Abstract
Background
GSK2256098 is a novel oral focal adhesion kinase (FAK) inhibitor. Preclinical studies demonstrate growth inhibition in glioblastoma cell lines. However, rodent studies indicate limited blood–brain barrier (BBB) penetration. In this expansion cohort within a phase I study, the safety, tolerability, pharmacokinetics (PK), and clinical activity of GSK2256098 were evaluated in patients with recurrent glioblastoma. Biodistribution and kinetics of [11C]GSK2256098 were assessed in a substudy using positron-emission tomography (PET).
Methods
Patients were treated with GSK2256098 until disease progression or withdrawal due to adverse events (AEs). Serial PK samples were collected on day 1. On a single day between days 9 and 20, patients received a microdose of intravenous [11C]GSK2256098 and were scanned with PET over 90 minutes with parallel PK sample collection. Response was assessed by MRI every 6 weeks.
Results
Thirteen patients were treated in 3 dose cohorts (1000 mg, 750 mg, 500 mg; all dosed twice daily). The maximum tolerated dose was 1000 mg twice daily. Dose-limiting toxicities were related to cerebral edema. Treatment-related AEs (>25%) were diarrhea, fatigue, and nausea. Eight patients participated in the PET substudy, with [11C]GSK2256098 VT (volume of distribution) estimates of 0.9 in tumor tissue, 0.5 in surrounding T2 enhancing areas, and 0.4 in normal brain. Best response of stable disease was observed in 3 patients, including 1 patient on treatment for 11.3 months.
Conclusions
GSK2256098 was tolerable in patients with relapsed glioblastoma. GSK2256098 crossed the BBB at low levels into normal brain, but at markedly higher levels into tumor, consistent with tumor-associated BBB disruption. Additional clinical trials of GSK2256098 are ongoing.
Keywords: focal adhesion kinase, glioblastoma, GSK2256098, PET
Importance of the study
This is the first clinical trial to evaluate a FAK inhibitor in glioblastoma. Increased FAK expression is observed in glioblastoma and is associated with a negative prognosis. While the FAK inhibitor GSK2256098 demonstrates preclinical activity in glioblastoma cell lines and xenografts, PK studies in rodents demonstrate limited central nervous system penetration in the presence of an intact BBB. We evaluated BBB penetration through assessment of [11C]GSK2256098 biodistribution and kinetics. Tumor penetration of [11C]GSK2256098 was observed in all participants with limited penetration into surrounding normal brain. Tumor concentrations of [11C]GSK2256098 exceeded those associated with antitumor activity in preclinical studies. GSK2256098 was tolerated in patients with recurrent glioblastoma. These data support further clinical trials of GSK2256098 in patients with central nervous system tumors, which are ongoing.
Glioblastoma is the most common malignant primary brain tumor. Median survival is less than a year and standard treatment with surgery followed by radiotherapy and temozolomide chemotherapy results in a median survival of 14.6 months and 5-year overall survival of less than 10%.1–3 Clinical trials of second-line therapies have yet to demonstrate a survival improvement.
Focal adhesion kinase (FAK) is a non–receptor tyrosine kinase that functions within dynamic protein complexes called focal adhesions that are present at the site of attachment between cells and the extracellular matrix. FAK supports the assembly of focal adhesions and their consolidation of signals from integrins and growth factor receptors.4,5 FAK is phosphorylated at tyrosine 397 during activation, and phosphorylated (p)FAK forms a binary complex with the Src family kinases. FAK can trigger multiple downstream intracellular signaling cascades required for cell survival, growth, adhesion, migration, and invasion.4–6 In addition, FAK is associated with the protection of cells from anoikis through the sequestration of receptor-interacting protein from the death-receptor machinery, and with regulation of chemokine transcription.4,7
Overexpression of FAK mRNA or protein is documented in many solid tumors, including glioblastoma.4,5,8–13 FAK expression is increased in metastatic disease compared with normal and early stage disease, indicating that it may be a marker of invasive potential.14,15 FAK protein expression is elevated in human glioblastoma relative to normal brain tissue.8,16 There is increased FAK and pFAK expression in higher-grade versus lower-grade gliomas; the former are associated with poorer survival.17 In addition, FAK expression is associated with angiogenesis in high-grade gliomas but not in normal brain tissue, and there is evidence that FAK promotes angiogenesis in glioma by activating endothelial cell migration.9
GSK2256098 is a potent, ATP-competitive, reversible inhibitor of FAK with an enzymatic half-maximal inhibitory concentration value of 1.5 nmol/L. It has approximately 1000-fold specificity for FAK over the closest family member, Pyk2.18 In assays of 95 cancer cell lines, glioblastoma cell lines were some of the most sensitive to GSK2256098 (GlaxoSmithKline internal data). In preclinical studies, dose- and time-dependent inhibition of pFAK was observed in subcutaneous U87MG (human glioma) xenograft models, with correlation between pFAK inhibition and blood concentration of GSK2256098.18 Preclinical studies in rats indicate limited central nervous system (CNS) penetration of GSK2256098 in the presence of an intact blood–brain barrier (BBB), with brain:plasma concentrations of 0.08, 0.06, and 0.07 at 20, 40, and 60 minutes after a single oral dose, and 0.12, 0.35, and 0.45 at 6 hours post dose following a 6-hour i.v. infusion (6 mL/kg/h). GSK2256098 is a substrate of p-glycoprotein (Pgp), an efflux pump implicated in poor BBB penetration of many drugs. In in vitro studies with cell lines of MDCKII-MDR1 (Madin-Darby canine kidney type II–multidrug resistance protein 1) heterologously expressing human Pgp, GSK2256098 (3 μM) was a Pgp substrate with a moderate efflux ratio of 5.0 and GSK2256098 (100 μM) inhibited transport of digoxin (probe Pgp substrate) by 47% of the control value.19 However, in glioblastoma the BBB is disrupted,20–22 potentially permitting tumor penetration of GSK2256098.
A phase I open-label clinical trial of orally administered GSK2256098 was conducted in patients with advanced non-CNS cancers.23 A total of 62 patients were enrolled into the study, comprising dose-escalation and expansion cohorts. GSK2256098 was well tolerated, and the declared maximum tolerated dose (MTD) was 1000 mg twice daily. Dose-limiting toxicities (DLTs) were grade 2 proteinuria (1000 mg), grade 2 nausea, vomiting, fatigue (1250 mg), and grade 3 asthenia and grade 2 fatigue (1500 mg). Minor responses were observed in 3 patients with mesothelioma and 1 patient with melanoma. These findings, combined with the encouraging preclinical evidence, raised the question of whether the drug was active in patients with glioblastoma. Here we report the safety and systemic and CNS pharmacokinetics (PK) of GSK2256098 in an expansion cohort of patients with glioblastoma. PET imaging was used to evaluate CNS and tumor penetration of [11C]GSK2256098 in participants.
Methods
Ethics
This study was conducted in accordance with the Declaration of Helsinki, the principles of Good Clinical Practice, and applicable clinical trials regulations. Study conduct was approved by the London (Chelsea) Research Ethics Committee (11/LO/0551) and the Medicines and Healthcare Regulatory Agency and the Administration of Radioactive Substances Advisory Committee (ARSAC research certificate #RPC: 630/3925/28196), UK. All participants provided written informed consent prior to participation in the study. The trial was registered with ClinicalTrials.gov (NCT01138033).
Study Design and Patients
FAK113517 was a phase I open-label, nonrandomized, multicenter study of GSK2256098. In this expansion cohort, patients were recruited at 3 sites within the United Kingdom. GSK2256098 was administered at the MTD of 1000 mg twice daily, and at a dose of 750 mg or 500 mg twice daily to explore doses below the MTD. Dose reductions for toxicity were permitted.23
Key eligibility criteria for recruitment: glioblastoma at first or second recurrence; progressive disease by Response Assessment in Neuro-Oncology (RANO) criteria; prior temozolomide-based chemoradiotherapy; measurable disease by RANO criteria; age ≥18 years; Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; and adequate hematologic, hepatic, and renal functions. Key exclusion criteria included active gastrointestinal disease that may impair drug absorption, prolonged QTc, and history of acute coronary syndrome or heart failure. CYP3A4, CYP2C8, CYP2C9, and OATP1B1 inhibitors and inducers were precluded prior to and during study participation.
Endpoints
The primary objectives of the study were to assess the safety and tolerability of GSK2256098 in patients with relapsed glioblastoma at the MTD determined in systemic cancers, with secondary objectives of characterizing PK and antitumor activity. The objectives of the PET imaging substudy were to assess the biodistribution and kinetics of [11C]GSK2256098 and to estimate the quantity of [11C]GSK2256098 in tumors and normal brain.
Assessments
Safety and tolerability
Pretreatment patient evaluation included medical history, physical examination, ECOG performance status, vital signs, blood tests (hematology, biochemistry, coagulation profiles), ECG, and urinalysis. These were repeated at specified timepoints throughout the study. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Toxicities occurring in the first 21 days of treatment were considered for determining DLTs.
Tumor assessment
Brain MRI with contrast was performed at baseline and every 6 weeks. Response evaluations were made according to the RANO criteria.24
Pharmacokinetics
Blood samples were collected on Dried Blood Spot (DBS) cards for PK assessment predose and at 1, 1.5, 2, 3, and 4 hours following day 1 dose; and for predose on days 8, 15, 22, and 43. GSK2256098 concentrations in blood were quantified using a validated analytical method based on extraction from DBS on Whatman FTA (Flinders Technology Associates) paper, followed by ultra-high-performance liquid chromatography–tandem mass spectrometry (assay range, 10 to 10000 ng/mL). The PK parameters were derived by noncompartmental methods.
Imaging substudy
A PET-CT scan was performed on a single day between day 9 and day 20, after intravenous injection of carbon-11 radiolabeled [11C]GSK2256098, about 2 hours after oral dosing of GSK2256098 (to coincide with expected peak plasma concentration). Initially, a CT scan of the head was performed to estimate tissue attenuation, immediately followed by a 90-minute cranial dynamic PET scan following intravenous administration of a bolus microdose (<10 μg) of [11C]GSK2256098. Continuous arterial blood sampling was performed for the first 15 minutes of the scan for blood radioactivity measurements. Additionally, arterial blood samples for radioactivity counting and radio-HPLC (high performance liquid chromatography) metabolite analysis were collected during the PET scan. Blood was collected for PK sampling prior to oral dosing and at 1, 2, and 4 hours post oral dosing. Whole blood and plasma radioactivity measurements were used to calculate time-activity curves (TACs) of radioactivity concentration in blood and plasma. Measured radiometabolite data were used to correct these curves and derive the time course of [11C]GSK2256098 in plasma. Supplementary Figures S1, S2, and S3 and Supplementary Table S1 give additional PET protocols.
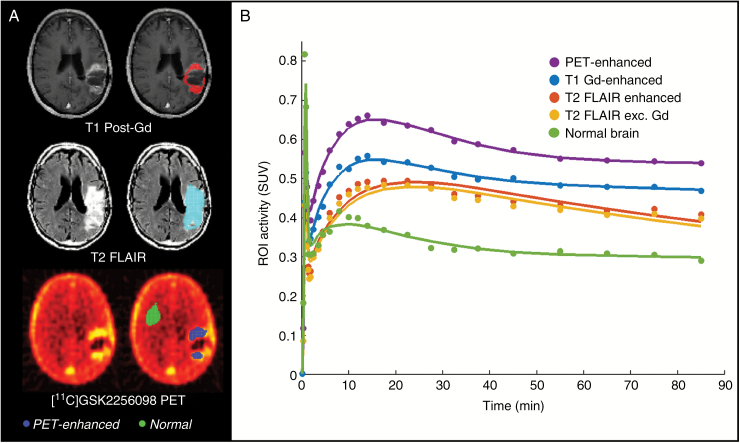
The dynamic PET images were reconstructed and corrected for attenuation and background randoms and scatter. The summed PET images for the full scan duration (0–90 min) was registered to each subject’s baseline MR images and corrected for motion using a frame-to-frame registration process with a normalized mutual information cost function. Regions of interest (ROIs) were manually delineated on the [11C]GSK2256098 PET scan corresponding visually to areas of [11C]GSK2256098 uptake in the PET scan (PET-enhanced regions) and in the normal brain tissue (normal brain region) away from the tumor. ROIs were also manually delineated on MR images obtained for clinical purposes on a day prior to the PET-CT scan and to correspond with gadolinium (Gd) enhancing regions on T1 postcontrast sequences (T1 Gd-enhanced region) and regions with increased T2 fluid attenuated inversion recovery (FLAIR) signal (T2-FLAIR enhanced region) indicating edematous regions. Given that the T1 Gd-enhanced region was typically a subset of the T2 FLAIR enhanced region, a third ROI was derived from the T2 FLAIR enhanced region that was not also enhanced on T1 post-Gd (T2 FLAIR exc. Gd region).
All the manually delineated regions were applied to the dynamic PET images to derive TACs for each of the regions defined. Tissue and plasma TACs were modeled to estimate the PET volume of distribution (VT), which is the equilibrium partition coefficient of radioactivity between tissue and plasma for the tissue regions delineated.
Statistical Analysis
Descriptive statistics were used for safety, response, and PK data. Sample size was based on feasibility and not on power to test a statistical hypothesis. PET imaging data were analyzed using MIAKAT software version 3.3.8.
Results
Patients
Thirteen patients (median age 53 y) from 3 hospitals were enrolled and received at least one dose of GSK2256098 (Table 1). Eleven patients had a histological diagnosis of glioblastoma, 1 had a histological diagnosis of gliomatosis cerebri, and 1 had a previous histological diagnosis of anaplastic oligoastrocytoma with radiological evidence of subsequent transformation to glioblastoma. The median time from diagnosis of glioblastoma to study entry was 17 months, with a median time from last progression to study entry of 42 days. The median number of lines of previous chemotherapy was 2 (range, 1–3). All patients discontinued study treatment, most frequently due to disease progression (69%).
Table 1.
Baseline patient characteristics
| 500 mg b.i.d. (N = 3) | 750 mg b.i.d. (N = 4) | 1000 mg b.i.d. (N = 6) | Total (N = 13) | |
|---|---|---|---|---|
| Age, y | ||||
| Mean | 52.0 | 43.0 | 52.0 | 49.2 |
| SD | 11.36 | 12.75 | 8.99 | 10.70 |
| Median (min, max) | 57.0 (39, 60) | 39.0 (33, 61) | 53.0 (40, 65) | 53.0 (33, 65) |
| Age group, n (%) | ||||
| 18–64 y | 3 (100) | 4 (100) | 5 (83) | 12 (92) |
| 65–74 y | 0 | 0 | 1 (17) | 1 (8) |
| Sex, n (%) | ||||
| Female | 0 | 2 (50) | 1 (17) | 3 (23) |
| Male | 3 (100) | 2 (50) | 5 (83) | 10 (77) |
| Race, n (%) | ||||
| White—Arabic/North African heritage | 1 (33) | 0 | 0 | 1 (8) |
| White—White/Caucasian/European heritage | 2 (67) | 4 (100) | 6 (100) | 12 (92) |
| Number of prior chemotherapy regimens, n (%) | ||||
| 1 | 0 | 0 | 2 (33) | 2 (15) |
| 2 | 2 (67) | 3 (75) | 3 (50) | 8 (62) |
| 3 | 1 (33) | 1 (25) | 1 (17) | 3 (23) |
| Receiving steroids at baseline, n (%) | ||||
| Yes | 3 (100) | 2 (50) | 5(83) | 10 (77) |
| No | 0 | 2 (50) | 1 (17) | 3 (23) |
Safety and Tolerability
Patients were treated with GSK2256098 at 1000 mg (n = 6), 750 mg (n = 4), or 500 mg (n = 3) twice daily. A single DLT of grade 4 cerebral edema with associated grade 3 somnolence was observed in the 1000 mg cohort, and 1000 mg was determined as the MTD as defined by protocol criteria. Due to the observed cerebral edema, the investigators decided to explore treatment at dose levels below the MTD, to further characterize safety and tolerability and to investigate tumor penetration of [11C]GSK2256098. One patient in the 750 mg cohort (n = 4) experienced grade 4 cerebral edema with associated grade 3 somnolence and grade 3 headache. There were no DLTs in patients in the 500 mg cohort (n = 3).
All patients reported at least one treatment-related adverse event (AE). The most frequent treatment-related AEs were diarrhea (38%), fatigue (31%), and nausea (31%). Treatment-related AEs by maximum toxicity grade are shown in Table 2. The majority of treatment-related AEs were grade 1 or 2. Brain edema was the only treatment-related grade 4 AE and was reported in 2 subjects. In both patients, the symptoms of brain edema improved following cessation of GSK2256098 and commencement of high-dose dexamethasone, but recurred following rechallenge with GSK2256098 at a dose reduction. In addition, there were 2 subjects with grade 3 AEs of fatigue and 2 with grade 2 AEs of somnolence that were reported as treatment related. All other treatment-related grades 3 and 4 AEs were reported for only one subject. No grade 3 or grade 4 treatment-related AEs occurred in more than one subject in the 500 mg cohort.
Table 2.
Treatment-related adverse events
| Preferred Term | Maximum CTCAE Toxicity Grade, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 500 mg b.i.d. (N = 3) | 750 mg b.i.d. (N = 4) | 1000 mg b.i.d. (N = 6) | |||||||
| 3 | 4 | Any Grade | 3 | 4 | Any Grade | 3 | 4 | Any Grade | |
| Any event | 1 (33) | 0 | 3 (100) | 1 (25) | 1 (25) | 4 (100) | 2 (33) | 1 (17) | 6 (100) |
| Diarrhea | 0 | 0 | 0 | 1 (25) | 0 | 3 (75) | 0 | 0 | 2 (33) |
| Fatigue | 0 | 0 | 1 (33) | 1 (25) | 0 | 1 (25) | 1 (17) | 0 | 2 (33) |
| Nausea | 0 | 0 | 1 (33) | 0 | 0 | 2 (50) | 0 | 0 | 1 (17) |
| Blood bilirubin increased | 0 | 0 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 2 (33) |
| Somnolence | 0 | 0 | 0 | 1 (25) | 0 | 1 (25) | 1 (17) | 0 | 2 (33) |
| Cerebral edema | 0 | 0 | 0 | 0 | 1 (25) | 1 (25) | 0 | 1 (17) | 1 (17) |
| Hypercholesterolemia | 0 | 0 | 0 | 0 | 0 | 1 (25) | 0 | 0 | 1 (17) |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 1 (25) | 0 | 0 | 1 (17) |
| ALT increased | 1 (33) | 0 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 |
| Aphasia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (17) |
| AST increased | 0 | 0 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 |
| Asthenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Blood cholesterol increased | 0 | 0 | 0 | 0 | 0 | 1 (25) | 0 | 0 | 0 |
| Headache | 0 | 0 | 0 | 1 (25) | 0 | 1 (25) | 0 | 0 | 0 |
| Hypokalemia | 0 | 0 | 0 | 0 | 0 | 1 (25) | 0 | 0 | 0 |
| Fall | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Lower respiratory tract infection | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (17) |
| Lymphopenia | 0 | 0 | 0 | 0 | 0 | 1 (25) | 0 | 0 | 0 |
| Muscular weakness | 0 | 0 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 |
| Myalgia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (17) |
| Myoclonus | 0 | 0 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 |
| Oropharyngeal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Seizure | 0 | 0 | 0 | 0 | 0 | 1 (25) | 0 | 0 | 0 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Discontinuation of GSK2256098 due to treatment-related AEs occurred in 2 patients due to grade 3 fatigue (n = 1) and grade 3 rise in alanine transaminase (n = 1). Dose reductions of GSK2256098 due to treatment-related AEs occurred in 1 patient in the 1000 mg cohort due to fatigue, and in 2 patients in the 1000 mg cohort (n = 1) and 750 mg cohort (n = 1) due to symptoms of cerebral edema. Two other patients had dose reductions (1 due to dosing error, and 1 per sponsor request). Twelve serious AEs occurred in 7 patients (54%); most were unrelated to GSK2256098 (58%). Treatment-related serious AEs included raised alanine transaminase (grade 3), somnolence (grade 3), lower respiratory tract infection (grade 3), and myalgia (grade 3).
Pharmacokinetics
All subjects participated in PK assessments on day 1 (Table 3). There was moderate to high intersubject variability. The results were not meaningfully different from those estimated for subjects in solid tumors in previous cohorts of this phase I trial with a difference in maximal concentration of 42% between the 2 groups following single or repeated doses across 750 mg and 1000 mg.23
Table 3.
Summary of GSK2256098 pharmacokinetic parameter values
| Dose (mg) | Cmax (ng/mL), Geometric Mean (%CVb) | Cavg (ng/mL) | tmax (h), Median (range) | AUC(0–4) (ng*h/mL), Geometric Mean (%CVb) | |
|---|---|---|---|---|---|
| Day 1 | 500 (n = 3) | 3075 (108) | 1.5 (1.0, 2.2) | 7038 (78.0) | |
| 750 (n = 4) | 4912 (14.7) | 3.0 (1.5, 4.0) | 8644 (49.0) | ||
| 1000 (n = 6) | 4079 (80.1) | 2.0 (1.5, 4.0) | 9330 (102) | ||
| Day of imaging procedure | 500 (n = 3) | 3262 (113) | 1548 (78.6) | 8608 (93.7) | |
| 750 (n = 2) | 5860 (32.9) | 2338 (40.3) | 10,639 (11.7) | ||
| 1000 (n = 3) | 3803 (82.8) | 1753 (60.3) | 10,286 (67.3) |
%CVb = between-subject coefficient of variation; AUC(0–4) = area under the DBS concentration-time curve to the last quantifiable concentration; Cavg = average concentration over the dose interval; Cmax = maximum observed concentration; DBS = dried blood spot.
Data reported as geometric mean (%CVb).
Clinical Activity
Eleven patients had imaging subsequent to commencing GSK2256098 and were evaluable for assessment of clinical activity. A best response of stable disease by RANO criteria was observed in 3 patients (27%), with progressive disease in 8 patients (73%). Median progression-free survival was 5.7 weeks (95% CI: 3.1–8.3 wk). Two patients were on study for over 90 days: 1 patient for 3.3 months (500 mg cohort) and 1 patient for 11.3 months (750 mg cohort).
[11C]GSK2256098 Biodistribution and Kinetics
Eight patients participated in the PET imaging substudy. Tracer metabolism was moderate, with around 30%–55% of radioactivity in plasma at 90 minutes attributable to intact parent radiotracer (Supplementary Figure S3). Estimates of the regional PET VT and images from a representative patient are shown in Table 4 and Fig. 1. Estimate of [11C]GSK2256098 VT was around 0.4 (range, 0.2‒0.6) in normal brain, 0.9 (range, 0.4‒1.7) in tumor tissue demonstrating [11C]GSK2256098 uptake (PET-enhanced regions), and 0.5 (range, 0.2‒0.7) in non–contrast enhancing disease (T2 FLAIR exc. Gd region). This indicates that at steady state, the concentration of GSK2256098 was approximately 0.4 times the corresponding concentration in plasma, and markedly higher (0.9) in tumor. Areas of high PET signal (indicating [11C]GSK2256098 concentration) were spatially consistent with areas that were Gd enhancing on T1 MRI. Based on an estimate of GSK2256098 blood-to-plasma ratio of 0.84 from in vitro experiments in human blood, the measured blood concentrations of GSK2256098 were combined with estimated VT values to produce estimates of tumor concentrations ranging from 448 ng/mL to 3482 ng/mL (Table 4). These drug concentrations do not take into account any potential contribution of radioactive metabolites to the PET tissue signal. No clear relationship was observed between dose of GSK2256098 and VT. There was no significant association between estimated tumor concentration of GSK2256098 and radiological response or time on study, although of note the patient on treatment for 11.3 months had the highest estimated tumor concentration.
Table 4.
Estimates of regional PET volume of distribution VT and tumor concentration
| Subject | Age (y) | Gender | Weight (kg) | Dose (mg) | Injected Radioactivity (MBq) | Injected mass (μg) | VT in Region of Interest (mL.cm−3) | Blood Concentration Cavg (ng/mL) | Estimated Tumor Concentration (ng/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 Gd-enhanced | T2 FLAIR- enhanced | T2 FLAIR exc. Gd | PET-enhanced | Normal Brain | |||||||||
| 409 | 53 | Male | 81.2 | 1000 | 468 | 6.65 | 0.63 | 0.44 | 0.42 | 0.83 | 0.39 | 2312 | 2273 |
| 410 | 54 | Female | 118.5 | 1000 | 95 | 1.55 | 0.64 | 0.64 | 0.64 | 0.82 | 0.55 | 2522 | 2449 |
| 411 | 61 | Male | 79.9 | 750 | 298 | 2.90 | 0.86 | 0.57 | 0.52 | 0.75 | 0.29 | 3076 | 2760 |
| 1002 | 57 | Male | 86.0 | 1000 | 429 | 5.17 | 1.68 | 0.67 | 0.74 | 1.72 | 0.44 | 923 | 1888 |
| 1008 | 34 | Female | 84.9 | 750 | 398 | 4.52 | 1.66 | 0.69 | 0.62 | 1.65 | 0.51 | 1777 | 3482 |
| 1012 | 61 | Male | 72.0 | 500 | 314 | 4.66 | 0.35 | 0.26 | 0.26 | 0.39 | 0.23 | 3147 | 1455 |
| 1015 | 40 | Male | 65.2 | 500 | 130 | 1.49 | 0.88 | 0.64 | 0.57 | 0.83 | 0.41 | 1496 | 1487 |
| 1017 | 57 | Male | 126.3 | 500 | 431 | 5.15 | 0.52 | 0.40 | 0.35 | 0.48 | 0.28 | 787 | 448 |
Fig. 1.
PET data. (A) Aligned PET and MR images in a representative patient with ROIs highlighted as colored overlays in images on right side. (B) Time-activity curves in a representative patient.
Discussion
This study demonstrates (i) that [11C]GSK2256098 penetrates the BBB at low levels into normal brain but markedly higher levels were observed in tumor and (ii) that GSK2256098 is tolerable in patients with recurrent glioblastoma. One patient with recurrent glioblastoma in the 750 mg twice daily cohort received GSK2256098 treatment for 11.3 months until disease progression.
The MTD of GSK2256098 in patients with recurrent glioblastoma was 1000 mg twice daily, consistent with that defined in previous cohorts of this trial which evaluated GSK2256098 in patients with advanced systemic cancer. The observed DLTs in patients with glioblastoma were both related to cerebral edema, which had not been observed in previous studies of GSK2256098. However, previous cohorts had excluded patients with primary CNS malignancy and patients with brain metastases who were symptomatic or untreated or required corticosteroids or P450-inducing anti-epileptics. Cerebral edema is common in patients with glioblastoma and is primarily vasogenic rather than cytotoxic in nature due to BBB disruption.25,26 Cerebral edema (both vasogenic and cytotoxic) is associated with focal treatments for glioblastoma, including radiotherapy and carmustine wafers.27,28 Given the potential for cerebral edema, in future studies in patients with glioblastoma we would advocate initiating treatment with GSK2256098 at 500 mg twice daily, and uptitrating to 1000 mg twice daily as tolerated.
As yet, no small-molecule targeted inhibitor therapy has demonstrated sufficient clinical efficacy in glioblastoma to be approved for routine use.29–37 Effective treatment of glioblastoma requires a target that is present throughout the tumor, and adequate drug penetration through the BBB and into the tumor (not just to cells adjacent to blood vessels).38,39 Despite preclinical data indicating limited penetration and distribution of GSK2256098 into the intact rodent brain, PET scans of participants in this study demonstrated significant penetration of [11C]GSK2256098 into tumors following microdose administration. There was limited penetration of [11C]GSK2256098 into non–contrast enhancing disease areas (representing infiltrative disease or peritumoral edema40), albeit at slightly higher levels than normal brain. The PET volume of distribution was not impacted by the different doses of oral GSK2256098 administered, suggesting no significant variation within the dose range explored in the saturation of membrane transporters (eg, Pgp) that prevent BBB penetration. Combined with colocalization of the Gd-enhancing tissue on MRI, the PET signal is consistent with penetration of GSK2256098 being associated with a locally compromised BBB. An intact BBB is likely to limit penetration of radioactive metabolites due to the relatively hydrophilic nature of the observed metabolites. However, in areas of BBB disruption, there is a greater possibility that metabolites might contribute to the PET signal and thus confound estimates of tumor drug concentrations. Given the highly infiltrative nature of glioblastoma, limited penetration of GSK2256098 outside of contrast-enhancing areas may limit clinical effectiveness. This, along with the cytostatic nature of FAK inhibitors, supports combination therapy in future studies of GSK2256098 in glioblastoma.
In conclusion, this is the first study of a FAK inhibitor in glioblastoma. GSK2256098 achieved high tumor penetration in study participants, consistent with tumor-related BBB disruption. It was present at lower levels in normal brain tissue. Diarrhea, fatigue, and nausea were the most frequently observed treatment-related adverse events. While the MTD of GSK2256098 in patients with glioblastoma was 1000 mg twice daily, due to the potential for cerebral edema we would advocate initiating treatment at 500 mg twice daily and uptitrating as tolerated. Clinical trials of GSK2256098 as monotherapy and in combination with other agents in patients with brain tumors and other cancers are currently ongoing (NCT02428270, NCT02523014).
Funding
This study was sponsored by GlaxoSmithKline.
Supplementary Material
Acknowledgments
We are thankful to staff at participating hospitals, Imanova, patients, and their families and carers for their contributions to the study. The study center at UCL is supported by the National Institute of Health Research/Wellcome University College Hospital Clinical Research Facility, the University College Hospital/University College London Biomedical Research Centre and UCL Experimental Cancer Medicine Centre, and the National Brain Appeal. Imperial College acknowledges infrastructural funding from the Experimental Cancer Medicine Centres, Cancer Research UK, and NIHR Biomedical Research Centre initiatives.
Conflict of interest statement.
J.T., L.Y., K.A., D.C. are employees of GlaxoSmithKline (GSK). R.F.R.F., L.L., A.S., C.P., Y.L., and G.S. are former employees of GSK. R.F.R.F., K.A., A.S., J.T. own stock in GSK. K.A. has a pending patent related to this study (WO 2013/003575 A1). C.P., G.S., Y.L., and A.S. are employees of Imanova UK (previously part of GSK), who performed and were paid for scanning services for this study and performed work for GSK outside of this study.
References
- 1. Brodbelt A, Greenberg D, Winters T, et al. Glioblastoma in England: 2007–2011. Eur J Cancer. 2015;51(4):533–542. [DOI] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 4. McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat Rev Cancer. 2005;5(7):505–515. [DOI] [PubMed] [Google Scholar]
- 5. Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28(1-2):35–49. [DOI] [PubMed] [Google Scholar]
- 6. Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71(3-4):435–478. [DOI] [PubMed] [Google Scholar]
- 7. Serrels A, Lund T, Serrels B, et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell. 2015;163(1):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Natarajan M, Hecker TP, Gladson CL. FAK signaling in anaplastic astrocytoma and glioblastoma tumors. Cancer J. 2003;9(2):126–133. [DOI] [PubMed] [Google Scholar]
- 9. Haskell H, Natarajan M, Hecker TP, et al. Focal adhesion kinase is expressed in the angiogenic blood vessels of malignant astrocytic tumors in vivo and promotes capillary tube formation of brain microvascular endothelial cells. Clin Cancer Res. 2003;9(6):2157–2165. [PubMed] [Google Scholar]
- 10. Jones G, Machado J Jr, Merlo A. Loss of focal adhesion kinase (FAK) inhibits epidermal growth factor receptor-dependent migration and induces aggregation of nh(2)-terminal FAK in the nuclei of apoptotic glioblastoma cells. Cancer Res. 2001;61(13):4978–4981. [PubMed] [Google Scholar]
- 11. Ozkal S, Paterson JC, Tedoldi S, et al. Focal adhesion kinase (FAK) expression in normal and neoplastic lymphoid tissues. Pathol Res Pract. 2009;205(11):781–788. [DOI] [PubMed] [Google Scholar]
- 12. Recher C, Ysebaert L, Beyne-Rauzy O, et al. Expression of focal adhesion kinase in acute myeloid leukemia is associated with enhanced blast migration, increased cellularity, and poor prognosis. Cancer Res. 2004;64(9):3191–3197. [DOI] [PubMed] [Google Scholar]
- 13. Giaginis CT, Vgenopoulou S, Tsourouflis GS, Politi EN, Kouraklis GP, Theocharis SE. Expression and clinical significance of focal adhesion kinase in the two distinct histological types, intestinal and diffuse, of human gastric adenocarcinoma. Pathol Oncol Res. 2009;15(2):173–181. [DOI] [PubMed] [Google Scholar]
- 14. Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342(8878):1024–1025. [DOI] [PubMed] [Google Scholar]
- 15. Owens LV, Xu L, Craven RJ, et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55(13):2752–2755. [PubMed] [Google Scholar]
- 16. Riemenschneider MJ, Mueller W, Betensky RA, Mohapatra G, Louis DN. In situ analysis of integrin and growth factor receptor signaling pathways in human glioblastomas suggests overlapping relationships with focal adhesion kinase activation. Am J Pathol. 2005;167(5):1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding L, Sun X, You Y, Liu N, Fu Z. Expression of focal adhesion kinase and phosphorylated focal adhesion kinase in human gliomas is associated with unfavorable overall survival. Transl Res. 2010;156(1):45–52. [DOI] [PubMed] [Google Scholar]
- 18. Auger KR, Smitheman KN, Korenchuk S, et al. 387 the focal adhesion kinase inhibitor GSK2256098: a potent and selective inhibitor for the treatment of cancer. Eur J Cancer. 2012;48:118. [Google Scholar]
- 19. Schinkel AH. P-glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36(2–3):179–194. [DOI] [PubMed] [Google Scholar]
- 20. Schneider SW, Ludwig T, Tatenhorst L, et al. Glioblastoma cells release factors that disrupt blood-brain barrier features. Acta Neuropathol. 2004;107(3):272–276. [DOI] [PubMed] [Google Scholar]
- 21. Roberts HC, Roberts TP, Brasch RC, Dillon WP. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol. 2000;21(5):891–899. [PMC free article] [PubMed] [Google Scholar]
- 22. Seitz RJ, Wechsler W. Immunohistochemical demonstration of serum proteins in human cerebral gliomas. Acta Neuropathol. 1987;73(2):145–152. [DOI] [PubMed] [Google Scholar]
- 23. Soria JC, Gan HK, Blagden SP, et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann Oncol. 2016;27(12):2268–2274. [DOI] [PubMed] [Google Scholar]
- 24. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 25. Papadopoulos MC, Saadoun S, Binder DK, Manley GT, Krishna S, Verkman AS. Molecular mechanisms of brain tumor edema. Neuroscience. 2004;129(4):1009–1018. [DOI] [PubMed] [Google Scholar]
- 26. Klatzo I. Evolution of brain edema concepts. In: Ito U, Baethmann A, Hossmann K-A, et al. eds. Brain Edema IX: Proceedings of the Ninth International Symposium Tokyo, May 16–19, 1993. Vienna: Springer Vienna; 1994:3–6. [Google Scholar]
- 27. Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH. Radiation necrosis following treatment of high grade glioma—a review of the literature and current understanding. Acta Neurochir (Wien). 2012;154(2):191–201; discussion 201. [DOI] [PubMed] [Google Scholar]
- 28. Westphal M, Ram Z, Riddle V, Hilt D, Bortey E; Executive Committee of the Gliadel Study G Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien). 2006;148(3):269–275; discussion 275. [DOI] [PubMed] [Google Scholar]
- 29. Brown N, McBain C, Nash S, et al. Multi-center randomized phase II study comparing cediranib plus gefitinib with cediranib plus placebo in subjects with recurrent/progressive glioblastoma. PLoS One. 2016;11(5):e0156369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mulholland PJ, Thirlwell C, Brock CS, Newlands ES. Emerging targeted treatments for malignant glioma. Expert Opin Emerg Drugs. 2005;10(4):845–854. [DOI] [PubMed] [Google Scholar]
- 31. Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stupp R, Hegi ME, Gorlia T, et al. ; European Organisation for Research and Treatment of Cancer (EORTC); Canadian Brain Tumor Consortium; CENTRIC study team Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 34. Wen PY, Chang SM, Lamborn KR, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02. Neuro Oncol. 2014;16(4):567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lassen U, Sorensen M, Gaziel TB, Hasselbalch B, Poulsen HS. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res. 2013;33(4):1657–1660. [PubMed] [Google Scholar]
- 36. Muhic A, Poulsen HS, Sorensen M, Grunnet K, Lassen U. Phase II open-label study of nintedanib in patients with recurrent glioblastoma multiforme. J Neurooncol. 2013;111(2):205–212. [DOI] [PubMed] [Google Scholar]
- 37. Chen R, Cohen AL, Colman H. Targeted therapeutics in patients with high-grade gliomas: past, present, and future. Curr Treat Options Oncol. 2016;17(8):42. [DOI] [PubMed] [Google Scholar]
- 38. Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–592. [DOI] [PubMed] [Google Scholar]
- 39. Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990;9(3):253–266. [DOI] [PubMed] [Google Scholar]
- 40. Ellingson BM, Wen PY, van den Bent MJ, Cloughesy TF. Pros and cons of current brain tumor imaging. Neuro Oncol. 2014;16(suppl 7):vii2–vii11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.