timmons MJ, Tallitsch RB (2009) Anatomia Humana. Porto Alegre: Artmed. [Google Scholar]
- McClearn D (1985) Anatomy of Raccoon (Procyon lotor) and Coati (Nasua narica and N. nasua) forearm and leg muscles: relations between fiber length, moment‐arm length, and joint‐angle excursion. J Morphol 183, 87–115. [DOI] [PubMed] [Google Scholar]
- Meachen‐Samuels J, Van Valkenburgh B (2009) Forelimb indicators of prey‐size preference in the Felidae. J Morphol 270, 729–744. [DOI] [PubMed] [Google Scholar]
- Meloro C, Elton S, Louys J, et al. (2013) Cats in the forest: predicting habitat adaptations from Humerus Morphometry in extant and Fossil Felidae (Carnivora). Paleobiology 39, 323–344. [Google Scholar]
- Mendez J, Keys A (1960) Density and composition of mammalian muscle. Metabolism 9, 184–188. [Google Scholar]
- Michilsens F, Vereecke EE, D'Août K, et al. (2009) Functional anatomy of the gibbon forelimb: adaptations to a brachiating lifestyle. J Anat 215, 335–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AL, Budny JE, Russell AP, et al. (2013) Architectural specialization of the intrinsic thoracic limb musculature of the American Badger (Taxidea taxus). J Morphol 274, 35–48. [DOI] [PubMed] [Google Scholar]
- Nickel R, Schummer A, Seiferle E, et al. (1986) The Locomotor System of the Domestic Mammals, 5th edn. Berlin: Verlag Paul Parey. [Google Scholar]
- Payne RC, Crompton RH, Isler K, et al. (2006) Morphological analysis of the hindlimb in apes and humans. I. Muscle architecture. J Anat 208, 709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira SG, Santos ALQ, Borges DCS, et al. (2016) Anatomia Óssea e Muscular da Escápula e Braço do Chrysocyon brachyurus (CARNIVORA, CANIDAE). Ci Anim Bras 17, 622–632. [Google Scholar]
- Perry JMG, Macneill KE, Heckler AL, et al. (2014) Anatomy and adaptations of the chewing muscles in Daubentonia (Lemuriformes). Anat Rec 316, 308–316. [DOI] [PubMed] [Google Scholar]
- Queirolo D, Kasper CB, Beisiegel BM (2013) Avaliação do risco de extinção do graxaim‐do‐campo Lycalopex gymnocercus (G. Fischer, 1814) no Brasil. Biodiv Bras 3, 172–178. [Google Scholar]
- Rose JA, Sandefur M, Huskey S, et al. (2013) Muscle architecture and out‐force potential of the thoracic limb in the Eastern Mole (Scalopus aquaticus). J Morphol 274, 1277–1287. [DOI] [PubMed] [Google Scholar]
- Sacks RD, Roy RR (1982) Architecture of the hind limb of muscles of cats: functional significance. J Morphol 173, 185–195. [DOI] [PubMed] [Google Scholar]
- Santos AC, Bertassoli BM, Oliveira VC, et al. (2010) Morfologia dos Músculos do Ombro, Braço e Antebraço do Quati (Nasua nasua Linnaeus, 1758). Biotemas 23, 167–173. [Google Scholar]
- Senter P, Moch JGA (2015) Critical survey of vestigial structures in the postcranial skeletons of extant mammals. PeerJ 3, e1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar R, Milgram J (2005) Morphometric and anatomic study of the forelimb of the dog. J Morphol 263, 107–117. [DOI] [PubMed] [Google Scholar]
- Sharir A, Milgram J, Shahar R (2006) Structural and functional anatomy of the neck musculature of the dog (Canis familiaris). J Anat 208, 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MFM, Souza WV, Almada RMF, et al. (2015) Anatomía Comparada de Los Músculos Supinador y Pronador Redondo de Tres Especies Carnívoras Silvestres. Rev Arg Anat Online 6, 117–122. [Google Scholar]
- Souza Junior P, Mattos K, Carvalho NC, et al. (2014) Topografia da Intumescência Lombar e do Cone Medular em Lycalopex gymnocercus (G. Fischer, 1814). Rev Bras Ci Vet 21, 173–177. [Google Scholar]
- Souza Junior P, Santos LMRP, Nogueira DMP, et al. (2015) Occurrence and morphometrics of the brachioradialis muscle in wild carnivorans (Carnivora: Caniformia, Feliformia). Zoologia 32, 23–32. [Google Scholar]
- Souza Junior P, Cruz NC, Mattos K, et al. (2016) Brachial plexus in the Pampas Fox (Lycalopex gymnocercus): a descriptive and comparative analysis. Anat Rec 300, 537–548. [DOI] [PubMed] [Google Scholar]
- Souza Junior P, Santos LMPR, Souza EC, et al. (2018) Osteologia do membro torácico de Lycalopex gymnocercus Fischer, 1814 (Carnivora, Mammalia): abordagens comparada, radiográfica e osteométrica. Pesq Vet Bras 38, 195–221. [Google Scholar]
- Spoor CF, Badoux DM (1986a) Descriptive and functional myology of the neck and forelimb of the striped Hyaena (Hyaena hyaena, L. 1758). Anat Anz 161, 375–387. [PubMed] [Google Scholar]
- Spoor CF, Badoux DM (1986b) Nomenclatural review of long digital forelimb flexors in carnivores. Anat Rec 216, 471–473. [DOI] [PubMed] [Google Scholar]
- Tchaicka L, Freitas TRO, Bager A, et al. (2016) Molecular assessment of the phylogeny and biogeography of a recently diversified endemic group of South American Canids (Mammalia: Carnivora: Canidae). Genet Mol Biol 39, 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viranta S, Lommi H, Holmala K, et al. (2016) Musculoskeletal anatomy of the Eurasian Lynx, Lynx lynx (Carnivora: Felidae) forelimb: adaptations to capture large prey? J Morphol 277, 753–765. [DOI] [PubMed] [Google Scholar]
- Ward S, Eng C, Smallwood L, et al. (2009) Are current measurements of lower extremity muscle architecture accurate? Clin Orthop Relat Res 467, 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster EL, Hudson PE, Channon SB (2014) Comparative functional anatomy of the epaxial musculature of dogs (Canis familiaris) bred for sprinting vs. fighting. J Anat 225, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Wilson AM, Daynes J, et al. (2008) Functional anatomy and muscle moment arms of the thoracic limb of an elite sprinting athlete: the racing greyhound (Canis familiaris). J Anat 213, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle B (1888) Notes on the limb myology of procyon cancrivorus and of the ursidae. J Anat Physiol 23, 81–89. [PMC free article] [PubMed] [Google Scholar]
- Windle B, Parsons F (1897) On the myology of the terrestrial carnivora. Part I: muscles of the head, neck, and fore‐limb. Proc Zool Soc London 65, 370–409. [Google Scholar]


