Abstract
Background
Meningiomas are mostly benign tumors tending to progress to higher-grade lesions. Mutations in the telomerase reverse transcriptase (TERT) gene promoter are comparably rare in meningioma, but were recently suggested to predict risk of recurrence and progression. Here we have analyzed a cohort of World Health Organization grades I–III meningiomas regarding the impact of TERT promoter mutations on patient prognosis and in vitro cell propagation feasibility.
Methods
From 110 meningioma patients, 128 tissue samples were analyzed for the TERT promoter mutations C228T and C250T by direct sequencing. Of the 128 samples, 121 were tested for cell propagation in vitro. Telomerase activity, TERT mRNA expression, and telomere lengths were investigated by telomeric repeat amplification protocol assay, reverse transcription PCR, and quantitative PCR, respectively. Impact of the E-twenty-six (ETS) transcription factor inhibitor YK-4–279 on cell viability and TERT promoter activity was analyzed.
Results
TERT promoter mutations were found in 5.5% of all samples analyzed and were associated with a significantly upregulated telomerase activity and TERT mRNA expression (P < 0.0001 both). Regarding telomere lengths, no significant difference between the TERT promoter wild-type and mutated subgroups was detected. Patients with TERT promoter mutated tumors exhibited significantly shorter overall survival (P = 0.0006; 53.8 vs 115.6 mo). The presence of TERT promoter mutations but not telomerase activity or TERT mRNA expression predicted indefinite cell growth in vitro. TERT promoter mutated meningioma cells were hypersensitive against the ETS transcription factor inhibitor YK-4–279, inducing a distinct downregulation of TERT promoter activity.
Conclusion
TERT promoter mutations drive meningioma aggressiveness, resulting in reduced patient survival, but might also open novel therapeutic options for progressive disease.
Keywords: cell immortalization, meningioma, prognosis, TERT promoter mutation
Importance of the study
Meningiomas are slow-growing, predominantly benign tumors but frequently recur as higher-grade lesions. However, the factors influencing this behavior are still unknown. In this study we show that activating mutations in the TERT promoter but not telomerase activity or TERT mRNA expression is the main driver of aggressiveness in meningiomas. Our findings underpin that TERT promoter mutations might serve as biomarkers for meningiomas to predict disease progression but also might open novel therapeutic options.
According to the World Health Organization (WHO) classification of tumors of the central nervous system (CNS) 2016, meningiomas represent with 36.1% the most frequent primary CNS tumor. Up to 90% of all meningiomas are slow-growing benign WHO grade I lesions, while WHO grades II and III meningiomas are classified as atypical (5%–15%) and malignant (1%–3%), respectively.1,2 Recurrence rates increase with tumor stage, being highest in WHO grade III tumors (50%–80%). Interestingly, only a subset of meningiomas recurs with progressive lesions.3 However, the factors underlying this behavior are widely unknown, and predictive biomarkers urgently need to be addressed in order to identify this unfavorable subgroup.
Recently, activating mutations in the promoter of the telomerase reverse transcriptase (TERT) gene were found to be associated with negative prognosis and tumor aggressiveness in human brain tumors, including meningiomas.4–6 Initially discovered in melanomas,7,8 these C>T transition mutations at chromosomal positions chr5:1 295 228 and 1 295 250 (C228T or C250T) were found—although at varying frequencies—in multiple cancer types, being highest in melanomas, gliomas, and bladder cancer. Interestingly, however, these mutations are missing in several major carcinomas like breast, lung, and colon cancer.9TERT promoter mutations have been associated with upregulation of telomerase activity (TA) and TERT mRNA expression in diverse cancer types.5,10,11 Furthermore, each of these noncoding mutations creates a novel binding site for members of the E-twenty-six (ETS)/ternary complex factor (TCF) transcription factor family, which are believed to participate in increased TERT promoter activity.7 Another factor known to be involved in the regulation of TERT mRNA expression is methylation at the cytosine-phosphate-guanine (CpG) cluster of the TERT promoter. Hypermethylation was associated with enhanced TERT mRNA expression for example, in pediatric brain tumors12 but also meningiomas.13
A few studies on meningiomas have analyzed the presence of TERT promoter mutations so far, indicating that these noncoding genetic alterations in a small patient subgroup might be associated with enhanced risk for recurrence and progression.4,14–16 Consequently, in the present study we have comprehensively analyzed the impact of the TERT promoter mutation compared with methylation status on telomerase activity, TERT mRNA expression, telomere lengths, and cancer cell immortalization as well as clinical outcome. We found that TERT promoter mutations are indicative for tumor cell aggressiveness, leading to dismal patient prognosis, and might even represent a biomarker for new therapeutic interventions.
Materials and Methods
Meningioma Tumor Samples
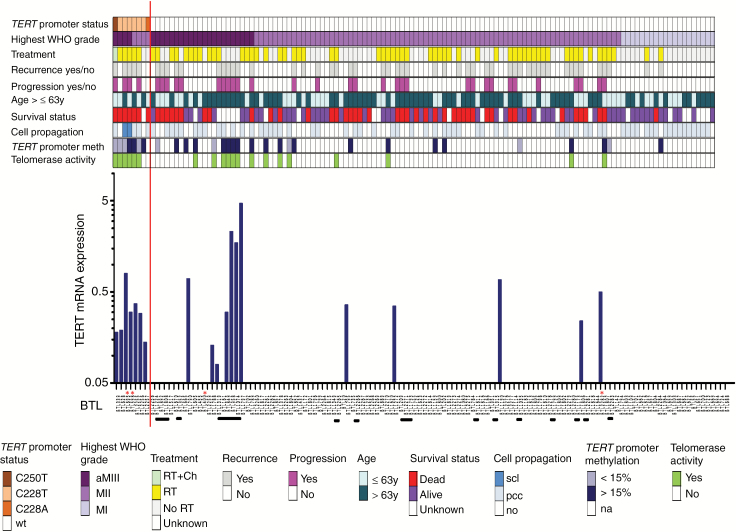
Out of the collection of meningiomas operated at the Neurosurgery Department at the Kepler University Hospital, 128 cases from 110 patients were selected according to availability of tumor tissue (Fig. 1). The tumors were histologically confirmed as meningioma WHO grade I (n = 32), atypical WHO grade II (n = 75), and anaplastic WHO grade III (n = 21). Patients relapsed with nonprogressive (n = 15) and progressive (n = 18) tumors. For all patients, clinical data were available and included sex, age at surgery, histology, treatment, and overall survival (OS). Tumor tissue and clinical information were obtained with written informed consent of patients, and the study was approved by the local ethics committee (E39-15). TERT promoter status linked to clinical and cell biological characteristics and TERT mRNA expression in 128 meningioma samples of 110 patients are depicted in Fig. 1.
Fig. 1 .
TERT promoter status linked to clinical and cell-biological characteristics and telomere stabilization parameters in 128 meningioma samples of 110 patients. Data on mutation status, tumor grade, treatment, tumor recurrence, tumor progression, patient age, survival status, cell propagation and TERT promoter methylation as well as telomerase activity are indicated in different colors. The respective legend is outlined below the graph. aMIII, anaplastic meningioma WHO grade III; secondary anaplastic meningiomas WHO III are indicated with white asterisks; MII, atypical meningioma WHO grade II; MI, meningothelial (47%; 15/32), transitional (31%; 10/32), fibroblastic (16%; 5/32), angiomatous (6%; 2/32) meningioma WHO grade I; RT, radiotherapy; Ch, chemotherapy; scl, stable cell line; pcc, primo-cell culture; TERT promoter methylation according to the cutoff of 15%; na, not analyzed. Patients with corresponding primary and recurrent tumor samples are highlighted with horizontal black bars below the graph depicting TERT mRNA expression levels by RT-PCR. The blue asterisks in the recurrence and progression lines highlight a special case of meningioma WHO III which recurred with a gliosarcoma 14 months after the first diagnosis. The respective BTL numbers of meningioma-derived cell cultures used for the in vitro experiments are marked with red asterisks.
Establishment of Meningioma Derived Primo-Cell Cultures
Cell cultures were established from surgery specimens, as published previously.17 In brief, freshly resected meningioma tissue was dissected mechanically and cultured in Roswell Park Memorial Institute medium 1640 supplemented with 7% fetal calf serum. Starting with 1% penicillin/streptomycin, cell cultivation was continued antibiotic/mycotic free after 14 days. Cells were periodically checked for mycoplasma contamination using a PCR-based approach (VenorGeM, Minerva Biolabs). Establishment of tumor derived primo-cell cultures was regarded as successful when living cell layers were obtained from tumor specimens allowing at least one passage. Tumor explants were considered as primo-cell cultures from passage 2 to passage 8. Afterward, cells displayed either increasing features of senescence (slower proliferation; enlarged, fibroblastoid cell shape) or developed into a stable immortalized cell line defined by propagation to >20 passages.
Telomere-Associated Parameters
Analyses of TA, TERT mRNA expression, and telomere lengths were performed in surgical specimen as recently published.18 To avoid distortion of TA and TERT mRNA expression data due to several consecutive specimens from one individual patient, generally only the first recurrence available was included in the analyses. For quantifying the impact of the ETS factor inhibitor YK-4–279 (Selleckchem) on TERT mRNA expression, quantitative reverse transcription polymerase chain reaction (qRT-PCR) experiments were performed as described by Lötsch et al.19 Quantitative RT-PCR was run on a C1000 Touch Thermal Cycler using the CFX96 Real-Time System (Biorad). The following primers were used: TERT fw (5ʹ-CCAAGTTCCTGCACTGG-3ʹ) and TERT rev (5ʹ-TTCCCGATGCTGCCTGAC-3ʹ); RPL41 fw (5ʹ-CAAGTGGAGGAAGAAGC-3ʹ) and RPL41 rev (5ʹ-TTACTTGGACCTCTGCCT-3ʹ). RPL41 served as a housekeeping gene and was used for normalization. All reactions were performed in triplicates and repeated at least twice.
DNA Extraction and TERT Promoter Mutation Analysis
The TERT promoter region of interest containing C228T, C250T,7,8 and C229A9 mutation sites as well as the single nucleotide polymorphism rs2853669 (c.-245T>C) were amplified using 25 ng genomic DNA, the HotStar Taq Mastermix Kit (Qiagen), the additive Q-solution (Qiagen), and primers S 5ʹ-AGTGGATTCGCGGGCACAGA-3ʹ and AS 5ʹ-CAGCGCTGCCTGAAACTC-3ʹ, resulting in a 235 bp PCR product. Quality confirmation was performed by polyacrylamide-gelelectrophoresis, followed by PCR cleanup using the Illustra ExoProStar 1-Step Kit (GE Healthcare Life Sciences). PCR products were sequenced using the BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems) and a 3130 Genetic Analyzer (Applied Biosystems) following standard procedures. All samples were checked in forward and reverse direction, and SeqScape software v3.0 (Applied Biosystems) was utilized for mutation analysis and fragment assembly.
TERT Promoter Methylation
Promoter hypermethylation was determined by pyrosequencing from snap-frozen meningioma tumor tissue. DNA was isolated and directly bisulfite converted with the EpiTect Bisulfite Kit (Qiagen) according to the manufacturer’s instructions. The specific region was previously published12 and analyzed on a PyroMark Q24 MDx pyrosequencing instrument (Qiagen). A cutoff of 15% was chosen to determine promoter hypermethylation.
Luciferase Reporter Assay
Reporter constructs containing either the wild-type or the C228T-mutant TERT promoter sequence were generated as published by Rachakonda et al.20 Cells (5 × 105) were seeded in 6-well plates, and 24 h later co-transfections with 1 μg of each reporter construct and 100 ng of phRG-TK plasmid (Addgene) for Renilla luciferase were performed using Lipofectamine 2000 (Invitrogen). The phRG-TK plasmid expressing Renilla luciferase under the thymidine kinase promoter served as internal control to determine transfection efficiency. In each experiment a negative (promoter-less, pGL3 basic vector) and positive (pGL-SV40 Luc; data not shown) control plasmid were included. Forty-eight hours post transfection, BTL695 cells were treated with 5 µM YK-4–279, and 18 h later proteins were harvested using lysis buffer (0.25 M Tris/HCl, 0.5% Triton X-100). Reporter expression (firefly luciferase) was analyzed using the Dual-Glo Luciferase assay system (Promega) in 96-well format following the manufacturer’s instructions. Relative luciferase activity was calculated as the ratio of firefly to Renilla luminescence. The experiments were repeated 3 times.
Chemosensitivity Testing
The impact on cell viability of the ETS factor inhibitor YK-4–279 (Selleckchem), originally developed for blocking downstream signaling of the EWS-FLI1 fusion protein in Ewing’s sarcoma,21 was tested using an MTT-based survival assay (EZ4U, Easy-for-You, Biomedica). Cell cultures analyzed were in all cases between passages 6 and 8. Cells in logarithmic growth phase were seeded in triplicates into 96-well plates (3 × 103/100 µL/well) and incubated for 24 h at 37°C. The drug was administered in increasing concentrations (0.3–15 µM) and the cell cultures left untouched for a 72 h continuous exposure. The surviving proportion of cells was determined using EZ4U according to the instructions of the manufacturer. Experiments were repeated 3 times, and half-maximal inhibitory concentration (IC50) values were calculated from whole dose response curves.
Statistical Analyses
Statistical analyses were carried out with GraphPad Prism v5.0 and IBM SPSS statistics v24.0. Parameter levels among subgroups were compared by Student’s t-test. Survival probabilities were estimated by the Kaplan–Meier method and log-rank test. Multivariate analysis was carried out via the Cox proportional hazards model.
Results
TERT Promoter Mutations Are Associated with a Significant Telomerase Upregulation
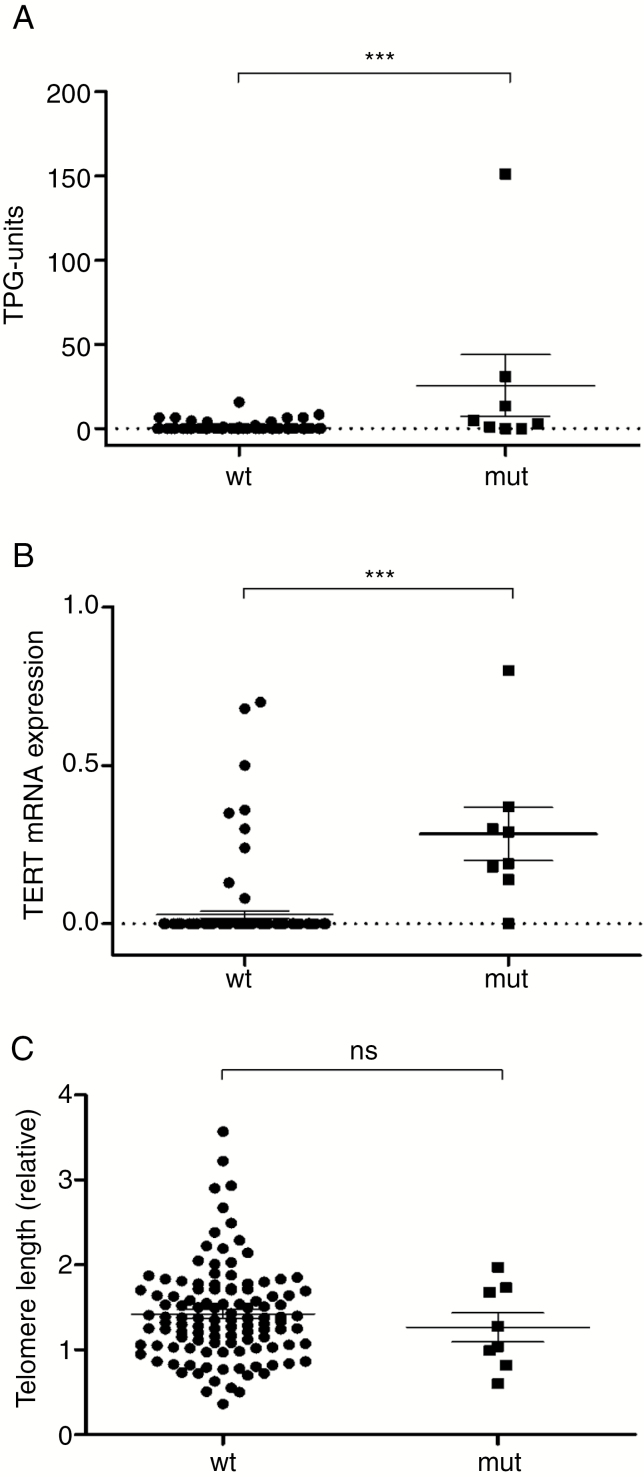
Using direct sequencing, meningioma tumor samples were screened for the TERT promoter hot spot mutations C228T and C250T. Mutations were found in 5.5% (7/128) of all samples analyzed. Within this collective of TERT promoter mutated (TERTp-mut) tumors, 6 (86%; 6/7) harbored a C228T mutation and 1 was positive for C250T. In addition to the 2 common TERT promoter mutations, one sample showed a C228A transition mutation (Fig. 1). Telomerase activity and TERT mRNA expression were detected in both TERT promoter wild-type (TERTp-wt) (TA 15/120 = 12.5%; TERT mRNA 12/120 = 10%) and TERTp-mut (TA 6/8 = 75%; TERT mRNA 7/8 = 88%) meningioma cohorts but were significantly higher in TERTp-mut compared with TERTp-wt tumors (P < 0.0001) (Fig. 2A, B). The difference in telomere lengths between TERTp-mut and -wt tumors failed to reach significance (Fig. 2C). Interestingly, the tumor harboring the C>A transition mutation was negative for TERT mRNA expression and TA.
Fig. 2.
Impact of TERT promoter mutations on telomere-associated parameters in meningiomas. (A) Telomerase activity was determined by the TRAP assay and results were expressed as total product generated units (TPG). (B) TERT mRNA expression levels were analyzed by semiquantitative RT-PCR (relative to glyceraldehyde 3-phosphate dehydrogenase mRNA) and (C) telomere lengths by real-time PCR (relative to the alternative lengthening of telomeres‒positive osteosarcoma cell line SA-OS) in TERTp-wt and TERTp-mut meningioma subgroups. Statistical analyses were performed by Student’s t-test, and significance (P < 0.001) was expressed using 3 asterisks.
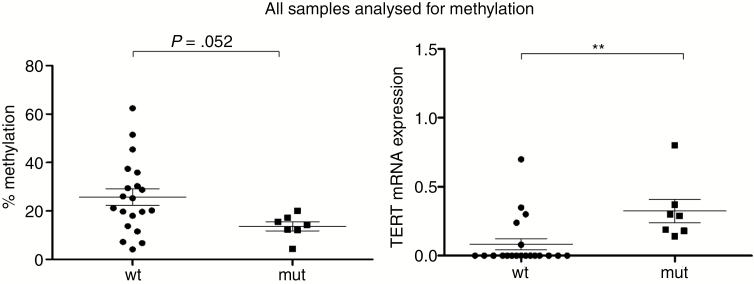
TERT Promoter Methylation
As mentioned before, telomerase activity was also found in 12% of TERTp-wt meningiomas, indicating that also other factors might drive telomerase reactivation. Therefore, we set out to test the association between TERT promoter methylation and mutation with TERT mRNA expression as well as TA. TERT promoter methylation was investigated by pyrosequencing in all TERTp-mut samples, in all TERTp-wt samples exerting TA (TA+), and in representative TA-negative (TA−) TERTp-wt samples. TERT promoter methylation was clearly higher with borderline significance (P = 0.052) in TERTp-wt compared with TERTp-mut meningiomas (Fig. 3). This suggests that promoter methylation might contribute to TERT gene reactivation specifically in cases lacking mutations. However, in TERTp-wt meningiomas no significant difference between the methylation pattern of TA+/TA− and TERT mRNA+/TERT mRNA− samples was observed (data not shown). Conversely, TERT promoter methylation was not associated with increased TERT mRNA expression in the TERTp-wt subgroup (data not shown). This strongly indicates that enhanced TERT mRNA expression is more associated with TERT promoter mutation rather than TERT promoter methylation.
Fig. 3 .
TERT promoter methylation in meningiomas and relation with TERT mRNA expression. (Left panel) TERT promoter methylation was analyzed by pyrosequencing in all tumors harboring a C228T or C250T mutation and compared with the methylation status of the TERTp-wt subgroup (comprising all TA-positive and selected TA-negative tumors). (Right panel) For comparison, TERT mRNA expression levels are depicted according to the TERT promoter methylation status in the respective sample subgroup. A cutoff of 15% was chosen to determine promoter hypermethylation.
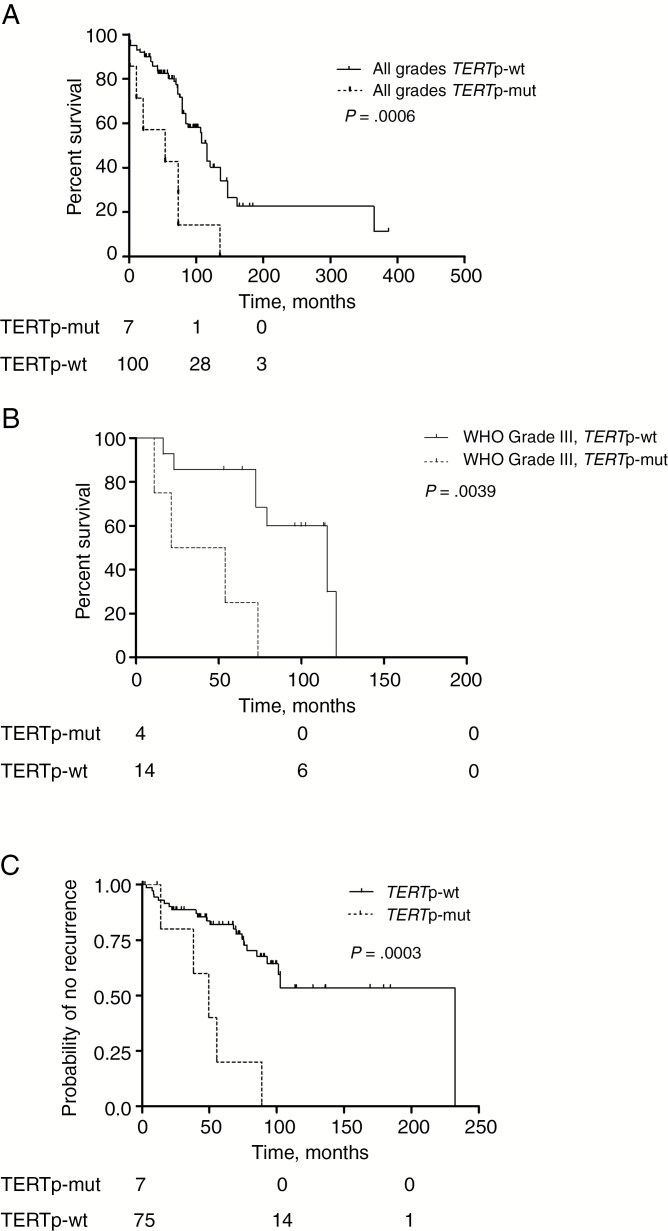
TERT Promoter Mutations Predict Poor Meningioma Patient Survival
To evaluate the effect of TERT promoter mutations on meningioma patient survival, a Kaplan–Meier survival analysis was performed considering OS times of patients between date of surgery and death by any reason. Kaplan–Meier estimates revealed a significantly shorter OS of meningioma patients with tumors harboring a TERT promoter mutation in both, the entire cohort (P = 0.0006; 53.8 vs 115.6 mo) (Fig. 4A) and the anaplastic meningioma WHO grade III subgroup (P = 0.0039; 37.6 vs 115.6 mo) (Fig. 4B). Regarding atypical meningioma WHO grade II (n = 75), a similar effect was detected. Although the histologic characteristics of TERTp-mut and -wt grade II meningiomas were widely similar (Ki-67, 12% in TERTp-mut vs 11.45% in TERTp-wt tumors; brain invasion 24% vs 33%, respectively), also in this cohort TERTp-mut was associated with a significantly reduced OS of patients (P = 0.0289). The prognostic power of this genomic alteration for OS also held true in multivariate Cox regression analyses (P = 0.003, hazard ratio [HR] 3.78). Moreover, TERTp-mut was associated with a higher hazard ratio compared with age (>63 y, P = 0.012, HR 2.44) and WHO grade (P = 0.08, HR 1.70), confirming TERTp-mut as an independent negative prognosticator in meningioma (Table 1). Additionally, TERTp-mut meningiomas recurred significantly earlier (P = 0.0003) than tumors harboring a wild-type TERT promoter (Fig. 4C).
Fig. 4 .
Impact of TERT promoter mutation on patient prognosis. (A, B) TERT promoter mutation status was compared with OS and (C) time to recurrence. (A) Kaplan–Meier plots for patient subgroups according to TERTp-wt and TERTp-mut variants are shown for the entire meningioma cohort and (B) for the anaplastic meningioma WHO grade III collective. (C) Comparison of meningioma patient subgroups with different TERT promoter status regarding “probability of no recurrence with time” (C). Numbers below the Kaplan–Meier survival plots (A, B, C) indicate patients at risk at timepoints 0, 100, and 200 months.
Table 1.
Multivariate Cox regression analysis for overall survival
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Age > ≤ 63 y | 2.44 | 1.214–4.916 | 0.012* |
| Highest tumor grade ever | 1.70 | 0.939–3.061 | 0.080 |
| TERT promoter mutation (mut vs wt) | 3.78 | 1.576–9.079 | 0.003* |
*Significant at P < 0.05 by multivariate Cox regression.
TERT Promoter Mutations but not TERT mRNA Expression or Telomerase Activity Predict Meningioma Cell Immortalization
To clarify cellular factors underlying meningioma aggressiveness, primo-cell cultures were established for viability/immortalization testing from almost all tumors (121/128). Irrespective of the TA, primo-cell cultures were able to be propagated with comparable success rates from TA+ and TA− meningiomas in 53% and 52% of TERTp-wt tumors, respectively. In contrast, within the cohort of tumors harboring a TERTp-mut background, 75% (6/8) of viable primo-cell cultures could be established (Fig. 1). Additionally, from the entire cohort only 2 out of these 6 viable TERTp-mut meningioma cell cultures (33.3%) developed into stable immortalized cell lines. This strongly indicates that aggressiveness of meningioma cells—reflected by the significantly worse OS of TERTp-mut meningioma patients—is closely related to TERT promoter mutations.
TERT Promoter Mutated Meningiomas Exhibit Hypersensitivity Against the ETS Transcription Factor Inhibitor YK-4–279
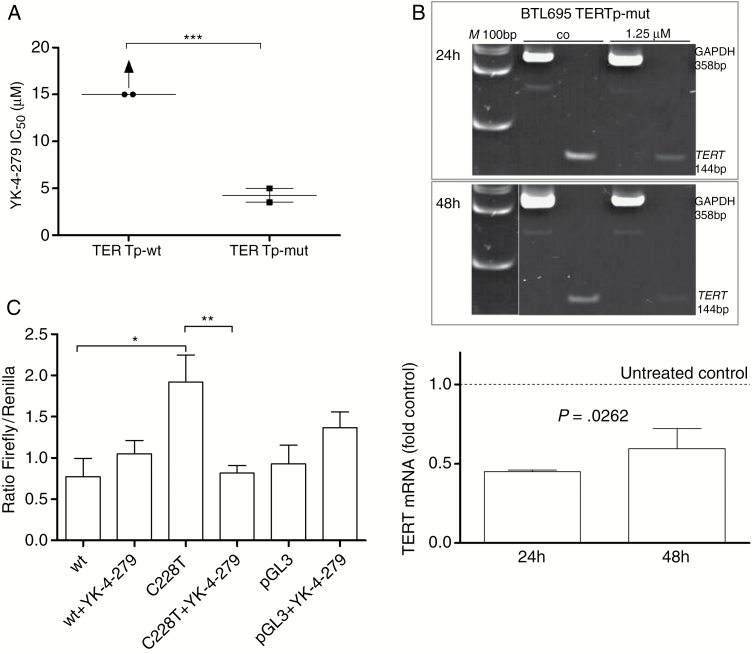
The C>T transition mutations in the TERT promoter region open up an ETS transcription factor consensus sequence whose binding by selected ETS family members might lead to enhanced promoter activity.22,23 Consequently, we have analyzed the impact of the ETS transcription factor inhibitor YK-4–279 on meningioma cells. Chemosensitivity testing revealed significantly enhanced YK-4–279 responsiveness of 2 meningioma-derived TERTp-mut primo-cell cultures compared with TERTp-wt cell models (P < 0.001; Fig. 5A). In the latter case, IC50 values were clearly above the highest applicable YK-4–279 concentration tested (15 µM). To enable statistical comparison, IC50 values for the TERTp-wt cell cultures were set at 15 µM, although clearly more than 50% of cells were viable at this concentration. These data suggest a central contribution of ETS transcription factors to meningioma cell growth solely in a TERTp-mut background. In line with expectations, ETS transcription factor inhibition resulted in a statistically significant downregulation of TERT mRNA expression as analyzed by PCR-based approaches (Fig. 5B). To analyze whether the observed TERT mRNA downregulation by YK-4–279 is indeed based on altered promoter activity, luciferase reporter assays were performed. In the TERTp-mut meningioma cell model BTL695, only the C228T but not the wt promoter plasmid led to significantly increased luciferase levels. Upregulation was completely blocked by presence of 5 µM YK-4–279 for 18 hours. This proves that the enhanced activity of a mutated TERT promoter in meningioma cells is dependent on ETS transcription factor interaction.
Fig. 5.
TERT promoter status and responsiveness to the ETS-factor inhibitor YK-4–279. (A) Meningioma primo-cell cultures with TERTp-wt (BTL400, BTL2282) and TERTp-mut (BTL695, BTL598) backgrounds were exposed to increasing concentrations of YK-4–279, and cell survival rates were determined by MTT assays. Mean IC50 values are given. In the case of TERTp-wt status, IC50 values were above the highest applicable YK-4–279 concentration (15 µM) but estimated at 15 µM for statistical analysis. (B) Downregulation of TERT mRNA expression analyzed by RT-PCR in the TERTp-mut BTL695 meningioma cell line after 24 and 48 h drug exposures as depicted. YK-4–279 treatment resulted in a marked decrease of TERT mRNA in the TERTp-mut BTL695 cell line (upper panel). M, 100 bp size marker. Additionally, quantitative real-time PCR was performed proving significant TERT mRNA downregulation by exposure to 1.25 µM YK-4–279. TERT mRNA expression levels are given relatively to the untreated control set as 1. Data are derived from 2 independent experiments performed in triplicates. (C) BTL695 cells were transfected with luciferase reporter constructs containing either the wild-type (wt) or the C228T TERT promoter sequence. Forty-eight hours post transfection, cells were treated with 5 µM YK-4–279 and 18 h later reporter expression was analyzed. Values are depicted as ratios firefly/Renilla. Negative control was pGL3 basic vector. Significant differences were calculated in panels A and C using Student’s t-test (***P < 0.001, **P < 0.01, *P < 0.05) and one-way ANOVA in panel B.
Discussion
Reactivation of telomerase is one of the hallmarks of malignant tumors, as it maintains telomere stability and, consequently, allows cellular immortalization. However, the precise mechanisms of telomerase reactivation are complex and multifaceted. Moreover, it is still unresolved whether telomerase and especially TERT might harbor, in addition to telomere stabilization, so-called noncanonical tumor-promoting functions, including hyperactivation of oncogenic signaling cascades like nuclear factor-kappaB and Wnt/c-myc.24 Recently, noncoding mutations in the promoter of the TERT gene were found to be associated with worse prognosis in multiple cancer types.25 We and others have demonstrated that this also holds true for CNS tumors like glioblastoma (GBM),6,26–30 where promoter mutations are associated with enhanced telomerase activity and shorter telomeres.5
Concerning meningioma, a few studies have reported presence of TERT promoter mutations in between 4.5% and 11% of cases affecting all clinical stages4,14,15 but enriched to between 14% and 23% in anaplastic lesions.4,16,31 Presence of mutations was clearly associated with disease recurrence or progression.4,14,15,31 In line with these previous reports, we detected TERT promoter mutations in 7.2% (8/110) of the entire and 19.0% (4/21) of the anaplastic meningioma cohort. Also in our study, presence of TERT promoter mutations significantly predicted probability for tumor recurrence. Additionally and in accordance with several previous reports,4,15TERT promoter mutations were persistent during tumor progression. In contrast, in the study by Juratli et al,16 several meningioma patients acquired TERT promoter mutations during disease progression. The authors describe remarkable intratumoral heterogeneity for TERT promoter mutations which might explain these results. Accordingly, within one progressing meningioma sample, presence of the C228T TERT promoter mutation was found in WHO grades II/III while missing in grade I areas,32 suggesting a direct role of this genetic alteration in malignant transformation. Additionally, we report here that TERTp-mut meningioma patients are characterized by a significantly reduced OS both in the entire cohort (P = 0.0006) as well as in the anaplastic patient subgroup (P = 0.0039). In agreement with Juratli et al,16 multivariate Cox regression analyses confirmed the independent prognostic power of this genomic alteration even exceeding the hazard ratio of other parameters like enhanced age and WHO stage. Survival analysis in our study was based on the parameter “death by all reasons,” due to higher patient age and presence of comorbidities characteristic of meningioma patient cohorts. Despite this limitation and the low percentage of TERTp-mut meningiomas, the impact on survival was highly significant and independent. This suggests a massive effect of TERT promoter status on the aggressiveness of this disease.
Interestingly, in our study and that of Sahm et al,4TERTp-mut was significantly associated with disease progression in both the entire and the anaplastic patient collectives. In contrast, Peyre et al31—analyzing only an anaplastic meningioma patient cohort—found an impact of the TERT promoter mutation status solely in the subgroup of secondary anaplastic disease, but neither in the entire collective nor in de novo meningioma cases. However, subgroup analysis in our study revealed a particularly strong association between presence of the mutation and short OS in the patient collective suffering from de novo meningioma (P = 0.006), while this comparison did not reach statistical significance in patients with secondary anaplastic disease (data not shown). These differences might be based on low TERTp-mut patient numbers and make analyses of larger patient cohorts necessary.
As in other malignancies, it remains unclear whether the worse prognosis of TERTp-mut meningioma patients is mediated solely by telomerase activation or by an additional yet undefined mechanism. To address this question, we first analyzed whether activating TERT promoter mutations are paralleled by enhanced TERT gene expression and enzyme activity, as observed in multiple other cancers, including GBM.5,23,33 In accordance with Goutagny et al,15 presence of these mutations correlated with enhanced TERT mRNA expression also in our meningioma collective. Additionally, telomerase enzyme activity was demonstrated to be upregulated. Interestingly, telomeres of TERT promoter-mutated cases have been reported to be shorter compared with wild-type cases in several malignancies, including GBM.5,34 However, in the case of our meningioma collection we found only an insignificant trend in this direction. It needs to be considered that, like all other telomere-associated parameters, telomere lengths were determined in the original tumor tissues. This might explain why especially in the low number of mutated cases telomere lengths were relatively variable with a high standard error of mean. Additionally, it might be hypothesized that signals are blurred by contamination with different amounts of nonmalignant cell types. Therefore we conclude that a higher number of cases would be necessary to obtain a statistically bulletproof result.
Nevertheless, TERT gene expression and telomerase activation were not restricted to TERTp-mut meningioma samples. TERT mRNA expression was detected in all meningiomas harboring one of the 2 classical mutations (C228T/C250T; 7/7) but also in 10% (12/121) of TERTp-wt samples. Interestingly, the highest TERT mRNA levels were found in 4 samples derived from one TERTp-wt progressive meningioma patient. This indicates that additional mechanisms of TERT gene activation exist and might explain the relatively high percentage (10% to 80% depending on the study) of TERT expression– and TA–positive meningiomas in earlier reports compared with the smaller subgroup with promoter mutations.35–38
Hypermethylation in the CpG cluster of the TERT promoter was detected in several tumor types and has been shown to be associated with increased TERT gene expression39 and progressive disease.12 Additionally, TERT promoter methylation was correlated with worse prognosis in pediatric brain tumors, gastric cancer, and acute myeloid leukemia.4,12,40,41 Corresponding data have been reported for meningiomas, where TERT promoter methylation was associated with increasing tumor grade and upregulation of TERT mRNA expression.13 In the latter study, a minor but significant impact of TERT promoter methylation on patient OS was detected in univariate analysis, which, however, lost significance in the multivariate setting. In our study, we have analyzed TERT promoter methylation in all TERTp-mut meningioma compared with a selection of TERTp-wt tumors positive and negative for telomerase reactivation. We observed a trend toward higher levels of TERT promoter methylation in TERTp-wt than TERTp-mut meningiomas. However, even in TERTp-wt tumors, elevated TERT promoter methylation levels did not associate with increased TERT mRNA expression. Conversely, samples positive or negative for TERT mRNA as well as TA did not show differences in the promoter methylation level. This clearly indicates that TERT promoter mutation rather than methylation dominates telomerase activation in anaplastic meningiomas. In contrast, Furtjes et al13 suggested enhanced TERT protein expression in methylated cases based on immunohistochemistry. This could be due to the different methods used for detection of TERT promoter methylation (methylation-specific PCR versus pyrosequencing) and gene expression (RT-PCR and telomeric repeat amplification protocol [TRAP] assay versus immunohistochemistry). Recently, chromosomal rearrangement resulting in a fusion involving the TERT gene (LPCAT1-TERT) was described for one meningioma case (1/28) and shown to be associated with TERT mRNA upregulation.16 However, the impact of LPCAT1-TERT gene fusion on TERT mRNA expression in TERTp-wt meningiomas needs to be addressed in larger patient cohorts.
Cell immortalization as a consequence of telomerase-mediated telomere stabilization is crucial for malignant transformation and affects more than 90% of all human tumors.42,43 However, despite telomerase activation in a relatively high proportion of meningiomas,35–38 immortalization of meningioma cells is comparatively rare and stable tumor-derived meningioma cell lines are sparse. Hence, we tested almost all tumors (121/128) from our meningioma cohort for unrestricted cell propagation in vitro. Successful establishment of viable and proliferating primo-cell cultures was independent of telomerase activity in the tumor sample (slightly more than 50% in both subgroups) but was moderately enhanced in TERTp-mut samples (75%). However, solely 2/121 (1.6%) meningioma samples developed into stable cell lines, both derived from TERTp-mut cases. Despite even high TERT mRNA expression and TA in selected cases, none of the TERTp-wt tumor-derived primo-cell cultures developed into a stable immortal cell line. In line with our observations, Johanns et al screened brain tumor cell models for TERT promoter mutations and found both immortalized meningioma cell lines to harbor TERT C228T mutations.44 This strongly supports a key role for activating mutations in the TERT promoter sequence in driving meningioma cell immortalization and aggressiveness. Interestingly, TERT promoter mutations were demonstrated to be sufficient to overcome the proliferative barrier of embryonic stem cells imposed by telomere shortening without additional tumor-selected mutations.45 Whether also noncanonical tumor-promoting functions of TERT underlie this enhanced cell aggressiveness is a matter of ongoing investigations in our research lab.
Both C>T transition mutations (C228T, C250T) of the TERT promoter generate a binding site for ETS/TCF transcription factors, which are known to be involved in the transcriptional regulation of the TERT gene.7,8,19,20 Hence, inhibition of ETS/TCF transcription factors might represent a novel and promising strategy to treat TERTp-mut cancer types. Against this background we have tested the impact of YK-4-279, a small molecule known to inhibit ETS-factor transactivation by blocking interaction with RNA helicase A,21 on TERTp-mut and TERTp-wt meningioma cells in vitro. Our cell viability data revealed significantly enhanced YK-4–279 sensitivity in cells harboring a TERTp-mut background compared with TERTp-wt tumors. Additionally, YK-4–279 exposure was accompanied by a significant downregulation of TERT mRNA expression in a TERTp-mut meningioma cell line. Accordingly, upregulated luciferase activity expressed from a plasmid driven by a C228T TERT promoter was completely abrogated in the presence of YK-4-279. Interestingly, expression of detectable luciferase levels from a wild-type promoter failed in the utilized meningioma cell culture. These data suggest that TERT promoter mutations might harbor quality as predictive markers for targeted therapy approaches, which should be investigated in multiple TERTp-mut–driven cancer types in depth.
In summary, activating mutations in the TERT promoter drive meningioma aggressiveness, also reflected by cell immortalization in vitro, resulting in worse prognosis for these meningioma patients. In addition, TERT promoter mutations might open novel therapeutic options for progressive disease.
Funding
This work was supported by the Jubiläumsfonds der Österreichischen Nationalbank (no. 16130 to W.B.), by the Austrian Science Fund (FWF, T906 to D.L. and P30105 to W.B.), and by the Fonds der Stadt Wien für Innovative Interdisziplinäre Krebsforschung (to W.B.).
Conflict of interest statement. No potential conflicts of interest were disclosed.
References
- 1. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Saraf S, McCarthy BJ, Villano JL. Update on meningiomas. Oncologist. 2011;16(11):1604–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sahm F, Schrimpf D, Olar A, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016;108(5):djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spiegl-Kreinecker S, Lötsch D, Ghanim B, et al. Prognostic quality of activating TERT promoter mutations in glioblastoma: interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro Oncol. 2015;17(9):1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Killela PJ, Pirozzi CJ, Healy P, et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;5(6):1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–961. [DOI] [PubMed] [Google Scholar]
- 8. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339(6122):957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15): 6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vinagre J, Almeida A, Pópulo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. [DOI] [PubMed] [Google Scholar]
- 11. Arita H, Narita Y, Fukushima S, et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126(2):267–276. [DOI] [PubMed] [Google Scholar]
- 12. Castelo-Branco P, Choufani S, Mack S, et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol. 2013;14(6):534–542. [DOI] [PubMed] [Google Scholar]
- 13. Fürtjes G, Köchling M, Peetz-Dienhart S, et al. hTERT promoter methylation in meningiomas and central nervous hemangiopericytomas. J Neurooncol. 2016;130(1):79–87. [DOI] [PubMed] [Google Scholar]
- 14. Koelsche C, Sahm F, Capper D, et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013;126(6):907–915. [DOI] [PubMed] [Google Scholar]
- 15. Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Juratli TA, Thiede C, Koerner MVA, et al. Intratumoral heterogeneity and TERT promoter mutations in progressive/higher-grade meningiomas. Oncotarget. 2017;8(65):109228–109237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spiegl-Kreinecker S, Pirker C, Marosi C, et al. Dynamics of chemosensitivity and chromosomal instability in recurrent glioblastoma. Br J Cancer. 2007;96(6):960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lötsch D, Ghanim B, Laaber M, et al. Prognostic significance of telomerase-associated parameters in glioblastoma: effect of patient age. Neuro Oncol. 2013;15(4):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lötsch D, Steiner E, Holzmann K, et al. Major vault protein supports glioblastoma survival and migration by upregulating the EGFR/PI3K signalling axis. Oncotarget. 2013;4(11):1904–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rachakonda PS, Hosen I, de Verdier PJ, et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci U S A. 2013;110(43):17426–17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erkizan HV, Kong Y, Merchant M, et al. A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing’s sarcoma. Nat Med. 2009;15(7):750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bell RJ, Rube HT, Kreig A, et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science. 2015;348(6238):1036–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heidenreich B, Kumar R. TERT promoter mutations in telomere biology. Mutat Res. 2017;771:15–31. [DOI] [PubMed] [Google Scholar]
- 24. Maida Y, Masutomi K. Telomerase reverse transcriptase moonlights: therapeutic targets beyond telomerase. Cancer Sci. 2015;106(11):1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu T, Yuan X, Xu D. Cancer-specific telomerase reverse transcriptase (TERT) promoter mutations: biological and clinical implications. Genes (Basel). 2016;7(7):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arita H, Yamasaki K, Matsushita Y, et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun. 2016;4(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Batista R, Cruvinel-Carloni A, Vinagre J, et al. The prognostic impact of TERT promoter mutations in glioblastomas is modified by the rs2853669 single nucleotide polymorphism. Int J Cancer. 2016;139(2):414–423. [DOI] [PubMed] [Google Scholar]
- 28. Labussière M, Boisselier B, Mokhtari K, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83(13):1200–1206. [DOI] [PubMed] [Google Scholar]
- 29. Mosrati MA, Malmström A, Lysiak M, et al. TERT promoter mutations and polymorphisms as prognostic factors in primary glioblastoma. Oncotarget. 2015;6(18):16663–16673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simon M, Hosen I, Gousias K, et al. TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015;17(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peyre M, Gauchotte G, Giry M, et al. De novo and secondary anaplastic meningiomas: a study of clinical and histomolecular prognostic factors. Neuro Oncol. 2017. doi: 10.1093/neuonc/nox231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abedalthagafi MS, Bi WL, Merrill PH, et al. ARID1A and TERT promoter mutations in dedifferentiated meningioma. Cancer Genet. 2015;208(6):345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mason PJ, Perdigones N. Telomere biology and translational research. Transl Res. 2013;162(6):333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simon M PT, Leuenroth S, Hans VH, Löning T, Schramm J. Telomerase activity and expression of the telomerase catalytic subunit, hTERT, in meningioma progression. J Neurosurg. 2000;92(5):832–840. [DOI] [PubMed] [Google Scholar]
- 36. Boldrini L, Pistolesi S, Gisfredi S, et al. Telomerase in intracranial meningiomas. Int J Mol Med. 2003;12(6):943–947. [DOI] [PubMed] [Google Scholar]
- 37. Kheirollahi M, Mehrazin M, Kamalian N, Mohammadi-asl J, Mehdipour P. Telomerase activity in human brain tumors: astrocytoma and meningioma. Cell Mol Neurobiol. 2013;33(4):569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maes L, Kalala JP, Cornelissen R, de Ridder L. Telomerase activity and hTERT protein expression in meningiomas: an analysis in vivo versus in vitro. Anticancer Res. 2006;26(3B):2295–2300. [PubMed] [Google Scholar]
- 39. Guilleret I, Yan P, Grange F, Braunschweig R, Bosman FT, Benhattar J. Hypermethylation of the human telomerase catalytic subunit (hTERT) gene correlates with telomerase activity. Int J Cancer. 2002;101(4):335–341. [DOI] [PubMed] [Google Scholar]
- 40. Gojo J, Lötsch D, Spiegl-Kreinecker S, et al. Telomerase activation in posterior fossa group A ependymomas is associated with dismal prognosis and chromosome 1q gain. Neuro Oncol. 2017;19(9):1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao X, Tian X, Kajigaya S, et al. Epigenetic landscape of the TERT promoter: a potential biomarker for high risk AML/MDS. Br J Haematol. 2016;175(3):427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43(2 Pt 1):405–413. [DOI] [PubMed] [Google Scholar]
- 43. Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–2015. [DOI] [PubMed] [Google Scholar]
- 44. Johanns TM, Fu Y, Kobayashi DK, et al. High incidence of TERT mutation in brain tumor cell lines. Brain Tumor Pathol. 2016;33(3):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chiba K, Johnson JZ, Vogan JM, Wagner T, Boyle JM, Hockemeyer D. Cancer-associated TERT promoter mutations abrogate telomerase silencing. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]