Abstract
The development of a positron emission tomography (PET)/magnetic resonance spectroscopy (MRS) hybrid imaging agent allows for functional imaging by both methods with a single imaging agent. Enzyme substrates that are cleaved to form two metabolites present an interesting opportunity, as the unique metabolites generated might each be detected by a different modality. To be successful, such enzyme substrates would require administration of doses that (a) reach the in vivo target tissue at concentrations necessary for MRS imaging, (b) do not show substrate inhibition of tissue uptake or enzymatic activity, and (c) provide PET images that still reflect the action of the enzyme. We report in vitro and in vivo proof-of-concept studies of a carbon-11 small molecule substrate for brain monoamine oxidases that, upon enzyme-mediated cleavage, produces two metabolites, one detectable by PET and the other by MRS.
Keywords: Hybrid imaging, carbon-11, positron emission tomography, magnetic resonance spectroscopy, neuroimaging
Positron emission tomography (PET) imaging is a noninvasive molecular imaging method that has been utilized for decades in studies of physiology and pharmacology in living animals and human subjects.1,2 Most applications of PET target high affinity but low capacity sites on proteins (e.g., receptors, enzymes, and transporters) and typically require high molar activities (radioactivity per mole), with clear decreases of specific binding as the mass of the injected radiotracer is increased.3 The molar activities commonly acceptable for PET imaging (>37 GBq/μmol; > 1000 Ci/mmol) are thus usually considered incompatible with the higher concentrations (mmol) of imaging agents required for alternate functional imaging methods like magnetic resonance spectroscopy (MRS).4
The recent development of PET/MR imaging instrumentation has made it possible to simultaneously acquire imaging data for both modalities.5−7 However, combining PET imaging with functional magnetic resonance spectroscopy (MRS) imaging has proven challenging. This would require a PET imaging agent that can function successfully at the high mass concentrations required for MRS imaging, despite the resulting low molar activity known to be problematic for PET radioligands. We hypothesized that the technique of metabolic trapping, whereby the product of an enzymatic reaction is trapped and retained in tissues at the site of the enzyme, might provide a suitable approach to combining PET and MRS: if the enzyme does not exhibit substrate inhibition in vivo, then a high mass/low molar activity PET radiotracer should still function well. To test this hypothesis, we have examined potential metabolically trapped substrates for monoamine oxidases (MAO-A and -B), enzymes of considerable interest in studies of brain biochemistry using PET, and report an example of a small molecule hybrid PET/MRS imaging agent.
Our prior radiotracer development efforts yielded several carbon-11 labeled N-methyl-4-aryloxy-1,2,3,6-tetrahydro-pyridines,8 based on the work of Castagnoli and co-workers,9,10 which are in vitro and in vivo substrates for MAO-A or MAO-B. These function in vivo as metabolic trapping PET agents, as the enzymatic reaction releases a carbon-11 labeled N-methylpyridinone that is retained in brain tissues. Inclusion of a coumarin group as the phenoxy substituent produced a radiotracer that released both radiolabeled and fluorescent metabolic products (Figure 1a), similar to a previous report of a MAO-B selective fluorogenic probe (which was not radiolabeled).11
Figure 1.
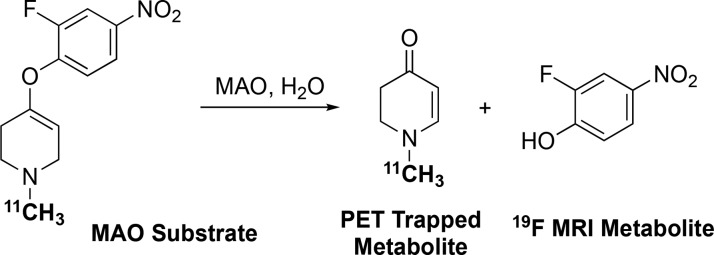
Design of a PET/MRS hybrid probe for monoamine oxidases, building on published examples of PET/fluorescence and chemical shift-switching in vitro MAO substrates.
To produce a combined PET/MRS radiotracer, we desired a 4-aryloxy substituent that would provide a metabolite with a unique signal visible by MRS following MAO oxidation. For this, we turned to the work of Yamaguchi et al., who reported an MAO substrate described as a chemical shift-switching agent (Figure 1b), as the 19F NMR signal of the substrate (a 2-fluoro-4-nitrophenyl ether) could be distinguished from that of the metabolite (2-fluoro-4-nitrophenol).12 We thus hypothesized that combining 2-fluoro-4-nitrophenol with the N-[11C]methyl-1,2,3,6-tetrahydro-pyridine moiety should provide a molecule suitable for testing our hypothesis that a combined PET/MRS radiotracer is feasible using the metabolic trapping concept (Figure 1c).
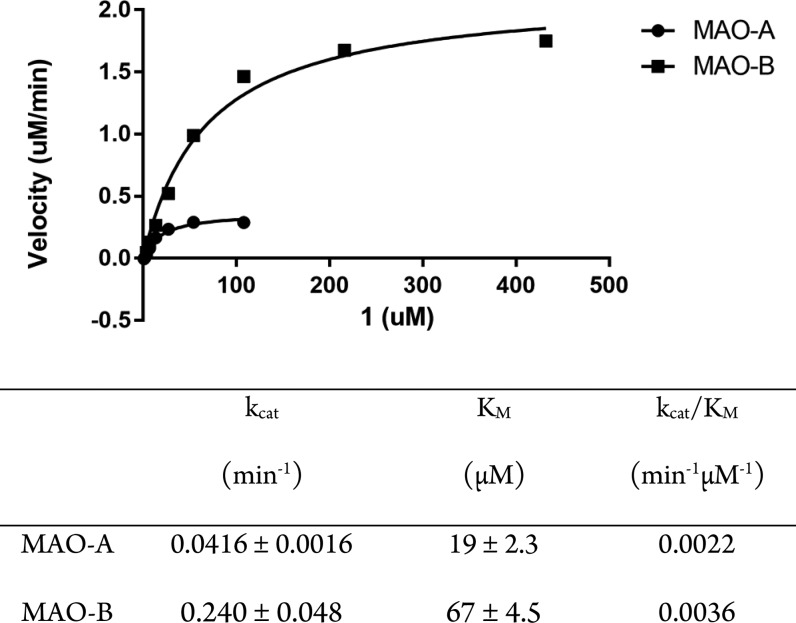
The authentic standard 4-(2-fluoro-4-nitrophenoxy)-1-methyl-1,2,3,6-tetrahydropyridine (1) and corresponding N-desmethyl compound (2) were prepared using methods previously reported for syntheses of 4-aryloxy substituted tetrahydropyridines.8,13 To first demonstrate that the proposed radiotracer was a substrate for monoamine oxidases, kinetic studies were done with solutions of human MAO (hMAO)-A or -B and using the change in absorbance from substrate to metabolite in a spectrophotometric assay. As shown in Figure 2, 1 is a substrate for both hMAO-A and -B, with similar catalytic efficiencies (kcat/KM) for both isozymes.
Figure 2.
Michaelis–Menten kinetic analysis data for MAO-A and -B by absorbance assay.
To next demonstrate that the compound would be useful as a chemical shift-switching agent for MRS, enzymatic reactions with hMAO-A and 1 were run for 90 min and quenched by addition of an excess of the irreversible inhibitor clorgyline. 19F NMR analyses showed that over the time period the signal for substrate 1 at −130 ppm was replaced by that of the phenol metabolite 4 at −136 ppm, as well as a secondary metabolite at −129 ppm (Figure 3).
Figure 3.
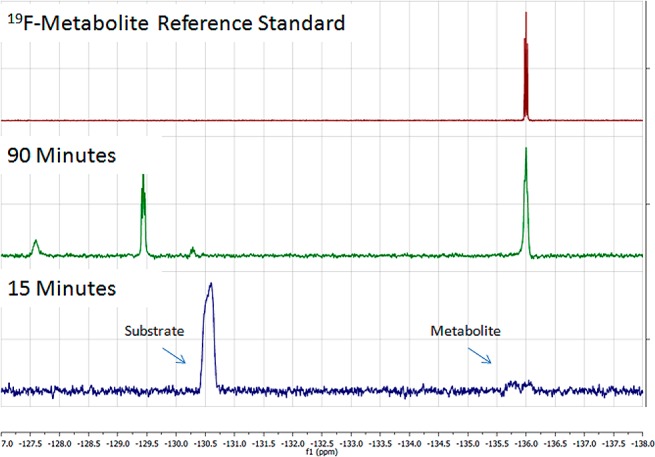
19F NMR experiments (376 MHz) demonstrating time-dependent formation of 2-fluoro-4-nitrophenol metabolite (−136 ppm) and secondary metabolite (−129 ppm) via oxidation of substrate 1 (−130 ppm) by MAO-A.
With 1 confirmed in vitro as a MAO chemical shift-switching agent, the last step was to radiolabel with carbon-11 and evaluate whether [11C]1 could be utilized as a PET imaging agent at low molar activity. The procedure to radiolabel [11C]1 was similar to the method previously described.8,13 Briefly, [11C]carbon dioxide was converted by standard procedure into [11C]CH3OTf and sparged through a solution of the pyridine precursor (1 mg) in ethanol (0.2 mL). The [11C]methylpyridinium intermediate was transferred to a conical vial containing NaBH4 (2 mg) in ethanol (0.3 mL) and stirred for 5 min. [11C]1 was then purified by HPLC and reformulated into a 10% ethanol in saline dose (392 ± 207 MBq; nondecay corrected 1.2% radiochemical yield based on starting [11C]CH3OTf; 30 min from end of bombardment) for use in animal imaging studies. Final product was obtained in high molar activities (>259 GBq/μmol), and to prepare doses of low molar activity [11C]1, unlabeled standard was added to the saline used for reformulation. Final molar activities were confirmed using HPLC analysis of the UV-254 nm absorbances.
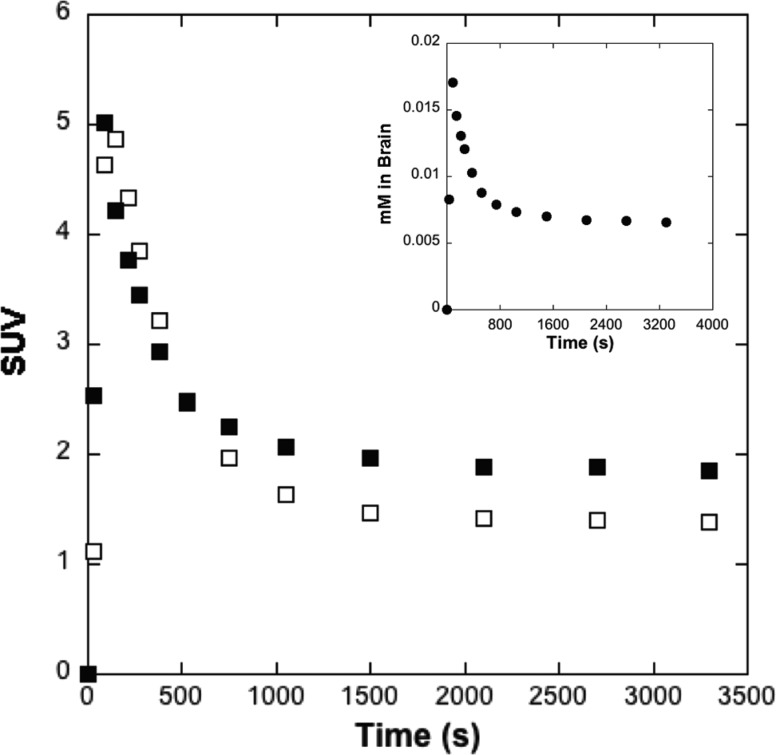
MAO is known to be a highly conserved enzyme between species with, for example, high sequence homology between hMAO and rat MAO (rMAO).14 Therefore, kinetic PET imaging studies were conducted in Sprague–Dawley rats. Experiments conducted with high (259 GBq/μmol) and low (0.0185 GBq/μmol) molar activity [11C]1 gave very similar kinetic curves for whole brain radioactivity (Figure 4). These data were also used in conjunction with specific activity to generate curves estimating mass of tracer in the brain (inset, Figure 4). For the low specific activity PET studies, 1.29 ± 0.86 μmol of 1 was administered, similar to the mass administered for prior rodent 19F-MRI studies,15 and peak uptake of 1 in the rodent brain was ∼0.02 mM. Given that the total concentration of MAO (A + B) in the rodent brain is ∼11 pmol/mg protein,16 and that rat brain is ∼10% protein,17 this corresponds to an estimated total rMAO concentration of ∼1.1 pmol/mg tissue (0.0011 mM). Thus, [1]/[rMAO] ≈ 18 and, given the high catalytic efficiency of MAO (Figure 2), we would not expect to overwhelm MAO at this concentration of 1 in the brain. Reflecting this, there was indeed no evidence that the MAO enzymes in the brain tissues were subject to in vivo substrate inhibition at this concentration.
Figure 4.
Main panel: averaged (n = 2) rodent whole brain time-radioactivity curves (0–60 min post-iv-injection of high (259 GBq/μmol, □) and low (0.0185 GBq/μmol, ■) molar activity doses of [11C]1). Inset: whole brain concentration curve (0–60 min post-iv-injection of low (●) molar activity doses of [11C]1).
In summary, enzyme substrates that form trapped metabolites appear as a promising way forward to design PET/MRS hybrid imaging agents. In the system evaluated here, the enzyme reaction produces two metabolites that are separately visible by PET or NMR. When considering potential in vivo applications of such a hybrid PET-MRS imaging agent in the future, if both metabolites are trapped in the tissue, they will yield a measure of the same parameter, MAO oxidation. Of more interest would be if the two methods gave different biochemical measures. How might that be achieved? Two possibilities come to mind. First, if the MRS-visible metabolite is freely diffusible across the BBB but is designed to have high affinity but reversible binding to a specific CNS site (e.g., receptor), then the retention and delayed clearance from the brain tissue might reflect the amount of specific binding (the pharmacokinetics of delivery are identical to the radiotracer trapping curve). As an alternative, if the metabolite is designed not to be BBB permeable but instead is a substrate for an efflux transporter (e.g., P-glycoprotein, Pgp), then the rate of clearance from the tissues will reflect the transporter function, as previously described for the glutathione-conjugated metabolites of radiolabeled purines.18 Future work will address the interesting possibilities of designing bifunctional PET/MRS brain imaging tracers based on MAO, and the extension to other enzymes that can be studied using the metabolic trapping principle.
Acknowledgments
We thank Phillip Sherman, Janna Arteaga, and Jenelle Stauff for conducting preclinical PET imaging.
Glossary
ABBREVIATIONS
- BBB
blood–brain barrier
- Bq
Becquerel
- CNS
central nervous system
- MAO
monoamine oxidase
- PET
positron emission tomography
- Pgp
P-glycoprotein
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00402.
Experimental procedures for chemistry and radiochemistry as well as spectra/chromatograms for all novel compounds synthesized; procedures for in vitro assays and in vivo rodent PET imaging (PDF)
Author Contributions
§ These authors contributed equally. The manuscript was written through contributions of all authors, and all authors have given approval to the final version of the manuscript.
We thank NIH (R21NS075553 (M.R.K.); T32GM007767 (P.J.H.S. and L.R.D.)) and the University of Michigan (Energy Institute/Michigan Memorial Phoenix Project (A.F.B.), Michigan Institute for Clinical and Health Research/TREC (L.R.D.), College of Pharmacy (L.R.D. and P.J.H.S.), Dept of Radiology (P.J.H.S.), and Rackham Graduate School (L.R.D.)) for financial support.
The authors declare no competing financial interest.
Supplementary Material
References
- For a general overview of PET imaging, see:; Ametamey S. M.; Honer M.; Schubiger P. A. Molecular Imaging with. Chem. Rev. 2008, 108, 1501–1516. 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- For general reviews covering PET radiochemistry, see:; a Miller P. W.; Long N. J.; Vilar R.; Gee A. D. Synthesis of 11C, 18F, 15O, and 13N Radiolabels for Positron Emission Tomography. Angew. Chem., Int. Ed. 2008, 47, 8998–9033. 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]; b Brooks A. F.; Topczewski J. J.; Ichiishi N.; Sanford M. S.; Scott P. J. H. Late-stage [18F]Fluorination: New Solutions to Old Problems. Chem. Sci. 2014, 5, 4545–4553. 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi J.; Zhang M. R.; Yanamoto K.; Nakao R.; Suzuki K. In Vitro Binding of [11C]Raclopride with Ultrahigh Specific Activity in Rat Brain Determined by Homogenate Assay and Autoradiography. Nucl. Med. Biol. 2008, 35, 19–27. 10.1016/j.nucmedbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Dietz C.; Ehret F.; Palmas F.; Vandergrift L. A.; Jiang Y.; Schmitt V.; Dufner V.; Habbel P.; Nowak J.; Cheng L. L. Applications of high-resolution magic angle spinning MRS in biomedical studies II-Human diseases. NMR Biomed. 2017, 30, e3784. 10.1002/nbm.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a recent review of PET-MRI in the context of brain imaging, see:; Chen Z.; Jamadar S. D.; Li S.; Sforazzini F.; Baran J.; Ferris N.; Shah N. J.; Egan G. F. From Simultaneous to Synergistic MR-PET Brain Imaging: A Review of Hybrid MR-PET Imaging Methodologies. Hum. Brain Mapp. 2018, 10.1002/hbm.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judenhofer M. S.; Wehrl H. F.; Newport D. F.; Catana C.; Siegel S. B.; Becker M.; Thielscher A.; Kneilling M.; Lichy M. P.; Eichner M.; Klingel K.; Reischl G.; Widmaier S.; Röcken M.; Nutt R. E.; Machulla H. J.; Uludag K.; Cherry S. R.; Claussen C. D.; Pichler B. J. Simultaneous PET-MRI: A New Approach for Functional and Morphological Imaging. Nat. Med. 2008, 14, 459–465. 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- Pichler B. J.; Wehrl H. F.; Kolb A.; Judenhofer M. S. Positron Emission Tomography/Magnetic Resonance Imaging: the Next Generation of Multimodality Imaging?. Semin. Nucl. Med. 2018, 38, 199–208. 10.1053/j.semnuclmed.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. F.; Shao X.; Quesada C. A.; Sherman P.; Scott P. J. H.; Kilbourn M. R. In Vivo Metabolic Trapping Radiotracers for Imaging Monoamine Oxidase-A and -B Enzymatic Activity. ACS Chem. Neurosci. 2015, 6, 1965–1971. 10.1021/acschemneuro.5b00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.; Dalvie D.; Naiman N.; Castagnoli K.; Castagnoli N. Jr. Design, Synthesis, and Biological Evaluation of Novel 4-Substituted 1-Methyl-1,2,3,6-tetrahydropyridine Analogs of MPTP. J. Med. Chem. 1992, 35, 4473–4478. 10.1021/jm00101a026. [DOI] [PubMed] [Google Scholar]
- Kalgutkar A. S.; Castagnoli K.; Hall A.; Castagnoli N. Jr. Novel 4-(Aryloxy)tetrahydropyridine Analogs of MPTP as Monoamine Oxidase A and B Substrates. J. Med. Chem. 1994, 37, 944–949. 10.1021/jm00033a012. [DOI] [PubMed] [Google Scholar]
- Long S.; Chen L.; Xiang Y.; Song M.; Zheng Y.; Zhu Q. An Activity-based Fluorogenic Probe for Sensitive and Selective Monoamine Oxidase-B Detection. Chem. Commun. 2012, 48, 7164–7166. 10.1039/c2cc33089j. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K.; Ueki R.; Nonaka H.; Sugihara F.; Matsuda T.; Sando S. Design of Chemical Shift-switching 19F Magnetic Resonance Imaging Probe for Specific Detection of Human Monoamine Oxidase A. J. Am. Chem. Soc. 2011, 133, 14208–14211. 10.1021/ja2057506. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for experimental details.
- Bai C.; Meng H.; Zhao W.; Pan Y. Molecular Cloning and Characterization of Monoamine Oxidase A and B Genes in Pig. DNA Sequence 2005, 16, 161–165. 10.1080/10425170500097925. [DOI] [PubMed] [Google Scholar]
- Ko I. O.; Jung K.-H.; Kim M. H.; Kang K. J.; Lee K. C.; Kim K. M.; Noh I.; Lee Y. J.; Lim S. M.; Kim J. Y.; Park J.-A. Preliminary 19F-MRS Study of Tumor Cell Proliferation with 3′-deoxy-3′-fluorothymidine and Its Metabolite (FLT-MP). Contrast Media Mol. Imaging 2017, 2017, 3981358. 10.1155/2017/3981358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J.; Kettler R.; Da Prada M.; Richards J. G. Quantitative Enzyme Radioautography with 3H-Ro 41–1049 and 3H-Ro 19–6327 in vitro: Localization and Abundance of MAO-A and MAO-B in Rat CNS, Peripheral Organs, and Human Brain. J. Neurosci. 1992, 12, 1977–1999. 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banay-Schwartz M.; Kenessey A.; DeGuzman T.; Lajtha A.; Palkovits M. Protein Content of Various Regions of Rat Brain and Adult and Aging Human Brain. AGE 1992, 15, 51–54. 10.1007/BF02435024. [DOI] [PubMed] [Google Scholar]
- Galante E.; Okamura T.; Sander K.; Kikuchi T.; Okada M.; Zhang M. R.; Robson M.; Badar A.; Lythgoe M.; Koepp M.; Årstad E. Development of Purine-derived 18F-labeled Pro-drug Tracers for Imaging of MRP1 Activity with PET. J. Med. Chem. 2014, 57, 1023–1032. 10.1021/jm401764a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.