Abstract
Whether the 1st segment of the human autopod 1st ray is a ‘true’ metapodial with loss of the proximal or mid phalanx or the original basal phalanx with loss of the metacarpal has been a long‐lasting discussion. The actual knowledge of the developmental pattern of upper autopod segments at a fetal age of 20–22 weeks, combined with X‐ray morphometry of normal long bones of the hand in the growing ages, was used for analysis of the parameters, percentage length, position of epiphyseal ossification centers and proximal/distal growth rate. The symmetric growth pattern in the fetal anlagen changed to unidirectional in the postnatal development in relation to epiphyseal ossification formation. The percentage length assessment, the distribution of the epiphyseal ossification centers, and differential proximal/distal growth rate among the growing hand segments supported homology of most proximal segment of the thumb with the 2nd–5th proximal phalanges and that of the proximal phalanx of the thumb with the 2nd–5th mid phalanges in the same hand. Published case reports of either metanalysis of ‘triphalangeal thumb’ and ‘proximal/distal epiphyseal ossification centers’ were used to support the applied morphometric methodology; in particular, the latter did not give evidence of growth pattern inversion of the proximal segment of the thumb. The presented data support the hypothesis that during evolution, the lost segment of the autopod 1st ray is the metacarpal.
Keywords: autopod fetal anlage growth, fetal ossification pattern, morphometric and patterning homology, postnatal ossification pattern
Introduction
During the fetal period, the long bone anlagen of the hand of modern humans undergo symmetric longitudinal growth of both the proximal and distal ends (Pazzaglia et al. 2017). However, this symmetric growth pattern changes with the onset of the epiphyseal ossification. This change is plainly evident in the postnatal age when the ossification centers can be routinely documented by X‐rays. In contrast, the symmetric growth pattern of the proximal and distal anlagen ends is maintained in the stylopod and zeugopod of the upper limb (arm and forearm) until the closure of the growth plate cartilages (Caffey, 1973; Christie, 1949). In the lower limb, the ossification pattern of the cartilage anlagen is similar to that of the upper limb.
X‐rays of normally developing hand and foot tubular bones show only one epiphyseal ossification center and the related growth plate cartilage, whereas the opposite end is described as undergoing direct ossification, as indicated by the term ‘pseudo‐epiphysis’ (Lee & Garn, 1967; Haines, 1938, 1974; Ogden et al. 1994). The distribution of the epiphyseal ossification centers is distal in metacarpals and metatarsals from the 2nd to 5th rays, but proximal in the 1st ray, similar to those of all the phalanges.
The 1st ray of the hand and foot has only two phalanges (ph. formula = 2–3–3–3–3); this similar patterning and the distribution of the epiphyseal ossification center in the autopods have engendered a long‐lasting debate about homology and phylogenetic evolution of this ray in mammalian and non‐therian tetrapods (Reno et al. 2013). In this discussion, there are two hypotheses. The first is that the 1st metacarpal/metatarsal is the original basal phalanx, and the corresponding metapodial has been lost during evolution. If this hypothesis is accepted, it may explain the discrepancy of the position of the epiphyseal center between the 1st and 2nd–5th metapodials. The second hypopthesis is that the metacarpal/metatarsal is a ‘true’ metapodial with the loss of one element of the 1st ray (the proximal or the mid phalanx). In this case, the 1st metapodial ossification pattern must have been reversed with respect to the patterns of the 2nd–5th rays.
Apart from these morphological considerations, other hypotheses consider the fusion between the thumb metacarpal and the proximal phalanx of the same ray (symbrachydactyly) or the fusion of the distal with the mid phalanx of the thumb (Guillem et al. 1999). The epiphyseal end growth asymmetry in autopod metapodials and phalanges has recently been addressed in a morphologic study by Reno et al. (2006) in an attempt to identify the cellular events underlying the induction of growth plate formation; this was followed by a comparative study in therian tetrapods (alligators), which form growth plates at both ends of their metapodials (Reno et al. 2007). These authors suggested in a recent review paper that an answer to the question needs to be considered in a larger phylogenetic context, and supported the view that the 1st ray proximal segment is a ‘true’ metapodial (Reno et al. 2013).
Anthropoids and hominins exhibit differential adaptation in the proportions and number of autopod segments. This differential adaptation is needed to satisfy similar functional demands related to climbing, suspension, bipedal posture and hand tool use (Marzke, 1997; Marzke & Marzke, 2000; Young & Hallgrimsson, 2005; Almecija et al. 2015). Both molecular and fossil evidence have had important consequences in the interpretation of the evolutionary history of the hand within the Hominidae family and the hominin tribe (Tocheri et al. 2008).
The histomorphology of fetal autopod segments (Uhthoff, 1990; Pazzaglia et al., 2017) and the postnatal morphometric study based on hand metacarpal and phalange X‐rays through the developmental age, can help integrate the knowledge derived from human and animal model histomorphology, developmental patterning studies and phylogenetic history. In this context, the present study offers several guiding points:
well‐established knowledge about the appearance of the ossification centers of tubular and carpal bones, which has been adapted to clinical use (Vogt & Vickers, 1938; Caffey, 1973; Christie, 1949);
the availability of normal hand X‐rays from hospital archives;
the wide documentation of congenital hand defects reported in radiology, hand and plastic surgery journals and the increasing number of gene analyses in syndromes that include hand development defects.
The aim of this study was to analyze the following: (1) X‐ray morphometry of normal long bones of the hand from postnatal age to 16 years old; (2) metanalyses of congenital human phenotypes consistent with the development of metacarpal and phalanges, such as the ‘triphalangeal thumb’ and ‘proximal/distal epiphyseal ossification centers’. The latter two phenotypes are related to autopod segment patterning, growth and genetically controlled morphogenesis. Specifically, the problem arising from the thumb biphalangeal pattern in the length measurement was determined setting the triphalangeal thumb metanalysis in the normal hand series as the reference ray for calculating the percentage length of the thumb segments. Otherwise, the distribution of the epiphyseal ossification centers, the epiphyseal shape and the proximal/distal growth rate index were evaluated and compared between the ray elements independently from the two or three phalangeal ray patterns.
The morphometric data of the study were limited to the development and ossification of the skeletal segments. To the best of our knowledge, combined metanalysis of human phenotypes with X‐ray morphometry of normal hand series in the developmental period represents an original methodology for the analysis of autopod segment variance and covariance in the more general context of the molecular control and the evolutionary phylogenetic line.
Materials and methods
X‐ray postnatal normal hands series
A total of 53 hand X‐rays of 47 normal children were selected from the Pediatric Radiology archives (Spedal I Civili di Brescia). The study protocol was approved by the DSMC Council of the University of Brescia.
The patients’ ages were between 8 months and 15 years and were equally distributed between the sexes; in three patients, both hands were available. For 30 hands, an X‐ray antero‐posterior view of both the whole hand and the 1st ray was taken. The radiographic survey was carried out on trauma of the wrist/fingers to exclude fracture or joint dislocation. Other X‐rays were taken for assessment of the skeletal age. X‐rays were taken in an a‐p projection of the hand, at a standard distance of 50 cm from the radiogenic tube. Those of the thumb were obtained while changing the position of the thumb on the X‐ray plate holder (Fig. 1). The selected 47 hand X‐rays (only one for the three subjects with right and left hand available) were divided for the morphometric analysis into six age groups: (A) 6 months–2 years; (B) 3–4 years; (C) 5–6 years; (D) 7–8 years; (E) 9–10 years; and (F) over 10 years.
Figure 1.
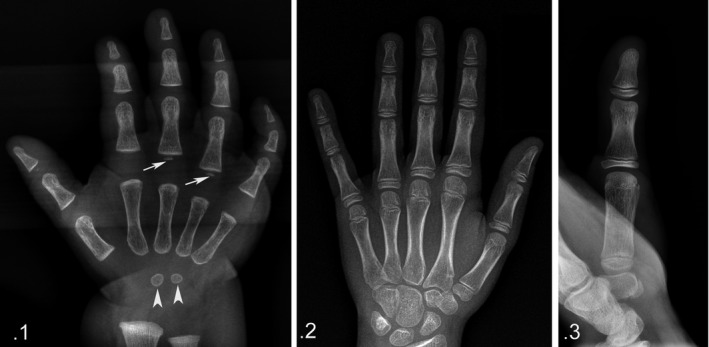
(1) Right‐hand X‐ray, a‐p projection (age 8 months, group A). Early stage of ossification with two centers of the carpal short bone anlagen and with basal, epiphyseal ossification centers of the 3rd and 4th ray proximal phalanges. The thumb bone segments are taken in an oblique projection, which is not comparable for shape analysis with those of the 2nd and 5th rays. (2) Right‐hand X‐ray, a‐p projection (age 13 years, group F). Advanced stage of ossification with all eight carpal bone ossification centers and the presence of all the long bone ossification centers: proximal position of the 1st–5th phalanges and inverted position of the thumb metacarpal to the 2nd–5th metacarpals. The shape of the thumb ossification center can be classified as flattened even if it is thicker than the phalangeal center, but it certainly is not similar to the round‐shaped distal epiphyses of the 2nd–5th metacarpals. The thumb bone segments are taken in oblique projection as in age group A. (3) Hand X‐ray, 1st ray a‐p projection (age 9 years, group E). Shape analysis of the thumb segments in this projection allows comparison with the other ray segments.
Length analysis
The length of each segment (metacarpals and phalanges) was assessed from the proximal to the distal end on the median axis; the epiphyseal ossification center (if present) was included in the measurement. The total ray length was calculated as the sum of the metacarpal and the corresponding phalanges. The absolute lengths were ordered transversally from the 1st to 5th ray. The percentage length of each element in the same hand was calculated based on the total length of the corresponding ray. The thumb metacarpal, proximal and distal phalanx percentage lengths were calculated either on the total length of the 1st or 3rd ray of the same hand. The purpose of performing two measurements of the percentage length of 1st ray elements was to consider the bias due to the biphalangism of this ray (see metanalysis of the triphalangeal thumb case report).
Two series of comparisons were carried out:
1 the thumb distal phalanx percentage length (calculated on the total length of the 1st ray and the 3rd ray of the same hand) vs. the percentage length of the 2nd–5th distal phalanges (calculated on its own ray);
2 the thumb metacarpal and proximal phalanx percentage length (calculated on the 3rd ray of the same hand) vs. the corresponding percentage lengths of the 2nd–5th metacarpals and proximal phalanges (each calculated on its own ray) or the thumb proximal phalanx vs. the proximal and mid phalanges of the 2nd–5th fingers, respectively.
In the first comparison, the difference between the thumb distal phalanx percentage length with regard to the 1st and 3rd rays quantified the bias due to the missing segment of the thumb (the 3rd ray length of the same hand was assumed to be that of a hypothetical, ancestral thumb with the regular number of phalanges). Indeed, the homology of all the distal phalanges cannot be questioned because of the apical tuft‐specific morphology.
In the second comparison, the degree of length homology was tested for the following: thumb metacarpal vs. the 2nd–5th metacarpals or the 2nd–5th proximal phalanges, and thumb proximal phalanx vs. the 2nd–5th proximal phalanges or the 2nd–5th mid phalanges.
Epiphyseal ossification centers distribution and shape analysis
The time of appearance and distribution of the epiphyseal ossification center were analyzed in the normal hand series separated into the earlier reported age groups by counting the mean number of ossification centers in the carpus and tubular bones.
The shape of the ossification centers was classified as ‘rounded’ when the ratio between the longitudinal and transverse diameter was 1.0–0.5, ‘flattened’ when it was 0.4–0.2, and ‘not‐assessable’ in the earlier phase of ossification.
Regarding the profile of the non‐epiphyseal ends and the geometry of the meta‐epiphysis, some typical patterns characterized proximal and distal extremity of each bone: (1) ‘rounded’, (2) ‘cone‐shaped’ and (3) ‘flat’. A further characterizing element was ‘metaphyseal flaring’ (4). This evaluation was not enforceable before the appearance and sufficient organization of the ossification center; therefore, this feature could be defined only in the older age groups D, E and F (Fig. 1, 2).
Figure 2.
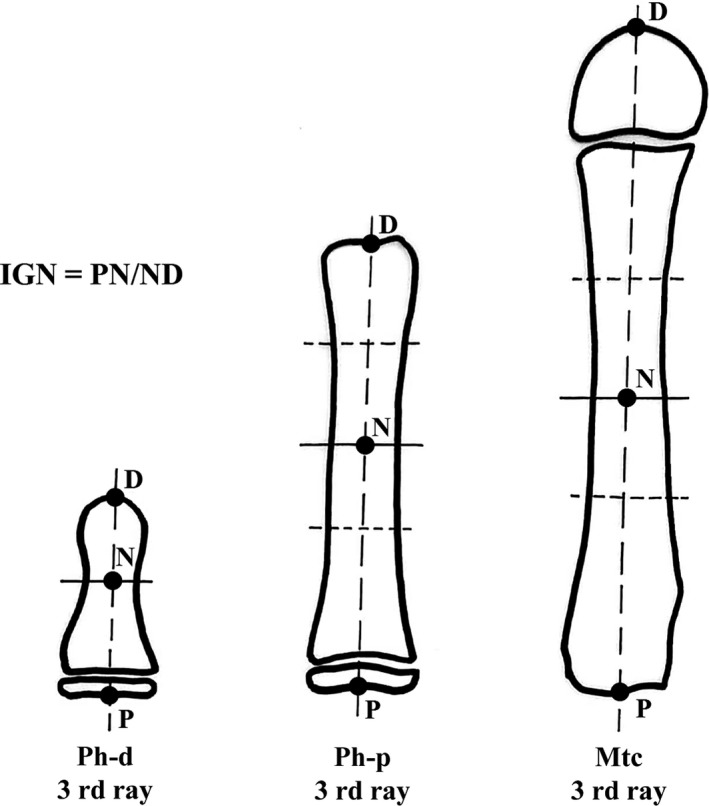
Graphic illustration of the IGR) measurement method in postnatal long bones (see details in Materials and methods). This assessment was applicable only in segments with a well‐developed epiphyseal ossification center (age groups D–F).
Proximal/distal growth rate index assessment
In all the analyzed phalanges and metacarpal X‐rays, the narrower part of the diaphysis did not correspond to the mid longitudinal length. Otherwise, in the early fetal period, the primary ossification center developed in the middle of the long bone cartilage anlage, which then provided the scaffold for the structuring diaphyseal cortex (Pazzaglia et al. 2016). Postnatally, the distance of the narrower, transverse diameter from the proximal and distal ends of each phalanx and metacarpal resulted from the longitudinal growth rate of the proximal and distal transition zone of the fetal anlage and from the metaphyseal growth plate when it was formed at the end of the fetal period. The ratio between these two measurements provided an index of the proximal and distal growth of the anlage.
To evaluate the normal hand series, the narrower, transverse diameter was traced in the diaphysis of the digitalized X‐ray images and the distance from the proximal and distal ends was measured with the program cell (Soft Imaging System GmbH, Munster, Germany). When the definition of the latter was uncertain, the proximal and distal boundaries of the narrower, central segment of the diaphysis were traced; the midpoint of the latter was assumed as the level of the narrower diameter (Fig. 2). The ratio between the proximal/distal longitudinal segments was determined and was expressed numerically (growth rate index, IGR). It represented the differential growth rate of the anlage during the fetal and the early postnatal periods: the value 1 corresponded to a proximal longitudinal growth rate equal to the distal; the values > 1 to a higher proximal growth rate, and those < 1 to a slower growth rate.
It was not possible to analyze the shape or determine the IGR of the thumb segments in the standard a‐p projection of the hand because the position of the 1st ray corresponded to an oblique projection. Appropriate a‐p thumb projections were available for 30 hands of the normal series. A further limitation of this evaluation was the insufficiently developed epiphyseal ossification centers. Therefore, a statistical comparison of IGR and shape analysis was restricted to a smaller population of hands than that used for percentage length assessment, which included only the older age groups D, E and F.
Metanalysis of triphalangeal thumbs and proximal/distal epiphyseal ossification centers
Triphalangeal thumbs with completely developed phalanges (a condition which excluded delta or severely underdeveloped phalanges) were an uncommon pattern; to the best of our knowledge, this has been documented only in the human species (Table 1). The morphometric analysis was carried out on a selected number of the published X‐ray images. The inclusion criterion was the quality and definition of the scanned image, which should allow reliable measurements of the total length of the ray, the percentage length of the segments and IGR. All the analyzed triphalangeal hands were in young adults. The thumb metacarpal percentage length (on its own ray) and that of the 2nd–5th fingers were compared with the proximal and mid phalanges of the corresponding rays. Further, the percentage length of each 2nd–5th ray segment was compared transversally with the corresponding segments of the 1st ray.
Table 1.
Case reports used for metanalysis of triphalangeal thumbs
| Reference | Case numbers | Hand number | Subject age | Parentage |
|---|---|---|---|---|
| Heiss (1953) | 1 | 2 | Adult | Mother |
| 1 | 2 | Newborn | Son | |
| Warm et al. (1988) | 1 | 1 | Adult | Father |
| 1 | 2 | Child | Son | |
| 1 | 2 | Infant | Son | |
| Zguricas et al. (1997) | 1 | 2 | Adult | |
| 1 | 1 | Adult | ||
| Zuidam et al. (2010) | 1 | 1 | Adult | |
| Wieczorek et al. (2010) | 1 | 2 | Adult | |
| Quazi & Kassner (1988) | 1 | 2 | Adult | |
| 1 | 1 | Child | ||
| Zuidam et al. (2016) | 1 | 1 | Adult | |
| Zguricas et al. (1994) | 1 | 2 | Adult | |
| Limb & Laughenbury (2012) | 1 | 2 | Infant | |
| Reynolds (1917) | 1 | 2 | Unknown |
Proximal and distal epiphyseal ossification centers (in the same bone) were also uncommonly reported phenotypes. In the former, one or more autopod segments presented a longitudinal growth pattern through a proximal and a distal epiphyseal ossification center (Zuidam et al. 2006). In one case, it was reported to be associated with a triphalangeal thumb; most frequently, however, it was seen in hands with normal digital patterning (Table 2). In the present series, the cases associated with polydactyly and those defined on the basis of the radiographic signs ‘notch’, ‘fissure’ or ‘incomplete pseudoepiphysis’ were not considered. The quality and definition of the scanned X‐ray images of this series did not allow reliable measurements of the morphometric parameters; therefore, the metanalysis was limited to the distribution in each hand of the double epiphyseal ossification centers. Only in the de Jong et al. (2014) case report could IGR be calculated and compared among all the hand segments.
Table 2.
Case reports used for metanalysis of proximal/distal epiphyseal ossification centers and distribution in hand long bone segments
| Reference | Case numbers | Hand numbers | Subject age | Hand segment |
|---|---|---|---|---|
| Milch (1951) J Bone Joint Surg A | 2 | 2 | 3 | 1st Mtc |
| 5 | 1st Mtc | |||
|
Caffey (1973) Pediatric X‐Ray diagnosis (book) |
1 | 1 | 5 | 1st Mtc (dx/sx) |
| Nakashima & Furukawa (1997) Ann Anatomy | 1 | 1 | 7 |
1st to 5th Mtc (dx/sx) 1st to 5th Mtc (dx/sx) |
| Limb & Laughenbury (2012) J Hand Surg Eur | 1 | 1 | 12 | 2nd Mtc |
| 1 | 1 | 8 | 1st Mtc | |
| de Jong et al. (2014)J Hand Surg Am | 1 | 1 | 6 |
1st Mtc 1st to 5th Ph‐p 2nd to 5th Ph‐m 2nd to 5th Ph‐p |
| 1 | 1 | 5 |
1 st Mtc 1st to 5th Ph‐p 1st to 5th Ph‐m 1st to 5th Ph‐p |
Statistical analysis
Repeated measurements of 380 hand segments were obtained independently by two investigators (A.G.S. and A.M.) from a sample equal to 40% of the total number of examined hands. Each dataset was measured twice with 1‐month interval in two series of paired measurements. The difference between each paired measurement (intra‐ and interobserver) was plotted against the difference in individual segments and total ray lengths. By analyzing the differences between the paired measurements, the only error was that which was likely to follow a normal distribution. The variation in the differences for the two series of measurements was wider in the interobserver paired dataset than in the corresponding intraobserver set, both with a degree of agreement above the 95% confidence interval (Bland & Altman, 2010).
The percentage of finger segment length, the IGRs and the number of ossification centers were expressed as the mean ± SEM. Statistical analysis was performed with a statistics package (Graph Pad prism 5, Graph Pad Software, San Diego, CA, USA). Non‐parametric data were analyzed by a Kruskal–Wallis test followed by Dunn's test or the Mann–Whitney test when appropriate.
The trend followed by the percentage measurements of finger segment lengths (each measured on its own ray) polled/age group over all age groups was analyzed by the area under the curve (AUC) calculated by trapezoidal approximation. Differences with P < 0.05 were considered significant (Table 3).
Table 3.
AUC (Area Under Curve) values calculated by trapezoidal rule of percentage length of metacarpal (Mtc), proximal (Ph‐p) and mid phalanx (Ph‐m) in different age groups (A‐F) as graphically presented in Fig. 4
| AUC (A‐F age groups) | |||||
|---|---|---|---|---|---|
| Segment | Ray | AUC | Segment | Ray | AUC |
| Mtc | 1 | 140.2 ± 10.8 | Ph‐p | 1 | 96.14 ± 0.71 |
| Mtc | 2 | 231.0 ± 1.3*** | Ph‐p | 2 | 138.1 ± 0.9**** |
| Mtc | 3 | 206,3 ± 1** | Ph‐p | 3 | 147.3 ± 0.7***** |
| Mtc | 4 | 196,3 ± 1.1* | Ph‐p | 4 | 149.1 ± 1***** |
| Mtc | 5 | 213,2 ± 4.5** | Ph‐p | 5 | 139.3 ± 0.6**** |
| Ph‐p | 2 | 138.1 ± 0.9 | Ph ‐m | 2 | 78.78 ± 0.86 |
| Ph‐p | 3 | 147.3 ± 0.7 | Ph ‐m | 3 | 90.49 ± 0.78 |
| Ph‐p | 4 | 149.1 ± 1 | Ph ‐m | 4 | 93.72 ± 0.63 |
| Ph‐p | 5 | 139.6 ± 0.6 | Ph ‐m | 5 | 77.17 ± 0.4 |
Thumb (R1) metacarpal and proximal phalanx percentage length was calculated on that of the 3rd ray of the same hand, whereas the percentage lengths of the 2nd–5th (R2‐R5) metacarpals and phalanges were calculated on their own rays. *P < 0.5, **P < 0.01, ***P < 0.001 vs. Mtc‐Ray 1 group; ****P < 0.01, *****P < 0.001 vs. Ph‐p Ray1 group.
Results
X‐ray postnatal, normal hand series
Percentage length of metacarpals and phalanges
The mean total length of the finger rays in the normal hand population increased from ray 1 to 3 and then decreased from ray 3 to 5 in all age groups, which represented the most common pattern of the species phenotype (Fig. 1.1 and 1.2). In the comparison of the ray segments, the percentage length assessment was further biased by the missing segment in the 1st ray. The percentage length calculation of the thumb segments on the total length of the 3rd ray, rather than on the 1st, produced the same percentage correction among all age groups. The adjusted percentage length of the thumb distal phalanx was significantly higher than that of the 2nd–3rd phalanges of the younger age groups (A and B) and of the 4th phalanges of the older age groups (C, D and F) (Fig. 3). However, the homology of the distal phalanges was not questionable because they share the unique apical tuft feature (Mittra et al. 2007).
Figure 3.
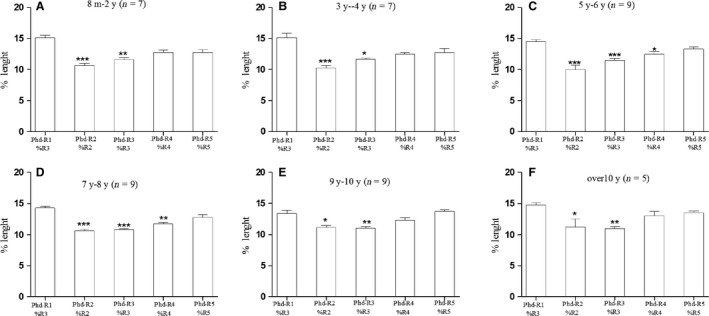
The 1st ray distal phalanx mean percentage length (measured on the total length of the 3rd ray) was compared with the mean percentage length of the 2nd–5th ray distal phalanges (measured on the total length of each ray). The result was significantly higher than that for the 2nd–3rd ray distal phalanges in all age groups A–F but not significantly different than that of the 4th–5th rays of age groups C–D. The typology of the 1st ray distal phalanx cannot be questioned because of the characterizing apical tuft morphology. Therefore, the observed differences documented a ‘true’ major growth of the latter segment vs. the 2nd–3rd rays; this is independent of the percentage measurement method, which assumed that the reference to the total length of the 3rd ray corrected the bias due to the missing segment of the thumb (* P < 0.05; ** P < 0.01; *** P < 0.001).
The profile (from age groups A–F) of the mean thumb metacarpal percentage length was lower than those detected in the 2nd–5th metacarpals (Fig. 4A) and superimposable on the profile of the 2nd–5th proximal phalanges (Fig. 4B). In line with these observations are the AUC data reported in Table 3, which show a significant difference in AUC percentage length of the thumb metacarpal throughout the age groups compared with the 2nd–5th metacarpals. The profile of the mean proximal phalanx percentage length was lower than those detected in the 2nd–5th proximal phalanges (Fig. 4C), reaching a high statistical significance as reported in the AUC analysis (Table 3), whereas it did not differ when compared with the 2nd–5th mid phalanges (Fig. 5D, Table 3).
Figure 4.
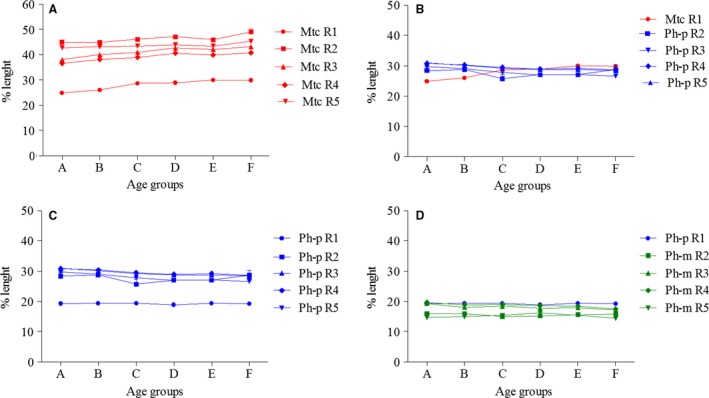
1 (A,B) Graphic profile of ray 1–5 total length of the metacarpal percentage length (ray 1 measured on ray 3 total length, rays 2–5 on the total length of each ray) in age groups A–F. This documents the percentage length dishomology of Mtc R1 (red) with respect to Mtcs R2‐R5 (red) and the homology of the same Mtc R1 (red) with respect to the percentage length of Ph‐p R2‐R5 (blue). (C,D) Corresponding graphic profile of R1‐R5 metacarpal percentage length (R1 measured on R3 total length, R2–R5 on the total length of each ray) documenting the percentage length dishomology of Ph‐p R1 (blue) with respect to Ph‐p R2‐R5 (blue) and the homology of the same Ph‐p R1 (green) with respect to percentage length of Ph‐m R2‐R5 (blue).
Figure 5.
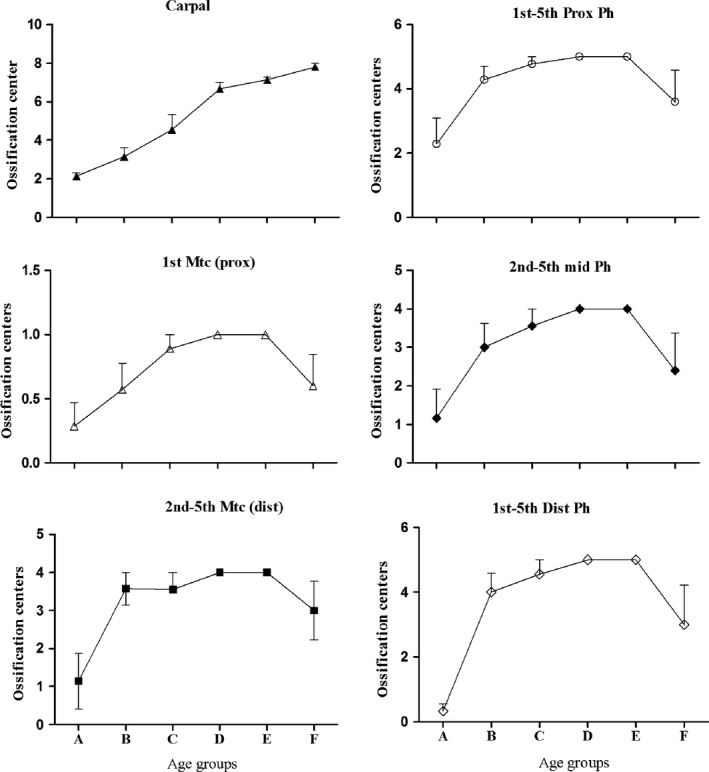
The regular progression of the number of carpal ossification centers with age confirmed the current use in the clinical assessment of skeletal age (Vogt & Vickers, 1938; Greunlich & Pyle, 1959). The different slope of the tubular bone epiphyseal ossification center number among the age groups is representative of variability of the time of appearance in epiphyseal center ossification. The reduction in number between age groups A and F corresponds to fusion with the ossified diaphyses.
These figures and data supported the percentage length parameter homology thumb metacarpal ≈ 2nd–5th proximal phalanges and thumb proximal phalanx ≈ 2nd–5th mid phalanges.
Distribution and shape of the epiphyseal ossification centers
The analyzed hand X‐ray series covered a range of ages from 8 months to 16 years. The appearance time of the carpals and long bone epiphyseal ossification centers had a variable agreement with chronological age; in the hands of the early age groups (A and B), few had appeared, but their number increased with age. In the older groups, some had undergone a partial fusion; these could also only be counted if the ossification center shape and morphology were still recognizable.
The first evidence of epiphyseal ossification in the group from 8 months to 2 years was observed in the central rays (2nd, 3rd and 4th) at the base of proximal phalanx; however, in two hands of this group, no evidence of ossification was present in any of the long bones. Two carpal ossification centers had developed in all hands, and three carpal ossification centers had developed in one hand. However, the sequence of the appearance of the long bone center did not follow a regular transverse or longitudinal order, so that occasionally one center might be absent or less developed either in the transverse line of the metacarpals and phalanges or along the digital ray. The mean number of centers increased in groups A and B and decreased later with the advancement of age, due to the fusion of the epiphyseal ossification center with the diaphysis. Only the number of carpal ossification centers showed a regular increment during the whole developmental period, validating their use for assessment of skeletal age (Fig. 5).
All the distal, mid and proximal phalanges ossification centers were of the ‘flattened’ type and were proximally positioned; those of the 2nd–5th metacarpals were of the ‘rounded’ type and were distally positioned (Fig. 1.2 and 1.3). The shape description of the thumb metacarpal and proximal phalanx was uncertain because in the standard hand X‐ray the thumb projection was a three‐quarters oblique, but the metacarpal ossification center was always proximal. The available thumb a‐p projections of age groups D–F documented the appearance sequence of the 1st ray ossification centers from the distal phalanx to the metacarpal and the apical tuft of all the distal phalanges. Both the proximal ossification centers of the 1st ray phalanx and metacarpal were classifiable as ‘flat’; however, the joint outline of the latter was unique because it was modeled on the shape of the saddle joint with the trapezius. All the segments of the thumb had larger transverse diameters than those of the other fingers (Figs 1).
Regarding the shape of the 2nd–5th ray segments, metaphyseal flaring characterized the proximal end of all phalanges, in contrast to the inverted cone shape of the distal end. Flaring was less evident in metacarpals before the appearance of the ossification centers than in the phalanges, but with the development of the distal centers and the cortical remodeling, the bone had an elongated, clepsydra‐like shape (Figs 1 and 2).
Proximal/distal growth rate index assessment
The proximal/distal growth rate of each thumb and finger segment class could be determined only in the older age groups D, E and F, because the definition of the narrower, transverse diameter was uncertain until the diaphysis was modeled. An IGR = 1 indicated a symmetric proximal/distal longitudinal growth rate. A comprehensive description of the distribution and growth rate difference among segments in the age groups is given in Fig. 6. All the phalanges showed an IGR > 1, with an increase from the age group D to the older ones. In the 2nd–5th metacarpals, the index documented a higher distal growth rate, whereas in the thumb there was a higher proximal growth rate. Significant differences were observed when comparing homologous segments in the three age groups and between the segments of each ray (Fig. 7).
Figure 6.
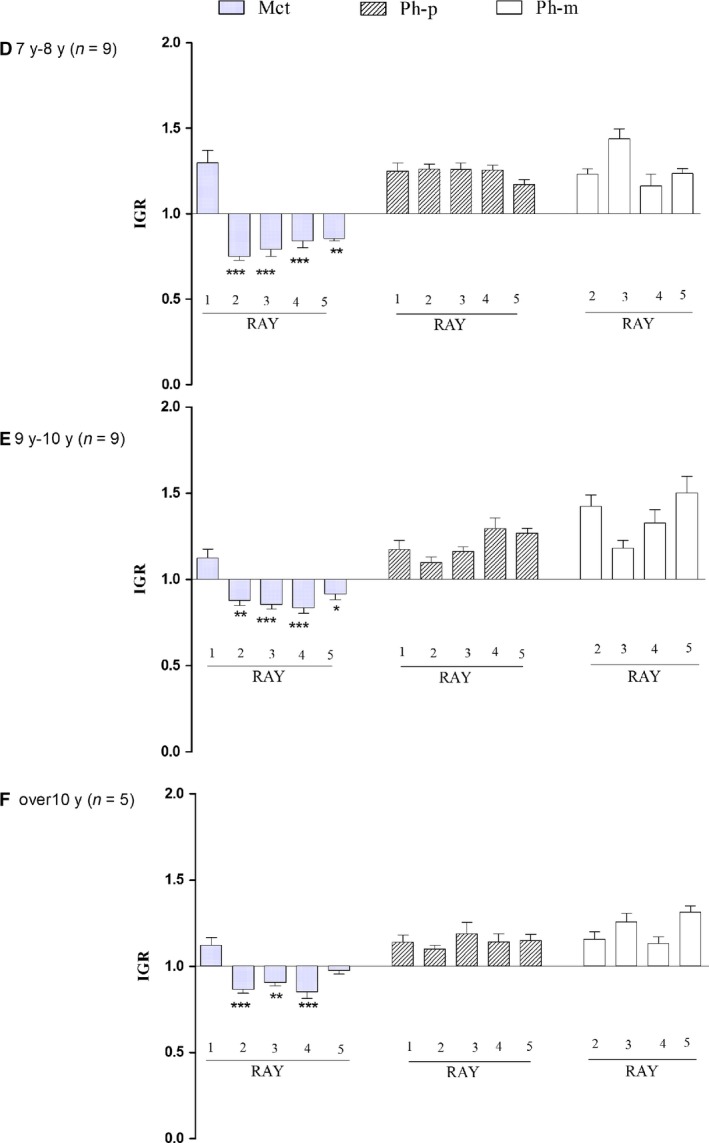
Proximal‐distal IGR) compared among R1–R5 metacarpals (Mtc), proximal phalanges (Ph‐p) and mid phalanges (Ph‐m) in age groups D–E. This parameter was not assessable in age groups A–C. With reference to IGR ≅ 1 corresponding to symmetric, bidirectional growth, the index was inverted at the passage from the 1st and the 2nd metacarpals with an evident relationship with the epiphyseal ossification center position (and later growth plate cartilage). Significant differences in proximal and mid phalanges (not reported in the histograms) but without inversion. *P < 0.05; **P < 0.01; *** P < 0.001 vs. R1 Mtc.
Figure 7.
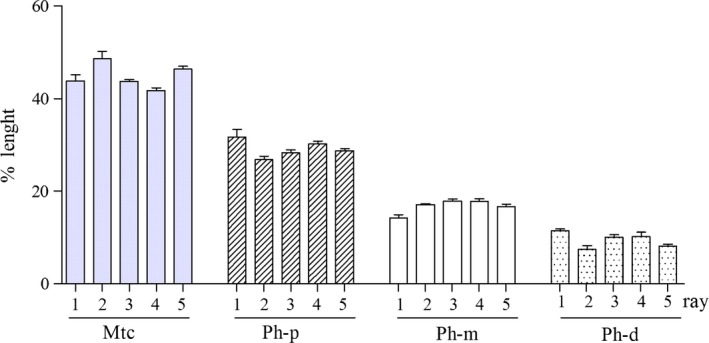
Triphalangeal thumb metanalysis. Comparison of the mean percentage length (measured on the total length of each ray) of 1st–5th ray metacarpals (Mtc), proximal (Ph‐p), mid (Ph‐m) and distal (Ph‐d) phalanges of TPT series (mean ± SEM of eight subjects). There was no significant difference when each segment type is considered in the transverse sequence R1–R5. The percentage length in all rays decreases from metacarpal to distal phalanges.
Metanalysis of triphalangeal thumb and proximal/distal epiphyseal ossification centers
Case reports of triphalangeal thumb and proximal and distal epiphyseal ossification centers were both uncommon phenotypes
The first cases may present with different degrees of expression such as hypoplastic or dysplastic supernumerary segments (known as delta phalanx), while they are rare when the extra‐phalanx is fully developed. In the metanalysis of ‘triphalangeal thumb’, we found 12 hands in nine reported cases, which were mostly young adults or adolescents. Based on the quality of the published X‐rays, eight hands were suitable for measurements (Table 1). These rare phenotypes were relevant for the aims of the study because the homology of the 1st metacarpal with the other four was not questionable.
The length of the hand segments was compared along each ray axis between the metacarpal and the proximal, mid and distal phalanx using the percentage length the segments in relation to the total length of each ray and between the series of the five segments in the transverse line (Fig. 7). The percentage length was significantly different between the ray segments in the longitudinal sequence metacarpal–proximal–mid–distal phalanx but not significant in the transverse line. Further, the mean of the 1st metacarpal IGR (calculated in eight hands) was not significantly different from the mean of the 2nd–5th metacarpals in the same hand. This suggested a homology between the 1st and the 2nd–5th metacarpals in this phenotype. Moreover, one case of this group (Heiss, 1953) documented that an autopod ray pattern = 4–4–4–4–4 in humans could occur through a genetic mutation, since it was present bilaterally in the mother and in her newborn (Fig. 8).
Figure 8.
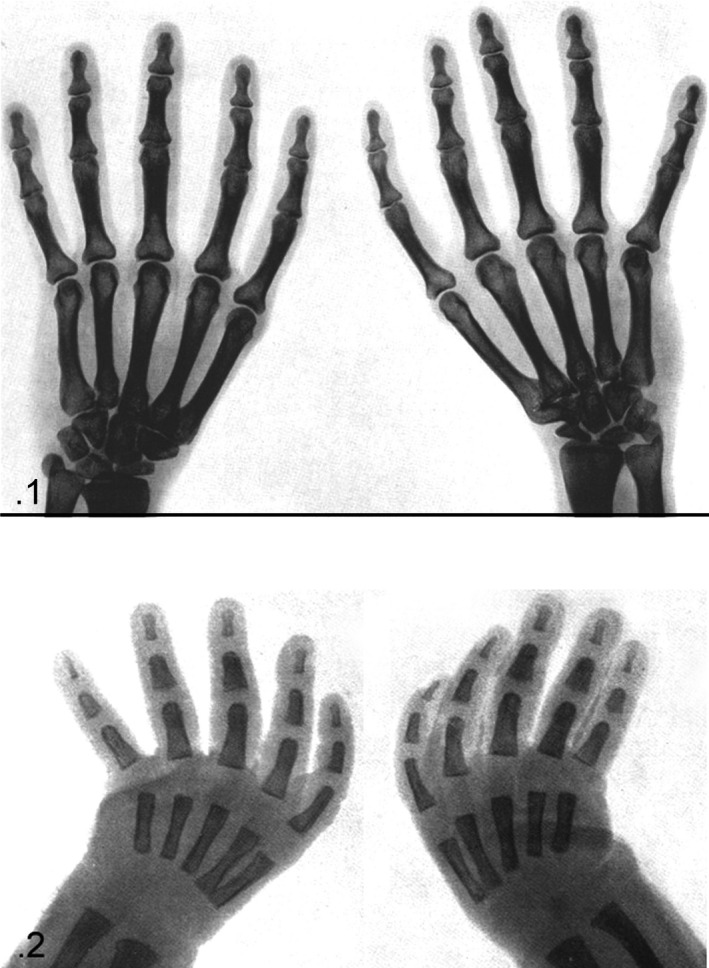
Image of triphalangeal thumb of the right and left hand of the mother (.1) and her newborn (.2) reported by Heiss (1953) and reproduced from Zeitschrift fur Anatomie und Entwicklungsgeschicte with permission of Springer Nature (licence no. 4334811065195).
The metanalysis of the case reports of complete double ossification centers included nine hands with an irregular distribution among the involved segments with a prevalence of metacarpals on the proximal and mid phalanges but never in the distal ones (Table 2 and Fig. 9). No percentage length measurements were enforceable in this hand series. However, in both hands of the case reported by de Jong et al. (2014), all the 1st–5th proximal and mid phalanges and the thumb metacarpal had double, well‐developed epiphyseal ossification centers, whereas in the 2nd–5th metacarpals, the ossification pattern was regular (Fig. 9), enabling IGR evaluation of this hand. It is worth pointing out that this case was also the result of a genetic mutation, because the younger sibling presented with the same bilateral pattern. The IGR of regular pattern hand segments (2nd–5th metacarpals and 1st–5th distal phalanges) and those of the double epiphyseal centers (all the other) documented (Fig. 9) a significantly higher index in the former (coherently with the position of the unique ossification center) compared with the double ossification center, where the mean IGR result was 0.74 for the 2nd–5th metacarpals and 1.38 for proximal and mid phalanges, and still higher (1.82) for the distal phalanges.
Figure 9.
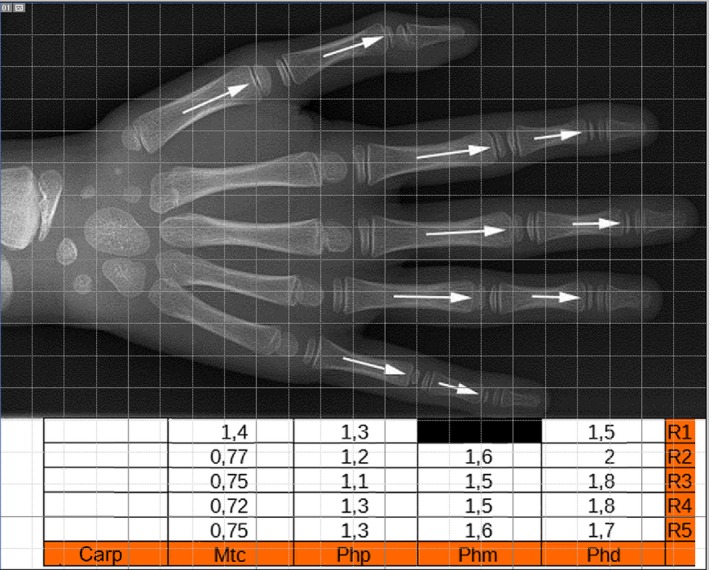
(top) Image of the hand with the widest distribution of proximal and distal epiphyseal ossification centers, reported in two siblings by de Jong et al. (2014) and reproduced from The Journal of Hand Surgery with permission of Elsevier (licence no. 4280070488758). (bottom) Table reporting the IGR calculation of each hand segment.
Discussion
Skeletal morphometry is a currently applied methodology in anthropology, paleontology, zoology and anatomy (Kivell, 2015). Since diversification is the key issue of biological development and evolution, homology, topology and typology represent basic concepts to deduce the phylogenetic history of the skeleton in the Kingdom Animalia (Riddle et al. 1993). Several parameters may be used to define the origin and the transformation of the vertebrate skeletal elements; they include size, shape, structural morphology, growth patterns, biochemistry, genetic transmission and control.
The autopod anlagen histomorphology and the X‐ray morphometry examined in this normal hand series during the postnatal developmental age address the question of homology of the thumb segments with the posterior metacarpals and phalanges. The answers to the above questions lead to the identification of the missing thumb segment and the different interpretations of the human autopod development given so far.
The comparative analysis of the homologous autopod segments and the measurement methodology required some statistical contrivances in relation to the developmental age of the studied population and to the somatic individual phenotypic variations. Regarding the first point, the lengths of the metacarpal and phalanges were divided into classes by age. For the second point, the metacarpal or phalanx length was expressed as the percentage of the corresponding ray total length in the same hand. However, a comparative evaluation of the homology of the thumb segments with that of the posterior fingers in the same hand was hampered by the yet unsolved question of the missing 1st ray segment.
To the best of our knowledge, the hand ray pattern = 4–4–4–4–4 (triphalangeal thumb) has seldom been reported in modern humans and never in therian tetrapods and anthropoids. However, this statement does not mean that this phenotype can be expressed only in the Family Hominidae, rather than in Homo sapiens (modern humans), the most monitored species in the Kingdom Animalia because of medical care. The metanalysis of reported triphalangeal thumb cases was used to reduce the missing element bias of the 1st ray measurements because this allowed extension to a more reliable percentage length comparison with the thumb segments. Beyond the methodological considerations, the triphalangeal thumb series provided evidence of a gene mutation that produced a phenotype with an evident length homology among the 1st segments of the hand rays (Swanson & Brown, 1962; Dobbs et al. 2000). The familiar transmission of this phenotype from the mother to the newborn was documented in the case report of Heiss (1953) and the genealogical tree of five families (Warm et al. 1988; Heutink et al. 1994; Wieczoreck et al. 2010; Girisha et al. 2014), where gene mutations were reported in the subtelomeric region of chromosome 7q or in the zone of polarizing activity regulatory sequence (ZRS) of Werner mesomelia.
The opinion that the thumb metacarpal is a modified phalanx was bolstered by many authors (Thompson, 1869; Shively, 1978; Guillem et al. 1999; Valenzuela et al. 2009), who considered primarily the parameter length and epiphyseal ossification center position (proximal in the phalanges and distal in metacarpals, respectively). The comparative percentage length analysis between the thumb and the posterior fingers in this study is original and allowed a crossed, statistical comparison of the thumb metacarpal with the 2nd–5th proximal phalanges, and the thumb proximal phalanx with the 2nd–5th mid phalanges. Regarding the epiphyseal ossification centers, we also considered the position in addition to the shape and the IGR of the bone segments. The first (shape) could be properly evaluated only with the a‐p projection X‐ray of the thumb because the standard hand a‐p projection gave an oblique and distorted image of the ossification centers. The second (IGR) was directly correlated to the growth pattern. allowing a quantitative evaluation of the growth process dynamics.
In the context of the debated question, the assignment of the thumb proximal segment to the metacarpal or phalanx class is a cornerstone in the understanding of autopod development and evolution; in particular, the epiphyseal ossification pattern deserves a thorough analysis. Reno et al. (2006), using an experimental model with mouse posterior metatarsals, observed the formation of a typical growth plate at one end, interposed between the primary and the epiphyseal ossification centers, whereas at the opposite end, a disorganized ossification replaced the cartilage epiphysis directly. The same pattern was also described in the growing bones of children (Haines, 1974; Ogden et al. 1994). Further, Reno et al. (2007) demonstrated the presence of growth plates at both cartilage anlage ends in alligator metapodials. More recently, the same authors (Reno et al. 2013) reviewed the literature reports of bidirectional growth in several therian tetrapod species and birds, and concluded that the latter was the ancestral condition, which was then lost in both placental and marsupial tetrapod mammals (therian synapomorphism). Their conclusions were that, despite the anatomic similarities shared by thumb metacarpal and phalanges, which continue to be the primary basis for a hypothesis of a modified phalanx, the question should be considered in a larger phylogenetic context because comparative developmental biology suggested that MP1 was not a phalanx.
The bidirectional growth as an ancestral condition of the autopod growth pattern, which changed to unidirectional in tetrapod mammals in the phylogenetic lineage, is not in disagreement with the histomorphology of human hand development. Indeed, up to the 23rd week of fetal age, growth was characterized by a symmetric proximal and distal ends length increment in metacarpals, proximal and mid phalanges (Pazzaglia et al. 2016, 2017). The data presented in this study confirmed that two different patterns of growth can be distinguished in human hand development related to age: the fetal phase with bidirectional and balanced growth in both metacarpals and phalanges, and the postnatal phase with growth in length restricted to the cartilage bone model extremity, where the epiphyseal center had initially formed. Later, the metaphyseal growth plate cartilage provided the remaining longitudinal growth up to skeletal maturity.
The IGR assessment in the normal hand series (age groups D–F) measured the whole growth period of fetal phase (growth bidirectional) and the postnatal period (growth unidirectional). This index documented the growth dynamics of metacarpals and phalanges, with full conformity to the position of the epiphyseal ossification centers. However, in terms of the aims of the present study, the relevant point was the documented, significant difference between the IGR of the 1st metacarpal and that of the 2nd–5th rays. Therefore, the hypothesis that the 1st segment of the thumb is a ‘true’ metacarpal indicates a need to explain the inversion of the unidirectional growth pattern of this segment.
In the detailed review of the evolutionary development and patterning digit identity, Reno et al. (2013) stated that the profound difference in selector gene expression territories during the 1st ray evolution had so altered the morphologies, growth patterns and responses of the 1st ray to the downstream gene expression that it was impossible to resolve the question of identity and homology of the mammalian 1st metacarpal. Further, they interpreted the triphalangeal thumb phenotype in humans as a complete homeotic transformation into an ancestral index finger associated with a proximal and distal ossification center and bidirectional growth.
In terms of developmental biology of autopod evolution in vertebrates, the number of digits and the digit segmentation varies between species (Wagner, 2005; Woltering & Duboule, 2010). The variability of the vertebrate autopod has so far raises unanswered questions, similar to that of the human 1st ray, such as digit loss, developmental variability and the origin of the avian hand (Vargas & Fallon, 2005; Bever et al. 2011; Young et al. 2011). Subtle Sox9 expression differences have been shown to be consistent with heterochrony detected in the stages of chondrification (Richardson et al. 2009; Montero et al. 2017) and may explain how digits differ in morphology. Hence, the analysis of phylogenetically related but phenotypically different species has provided important clues accounting for limb morphogenesis and the autopod development in particular (Moore et al. 2015). The studied differences support mechanisms of skeletal diversification based on a combination of a distinct distribution of finely tuned signals by the regulatory genes responsible for growth (Montero et al. 2017).
The triphalangeal thumb (TPT) phenotype in humans is an expression of a transmittable mutation producing an epiphyseal ossification and growth pattern of the anlage (abridged by the parametric length) similar to that of the other four ray segments. It is also worth emphasizing that the latter was associated in almost all cases with a trapezius 1st metacarpal saddle joint dysmorphism and with failure of the related muscle and tendon system development, which produced a non‐opposable 1st ray (also indicated by the term ‘five‐fingered hand’). The metanalysis for morphometry required selection of published X‐rays that satisfied the basic conditions of having fully developed hand segments, absence of other congenital defects, and good quality of the X‐ray reproduced image. All the analyzed cases were young adults with ossified epiphyses; therefore, the position of the ossification center or the presence of a proximal and distal center was not assessable. However, the IGR of the TPT 1st metacarpal showed the same growth pattern as those of the 2nd–5th hand metacarpals (IGR < 1), in contrast to that of the five proximal phalanges of the corresponding rays (IGR > 1). Therefore, these data did not give useful insights to explain the proximal location of the metacarpal ossification center in the normal hand.
Proximal and distal epiphyseal ossification centers were seldom reported in metacarpals or phalanges of otherwise normal hands, without an exclusive localization in the 1st ray metacarpal. De Jong et al.'s (2014) report of two siblings with the widest distribution so far documented of true, double ossification centers in both the hands and feet suggests a mutation that did not change the patterning of the autopod segments, but whose expression was limited to the anlage epiphyseal ossification and longitudinal growth pattern. From the metanalysis carried out in this study, the number of true, double ossification centers was difficult to ascertain because the earlier papers (Posener et al. 1939; Brailsford, 1943; Snodgrasse et al. 1955; Dreizen et al. 1965; Garn et al. 1972) also included features such as epiphyseal notches or partial clefts, which were interpreted as an incomplete or a late phase of the supernumerary ossification center fusion. The variability of the time of appearance of the epiphyseal center and the age of the child when the X‐rays were taken contributed to the uncertainty of frequency figures in the tubular bones of the hand. In general, proximal and distal epiphyseal centers in the same bone were rare observations that had a variable distribution in the autopod segments. Zuidam et al. (2006) calculated the length ratio between six metacarpals (with double ossification centers) and the corresponding 2nd metacarpal in the same hand. This ratio was compared with the values of the normal population given by Garn et al. (1972), resulting an increase in the 1st group compared with the normal population. More extensive research based on X‐ray IGR assessment in a normal hand series could give a more reliable incidences of this growth pattern, since an IGR ≈ 1 should correspond to a bidirectional, longitudinal growth pattern.
In the discussion of the anatomic definition of 1st ray segments, the TPT and bidirectional growth pattern are of particular interest. To the best of our knowledge, there have been no reports of a ray patterning = 4–4–4–4–4 in the evolutive lineage of therian tetrapods and anthropoids, which suggests that possible gene mutations similar to those documented in modern humans did not give a reproductive advantage and did not survive natural selection. Exclusive reports among human subjects can be explained by the wide diffusion of research and medical care in this species, prohibiting comparison. Beside TPT and the hand segment bidirectional growth pattern, the congenital hand malformations extensively studied in modern humans in general express mutations involving the Hox genes and the signaling pattern through overexpression or repression of Shh regulatory region of the limb bud (Tickle et al. 1975; Burke et al. 1995; Reno et al. 2008; Rosello‐Diez et al. 2011). The oldest classifications were exclusively based on the appearance of the clinical defect (Swanson & Brown, 1962; Swanson, 1964). Increased knowledge of the molecular basis of limb development prompted new classification schemes that also considered genetic and molecular pathways involved in skeletal segment patterning (Oberg et al. 2010; Oberg, 2014). In relation to the present discussion and the point concerning the missing thumb segment, the thumb hypoplasia (radial longitudinal deficiency) of the Blauth (1967) classification, may be relevant. This was updated by Manske and McCarrol (1992) who provided examples of severe metacarpal underdevelopment or absence as a specific entity.
In conclusion, the normal hand X‐ray morphometric study suggested that the missing thumb segment was the metacarpal. The proportion of the hand segments along each ray was respected if a correction for the missing segment of the 1st ray was introduced. The ray formula 3–4–4–4–4, the directional growth pattern, and the shape of the epiphyseal ends (including apical tufts of distal phalanges) remained remarkably constant in the tetrapod evolutionary lineage with only two examples of a different formula 2–4–4–4–4 in extant primates (Patel & Maiolino, 2016).
Variations of segment length and width occurred among taxa as an evolutive adaptation to tetrapedal and bipedal walking, climbing and suspension up to upper limb and tool manipulation. Otherwise, the lack of a triphalangeal thumb in the phylogenetic lineage and other human phenotypes did not seem sufficient to support the opposing theory of the proximal thumb segment as a modified metacarpal as opposed to the data provided by morphometry.
Conflict of interests
None of the authors had conflict of interests.
Acknowledgements
The study was supported by funds for current research of the DSMC of the University of Brescia. The postnatal X‐ray images were provided by Mariapia Bondioni, M.D. (Brescia Children Hospital), who provided valuable assistance in the selection, morphometry and analysis of radiographs. Francesca Pagani, Biol.D. (University of Milan) provided valuable assistance in statistical analysis, help and criticism at all stages of the drafting this paper.
References
- Almecija S, Smaers IB, Jungers WL (2015) The evolution of human ape hand proportion. Nat Commun. 6, 8717 10.1038/ncomms8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever GS, Gauthier JA, Wagner GP (2011) Finding the frame shift, digit loss developmental variability, and the origin of the avian hand. Evol Dev 13, 269–279. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG (2010) Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud 47, 931–936. [PubMed] [Google Scholar]
- Blauth W (1967) Der hypoplastischeDaumen. Arch OrthopUnfallchir 62, 225–246. [Google Scholar]
- Brailsford JF (1943) Variations in the ossifications of the one of the hand. J Anat 77, 170–175. [PMC free article] [PubMed] [Google Scholar]
- Burke AC, Nelson CE, Morgan BA, et al. (1995) Hox genes and the evolution vertebrate axial morphology. Development 121, 333–346. [DOI] [PubMed] [Google Scholar]
- Caffey J (1973) Pediatric X‐rays diagnosis. London, UK: Lloyd‐Luke (Medical Books). [Google Scholar]
- Christie A (1949) Prevalence and distribution of ossification centers in the new born infants. Am J Dis Child 77, 305–336. [DOI] [PubMed] [Google Scholar]
- Dobbs MB, Dietz FR, Gurnett CA, et al. (2000) Localization of dominantly inherited isolated triphalangeal thumb to chromosomal region 7q36. J Orthop Res 18, 398–406. [DOI] [PubMed] [Google Scholar]
- Dreizen S, Spirakis CN, Stone RE (1965) The distribution and disposition of anomalous notches in the non‐epiphyseal ends of human metacarpal shafts. Am J Phys Anthropol 23, 131–137. [DOI] [PubMed] [Google Scholar]
- Garn SM, Hertzog KP, Poznansky AK, et al. (1972) Metacarpophalangeal length in the evaluation of skeletal malformation. Radiology 105, 375–381. [DOI] [PubMed] [Google Scholar]
- Girisha KM, Bidchol AM, Kamath PS, et al. (2014) A novel mutation (9.1067377T) in zone of polarizing activity regulatory sequence (ZRS) causes variable limb phenotypes in Werner mesomelia. Am J Med Genet A 16A, 898–906. [DOI] [PubMed] [Google Scholar]
- Greunlich WW, Pyle JS (1959) Radiographic Atlas of Skeletal Development of Hand and Wrist. 2nd edn Stanford: Stanford University Press. [Google Scholar]
- Guillem P, Demondion X, Drizenko A, et al. (1999) La biophalange du pouce. Revue générale de la literature. Morphologie 83, 27–31. [PubMed] [Google Scholar]
- Haines RW (1938) The primitive form of epiphysis in the long bones of tetrapods. J Anat 72, 303–343. [PMC free article] [PubMed] [Google Scholar]
- Haines RW (1974) The pseudoepiphysis of the first metacarpal of man. J Anat 117, 145–158. [PMC free article] [PubMed] [Google Scholar]
- Heiss H (1953) Beiderseitigen kongenitaledaumenlose Funffigerhandbei Mutter und Kind. Zeithschr Anat Entwicklungsgeschicte 120, 488–492. [Google Scholar]
- Heutink P, Zguricas J, von Osteont L, et al. (1994) The gene for triphalangeal thumb maps to the subtelomeric region of chromosome 7q. Nat Genet 6, 287–292. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Melenhorst WP, Houpt P (2014) Complete pseudoepiphyses with associated enhanced growth in hands and feet: a report of two siblings. Case report. J Hand SurgAm 39, 488–492. [DOI] [PubMed] [Google Scholar]
- Kivell TL (2015) Evidence in hand: recent discoveries and early evolution of human manual manipulation. Philos Trans R Soc., band B Biol Sci, 370, 101098 10.1098/rstb.2015.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MMC, Garn SM (1967) Pseudoepiphyses or notches in the non‐epiphyseal end of metacarpal bones in healthy children. An Rec 152, 263–272. [Google Scholar]
- Limb D, Laughenbury PR (2012) The prevalence of pseudoepiphyses in metacarpals of the growing hand. J Hand SurgEur. 37, 678–681. [DOI] [PubMed] [Google Scholar]
- Manske PR, McCarrol HR Jr (1992) Reconstruction of the congenital deficient thumb. Hand Clin 8, 177–196. [PubMed] [Google Scholar]
- Marzke MW (1997) Precision grip, hand morphology, and tools. Am J Phys Anthropol 102, 91–110. [DOI] [PubMed] [Google Scholar]
- Marzke MW, Marzke RF (2000) Evolution of the human hand: approaches to acquiring, analysing and interpreting the anatomical evidence. J Anat 197, 121–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milch H (1951) Triphalangeal thumb (TPT). J Bone Joint Surg 33A, 692–697. [PubMed] [Google Scholar]
- Mittra ES, Smith HF, Lemelin P, et al. (2007) Comparative morphometrics of primate apical tufts. Am J Phys Anthropol 134, 449–459. [DOI] [PubMed] [Google Scholar]
- Montero JA, Lorda‐Diez CI, Francisco‐Morcillo J, et al. (2017) Sox9 expression in amniotes: differences in the formation of digits. Front Cell Dev Biol 5, 23 10.3389/fcell.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TY, Organ CL, Edwards SW, et al. (2015) Multiple phylogenetically distinct events shaped the evolution of limb skeletal morphogenesis associated with bipedalism in the jerboas. Curr Biol 25, 2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Furukawa H (1997) A rare case of complete proximal epiphyses (so‐called pseudoepiphyses) of the metacarpal and metatarsal bones in the human. Ann Anat 179, 549–551. [DOI] [PubMed] [Google Scholar]
- Oberg KC (2014) Review of the molecular development of the thumb: digit primera. Clin Orthop Relat Res 472, 1101–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg KC, Feenstra JM, Manske PR, et al. (2010) Developmental biology and classification of congenital anomalies of the hand and upper extremity. J Hand Surg Am 35, 2066–2076. [DOI] [PubMed] [Google Scholar]
- Ogden JA, Ganey TM, Light TR, et al. (1994) Ossification and pseudoepiphysis formation in the ‘nonepiphyseal’ ends of bones of the hands and feet. Skel Radiol 23, 3–13. [DOI] [PubMed] [Google Scholar]
- Patel BA, Maiolino SA (2016) Morphological diversity in the digital rays of primate hands In: The Evolution of the Primate Hand (eds. Kivell TL, Lamelin P, Richmond BG, Schmitt D.), pp. 55–100. New York: Springer Science + Business Media. [Google Scholar]
- Pazzaglia UE, Congiu T, Sibilia V, et al. (2016) Relationship between chondrocyte maturation cycle and the endochondral ossification in the diaphyseal and epiphyseal ossification centers. J Morphol 277, 1187–1198. [DOI] [PubMed] [Google Scholar]
- Pazzaglia UE, Congiu T, Sibilia V, et al. (2017) Growth and shaping of metacarpal and carpal cartilage anlagen: application of morphometry to the development of short and long bone. A study of human hand anlagen in the fetal period. J Morphol 278, 887–895. [DOI] [PubMed] [Google Scholar]
- Posener K, Wolker E, Weddel G (1939) Radiographic studies of the metacarpal and metatarsal bones in children. J Anat 74, 76–79. [PMC free article] [PubMed] [Google Scholar]
- Reno PL, McBurney DL, Lovejoy CO, et al. (2006) Ossification of the mouse metatarsal: differentiation and proliferation in the presence/absence of a defined growth plate. An Rec 288A, 104–118. [DOI] [PubMed] [Google Scholar]
- Reno PL, Horton WE Jr, Elsey RM, et al. (2007) Growth plate formation and development in alligator and mouse metapodials: evolutionary and functional implications. J ExpZool (MolDevEvol) 308B, 283–296. [DOI] [PubMed] [Google Scholar]
- Reno PL, McCollum MA, Cohn MJ, et al. (2008) Patterns of correlation and covariation of anthropoid distal forelimb segments correspond to Hoxd expression territories. J Exp Zool B Mol Dev Evol 310B, 240–258. [DOI] [PubMed] [Google Scholar]
- Reno PL, Horton WE Jr, Lovejoy CW (2013) Metapodial or phalanx? Evolutionary and developmental perspective on the homology of the first ray's proximal segment. J Exp Zool B Mol Dev Evol 320B, 276–285. [DOI] [PubMed] [Google Scholar]
- Reynolds LR (1917) Hyperphalagism accompanied by supernumerary epiphyses and muscular deficiencies. Anat Rec 13, 113–126. [Google Scholar]
- Richardson MK, Gobes SM, van Leeuwen AC, et al. (2009) Heterochrony in limb evolution: development mechanisms and natural selection. J Exp Zool B Mol Dev Evol 312, 639–664. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Lanfer E, et al. (1993) Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 57, 1401–1416. [DOI] [PubMed] [Google Scholar]
- Rosello‐Diez A, Ros MA, Torres M (2011) Diffusable signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science 332, 1086–1088. [DOI] [PubMed] [Google Scholar]
- Shively MJ (1978) First metacarpal bone or proximal phalanx? Vet Radiol Ultrasound 19, 50–52. [Google Scholar]
- Snodgrasse RM, Dreizen S, Parker GS, et al. (1955) Serial sequential development of anomalous metacarpals and phalangeal ossification centers in human hand. Growth 19, 307–322. [PubMed] [Google Scholar]
- Swanson AB (1964) A classification for congenital malformation of the hand. N Y Bull Acad Med 10, 166–169. [Google Scholar]
- Swanson AB, Brown K (1962) Hereditary triphalangeal thumb. J. Hand 53, 259–265. [Google Scholar]
- Thompson A (1869) On the differences in the mode of ossification of the first and other metacarpals and metatarsal bones. J Anat 3, 131–146. [PMC free article] [PubMed] [Google Scholar]
- Tickle C, Summerbell D, Wolpert L (1975) Positional signalling and specification of digits in chick limb morphogenesis. Nature 254, 199–202. [DOI] [PubMed] [Google Scholar]
- Tocheri MW, Orr CM, Jakorsky MC, et al. (2008) The evolution history of the hominin hand since the last common ancestor of Pan and Homo. J Anat 212, 644–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhthoff HK (1990) The embryology of the human locomotor system Heidelberg: Springer Verlag. [Google Scholar]
- Valenzuela CY, Berrios‐Loyola R, Canals A (2009) The first metacarpal in the thumb in the first phalange. Evo‐devo implications. Int J Morphol 27, 985–988. [Google Scholar]
- Vargas AQ, Fallon JF (2005) The digits of the wing of birds are 1, 2, and 3. A review. J Exp Zool B Mol Dev Evol 304, 206–219. [DOI] [PubMed] [Google Scholar]
- Vogt EC, Vickers VS (1938) Osseous growth and development. Radiology 31, 441–444. [Google Scholar]
- Wagner GP (2005) The developmental evolution of avian digit homology: an update. Theory Biosci 124, 165–183. [DOI] [PubMed] [Google Scholar]
- Warm A, Di Pietro C, D'Agrose F, et al. (1988) Non‐opposable triphalangeal thumb in an Italian family. J Med Gen 25, 337–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczoreck D, Pawlik B, Akarsu NA, et al. (2010) A specific mutation in the distal sonic hedgehog (SHH) cis‐regulator (ZRS) causes Werner mesomelic syndrome (WMS) while complete ZRS duplications underlie Haas type polysyndactyly (PPD) with or without triphalangeal thumb. Hum Mut 31, 81–89. [DOI] [PubMed] [Google Scholar]
- Woltering JM, Duboule D (2010) The origin of digits: expression patterns versus regulatory mechanisms. Dev Cell 18, 526–532. [DOI] [PubMed] [Google Scholar]
- Young NM, Hallgrimsson E (2005) Serial homology and the evolution of mammalian limb covariation structure. Evolution 59, 2691–2704. [PubMed] [Google Scholar]
- Young RL, Bever GS, Wang Z, et al. (2011) Identity of the avian wing digits: problems resolved and unsolved. Dev Dynam 240, 1042–1053. [DOI] [PubMed] [Google Scholar]
- Zguricas J, Snijders PJ, Horius SC, et al. (1994) Phenotypic analysis of triphalangeal thumb and associated hand malformations. J Med Gen 31, 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zguricas J, Dijustra PF, Gelsema ES, et al. (1997) Metacarpal phalangeal pattern (MCPP) profile analysis in a family with triphalangeal thumb. J Med Gen 34, 55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidam JM, Dees EEC, Lequin MH, et al. (2006) The effects of the epiphyseal growth plate on the length of the first metacarpal in triphalangeal thumb. J Hand Surg Am 31A, 353–360. [DOI] [PubMed] [Google Scholar]
- Zuidam JM, Selles RW, deKraker M, et al. (2016) Outcome of two types of surgical correction of the extra phalanx in triphalangeal thumb: is there a difference? J Hand Surg Eur 41, 253–257. [DOI] [PubMed] [Google Scholar]


