Abstract
Background
Aerobic glycolysis confers several advantages to tumor cells, including shunting of metabolites into anabolic pathways. In glioblastoma cells, hypoxia induces a flux shift from the pentose phosphate pathway toward glycolysis and a switch from proliferation to migration. The mechanistic link between glycolysis and migration is poorly understood. Since glucose-6-phosphate isomerase (GPI) is identical to the secreted cytokine autocrine motility factor (AMF), we investigated whether GPI/AMF regulates glioblastoma cell invasion.
Methods
The expression and hypoxic regulation of GPI/AMF and its receptor AMFR were analyzed in glioblastoma tissue and cell lines. Functional effects were studied in vitro and in xenograft models.
Results
High GPI/AMF expression in glioblastomas was found to be associated with a worse patient prognosis, and levels were highest in hypoxic pseudopalisades. Hypoxia upregulated both GPI/AMF and AMFR expression as well as GPI/AMF secretion in vitro. GPI/AMF stimulated cell migration in an autocrine fashion, and GPI/AMF expression was upregulated in migratory cells but reduced in rapidly proliferating cells. Knockdown or inhibition of GPI/AMF reduced glioblastoma cell migration but in part stimulated proliferation. In a highly invasive orthotopic glioblastoma model, GPI/AMF knockdown reduced tumor cell invasion but did not prolong survival. In a highly proliferative model, knockdown tumors were even larger and more proliferative than controls; however, perivascular invasion, provoked by simultaneous bevacizumab treatment, was reduced.
Conclusions
GPI/AMF is a potent motogen for glioblastoma cells, explaining in part the association between glycolysis and migration. Targeting GPI/AMF is, however, problematic, since beneficial anti-invasive effects may be outweighed by unintended mitogenic effects.
Key Points
1.Increased glycolysis is linked with increased cell migration and invasion in glioblastoma cells.
2.The glycolysis enzyme GPI/AMF may serve as a target for antimetabolic and anti-invasive therapy.
3.Despite reducing tumor invasion, GPI/AMF targeting may have unwanted growth stimulatory effects.
Keywords: glioma, glycolysis, in vivo, metabolism, Warburg effect
Importance of the Study
Deregulated cancer metabolism offers selective targeting opportunities, and ongoing clinical trials attempt to interfere with glucose metabolism in glioblastoma patients. This strategy may be particularly favorable in combination with anti-angiogenic treatment, which was shown to enhance the glycolytic pathway via hypoxia. Even in the absence of hypoxia, however, increased glycolysis is associated with accelerated migration, but little is known about the mechanisms of this link. The glycolytic enzyme GPI is identical to the secreted cytokine AMF and can exert moonlighting functions, including pro-invasive effects in other types of cancer. Our study demonstrates that GPI/AMF is prognostically relevant in human glioblastomas and strongly promotes tumor cell migration and invasion. However, GPI/AMF also has some significant anti-proliferative effects, consistent with the “go or grow” hypothesis. Our findings raise caution as to whether GPI/AMF is a suitable molecular target for interfering with glycolysis and/or for inhibiting glioblastoma cell invasion.
Metabolic reprogramming is a hallmark of cancer cells and is required for cellular transformation.1 In most types of tumors, including glioblastoma, the rate of glucose uptake is greatly increased and glucose is mostly metabolized to lactate even in the presence of abundant oxygen, a process known as aerobic glycolysis (Warburg effect).2 This metabolic deregulation distinguishes tumor cells from normal differentiated cells, which rely primarily on mitochondrial oxidative phosphorylation (OXPHOS) for energy production, and thus offers therapeutic targeting opportunities.
Although aerobic glycolysis is energetically less efficient than OXPHOS, it confers several advantages to cancer cells, including the shunting of carbon from glycolysis into subsidiary anabolic pathways, such as the pentose phosphate pathway (PPP) or the serine synthesis pathway. The PPP is highly important for cell division, as it produces ribose-5-phosphate and NADPH, which are required for nucleotide and fatty acid synthesis. In glioblastoma cells, PPP enzyme expression and metabolic flux are high when oxygen is abundant and cells proliferate at a high rate. In response to acute hypoxia, a metabolic shift occurs away from the PPP toward increased glycolysis, and this switch is further associated with a functional shift from high cell proliferation toward increased migration.3 Even without hypoxia, however, several key glycolysis enzymes are upregulated in rapidly migrating cells, while PPP enzymes are downregulated, and an inverse regulation pattern is present in rapidly proliferating cells.4 These findings indicate that cell function and metabolic state are coupled, independent of hypoxia. Whereas the relevance of the PPP for cell division is obvious, the association between glycolysis and migration is less straightforward to explain, and only little is known about the mechanistic involvement of glycolysis in cell migration.
Interestingly, several glycolytic enzymes can exhibit additional noncanonical functions, some of which are related to apoptosis, transcription, or migration.5 Glucose-6-phosphate isomerase (GPI), which catalyzes the second step of glycolysis, is genetically identical to autocrine motility factor (AMF), a secreted 55 kDa extracellular cytokine that can accelerate the migration of melanoma cells and other types of cancer cells.6,7 GPI/AMF binds to a 78 kDa seven-transmembrane domain G-protein coupled glycoprotein receptor, the AMF receptor (AMFR, gp78).8 AMFR activation leads to stress-fiber formation, activation of RhoA, Rac1, and the mitogenic and anti-apoptotic mitogen-activated protein kinase/extracellular signal-regulated kinase and phosphatidylinositol-3 kinase/Akt pathways, similar to the signaling mode of growth factors.6,8 GPI/AMF is not secreted by normal cells, but GPI/AMF levels are elevated in the serum or urine of patients with malignant tumors, such as colorectal, lung, kidney, and breast carcinomas, and correlate with a poor prognosis.6,8
The goal of this study was to elucidate the role of secreted GPI/AMF-AMFR in glioblastoma progression. We demonstrate that GPI/AMF secretion is a mechanism which can explain the increased tumor cell migration observed in association with elevated glycolysis and hypoxia. High expression of GPI/AMF in glioblastoma tissue is associated with a worse patient prognosis. However, our results also show that interfering with GPI/AMF expression is an ambiguous strategy, as the advantage of reducing tumor cell invasion can be outweighed by an enhanced proliferative growth in vivo.
Materials and Methods
Cell Culture
All cell lines were published previously, and details of their origin and authentication are provided in the Supplementary material. Glioblastoma stemlike (GS) cell lines GS-11, GS-12, and BT112 were cultured in serum-free Neurobasal medium (NBM) as described previously.9,10 G55 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (Life Technologies).11
Quantitative PCR Analysis
Real-time PCR analysis was performed as detailed in the Supplementary material and as described previously,3 using TaqMan Gene Expression Assays (Applied Biosystems).
Immunoblot Analysis
Western blot analysis was performed as detailed in the Supplementary material and as described previously.3 Western blot scans of X-ray films were quantified by densitometry using the ImageJ program.
Immunohistochemical Analyses
Paraffin sections of human glioblastomas and a previously constructed glioblastoma tissue microarray (TMA) as well as xenograft tumors were immunostained for AMF, AMFR, Ki-67, and CD34 as described previously4 and as detailed in the Supplementary material.
Migration Assay
Glioblastoma cell migration was analyzed using modified Boyden chamber assays as described previously10 and as detailed in the Supplementary material.
Cell Proliferation Assays
Glioblastoma cell proliferation was analyzed by manual cell counting (GS lines) or a crystal violet assay as described previously4 and as detailed in the Supplementary material.
PKH67 Staining and Cell Separation
To isolate cell subpopulations that proliferate rapidly versus slowly, cells were labeled with PKH67 and separated by fluorescence activated cell sorting (FACS) at optimized time points into populations with either high or low dye retention as described previously4 and as detailed in the Supplementary material.
Separation of Migrated and Nonmigrated Cells
Transwell Permeable Supports (Corning) were coated with laminin (5 µg/mL) or collagen (0.875%) for GS-11 or G55 cells, respectively, and installed in 6-well plates. We seeded 2 × 106 cells into each transwell, and after 6 hours (G55) or 28 hours (GS-11) of incubation, nonmigrated cells from the upper side of the transwell membrane as well as migrated cells from the underside were scraped off and collected separately.
AMF Immunodepletion from Conditioned Medium
We seeded 1 × 106 cells in 20 mL of medium without supplements and cultured them for 24 hours at 1% O2. Supernatants were concentrated 30-fold using 10 kDa Amicon Ultra-15 filter units (Merck Millipore). To immunodeplete AMF, 12.5 µL anti-AMF antibody solution (rabbit pAbm H00002821-D01; Novus) was added to 500 µL concentrated conditioned medium, followed by rotating incubation overnight at 4°C. Protein A-agarose beads were added, and after 2 hours beads with absorbed immune complexes were precipitated by centrifugation. Two additional rounds of Protein A binding were performed and all supernatants were collected.
Short Hairpin RNA Transduction
Lentiviral transduction of short hairpin (sh)RNAs targeting GPI/AMF or nonsilencing control shRNA was performed as outlined in the Supplementary material. ShRNA-expressing cells were selected using puromycin.
Orthotopic In Vivo Model
Animal experiments were approved by the local authority in Hamburg. Tumor xenografts were generated as described previously.10,11 Briefly, dissociated G55 cells (4 × 104) or BT112 cells (1 × 105) were injected stereotactically into the striatum of 6- to 8-week-old anesthetized NMRI/Foxn1nu mice (10 mice per group; Harlan). Endpoint of the BT112 model was the occurrence of symptoms, such as weight loss ≥10% or neurological symptoms. In the G55 model, treatment with bevacizumab (Bev) (250 µg/100 µL i.p.) was initiated one day after tumor cell implantation and continued every 3 days until animals were humanely euthanized at day 11. Serial sections from formalin-fixed, paraffin-embedded brains were stained with hematoxylin and eosin. Quantification of invasive growth in the GS-11 model was performed as described previously10 and as detailed in the Supplementary material.
Database Analyses
Analyses of the databases of the Repository of Molecular Brain Neoplasia Data (REMBRANDT), The Cancer Genome Atlas (TCGA), the Glioma-French-284-MAS5.0-u133p2, and Ivy Glioblastoma Atlas Project (GAP) were performed as detailed in the Supplementary material.
Statistical Analyses
Differences in gene expression and functional assays were analyzed using the unpaired t-test, one-way ANOVA, and the SigmaPlot program (version 12.3). Kaplan–Meier analyses of animal survival were carried out using the MedCalc program.
Results
Expression and Hypoxic Regulation of GPI/AMF and AMFR in Glioblastoma Cell Lines and Tissue
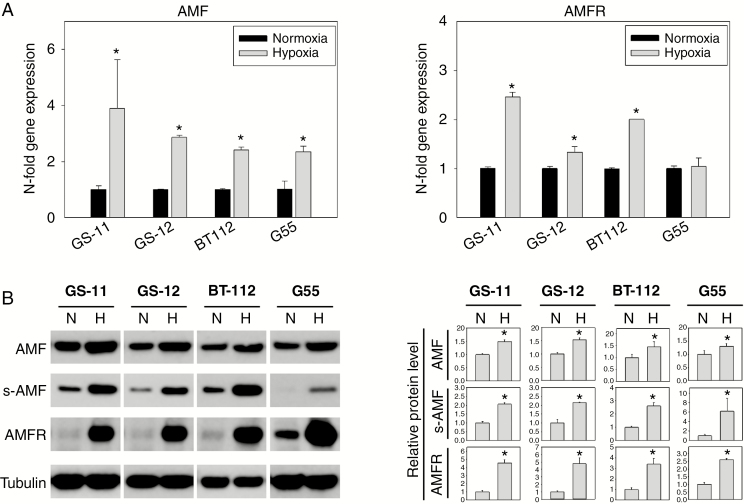
GPI/AMF and AMFR were expressed in all cell lines analyzed, including glioblastoma stemlike (GS-11, GS-12, BT112) and adherent cells (G55) (Fig. 1A, B). Hypoxia markedly increased GPI/AMF and AMFR transcript and protein levels. Furthermore, extracellular secretion of GPI/AMF was enhanced by hypoxia (Fig. 1B), indicating that under hypoxic conditions GPI/AMF not only is elevated intracellularly to serve its glycolytic function, but also becomes increasingly available extracellularly for autocrine and paracrine receptor binding.
Fig. 1.
Induction of GPI/AMF and AMFR in glioblastoma cell lines by hypoxia. (A) Cells were incubated under normoxic or hypoxic conditions for 48 hours. GPI/AMF and AMFR transcripts were quantified by quantitative PCR, and relative expression values were normalized to normoxic controls. (B) For western blot analysis of cellular GPI/AMF and AMFR, cells were lysed after 48 hours of incubation under hypoxia (H) or normoxia (N). To detect secreted GPI/AMF (s-AMF), supernatants were collected from serum-free cultures after 24 hours of incubation. Signals were quantified by densitometry, and pixel intensities for cellular GPI/AMF and AMFR were normalized to tubulin. Data are means ± SD of triplicate determinations. Asterisks indicate significance (P < 0.05).
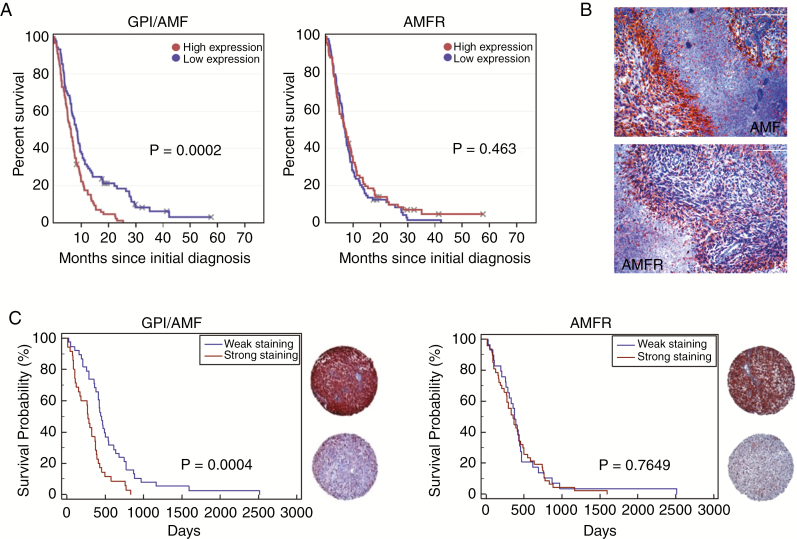
To assess the clinical relevance of GPI/AMF and AMFR expression in glioblastoma, we first interrogated the REMBRANDT database. Glioblastoma patients with high GPI/AMF mRNA expression were found to carry a significantly worse prognosis than patients with low levels (P < 0.001), whereas AMFR expression was not associated with survival (Fig. 2A). To assess GPI/AMF and AMFR protein distribution in situ, we immunostained glioblastoma tissue sections and a TMA. The majority of tumor cells displayed immunoreactivity for both GPI/AMF and AMFR, and staining was particularly strong in hypoxic pseudopalisades (Fig. 2B), consistent with the observed upregulation of GPI/AMF and AMFR by hypoxia in vitro. GPI/AMF was further detected in all 73 glioblastoma TMA spots available for analysis, and AMFR was detected in all except 2 spots. Consistent with the REMBRANDT analysis, patients with high intratumoral GPI/AMF immunoreactivity (n = 35) had a significantly shorter survival (median: 276 days) than those with low expression (n = 38, median: 458 days) (Fig. 2C). Survival of patients with high versus low AMFR expression did not differ significantly.
Fig. 2.
GPI/AMF and AMFR expression in human glioblastomas. (A) REMBRANDT analysis showed that glioblastoma patients (n = 178) with high GPI/AMF expression survived shorter. (B) Immunoreactivity for GPI/AMF and AMFR was particularly strong in pseudopalisading regions (size bars, 200 µm). (C) TMA analysis confirmed the negative association between GPI/AMF expression and survival. Representative TMA samples with strong versus weak staining intensity are shown.
To further validate these observations, we queried the Ivy GAP database, which confirmed that GPI/AMF expression is significantly elevated in hypoxic perinecrotic regions and is also increased in invading tumor cells compared with central solid cellular tumor areas (Supplementary Figure. 1). TCGA database analysis showed that the expression of GPI/AMF correlates with other known markers of hypoxia, including carbonic anhydrase 9, vascular endothelial growth factor A, solute carrier family 2 member 1, lactate dehydrogenase A, and hexokinase 2 (HK2) (Supplementary Figure 2). In addition, TCGA analysis revealed that GPI/AMF is significantly overexpressed in the classical and mesenchymal glioblastoma subtypes, which carry a worse prognosis,12 and confirmed the negative prognostic value of high GPI/AMF expression levels (Supplementary Figure 3A, B). Analysis of the Glioma-French-284-MAS5.0-u133p2 dataset showed that GPI/AMF expression is significantly higher in glioblastomas than in astrocytomas, oligodendrogliomas, and oligoastrocytomas (World Health Organization grade II or III) as well as in isocitrate dehydrogenase 1 mutated versus nonmutated glioblastomas (Supplementary Figure 3C, D).
GPI/AMF Stimulates Glioblastoma Cell Migration in an Autocrine Fashion but Has Opposite Effects on Proliferation
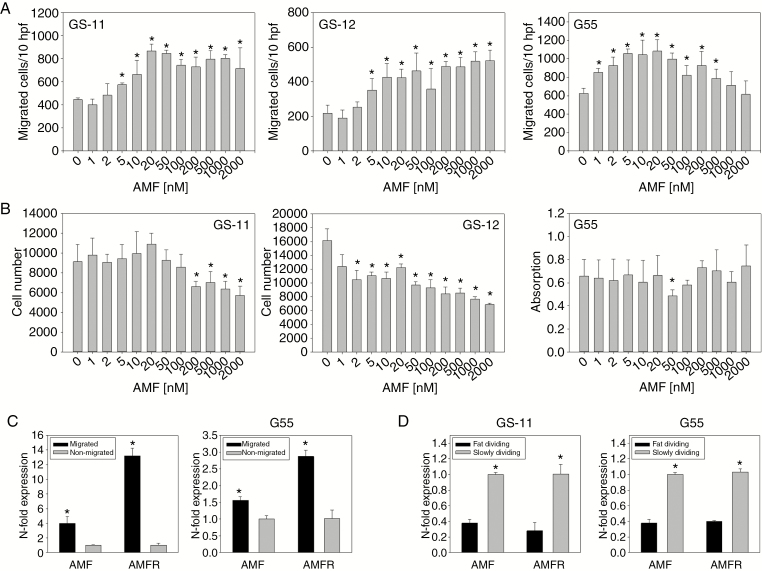
Effects of recombinant and autocrine GPI/AMF on cell migration were studied using modified Boyden chamber assays. Human recombinant GPI/AMF stimulated glioblastoma cell migration in a concentration-dependent fashion, and effects were significant at concentrations between 5 nM and 500 nM (Fig. 3A). In contrast, GPI/AMF did not stimulate cell proliferation, and especially at higher concentrations proliferation of the 2 GS cell lines was even significantly inhibited (Fig. 3B). These reciprocal effects are similar to those observed previously for the same cell lines in response to hypoxia.3,4
Fig. 3.
Glioblastoma cell migration and proliferation in response to GPI/AMF. (A) Migration was analyzed using modified Boyden chamber assays. After 5 hours (G55) or 24 hours (GS-11, GS-12) of incubation, migrated cells were counted in 10 high power fields (hpf). Values are means ± SD of sextuplicate determinations. (B) Proliferation of G55 cells in medium with 2% fetal calf serum was quantified after 3 days of growth using a colorimetric assay, and proliferation of GS cells was quantified by manual cell counting after 14 days. Values are means ± SD of quintuplicate determinations. (C) Cells were separated using transwell assays, and quantitative PCR analysis showed upregulation of GPI/AMF and AMFR in migrated versus nonmigrated subpopulations. (D) Fast and slowly dividing subpopulations were separated by FACS from PKH67-labeled cells. Expression of GPI/AMF and AMFR was decreased in fast dividing cells. Asterisks in (A)–(D) indicate significance (P < 0.05).
We further measured GPI/AMF and AMFR expression in cell subpopulations with different migratory or proliferative activity. Migrating versus nonmigrating G55 and GS-11 cells were separated using transwell assays. Expression of both GPI/AMF and AMFR was significantly higher in migrated than in nonmigrated cells (Fig. 3C). To isolate cell subpopulations that proliferate rapidly versus slowly, cells were labeled with PKH67 and were FACS-separated into populations with either high dye retention (PKHhigh, slowly proliferating) or low retention (PKHlow, fast proliferating), (Supplementary Figure 4A, B). Expression of GPI/AMF and AMFR was significantly lower in rapidly proliferating versus slowly proliferating cells (Fig. 3D).
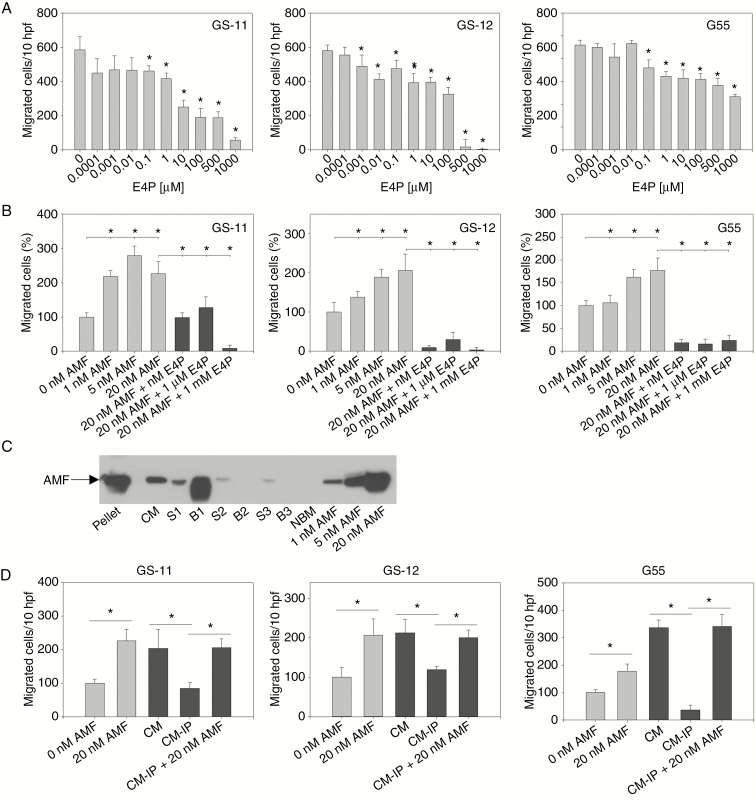
Erythrose 4-phosphate (E4P) is a potent competitive inhibitor of both the cytokine and enzymatic activities of GPI/AMF. We analyzed whether it can inhibit both random and GPI/AMF-stimulated glioblastoma cell migration. E4P had a significant concentration-dependent antimigratory effect on all cell lines at concentrations ≥0.1 µM (Fig. 4A). The extremely strong inhibitory effect, especially on GS cell lines, at high concentrations suggested that nonspecific toxicity might add to the effect. Indeed, proliferation assays showed that while cell growth was not reduced or even moderately increased at concentrations <0.1 µM E4P, GS cell proliferation was inhibited by >90% at 500 µM and 1000 µM, indicating cytotoxic effects (Supplementary Figure 5), which could be due to the fact that E4P is also a precursor of several amino acids and an intermediate in the PPP. We next studied the effect of E4P on GPI/AMF-stimulated cells. In all cell lines, E4P at ≥1 nM abrogated the chemotactic effect of 20 nM GPI/AMF (Fig. 5B).
Fig. 4.
Autocrine stimulation of migration by GPI/AMF. (A) Inhibition of random cell migration by E4P. Modified Boyden chamber assays were performed as described in Fig. 3. (B) E4P abrogated the chemotactic effect of GPI/AMF. (C) Immunodepletion of GPI/AMF from GS-11 cells. Concentrated conditioned medium (CM) was centrifuged to remove cellular debris and incubated with an α-AMF antibody. After incubation with Protein A-agarose beads, beadpellets (B1) were precipitated. Fresh beads were added to the supernatant (S1), and 2 additional rounds of Protein A binding were performed resulting in beadpellets B2 and B3 and supernatants S2 and S3. As control, cell-free medium (NBM) and medium supplemented with AMF (1–20 nM) were loaded. (D) Depletion of GPI/AMF from CM (CM-IP, corresponding to S3 in (C)) reduced autocrine stimulation of cell migration, and the effect was rescued by addition of recombinant GPI/AMF. Asterisks in (A)–(D) indicate significance (P < 0.05).
Fig. 5.
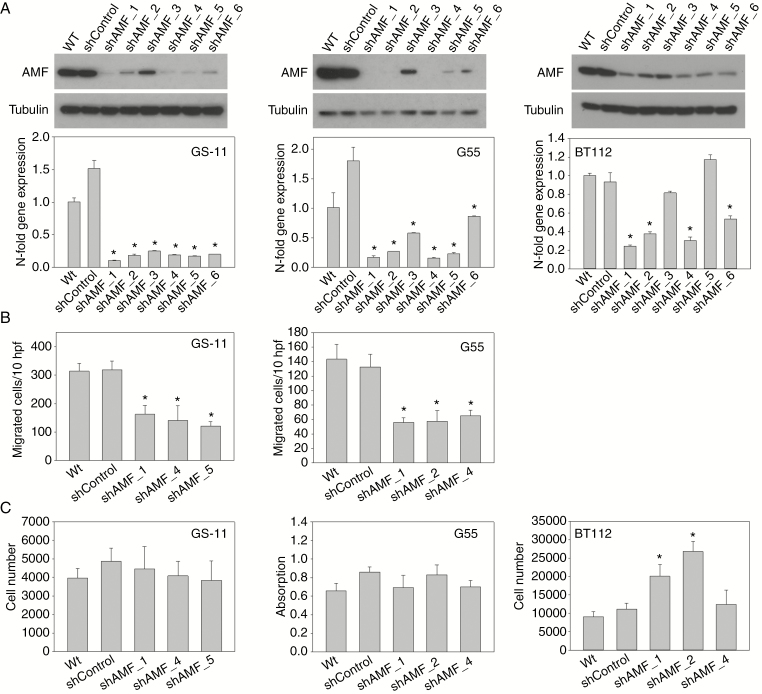
GPI/AMF knockdown in vitro. (A) Quantification of GPI/AMF expression by immunoblot and quantitative PCR analysis in GS-11, G55, and BT112 cells transduced with 6 different AMF shRNAs or nonsilencing shRNA (shControl) and in wild-type (Wt) cells. α-Tubulin served as loading control. Gene expression values are means ± SD of triplicate determinations. (B) Reduction of random migration in GPI/AMF knockdown cells. Modified Boyden chamber assays were performed as described in Fig. 3 (BT112 cells did not attach to the assay membrane). (C) Proliferation of G55 cells was quantified after 3 days of growth, using a colorimetric assay, and proliferation of GS-11 and BT112 cells was quantified by manual cell counting after 7 days. Values are means ± SD of quintuplicate determinations. Asterisks indicate significance (P < 0.05).
To determine whether an autocrine stimulatory loop of migration is present, cell supernatants were immunodepleted of GPI/AMF (Fig. 4C). While undepleted concentrated conditioned medium stimulated chemotactic migration at least 2-fold, GPI/AMF depletion reduced migration to levels similar to unstimulated controls. Addition of 20 nM GPI/AMF to immunodepleted medium rescued the motogenic effects, indicating that GPI/AMF stimulates migration in an autocrine fashion.
Knockdown of GPI/AMF Decreases Migration but Not Proliferation
To obtain further evidence for GPI/AMF acting as autocrine stimulator of glioblastoma cell migration as well as to evaluate its role in vivo, we silenced GPI/AMF expression. Initially, GS-11, GS-12, and G55 cells were transduced with 6 different shRNAs targeting GPI/AMF (shAMF) or with nonsilencing control shRNA (shControl). Stable knockdown of GPI/AMF mRNA and protein was achieved in GS-11 and G55 cells, whereas silencing was not successful in GS-12, therefore we alternatively transduced the BT112 cell line (Fig. 5A). The morphological phenotype of the knockdown cells did not differ from controls. Functional assays were performed using shAMF sublines that exhibited the strongest GPI/AMF expression reduction. All GS-11 and G55 knockdown sublines displayed significantly decreased random cell motility compared with controls (Fig. 5B), and the response to hypoxia was significantly mitigated in knockdown cells (Supplementary Figure 6). BT112 cells failed to attach to matrix-coated filters in the Boyden chamber assays (despite a broad variety of substrates tested), so that they could not be assayed. In proliferation experiments, growth of GS-11 and G55 GPI/AMF knockdown sublines did not differ from controls, while proliferation of 2 BT112 shAMF sublines was significantly increased (Fig. 5C).
GPI/AMF Knockdown Effects on Tumor Growth and Invasion In Vivo
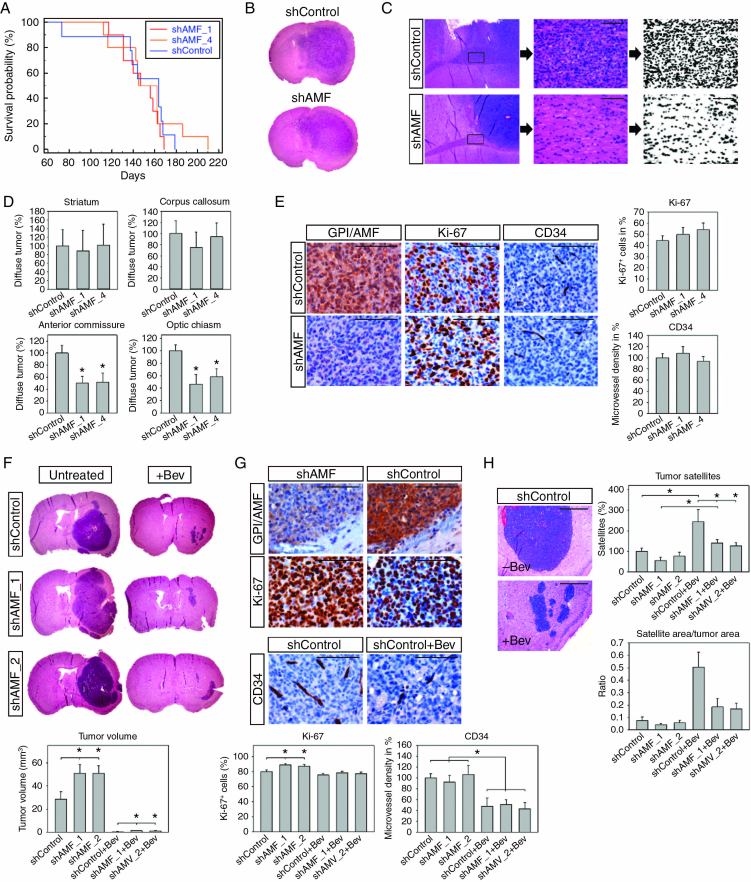
To analyze the effect of the GPI/AMF knockdown in vivo, we used 2 different models. BT112 cells typically form highly invasive tumors that also infiltrate the contralateral hemisphere. In contrast, G55 cells generate rapidly proliferating nodular tumors that are highly angiogenesis dependent.11 In the first model, we injected clones BT112_shAMF_1, BT112_shAMF_4, and BT112_shControl intracerebrally into nude mice and sacrificed the animals when they developed tumor-related symptoms. The median survival of mice with shAMF_1 tumors (147 days) or shAMF_4 tumors (145 days) did not differ significantly from controls (164 days) (Fig. 6A). Histologically, large tumors with variable degrees of invasion were present in all experimental groups (Fig. 6B). To quantify tumor invasion in different areas, we used a modified technique established previously for evaluating diffuse intracerebral glioblastoma growth.10 Four different landmark areas distant from the injection site were selected on coronal sections, including the contralateral corpus callosum and striatum (both anterior to injection level), the anterior commissure, and the optic chiasm (both posterior to injection level). Tumor cell burden in these areas was quantified by image analysis (Fig. 6C). While no differences were observed in the corpus callosum and contralateral striatum, which are both in relatively close proximity to the injection site, the more distant basal regions (ie, anterior commissure and optic chiasm) were less extensively infiltrated in mice with GPI/AMF knockdown tumors compared with controls (P < 0.05), suggesting that GPI/AMF silencing had compromised tumor cell invasion (Fig. 6C, D). Tumor cell proliferation rate and microvessel density did not differ significantly (Fig. 6E).
Fig. 6.
In vivo effects of GPI/AMF knockdown. (A) Survival of mice with BT112_shAMF xenografts did not differ from controls. (B) Large tumors were present in GPI/AMF knockdown and control mice. (C) Invasion was quantified by analyzing 4 different landmark areas at 3 coronal levels. Exemplary analysis of diffuse invasion is shown for level 3, anterior commissure. Digital images were acquired from sections stained with hematoxylin and eosin. Pictures were transformed into black and white images, and the percentage of black pixels was quantified. Values acquired for identical regions in normal murine brain were subtracted, resulting in the net area occupied by tumor cell nuclei. (D) GPI/AMF knockdown tumors showed less invasion in the anterior commissure and the optic chiasm. (E) Tumor cell proliferation (Ki-67) and microvessel density (CD34) did not differ significantly between groups. (F) Overview of untreated and Bev-treated G55 shAMF and shControl tumors and quantification of tumor volumes. Bev treatment strongly inhibited tumor growth. However, untreated as well as Bev-treated shAMF tumors were larger than the respective shControl tumors. (G) Tumor cell proliferation was increased in untreated shAMF versus shControl tumors and microvessel density was reduced in all Bev-treated groups versus respective controls. (H) Bev-treated tumors were surrounded by numerous small satellite tumors. Satellite numbers were significantly increased by Bev treatment in shControls and in shAMF_1 tumors and were reduced in Bev-treated shAMF tumors compared with Bev-treated shControls. The ratio between cumulative satellite area and tumor area was higher in Bev-treated versus untreated tumors and was reduced in Bev-treated shAMF tumors compared with treated shControls. Asterisks indicate significance (P < 0.05), values are means ± SEM. Size bars in (C), (E), and (G) are 100 µm and in (H) 1 mm.
In the G55 model, we had previously demonstrated that anti-angiogenic treatment highly effectively inhibits tumor growth but provokes tumor cell invasion along the preexisting host vasculature.11 In this present study, we injected clones G55_shAMF_1, G55_shAMF_2, and G55_shControl intracerebrally, and in order to provoke invasion we also treated mice with Bev. All animals were euthanized after 11 days, when several of the untreated animals developed weight loss, in order to facilitate comparisons of tumor invasiveness at a single identical time point. Bev treatment reduced tumor growth and microvessel density by >95% and >48%, respectively, in all experimental groups (Fig. 6F, G). Unexpectedly, in untreated animals GPI/AMF knockdown tumors were about 1.8-fold larger than shControl tumors (P < 0.05%). Moreover, in Bev-treated mice, GPI/AMF knockdown tumors were about 1.5-fold larger than controls (P < 0.05%). Proliferation was high in all tumors but even slightly higher in untreated shAMF tumors than in controls (P < 0.05) (Fig. 6G).
Invasion in the G55 model mainly occurs perivascularly, and on cross sections blood vessels cuffed by invading tumor cells appear as satellite-like structures (Fig. 6H). To quantify invasion, satellite counts were determined at levels of maximum tumor extension.11 Satellite numbers were significantly increased by Bev treatment in shControl tumors (by 144%) and in G55_shAMF_1 tumors (by 153%) (P < 0.05), while a similar trend in shAMF_2 tumors did not reach significance (Fig. 6H). Notably, the Bev-provoked satellite increase was significantly reduced in GPI/AMF knockdown tumors compared with treated shControl tumors (42.4% in shAMF_1+Bev tumors and 48.5% in shAMF_2+Bev tumors). In untreated groups, a trend toward reduced satellite numbers was observed in shAMF_1 tumors (P = 0.076). We further quantified the cumulative area taken up by tumor satellites and calculated the ratio between this area and the area taken up by the main tumor mass on coronal sections. The satellite/tumor area ratio was 6.8-fold higher in shControl+Bev tumors, 4.6-fold higher in shAMF_1+Bev tumors, and 2.9-fold higher in shAMF_2+Bev tumors compared with the respective untreated controls (Fig. 6H). The ratio was reduced by 63.6% and 66.6% in shAMF_1+Bev and shAMF_2+Bev tumors, respectively, compared with shControl+Bev tumors, confirming the relative shift from mononodular tumor growth to multisatellite invasive growth in Bev-treated tumors and the reduction of Bev-provoked invasive growth by the GPI/AMF knockdown.
Discussion
Previous studies suggested a link between glycolysis and tumor cell migration independent of hypoxia. Glycolytic enzymes are enriched in pseudopodia, and glycolytic energy rather than OXPHOS is the primary energy source for cancer cell motility and cytoskeletal rearrangement.13,14 Furthermore, lactate not only promotes tumor invasion by acidification of the microenvironment, but can also be directly motogenic for tumor cells.15 Our present study extends these observations, by showing that GPI/AMF is secreted from glioblastoma cells and stimulates migration in an autocrine fashion. GPI/AMF expression and secretion are further upregulated by hypoxia, consistent with the known regulation of the enzyme by hypoxia-inducible factor 1.6 AMFR expression was also found to be upregulated by hypoxia, which to our knowledge was not reported before. Moreover, we detected a significantly elevated expression of GPI/AMF and AMFR in migrated versus nonmigrated cells, even under normoxic conditions, as well as reduced migration of GPI/AMF knockdown cells. These findings corroborate the evidence for a hypoxia-independent intrinsic link between glycolysis and migration and suggest that autocrine stimulation by GPI/AMF is particularly relevant in an actively migrating subpopulation of glioblastoma cells.
Concerning proliferation, we found that GPI/AMF and AMFR expression was reduced in rapidly versus slowly dividing glioblastoma cells and that recombinant GPI/AMF tended to inhibit proliferation, whereas GPI/AMF silencing increased proliferation in some instances. These findings suggest that GPI/AMF can act as a regulator of the “go versus grow” behavior. Several previous studies described a dichotomous activation of either proliferative or migratory functional programs in glioblastoma cells, and a variety of regulators of this dichotomy have been identified, including extracellular matrix components, growth factors, microRNAs, and transcription factors.16–20 Moreover, we previously reported that knockdown of aldolase C (ALDOC) resulted in reduced migration but increased proliferation.4 ALDOC can also be released from cells but has no known receptor, so that the mechanism of its motogenic effect remains elusive. Collectively, our findings indicate that at least 2 glycolysis enzymes are causatively involved in enhancing glioblastoma cell migration.
In glioblastoma tissue, GPI/AMF and AMFR immunoreactivity was highest in hypoxic pseudopalisades, and a similar expression pattern was reported previously in a small in situ hybridization study.21 These findings are consistent with upregulation of both ligand and receptor by hypoxia and with their elevated expression in actively migrating cells, since pseudopalisades most likely represent a wave of tumor cells that migrate away from a necrotic area arising after a microvascular insult.22 Our TMA analysis further showed that high GPI/AMF expression is associated with poorer survival, confirming the database analyses and the previous in situ hybridization study.21 The negative association with prognosis suggests that GPI/AMF could be a potential target for inhibiting glioblastoma progression.
To evaluate the usefulness of GPI/AMF as a therapeutic target, we used 2 different in vivo models. BT112-derived tumors grow highly invasively but slowly. In this model, GPI/AMF silencing reduced tumor cell invasion, and tumors were histomorphologically more compact than controls; however, survival was not prolonged. In the G55 model, GPI/AMF knockdown tumors were even larger and more proliferative than controls. Notably, when we previously evaluated the growth of ALDOC-silenced G55 cells we found that ALDOC knockdown tumors were also more proliferative and mice survived shorter than controls,4 indicating that interference with 2 different enzymes of the preparatory phase of glycolysis produces similar results. In the present study, Bev treatment inhibited G55 tumor growth but provoked invasion along the host vasculature, reconfirming previous findings.11 Interestingly, GPI/AMF-silenced tumors displayed significantly less perivascular invasion upon Bev treatment than controls. Collectively, our findings indicate that GPI/AMF silencing can inhibit glioblastoma invasion, which alone, however, is not sufficient to translate into a survival-prolonging effect. Few studies evaluated GPI/AMF targeting in other in vivo cancer models. In an endometrial carcinoma model, GPI/AMF silencing caused inhibition of tumor progression and prolonged survival,23 and in a pancreatic cancer model GPI/AMF overexpression promoted tumor growth and metastasis.24 Of note, in addition to its glycolytic and motogenic effects, GPI/AMF possesses other moonlighting functions and is further identical to the neurotrophic factor neuroleukin and to maturation factor, which can mediate differentiation of myeloid cells.6 Hence, the pleiotropic effects of GPI/AMF depend on cell type and microenvironmental conditions, which may in part explain the differences observed in our BT112 and G55 in vivo and in vitro models.
Our rationale for combining anti-angiogenic treatment with GPI/AMF targeting was based not only on the observation that Bev treatment can enhance glioblastoma invasion in xenograft models, but also on the fact that Bev aggravates tumor hypoxia, resulting in an even more glycolysis-dependent phenotype in glioblastoma patients.25 Combining Bev treatment with targeting glycolysis could therefore have dual beneficial effects, and some studies already tested this strategy. In a subcutaneous glioma model, the combination of Bev with dichloroacetate produced synergistic effects.26 In an orthotopic model, a ketogenic diet was found to further extend the survival-prolonging effect of Bev treatment; moreover, a pilot trial with recurrent glioblastoma patients suggested that a ketogenic diet can increase the activity of Bev treatment.27 Moreover, the knockdown of some glycolytic enzymes, such as HK2, phosphofructokinase platelet, and pyruvate kinase M2 isoform could inhibit glioblastoma growth in some xenograft models.28–30 However, concerning GPI/AMF, our findings raise caution as to whether this molecule is a suitable target for interfering with glycolysis and/or for inhibiting glioblastoma cell invasion, as desired anti-invasive effects could potentially be outweighed by unintended mitogenic effects. The inconsistent response pattern seen already in our 2 model systems nevertheless allows speculation that GPI/AMF inhibition may be effective in a certain cellular context for which the determinants have yet to be defined.
Funding
This work was supported by the Anni Hofmann Stiftung (K.L.), the Forschungsförderungsfonds des Universitätsklinikums Hamburg-Eppendorf (A.K-B.), and the Rudolf Bartling Stiftung (K.L.).
Supplementary Material
Acknowledgments
We thank Svenja Zapf and Katharina Kolbe for expert technical assistance and the FACS core facility and Mouse Pathology Core Facility of the UKE for help with cell sorting and tissue embedding, respectively.
Conflict of interest statement. None.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 2. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kathagen A, Schulte A, Balcke G, et al. Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol. 2013;126(5):763–780. [DOI] [PubMed] [Google Scholar]
- 4. Kathagen-Buhmann A, Schulte A, Weller J, et al. Glycolysis and the pentose phosphate pathway are differentially associated with the dichotomous regulation of glioblastoma cell migration versus proliferation. Neuro Oncol. 2016;18(9):1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lincet H, Icard P. How do glycolytic enzymes favour cancer cell proliferation by nonmetabolic functions?Oncogene. 2015;34(29):3751–3759. [DOI] [PubMed] [Google Scholar]
- 6. Funasaka T, Raz A. The role of autocrine motility factor in tumor and tumor microenvironment. Cancer Metastasis Rev. 2007;26(3-4):725–735. [DOI] [PubMed] [Google Scholar]
- 7. Liotta LA, Mandler R, Murano G, et al. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986;83(10):3302–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fairbank M, St-Pierre P, Nabi IR. The complex biology of autocrine motility factor/phosphoglucose isomerase (AMF/PGI) and its receptor, the gp78/AMFR E3 ubiquitin ligase. Mol Biosyst. 2009;5(8):793–801. [DOI] [PubMed] [Google Scholar]
- 9. Mehta S, Huillard E, Kesari S, et al. The central nervous system-restricted transcription factor Olig2 opposes p53 responses to genotoxic damage in neural progenitors and malignant glioma. Cancer Cell. 2011;19(3):359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schulte A, Günther HS, Phillips HS, et al. A distinct subset of glioma cell lines with stem cell-like properties reflects the transcriptional phenotype of glioblastomas and overexpresses CXCR4 as therapeutic target. Glia. 2011;59(4):590–602. [DOI] [PubMed] [Google Scholar]
- 11. Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61(18):6624–6628. [PubMed] [Google Scholar]
- 12. Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beckner ME, Chen X, An J, Day BW, Pollack IF. Proteomic characterization of harvested pseudopodia with differential gel electrophoresis and specific antibodies. Lab Invest. 2005;85(3):316–327. [DOI] [PubMed] [Google Scholar]
- 14. Beckner ME, Stracke ML, Liotta LA, Schiffmann E. Glycolysis as primary energy source in tumor cell chemotaxis. J Natl Cancer Inst. 1990;82(23):1836–1840. [DOI] [PubMed] [Google Scholar]
- 15. Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39(2):453–463. [DOI] [PubMed] [Google Scholar]
- 16. Dhruv HD, McDonough Winslow WS, Armstrong B, et al. Reciprocal activation of transcription factors underlies the dichotomy between proliferation and invasion of glioma cells. PLoS One. 2013;8(8):e72134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghosh P, Beas AO, Bornheimer SJ, et al. A G{alpha}i-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Mol Biol Cell. 2010;21(13):2338–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21(8):1624–1636. [DOI] [PubMed] [Google Scholar]
- 19. Godlewski J, Bronisz A, Nowicki MO, Chiocca EA, Lawler S. MicroRNA-451: a conditional switch controlling glioma cell proliferation and migration. Cell Cycle. 2010;9(14):2742–2748. [PubMed] [Google Scholar]
- 20. Höring E, Harter PN, Seznec J, et al. The “go or grow” potential of gliomas is linked to the neuropeptide processing enzyme carboxypeptidase E and mediated by metabolic stress. Acta Neuropathol. 2012;124(1):83–97. [DOI] [PubMed] [Google Scholar]
- 21. Tanizaki Y, Sato Y, Oka H, et al. Expression of autocrine motility factor mRNA is a poor prognostic factor in high-grade astrocytoma. Pathol Int. 2006;56(9):510–515. [DOI] [PubMed] [Google Scholar]
- 22. Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Jia Y, Che Q, Zhou Q, Wang K, Wan XP. AMF/PGI-mediated tumorigenesis through MAPK-ERK signaling in endometrial carcinoma. Oncotarget. 2015;6(28):26373–26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsutsumi S, Yanagawa T, Shimura T, Kuwano H, Raz A. Autocrine motility factor signaling enhances pancreatic cancer metastasis. Clin Cancer Res. 2004;10(22):7775–7784. [DOI] [PubMed] [Google Scholar]
- 25. Fack F, Espedal H, Keunen O, et al. Bevacizumab treatment induces metabolic adaptation toward anaerobic metabolism in glioblastomas. Acta Neuropathol. 2015;129(1):115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kumar K, Wigfield S, Gee HE, et al. Dichloroacetate reverses the hypoxic adaptation to bevacizumab and enhances its antitumor effects in mouse xenografts. J Mol Med (Berl). 2013;91(6):749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rieger J, Bähr O, Maurer GD, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukherjee J, Phillips JJ, Zheng S, Wiencke J, Ronen SM, Pieper RO. Pyruvate kinase M2 expression, but not pyruvate kinase activity, is up-regulated in a grade-specific manner in human glioma. PLoS One. 2013;8(2):e57610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanzey M, Abdul Rahim SA, Oudin A, et al. Comprehensive analysis of glycolytic enzymes as therapeutic targets in the treatment of glioblastoma. PLoS One. 2015;10(5):e0123544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolf A, Agnihotri S, Micallef J, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208(2):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.