Abstract
Background
Plexiform neurofibromas (PN) in neurofibromatosis 1 (NF1) can cause substantial morbidities. Clinical trials targeting PN have recently described decreases in PN volumes. However, no previous study has assessed the association between changes in PN volumes and PN-related morbidities. Our objective was to assess if increasing PN volume in NF1 is associated with increasing PN-related morbidity.
Methods
This is a retrospective review of patients enrolled on the NCI NF1 natural history study with ≥7 years of data available. Morbidities including pain, motor dysfunction, vision loss, and PN-related surgery were assessed at time of baseline PN MRI with volumetric analysis and time of MRI with maximum PN volume.
Results
Forty-one patients (median age at baseline 8 y) with 57 PN were included. At baseline, 40 PN had at least 1 PN-associated morbidity. During the observation period, 27 PN required increasing pain medication, and these PN grew faster per year (median difference 8.3%; 95% CI: 2.4, 13.8%) than those PN which did not. PN resulting in motor impairment at baseline (n = 11) had larger volumes compared with those that did not (median difference 461 mL; 95% CI: 66.9, 820).
Conclusions
Many NF1 PN were associated with clinically significant morbidity at baseline, highlighting the need for longitudinal morbidity evaluations starting at an early age to capture changes in PN-associated morbidities. Prospective evaluation of standardized patient reported and functional outcomes in clinical trials are ongoing and may allow further characterization of the association of PN volume increase or decrease and clinical changes.
Keywords: function, morbidity, neurofibromatosis type 1, plexiform neurofibroma
Importance of the study
Plexiform neurofibromas in NF1 can be a cause of substantial morbidity, with no effective medical therapy currently available. Clinical trials targeting PN recently described PN volume decreases. Understanding the relationship of PN volume changes and changes in PN-related morbidity will be critical to regulatory approval of agents targeting PN. This is the first study to assess changes in PN volume and the development of clinical morbidities over extended time periods in children with NF1 PN. Our study demonstrates that the majority of PN are associated with morbidities, which manifest at an early age and almost never resolve or improve spontaneously. We also demonstrate that increases in PN volumes are associated with increases in pain and motor morbidity. Ongoing clinical trials are assessing if decreases in PN volumes are associated with improvement in morbidity.
Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder caused by a mutation in the NF1 tumor suppressor gene. NF1 has a prevalence of approximately 1 in 2700 people1,2 in the United States. Plexiform neurofibromas (PN), histologically benign tumors of peripheral nerves which arise from Schwann cells,3 occur in 20%–50% of all patients with NF14,5 and can be a major source of morbidity. Since PN can arise along any peripheral nerve, associated morbidities can range from mild disfigurement and pain to life-threatening complications such as complete airway obstruction.6–8
To date, surgical resection has been the only potentially effective treatment for PN; however, due to PN’s infiltrative nature, the risks of surgery often outweigh the potential benefits.9 In addition, many PN cannot be completely resected due to their location, and partially removed PN often regrow.10 Given the great need for medical therapies for PN, many clinical trials over the past decade have attempted to target PN growth. In contrast to malignant solid tumors, PN grow relatively slowly, with the most rapid growth typically observed in younger children.11 For this reason, volumetric MRI assessment was developed to sensitively and reproducibly measure changes in PN over time, and this has become the standard method of response assessment in clinical trials.12,13 However, most studies have not shown clinically significant impact on tumor progression even as measured by volumetric MRI analysis, and only few PN had a decrease in tumor volume.14–17 In 2014, Robertson et al reported that imatinib resulted in shrinkage of 6 of 36 PN,18 though this shrinkage was seen only in tumors that were less than 20 mL at baseline. A recent phase I trial of the mitogen-activated protein kinase kinase (MEK) inhibitor selumetinib (AZD6244) for pediatric patients with NF1 and inoperable PN showed promising results, with 17 of 24 patients achieving ≥20% shrinkage in PN volume, with all of the PN being larger than 20 mL at baseline (median 1205 mL).19 Several patients reported anecdotal improvement of symptoms such as pain or function with tumor shrinkage. Prospective evaluation of functional improvement and patient reported outcomes in addition to response based on volumetric MRI analysis are being investigated in 2 ongoing phase II clinical trials of selumetinib (NCT01362803 and NCT02407405).
Previous studies have demonstrated that patients with PN are at increased risk for mortality from NF1-related complications and that PN frequently cause clinical deficits even in young children.7 Nguyen et al showed that symptomatic PN tended to be larger than asymptomatic ones; however, it is unclear what, if any, relationship exists between the presence of specific clinical morbidities and PN volume change or growth rate.7 Demonstration of clinical benefit will be crucial for regulatory approval of novel agents. While the functional impact of shrinkage of PN is being assessed prospectively on clinical trials, we propose that tumor growth may be correlated with worsening functional morbidity. Therefore, stopping tumor growth could potentially prevent morbidity development. Our goal was to further elucidate the relationship between PN size, growth rate, and PN-related morbidity.
Methods
We performed a retrospective review of patients enrolled on the NCI Natural History Study of Patients with Neurofibromatosis Type 1 (NCT00924196). This study follows patients with NF1 with comprehensive evaluations at least yearly until age 18 and thereafter at least every 3 years to characterize NF1-related manifestations. This includes clinical, MRI, endocrine, and neuropsychological evaluations. Due to our patient referral pattern, most patients referred to the natural history study have PN. These patients were thus often co-enrolled on clinical trials for treatment of PN at the NIH and therefore have a higher proportion of PN burden than the general NF1 population. However, prior to MEK inhibitors, none of the clinical trials resulted in significant PN shrinkage and therefore patients on these trials were still considered eligible for this analysis. This protocol was approved by the institutional review board and included permission to utilize clinical data from prior to the date of study enrollment. All patients or their legal guardians provided written informed consent.
Eligibility
To be included in this analysis, patients had to have at least one PN amenable to volumetric analysis, with at least 2 data points. Given that disease progression occurs relatively slowly, only patients with at least 7 years of clinical data as of July 2015 were included in this analysis to increase the chances of capturing clinical changes in morbidity over time. Patients who had been on clinical trials for their PN with the exception of MEK inhibitors were included in this analysis, since none of the other agents resulted in tumor shrinkage (PN volume decrease ≥20%). Plexiform neurofibromas which were known to have subsequently transformed to malignant peripheral nerve sheath tumors were excluded from this study as the goal was to assess the morbidity caused by the nonmalignant lesions.
Evaluations
Patients had serial evaluations of their PN with volumetric MRI analysis as part of their participation in the NF1 natural history study. In addition, patients were assessed for presence of clinical morbidities at least every 12 months until age 18 years and then every 1–3 years. Most patients were followed at the NIH on clinical trials prior to enrollment on the NCI NF1 natural history study. The date from their first NIH history and physical examination was used to determine overall length of follow-up rather than the date of natural history study enrollment.
Volumetric MRI analysis of PN
For each PN tumor that met the above criteria, available volumetric measurements were reviewed. The date of the first MRI with volumetric analysis was defined as the baseline assessment for that PN tumor, and the date of the MRI where the tumor reached its largest volume was considered the maximum assessment. PN with <20% volume change between the baseline and maximum assessments were considered to be stable in size, and for these tumors a third timepoint was defined as the date of the most recent visit prior to July 2015.
Assessment of morbidities
For each of these timepoints (baseline, maximum, and most recent [when applicable]), a corresponding clinical note within 6 months of each date was identified in the patient’s chart. If more than one note was within this time frame, the clinical note dated most closely to the corresponding MRI was used.
At each timepoint, PN-related morbidity was systemically determined from the clinical note using 6 separate categories: pain, motor, vision, bowel, bladder, and airway function. The use of pain medications for pain caused by the PN was used as a surrogate to assess PN-related pain, and the number, type (over the counter, opioid, or neuropathic), and timing (scheduled vs as needed) of pain medications were noted. For motor function, muscle strength (manual muscle testing; 0–5 scale), range of motion (normal vs abnormal), and need for a mechanical brace or support (yes or no) were recorded. Vision morbidity was defined as any degree of visual deficit related to the PN. To qualify for presence of a bowel or bladder morbidity, the patient had to have severe obstructive or incontinence symptoms related to PN location. Mild constipation or age-appropriate isolated nocturnal enuresis alone are common in this population and not considered a bowel or bladder morbidity, regardless of PN location. Presence of a PN-related airway morbidity included snoring that was noted by the clinician to be of concern to the family or need for any type of mechanical support (including need for intermittent positive airway pressure, tracheostomy, and/or ventilator dependence). If there was no specific documentation in the chart of any of the above functional evaluations (eg, range of motion or bladder function), it was presumed to be normal (no morbidity present). For patients with ≥2 PN followed in this analysis, the clinical information provided in the medical record was used to determine which PN was most likely responsible for each morbidity and, in some cases, one morbidity could be assigned to more than one PN (eg, pain). In addition to these functional morbidities, the total number of PN-related surgeries prior to each timepoint was recorded.
A sub-analysis was performed on PN that had the potential for causing motor morbidity to evaluate for the development of motor morbidity over time. Each PN was determined as having the potential to result in a motor morbidity based on its location prior to any chart review or analysis. Some PN, such as orbital tumors, are not considered to be at risk to cause range-of-motion or strength defects and therefore were excluded from this sub-analysis.
Statistical Analysis
PN volume changes analysis
In addition to absolute size (mL) calculated at each timepoint (eg, baseline, maximum, most recent assessments), PN volume was calculated in relation to the patient’s body weight (kg) to account for differing patient sizes and patient growth over time. Each of these values were also converted to growth rates (absolute change in size over time [mL/y] and PN percentage of body weight over time [y]). In addition, the absolute change in volume from baseline to maximum was calculated and compared with the baseline to create a relative percent volume change from baseline and a relative percent volume change per year. For any PN where the relative percent volume change was <20% between the baseline and maximum assessment, each of these volumetric measurements was also performed at the most recent timepoint clinically available. Thus, a total of 10 PN volume variables were analyzed (see Supplementary Table S1).
Analysis of morbidities
To evaluate the association between tumor volume and presence of functional morbidity, a dichotomous variable was created to assess for presence or absence of each morbidity at each timepoint (baseline, maximum, and most recent assessment).
For pain morbidities, PN which required a change in the absolute number and/or type of pain medication (scheduled vs as needed) between baseline and maximum assessments were compared with those that did not require a change. For surgeries, patients were classified as either having had a PN-related surgery between baseline and maximum assessments or not having an additional surgery during that time. For motor morbidities, PN that had any limitation in range of motion or strength or required a brace were considered to have a present motor morbidity, and those that developed motor morbidities after the baseline assessment were compared with those that did not. Given the relatively few incidences recorded of nonmotor functional morbidities (airway, bowel, bladder, and vision), these were grouped as either “present” or “absent” at both baseline and maximum assessment, and the tumor volumes and growth rate of those that developed a nonmotor morbidity during this period were compared with those that did not. Thus, a total of 10 dichotomous variables were created for use as classification variables.
In addition to performing analyses using all 57 PN, 3 subgroup analyses were performed: first, restricting the analysis to non-orbit PN (n = 49); second, restricting the analysis to those PN with the potential to cause motor dysfunction (n = 42); and third, restricting the analysis to the PN with >20% increase in size (n = 49).
Wilcoxon rank sum tests were used to test the hypothesis of no difference between distributions of the PN volume variables with the dichotomous morbidity variables defined above. The Hodges–Lehmann estimate of the median difference (and 95% CI) for each variable was also calculated and used to estimate the effect size between the dichotomized groups. Clinically important results were ascertained by using both the rank sum test P-values and the effect sizes. Unless indicated otherwise, the median is used to estimate central tendency and the minimum and maximum values are used to indicate the range; lower and upper quartiles are reported in Table 1 to illustrate dispersion of these continuous variables.
Table 1.
Patient and PN characteristics at baseline and maximum volumetric assessments
| Patient Characteristics (n = 41) | |
|---|---|
| Age at enrollment, y | |
| Median (min, max) | 13 (5.2, 31.3) |
| Lower quartile, upper quartile | 9.9, 17.0 |
| Number of PN per patient | |
| Median (min, max) | 1 (1, 3) |
| Sex, n (%) | |
| Male | 26 (63) |
| Female | 15 (37) |
| Time between baseline and maximum assessments, y | |
| Median (min, max) | 6.5 (0.7, 12.6) |
| Lower quartile, upper quartile | 3.8, 9.1 |
| PN Characteristics (n = 57) | |
| Baseline PN volume, ml | |
| Median (min, max) | 238 (2.3, 4895) |
| Lower quartile, upper quartile | 65.4, 674 |
| Baseline PN weight adjusted volume, mL/kg | |
| Median (min, max) | 9.9 (0.1, 164.8) |
| Lower quartile, upper quartile | 2.36, 30.0 |
| Maximum PN volume, mL | |
| Median (min, max) | 489 (10.2, 7210) |
| Lower quartile, upper quartile | 152, 1329 |
| Max PN weight adjusted volume, mL/kg | |
| Median (min, max) | 11.2 (0.3, 198.3) |
| Lower quartile, upper quartile | 2.8, 34 |
| Potential motor PN, n (%) | 42 (74) |
| Orbital PN, n (%) | 8 (14) |
| PN >20% volume change, n (%) | 49 (86) |
Results
Forty-one patients (26 male, 15 female) with 57 distinct PN with at least 7 years of clinical data and with volumetric MRI measurements from at least 2 different timepoints are included in this analysis. The median patient age at baseline volumetric assessment was 8 years (range, 3–25), though notably the median age at enrollment on the NF1 natural history study was 13 years (5–31). At baseline, the median PN volume was 238 mL (2.3–4895) and median PN volume adjusted for body weight was 9.9 mL/kg (0.1–164.8). The median time between baseline and maximum assessments was 6.5 years (0.7–12.6) (Table 1).
The physical distribution of PN throughout the body was consistent with that reported in previous studies, with 31 (56%) of PN located in either the trunk or extremity, and the remaining 26 (44%) in the head, neck, or upper chest. Eight PN were located in the orbital region. Of the 57 total PN, 42 were considered to have the potential to cause motor-related morbidities based on their locations.
Of the 57 PN evaluated, 49 (86%) had >20% increase in tumor volume from baseline to maximum assessment. The median PN volume change between baseline and maximum assessments was 108.9% (−2.15%, 790%) with a median growth rate of 15.9% per year (lower quartile 10.1%, upper quartile 28.0%). PN volume adjusted for body weight increased by a median of 0.016% of body weight per year (lower quartile 0.00%, upper quartile 0.07%). The most rapid growth was seen in the youngest age quartile (3–5 y) with a median rate of 35.1% per year, in contrast with the oldest age quartile (11–25 y), where the median growth rate was only 13.1% per year. At maximum PN volume assessment, the median PN volume was 489 mL (range, 10.2–7210).
Eight PN had less than 20% relative percent volume difference between baseline and maximum assessments and therefore were considered stable in size. These 8 PN had a median of 14.2% growth (5.7% per year). Only one PN, in a 25-year-old patient, had negative volume difference after baseline assessment, decreasing from 3541 mL to 3465 mL (−2.1%) in 6 months, which is well within the variability observed with this method (coefficient of variation 0.6–5.6%).13 This patient never received any medical therapy targeting PN. At the most recent volumetric measurement available for this PN, 13 years later, it remained effectively stable in size (−1.4% from baseline).
Baseline PN-Related Morbidities
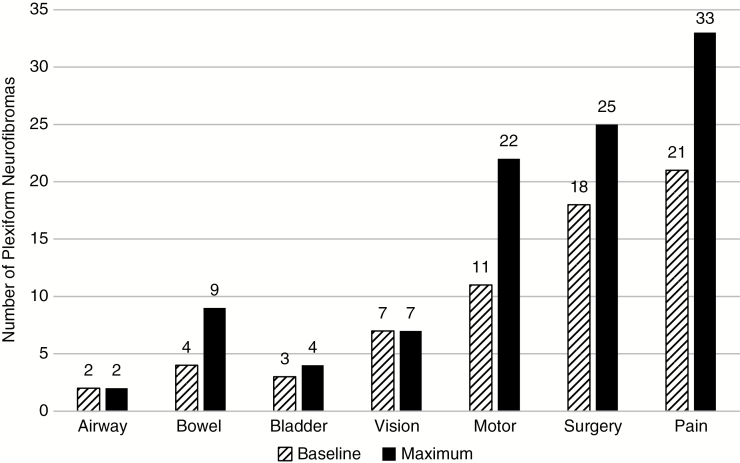
Forty of 57 PN (70%) and 36 of 41 patients had at least 1 associated morbidity at baseline assessment, 21 PN (37%) had 1 associated morbidity, 13 PN (23%) had 2 morbidities, and 6 PN (10%) had 3 or 4 morbidities. At baseline, the most common morbidity was pain (n = 21, 37% of PN), followed by having had PN-related surgery (n = 18, 32%) and motor dysfunction (n = 11, 19%). Vision, airway, bowel, and bladder morbidities were the least frequent. There were a total of 8 orbital PN, and 7 of the 8 already had visual deficits related to the PN at baseline assessment. Four patients had PN-related bowel obstruction and 3 had urinary incontinence. The 2 patients with airway morbidity on the study both had this morbidity present at baseline (Fig. 1).
Fig. 1.
PN-related morbidities and surgeries at baseline and maximum assessment. The number of PN with each type of PN-related morbidity at baseline and maximum volume assessments.
Relationship of PN Size, Growth Rate, and Functional Morbidity
All PN with a morbidity present at baseline still had morbidity present at the time of maximum assessment. Thirty of the 57 PN had an increase in the number of functional morbidities present between baseline and maximum. The only patient who had a decrease in overall morbidity between these assessments had resolution of PN-related pain as a result of surgical resection of a nodular portion of the tumor along the sciatic nerve that was causing the majority of his discomfort.
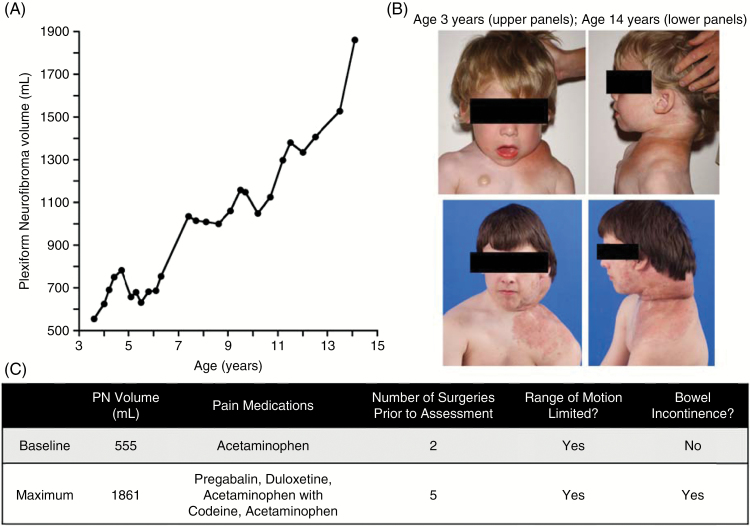
Eight PN went from no associated functional morbidities at baseline to resulting in one or more at the time of maximum assessment. These PN had varied locations, including the neck (n = 2), back (n = 2), chest (n = 1), and pelvis (n = 3). Their volumes varied widely, as did volume change between baseline and maximum assessments with a median increase of 119% (8%, 590%). Figure 2 illustrates one example of a patient who had 235% increase in the volume of his cervical spine PN between the ages of 3 and 14, and over that time period also had significant increase in PN-related morbidity.
Pain morbidity
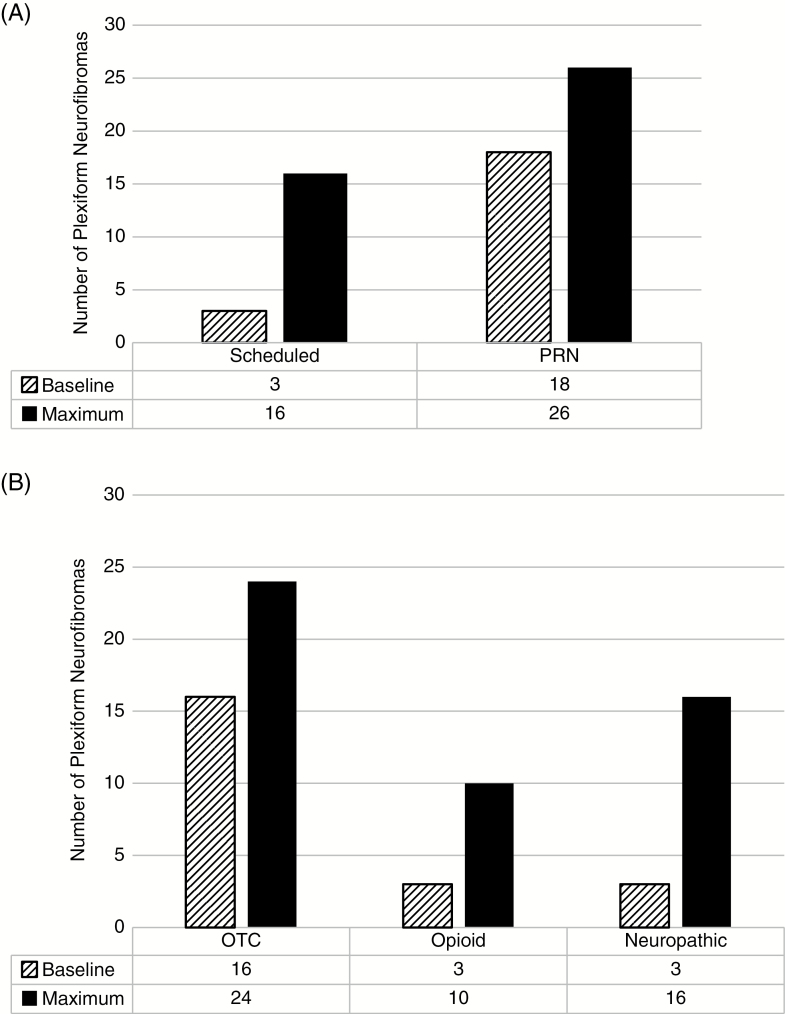
Of the PN being treated with pain medication at baseline (n = 21), 3 required at least one scheduled pain medication, and 3 needed at least one opioid pain medication. At the time of maximum PN volume, 33 of 57 PN were being treated with pain medication, and the scheduled and opioid pain medication use had both more than tripled to 16 and 10 PN, respectively, requiring them (Fig. 3). Eighteen of 41 (43%) patients required one or more pain medications at their baseline evaluation, which increased to 26 (63%) at the time of maximum assessment. As noted above, only one patient reported resolution of PN-related pain between baseline and maximum assessments, as a result of resection of a nodular thigh tumor.
Fig. 3.
Pain morbidity at baseline and maximum assessments. Pain medication was used as a surrogate marker for PN related pain at both timepoints. There was an increase in the amount of scheduled and PRN (as needed) pain medications used at maximum assessment (A, solid bars) compared with baseline (A, striped bars). Similarly, the use of opioid, neuropathic and over-the-counter (OTC) pain medications all increased between the baseline and maximum assessments (B).
Twenty-seven PN required an increase in the number of pain medications needed between the baseline and maximum assessments. Those PN with an increase in pain medications from baseline to maximum generally grew faster (median 21% growth per year, n = 27) than those PN that did not result in an increase in pain medication (median 13% growth per year, n = 30) with the median difference between their growth rates of 8.34% more per year (95% CI: 2.37%, 13.8%, P = 0.016). There was only weak evidence for a difference between these groups in absolute volume, with the PN resulting in an increase in pain medication use growing more than those that did not (median difference, 106 mL; 95% CI: −3.9, 457; P = 0.058) between baseline and maximum assessments. Furthermore, when the PN were divided into 2 groups based on the baseline volume (at the median of 238 mL), those PN with baseline volume at or below the median (n = 29) had a median difference in their growth rates of 8.68 mL; 95% CI: −1.81, 24.0; P = 0.097), whereas those with baseline volume greater than the median (n = 28) had a median difference in their growth rates of 8.62 mL (95% CI: −0.27, 15.1; P = 0.045). Thus, baseline volume did not impact the association of pain on the difference of growth rates.
Motor morbidity
The number of PN with any motor-related morbidity doubled from 11 to 22 between time of baseline and maximum assessment. The most common motor deficit was weakness, which affected 9 and 14 PN at baseline and maximum assessments, respectively.
Of the 42 PN with potential to cause motor morbidities, the PN with motor morbidity at baseline generally had larger volumes (median = 818 mL, n = 11) than those without morbidity (median = 238 mL, n = 31), with the median difference being 461 mL (95% CI: 66.9, 820; P = 0.013). They also made up a larger percentage of the patients’ body weight at baseline (median difference 2.62%; 95% CI: 0.468%, 3.87%; P = 0.005). Similarly, the PN with motor morbidity at maximum assessment continued to make up a larger percent of patient body weight (median difference 1.08%; 95% CI: 0.043%, 3.19%; P = 0.045) at maximum. There was also a suggestion that those PN with motor morbidity at maximum were generally larger (median = 1240 mL, n = 20) than those without motor morbidity (median = 664 mL, n = 22), with the median difference being 435 mL (95% CI: −68, 1260; P = 0.072).
Twelve PN resulted in an increase in motor morbidity from baseline to maximum. There was a weak trend toward more growth per year in PN with an increase in motor morbidity, as they grew by a median of 6.5% more per year than those that did not (95% CI: −1.95%–16.3%; P = 0.14).
Airway morbidity
One of the 2 patients with airway morbidity at baseline had worsening of symptoms over time, progressing from snoring to requiring continuous positive airway pressure for obstructive sleep apnea over 11 years, with the PN growing from 238 to 1157 mL during that time. The other patient with airway morbidity had a tracheostomy at the time of baseline assessment, when she was 8 years old, and she continued to require this as her facial tumor more than doubled in size over the next 9 years from 289 to 754 mL
Other functional morbidities
One patient developed PN-related urinary incontinence and 5 additional patients developed PN-related bowel obstruction between the baseline and maximum assessments. Only weak associations with relatively small differences in size and growth were found between PN size or growth rate and the presence or absence of vision, airway, bowel, or bladder morbidities, and none of these were statistically significant.
Changes in morbidity in stable tumors
Eight PN had less than 20% relative percent volume change between their baseline and maximum assessments. Of these, 5 had morbidity at baseline, maximum, and most recent clinical assessments, one PN remained without any reported clinical morbidities at any timepoint, one developed PN-related morbidity after baseline (present at both maximum and most recent assessments), and one had a decrease in morbidity at the most recent assessment only. The PN with a decrease in morbidity was located in the pelvis and associated with difficulty urinating reported at baseline. This morbidity had resolved at her most recent visit 13 years after her baseline, at which time the PN was 1.4% smaller than it had been at baseline, which is considered to be stable in size.
Discussion
The recent identification of targeted therapies that can decrease the size of large NF1-related PN19 brings the potential regulatory approval of therapies for PN in closer reach. However, in addition to PN shrinkage, a detailed understanding of the natural history of the growth of PN and its relationship to the development of PN-related morbidities is required in order to meaningfully analyze clinical benefit on clinical trials. This retrospective analysis is the first to analyze the relationship between the development of PN-related clinical morbidities and changes in PN volume. PN are slow-growing, histologically benign tumors that can result in substantial clinical morbidity. While it may be intuitive that larger and faster-growing PN would lead to increased morbidity, this study attempted to analyze these relationships using the NCI NF1 natural history data with volumetric MRI analysis of PN. The population of patients on the NCI NF1 natural history study is similar to those included on clinical trials for NF1-related PN,8 with a higher tumor and overall disease burden than the general NF1 population.
Comparing the presence of pain and functional morbidities with PN volumetric assessments demonstrated several key points. First, the majority of PN had already resulted in PN-related morbidity at the baseline assessment, at which time the median patient age was 8 years old. For example, Fig. 2 shows a patient who had significant morbidity even at his baseline evaluation when he was 3 years old. Of the 4 PN that resulted in urinary incontinence or retention at maximum assessment, 3 were already causing issues at the time of baseline evaluation. Similarly, 4 of the 9 PN causing bowel obstruction or incontinence were doing so at baseline evaluation. This finding is consistent with what has been observed in clinical practice and in other PN-related morbidity analyses,6–8 and reinforces the need for early intervention to prevent the development of PN-related morbidities. Nguyen et al showed in their 2011 analysis that symptomatic PN tended to be larger in volume,7 which was consistent with our results for both pain and motor morbidities. They also noted that smaller tumors can cause morbidity based on their location, which is consistent with what we found for nonmotor morbidities such as vision or bowel/bladder dysfunction, where tumor size and growth rate may be less predictive of degree of impairment. Orbital tumors in particular had a high degree of associated morbidity despite their relatively small size, and this morbidity was already present for most patients at their baseline assessment.
Fig. 2.
Example of worsening PN related morbidity over time. This patient has a PN of the cervical spine which grew 235% in the 10.5 years between the baseline and maximum volumetric assessments (A). At his baseline assessment, he already had mild limitation in his neck range of motion and had required 2 debulking procedures. Between his baseline and maximum assessments, he required 3 more surgeries, as well as developing worsening disfigurement (B), increased pain requiring the addition of multiple pain medications, speech and swallowing difficulties, intermittent bowel incontinence, and increasing limitation in his neck and shoulder range of motion (C). The decrease in tumor volume at age 5 was the result of a debulking procedure.
Secondly, once a clinical morbidity develops in patients with PN that are growing, it is extremely unlikely to resolve spontaneously. No stable or growing PN in our cohort had resolution of functional morbidities between baseline and maximum assessments. One patient had resolution of his PN-related pain after surgical resection; however, he continued to have other PN-related functional morbidities, including motor and bowel at the time of maximum assessment. Only one PN that was stable in size had improvement of clinical morbidity (difficulty urinating) between the baseline and most recent assessment. Though this latter PN had a very small spontaneous volume decrease over a 13-year time period (−1.4%), such minor variability is unlikely to have directly caused this improvement. Of note, slow spontaneous tumor shrinkage has been reported in few older adolescents and adults,11 and the patient was 25 years old at the time of her baseline evaluation. The overwhelming trend in patients with stable or growing PN which cause clinical morbidity is that this morbidity will remain stable or worsen over time. Therefore, if improvement in clinical morbidity is demonstrated in patients receiving any future PN-directed therapies, it is likely to be related to treatment rather than the natural disease course.
Thirdly, larger tumors were more likely to have associated motor dysfunction which, though intuitively obvious, had not previously been demonstrated. In addition, we found a relationship between PN growth rate and increasing need for pain medication, with PN requiring escalation in pain medications growing by a median of 8.3% more per year than those that did not. This finding correlates with our clinical experience, as more rapid tumor growth appears to be associated with increased pain. The median growth rate of PN that required escalation in pain medication was 21% per year, which reinforces that 20% PN growth may be a reasonable marker of progressive disease for clinical trials.20 However, for many of the nonmotor morbidities, PN with a slower growth rate may still have clinical significance for the patient; and conversely, PN shrinkage of <20% may also reasonably have a clinically significant impact on morbidity.
This analysis had several strengths, including access to long-term follow-up in a patient population where PN growth is slow compared with malignant tumors. In addition, the comprehensive clinical exams performed at the NCI at the time of volumetric MRI measurements allowed us to assess the relationship between tumor volume and morbidities in a manner that has not previously been feasible. However, the fact that many patients had their baseline MRI prior to enrollment on the NF1 natural history study meant that the prospective evaluations of functional morbidity being used in that study could not be consistently utilized in our patient population, as these assessments were not available for the majority of baseline clinical assessments. In addition, though this analysis contains the largest pool of patients with NF1 and PN for whom there is both clinical and volumetric MRI data to date, sample size is still relatively small and the significance of the findings may be limited by the multiple comparisons in this exploratory analysis.
This retrospective analysis illustrates that PN-related functional morbidity is a clinically significant problem for the NF1 population. These morbidities tend to develop early in a patient’s life, and patients with growing tumors are extremely unlikely to have spontaneous improvement of symptoms. Our study reinforces the need for early prospective evaluations of both functional and patient reported outcomes for patients with PN on clinical trials. The Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) international working group has developed a series of recommendations, which can be found on the REiNS website (https://ccrod.cancer.gov/confluence/display/REINS/Home). These prospective evaluations are also being performed in ongoing phase II trials of selumetinib for children (NCT01362803) and adults (NCT02407405) with NF1 and inoperable PN.
Funding
This work was supported by the Center for Cancer Research, National Cancer Institute, Intramural Research Program and funding from the Neurofibromatosis Therapeutic Acceleration Program (NTAP).
Conflict of interest statement. None declared.
Supplementary Material
References
- 1. Gutmann DH, Blakeley JO, Korf BR, Packer RJ. Optimizing biologically targeted clinical trials for neurofibromatosis. Expert Opin Investig Drugs. 2013;22(4):443–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monroe CL, Dahiya S, Gutmann DH. Dissecting clinical heterogeneity in neurofibromatosis type 1. Annu Rev Pathol. 2017;12:53–74. [DOI] [PubMed] [Google Scholar]
- 3. Carroll SL, Ratner N. How does the Schwann cell lineage form tumors in NF1?Glia. 2008;56(14):1590–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999;89(1):31–37. [DOI] [PubMed] [Google Scholar]
- 5. Mautner VF, Asuagbor FA, Dombi E, et al. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008;10(4):593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prada CE, Rangwala FA, Martin LJ, et al. Pediatric plexiform neurofibromas: impact on morbidity and mortality in neurofibromatosis type 1. J Pediatr. 2012;160(3):461–467. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen R, Kluwe L, Fuensterer C, Kentsch M, Friedrich RE, Mautner VF. Plexiform neurofibromas in children with neurofibromatosis type 1: frequency and associated clinical deficits. J Pediatr. 2011;159(4):652–655.e2. [DOI] [PubMed] [Google Scholar]
- 8. Kim A, Gillespie A, Dombi E, et al. Characteristics of children enrolled in treatment trials for NF1-related plexiform neurofibromas. Neurology. 2009;73(16):1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Needle MN, Cnaan A, Dattilo J, et al. Prognostic signs in the surgical management of plexiform neurofibroma: the Children’s Hospital of Philadelphia experience, 1974-1994. J Pediatr. 1997;131(5):678–682. [DOI] [PubMed] [Google Scholar]
- 10. Canavese F, Krajbich JI. Resection of plexiform neurofibromas in children with neurofibromatosis type 1. J Pediatr Orthop. 2011;31(3): 303–311. [DOI] [PubMed] [Google Scholar]
- 11. Dombi E, Solomon J, Gillespie AJ, Fox E, Balis FM, Patronas N. Relationship to age and body weight NF1 plexiform neurofibroma growth rate by volumetric MRI relationship to age and body weight. Neurology. 2007;68:643–647. [DOI] [PubMed] [Google Scholar]
- 12. Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. ; REiNS International Collaboration Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21 Suppl 1):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Solomon J, Warren K, Dombi E, Patronas N, Widemann B. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput Med Imaging Graph. 2004;28(5):257–265. [DOI] [PubMed] [Google Scholar]
- 14. Kim A, Dombi E, Tepas K, et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas. Pediatr Blood Cancer. 2013;60(3):396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Widemann BC, Dombi E, Gillespie A, et al. Phase 2 randomized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipifarnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro Oncol. 2014;16(5):707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weiss B, Widemann BC, Wolters P, et al. Sirolimus for non-progressive NF1-associated plexiform neurofibromas: an NF clinical trials consortium phase II study. Pediatr Blood Cancer. 2014;61(6):982–986. [DOI] [PubMed] [Google Scholar]
- 17. Widemann BC, Babovic-Vuksanovic D, Dombi E, et al. Phase II trial of pirfenidone in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Pediatr Blood Cancer. 2014;61(9):1598–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robertson KA, Nalepa G, Yang FC, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 2012;13(12):1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. ; REiNS International Collaboration Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21 Suppl 1):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.