Abstract
Running biomechanics and its evolution that occurs over intensive trials are widely studied, but few studies have focused on the reproducibility of stride evolution in these runs. The purpose of this investigation was to assess the reproducibility of changes in eight biomechanical variables during exhaustive runs, using three-dimensional analysis. Ten male athletes (age: 23 ± 4 years; maximal oxygen uptake: 57.5 ± 4.4 ml02·min-1·kg-1; maximal aerobic speed: 19.3 ± 0.8 km·h-1) performed a maximal treadmill test. Between 3 to 10 days later, they started a series of three time-to-exhaustion trials at 90% of the individual maximal aerobic speed, seven days apart. During these trials eight biomechanical variables were recorded over a 20-s period every 4 min until exhaustion. The evolution of a variable over a trial was represented as the slope of the linear regression of these variables over time. Reproducibility was assessed with intraclass correlation coefficients and variability was quantified as standard error of measurement. Changes in five variables (swing duration, stride frequency, step length, centre of gravity vertical and lateral amplitude) showed moderate to good reproducibility (0.48 ≤ ICC ≤ 0.72), while changes in stance duration, reactivity and foot orientation showed poor reproducibility (-0.71 ≤ ICC ≤ 0.04). Fatigue-induced changes in stride biomechanics do not follow a reproducible course across the board; however, several variables do show satisfactory stability: swing duration, stride frequency, step length and centre of gravity shift.
Key words: kinematics, 3D, treadmill, exhaustion, running, evolution
Introduction
Running is an increasingly widespread form of exercise due to the naturalness of the movements required and health benefits (Dugan and Bhat, 2005). The growing interest in this sport has been mirrored by technical improvements in research facilities and diversification of research into running biomechanics. As the number of recreational and competitive runners increases, professionals such as sports physicians, physical therapists and trainers have a key role to play in improving our understanding of the mechanisms underlying sports injuries as well as in developing performance enhancement strategies.
To this end a lot of equipment for recording temporal, spatial and angular variables has been developed in recent decades and is used for both clinical and practical purposes. These devices include force platforms, pressure sensors, electromyography, accelerometers, electrogoniometers and motion analysis systems (Higginson, 2009). Motion analysis systems are used mainly by the research community due to their cost and weight. All these tools have their pros and cons; three-dimensional (3D) motion capture is powerful and is extensively used by investigators (although the validity of the data is sometimes questionable due to skin movement artefacts) (Leardini et al., 2005; Reinschmidt et al., 1997). It is suited to assess biomechanical patterns of the normal gait (Deflandre et al., 2016; Pietraszewski et al., 2012), to investigate abnormal movements that cause injuries (Bruderer-Hofstetter et al., 2015) and to estimate the success of interventions intended to correct gait anomalies (Ferber et al., 2005) or enhance performance (Snyder et al., 2009).
There are many features that distinguish running from walking (e.g. increased velocity, float phase, decreased centre of gravity (CG) and variation in initial foot contact) (Dugan and Bhat, 2005) and this is why stride biomechanics are often described in terms of variables that are sensitive to an increase in velocity, such as stride frequency (Avogadro et al., 2003; Schache et al., 2014), step length (Cho, 2015; Hayes et al., 2014), stance and swing duration (Kivi et al., 2002; Ogueta-Alday et al., 2013) as well as running economy (Gruber et al., 2013; Lacour and Bourdin, 2015) (i.e. oxygen uptake at a given velocity; Anderson, 1996). Some patterns are known to alter running economy, for example an increase in the vertical or lateral shift in the CG during running (Dugan and Bhat, 2005; Saunders et al., 2004). Some authors focus on the foot (initial contact location and toe-off position for instance) (Delgado et al., 2013; Lieberman et al., 2010) or the knee pattern (leg stiffness or range of motion) (Abt et al., 2011; Hayes et al., 2014), whereas others take a holistic approach to the running gait. The latter group has described various patterns of stride biomechanics, but under differing conditions. Some have focused on the evolution of kinematic variables during long runs at constant speed (Hunter and Smith, 2007), whereas others have compared the evolution of variables across runs at different speeds (Dorn et al., 2012) or over the course of an accelerating run (Schache et al., 2014). Other studies have investigated elite athletes running at high intensities or until exhaustion (Fourchet et al., 2014). Interesting biomechanical changes emerge during these exhaustive runs: swing duration reduces and stance duration increases, peak vertical ground reaction force decreases and so does leg stiffness (Gazeau et al., 1997; Rabita et al., 2013). Although the evolution of these kinematic variables during an exhaustive run seems to have been widely investigated, there is a lack of knowledge about the reproducibility of the pattern of changes over repeated exhaustive runs.
This may be partly explained by the difficulty to assess the reproducibility of various types of efforts through 3D analysis, given the complexity of marker placement on the anatomical landmarks. Inter-investigator variability in marker placement is a major issue; it appears to be high and dependent on the investigator’s familiarity with the procedures (Sinclair et al., 2014). It is therefore recommended that the markers should always be placed by the same person throughout an investigation. Several other variables must also be kept constant to ensure the reproducibility of the measurements including time of day (Saunders et al., 2004), shoes (Nigg et al., 2003; Rose et al., 2011), sportswear (Zhang et al., 2002) and the treadmill slope (Jones and Doust, 1996). To determine whether a given runner’s biomechanical pattern always evolves in the same way during an exhaustive effort, it needs to be measured repeatedly under the same conditions and, to the authors’ knowledge, this kind of study has never been carried out with respect to long, exhaustive runs. We would like to fill the gap in the existing literature regarding the repetition of exhaustion-induced biomechanical events to see whether the potential adaptations of the running gait are reproducible from one run to another.
The aim of this study was, therefore, to investigate whether the evolution of the main biomechanical variables used to describe a running gait is reproducible.
Methods
Participants
Ten well-trained male runners (age: 23 ± 4 years; body mass: 69 ± 7 kg; body height: 181 ± 5 cm; maximal oxygen uptake (VO2max): 57.5 ± 4.4 ml02·min-1·kg-1; maximal aerobic speed (MAS): 19.3 ± 0.8 km·h-1) were recruited for this study. The inclusion criteria were (1) being an active, male runner training at least three times a week; (2) VO2max > 55 ml·min-1·kg-1; (3) MAS > 18 km·h-1. All participants were healthy and pain-free during the testing period. None of them reported any musculoskeletal injury or dysfunction in the lower limbs over the six weeks preceding the study. All runners participated on a voluntary basis and were fully informed about the nature of the experimental protocol. An institutional ethical committee approved the study.
Measures
We chose to monitor eight biomechanical variables which have been extensively studied and/or are sensitive to gait adjustments over time: stance duration, swing duration, reactivity, step length (Lienhard et al., 2013), stride frequency (Dorn et al., 2012), foot orientation at impact (Rooney and Derrick, 2013), and variations in vertical (Morin et al., 2006) and lateral amplitude of the CG (Table 1).
Table 1.
Biomechanical variables considered
| Variable | Units | Definition | Determination |
|---|---|---|---|
| Stance duration | Seconds | Interval between foot landing and take-off | Interval between the vertical negative velocity peak for the centre of the foot (computed as the barycentre of the big toe and heel markers) and the point of minimal big toe contact |
| Swing duration | Seconds | Take-off duration of one foot and landing of the other | Interval between minimal big toe contact with one foot and vertical negative velocity peak for the centre of the other foot |
| Reactivity | None | Ratio between swing duration and stance duration | |
| Stride frequency | Hertz | Number of strides executed in 1 s | |
| Step length | Metres | Ratio between distance covered and total number of steps | |
| Foot orientation at impact | Degrees | Angle between the vector linking the heel marker to the 5th metatarsal marker and the horizontal axis in the sagittal plane. Positive angles represent dorsiflexion | |
| Vertical amplitude of CG | Metres | Vertical motion of the CG during one whole stride | CG defined as the barycentre of the four pelvic markers |
| Lateral amplitude of CG | Metres | Lateral of the CG during one whole stride | CG defined as the barycentre of the four pelvic markers |
In every trial, all biomechanical variables were recorded over a 20 s period every 4 min. Sessions lasted until exhaustion was reached.
Design and Procedures
The experimental design consisted of four sessions: the first one was used to determine the participant’s MAS and the remaining three to assess reproducibility of the evolution of the stride at 90% of this intensity. Participants were instructed to keep their usual training pace between sessions and to avoid major competitions during the study period. The first test consisted of an incremental run to exhaustion on a calibrated treadmill (SportsArt T650, SportsArt, Taiwan) comparable to the Bruce protocol (McDonough and Bruce, 1969) to assess VO2max. Briefly, participants started exercising at a treadmill speed of 8 km·h-1. Speed was subsequently increased by 2 km·h-1 every 3 min until exhaustion was reached. Oxygen uptake (VO2), minute ventilation (VE), respiratory exchange ratio (RER) (Ergostick, Geratherm Respiratory, Germany), heart rate (Polar Belt, Polar, USA) and lactataemia (1500 Sport L-Lactate, YSI, USA) were measured. The test was stopped when the participant could not maintain the required pace or had reached voluntary exhaustion. The criteria used to assess VO2max were a RER ≥ 1.10, a heart rate in excess of 90% of the age-predicted maximum (i.e. 220 - age) and identification of a VO2max plateau (< 150 ml/min increase despite a further velocity increase). In all tests two of the three criteria were met. MAS was defined as the lowest speed that elicited VO2max and was used in the next three sessions, which were carried out 3 to 15 days later, as shown in Figure 1.
Figure 1.
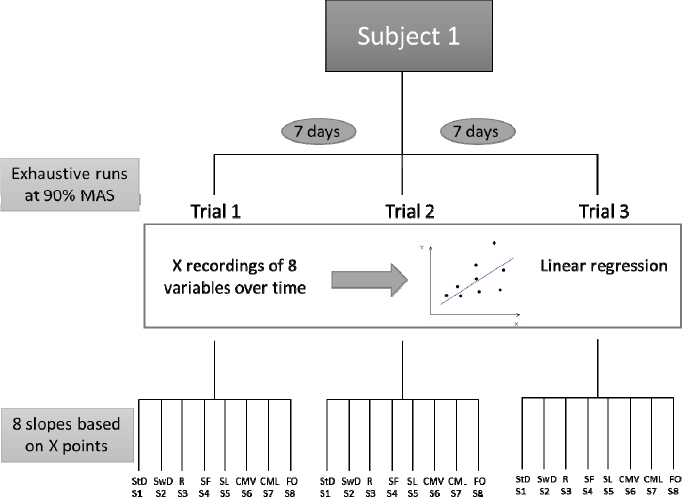
Study design
Each session consisted of running to exhaustion on an equivalent treadmill with a 1% gradient. The running speed was set at 90% of the individual’s MAS (90% MAS). Cardiac frequency was monitored with a Polar® belt. All the subjects ran in the same model of shoes (Neutral Asics shoes, Asics Corporation, Japan) wearing just their underwear. A three-dimensional optoelectronic system (CX1, Codamotion™, Charnwood Dynamics, Rothley, UK) was used to track four active markers fixed to the traditional anatomical landmarks (i.e, right and left anterosuperior iliac spine and posterosuperior iliac spine) and four attached directly to the shoe (i.e. right and left heel and great toe distal end) as described by Fellin et al. (2010) and Pohl et al. (2010); the marker locations are depicted in Figure 2. Data were acquired over 20-s periods every 4 min at a rate of 200 Hz. Markers were always attached by the same investigator, with the subject in an upright position. The three tests were performed at the same time of the day at 7-day intervals.
Figure 2.
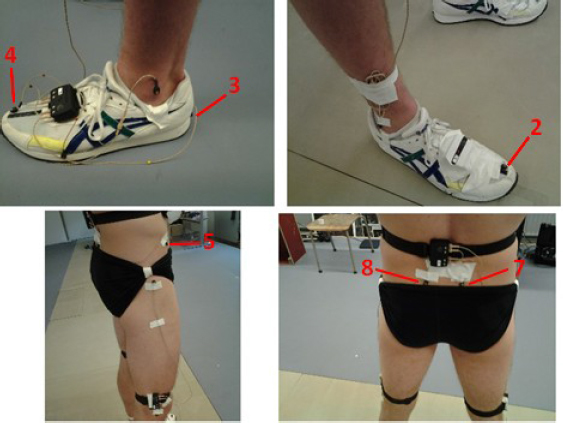
Marker placement
Statistical Analysis
Mean values were calculated for all 20-s recordings. At the end of a session, the 20-s means were averaged and their standard deviation (SD) computed. A linear regression line was fitted to the means and its slope estimated to assess any within-trial time effect. The coefficient of determination (R2) was also calculated to assess the fit of the regression. Reproducibility was assessed using the intraclass correlation coefficient (ICC – two-way random model) and its 95% confidence interval (95%CI), using SAS software (SAS© Institute Inc., Cary, NC, USA). Reliability was expressed as standard error of measurement (SEM) using ICC values Reproducibility refers to the proportion of variance attributable to the true variation (Shrout et al., 1979; Maszczyk et al, 2012), whereas variation due to measurement error was evaluated as reliability (De Vet et al., 2011; Maszczyk et al., 2014).
We assessed the reproducibility and reliability of the biomechanical variables and their time courses (slopes). We also evaluated the reproducibility of the athletes’ performance (expressed as running time and distance). We used the standard Fleiss criteria (Fleiss, 2011) to categorise reproducibility (i.e. poor: ICC < .4; moderate to good: .41 < ICC < .74; excellent ICC > .75). SEM values were interpreted in relation to the mean values of each variable; the lower the SEM, the better.
Results
Physiological Data
The first incremental exercise to exhaustion session was used to obtain physiological data for the whole sample, VO2max: 57.5 ± 4.4 mL/kg/min; MAS: 19.3 ± 0.8 km/h; lactate thresholds: 15.3 ± 2.2 and 17.6 ± 1.4 km/h; maximal lactataemia: 7.5 ± 1.3 mmol/L; maximal heart rate: 191 ± 8 bpm. Table 2 shows the 10 runners’ performance during the three runs to exhaustion at 90% MAS (17.3 ± 0.7 km/h). The ICC values suggest that both running time and distance had excellent reproducibility.
Table 2.
Mean values, standard deviations, intraclass correlation coefficients with 95% confidence intervals and standard errors of measurement for performance over the three 90% MAS trials
| Variable | Mean± SD | ICC | 95%CI | SEM |
|---|---|---|---|---|
| Running time (min) | 30 ± 10 | 0.80 | 0.58 – 0.91 | 4.5 |
| Covered distance (km) | 8.7 ± 2.9 | 0.81 | 0.59 – 0.92 | 1.3 |
Biomechanical Variables
Table 3 shows the grand averages over the three runs to exhaustion for all variables. The mean running time for the 30 runs (30 ± 10minutes) yielded 8 ± 3 recordings/session from which the slope of the regression line was estimated. Based on the ICCs all variables showed excellent reproducibility. The SEM was low for all variables except for foot orientation at impact.
Table 3.
Mean values and standard deviations for each trial, intraclass correlation coefficient with 95% confidence interval and standard error of measurement of 8 biomechanical variables over the three trials at 90% MAS
| Variable | Trial 1 | Trial 2 | Trial 3 | ICC | 95%CI | SEM |
|---|---|---|---|---|---|---|
| Stance duration (s) | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.99 | 0.99-0.99 | 0.001 |
| Swing duration (s) | 0.48 ± 0.02 | 0.48 ± 0.02 | 0.48 ± 0.01 | 0.99 | 0.99-0.99 | 0.002 |
| Reactivity | 2.13 ± 0.17 | 2.07 ± 0.11 | 2.08 ± 0.11 | 0.99 | 0.99-0.99 | 0.013 |
| Stride frequency (Hz) | 1.41 ± 0.04 | 1.40 ± 0.04 | 1.41 ± 0.05 | 0.99 | 0.99-0.99 | 0.004 |
| Step length (m) | 1.71 ± 0.08 | 1.72 ± 0.08 | 1.72 ± 0.08 | 0.99 | 0.99-0.99 | 0.008 |
| CG vertical amplitude (m) | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.99 | 0.99-0.99 | 0.001 |
| CG lateral amplitude (m) | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.99 | 0.99-0.99 | 0.002 |
| Foot orientation at impact (°) | 11.9 ± 5.0 | 13.0 ± 4.3 | 12.3 ± 3.7 | 0.99 | 0.98-0.99 | 0.425 |
Reproducibility of Evolution of Parameters
Table 4 displays, for each biomechanical variable and each trial, the means and SDs of the slopes calculated from the 10 athletes. The corresponding mean coefficients of determination (r2), ICCs (95%CI) and SEMs are also given. Five out of the eight variables evolved in a way that showed moderate to good reproducibility across the three trials (swing duration; stride frequency; step length; vertical amplitude of CG; lateral amplitude of CG), but the time courses of the remaining three (stance duration; reactivity; foot orientation at impact) had poor reproducibility. All SEMs were high relative to the corresponding mean.
Table 4.
Means and standard deviations of the slopes with mean coefficient of determination ( r2) for each trial, intraclass correlation coefficient with 95% confidence interval and standard error of measurement for eight biomechanical variables over the three trials at 90% MAS.
| Variable slope | Test 1 | Test 2 | Test 3 | r2 | ICC | 95% CI | SEM |
|---|---|---|---|---|---|---|---|
| Stance duration | 1.5 ± 0.8 | - 1.9 ± 8.7 | 0.7 ± 1 | 0.55 | -0.18 | -0.56-0.25 | 5.4 |
| (s·rec-1a·103) | |||||||
| Swing duration | 0.8 ± 4.7 | 1.3 ± 1.5 | 0.5 ± 3 | 0.38 | 0.57 | 0.2-0.8 | 2.4 |
| (s·rec-1·103) | |||||||
| Reactivity | -12 ± 23 | 13 ± 47 | -3.5 ± 27 | 0.33 | -0.71 | -0.87--0.41 | 40.0 |
| (s·rec-1·103) | |||||||
| Stride frequency | -4.5 ± 9.7 | -0.3 ± 13.3 | -2.5. ± 4.3 | 0.49 | 0.67 | 0.35-0.85 | 6.4 |
| (s·Hz-1·103) | |||||||
| Step length | 5.3 ± 11.6 | -0.1 ± 17.4 | 3.0 ± 5.2 | 0.49 | 0.65 | 0.31-0.84 | 8.4 |
| (m·rec-1·103) | |||||||
| vertical ampl· CG b | 0.4 ± 1.0 | 0.2 ± 0.9 | 0.4 ± 0.5 | 0.37 | 0.72 | 0.44-0.88 | 0.5 |
| (m·rec-1·103) | |||||||
| lateral ampl· CG | 1.1 ± 1.1 | 1.3 ± 1.5 | 0.8 ± 0.9 | 0.54 | 0.48 | 0.084-0.75 | 0.9 |
| (m·rec-1·103) | |||||||
| Foot orientation at | 27.6 ± 263 | 88.4 ± 542 | -102 ± 177 | 0.31 | 0.04 | -0.38-0.45 | 354.0 |
| impact (°·rec-1·103) |
:recordings
:centre of gravity
Discussion
The aim of this study was to investigate whether changes in the biomechanical pattern, as described by eight variables (related to foot strike, movement of CG or stride), during performance were similar from one 90% MAS trial to another in a sample of 10 regular runners. Well-trained runners are known to have a more stable running pattern than novice runners (Hopkins et al., 2001; Pollock et al., 1976) and hence are better subjects for investigations into reproducibility.
The selected intensity (90% of the individual’s MAS) had to be high enough to induce fatigue-related changes yet allow us a recording period long enough to observe biomechanical adjustments to the running gait before exhaustion was reached. Several earlier studies had shown that major biomechanical alterations tended to appear shortly before exhaustion (Derrick et al., 2002; Gazeau et al., 1997; Rabita et al., 2013). The time course of changes over higher intensity runs to exhaustion (100% of the MAS) appears to be reproducible, but this is based on assessments of a relatively small number of recordings per trial, since running times at this speed are rather short, ranging from 5 (Gazeau et al., 1997) to 7 minutes (Billat et al., 1994) and do not allow high volumes of biomechanical data to be recorded.
We chose to use 3D analysis as it provides a precise record of the runner’s kinematics (Deflandre et al., 2016) and we needed accurate data on biomechanics of the entire stride. This method of analysis requires the subject to run on a treadmill; however, running reliability should be similar in the field and on a treadmill (Hopkins et al., 2001).
Since the volume of 3D data collected depended directly on the duration of effort (which varied between participants), the main analytical challenge was to find an appropriate way of quantifying the evolution of the variables of interest. For this purpose, we drew on research by Bosquet et al. (2010) and Pincivero et al. (2001). These scientists studied the reproducibility of muscle fatigue using the slope of the linear regression of the performance on the amount of contractions to track its course. This method allows one to maximise use of data obtained between the first and last recordings. The reproducibility and reliability of the regression slopes were assessed using the ICC and SEM, in accordance with the work of the above-mentioned groups and existing sports science literature on reproducibility (Ford et al., 2007; Karamanidis et al., 2003; Lienhard et al., 2013; Schabort et al., 1998; Sinclair et al., 2014; Wilken et al., 2011).
Reproducibility of Changes in Physiological and Biomechanical Variables
Running time and distance covered showed excellent reproducibility; in other words, the participants performed similarly in their three high-intensity trials to exhaustion. In contrast the SEMs were quite high. In the case of running time the mean was 30 min and the SEM 4.5 min, meaning that only changes of at least 9 min can be attributed to factors other than random measurement error (Gouttebarge et al., 2015).
It was not the primary focus of this study, but we observed that the means for all biomechanical variables showed excellent reproducibility (all ICCs were .99), indicating that at this intensity an individual running style appears to be stable. Several authors have already demonstrated that many biomechanical variables (stance duration, swing duration, step length, vertical CG amplitude) have satisfactory reproducibility at velocities ranging from 9 to 14 km·h-1 (Diss, 2001; Karamanidis et al., 2003; Morgan et al., 1991). The average speed of our sample was 17.3 ± 0.7 km·h-1 and to our knowledge reproducibility has not been investigated at this velocity. The SEMs were small relative to the means in the case of all variables, indicating relatively little random measurement error. We can therefore assume that the position of the markers and the recording technology were both highly reliable. However, the reproducibility of a mean value only provides an estimate of where the markers are placed but lacks specificity regarding the evolution of their location with exercise.
Reproducibility of Evolution
We therefore analysed the reproducibility of the evolution of biomechanical variables during one exhaustion trial. Evolution was estimated as the slope of the recorded values of a given variable (8 ± 3 recordings per trial) over time. First, inspection of the SEMs for the slopes made it clear that not all were acceptably small relative to the mean. The worst example was the evolution of foot orientation at impact for which the mean was -0.29 and the SEM 0.351; this indicates that the reliability of these measurements was extremely poor; in effect, they lack precision. This may be partly because the raw data were converted into slopes based on linear regressions that sometimes had very small coefficients of determination in the case of time courses that were not in fact linear. Nevertheless, as explained above, we chose this method to retain as much of the recorded data as possible for analysis. Further studies of the reproducibility of temporal changes in biomechanical variables should tackle this issue.
Nonetheless our results showed that the time course of changes in the following variables had moderate to good reproducibility: swing duration, stride frequency, step length and both CG amplitudes; the reliability of vertical CG amplitude approached excellence (ICC = .72). Stance duration, reactivity (calculated on basis of stance duration) and foot orientation at impact showed poor reproducibility. The evolution of these stride-related variables thus appears to be rather steady with the significant exception of stance duration (ICC = -.18).
The sign of the slope for stance duration indicated that in all participants, stance duration increased over the course of the first and third trials (positive slope) but decreased during the second trial (negative slope), which suggests that there is high variability in the evolution of stance duration over time. This is not that surprising, since the literature on evolution of stance duration during a single session is not consistent. Some authors have reported that stance duration increases over time at high intensities, i.e. between 90% and 100% MAS (Avogadro et al., 2003; Gazeau et al., 1997; Hobara et al., 2010; Slawinski et al., 2008). Several explanations for this increase have been proposed namely that it decreases the energetic cost of running as fewer muscle fibres are recruited and that it reduces leg stiffness and the decline in muscular propulsion capacity (Fourchet et al., 2013). However, other authors have reported the opposite pattern, namely a decrease in stance duration. Borrani et al. (2003) reported that stance duration decreased over time in a 95% MAS trial, attributing this result to activation of fast twitch fibres to prevent loss of strength. Stance duration also seems to decrease over long (several hours) runs (Degache et al., 2013; Morin et al., 2011a).
The stance phase is actually the only stride phase in which the runner is able to adjust his or her running pattern. Our results suggest that the time courses of all the variables directly related to this phase (i.e. stance duration, reactivity and foot orientation at impact) have poor reproducibility, which suggests that adjustments to the gait vary considerably from one similar effort to another, perhaps partly due to use of multiple strategies to tackle fatigue onset (e.g. reduction in stride frequency (Hunter and Smith, 2007), reduction in stance duration (Degache et al., 2013) and increase in knee flexion at impact (Kellis and Liassou, 2009; Mizrahi et al., 2000)). It has previously been shown that foot location at impact varies considerably between individuals (Delgado et al., 2013; Lieberman et al., 2010); for instance, a distinction has been drawn between heel-striking and toe-striking runners (Deflandre et al., 2016; Gruber et al., 2013). Our results suggest that the evolution of impact location is also highly individually variable and does not follow the same pattern in each trial (ICC = .04; positive slopes for the first and second trials and a negative slope for the third trial).
On the other hand, the evolution of the other stride-related variables (swing duration, stride frequency and step length) showed satisfactory reproducibility (ICCs of .57, .67 and .65, respectively), indicating that the evolution of the flight phase followed a consistent pattern across trials involving similar effort. The extant evidence on changes in swing duration during a single session suggests that it depends on effort, with reports that it remains constant (Gazeau et al., 1997; Hobara et al., 2010; Slawinski et al., 2008), increases (Avogadro et al., 2003) or decreases (Fourchet et al., 2013; Morin et al., 2011b) depending on the type of effort. We found that although inter-subject variability in the evolution of swing duration was high, over three trials intra-subject variation was low.
The same applies to the evolution of stride frequency, which is generally reported to decrease over time (Borrani et al., 2003; Dutto and Smith, 2002; Hobara et al., 2010; Hunter and Smith, 2007). This gradual decrease appears to follow a similar course in similar runs. Step length is directly related to stride frequency for a given velocity, so it is not surprising that the reproducibility of their evolution appears to be similar. There is a consensus that step length is constant over both exhaustive (Fourchet et al., 2014; Derrick et al., 2002) and endurance (Degache et al., 2013; Morin et al., 2011b) runs. Based on our results (ICC = .65), we assume that this regularity is reproducible from one effort to another.
Turning to CG amplitudes, the reproducibility of the evolution of vertical amplitude of CG was close to excellent (ICC = .72) and that of lateral amplitude of CG was moderate (ICC = .48). The incremental increase in vertical amplitude over an effort (Borrani et al., 2003; Fourchet et al., 2013) is known to have a deleterious effect on running economy (Anderson, 1996). Anderson (1996) also reported that the vertical amplitude of CG was negatively related to stride frequency. Unsurprisingly the reproducibility of the evolution of both stride frequency and CM vertical amplitude was, therefore, satisfactory. The lateral amplitude of CG has been much less widely studied, but its evolution appeared to be consistent over three trials (ICC = .48).
In summary, five out of the eight biomechanical variables studied evolved in a way that showed moderate to good reproducibility, with ICCs ranging from .48 to .72 despite poor reliability indicated by relatively large SEMs. This means that the overall reproducibility of the evolution of stride biomechanics is not homogeneous, even in experienced runners. Although variables linked to the stance phase (i.e. stance duration, reactivity and foot orientation at impact) seem to evolve differently across trials, those related to the swing phase (i.e. swing duration, stride frequency and step length) and the vertical and lateral amplitude of CG appear to evolve consistently across trials; in other words, the evolution of these variables over an exhaustive trial is consistent in regular runners. The poor reproducibility of the variables linked to the stance phase suggests that this critical period when the foot is in contact with the treadmill is highly sensitive to changes regarding the biomechanical pattern. These changes may be the results of the runner’s progressive adaptation in his stride along with the apparition of fatigue and might affect running economy.
In this study the variables which evolved in the most reproducible way were vertical amplitude of the CG and stride frequency. Hence several individual strategies to adapt the stride incrementally during this stance phase could be identified in further studies.
Conclusion
Temporal evolution of the running gait appears to be consistent in well-trained runners performing an extended, high-intensity effort; nevertheless, there are intra-individual fluctuations especially with respect to stance duration and reactivity. This shows the holistic approach of running biomechanics is reductive and that each recorded variable should be considered separately.
Acknowledgements
The authors would like to thank all the runners for their participation in the study.
References
- Abt JP, Sell TC, Chu Y, Lovalekar M, Burdett RG, Lephart SM. Running kinematics and shock absorption do not change after brief exhaustive running. J Strength Cond Res. 2011;25:1479. doi: 10.1519/JSC.0b013e3181ddfcf8. –. [DOI] [PubMed] [Google Scholar]
- Anderson T. Biomechanics and running economy. Sports Medicine. 1996;22:76. doi: 10.2165/00007256-199622020-00003. –. [DOI] [PubMed] [Google Scholar]
- Avogadro P, Dolenec Belli A. Changes in mechanical work during severe exhausting running. Eur J Appl Physiol. 2003;90:165. doi: 10.1007/s00421-003-0846-y. –. [DOI] [PubMed] [Google Scholar]
- Billat V, Renoux JC, Pinoteau J, Petit B, Koralsztein JP. Reproducibility of running time to exhaustion at VO2max in subelite runners. Med Sci Sports Exerc. 1994;26:254. doi: 10.1249/00005768-199402000-00018. –. [DOI] [PubMed] [Google Scholar]
- Borrani F, Candau R, Perrey S, Millet GY, Millet GP, Rouillon JD. Does the Mechanical Work in Running Change during the VO2 Slow Component? Med Sci Sports Exerc. 2003;35:50. doi: 10.1097/00005768-200301000-00009. –. [DOI] [PubMed] [Google Scholar]
- Bosquet L, Maquet D, Forthomme B, Nowak N, Lehance C, Croisier JL. Effect of the lengthening of the protocol on the reliability of muscle fatigue indicators. Int J Sports Med. 2010;31:82. doi: 10.1055/s-0029-1243168. –. [DOI] [PubMed] [Google Scholar]
- Bruderer-Hofstetter M, Fenner V, Payne E, Zdenek K, Klima H, Wegener R. Gait deviations and compensations in pediatric patients with increased femoral torsion. J Orthop Res. 2015;33:155. doi: 10.1002/jor.22746. –. [DOI] [PubMed] [Google Scholar]
- Cho M. Effects of running in place accompanied by abdominal drawing-in on gait characteristics of healthy adults. J Phys Ther Sci. 2015;27:87. doi: 10.1589/jpts.27.87. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vet HC, Terwee CB, Mokkink LB, Knol DL. Measurement in medicine: a practical guide. Cambridge University Press. 2011.
- Deflandre D, Schwartz C, Weerts JP, Croisier JL, Bury T. A Comparison of 3D Methods for Identifying the Stance Phase in Treadmill Running for Both Rearfoot and Forefoot Runners. J Sports Science. 2016;4:124. –. [Google Scholar]
- Degache F, Guex K, Fourchet F, Morin JB, Millet GP, Tomazin K, Millet GY. Changes in running mechanics and spring-mass behaviour induced by a 5-hour hilly running bout. J Sports Sci. 2013;31:299. doi: 10.1080/02640414.2012.729136. –. [DOI] [PubMed] [Google Scholar]
- Delgado TL, Kubera-Shelton E, Robb RR, Hickman R, Wallmann HW, Dufek JS. Effects of foot strike on low back posture, shock attenuation, and comfort in running. Med Sci Sports Exerc. 2013;45:490. doi: 10.1249/MSS.0b013e3182781b2c. –. [DOI] [PubMed] [Google Scholar]
- Derrick TR, Dereu D, McLean SP. Impacts and kinematic adjustments during an exhaustive run. Med Sci Sports Exerc. 2002;34:998. doi: 10.1097/00005768-200206000-00015. –. [DOI] [PubMed] [Google Scholar]
- Diss CE. The reliability of kinetic and kinematic variables used to analyse normal running gait. Gait Posture. 2001;14:98. doi: 10.1016/s0966-6362(01)00125-4. –. [DOI] [PubMed] [Google Scholar]
- Dorn TW, Schache AG, Pandy MG. Muscular strategy shift in human running: dependence of running speed on hip and ankle muscle performance. J Exp Biol. 2012;215:1944. doi: 10.1242/jeb.064527. –. [DOI] [PubMed] [Google Scholar]
- Dugan SA, Bhat KP. Biomechanics and analysis of running gait. Phys Med Rehabil Clin N Am. 2005;16:603621. doi: 10.1016/j.pmr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Dutto DJ, Smith GA. Changes in spring-mass characteristics during treadmill running to exhaustion. Med Sci Sports Exerc. 2002;34:1324. doi: 10.1097/00005768-200208000-00014. –. [DOI] [PubMed] [Google Scholar]
- Fellin RE, Rose WC, Royer TD, Davis IS. Comparison of methods for kinematic identification of footstrike and toe-off during overground and treadmill running. J Sci Med Sport. 2010;13:646. doi: 10.1016/j.jsams.2010.03.006. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber R, Davis IM, Williams Iii DS. Effect of foot orthotics on rearfoot and tibia joint coupling patterns and variability. J Biomech. 2005;38:477. doi: 10.1016/j.jbiomech.2004.04.019. –. [DOI] [PubMed] [Google Scholar]
- Fleiss JL. Design and analysis of clinical experiments. John Wiley & Sons. 2011.
- Ford KR, Myer GD, Hewett TE. Reliability of landing 3D motion analysis: Implications for longitudinal analyses. Med Sci Sports Exerc. 2007;39:2021. doi: 10.1249/mss.0b013e318149332d. –. [DOI] [PubMed] [Google Scholar]
- Fourchet F, Girard O, Kelly L, Horobeanu C, Millet G. Changes in leg spring behaviour, plantar loading and foot mobility magnitude induced by an exhaustive treadmill run in adolescent middle-distance runners. J Sci Med Sport. 2014;18:199. doi: 10.1016/j.jsams.2014.01.007. –. [DOI] [PubMed] [Google Scholar]
- Fourchet F, Taiar R, Millet G. Influence de la fatigue sur la biomécanique de la course à pied chez les jeunes athlètes. Ann Phys Rehab Med. 2013;56:e210. [Google Scholar]
- Gazeau F, Koralsztein JP, Billat V. Biomechanical events in the time to exhaustion at maximum aerobic speed. Arch Physiol Biochem. 1997;105:583. doi: 10.1076/apab.105.6.583.3272. –. [DOI] [PubMed] [Google Scholar]
- Gouttebarge V, Wolfard R, Griek N, de Ruiter CJ, Boschman JS, van Dieën JH. Reproducibility and validity of the myotest for measuring step frequency and ground contact time in recreational runners. J Hum Kinet. 2015;45:19. doi: 10.1515/hukin-2015-0003. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AH, Umberger BR, Braun B, Hamill J. Economy and rate of carbohydrate oxidation during running with rearfoot and forefoot strike patterns. J Appl Physiol. 2013;115:194. doi: 10.1152/japplphysiol.01437.2012. –. [DOI] [PubMed] [Google Scholar]
- Hayes PR, Caplan N. Leg stiffness decreases during a run to exhaustion at the speed at VO2max. Eur J Sport Sci. 2014;14:556. doi: 10.1080/17461391.2013.876102. –. [DOI] [PubMed] [Google Scholar]
- Higginson BK. Methods of running gait analysis. Curr Sports Med Rep. 2009;8:136. doi: 10.1249/JSR.0b013e3181a6187a. –. [DOI] [PubMed] [Google Scholar]
- Hobara H, Inoue K, Gomi K, Sakamoto M, Muraoka T, Iso S, Kanosue K. Continuous change in spring-mass characteristics during a 400m sprint. J Sci Med Sport. 2010;13:256. doi: 10.1016/j.jsams.2009.02.002. –. [DOI] [PubMed] [Google Scholar]
- Hopkin W, Schabort E, Hawley J. Reliability of power in physical performance tests. Sports Med. 2001;31:211. doi: 10.2165/00007256-200131030-00005. –. [DOI] [PubMed] [Google Scholar]
- Hunter I, Smith GA. Preferred and optimal stride frequency, stiffness and economy: changes with fatigue during a 1-h high-intensity run. Eur J Appl Physiol. 2007;100:653. doi: 10.1007/s00421-007-0456-1. –. [DOI] [PubMed] [Google Scholar]
- Jones AM, Doust JH. A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J Sports Sci. 1996;14:321. doi: 10.1080/02640419608727717. –. [DOI] [PubMed] [Google Scholar]
- Karamanidis K, Arampatzis Bruggemann GP. Symmetry and reproducibility of kinematic parameters during various running techniques. Med Sci Sports Exerc. 2003;35:1009. doi: 10.1249/01.MSS.0000069337.49567.F0. –. [DOI] [PubMed] [Google Scholar]
- Kellis E, Liassou C. The effect of selective muscle fatigue on sagittal lower limb kinematics and muscle activity during level running. J Orthop Sports Phys Ther. 2009;39:210. doi: 10.2519/jospt.2009.2859. –. [DOI] [PubMed] [Google Scholar]
- Kivi DM, Maraj BK, Gervais P. A kinematic analysis of high-speed treadmill sprinting over a range of velocities. Med Sci Sports Exerc. 2002;34:662. doi: 10.1097/00005768-200204000-00016. –. [DOI] [PubMed] [Google Scholar]
- Lacour JR, Bourdin M. Factors affecting the energy cost of level running at submaximal speed. Eur J Appl Physiol. 2015;115:651. doi: 10.1007/s00421-015-3115-y. –. [DOI] [PubMed] [Google Scholar]
- Leardini A, Chiari L, Della Croce U, Cappozzo A. Human movement analysis using stereophotogrammetry: Part 3. Soft tissue artifact assessment and compensation. Gait Posture. 2005;21:212. doi: 10.1016/j.gaitpost.2004.05.002. –. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Venkadesan M, Werbel WA, Daoud AI, D'Andrea S, Davis IS, Mang'eni RO, Pitsiladis Y. Foot strike patterns and collision forces in habitually barefoot versus shod runners. Nature. 2010;463:531. doi: 10.1038/nature08723. –. [DOI] [PubMed] [Google Scholar]
- Lienhard K, Schneider D, Maffiuletti NA. Validity of the Optogait photoelectric system for the assessment of spatiotemporal gait parameters. Medl Eng Physics. 2013;35:500. doi: 10.1016/j.medengphy.2012.06.015. –. [DOI] [PubMed] [Google Scholar]
- Maszczyk A, Roczniok R, Waśkiewicz Z, Czuba M, Mikołajec K, Zajac A, Stanula A. Application of regression and neural models to predict competitive swimming performance. Percept Mot Skills. 2012;114(2):610. doi: 10.2466/05.10.PMS.114.2.610-626. –. [DOI] [PubMed] [Google Scholar]
- Maszczyk A, Gołaś A, Pietraszewski P, Roczniok R, Zając A, Stanula A. Application of Neural and Regression Models in Sports Results Prediction. Procedia - Soci Behavio Sci. 2014;117:482. –. [Google Scholar]
- McDonough JR, Bruce R. Maximal exercise testing in assessing cardiovascular function. J S Carolina Med Assoc. 1969;65:25. [PubMed] [Google Scholar]
- Mizrahi J, Verbitsky O, Isakov E, Daily D. Effect of fatigue on leg kinematics and impact acceleration in long distance running. Hum Movt Sci. 2000;19:139. –. [Google Scholar]
- Morgan DW, Martin PE, Krahenbuhl GS, Baldini FD. Variability in running economy and mechanics among trained male runners. Med Sci Sports Exerc. 1991;23:378. –. [PubMed] [Google Scholar]
- Morin JB, Jeannin T, Chevallier B, Belli A. Spring-mass model characteristics during sprint running: correlation with performance and fatigue-induced changes. Int J Sports Med. 2006;27:158. doi: 10.1055/s-2005-837569. –. [DOI] [PubMed] [Google Scholar]
- Morin JB, Samozino P, Millet GY. Changes in running kinematics, kinetics, and spring-mass behavior over a 24-h run. Med Sci Sports Exerc. 2011a;43:829. doi: 10.1249/MSS.0b013e3181fec518. –. [DOI] [PubMed] [Google Scholar]
- Morin JB, Tomazin K, Edouard P, Millet GY. Changes in running mechanics and spring-mass behavior induced by a mountain ultra-marathon race. J Biomech. 2011b;44:1104. doi: 10.1016/j.jbiomech.2011.01.028. –. [DOI] [PubMed] [Google Scholar]
- Nigg BM, Stergiou P, Cole G, Stefanyshyn D, Mündermann A, Humble N. Effect of shoe inserts on kinematics, centre of pressure, and leg joint moments during running. Med Sci Sports Exerc. 2003;35:314. doi: 10.1249/01.MSS.0000048828.02268.79. –. [DOI] [PubMed] [Google Scholar]
- Ogueta-Alday A, Morante JC, Rodriguez-Marroyo JA. Garcia-Lopez J. Validation of a new method to measure contact and flight times during treadmill running. J Strength Cond Res. 2013;27:1455. doi: 10.1519/JSC.0b013e318269f760. and. –. [DOI] [PubMed] [Google Scholar]
- Pietraszewski B, Winiarski S, Jaroszczuk S. Three-dimensional human gait pattern - reference data for normal men. Acta Bioeng Biomech. 2012;14:9. –. [PubMed] [Google Scholar]
- Pincivero DM, Gear WS, Sterner RL. Assessment of the reliability of high-intensity quadriceps femoris muscle fatigue. Med Sci Sports Exerc. 2001;33:334. doi: 10.1097/00005768-200102000-00025. –. [DOI] [PubMed] [Google Scholar]
- Pohl MB, Lloyd C, Ferber R. Can the reliability of three-dimensional running kinematics be improved using functional joint methodology? Gait Posture. 2010;32:559. doi: 10.1016/j.gaitpost.2010.07.020. –. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, Linnerud AC. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39. doi: 10.1016/s0002-8703(76)80401-2. –. [DOI] [PubMed] [Google Scholar]
- Rabita G, Couturier A, Dorel S, Hausswirth C, Le Meur Y. Changes in spring-mass behavior and muscle activity during an exhaustive run at VO2max. J Biomech. 2013;46:2011. doi: 10.1016/j.jbiomech.2013.06.011. –. [DOI] [PubMed] [Google Scholar]
- Reinschmidt C, van den Bogert AJ, Nigg BM, Lundberg A, Murphy N. Effect of skin movement on the analysis of skeletal knee joint motion during running. J Biomech. 1997;30:729. doi: 10.1016/s0021-9290(97)00001-8. –. [DOI] [PubMed] [Google Scholar]
- Rooney BD, Derrick TR. Joint contact loading in forefoot and rearfoot strike patterns during running. J Biomech. 2013;46:2201. doi: 10.1016/j.jbiomech.2013.06.022. –. [DOI] [PubMed] [Google Scholar]
- Rose A, Birch I, Kuisma R. Effect of motion control running shoes compared with neutral shoes on tibial rotation during running. Physiother. 2011;97:250. doi: 10.1016/j.physio.2010.08.013. –. [DOI] [PubMed] [Google Scholar]
- Saunders PU, Pyne DB, Telford RD, Hawley JA. Factors affecting running economy in trained distance runners. Sports Med. 2004;34:465. doi: 10.2165/00007256-200434070-00005. –. [DOI] [PubMed] [Google Scholar]
- Schabort EJ, Hopkins WG, Hawley JA. Reproducibility of self-paced treadmill performance of trained endurance runners. Int J Sports Med. 1998;19:48. doi: 10.1055/s-2007-971879. –. [DOI] [PubMed] [Google Scholar]
- Schache AG, Dorn TW, Williams GP, Brown NA, Pandy MG. Lower-limb muscular strategies for increasing running speed. J Orthop Sports Phys Ther. 2014;44:813. doi: 10.2519/jospt.2014.5433. –. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psy Bull. 1979;86:420. doi: 10.1037//0033-2909.86.2.420. –. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Hebron J, Taylor PJ. The influence of tester experience on the reliability of 3D kinematic information during running. Gait Posture. 2014;40:707. doi: 10.1016/j.gaitpost.2014.06.004. –. [DOI] [PubMed] [Google Scholar]
- Slawinski J, Heubert R, Quievre J, Billat V, Hanon C. Changes in spring-mass model parameters and energy cost during track running to exhaustion. J Strength Cond Res. 2008;22:930. doi: 10.1519/JSC.0b013e31816a4475. –. [DOI] [PubMed] [Google Scholar]
- Snyder KR, Earl JE, O’Connor KM, Ebersole KT. Resistance training is accompanied by increases in hip strength and changes in lower extremity biomechanics during running. Clin Biomech. 2009;24:26. doi: 10.1016/j.clinbiomech.2008.09.009. –. [DOI] [PubMed] [Google Scholar]
- Wilken JM, Rodriguez KM, Brawner M, Darter BJ. Reliability and minimal detectible change values for gait kinematics and kinetics in healthy adults. Gait Posture. 2011;35:301. doi: 10.1016/j.gaitpost.2011.09.105. –. [DOI] [PubMed] [Google Scholar]
- Zhang P, Gong R, Yanai Y, Tokura. Effects of clothing material on thermoregulatory responses. Textile Res J. 2002;72:83. –. [Google Scholar]


