Abstract
Background
The downregulation of tropomyosin 1 (TPM1) has been observed in various tumors, but few studies have focused on the clinical significance of TPM1 in intrahepatic cholangiocarcinoma (ICC). In the present study, we investigated the prognostic significance of TPM1 in ICC.
Material/Methods
A total of 124 patients with ICC were enrolled in this study. Quantitative real-time polymerase chain reaction (qRT-RCR) was performed to examine the mRNA levels of TPM1 in ICC tissue samples and adjacent noncancerous tissue specimens, while the protein level of TPM1 in tissue specimens were investigated using immunohistochemistry assay. The correlation of TPM1 with clinicopathological features of ICC was analyzed by chi-square test. Survival analysis was performed with Kaplan-Meier method. The Cox proportional hazards model was used to evaluate the prognostic value of TPM1 in patients with ICC.
Results
TPM1 expression was significantly downregulated in ICC tissues at mRNA and protein levels (P<0.001 for both). Downregulated TPM1 mRNA was negatively associated with tumor size (P=0.001) and TNM stage (P=0.007). Moreover, survival analysis demonstrated that patients with low TPM1 expression had a shorter overall survival (OS) (P<0.001) and recurrence-free survival (RFS) (P<0.001) than those with high TPM1 expression. Additionally, multivariate analysis showed that TPM1 could be a potential biomarker for predicting the recurrence (HR=4.632, 95% CI: 3.832–10.368, P<0.001) and survival outcome (HR=5.320, 95% CI: 2.627–11.776, P<0.001) of ICC.
Conclusions
TPM1 may serve as a useful biomarker for predicting tumor recurrence and prognosis in patients with ICC.
MeSH Keywords: Biological Markers, Cholangiocarcinoma, Prognosis, Tropomyosin
Background
Cholangiocarcinoma (CCA), originating from the bile duct epithelium, is known as one of the most aggressive malignant tumors, with high risk of recurrence and metastasis [1]. CCA can be classified into 3 broad categories: intrahepatic, perihilar, and distal tumors [2]. Intrahepatic cholangiocarcinoma (ICC) accounts for around 20% to 25% of all CCA cases [3]. Although the incidence of ICC is relatively low, it has been progressively and significantly increasing over the last 30 years [4]. Surgical resection offers the only hope of cure for patients with ICC and can improve median survival compared with conservative therapy alone (1.8 months), but the outcome is still poor [5–7]. The dismal prognosis of ICC may be attributed to various reasons, including late onset of symptoms, heterogeneous tumor differentiation, aggressive infiltration, and rapid metastasis, delay in early diagnosis [8]. Therefore, identification of novel biomarkers for predicting tumor recurrence and patient outcomes is crucial to finding effective therapeutic strategies for ICC.
Tropomyosin proteins (TMs) belong to a family of highly conserved actin-binding proteins that are generated by 4 distinct genes designated as tropomyosin 1 (TPM1), TPM2, TPM3, and TPM4 [9]. As a member of the TM family, TPM1 encodes isoforms of the high molecular weight (HMW) TMs [10], which can regulate the proliferation, invasion, metastasis, and motility of tumor cells [11]. Research shows that the expression of TPM1 is downregulated in numerous carcinomas, such as breast carcinoma [12], neuroblastoma [13], and bladder cancer [14].
Previous studies have identified TPM1 as a tumor suppressor and a potential candidate biomarker for multiple malignancies. However, the clinical significance of TPM1 in ICC remains unclear. Therefore, the aim of the present study was to determine the expression of TPM1 in ICC as well as its clinical and prognostic values in ICC patients.
Material and Methods
Patients and specimens
The study was approved by the Ethics Committee of Beijing 302 Hospital, and all patients signed informed consent. From February 2010 to January 2013, 124 ICC tissue specimens and matched adjacent noncancerous tissue samples were obtained from patients who underwent curative surgery at Beijing 302 Hospital. All patients were histologically confirmed to have ICC by 2 pathologists, and none of them had received any prior treatment (chemotherapy and radiotherapy). Clinical tumor stage was determined according to the American Joint Committee on Cancer (AJCC) 7th TNM staging system. The median follow-up period for these patients was 15 months (range, 1–60 months). All the clinical information obtained is summarized in Table 1.
Table 1.
The relationship between TPM1 expression and the clinicopathological characteristics in ICC.
| Variables | N | TPM1 mRNA level | P value | |
|---|---|---|---|---|
| High | Low | |||
| Age | 0.472 | |||
| ≥60 | 63 | 35 | 28 | |
| <60 | 61 | 29 | 32 | |
| Gender | 0.281 | |||
| Male | 63 | 36 | 27 | |
| Female | 61 | 28 | 33 | |
| Tumor size | 0.001 | |||
| ≥3 cm | 81 | 33 | 48 | |
| <3cm | 43 | 31 | 12 | |
| Lymph node metastasis | 0.063 | |||
| Absent | 78 | 35 | 43 | |
| Present | 46 | 29 | 17 | |
| Differentiation | 0.470 | |||
| Well or moderate | 72 | 35 | 37 | |
| Poor | 52 | 29 | 23 | |
| TNM stage | 0.007 | |||
| III, IV | 71 | 29 | 42 | |
| I, II | 53 | 35 | 18 | |
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol (Invitrogen) reagent according to the manufacturer’s protocol. For measurement of the TPM1 transcript from total RNA, cDNA was synthesized using the PrimeScript RT Master Mix (Takara). The qRT-PCR was carried out using the Power SYBR Green PCR Master Mix based on the manufacturer’s instructions, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. The relative expression level of TPM1 normalized to GAPDH was calculated by the 2ΔΔCT method. Experiments were repeated at least 3 times.
Immunohistochemistry assay
We detected TPM1 protein expression in isolated tissue specimens using immunohistochemistry (IHC) assay. Samples were cut into 4-μm-thick sections and baked at 65°C for 1 h. Then, deparaffinization and rehydration were performed with gradient series alcohol. Next, the sections were incubated with 0.01M citric acid buffer (pH 6.0) at 98°C for 10 min and then air-dried at room temperature, after which the sections were mixed with primary antibody at 37°C for 1 h or at 4°C overnight. PBS buffer was used to wash the sections 3 times. After that, biotin-labeled secondary antibody was added to each section at 37°C for 30 min. Finally, staining signaling was conducted with DAB. The IHC results are expressed as the staining percentage of cells (0% to 100%). Staining of under 10% of the cells or no staining was considered to be negative. Staining of 10% to 20% of the cells indicated moderate immunopositivity and staining of more than 20% of cells showed strong immunopositivity. Both moderate and strong immunopositivity were classified as positive. The sections were blocked and preserved for further use.
Statistical analyses
Statistical analyses were carried out using SPSS Statistical Software version 18.0 (SPSS, Inc.) and GraphPad Prism 5.0 (GraphPad Software, Inc.). All descriptive statistical variables are presented as mean ± standard deviation (SD). The differences between variable was tested using the t test and the chi-square test was applied to measure the differences between quantitative variables. The Kaplan-Meier method with log-rank test was used to estimate survival rates and the Cox proportional hazards model for multivariate survival analysis was used to evaluate the predictive value of markers for survival and recurrence. For each analysis, a P value less than 0.05 was considered statistically significant.
Results
Decreased TPM1 expression levels in ICC
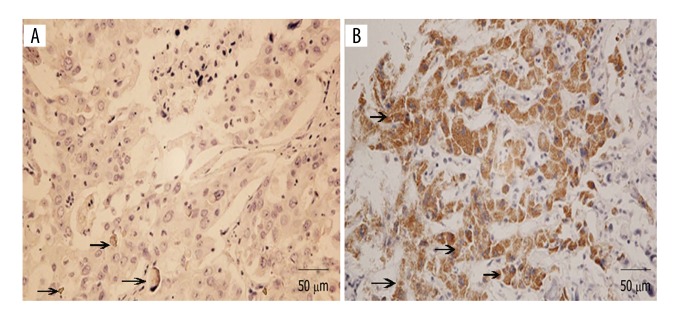
The mRNA level of TPM1 expression was assessed in 124 ICC tissue specimens and matched adjacent noncancerous tissue samples using qRT-PCR method. As shown in Figure 1, the expression of TPM1 was significantly decreased in ICC tissues compared to that in noncancerous samples (0.340±0.151 vs. 0.821±0.303, P<0.001). The results of IHC showed that the positive expression rate of TPM1 was only 16.1% in ICC tissues, but was 79.0% in the adjacent normal tissues (Table 2, Figure 2). The above results indicate that TPM1 can act as a tumor-suppressor gene in the development of ICC.
Figure 1.

Relative TPM1 mRNA expression levels detected by qRT-PCR. TPM1 expression was significantly decreased in ICC tissue samples compared to that in adjacent noncancerous tissue specimens (P<0.001).
Table 2.
Different TPM1 expression in ICC tissues and normal tissues.
| Tissue | No. | Expression | Positive rate | P value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| ICC tissues | 124 | 20 | 104 | 16.1% | P<0.001 |
| Adjacent normal tissues | 124 | 98 | 26 | 79.0% | |
Figure 2.
The expression of TPM1 protein in ICC tissue specimens detected using IHC. (A) The expression of TPM1 in ICC tissues. (B) The expression of TPM1 in adjacent noncancerous tissues. The expression of TPM1 was lower in ICC tissues than in adjacent normal tissues. Arrows represent the signal of TPM1.
Expression levels of TPM1 and clinicopathological characteristics of ICC
To test the hypothesis that TPM1 plays an important role in the progression and development of ICC, we further examined the association of TPM1 expression with its clinicopathological characteristics. As shown in Table 1, the TPM1 levels were classified into 2 groups (high and low) according to the mean value. Low expression of TPM1 was obviously correlated with tumor size (P=0.001) and TNM stage (P=0.007). However, no significant relationship was observed between TPM1 expression and age, sex, lymph node metastasis, or differentiation.
Correlation of TPM1 expression with overall survival and recurrence in ICC patients
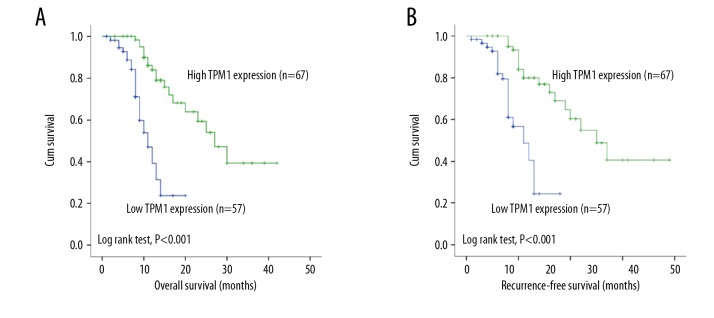
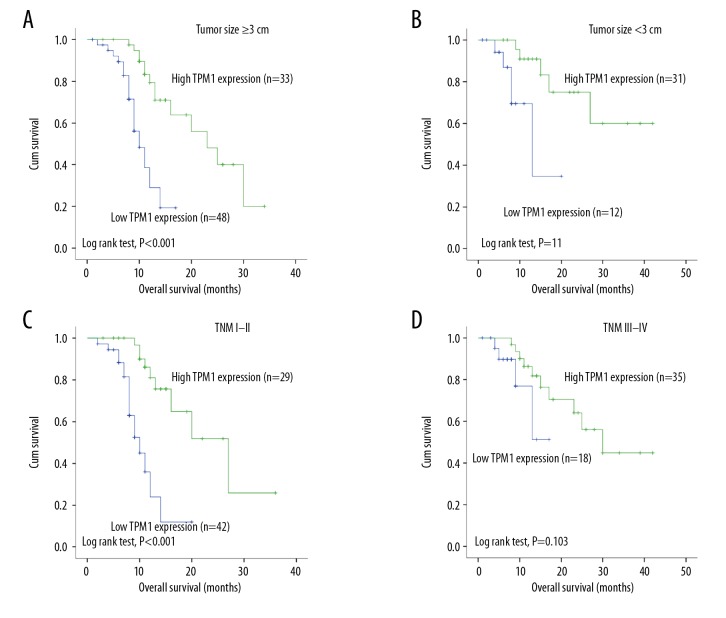
As TPM1 level was significantly decreased in patients with ICC, we assessed the association between TPM1 expression and survival situation. The mean overall survival (OS) of the 124 ICC patients was 12 months, while the mean recurrence-free survival (RFS) was 10 months. Patients with high TPM1 expression exhibited a significantly longer OS than those with low expression (P<0.001; Figure 3A). Univariate survival analysis showed various factors were associated with tumor OS, including tumor size (P=0.013), TNM stage (P=0.008), lymph node metastasis (P=0.048), and differentiation (P=0.036) (Table 3). RFS was similar to OS in that the TPM1 high-expression group showed a significantly longer RFS (P<0.001; Figure 3B). Other factors significantly related to RFS were tumor size (P=0.012) and TNM stage (P=0.003) (Table 4). Furthermore, the subgroup analyses of TPM1 expression stage revealed that patients with high TPM1 expression had better OS, not only in the tumor size ≥3 cm group (P<0.001; Figure 4A), but also in the tumor size <3 cm group (P=0.011; Figure 4B), whereas patients with high TPM1 showed a longer OS in stages III–IV (P<0.001; Figure 4C) but not in stages I and II (P=0.103; Figure 4D).
Figure 3.
Survival analysis of TPM1 in ICC. (A) Patients with high TPM1 levels had better OS than those with low TPM1 expression (P<0.001). (B) Patients with high TPM1 expression had longer RFS than those with low TPM1 expression (P<0.001).
Table 3.
Univariate and multivariate analysis of variables associated with OS in patients with ICC.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.195 (0.638–2.237) | 0.264 | – | – |
| Gender | 1.921 (1.019–3.621) | 0.084 | – | – |
| Tumor size | 2.613 (1.932–3.438) | 0.013 | – | – |
| TNM stage | 1.936 (0.841–4.754) | 0.008 | 1.521 (1.271–2.005) | 0.012 |
| Lymph node metastasis | 1.582 (1.020–3.533) | 0.048 | 1.211 (0.963–2.163) | 0.036 |
| Differentiation | 1.544 (1.288–4.360) | 0.036 | – | – |
| TPM1 expression | 5.153 (2.544–10.436) | <0.001 | 5.320 (2.627–11.776) | <0.001 |
Table 4.
Univariate and multivariate analysis of variables associated with recurrence in patients with ICC.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.266 (0.676–2.372) | 0.464 | – | – |
| Gender | 1.926 (1.021–3.632) | 0.053 | – | – |
| Tumor size | 1.919 (1.488–2.729) | 0.012 | – | – |
| TNM stage | 2.636 (1.841–5.754) | 0.003 | 1.963 (1.265–4.327) | 0.024 |
| Lymph node metastasis | 2.701 (2.020–4.344) | 0.098 | – | – |
| Differentiation | 1.570 (1.296–3.098) | 0.056 | – | – |
| TPM1 expression | 3.653 (2.252–8.746) | <0.001 | 4.632 (3.832–10.368) | <0.001 |
Figure 4.
Subgroup survival analysis of TPM1. (A, B) Patients with high TPM1 expression had a better OS, not only in the size ≥3 cm group (P<0.001), but also in the size <3 cm group (P=0.011). (C, D) Patients with high TPM1 expression had a better OS in stages III–IV (P<0.001), but not in stages I–II (P=0.103).
TPM1 as an independent prognostic factor in ICC patients
Based on multivariate analysis with the Cox proportional hazards model for the significant clinical features in univariate analysis, low TPM1 level was identified as an independent prognostic factor (HR=5.320; 95% CI: 2.627–11.776, P<0.001; Table 3) and an independent predictive factor of recurrence (HR=4.632; 95% CI 3.832–10.368, P<0.001; Table 4). Moreover, the results demonstrated that both TNM stage (P=0.012) and lymph node metastasis (P=0.036) were independent prognostic factors, and TNM stage (P=0.024) was also identified as an independent predictive factor of recurrence. Therefore, all these results suggest that TPM1 is a potential biomarker for predicting the recurrence and survival outcome of ICC.
Discussion
In the present study we observed that the expression of TPM1 was downregulated in ICC tissues compared with matched adjacent noncancerous tissues and that TPM1 expression can be an independent prognostic indicator of OS and recurrence for ICC patients.
ICC is an aggressive tumor that continues to be one of the most common causes of cancer-related mortality. There are various available treatments for patients with ICC, but the therapeutic effects are unsatisfactory [15]. To improve the survival outcome of ICC patients, it is essential to identify prognostic indicators to guide treatments and to predict disease progression in ICC. The tumorigenesis of ICC is a complex process regulated by multiple genetic and environmental factors and their interactions. With the development of molecular sequencing techniques, various studies have explored novel molecular biomarkers for management of ICC. For example, a study by Wu et al. reported that miR-122 regulates ICC cell proliferation and apoptosis via the p53 pathway, which might be a potential therapeutic target [16]. Although various molecular biomarkers have been identified for ICC, few of them had been used clinically. Thus, novel and reliable molecular biomarkers are urgently needed for ICC.
As a microfilament-associated protein, TPM1 can be abundantly expressed in different human cells, such as epithelia, fibroblasts, and smooth muscle cells [17,18]. Previous studies have found that the expression of TPM1 is decreased in many transformed cell lines and multiple carcinomas, including breast carcinoma [12,19,20], neuroblastoma cancer [13], bladder cancer [14], tongue squamous cell carcinoma [21], and high-metastatic Lewis lung carcinoma [22]. In ICC, TPM1 expression has been found to be significantly downregulated in ICC cells (HuCCT1) compared with normal intrahepatic biliary epithelial cells (HIBEC) [2]. In the present study, we found that TPM1 expression in ICC tissues was significantly decreased compared to adjacent noncancerous tissues, which was consistent with the above-mentioned study. Furthermore, the downregulation of TPM1 was negatively associated with TNM stage and tumor size. All our data revealed that TPM1 acts as a tumor suppressor, and its decreased expression might contribute to aggressive progression of ICC. An increasing number of studies have suggested that TPM1 can suppress anchorage-independent growth and restore anoikis in cancer cells, which has been generally regarded as a tumor suppressor [12,23,24]. Growing evidence has demonstrated that the decreased expression of TPM1 in carcinogenesis is regulated by microRNAs or several growth factors such as vascular endothelial cell growth factor (VEGF) and fibroblast growth factor (FGF) [24–27]. In ICC, it had been reported that downregulation of TPM1 might be regulated by DNA methylation, histone deacetylation, and upregulating of miR-21 [2]. All these studies can guide further research on the specific molecular mechanisms underlying the regulatory roles of TPM1 in ICC.
Li et al. reported that tongue squamous cell carcinoma patients with high TPM1 expression had better survival [21], and Wang et al. demonstrated TPM1 expression was associated with prognosis in patients with renal cell carcinoma (RCC) [28]. However, the potential significance of TPM1 for survival evaluation in patients with ICC remained unclear. In the present study, our results showed that patients with low TPM1 expression had shorter OS n and RFS than those with high TPM1 expression. Our results also show that the OS of patients with high TPM1 expression was significantly better in different sizes (size ≥3 cm or size <3 cm) than that of the patients with low TPM1 expression. However, according to TNM staging, patients with high TPM1 expression had better OS in stages III–IV, but not in stages I and II, suggesting that TPM1 was more sensitive in advanced stages of ICC. Both univariate and multivariate analyses indicated that low TPM1 expression increased the risk of death in ICC patients, and TPM1 could be an independent predictor for the recurrence and survival outcome of ICC. Recent evidence supports that TPM1 expression is correlated with some clinical features such as tumor size, smoking status, and tumor grade [28]. In our study, the low expression of TPM1 was found to be correlated with tumor size and TNM stage. Although we studied the clinical role of TPM1 in ICC patients, there are several limitations to our study. First, this was a single-center study with a relatively small sample size. Second, the underlying mechanism of TPM1 in ICC was not explored, and this needs further research.
Conclusions
In summary, TPM1 expression was significantly decreased in human ICC tissues, and survival analysis demonstrated that TPM1 could be a promising biomarker for predicting recurrence and OS in patients with ICC.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Farley DR, Weaver AL, Nagorney DM. “Natural history” of unresected cholangiocarcinoma: Patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70:425–29. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Wang X, Zheng W, et al. Genetic and epigenetic alterations are involved in the regulation of TPM1 in cholangiocarcinoma. Int J Oncol. 2013;42:690–98. doi: 10.3892/ijo.2012.1741. [DOI] [PubMed] [Google Scholar]
- 3.Ma KW, Cheung TT, She WH, et al. The effect of wide resection margin in patients with intrahepatic cholangiocarcinoma: A single-center experience. Medicine (Baltimore) 2016;95:e4133. doi: 10.1097/MD.0000000000004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: A true increase? J Hepatol. 2004;40:472–77. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Farges O, Fuks D. Clinical presentation and management of intrahepatic cholangiocarcinoma. Gastroenterol Clin Biol. 2010;34:191–99. doi: 10.1016/j.gcb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–25. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 7.Soares KC, Kamel I, Cosgrove DP, et al. Hilar cholangiocarcinoma: Diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014;3:18–34. doi: 10.3978/j.issn.2304-3881.2014.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao ZY, Guo XC, Su D, et al. Prognostic factors of cholangiocarcinoma after surgical resection: A retrospective study of 293 patients. Med Sci Monit. 2015;21:2375–81. doi: 10.12659/MSM.893586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JJ, Eppinga RD, Warren KS, McCrae KR. Human tropomyosin isoforms in the regulation of cytoskeleton functions. Adv Exp Med Biol. 2008;644:201–22. doi: 10.1007/978-0-387-85766-4_16. [DOI] [PubMed] [Google Scholar]
- 10.Schevzov G, Whittaker SP, Fath T, et al. Tropomyosin isoforms and reagents. Bioarchitecture. 2011;1:135–64. doi: 10.4161/bioa.1.4.17897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi C, Kim D, Kim S, et al. From skeletal muscle to cancer: Insights learned elucidating the function of tropomyosin. J Struct Biol. 2012;177:63–69. doi: 10.1016/j.jsb.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Raval GN, Bharadwaj S, Levine EA, et al. Loss of expression of tropomyosin-1, a novel class II tumor suppressor that induces anoikis, in primary breast tumors. Oncogene. 2003;22:6194–203. doi: 10.1038/sj.onc.1206719. [DOI] [PubMed] [Google Scholar]
- 13.Yager ML, Hughes JA, Lovicu FJ, et al. Functional analysis of the actin-binding protein, tropomyosin 1, in neuroblastoma. Br J Cancer. 2003;89:860–63. doi: 10.1038/sj.bjc.6601201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawlak G, McGarvey TW, Nguyen TB, et al. Alterations in tropomyosin isoform expression in human transitional cell carcinoma of the urinary bladder. Int J Cancer. 2004;110:368–73. doi: 10.1002/ijc.20151. [DOI] [PubMed] [Google Scholar]
- 15.Schicho A, Pereira PL, Putzler M, et al. Degradable starch microspheres transcatheter arterial chemoembolization (DSM-TACE) in intrahepatic cholangiocellular carcinoma (ICC): Results from a national multi-center study on safety and efficacy. Med Sci Monit. 2017;23:796–800. doi: 10.12659/MSM.902901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C, Zhang J, Cao X, et al. Effect of Mir-122 on Human Cholangiocarcinoma Proliferation, Invasion, and Apoptosis Through P53 Expression. Med Sci Monit. 2016;22:2685–90. doi: 10.12659/MSM.896404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad GL, Meissner S, Sheer DG, Cooper HL. A cDNA encoding a muscle-type tropomyosin cloned from a human epithelial cell line: Identity with human fibroblast tropomyosin TM1. Biochem Biophys Res Commun. 1991;177:1068–75. doi: 10.1016/0006-291x(91)90647-p. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger MF, Kazzaz JA, Helfman DM. Functional properties of non-muscle tropomyosin isoforms. Curr Opin Cell Biol. 1994;6:96–104. doi: 10.1016/0955-0674(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 19.Hughes JA, Cooke-Yarborough CM, Chadwick NC, et al. High-molecular-weight tropomyosins localize to the contractile rings of dividing CNS cells but are absent from malignant pediatric and adult CNS tumors. Glia. 2003;42:25–35. doi: 10.1002/glia.10174. [DOI] [PubMed] [Google Scholar]
- 20.Da Costa GG, Gomig TH, Kaviski R, et al. Comparative proteomics of tumor and paired normal breast tissue highlights potential biomarkers in breast cancer. Cancer Genomics Proteomics. 2015;12:251–61. [PubMed] [Google Scholar]
- 21.Li J, Huang H, Sun L, et al. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15:3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- 22.Takenaga K, Nakamura Y, Sakiyama S. Differential expression of a tropomyosin isoform in low- and high-metastatic Lewis lung carcinoma cells. Mol Cell Biol. 1988;8:3934–37. doi: 10.1128/mcb.8.9.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helfman DM, Flynn P, Khan P, Saeed A. Tropomyosin as a regulator of cancer cell transformation. Adv Exp Med Biol. 2008;644:124–31. doi: 10.1007/978-0-387-85766-4_10. [DOI] [PubMed] [Google Scholar]
- 24.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–36. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 25.Kieffer-Kwon P, Happel C, Uldrick TS, et al. KSHV MicroRNAs repress tropomyosin 1 and increase anchorage-independent growth and endothelial tube formation. PLoS One. 2015;10:e0135560. doi: 10.1371/journal.pone.0135560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker AH. MicroRNA 21 “shapes” vascular smooth muscle behavior through regulating tropomyosin 1. Arterioscler Thromb Vasc Biol. 2011;31:1941–42. doi: 10.1161/ATVBAHA.111.231985. [DOI] [PubMed] [Google Scholar]
- 27.Zibert JR, Lovendorf MB, Litman T, et al. MicroRNAs and potential target interactions in psoriasis. J Dermatol Sci. 2010;58:177–85. doi: 10.1016/j.jdermsci.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Guan J, Lu Z, et al. Clinical and tumor significance of tropomyosin-1 expression levels in renal cell carcinoma. Oncol Rep. 2015;33:1326–34. doi: 10.3892/or.2015.3733. [DOI] [PubMed] [Google Scholar]