Abstract
Background
Noninvasive and accurate modality to predict isocitrate dehydrogenase (IDH) mutant glioma may have great potential in routine clinical practice. We aimed to investigate the diagnostic performance of 2-hydroxyglutarate (2HG) magnetic resonance spectroscopy (MRS) for prediction of IDH mutant glioma and provide an optimal cutoff value for 2HG.
Methods
A systematic literature search of Ovid-MEDLINE and EMBASE was performed to identify original articles investigating the diagnostic performance of 2HG MRS up to March 20, 2018. Pooled sensitivity and specificity were calculated using a bivariate random-effects model. Subgroup analysis and meta-regression were performed to explain heterogeneity effects. An optimal cutoff value for 2HG was calculated from studies providing individual patient data.
Results
Fourteen original articles with 460 patients were included. The pooled sensitivity and specificity for the diagnostic performance of 2HG MRS for prediction of IDH mutant glioma were 95% (95% CI, 85–98%) and 91% (95% CI, 83–96%), respectively. The Higgins I2 statistic demonstrated that heterogeneity was present in the sensitivity (I2 = 50.69%), but not in the specificity (I2 = 30.37%). In the meta-regression, echo time (TE) was associated with study heterogeneity. Among the studies using point-resolved spectroscopy (PRESS), a long TE (97 ms) resulted in higher sensitivity (92%) and specificity (97%) than a short TE (30–35 ms; sensitivity of 90%, specificity of 88%; P < 0.01). The optimal 2HG cutoff value of 2HG using individual patient data was 1.76 mM.
Conclusion
2HG MRS demonstrated excellent specificity for prediction of IDH mutant glioma, with TE being associated with heterogeneity in the sensitivity.
Key Points
1. HG MRS has excellent diagnostic performance in the prediction of IDH mutant glioma.
2. The pooled sensitivity was 95% and the pooled specificity was 91%.
3. Echo time was associated with study heterogeneity in the meta-regression.
Keywords: 2-hydroxyglutarate, glioma, isocitrate dehydrogenase mutation, magnetic resonance spectroscopy
Importance of the Study
Noninvasive and accurate modality to predict IDH mutant glioma may have great potential in routine clinical practice, and could help with the implementation of appropriate management in patients with glioma. This study revealed that 2HG MRS has excellent diagnostic performance in the prediction of IDH mutant glioma. The pooled sensitivity was 95% (95% CI, 85–98%), the pooled specificity was 91% (95% CI, 83–96%), and the area under the hierarchical summary receiver operating characteristics curve was 0.96 (95% CI, 0.94–0.98). Among the studies using PRESS, those using a long TE (97 ms) showed higher diagnostic performance than those using a short TE. Using individual patient data, the optimal cutoff value for 2HG was found to be 1.76 mM. Further validation of this optimal cutoff value for 2HG is critical for its application to glioma management in daily practice.
After the 2016 World Health Organization (WHO) classification was announced,1 isocitrate dehydrogenase (IDH) mutation status became one of the most important prognostic biomarkers in glioma management.2–4 Recent large-scale molecular studies of adult lower-grade gliomas clearly defined the IDH mutation as being crucial for determining the prognosis.2,3 Immunohistochemistry and genomic sequence analysis are considered to be gold standard methods for detecting IDH mutant glioma.5 However, these approaches are invasive, and a biopsy may also lead to an incorrect result because of intratumoral heterogeneity, which may reduce the value of invasive biopsy-based genomic analysis. Therefore, a noninvasive and accurate modality to predict IDH mutant glioma may have great potential in routine clinical practice, and could help with the implementation of appropriate management in patients with glioma.
Magnetic resonance imaging (MRI) may have such a role as an important noninvasive tool for prediction of IDH mutant glioma; however, no MRI techniques have shown impressive results for predicting IDH mutant glioma except for 2-hydroxyglutarate (2HG) MR spectroscopy (MRS). 2HG MRS was introduced to detect the oncometabolite 2HG,6–19 which occurs as a direct consequence of an IDH mutation. In previous studies, 2HG MRS demonstrated higher diagnostic performance (high sensitivities of 89–100% and high specificities of 81–88%)10,18 than conventional MRI (sensitivities of 71–100% and specificities of 51–100%)20–22 and diffusion-weighted imaging and perfusion-weighted imaging (sensitivities of 56–100% and specificities of 63–100%).23–30 Therefore, the imaging prediction of IDH mutation status using 2HG MRS could be a valuable method for clinical decision making. Moreover, 2HG MRS is likely to become more valuable when IDH mutant inhibitors become clinically available.31
However, the diagnostic performance of 2HG MRS for prediction of IDH mutant glioma has not yet been systematically evaluated. In addition, an optimal 2HG cutoff value obtained from individual patient data has not been evaluated. Moreover, if heterogeneity is present in the diagnostic use of 2HG MRS, any covariates affecting the diagnostic performance should be identified. Therefore, this systematic review and meta-analysis aimed to investigate the diagnostic performance of 2HG MRS for prediction of IDH mutant glioma, and provide an optimal 2HG cutoff value.
Materials and Methods
This study was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).32
Literature Search
A systematic literature search of Ovid-MEDLINE and EMBASE was performed to identify original articles investigating the diagnostic performance of 2HG MRS for prediction of IDH mutant glioma published up to March 20, 2018. The search terms were as follows: ((“Isocitrate dehydrogenase”) OR (IDH)) AND ((2-hydroxyglutarate) OR (2HG)) AND ((“magnetic resonance spectroscopy”) OR (“MR spectroscopy”)). The search was not limited by language, by whether studies were human or animal, or by search date. Any additional studies identified were screened to expand the extent of the search.
Eligibility Criteria
Studies were selected if all of the following inclusion criteria were met: (1) patients with histopathologically confirmed WHO grade II, III, or IV gliomas; (2) patients with preoperative in vivo 2HG MRS before treatment; (3) a reference standard based on immunohistochemistry analysis for IDH1 mutation (R132H) or genomic sequencing analysis for IDH1 and IDH2 genes; and (4) sufficient information for the reconstruction of 2 × 2 tables for determination of the diagnostic performance of 2HG MRS for prediction of IDH mutant glioma.
Studies were excluded if any of the following exclusion criteria were met: (1) a review article; (2) case reports or case series including fewer than 5 patients; (3) conference abstracts; (4) letters, editorials, and comments; (5) animal or phantom studies; and (6) studies with a partially overlapping patient cohort. For studies with an overlapping study population, the study with the largest population was selected. Authors of papers not providing sufficient information for the construction of 2 × 2 tables were contacted for the provision of further data.
Data Extraction and Quality Assessment
A standardized extraction form was used to obtain the following information from the selected studies: (1) study characteristics: institution, study period, study design, consecutive or non-consecutive enrollment, reference standard, interval between MRS and the reference standard, and blinding to the reference standard; (2) demographic and clinical characteristics: total number of patients, number of patients with IDH mutant glioma, mean age (range), and male to female ratio; (3) technical characteristics of MRI: magnetic field strength, vendor, model, head coil channels, 2HG MRS techniques or sequences, echo time (TE; ms), and cutoff values for the specific technical parameters used to diagnose IDH mutant glioma.
The quality assessment of selected studies was investigated using the Quality Assessment of Diagnostic Accuracy Studies–2 (QUADAS-2) tool.33 The literature search, study selection, data extraction, and quality assessment were conducted independently by 2 reviewers (C.H.S. and H.S.K.). If disagreement was present, a third reviewer (S.J.K.) was consulted to reach a consensus.
Data Synthesis and Analysis
Two-by-two tables were reconstructed for each study, choosing the results with the highest performance if the diagnostic performance of multiple MRS sequences was separately evaluated. The pooled sensitivity and pooled specificity and their 95% CIs were calculated using a bivariate random effects model, and a coupled forest plot was obtained.34–38 The pooled positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were also calculated. The PLR was defined as the likelihood that a 2HG MRS result positive for differentiating IDH mutant glioma from IDH wild-type glioma would occur in patients with IDH mutant glioma. The NLR was defined as the likelihood that a 2HG MRS result negative for differentiating IDH mutant glioma from IDH wild-type glioma would occur in patients without IDH mutant glioma. The DOR was defined as the odds of having a positive 2HG MRS result in patients with IDH mutant glioma compared with the odds of having a positive 2HG MRS result in patients without IDH mutant glioma. In addition, a hierarchical summary receiver operating characteristic (HSROC) curve with 95% confidence and prediction regions was plotted.
Heterogeneity was assessed using the following: (1) Cochran’s Q-test with P < 0.05 indicating the presence of heterogeneity; (2) Higgins inconsistency index (I2) test with a value >50% indicating the presence of heterogeneity;39 (3) visual assessment of the difference between the 95% confidence region and prediction region in the HSROC curve (a large difference indicating heterogeneity); (4) visual assessment of the coupled forest plot to assess the presence of a threshold effect (ie, a positive correlation between sensitivity and false positive rate among the selected studies); and (5) a Spearman correlation coefficient >0.6 revealing a threshold effect.40 A Deeks funnel plot was constructed to test for publication bias, with statistical significance being assessed using Deeks’s asymmetry test.41 A subgroup analysis of studies using point-resolved spectroscopy (PRESS) as the MRS sequence and a meta-regression were performed to explain the effects of heterogeneity. The following covariates were considered for the bivariate meta-regression model: (1) study design; (2) study enrollment; (3) the percentage of IDH mutant gliomas (<49% [median value of the selected studies] vs ≥49%); (4) TE 97 ms vs 30–35 ms; and (5) TE 97 ms vs 110 ms.
An optimal cutoff value for 2HG was calculated from those studies providing individual patient data. The sensitivity and specificity of 2HG MRS and the corresponding cutoff were estimated using the Youden index, which is defined as sensitivity + specificity − 1.42 The Youden index has a minimum value of −1 and a maximum value of +1, and a value of +1 represents the optimal value for an algorithm. Statistical analyses were conducted by one of the authors (C.H.S., with 5 years of experience in performing systematic reviews and meta-analyses) using the “metandi” and “midas” modules in Stata 15.0 (StataCorp), the “mada” package in R version 3.4.1, and MedCalc Software. A value of P < 0.05 was taken to indicate statistical significance.
Results
Literature Search
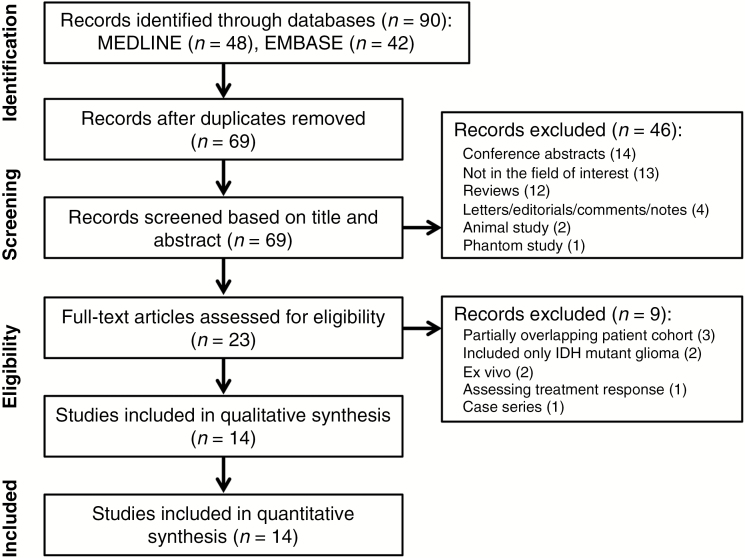
The study selection process is described in Fig. 1. The systematic search found 90 articles. After removal of 21 duplicates, screening of the abstracts of the 69 remaining articles was performed, and 46 articles were excluded for the following reasons: 14 conference abstracts, 13 articles that were not in the field of interest, 12 reviews, 4 editorials/letters/comments/notes, 2 animal studies, and 1 phantom study. Full-text reviews of 23 potentially eligible articles were performed, and a further 9 articles were excluded for the following reasons: 3 articles with partially overlapping patient cohorts,43–45 2 articles including only IDH mutant glioma,46,47 2 ex vivo studies,48,49 1 article assessing treatment response,50 and 1 case series.51 Finally, 14 original articles investigating the diagnostic performance of 2HG MRS for prediction of IDH mutant glioma, with a total of 460 patients, were included in our meta-analysis.6–19
Fig. 1.
Flow diagram of the study selection process.
Characteristics of the Included Studies
The patient and study characteristics are described in Table 1. Six studies had a prospective design,8,10,11,14,17,19 with the other studies not reporting the design. Patient enrollment was performed in a consecutive fashion in 5 studies,11,12,14,16,17 but was not reported in the others. Eleven of the 14 studies included gliomas of WHO grades II, III, and IV,7–14,17–19 1 study included gliomas of grades II and III,15 1 study included gliomas of grades III and IV,6 and 1 study included only glioblastoma.16 All the selected studies used immunohistochemistry and/or genomic sequence analysis as a reference standard, with immunohistochemistry being conducted for mutated IDH1 (R132H) protein expression, and genomic sequence analysis for mutations in IDH1 or IDH2 genes.25
Table 1.
Study and patient characteristics of the selected studies
| Author (year of publication) |
Institution | Duration of Patient Recruitment | No. of Patients (n) | IDH Mutant Glioma (n) | IDH Wild- type (n) | IDH Mutant Glioma (%) | Mean age, y | Range | Male: Female | Study Design | Consecutive Enrollment | Reference Standard |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andronesi OC, et al6 (2012) | Massachusetts General Hospital, USA | NA | 6 | 2 | 4 | 33.3 | 47.7 | 39–70 | 2:4 | NA | NA | Immunohistochemistry |
| Bisdas S, et al7 (2016) | Eberhard-Karls University Tübingen, Germany | NA | 16 | 11 | 5 | 68.8 | NA | NA | NA | NA | NA | Immunohistochemistry + genomic sequencing analysis |
| Branzoli F, et al8 (2017) | Pitié-Salpetrière and Foch Hospitals, France | NA | 24 | 20 | 4 | 83.3 | median 38 | 22–63 | 13:11 | Prospective | NA | Immunohistochemistry + genomic sequencing analysis |
| Choi C, et al9 (2012) | University of Texas Southwestern Medical Center, USA | NA | 30 | 15 | 15 | 50.0 | NA | NA | NA | NA | NA | Immunohistochemistry + genomic sequencing analysis |
| Choi C, et al10 (2016) | University of Texas Southwestern Medical Center, USA | 2.5 year period | 42 | 34 | 8 | 81.0 | 37.2 | 18–62 | 25:17 | Prospective | NA | Immunohistochemistry + genomic sequencing analysis |
| Crisi G, et al11 (2017) | University Hospital of Parma, Italy | 2014.4–2017.5 | 82 | 26 | 56 | 31.7 | 53.7 | NA | 56:26 | Prospective | yes | Immunohistochemistry + genomic sequencing analysis |
| de la Fuente MI, et al12 (2016) | Memorial Sloan Kettering Cancer Center, USA | NA | 28 | 22 | 6 | 78.6 | NA | NA | NA | NA | yes | Immunohistochemistry + genomic sequencing analysis |
| Emir UE, et al13 (2016) | University of Oxford, John Radcliffe Hospital, UK | NA | 14 | 10 | 4 | 71.4 | 45 | 29–68 | 8:6 | NA | NA | Immunohistochemistry + genomic sequencing analysis |
| Nagashima H, et al14 (2016) | Kobe University Hospital | 2013.8–2015.8 | 47 | 18 | 29 | 38.3 | 52.2 | 27–73 | 28:19 | Prospective | yes | Immunohistochemistry + genomic sequencing analysis |
| Natsumeda M, et al15 (2014) | University of Niigata, Japan | 2006.12–2013.3 | 52 | 25 | 27 | 48.1 | 53.3 | 26–77 | 28:24 | NA | NA | Immunohistochemistry + genomic sequencing analysis |
| Natsumeda M, et al16 (2018) | University of Niigata, Japan | 2007.7–2015.9 | 44 | 6 | 38 | 13.6 | 61 | 12–81 | 32:12 | NA | yes | Immunohistochemistry + genomic sequencing analysis |
| Pope WB, et al17 (2012) | University of California, Los Angeles | 2009.11–2010.3 | 24 | 9 | 15 | 37.5 | 53 | 22–87 | 12:12 | Prospective | yes | Genomic sequencing analysis |
| Tietze A, et al18 (2018) | Aarhus University Hospital, USA | NA | 34 | 19 | 16 | 55.9 | NA | 18–65 | NA | NA | NA | Immunohistochemistry + genomic sequencing analysis |
| Zhou M, et al19 (2018) | Brigham and Women’s Hospital | 2014.4–2017.11 | 17 | 7 | 10 | 41.2 | median 39 | 20–83 | 10:07 | Prospective | NA | Immunohistochemistry + genomic sequencing analysis |
NA = not available.
The MRI characteristics are shown in Table 2. Twelve studies used 3T scanners,6,8–12,14–19 1 study used a 7T scanner,13 and 1 study used a 9.4T scanner.7 In terms of MRS sequences, 10 studies used PRESS,9–12,14–19 1 study used Mescher–Garwood PRESS (MEGA-PRESS),8 1 study used stimulated echo acquisition mode (STEAM),7 1 study used localization by adiabatic selective refocusing (LASER),6 and 1 study used semi-LASER.13 Among the 10 studies using PRESS, 5 used a long TE (97 ms)9,10,12,18,19 and 5 used a short TE (30–35 ms).11,14–17 All included studies used a single voxel spectroscopy approach to obtain 2HG concentration. In terms of voxel size, 6 of 14 studies used 2 cm × 2 cm × 2 cm.9,10,13,17–19 Nine of the 14 studies used the 2HG concentration with a peak at 2.25 ppm as a cutoff parameter, with the cutoff values for 2HG concentration varying from 0.897 to 2 mM.9–13,15–18
Table 2.
MRI characteristics of the selected studies
| Author (year of publication) |
Magnet Strength (T) | Vendor | Scanner | Head Coil | MRS Techniques | TE (ms) | Voxel Size (cm) | Software | Parameter | Cutoff | Range of 2HG (mM) in IDH Mutant Glioma | Range of 2HG (mM) in IDH Wild- type Glioma |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andronesi OC, et al6 (2012) | 3 | Siemens | Tim Trio | 32 | 2D localization by adiabatic selective refocusing (LASER)-COSY, 1D MEGA-LASER, and 1D LASER spectra | 45 | 3 × 3 × 3 | LCModel | 2HG/(Glu+Gln) | NA | NA | NA |
| Bisdas S, et al7 (2016) | 9.4 | Siemens | NA | NA | Stimulated echo acquisition mode (STEAM) | 20 | 2 × 2 | LCModel | 2HG/tCr (CRLB) | NA | NA | NA |
| Branzoli F, et al8 (2017) | 3 | Siemens | Magnetom Verio | 32 | Mescher–Garwood point-resolved spectroscopy (MEGA-PRESS) |
68 | NA | LCModel | CRLB | 999% | 0–6.28 | 0–1.61 |
| Choi C, et al9 (2012) | 3 | Philips | NA | 8 | Optimized point-resolved spectroscopy (PRESS) | 97 | 2 × 2 × 2 | LCModel | 2HG concentration | NA | 1.7–8.9 | 0 |
| Choi C, et al10 (2016) | 3 | Philips | NA | 8 | PRESS | 97 | 2 × 2 × 2 | LCModel | 2HG concentration | 1 mM | 0.5–11 | 0–0.5 |
| Crisi G, et al11 (2017) | 3 | GE | Discovery MR750 | 8 | PRESS | 35 | NA | LCModel | 2HG concentration | NA | NA | NA |
| de la Fuente MI, et al12 (2016) | 3 | GE | MR750 | 8 | PRESS | 97 | NA | LCModel | 2HG concentration | NA | NA | NA |
| Emir UE, et al13 (2016) | 7 | Siemens | NA | 32 | Semi-localization by adiabatic selective refocusing (semi-LASER) | 110 | 2 × 2 × 2 | LCModel | 2HG concentration | NA | NA | NA |
| Nagashima H, et al14 (2016) | 3 | Philips | NA | 8 | PRESS | 35 | 1.5 × 1.5 × 1.5 | LCModel | 2HG+glutamate | 2HG >1.8 mM, glutamate <3.9 | 0–13.029 | 0–2.84 |
| Natsumeda M, et al15 (2014) | 3 | GE | Signa LX | 8 | PRESS | 30 | 1.2–2 × 1.2–2 × 1.2–2 | LCModel | 2HG concentration | 1.489 mM | NA | NA |
| Natsumeda M, et al16 (2018) | 3 | GE | Signa LX | 8 | PRESS | 30 | 1.2–2 × 1.2–2 × 1.2–2 | LCModel | 2HG concentration | 0.897 mM | 1.309–6.041 | 0–1.993 |
| Pope WB, et al17 (2012) | 3 | Siemens | Trio-Tim | 12 | PRESS | 30 | 2 × 2 × 2 | LCModel | 2HG concentration | NA | NA | NA |
| Tietze A, et al18 (2018) | 3 | Philips | Achieva | 8 | PRESS | 97 | 2 × 2 × 2 | LCModel | 2HG concentration | 2 mM | 0.35–13.93 | 0–5.11 |
| Zhou M, et al19 (2018) | 3 | Siemens | TIM Skyra | 32 | PRESS | 97 | 2 × 2 × 2 | LCModel | 2HG/Cr | 0.11 | NA | NA |
NA = not available.
Quality Assessment
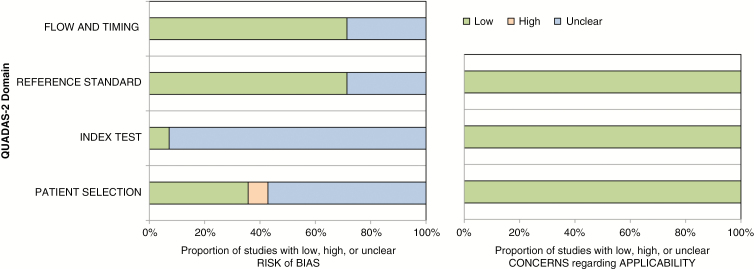
The results of the quality assessment based on QUADAS-2 criteria are illustrated in Fig. 2. Eight of 14 studies were regarded as having an unclear risk of bias in the patient selection domain because of non-consecutive enrollment.6–10,13,15,18,19 Thirteen of 14 studies were regarded as having an unclear risk of bias in the index test domain, as it was unclear whether 2HG MRS was performed blinded to the reference standard.6–8,10–19 Ten of 14 studies were regarded as having an unclear risk of bias in the reference standard domain, because it was unclear whether evaluation of the reference standard was performed blinded to the 2HG MRS.6–8,11,13,15–19 Additionally, 10 of 14 studies were regarded as having an unclear risk of bias as the time intervals between 2HG MRS and the reference standard were not mentioned.6,7,9–13,15,18,19 However, there were no concerns on the applicability of any of the studies.
Fig. 2.
QUADAS-2 criteria for the 14 included studies.
Diagnostic Performance of 2HG MRS for Prediction of IDH Mutant Glioma
In all 14 studies, IDH mutant gliomas consistently showed a significantly higher accumulation of 2HG than did IDH wild-type gliomas. The sensitivities and specificities of the individual selected studies ranged from 72% to 100% and 73% to 100%, respectively.
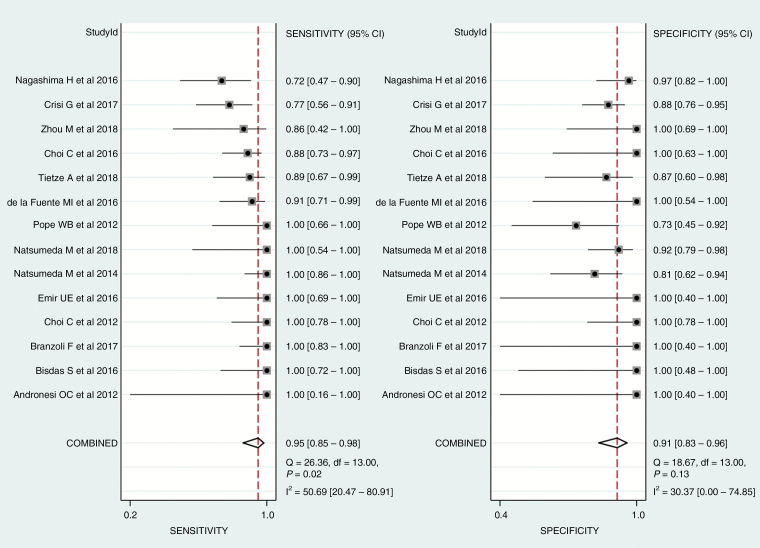
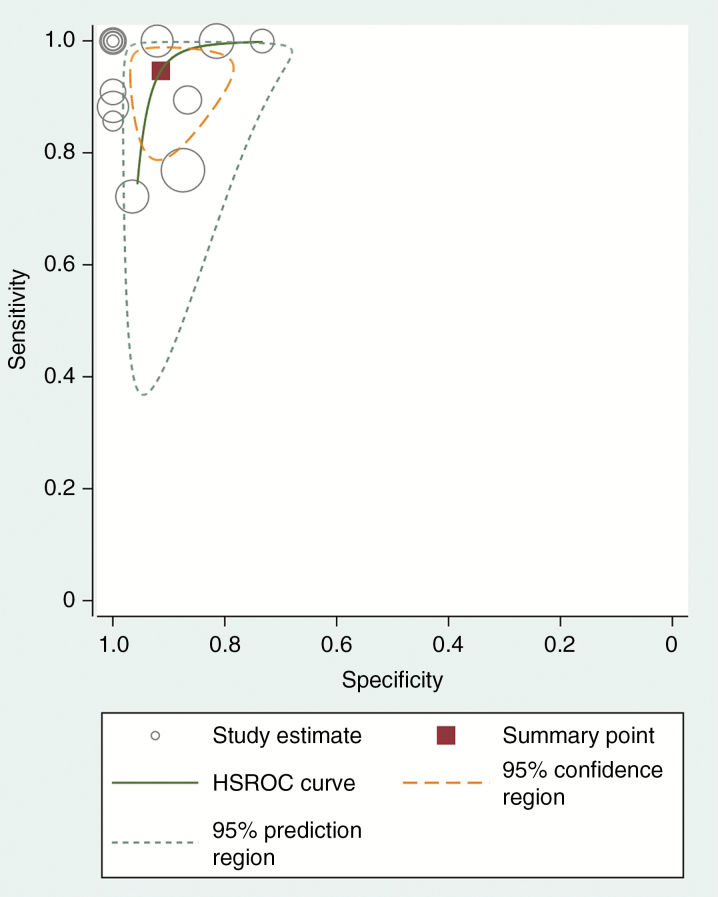
The pooled sensitivity and specificity for the diagnostic performance of 2HG MRS in the prediction of IDH mutant glioma were 95% (95% CI, 85–98%) and 91% (95% CI, 83–96%; Fig. 3), respectively. The pooled PLR, NLR, and DOR were 11 (95% CI, 5.5–21.9), 0.06 (95% CI, 0.02–0.17), and 189 (95% CI, 51–693), respectively. The area under the HSROC curve was 0.96 (95% CI, 0.94–0.98), which suggests high diagnostic performance (Fig. 4).
Fig. 3.
Coupled forest plots of the pooled sensitivity and specificity for the diagnostic performance of 2-hydroxyglutarate MRS for prediction of IDH mutant glioma. Numbers are pooled estimates with 95% CIs in parentheses; horizontal lines indicate 95% CIs.
Fig. 4.
HSROC curve of the diagnostic performance of 2HG MRS for prediction of IDH mutant glioma. There is a small difference between the 95% confidence and 95% prediction regions, indicating a low possibility of heterogeneity between the studies.
The Q-test demonstrated that heterogeneity was present across the studies (Q = 4.716, P = 0.047), and the Higgins I2 statistic demonstrated that heterogeneity was present in the sensitivity (I2 = 50.69%), but not in the specificity (I2 = 30.37%). However, there was a small difference between the 95% confidence region and the 95% prediction region in the HSROC curve, indicating a low possibility of heterogeneity across the studies (Fig. 4). The coupled forest plot of the sensitivity and specificity indicated no evidence of a threshold effect (Fig. 3), and the Spearman correlation coefficient between the sensitivity and false positive rate was 0.289 (95% CI, −0.285–0.711), also indicating no threshold effect. The Deeks funnel plot revealed that the likelihood of publication bias was low (P = 0.25; Supplementary Figure. S1).
Subgroup Analysis and Meta-Regression
In the subgroup analysis, studies using PRESS as the MRS sequence also showed high sensitivity (91% [95% CI, 82–96%]) and specificity (90% [95% CI, 83–95%]), with the area under the HSROC curve being 0.95 (95% CI, 0.93–0.97). The heterogeneity in the sensitivity was resolved (I2 = 43.73%) and remained absent from the specificity (I2 = 32.19%).
Among the various potential covariates, the TE was associated with study heterogeneity (Supplementary Table S1). Although studies using PRESS as the MRS sequence showed high sensitivity (91% [95% CI, 84–98%]) and specificity (90% [95% CI, 85–96%]), those studies using PRESS with a long TE (97 ms) showed higher sensitivity (92% [95% CI, 85–99%]) and specificity (97% [95% CI, 92–100%]) than those using a short TE (30–35 ms; sensitivity of 90% [95% CI, 79–100%], specificity of 88% [95% CI, 83–93%]; P < 0.01). Otherwise, the study design, study enrollment, and percentage of IDH mutant glioma were not significant factors affecting the heterogeneity.
Diagnostic Performance Using Individual Patient Data
Five studies provided individual patient data including IDH mutation status and 2HG concentration (mM) measured on MRS.8–10,14,18 These studies included a total of 173 patients, with 106 patients (61.4%) having IDH mutant gliomas. The area under the ROC of 2HG MRS for prediction of IDH mutant glioma was 0.903 (95% CI, 0.85–0.94; Supplementary Figure S2). The optimal cutoff value was 1.76 mM using the Youden index, resulting in a sensitivity of 75% (95% CI, 65–83%) and specificity of 94% (95% CI, 85–98%).
After excluding one study using short TE,14 individual patient data using 4 studies were calculated.8–10,18 These studies included a total of 126 patients, with 88 patients (70.0%) having IDH mutant gliomas. The area under the ROC of 2HG MRS for prediction of IDH mutant glioma was 0.921 (95% CI, 0.859–0.962). The optimal cutoff value was 2.0 mM using the Youden index, resulting in a sensitivity of 80% (95% CI, 70–87%) and specificity of 95% (95% CI, 82–99%).
Treatment Response
Among the included studies, 2 mentioned the use of 2HG in monitoring treatment response.10,12 One study showed that 2HG concentration levels rapidly decreased in oligodendroglioma and with a slower time course in astrocytoma and mixed glioma in response to cytotoxic therapy.10 Another study also reported a decrease in 2HG concentration levels after cytoreductive therapy in IDH mutant glioma patients.12
Discussion
This study revealed that 2HG MRS has excellent diagnostic performance in the prediction of IDH mutant glioma. The pooled sensitivity was 95% (95% CI, 85–98%), the pooled specificity was 91% (95% CI, 83–96%), and the area under the HSROC curve was 0.96 (95% CI, 0.94–0.98). Heterogeneity was present in the sensitivity (I2 = 50.69%), but not in the specificity (I2 = 30.37%). The meta-regression revealed that TE was associated with study heterogeneity. Among the studies using PRESS, those using a long TE (97 ms) showed higher diagnostic performance than those using a short TE. Using individual patient data, the optimal cutoff value for 2HG was found to be 1.76 mM. Further validation of this optimal cutoff value for 2HG is critical for its application to glioma management in daily practice.
There are several potential clinical applications for 2HG MRS in glioma management. First, 2HG MRS could be used to accurately predict IDH mutation status, even in those patients who cannot undergo surgical procedures or biopsy, particularly those with brainstem glioma. Second, 2HG MRS could be used to indirectly predict IDH mutation status in those gliomas classified as “not otherwise specified” according to the 2016 WHO classification when laboratory IDH testing is not available. Third, 2HG detection by MRS could be utilized as a pharmacodynamic marker to monitor treatment response.10,12,50 Concentration of 2HG increases sharply with tumor progression, whereas it decreases in response to treatment.10 Other studies also reported a decrease in 2HG concentration levels after cytoreductive therapy in IDH mutant glioma patients.12,50 Our study highlights the use of 2HG for points in clinical management of glioma beyond simply measuring 2HG for diagnostic purpose. Recently, a phase I clinical trial used 2HG MRS to document treatment response to a mutant-IDH1 inhibitor and showed a decrease of 2HG levels by 70% after one week of treatment.52 There was preclinical data from studies using AG-120, a potent orally available mutant-IDH1 inhibitor, and more than 77% inhibition of 2HG production in a mouse model with IDH1 mutant glioma was observed.53 Treatment monitoring of a mutant-IDH1 inhibitor using 2HG MRS may be used for personalized and precision medicine and early treatment response in clinical trials. Fourth, 2HG MRS might help to distinguish tumor recurrence from treatment-related changes in patients who have undergone surgery and/or concurrent chemoradiotherapy. Although laboratory IDH testing remains the gold standard for detecting IDH mutation, 2HG MRS could contribute to glioma management.
An advantage of 2HG MRS over other MRI techniques is that it can be used as direct evidence of increased levels of 2HG, an oncometabolite that is a direct consequence of an IDH mutation.54 In addition, 2HG MRS shows higher diagnostic performance (pooled sensitivity of 95% and specificity of 91%) for the prediction of IDH mutant gliomas than other MRI techniques, with conventional MRI having shown a wide range of sensitivities (71–100%) and specificities (51–100%).20–22 Diffusion-weighted imaging and perfusion-weighted imaging have also demonstrated wide ranges of sensitivities (56–100%) and specificities (63–100%).23–30In addition, 2HG concentration is known to correlate positively with tumor cellularity.10,12 High cellularity tumors demonstrated significantly higher 2HG concentration than low cellularity tumors.10,12
However, 2HG MRS has not been widely used because of the difficulty in optimizing 2HG MRS sequences, the challenging post-processing, the lack of commercialized sequences or post-processing software, and the shortage of large-scale cohort studies. Therefore, standardization of 2HG MRS is crucial. We found that the PRESS sequence was the most commonly used MRS sequence (10 of 14), and among those studies using PRESS, 5 used a long TE (97 ms)9,10,12,18,19 and 5 used a short TE (30–35 ms).11,14–17 In the meta-regression, the studies using a long TE (97 ms) showed higher diagnostic performance (sensitivity, 92%; specificity, 97%) than those using a short TE (30–35 ms; sensitivity, 90%; specificity, 88%). Therefore, we cautiously recommend a long TE PRESS sequence for 2HG MRS, although further clinical validation of 2HG MRS should be performed. Another consideration is voxel size of MRS and tumor volume. The voxel size should be adapted to tumor size to minimize partial volume effects. In our study, voxel size of 2 cm × 2 cm × 2 cm was most commonly used. One recent study reported that detection of 2HG by MRS is highly dependent on tumor volume, and that diagnostic performance was higher for large tumors (>8 mL) than for small tumors (<3.4 mL).12 In addition, all included studies that have measured 2HG concentration in patients have advanced expertise in MRS and used custom-designed software (LCModel) for 2HG quantitation. In case of a clinical environment where this expertise is not available, manufacturers of MR systems need to develop push-button applications to allow assessment of IDH status.
Determination of a standard cutoff value is clinically important if standardized 2HG MRS is to be used in daily clinical practice. We found that 9 studies used the 2HG concentration with a peak at 2.25 ppm as a cutoff parameter, with the cutoff values for 2HG concentration varying from 0.897 to 2 mM. Using the individual patient data, we found an optimal cutoff value for 2HG of 1.76 mM. However, one study reported that a combined measurement of 2HG and glutamate had a higher diagnostic performance (sensitivity, 88%; specificity, 100%) than 2HG concentration alone (sensitivity, 75%; specificity, 100%).14 Another study reported that MEGA-PRESS measuring 2HG at 4.02 ppm showed higher diagnostic performance (sensitivity, 100%; specificity, 100%) than optimized PRESS (sensitivity, 72%; specificity, 100%).8 Therefore, further large cohort studies are needed to determine optimal cutoff values.
Several limitations to this study are of note. First, only 6 of 14 studies used a prospective design, and most studies included only a small number of patients and were therefore vulnerable to selection bias. Further large-scale prospective studies are needed. Second, we could not perform subgroup analysis for lower-grade gliomas (glioma grades II and III) and glioblastoma (grade IV) because of the paucity of information. The prediction of IDH mutation is more important in lower-grade glioma and has a great impact on survival.2 Further studies evaluating the covariates affecting the diagnostic accuracy of 2HG MRS are required. Third, heterogeneity was present in the sensitivity. To overcome this heterogeneity, we performed subgroup analysis and meta-regression. The heterogeneity was resolved in the subgroup analysis of the studies using a PRESS sequence. In the meta-regression, TE was associated with heterogeneity. Fourth, we used 2HG/creatine ratio rather than absolute 2HG concentrations to diagnose IDH mutations in 2 studies because there was no information regarding 2HG concentrations.7,19
In conclusion, 2HG MRS demonstrated excellent specificity for prediction of IDH mutant glioma, with the TE value being associated with heterogeneity in the sensitivity.
Funding
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1720030).
Conflict of interest statement. The authors report no conflict of interest, and the present study has not been presented elsewhere.
Authorship statement. C.H.S.: experimental design, its implementation, analysis and interpretation of the data, writing of the manuscript at draft, and approval of the final version. H.S.K.: experimental design, its implementation, analysis and interpretation of the data, writing of the manuscript at revision stage, and approval of the final version.
S.C.J., C.G.C., S.J.K.: analysis and interpretation of the data, writing of the manuscript at revision stage, and approval of the final version.
Supplementary Material
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller JJ, Shih HA, Andronesi OC, Cahill DP. Isocitrate dehydrogenase-mutant glioma: evolving clinical and therapeutic implications. Cancer. 2017;123(23):4535–4546. [DOI] [PubMed] [Google Scholar]
- 5. Kickingereder P, Sahm F, Radbruch A, et al. IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci Rep. 2015;5:16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andronesi OC, Kim GS, Gerstner E, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012;4(116):116ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bisdas S, Chadzynski GL, Braun C, et al. MR spectroscopy for in vivo assessment of the oncometabolite 2-hydroxyglutarate and its effects on cellular metabolism in human brain gliomas at 9.4T. J Magn Reson Imaging. 2016;44(4):823–833. [DOI] [PubMed] [Google Scholar]
- 8. Branzoli F, Di Stefano AL, Capelle L, et al. Highly specific determination of IDH status using edited in vivo magnetic resonance spectroscopy. Neuro Oncol. 2018;20(7):907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18(4):624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi C, Raisanen JM, Ganji SK, et al. Prospective longitudinal analysis of 2-hydroxyglutarate magnetic resonance spectroscopy identifies broad clinical utility for the management of patients with IDH-mutant glioma. J Clin Oncol. 2016;34(33):4030–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crisi G, Filice S, Michiara M, Crafa P, Lana S. 2-Hydroxyglutarate detection by short echo time magnetic resonance spectroscopy in routine imaging study of brain glioma at 3.0 T. J Comput Assist Tomogr. 2018;42(3):469–474. [DOI] [PubMed] [Google Scholar]
- 12. de la Fuente MI, Young RJ, Rubel J, et al. Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol. 2016;18(2):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emir UE, Larkin SJ, de Pennington N, et al. Noninvasive quantification of 2-hydroxyglutarate in human gliomas with IDH1 and IDH2 mutations. Cancer Res. 2016;76(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagashima H, Tanaka K, Sasayama T, et al. Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro Oncol. 2016;18(11):1559–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Natsumeda M, Igarashi H, Nomura T, et al. Accumulation of 2-hydroxyglutarate in gliomas correlates with survival: a study by 3.0-tesla magnetic resonance spectroscopy. Acta Neuropathol Commun. 2014;2:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Natsumeda M, Motohashi K, Igarashi H, et al. Reliable diagnosis of IDH-mutant glioblastoma by 2-hydroxyglutarate detection: a study by 3-T magnetic resonance spectroscopy. Neurosurg Rev. 2018;41(2):641–647. [DOI] [PubMed] [Google Scholar]
- 17. Pope WB, Prins RM, Albert Thomas M, et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol. 2012;107(1):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tietze A, Choi C, Mickey B, et al. Noninvasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting. J Neurosurg. 2018;128(2):391–398. [DOI] [PubMed] [Google Scholar]
- 19. Zhou M, Zhou Y, Liao H, et al. Diagnostic accuracy of 2-hydroxyglutarate magnetic resonance spectroscopy in newly-diagnosed brain mass and suspected recurrent gliomas. Neuro Oncol. 2018;doi: 10.1093/neuonc/noy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carrillo JA, Lai A, Nghiemphu PL, et al. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am J Neuroradiol. 2012;33(7):1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lasocki A, Tsui A, Gaillard F, Tacey M, Drummond K, Stuckey S. Reliability of noncontrast-enhancing tumor as a biomarker of IDH1 mutation status in glioblastoma. J Clin Neurosci. 2017;39:170–175. [DOI] [PubMed] [Google Scholar]
- 22. Zhou H, Vallières M, Bai HX, et al. MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro Oncol. 2017;19(6):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee S, Choi SH, Ryoo I, et al. Evaluation of the microenvironmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. J Neurooncol. 2015;121(1):141–150. [DOI] [PubMed] [Google Scholar]
- 24. Leu K, Ott GA, Lai A, et al. Perfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II-III diffuse gliomas. J Neurooncol. 2017;134(1):177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Price SJ, Allinson K, Liu H, et al. Less invasive phenotype found in isocitrate dehydrogenase-mutated glioblastomas than in isocitrate dehydrogenase wild-type glioblastomas: a diffusion-tensor imaging study. Radiology. 2017;283(1):215–221. [DOI] [PubMed] [Google Scholar]
- 26. Tan W, Xiong J, Huang W, Wu J, Zhan S, Geng D. Noninvasively detecting isocitrate dehydrogenase 1 gene status in astrocytoma by dynamic susceptibility contrast MRI. J Magn Reson Imaging. 2017;45(2):492–499. [DOI] [PubMed] [Google Scholar]
- 27. Wasserman JK, Nicholas G, Yaworski R, et al. Radiological and pathological features associated with IDH1-R132H mutation status and early mortality in newly diagnosed anaplastic astrocytic tumours. PLoS One. 2015;10(4):e0123890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xing Z, Yang X, She D, Lin Y, Zhang Y, Cao D. Noninvasive assessment of IDH mutational status in World Health Organization grade II and III astrocytomas using DWI and DSC-PWI combined with conventional MR imaging. AJNR Am J Neuroradiol. 2017;38(6):1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiong J, Tan W, Wen J, et al. Combination of diffusion tensor imaging and conventional MRI correlates with isocitrate dehydrogenase 1/2 mutations but not 1p/19q genotyping in oligodendroglial tumours. Eur Radiol. 2016;26(6):1705–1715. [DOI] [PubMed] [Google Scholar]
- 30. Yamashita K, Hiwatashi A, Togao O, et al. MR imaging-based analysis of glioblastoma multiforme: estimation of IDH1 mutation status. AJNR Am J Neuroradiol. 2016;37(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dang L, Yen K, Attar EC. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol. 2016;27(4):599–608. [DOI] [PubMed] [Google Scholar]
- 32. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. [DOI] [PubMed] [Google Scholar]
- 33. Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. [DOI] [PubMed] [Google Scholar]
- 34. Suh CH, Park SH. Successful publication of systematic review and meta-analysis of studies evaluating diagnostic test accuracy. Korean J Radiol. 2016;17(1):5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim KW, Lee J, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers–part I. General guidance and tips. Korean J Radiol. 2015;16(6):1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers–part II. Statistical methods of meta-analysis. Korean J Radiol. 2015;16(6):1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–990. [DOI] [PubMed] [Google Scholar]
- 38. Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–2884. [DOI] [PubMed] [Google Scholar]
- 39. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Devillé WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–893. [DOI] [PubMed] [Google Scholar]
- 42. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. [DOI] [PubMed] [Google Scholar]
- 43. Berrington A, Voets NL, Larkin SJ, et al. A comparison of 2-hydroxyglutarate detection at 3 and 7 T with long-TE semi-LASER. NMR Biomed. 2018;31(3):doi: 10.1002/nbm.3886. [DOI] [PubMed] [Google Scholar]
- 44. Berrington A, Voets NL, Plaha P, et al. Improved localisation for 2-hydroxyglutarate detection at 3T using long-TE semi-LASER. Tomography. 2016;2(2):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi C, Ganji S, Hulsey K, et al. A comparative study of short- and long-TE ¹H MRS at 3 T for in vivo detection of 2-hydroxyglutarate in brain tumors. NMR Biomed. 2013;26(10):1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. An Z, Ganji SK, Tiwari V, et al. Detection of 2-hydroxyglutarate in brain tumors by triple-refocusing MR spectroscopy at 3T in vivo. Magn Reson Med. 2017;78(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ganji SK, An Z, Tiwari V, et al. In vivo detection of 2-hydroxyglutarate in brain tumors by optimized point-resolved spectroscopy (PRESS) at 7T. Magn Reson Med. 2017;77(3):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Elkhaled A, Jalbert LE, Phillips JJ, et al. Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci Transl Med. 2012;4(116):116ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kalinina J, Carroll A, Wang L, et al. Detection of “oncometabolite” 2-hydroxyglutarate by magnetic resonance analysis as a biomarker of IDH1/2 mutations in glioma. J Mol Med (Berl). 2012;90(10):1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andronesi OC, Loebel F, Bogner W, et al. Treatment response assessment in IDH-mutant glioma patients by noninvasive 3D functional spectroscopic mapping of 2-hydroxyglutarate. Clin Cancer Res. 2016;22(7):1632–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. An Z, Tiwari V, Ganji SK, et al. Echo-planar spectroscopic imaging with dual-readout alternated gradients (DRAG-EPSI) at 7 T: application for 2-hydroxyglutarate imaging in glioma patients. Magn Reson Med. 2018;79(4):1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Andronesi OC, Arrillaga-Romany IC, Ly KI, et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat Commun. 2018;9(1):1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nicolay B, Narayanaswamy R, Aguado E, et al. The IDH1 mutant inhibitor AG-120 shows strong inhibition of 2-HG production in an orthotopic IDH1 mutant glioma model in vivo. Neuro Oncol. 2017;19:vi86. [Google Scholar]
- 54. Choi C, Ganji SK, DeBerardinis RJ, et al. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18(4):624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.