Abstract
Klippel–Feil syndrome is a congenital vertebral anomaly, which is characterised by the fusion of at least two cervical vertebrae and a clinically broad set of symptoms, including congenital scoliosis and elevated scapula (Sprengel's deformity). Klippel–Feil syndrome is associated with mutations in MEOX1. The zebrafish mutant choker (cho) carries a mutation in its orthologue, meox1. Although zebrafish is being increasingly employed as fidelitous models of human spinal disease, the vertebral column of Meox1‐deficient fish has not been assessed for defects. Here, we describe the skeletal defects of meox1 cho mutant zebrafish utilising alizarin red to stain bones and chemical maceration of soft tissue to detect fusions in an unbiased manner. Obtained data reveal that meox1 cho mutants feature aspects of a number of described symptoms of patients who suffer from Klippel–Feil syndrome and have mutations in MEOX1. These include vertebral fusion, congenital scoliosis and an asymmetry of the pectoral girdle, which resembles Sprengel's deformity. Thus, the meox1 cho mutant zebrafish may serve as a useful tool to study the pathogenesis of the symptoms associated with Klippel–Feil syndrome.
Keywords: disease modelling, Klippel–Feil syndrome, MEOX1, mesenchyme homeobox 1, spinal disease, zebrafish
Introduction
Klippel–Feil syndrome is a congenital vertebral anomaly, which is diagnosed by the fusion of at least two cervical vertebrae (Klippel & Feil, 1912). Clinically, there is a broad spectrum of symptoms including Sprengel's deformity (congenitally elevated scapula), congenital scoliosis, kyphosis and a range of visceral defects. Klippel–Feil syndrome is reported in 1 : 40 000 live births; however, this is likely to be lower than the actual incidence, as the manifestation of Klippel–Feil syndrome is broad, such that disease severity ranges from seemingly asymptomatic to requiring surgical intervention (Tracy et al. 2004; Krakow, 2018). As there currently is no cure, treatment is focused on symptoms, which are managed through either surgical or lifestyle interventions (Krakow, 2018). Reflecting its clinical heterogeneity, mutations in different genes have been associated with Klippel–Feil syndrome, including MEOX1, GDF3/6, RIPPLY2 and PAX1 (McGaughran et al. 2003; Tassabehji et al. 2008; Ye et al. 2010; Mohamed et al. 2013; Karaca et al. 2015).
MEOX1 encodes mesenchyme homeobox 1, a transcription factor expressed in somites and known to be involved in the development of vertebral elements (Candia et al. 1992; Candia & Wright, 1996; Rodrigo et al. 2004; Skuntz et al. 2009; Nguyen et al. 2014). In addition to its DNA‐binding homeobox domain, the murine Meox1 orthologue has also been shown to directly bind Pax1 in vitro (Stamataki et al. 2001). Furthermore, it has been demonstrated that Meox1 together with Pax1 and Pax9 directly regulate Nkx3‐2, which impacts on axial skeleton development (Lettice et al. 1999; Rodrigo et al. 2004; Skuntz et al. 2009). In addition, the zebrafish orthologue, Meox1, is known to directly inhibit the cell cycle regulator Ccnb1 (Nguyen et al. 2017). Despite the clinical heterogeneity of Klippel–Feil syndrome, patients who harbour mutations in MEOX1 have a set of common reported symptoms. These common symptoms include congenital scoliosis, Sprengel's deformity and the presence of an ectopic omovertebral bone, which connects the scapula with a vertebra (Bayrakli et al. 2013; Mohamed et al. 2013).
Meox1‐deficient mice share cervical vertebral fusions with human Klippel–Feil syndrome patients, who harbour mutations in MEOX1 (Skuntz et al. 2009). However, in contrast to human patients, Meox1 loss‐of‐function mice neither develop pectoral girdle asymmetry nor exhibit congenital scoliosis (Skuntz et al. 2009; Bayrakli et al. 2013; Mohamed et al. 2013). In addition, meox1‐deficient mice occasionally feature malsegmentation defects in more posterior regions of the spine, not reported in humans. Zebrafish (Danio rerio) has become a popular model system due to their genetic tractability, ex utero development and amenability to microscopy (Lieschke & Currie, 2007). Furthermore, zebrafish are increasingly employed to model human spinal diseases with a high degree of fidelity (Boswell & Ciruna, 2017). The zebrafish mutant choker (cho) carries a loss‐of‐function mutation in its orthologue of MEOX1, meox1 (Nguyen et al. 2014). meox1 cho zebrafish have been previously shown to have somite defects in the posterior hindbrain region and various stem cell defects (van Eeden et al. 1996; Kelsh et al. 1996; Nguyen et al. 2014, 2017). Here, we describe bone defects of meox1 cho mutants using alizarin red to stain bone and assess the suitability of meox1 cho zebrafish as a disease model of Klippel–Feil syndrome. We characterise distinct bone defects in meox1 cho zebrafish, including vertebral fusion, congenital scoliosis and an asymmetry of the pectoral girdle, suggesting that Meox1‐deficient zebrafish might be used to model Klippel–Feil syndrome.
Materials and methods
Animal husbandry
The meox1 cho (choker) line was used in the Tübingen genetic background for all experiments (van Eeden et al. 1996; Kelsh et al. 1996). Genotyping for the meox1 cho allele was performed as described (Nguyen et al. 2014). All zebrafish used were bred and raised as approved by MARP 2017/240.
Alizarin red staining
For whole‐mount preparations, adult zebrafish were killed in tricaine and staged by standard length prior to fixation in 4% paraformaldehyde (w/v) for 8–72 h, where fixation duration depended upon specimen size. Adult zebrafish were descaled, skinned and eviscerated before fixation. Larval zebrafish were fixed in ethanol for 4 h to overnight. Samples fixed in paraformaldehyde were dehydrated first in a solution containing 1 part ethanol to 1 part phosphate‐buffered saline for 4 h to overnight, followed by ethanol for 4 h to overnight. After dehydration or ethanol fixation, samples were degreased in acetone for a period similar to their fixation period. Subsequently, samples were stained with alizarin red S, bleached and then macerated following established methodology.
To obtain individual and digested adult bones, adults were descaled, skinned and eviscerated prior to fixation in ethanol. Samples were then directly transferred from the fixative to the staining solution. Thereafter, soft tissues were completely macerated in 1% KOH (w/v) at 37 °C, yielding individual bones. The bones were briefly rinsed in water immediately prior to imaging.
Macrophotography
Whole‐mount adult zebrafish were photographed using a NEX‐5 camera equipped with an E 18–55 mm f/3.5–5.6 OSS objective (Sony). Samples were illuminated with a lightbox. Photographs were subsequently processed in Darktable (v. 2.2.5) software.
Fluorescence microscopy
Dissected pectoral girdles dissected from adult whole‐mount alizarin red S‐stained preparations were imaged using an MVX10 stereomicroscope equipped with an DP72 camera, MV PLAPO 1 × objective and an RFP filter (Olympus). All images were processed using the GNU Image Manipulation Programme (v. 2.9.6) and Darktable (v. 2.2.5).
Optical slices of whole‐mount larval preparations and dissected bones were obtained using an ImagerZ1 microscope with ApoTome and AxioCam HRm camera (Zeiss). A combination of 5 ×, 10 × and 20 × dry objectives with a Texas Red filter cube were used. Maximum intensity and sum slices Z‐projections were obtained using Fiji Is Just ImageJ. Where necessary, image tiles were combined using Hugin (v. 2017.0). Images were further processed using the GNU Image Manipulation Programme (v. 2.9.6) and Darktable (v. 2.2.5).
Results
The majority of adult meox1 cho homozygotes exhibit scoliosis
Externally visible manifestations of spinal malformation were not observed in live adult sibling zebrafish. In contrast, scoliosis occurred in two‐thirds of adult meox1 cho homozygotes across two independent clutches (Fig. S1; clutch 1: n = 20/30; clutch 2: n = 14/22). Thus, a lack of Meox1 is associated with the development of scoliosis in adult zebrafish.
Adult meox1 cho zebrafish feature fused vertebrae
To visualise bones of adult meox1 cho homozygotes and siblings, whole‐mount alizarin red staining was performed. Although two‐thirds of adult meox1 cho homozygous zebrafish develop externally discernible congenital scoliosis, all examined homozygotes exhibited bone defects in the axial skeleton. Bone defects in meox1 cho homozygotes were largely confined to the anterior axial skeleton. The exoccipital bone, which is readily distinguishable in siblings on account of the exoccipital foramen (Fig. 1A′, arrowhead; n = 6/6), was reduced in all analysed meox1 cho mutants (Fig. 1B′, arrowhead; n = 6/6). In siblings, neural arches appeared as long, slender, dorsoposterior projections from centra (Fig. 1A′; n = 6/6); however, in meox1 cho mutants, the neural arches assumed an altered morphology, where neural arches were anteroposteriorly broadened and dorsoventrally shortened in comparison to their siblings (Fig. 1B′; n = 6/6). In meox1 cho mutant zebrafish, vertebral fusions were observed between variable vertebrae at the anterior end of the vertebral column between neural arches (Fig. 1B′, arrowheads; n = 6/6).
Figure 1.
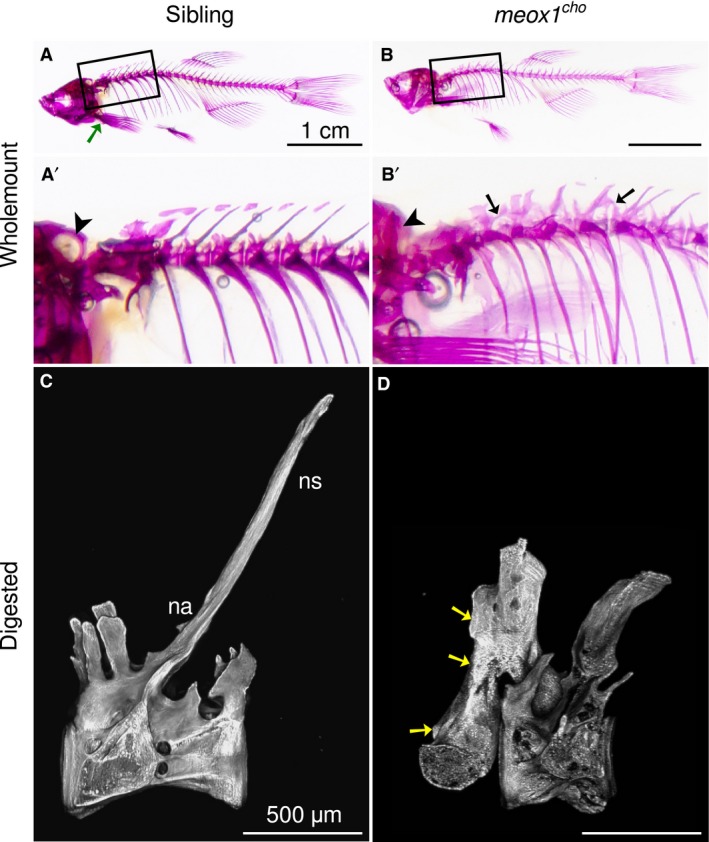
Bone defects coalesce anteriorly in meox1 cho mutant zebrafish. (A,B) Lateral views of whole‐mount alizarin red‐stained 32‐mm standard length adult siblings and homozygotes reveal that bone defects within meox1 cho homozygotes are confined to the axial skeleton. The boxed regions are magnified in (A′) and (B′). A green arrow indicates the location of the pectoral girdle. (A′,B′) The exoccipital foramen is readily discerned in siblings (A′, arrowhead, n = 6/6) and is not evident in whole‐mount meox1 cho homozygous mutants (B′, arrowhead, n = 6/6). Vertebral fusion is evident in vertebræ across neural arches (B′, arrows, n = 6/6). (C) Lateral maximum intensity Z‐projection of a chemically isolated vertebra shows the morphology typical of a vertebra from the anterior region of the wild‐type zebrafish spine. (D) Lateral view of a chemically isolated vertebra involved in an apparent anterior fusion in a meox1 cho adult mutant. Fusion occurred between neural arches (arrows); however, only one vertebral centrum was incorporated. na, neural arch; ns, neural spine. Scale bars: 1 cm (A,B); 500 μm (C,D).
To further assess whether these defects are bona fide fusions, whole, alizarin red‐stained adult fish were subjected to total chemical digestion. This allowed for the removal of all soft tissues leaving only mineral bone remaining. Complete digestion of soft tissues confirmed the anterior fusions observed in whole‐mount preparations. Furthermore, these vertebral fusions were partial, i.e. a single vertebra was fused to its own neural arches in addition to that of its neighbouring vertebra (Fig. 1D, arrows).
Vertebrae 1–4 in the zebrafish comprise the Weberian vertebrae, which are distinguishable by specialised modifications and form part of the Weberian apparatus. The Weberian apparatus enables the transduction of vibrations from the swimbladder to the fish's inner ear. In neither meox1 cho homozygotes nor siblings were these vertebrae readily discernible in whole‐mount preparations due to the highly specialised and spatially overlapping Weberian ossicles surrounding the vertebral centra. However, chemical digestion of soft tissues enabled the isolation of these fragile and deep bones with minimal handling. In all siblings, the Weberian neural arches of vertebrae 3 and 4 did not form a contiguous bony element; rather, they were conterminous and separable by maceration of connective soft tissues (Fig. 2A–E). In homozygous meox1 cho mutant zebrafish, the corresponding centra and its neural arches were observed to be fused as a single bony unit (Fig. 2F, dotted line). Occasionally, fused elements consisted of a centrum, its neural arches and a neural arch of its immediate neighbour (Fig. 2G), or these elements were completely fused and consisted of two centra and their neural arches (Fig. 2H). Where vertebral fusion did occur, synostoses were between neural arches and not between centra, as the intervertebral space remained perceptible (Fig. 2H, arrowhead).
Figure 2.
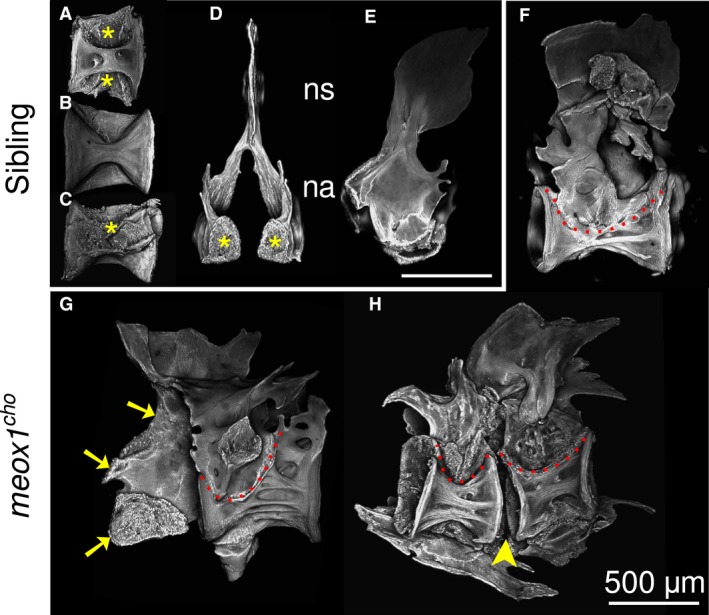
The anterior‐most vertebral elements in meox1 cho mutants have fusions within and across vertebrae. (A–E) Maximum intensity Z‐projections of Weberian centra (respectively, dorsal, ventral and lateral views) and neural arches (respectively, medioanterior and lateral views) isolated from siblings reveal that centra and neural arches are discrete and conterminous bony elements within the Weberian apparatus. Articular surfaces are apparent (asterisks). (F–H) In meox1 cho homozygotes, neural arches are fused to centra (dotted lines), excepting where a centrum has fused to its arches and an arch of its neighbour (G, arrows). Where fusion incorporated two centra into a single bony element, the intervertebral gap was discernible (arrowheads). (F–H) Lateral views, where anterior is left. na, neural arch; ns, neural spine. Scale bars: 500 μm.
In addition to fusions in anterior regions of the meox1 cho mutant vertebral column, fusions were sporadically detected throughout the entirety of mutant spines following digestion (Fig. 3B,D). Excluding the specialised Weberian vertebrae, all precaudal and caudal vertebrae in digested sibling preparations were discrete bony elements broadly comprising of a centrum, neural arches and haemal arches (Fig. 3A,C). In digests of meox1 cho mutant zebrafish, fused vertebrae were sporadically present in all regions of the spine, where fusion was between either neural (Fig. 3D, arrows) or haemal arches (Fig. 3B,B′, arrow). Similar to fusions of more anterior vertebrae, an intervertebral space was detectable (Fig. 3B,D,D′, arrowheads). Thus, vertebral fusions in meox1 cho mutants were confined between vertebral arches.
Figure 3.
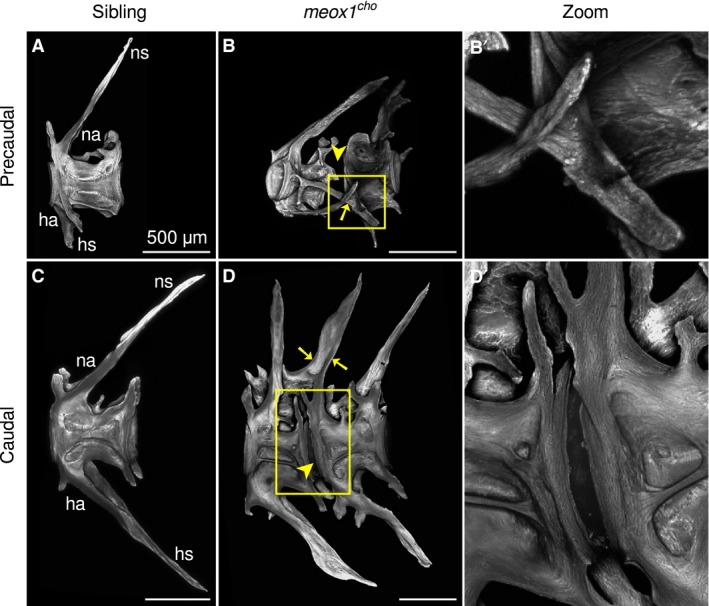
Adult meox1 cho zebrafish have fused precaudal and caudal vertebrae. (A,C) Maximum intensity Z‐projections of chemically isolated precaudal (A) and caudal (C) sibling vertebrae. (B,D) In meox1 cho homozygotes, vertebral fusion occurs between haemal (B, arrow) and neural (D, arrows) arches; however, the intervertebral space between centra remains appreciable (arrowheads). Boxed regions are magnified in (B′) and (D′). (B′) The right haemal arch of the more anterior vertebra has extended more leftward than the right haemal arch, which has fused to the right haemal arch of the neighbouring vertebra. (D′) A magnified view of the intervertebral region shows that the vertebral centra are discrete despite their proximity in meox1 cho homozygotes. All panels are lateral views, where anterior is left. ha, haemal arch; hs, haemal spine; na, neural arch; ns, neural spine. Scale bars: 500 μm.
Meox1 is involved in neural arch fusion at the midline
Excepting the distinct Weberian vertebrae, adult zebrafish vertebrae comprise broadly of a centrum, neural arches, a neural spine and, where applicable, haemal arches and a haemal spine; Fig. 4A,B,E,F). In contrast to siblings, the vertebrae of meox1 cho mutants, which are not involved in a vertebral fusion, comprise these same elements. However, in the case of more posterior precaudal and caudal vertebrae, the neural arches and occasionally the haemal arches fail to join at the midline and project two spines, one for each arch (Fig. 4C,D,G,H, arrowheads, boxes). Moreover, the right neural arch was observed to be shorter and projected less posteriorly than left neural arches (Fig. 4C′,D′,G′,H′), indicating that Meox1 is involved in neural arch fusion at the midline.
Figure 4.
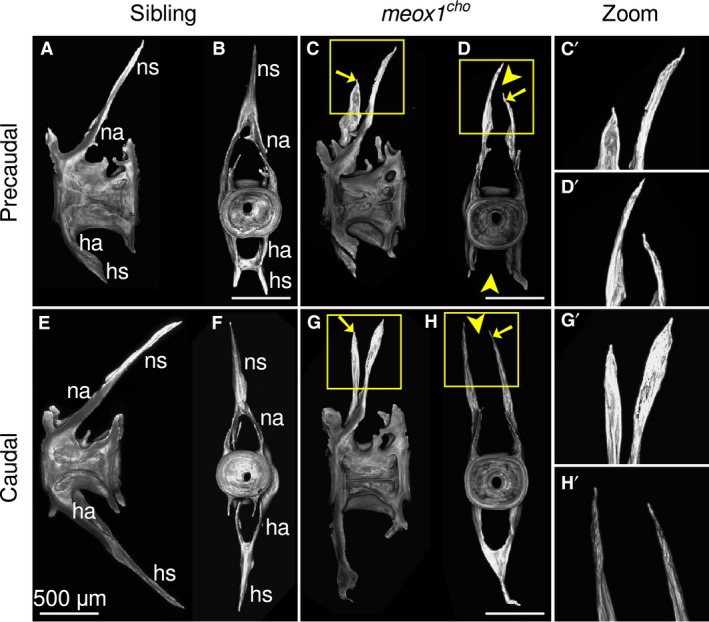
Neural arches in meox1 cho mutants are typically unfused at the midline and are asymmetric. (A,B,E,F) Maximum intensity Z‐projections of precaudal and caudal vertebrae both laterally (A,E) and posteriorly (B,F) show that vertebrae are symmetric, and that vertebral arches fuse at the midline and project a single neural or haemal spine. (C,D,G,H) Exemplar precaudal and caudal vertebrae of an adult meox1 cho mutant viewed both laterally (C,C′,G,G′) and posteriorly (D,D′,H,H′) show that the neural arches and occasionally the haemal arch is unfused at the midline (arrowheads). The left neural arch is more anterior and shorter than the right (arrows). (C′,D′,G′,H′) Magnified views of the boxed regions in (C,D,G,H). ha, haemal arch; hs, haemal spine; na, neural arch; ns, neural spine. Scale bars: 500 μm.
meox1 cho mutant zebrafish have pectoral asymmetry
Patients suffering from Klippel–Feil syndrome are often diagnosed with Sprengel's deformity, where one scapula is elevated relative to the other. Given the asymmetry in meox1 cho mutant neural arches described above and that patients with Klippel–Feil syndrome with mutations in MEOX1 have the asymmetry that is Sprengel's deformity (Bayrakli et al. 2013; Mohamed et al. 2013), the pectoral girdle was assessed. Despite the anatomical differences between fish and human pectoral girdle architecture, such as the presence of a cleithra in zebrafish but not humans, we find that in the dissected pectoral girdles of meox1 cho homozygotes, the left side was observed to be more anterior than the right (Fig. 5B; n = 3/3). Those of sibling zebrafish were symmetrical (Fig. 5A; n = 3/3). In all analysed fish, it was noted that the left postcleithra of mutant pectoral girdles had projected anteriorly, whereas the right postcleitha were unremarkable (Fig. 5B, arrow). Thus, meox1 cho mutant zebrafish feature defects similar to the human Sprengel's deformity.
Figure 5.
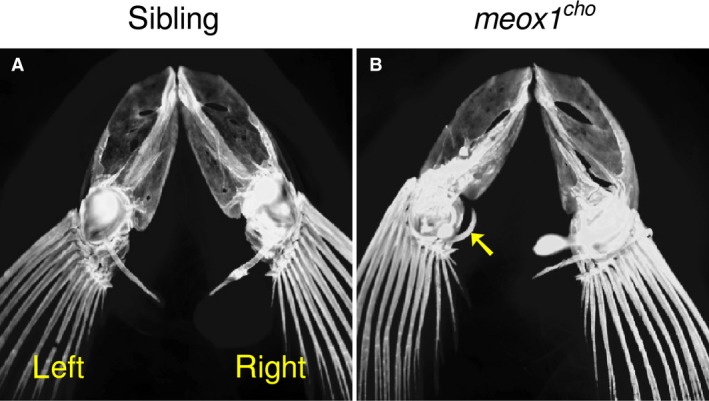
The pectoral girdle of meox1 cho mutant zebrafish is more anterior on the left than the right. (A) A dorsal view of pectoral girdles of cleared, alizarin red‐stained sibling zebrafish shows that they are symmetrical (n = 3/3). (B) In meox1 cho homozygotes, the left side is more anterior than the right (n = 3/3). The left postcleithrum has extended anteriorly (arrowhead).
Vertebral fusions within meox1 cho larvae occur at the onset of vertebral development
In zebrafish, the onset of vertebral formation is initiated at 4.6 mm standard length, where vertebral centra arise as direct ossifications of the notochord (Bird & Mabee, 2003; Inohaya et al. 2007). To assess defects at the onset of vertebral development, the early larval centra of both siblings and meox1 cho homozygotes were subjected to whole‐mount alizarin red staining (Fig. 6). At 4.6–4.9 mm standard length, centra of sibling zebrafish were discrete with well‐defined borders (Fig. 6A; n = 4/4), whereas fusions were observed in meox1 cho homozygotes variably between vertebrae 1–4 (Fig. 6B, arrows; n = 4/5). At earlier stages, all centra are discrete in meox1 cho larvae and are unaffected upon comparison with their siblings (data not shown). All larval centra posterior to vertebra 4 were unremarkable in analysed meox1 cho mutant larvae in comparison to their siblings of similar standard length (data not shown). In conclusion, Meox1 is involved in the early segmentation of the anterior vertebrae.
Figure 6.
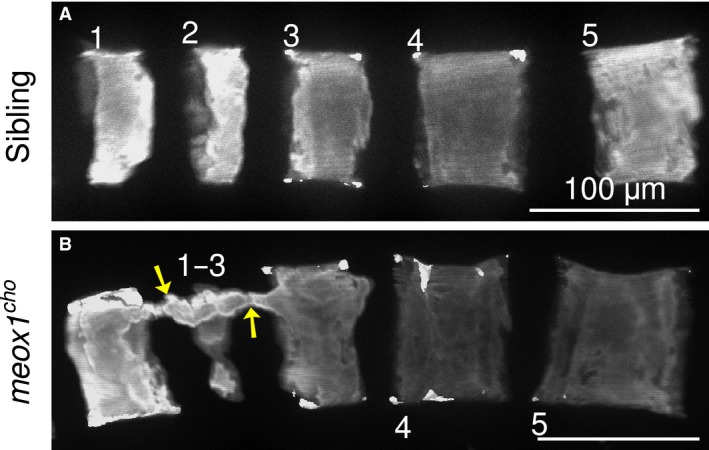
Vertebral fusion is observed shortly after the formation of the anterior‐most vertebrae. (A) Lateral maximum intensity Z‐projection of the anterior‐most vertebrae of a 4.6–4.9‐mm standard length larval sibling reveals that centra are discrete and well defined (n = 4/4). (B) Fusion between vertebrae 1–3 is evident as a bony bridge spanning the dorsum of developing centra in this 4.6–4.9‐mm standard length meox1 cho mutant (arrows). Fusions were variably observed between vertebrae 1–4 in mutants of the same standard length (n = 4/5). Numbers indicate vertebrae. Scale bars: 100 μm.
Discussion
Given the differences between the proclivities of bipedal humans and quadrupedal animals to the development of spinal disease, the use of some popular model organisms, such as mice, to fideliously model human spinal diseases may prove challenging. For example, Meox1 −/− mice share only anterior axial synostoses with human Klippel–Feil syndrome patients, who have mutations in MEOX1 (Skuntz et al. 2009; Bayrakli et al. 2013; Mohamed et al. 2013). Despite their lack of a load‐bearing spinal column and being more phylogenically removed from humans than mice, fish such as zebrafish are frequently used to model human spinal disease (Gorman & Breden, 2007; Boswell & Ciruna, 2017).
The hallmark of Klippel–Feil syndrome is the fusion of cervical vertebrae. Cervical vertebrae are unique to tetrapods and are not present in fish. Nonetheless, meox1 cho mutant zebrafish maintain skeletal defects in an equivalent anterior region immediately posterior to the cranium at both larval and adult stages of life. Human Klippel–Feil syndrome patients with mutations in MEOX1 have deformities in the occipital bone, such that the occipital bone is fused to parts of the atlas and axis, or is deformed (Bayrakli et al. 2013; Mohamed et al. 2013). Therefore, the synostoses associated with Klippel–Feil syndrome are not confined to cervical vertebrae; rather, they are confined to an anterior region that includes the cervical vertebrae and posterior occipital bones in humans. In agreement with patient observations, the exoccipital bone in meox1 cho mutant zebrafish was also misshapen. Similar in Meox1 −/− mice, synostoses affect both occipital bones and cervical vertebrae (Skuntz et al. 2009). These across‐species data suggest that MEOX1 and its orthologues are involved in patterning an anterior region of the axial skeleton, which includes the anterior‐most vertebrae and the posterior exoccipital bones across vertebrates. In addition to occipital defects detected in human patients, meox1 cho mutant zebrafish have synostoses within and between anterior vertebrae. Therefore, notwithstanding their lack of cervical vertebrae, homozygous meox1 cho zebrafish feature the characteristic localised fusions associated with Klippel–Feil syndrome.
Although autonomous segmentation of the notochord was suggested as the developmental origin of segmental patterning of centra in teleosts, an influence by the paraxial mesoderm was demonstrated (Lleras‐Forero et al. 2018). Interestingly, loss of Meox1 function within the paraxial mesoderm, as seen within meox1 cho mutants (Nguyen et al. 2014), leads to vertebrae defects. Therefore, the signal from the paraxial mesoderm that influences the segmentation of centra might be disrupted in the absence of Meox1 within the anterior region.
In addition to cervical fusion and congenital scoliosis, patients suffering from Klippel–Feil syndrome are also diagnosed with Sprengel's deformity (congenitally elevated scapula) and ectopic omovertebral bone formation. Adult meox1 cho homozygotes displayed comparable bone defects, with the exception of an ectopic bony connection between the pectoral girdle and a vertebra. This is striking, as there are a number of anatomical differences between the zebrafish and human pectoral girdle, such as the location of the girdle relative to the axial skeleton and whether dermal bones are present (McGonnell, 2001). However, with the embryological origins of the omovertebral bone being contentious (Matsuoka et al. 2005; Dhir et al. 2018), determination of the structure homologous to the omovertebral bone would be controversial. Klippel–Feil syndrome is noted for its heterogeneity in severity. Accordingly, two‐thirds of adult meox1 cho homozygous zebrafish developed congenital scoliosis. Furthermore, pectoral girdle asymmetry was observed in meox1 cho, which is reminiscent of Sprengel's deformity. In patients suffering from Sprengel's deformity, the left shoulder is more often affected than the right, with bilateral scapular elevation occurring rarely (Di Gennaro et al. 2012). Zebrafish meox1 cho mutants are also characterised by a left–right bias in the pectoral girdle, with the left side being positioned more anteriorly than the right. Therefore, meox1 cho mutants feature aspects of a number of symptoms, which are characteristic of Klippel–Feil syndrome associated with mutations in MEOX1. Interestingly, expression of Nkx3‐2, whose expression is directly regulated by Meox1, exhibits transient left–right asymmetry in mice and chick (Schneider et al. 1999). Although the role of Nkx3‐2 in axial skeletal development and chondrogenesis has been demonstrated, the significance of its asymmetric expression is hereunto unknown (Schneider et al. 1999). Therefore, it could be speculated that the aetiology of Sprengel's deformity is associated with developmental dysregulation of NKX3‐2. A future study could explore what role Meox1 has in left–right patterning using the meox1 cho mutant zebrafish model.
Although meox1 cho zebrafish recapitulate aspects of Klippel–Feil syndrome, these mutant zebrafish conspicuously harbour fusions throughout the vertebral column. Additionally, meox1 cho homozygotes exhibit a failure of the left and right vertebral arches of precaudal and caudal vertebrae to fuse at the midline, with neural arches being more affected than haemal arches. Within the zebrafish precaudal and caudal vertebrae, the aforementioned vertebral synostoses and failure of fusion are likely different phenotypes arising from the same underlying developmental defect. In meox1 cho mutants, left and right neural arches fail to fuse and are asymmetric in meox1 cho mutant zebrafish, such that left arches are more anterior than right arches. Considering this and the thinness of zebrafish vertebral arches, it is likely that fusion has arisen as the result of defective growth of neighbouring arches, such that these arches are within proximity of each other. This is in contrast to the aforementioned anterior fusion, which in meox1 cho mutants occurs where in zebrafish the neural arches are thickest and likely arose through a differing mechanism, as evidenced by the larval fusion observed in early centra development at this region. A similar neural arch fusion defect has been reported in patients with Klippel–Feil syndrome, where the left and right neural arches of some cervical vertebrae fail to fuse at the midline (Bayrakli et al. 2013). Interestingly, mice deficient for Meox1 occasionally exhibit fusion defects in posterior spinal regions; however, these defects were reported as split centra as opposed to neural arch defects (Skuntz et al. 2009). In both humans and zebrafish lacking meox1 orthologues, this neural arch fusion defect occurs in the least substantial neural arches within respective vertebral columns. Therefore, although meox1 cho zebrafish have fusions throughout its vertebral column, this defect remains relevant to modelling Klippel–Feil syndrome.
In summation, meox1 cho mutant zebrafish feature aspects of a range of symptoms associated with patients suffering from Klippel–Feil syndrome, such as ‘cervical’ fusion, congenital scoliosis, Sprengel's deformity and neural arch fusion defects. When considering the innate differences in vertebral anatomy between zebrafish and humans, the bone defects that characterise meox1 cho fish were remarkably comparable to human patients who carry mutations in MEOX1. This suggests that the role of MEOX1 orthologues in patterning vertebral arches and an anterior region of the axial skeleton is highly conserved across species. Thus, meox1 cho zebrafish are poised to serve as a useful tool to further our understanding of the pathogenesis of Klippel–Feil syndrome and the relatedness of its various symptoms.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
M.V.P.D. performed the experiments and analysis of data. J.B. and P.D.C. conceived experiments and performed data analysis. All authors wrote and edited the manuscript.
Supporting information
Fig. S1 Scoliosis occurs in two‐thirds of meox1 cho adult homozygotes.
Acknowledgements
The authors thank Monash Micro Imaging for technical support. P.D.C. and J.B. were supported by the National Health and Medical Research Council of Australia (APP1144159 and APP1084944 to P.D.C and J.B; APP1136567 to P.D.C.). The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government.
Contributor Information
Peter D. Currie, Email: Peter.Currie@monash.edu
Joachim Berger, Email: Joachim.Berger@monash.edu.
References
- Bayrakli F, Guclu B, Yakicier C, et al. (2013) Mutation in MEOX1 gene causes a recessive Klippel–Feil syndrome subtype. BMC Genet 14, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird NC, Mabee PM (2003) Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev Dyn 228, 337–357. [DOI] [PubMed] [Google Scholar]
- Boswell CW, Ciruna B (2017) Understanding idiopathic scoliosis: a new zebrafish school of thought. Trends Genet 33, 183–196. [DOI] [PubMed] [Google Scholar]
- Candia AF, Wright C (1996) Differential localization of Mox‐1 and Mox‐2 proteins indicates distinct roles during development. Int J Dev Biol 40, 1179–1184. [PubMed] [Google Scholar]
- Candia AF, Hu J, Crosby J, et al. (1992) Mox‐1 and Mox‐2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development 116, 1123–1136. [DOI] [PubMed] [Google Scholar]
- Dhir R, Chin K, Lambert S (2018) The congenital undescended scapula syndrome: sprengel and the cleithrum: a case series and hypothesis. J Shoulder Elbow Surg 27, 252–259. [DOI] [PubMed] [Google Scholar]
- Di Gennaro G, Fosco M, Spina M, et al. (2012) Surgical treatment of Sprengel's shoulder: experience at the Rizzoli Orthopaedic Institute 1975‐2010. J Bone Joint Surg Br 94, 709–712. [DOI] [PubMed] [Google Scholar]
- van Eeden F, Granato M, Schach U, et al. (1996) Mutations affecting somite formation and patterning in the zebrafish, Danio rerio . Development 123, 153–164. [DOI] [PubMed] [Google Scholar]
- Gorman KF, Breden F (2007) Teleosts as models for human vertebral stability and deformity. Comp Biochem Physiol C Toxicol Pharmacol 145, 28–38. [DOI] [PubMed] [Google Scholar]
- Inohaya K, Takano Y, Kudo A (2007) The teleost intervertebral region acts as a growth center of the centrum: in vivo visualization of osteoblasts and their progenitors in transgenic fish. Dev Dyn 236, 3031–3046. [DOI] [PubMed] [Google Scholar]
- Karaca E, Yuregir OO, Bozdogan ST, et al. (2015) Rare variants in the notch signaling pathway describe a novel type of autosomal recessive Klippel‐Feil syndrome. Am J Med Genet A 167, 2795–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh R, Brand M, Jiang Y, et al. (1996) Zebrafish pigmentation mutations and the processes of neural crest development. Development 123, 369–389. [DOI] [PubMed] [Google Scholar]
- Klippel M, Feil A (1912) Un cas d'absence des vertèbres cervicales avec cage thoracique remontant jusqu’à la base du crâne (cage thoracique cervicale). Nouv Iconog Salpêtrière 25, 223–250. [Google Scholar]
- Krakow D (2018) Spinal abnormalities and Klippel–Feil syndrome In: Obstetric Imaging: Fetal Diagnosis and Care. (eds Copel JA, D'Alton ME, Feltovich H, Gratacós E, Krakow D, Odibo AO, Platt LD, Tutschek B.), pp. 295–297. Philadelphia: Elsevier. [Google Scholar]
- Lettice LA, Purdie LA, Carlson GJ, et al. (1999) The mouse bagpipe gene controls development of axial skeleton, skull, and spleen. Proc Natl Acad Sci USA 96, 9695–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD (2007) Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8, 353–367. [DOI] [PubMed] [Google Scholar]
- Lleras‐Forero L, Narayanan R, Huitema LFA, et al. (2018) Segmentation of the zebrafish axial skeleton relies on notochord sheath cells and not on the segmentation clock. ELife 7, e33843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, et al. (2005) Neural crest origins of the neck and shoulder. Nature 436, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughran J, Oates A, Donnai D, et al. (2003) Mutations in PAX1 may be associated with Klippel‐Feil syndrome. Eur J Hum Genet 11, 468–474. [DOI] [PubMed] [Google Scholar]
- McGonnell I (2001) The evolution of the pectoral girdle. J Anat 199, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed JY, Faqeih E, Alsiddiky A, et al. (2013) Mutations in MEOX1, encoding mesenchyme homeobox 1, cause Klippel‐Feil anomaly. Am J Hum Genet 92, 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PD, Hollway GE, Sonntag C, et al. (2014) Haematopoietic stem cell induction by somite‐derived endothelial cells controlled by meox1 . Nature 512, 314–318. [DOI] [PubMed] [Google Scholar]
- Nguyen PD, Gurevich DB, Sonntag C, et al. (2017) Muscle stem cells undergo extensive clonal drift during tissue growth via Meox1‐mediated induction of G2 cell‐cycle arrest. Cell Stem Cell 21, 107–119. [DOI] [PubMed] [Google Scholar]
- Rodrigo I, Bovolenta P, Mankoo BS, et al. (2004) Meox homeodomain proteins are required for Bapx1 expression in the sclerotome and activate its transcription by direct binding to its promoter. Mol Cell Biol 24, 2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Mijalski T, Schlange T, et al. (1999) The homeobox gene it NKX3.2 is a target of left–right signalling and is expressed on opposite sides in chick and mouse embryos. Curr Biol 9, 911–914. [DOI] [PubMed] [Google Scholar]
- Skuntz S, Mankoo B, Nguyen M‐TT, et al. (2009) Lack of the mesodermal homeodomain protein MEOX1 disrupts sclerotome polarity and leads to a remodeling of the cranio–cervical joints of the axial skeleton. Dev Biol 332, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamataki D, Kastrinaki M‐C, Mankoo BS, et al. (2001) Homeodomain proteins Mox1 and Mox2 associate with Pax1 and Pax3 transcription factors. FEBS Lett 499, 274–278. [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Fang ZM, Hilton EN, et al. (2008) Mutations in GDF6 are associated with vertebral segmentation defects in Klippel‐Feil syndrome. Hum Mutat 29, 1017–1027. [DOI] [PubMed] [Google Scholar]
- Tracy M, Dormans J, Kusumi K (2004) Klippel‐Feil syndrome: clinical features and current understanding of aetiology. Clin Orthop Relat Res 424, 183–190. [PubMed] [Google Scholar]
- Ye M, Berry‐Wynne KM, Asai‐Coakwell M, et al. (2010) Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Hum Mol Genet 19, 287–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Scoliosis occurs in two‐thirds of meox1 cho adult homozygotes.


