Abstract
A randomized, double-blind, placebo-controlled study was conducted, in order to evaluate if Lactobacillus helveticus Lafti® L10 (Lallemand Health Solutions, Montreal, Canada) supplementation during three months could influence oxidative markers in the population of elite athletes: triathletes, cyclists and endurance athletes. Twenty-two elite athletes were randomized to either placebo (n = 12) or probiotic (n = 10) groups. The probiotic group received 2x1010 colony forming units of Lafti® L10. Before and after the supplementation serum samples were collected. Markers of oxidative stress and anti-oxidative defense: superoxide dismutase (SOD), paraoxonase (PON), advanced oxidation protein products (AOPP), malondialdehyde (MDA), total antioxidant status, total oxidant status, pro-oxidant-antioxidant balance, oxidative stress index, bilirubin, uric acid and albumin were determined in serum. Parameters of lipid status, as well as susceptibility to copper-induced oxidation of LDL particles in vitro were also determined. There was a significant interaction effect for MDA (p = 0.039), with a decrease in MDA in the probiotic group only (p = 0.049). There was a significant interaction effect for AOPP (p = 0.037), with a significant decrease in the probiotic group (p = 0.045). Interaction effect for SOD was approaching to formal significance (p = 0.108) and the post-hoc test showed a significant decrease in the probiotic group (p = 0.041) only. A significant correlation between AOPP and SOD (p = 0.012, r = -0.40) was found in the probiotic group at the end of the study. PON1 activity was decreased in both the probiotic (p = 0.032) and placebo group (p = 0.035). No significant changes in the remainder of the evaluated parameters were noted. In conclusion, probiotic strain Lafti® L10 exerts certain antioxidant potential, but further research is needed.
Key words: exercise, Lactobacillus, antioxidants, endurance athletes
Introduction
Strenuous exercise leads to increased generation of free radicals which can cause oxidative damage to proteins, lipids and nucleic acids and consequently result in a detrimental impact on individual’s health (Teixeira et al., 2009). A growing number of chronic conditions, such as neurodegenerative and cardiovascular diseases and cancer may be linked to overproduction of free radicals (Peternelj and Coombes, 2011). In order to minimize the deleterious impact of oxidative stress, enzymatic and non-enzymatic antioxidants are strategically located in both intracellular and extracellular compartments (Teixeira et al., 2009). Athletes are reported to have a strong antioxidant defense system since repeated bouts of exercise result in increased amounts of free radicals which upregulate antioxidant enzyme expression (Peternelj and Coombes, 2011; Teixeira et al., 2009; Czuba et al., 2014). However, excessive and prolonged exercise can augment athletes’ susceptibility to exercise-induced oxidative damage (Teixeira et al., 2009). Overtraining syndrome, a condition characterized by an unanticipated decline in performance, is concomitant with increased levels of oxidative stress biomarkers (Margaritelis et al., 2016). To circumvent this antioxidant supplementation has been reported to support endogenous defense by reducing free radical production (Peternelj and Coombes, 2011). Despite being popular in athletes, antioxidant supplementation has become controversial in recent years, as huge dispute exists regarding whether it provides more benefit or harm if used during intensive physical training as it could suppress training-induced up-regulation of antioxidant enzyme expression (Peternelj and Coombes, 2011). Keeping this in mind, antioxidant supplementation should be restricted only to individuals with high levels of oxidative stress and an impaired antioxidant defense system (Margaritelis et al., 2016).
Probiotics are appealing nutraceuticals with promising applications in various aspects of human health. They are widely used in different populations to improve diverse health conditions such as allergies, eczema, food intolerance, respiratory and gastrointestinal infections (Mishra et al., 2015). Probiotic supplements have gained interest as potential antioxidant agents due to stimulated production of a variety of bioactive peptides, anti-oxidative enzyme upregulation and re-establishment of gut flora (Mishra et al., 2015).
To the best of our current knowledge, there are only 3 studies examining antioxidant potential of probiotics in athletes. Supplementation with L. rhamnosus and L. paracasei over 4 weeks increased plasma antioxidant levels (Martarelli et al., 2011), while multi-species probiotics exerted a beneficial effect on exercise-induced protein oxidation (Lamprecht et al., 2012). These results provided the first evidence of probiotics support for endogenous antioxidant defense against exercise-induced over-production of free radicals. On the other hand, LGG supplementation did not affect oxidized-LDL particles, serum antioxidant potential (s-TRAP) or serum antioxidants levels in runners during a 3-month period (Valimaki et al., 2012). Taken into account the significance of enhanced oxidative stress during strenuous exercise, it would be useful to ensure new proofs of antioxidant properties of probiotics. Accordingly, we chose to test the effects of L. helveticus Lafti® L10 supplementation on the oxidative status markers, along with the biochemical profile, in a cohort of elite athletes. The probiotic strain Lactobacillus helveticus Lafti® L10 was previously reported to have inherent immunological enhancing properties. Namely, supplementation with L. helveticus Lafti® L10 reduced the duration of respiratory infection, increased the CD4+/CD8+ (T helper/T suppressor) cell ratio and enhanced mucosal and humoral immunity in a cohort of elite athletes (Michalickova et al., 2016, 2017).
Methods
Experimental design
A randomized, double-blind, placebo-controlled parallel-groups study was conducted following the guidelines laid down in the Declaration of Helsinki. The trial procedure was approved by the ethics committee of SMAS (Sport Medicine Association of Serbia). Written consent from the participants was obtained before initiating the study.
Athletes were randomly allocated to one of the two groups. The experimental group received daily L. helveticus Lafti® L10 (2 x 1010 CFU) capsules for 14 weeks, while the control group received placebo capsules. Placebo and probiotic capsules were identical in taste and appearance (they were composed of hydroxypropylmethylcellulose and covered by titanium dioxide). The placebo capsules contained 1% magnesium stearate and 99% maltodextrin and the probiotic capsules contained 72.2% of bacterial mass, 26.7% maltodextrin and 1% magnesium stearate. Both probiotic and placebo capsules were kept in the fridge (2°C to 8°C).
Both athletes and the study team were blinded to the intervention until the statistical analyses were finished. Prior to the onset of the study athletes kept food diaries for three days and were required to hold a steady training regimen and diet during the study. Estimation of nutrient and energy intake was evaluated by original nutritional software made by SMAS (based on EuroFIR food database for Serbia). Athletes were asked not only to avoid dietary supplements (antioxidants, multivitamins and multiminerals) but also fermented milk products.
Participants
A total of 22 elite athletes finished the study: males, aged 20-24 years, non-smokers and with training volume of >11 hours/week (considered to be a high training load (Gleeson et al., 2013)). Exclusion criteria were as follows: sensitivity to the ingredients of probiotics, use of probiotics and antibiotics a month before the beginning of the study, recent surgical intervention and presence of chronic diseases (diabetes mellitus, rheumatoid arthritis, neurological, renal, pulmonary, psychiatric diseases).
Athletes included in the study were training: triathlon, cycling as well as middle and long distance running. They had 5 to 7 training sessions per week with an average weekly training volume of 11 to 15 hours. The participants in this study were the winners of National or European and World Championships in their categories and sport. There were no differences in the physical and anthropometric characteristics of the participants (Table 1), as well as in nutrient intake (Table 2) between the groups.
Table 1.
Physical and anthropometric characteristics of the participants
| Probiotic | Placebo | p | |
|---|---|---|---|
| Number | 10 | 12 | |
| Age (years) | 22 ± 3.4 | 21 ± 1.5 | 0.732 |
| Body height (cm) | 178 ± 10 | 179 ± 12 | 0.545 |
| Body mass (kg) | 75 ± 11 | 77 ± 8 | 0.412 |
| FAT % | 12 ± 2 | 11 ± 5 | 0.351 |
| BMI | 23 ± 1.2 | 22 ± 0.5 | 0.820 |
| Training loads (MET-hr/week) | 94 ± 53 | 98 ± 51 | 0.903 |
| VO2max | 65 ± 12 | 67 ± 14 | 0.235 |
BMI, Body-Mass Index. Results are expressed as means ± standard deviations. Significance was considered for p < 0.05. Results are derived from the unpaired T-test.
Table 2.
Estimation of energy and nutrient intake among the participants during the study
| Nutrient | Probiotic | Placebo | p |
|---|---|---|---|
| Energy intake (kcal) | 2076 ± 988 | 2029 ± 636 | 0.912 |
| Proteins (% EI) | 18 ± 6 | 19 ± 7 | 0.784 |
| Carbohydrates (% EI) | 49 ± 25 | 47 ± 16 | 0.655 |
| Lipids (% EI) | 30 ± 14 | 31 ± 12 | 0.458 |
| Cholesterol (mg) | 372 ± 149 | 341 ± 91 | 0.348 |
| SFA (% EI) | 11 ± 5 | 10 ± 5 | 0.369 |
| MFA (% EI) | 10 ± 5 | 10 ± 5 | 0.874 |
| PUFA (% EI) | 9 ± 5 | 10 ± 4 | 0.545 |
| Dietary fibers (g) | 16 ± 10 | 15 ± 7 | 0.889 |
| Vitamin A (μg) | 845 ± 777 | 862 ± 550 | 0.413 |
| Vitamin C (mg) | 70 ± 26 | 77 ± 55 | 0.771 |
| Vitamin E (mg) | 9.85 ± 2.20 | 10.0 ± 5.2 | 0.124 |
| Chromium (μg) | 30 ± 19 | 35 ± 25 | 0.670 |
| Selenium (μg) | 50 ± 56 | 55 ± 34 | 0.657 |
| Zinc (mg) | 10.3 ± 3.23 | 9.69 ± 6.07 | 0.836 |
EI-energy intake, SFA- saturated fatty acids, MFA-mono-saturated fatty acids, PUFA-poly-saturated fatty acids Results are expressed as means ± standard deviations. Significance was considered for p < 0.05. Results are derived from the unpaired T-test.
Sample collection
Twelve-hour fasting blood samples (10 mL) were taken from the antecubital vein and collected in serum sample tubes (Vacutainer, Becton Dickinson, USA). All the samples were collected twice: before and after the study and at the same time (between 09:30 and 10:30 am), in order to avoid diurnal changes. Serum was separated by centrifugation (1500xg, 15 min) and stored frozen at -20°C until analysis.
Evaluation of oxidative stress status
We assessed several pro-oxidative and anti-oxidative parameters, as specific markers of free radical damaging activity. As markers of oxidative stress we measured total oxidative status (TOS), pro-oxidative-anti-oxidative balance (PAB), malondialdehyde (MDA) and advanced oxidation protein products (AOPP). As markers of anti-oxidative status, total anti-oxidative status (TAS), serum paraoxonase (PON1) and superoxide dismutase activity (SOD) were estimated (Kotur-Stevuljevic et al., 2008). The predictor of antioxidant/prooxidant balance, oxidative stress index (OSI), was calculated as the ratio between TOS and TAS. The LDL fraction was isolated by precipitating reagents (Biosystem Chol - LDL kit, code 11579), incubated at 4°C for 30 min and centrifuged for 5 min at 4000 rpm. The supernatant was discarded and the precipitate was dissolved in 0.1% Triton X-100/NaCl. This fraction was exposed to Cu2+-induced oxidation, thereafter electrophoretic mobility of LDLs and MDA level were measured.
The MDA level was determined by the thiobarbituric acid-reactive substances (TBARS) assay spectrophotometrically as previously described by Girotti et al. (1991). The AOPP level was determined according to the Witko-Sarsat method, using a reaction with glacial acetic acid and potassium iodide (Witko-Sarsat et al., 1996). Total oxidative status (TOS) was determined by a spectrophotometric method optimized by Erel (2005). Prooxidant-antioxidant balance (PAB) was determined according to a previously published method (Alamdari et al., 2007). Serum SOD activity was assessed by a slightly modified method of Misra and Fridovich (1972). Total antioxidative status (TAS) was determined by a spectrophotometric method using 10 mmol/L ABTS as a chromogen (Erel, 2004). Serum PON1 activity was measured kinetically using paraoxon as substrate (Chem Service, Pennsylvania, USA), by method of Richter and Furlong (1999). All spectrophotometric methods which do not include precipitation and centrifugation steps were slightly modified in order to be compatible with the Ilab 300 plus auto-analyzer (Instrumentation Laboratory, Milan, Italy). Chemicals were purchased from Sigma-Aldrich (Munich, Germany).
Cu2+-induced oxidation of LDL particles
Susceptibility to oxidation was assessed as previously described in detail (Scoccia et al., 2001). REM was defined as the ratio of the distances migrated from the origin by oxidized LDL vs. native LDL.
Biochemical analyses
Frozen serum samples were sent to a certified human diagnostics laboratory (Laboratorija Beograd, Belgrade, Serbia) for the quantification of total cholesterol (TC), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), triglycerides (TGC), glucose, uric acid, bilirubin and albumin.
Physical activity and training loads
Athletes were obliged to report their training loads weekly by filling in a standard short form (International Physical Activity Questionnaire, IPAQ). Training loads in metabolic equivalents (MET)-hour/week were calculated on the basis of completed questionnaires (Ainsworth et al., 2011).
Statistical analyses
All statistical analyses were performed with SPSS software (SPSS v18.0; SPSS Inc., Chicago, IL, USA). The normality of the data was checked using the Shapiro-Wilk test. A mixed (between-within subjects) analysis of variance was used to determine the main effects, as well as interaction effect between the two independent variables of time (baseline and after 14 weeks; within subjects factor) and group (placebo and probiotic; between subjects factors). A T test with Bonferroni correction was applied for any significant main effect or interaction effect; p < 0.05 was considered significant. The strength of the correlation between two investigated parameters was expressed by the Pearson’s and Spearman’s correlation coefficients. The results for normally distributed variables data are expressed as mean values and standard deviation (SD) or mean and standard error. When distribution was not normal, medians and 95% confidence intervals (CI) were provided.
Results
Biochemical variables
All the biochemical biomarkers were within the reference range (Table 3). There were no significant interaction effects for any of biochemical parameters.
Table 3.
Biochemical profile of the athletes at baseline and at the end of the study (after 14 weeks)
| Treatment | time | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 14 weeks | Reference values | TxG | T | G | ||
| probiotic | |||||||
| TC | 4.3 ± 0.60 | 4.0 ± 0.61 | ˂ 5.2 | 0.172 | 0.992 | 0.045a | |
| (mmol/L) | placebo | 4.6 ± 0.52 | 4.8 ± 0.62 | ||||
| probiotic | |||||||
| HDL-C | 1.2 ± 0.25 | 1.5 ± 0.33 | 0.011a | ||||
| ˃1.17 | 0.263 | 0.711 | |||||
| (mmol/L) | placebo | 1.2 ± 0.26 | 1.4 ± 0.34 | b | |||
| probiotic | |||||||
| LDL-C | 2.8 ± 0.63 | 2.2 ± 0.57 | ˂3.3 | 0.262 | 0.004 | 0.168 | |
| (mmol/L) | placebo | 3.0 ± 0.49 | 2.7 ± 0.85 | c | |||
| probiotic | |||||||
| TGC (mmol/L) | placebo | 0.63 ± 0.19 | 0.92 ± 0.37 | ˂1.7 | 0.438 | 0.037 d | 0.163 |
| 0.77 ± 0.31 | 1.4 ± 1.2 | ||||||
| probiotic | |||||||
| 4.4 ± 0.54 | 4.4 ± 0.29 | ||||||
| Glucose | |||||||
| 3.9–5.5 | 0.195 | 0.063 | 0.632 | ||||
| (mmol/L) | placebo | ||||||
| 4.3 ± 0.42 | 4.7 ± 0.68 | ||||||
| probiotic | 4.1 (3.5- | 9.0 (5.5-17) | |||||
| Bilirubin | 8.1) | 0.031 | |||||
| <26 | 0.927 | 0.749 | |||||
| (μmol/L) | placebo | 5.4 (3.5- | 11 (5.9-18) | e | |||
| 10) | |||||||
| probiotic | 239 ± 46 | 235 ± 84 | |||||
| Uric acid | 180-420 | 0.867 | 0.606 | 0.818 | |||
| (μmol/L) | placebo | 268 ± 66 | 259 ± 74 | ||||
| probiotic | 41 ± 3.01 | 42 ± 3.05 | |||||
| Albumin | |||||||
| 35-50 | 0.764 | 0.230 | 0.140 | ||||
| (g/L) | placebo | 43 ± 2.84 | 42 ± 1.49 |
TC-total cholesterol, HDL-C-high density lipoprotein cholesterol, LDL-low density lipoprotein cholesterol, TGC- triglycerides, T-time effect, TxG-interaction effect, G-group effect Results are expressed as mean ± standard deviation. Significance was considered for p < 0.05. Results are derived from mixed ANOVA analyses. A t test with Bonferroni correction was applied for any significant main effect or interaction effect.
a probiotic vs. placebo: baseline: p = 0.219, 14 weeks: p = 0.024;
probiotic vs. placebo: baseline: p = 0.219, 14 weeks: p = 0.024;
pre/post:probiotic: p = 0.008, placebo: p = 0.261;
c pre/post:probiotic: p = 0.004, placebo: p = 0.159;
d pre/post:probiotic: p = 0.283, placebo: p = 0.056;
e pre/post: probiotic: p = 0.084, placebo: p = 0.148
Parameters of oxidative damage and biomarkers of antioxidant defense in serum
There was a significant interaction effect for MDA (p = 0.039). The post-hoc comparison showed a decrease in MDA in the probiotic group only (p = 0.049). There was also a significant interaction effect for AOPP (p = 0.037), with a significant decrease in the probiotic group (p = 0.045). Interaction effect for SOD was approaching to formal significance (p = 0.108) and the post-hoc test showed a significant decrease in the probiotic group (p = 0.041), but not in the placebo group. PON1 activity was decreased (time effect: p = 0.012) in both the probiotic (p = 0.032) and placebo group (p = 0.035). There were no observable time, group and interaction effects for REM, TAS, TOS, PAB and OSI (Table 4, Figure 1).
Table 4.
Variables of oxidative damage and biomarkers of anti-oxidative defense
| Time | ANOVA | |||||
|---|---|---|---|---|---|---|
| Variables | Group | Baseline | 14 weeks | TxG | T | G |
| probiotic | 2.6 ± 0.34 | 1.9 ± 0.65 | ||||
| MDA (μmol/L) | placebo | 2.5 ± 0.57 | 2.4 ± 0.69 | 0.039a | 0.301 | 0.714 |
| MDA in oxLDL | probiotic | 3.5 (3.4-3.7) | 3.5 (3.3-3.8) | |||
| 0.229 | 0.336 | 0.883 | ||||
| (μmol/L) | placebo | 3.5 (3.4-3.6) | 3.4 (3.3-3.6) | |||
| probiotic | 16 ± 9.1 | 11 ± 3.2 | ||||
| TOS | ||||||
| 0.738 | 0.080 | 0.103 | ||||
| (μmol/L) | placebo | 14 ± 4.5 | 15 ± 7.5 | |||
| probiotic | 705 ± 186 | 807 ± 175 | ||||
| TAS | ||||||
| 0.840 | 0.065 | 0.578 | ||||
| (μmol/L) | placebo | 616 ± 176 | 702 ± 166 | |||
| probiotic | 40 ± 10 | 26 ± 11 | ||||
| AOPP | ||||||
| 0.037b | 0.102 | 0.325 | ||||
| (μmol/L) | placebo | 38 ± 9.1 | 46 ± 5.6 | |||
| probiotic | 301 (243-523) | 183 (157-343) | ||||
| PON | ||||||
| (U/L) | placebo | 381 (361-681) | 190 (150-370) | 0.660 | 0.012c | 0.594 |
| probiotic | 30 (19-41) | 43 (37-49) | ||||
| SOD | 0.108d | 0.065 | 0.102 | |||
| (U/L) | placebo | 35 (27-57) | 30 (23-37) | |||
| probiotic | 96 (70-112) | 79 (58-95) | ||||
| PAB | 0.208 | 0.235 | 0.094 | |||
| (U/L) | placebo | 95 (75-118) | 89 (80-96) | |||
| probiotic | 0.028 ± 0.002 | 0.016 ± 0.003 | ||||
| OSI | 0.145 | 0.098 | 0.512 | |||
| placebo | 0.025 ± 0.002 | 0.017 ± 0.002 |
T-time effect, TxG-interaction effect, G-group effect The results are expressed as mean + standard deviation or median (95%CI). Significance was considered for p < 0.05. Results are derived from mixed ANOVA analyses. t test with Bonferroni correction was applied for any significant main effect or interaction effect.
apre/post: probiotic: p = 0.049, placebo: p = 0.834;
bpre/post: probiotic: p = 0.045, placebo: p = 0.055
cpre/post: probiotic: p = 0.032, placebo: p = 0.035
dpre/post: probiotic: p = 0.041, placebo: p = 0.198
Figure 1.
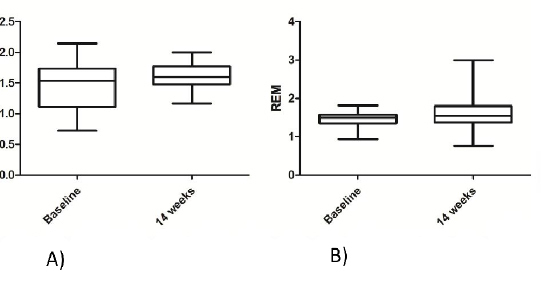
REM in: A) the probiotic group B) the placebo group Results are expressed as mean ± standard deviation
Correlations
There were significant correlations between: uric acid and AOPP (p = 0.049, r = -0.35) in the placebo group at the end of the study, as well as AOPP and SOD (p = 0.012, r = -0.40) in the probiotic group at the end of the study.
Discussion
The most important result of the present study is the attenuation of the markers of oxidative stress MDA and AOPP in the probiotic group, suggesting antioxidant potential of probiotic Lafti®. Probiotics have been reported to decrease oxidative stress by reinforcement of antioxidant defense systems, production of antioxidant biopeptides and reestablishment of gut microbiota (Mishra et al., 2015). These antioxidative properties were shown also in human studies, mostly in subjects with metabolic disorders (Ejtahed et al., 2012) and mental diseases, such as depression (Akkasheh et al., 2016). However, to the best of our knowledge, there are only two clinical studies indicating probiotic antioxidant potential in athletes (Lamprecht et al., 2012; Martarelli et al., 2011). Therefore, this trail contributes to the growing body of evidence of the probiotic antioxidant potential. Moreover, we found an increasing trend in SOD activity in the probiotic group. We suppose that upregulation of SOD contributed to the decrease in MDA and AOPP in the probiotic group. Upregulation of the activity of antioxidant enzymes, such as SOD, catalase and glutathione reductase by probiotic supplementation was previously reported (Ejtahed et al., 2012). Therefore, future studies should examine the effect of Lafti® on other antioxidant enzymes.
We also investigated the effects of Lafti® on the activity of PON1, an esterase with antioxidant properties located on the surface of HDL particles. PON1 prevents lipid peroxide accumulation on LDL, hydrolyses lipid peroxides in oxidized LDL particles and consequently hinders the formation of foam cells (Kullisaar et al., 2003). We found a substantial decrease of PON1 activity in both groups. This result contradicts most of the findings in other studies that demonstrated enhanced (Senti et al., 2003) or no effect on PON1 activity after intense physical activity (Tomas et al., 2002). It seems that the PON1’s ability to resist constantly repeated bouts of ROS is dependent on training, as approximately 8 to 10 years of training permitted PON1 activity to reach its maximum (Martinovic et al., 2009). We can speculate that the cohort of athletes in the present study did not achieve yet the full capacity of PON1 adaptation to exercise-induced oxidative stress.
Although the PON1 activity was decreased, the susceptibility of LDL particles to oxidation did not change along the study in both groups. Oxidized LDL particles constitute a recognized risk factor for atherosclerosis development, due to an ability to directly damage the endothelial cells, facilitate conversion of monocytes to macrophages and macrophages to foam cells (Kullisaar et al., 2003). The results of studies regarding probiotic ability to affect oxidized LDL levels are however not unequivocal and conclusive. Probiotic L. fermentum, for instance, exerted an anti-atherogenic character, since it decreased the amount of oxidized LDL in the subjects’ blood, and increased oxidation resistance of lipoprotein fraction (Kullisaar et al., 2003). On the other hand, our results are in accordance with another study, where L. rhamnosus GG supplementation did not affect the level of oxidized LDL particles in recreational marathon runners (Valimaki et al., 2012).
Given the physical relationship of PON1 with HDL particles and the existence of cholesterol pathway regulatory elements at the PON1 locus, which indicate an additional connection between PON1 and lipoproteins (Kotur-Stevuljevic et al., 2008), we evaluated the variables of the lipid profile. Some variables (HDL-C, LDL-C, TC) seemed to be altered by time, yet regardless of treatment. In other words, the results of ANOVA did not indicate any significant interaction effect for any marker of lipid status. In fact, these results could have been expected, since the study participants were young and physically active healthy individuals, with lipid profile parameters within the normal reference range at both measurement points. Interestingly, we did not find a significant correlation between the HDL-C level and PON1 activity, probably because only discrete subpopulation of HDL particles is connected to PON1 (James and Deakin, 2004).
Levels of different oxidant species in serum are usually measured separately or as TOS due to the additive nature of oxidant effects of different molecules (Erel, 2004). We noted only non-significantly attenuated TOS values in the probiotic group. Another study evaluating probiotic effects in athletes found similar results (Lamprecht et al., 2012). In line with this, PAB and OSI, variables which provide a more detailed picture of the balance between pro-oxidants and antioxidants in the samples, were also not significantly changed.
Non-enzymatic antioxidant levels, measured as TAS, can be modified by aerobic exercise, but results are contradictory and usually depend on blood sampling time. Previous studies evaluating effects of antioxidant supplementation found no effects on TAS at rest (Teixeira et al., 2009; Valimaki et al., 2012). In the present study, the TAS level in serum was non-significantly increased in both groups. Total antioxidant capacity usually elevates in response to acute strenuous exercise, in order to maintain the antioxidant status and to protect the body against ROS (Andersson et al., 2010; Teixeira et al., 2009). This augmentation is due to the recruitment of different antioxidants from the tissues into the serum or significantly augmented UA synthesis following enhanced activation of xanthine oxidase (Andersson et al., 2010). We observed no differences in the levels of UA along the study, since the blood samples in this study were collected at rest. Namely, it seems that UA plays an important role during and immediately after the strenuous exercise: increased serum values were found within 40 minutes after the intense physical activity (Andersson et al., 2010). Moreover, we found a small increase in the level of antioxidant bilirubin, the end product of hem degradation. Strenuous exercise was found to induce elevation of bilirubin, which exerts antioxidant effects due to a redox cycle in which it is oxidized to biliverdin by hydrophobic ROS and then recycled by biliverdin-reductase (Swift et al., 2012). Finally, the level of an important circulating antioxidant, albumin, was not changed neither in the placebo, nor in the probiotic group. These results are in accordance with previously published results (Melville et al., 2017).
One limitation of our study is that we measured a relatively small number of variables of prooxidant-antioxidant balance. The levels of other markers (glutathione, alpha-lipoic acid, S-ubiquinone, vitamins C, A and E, carotenoids, catalase and glutathione reductase) would provide a more detailed picture of oxidative stress and antioxidant status in the present cohort of athletes. A specific strength of the current study is the fact that only athletes with high training loads (>11 h training per week) were included into the study and that they were followed during a habitual competitive season, under regular dietary and training conditions.
Conclusion
Probiotic supplementation decreases markers of cumulative oxidation of lipids (MDA) and proteins (AOPP), probably by augmentation of antioxidant enzyme SOD activity. Lafti® might be of particular interest for the athletes in the periods of strenuous exercise before or during the competitions and/or insufficient antioxidants intake. However, studies including larger samples of athletes are needed to confirm our findings and elucidate the mechanisms regarding the antioxidant effect of Lafti® supplementation in athletes.
Acknowledgements
The authors gratefully acknowledge support of Lallemand Health Solutions, Montreal, Canada (probiotic and placebo capsules). This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project No. III 46009 and 175035) and by the Charles University Project Progress Q25.
References
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2001;43:1575. doi: 10.1249/MSS.0b013e31821ece12. –. [DOI] [PubMed] [Google Scholar]
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, Memarzadeh MR, Asemi Z, Esmaillzadeh A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32:315. doi: 10.1016/j.nut.2015.09.003. –. [DOI] [PubMed] [Google Scholar]
- Alamdari DH, Paletas K, Pegiou T, Sarigianni M, Befani C, Koliakos G. A novel assay for the evaluation of the prooxidant-antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin Biochem. 2007;40:248. doi: 10.1016/j.clinbiochem.2006.10.017. –. [DOI] [PubMed] [Google Scholar]
- Czuba M, Maszczyk A, Gerasimuk D, Roczniok R, Fidos-Czuba O, Zając A, Gołaś A, Mostowik A, Langfort J. The effects of hypobaric hypoxia on erythropoiesis, maximal oxygen uptake and energy cost of exercise under normoxia in elite biathletes. J Sports Sci Medic. 2014;13(4):912. –. [PMC free article] [PubMed] [Google Scholar]
- Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539. doi: 10.1016/j.nut.2011.08.013. –. [DOI] [PubMed] [Google Scholar]
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103. doi: 10.1016/j.clinbiochem.2005.08.008. –. [DOI] [PubMed] [Google Scholar]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277. doi: 10.1016/j.clinbiochem.2003.11.015. –. [DOI] [PubMed] [Google Scholar]
- Girotti MJ, Khan N, McLellan BA. 1991. Early measurement of systemic lipid peroxidation products in the plasma of major blunt trauma patients. J Trauma. 1991;31:32. doi: 10.1097/00005373-199101000-00007. –. [DOI] [PubMed] [Google Scholar]
- Gleeson M, Bishop N, Oliveira M, Tauler P. Influence of training load on upper respiratory tract infection incidence and antigen-stimulated cytokine production. Scand J Med Sci Sports. 2013;23:451. doi: 10.1111/j.1600-0838.2011.01422.x. –. [DOI] [PubMed] [Google Scholar]
- James RW, Deakin SP. The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activity. Free Radic Biol Med. 2004;37:1986. doi: 10.1016/j.freeradbiomed.2004.08.012. –. [DOI] [PubMed] [Google Scholar]
- Kotur-Stevuljevic J, Spasic S, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V, Stefanovic A, Vujovic AL, Memon L, Kalimanovska-Ostric D. PON1 status is influenced by oxidative stress and inflammation in coronary heart disease patients. Clin Biochem. 2008;41:1067. doi: 10.1016/j.clinbiochem.2008.06.009. –. [DOI] [PubMed] [Google Scholar]
- Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M. Antioxidative probiotic fermented goats' milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr. 2003;90:449. doi: 10.1079/bjn2003896. –. [DOI] [PubMed] [Google Scholar]
- Lamprecht M, Bogner S, Schippinger G, Steinbauer K, Fankhauser F, Hallstroem S, Schuetz B, Greilberger JF. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J Int Soc Sports Nutr. 2012;9:45. doi: 10.1186/1550-2783-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaritelis NV, Cobley JN, Paschalis V, Veskoukis AS, Theodorou AA, Kyparos A. Nikolaidis MG. Going retro: Oxidative stress biomarkers in modern redox biology. Free Radic Biol Med. 2016;98:2. doi: 10.1016/j.freeradbiomed.2016.02.005. and. –. [DOI] [PubMed] [Google Scholar]
- Martarelli D, Verdenelli MC, Scuri S, Cocchioni M, Silvi S, Cecchini C, Pompei P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr Microbiol. 2011;62:1689. doi: 10.1007/s00284-011-9915-3. –. [DOI] [PubMed] [Google Scholar]
- Martinovic J, Dopsaj V, Dopsaj MJ, Kotur-Stevuljevic J, Vujovic A, Stefanovic A, Nesic G. Long-term effects of oxidative stress in volleyball players. Int J Sports Med. 2009;30:851. doi: 10.1055/s-0029-1238289. –. [DOI] [PubMed] [Google Scholar]
- Melville GW, Siegler JC, Marshall PWM. The effects of d-aspartic acid supplementation in resistance-trained men over a three month training period: A randomised controlled trial. PLoS One. 2017;12:e0182630. doi: 10.1371/journal.pone.0182630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalickova DM, Kostic-Vucicevic MM, Vukasinovic-Vesic MD, Stojmenovic TB, Dikic NV, Andjelkovic MS, Djordjevic BI, Tanaskovic BP, Minic RD. Lactobacillus helveticus Lafti L10 supplementation modulates mucosal and humoral immunity in elite athletes: A Randomized, double-blind, placebo-controlled trial. J Strength Cond Res. 2017;31:62. doi: 10.1519/JSC.0000000000001456. –. [DOI] [PubMed] [Google Scholar]
- Michalickova D, Minic R, Dikic N, Andjelkovic M, Kostic-Vucicevic M, Stojmenovic T, Nikolic I. Djordjevic B. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. 2016;41:782. doi: 10.1139/apnm-2015-0541. and. –. [DOI] [PubMed] [Google Scholar]
- Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J. Probiotics as potential antioxidants: a systematic review. J Agric Food Chem. 2015;63:3615. doi: 10.1021/jf506326t. –. [DOI] [PubMed] [Google Scholar]
- Misra HP. Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170. and. –. [PubMed] [Google Scholar]
- Otocka-Kmiecik A, Orlowska-Majdak M. The role of genetic (PON1 polymorphism) and environmental factors, especially physical activity, in antioxidant function of paraoxonase. Postepy Hig Med Dosw. 2009;63:668. –. [PubMed] [Google Scholar]
- Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med. 2011;41:1043. doi: 10.2165/11594400-000000000-00000. –. [DOI] [PubMed] [Google Scholar]
- Richter RJ, Furlong CE. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics. 1999;9:745. –. [PubMed] [Google Scholar]
- Scoccia AE, Molinuevo MS, McCarthy AD, Cortizo AM. A simple method to assess the oxidative susceptibility of low density lipoproteins. BMC Clin Pathol. 2001;1:1. doi: 10.1186/1472-6890-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senti M, Tomas M, Anglada R, Elosua R, Marrugat J, Covas MI, Fito M. Interrelationship of smoking, paraoxonase activity, and leisure time physical activity: a population-based study. Eur J Intern Med. 2003;14:178. doi: 10.1016/s0953-6205(03)00041-4. –. [DOI] [PubMed] [Google Scholar]
- Swift DL, Johannsen NM, Earnest CP, Blair SN, Church TS. Effect of different doses of aerobic exercise training on total bilirubin levels. Med Sci Sports Exerc. 2012;44:569. doi: 10.1249/MSS.0b013e3182357dd4. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira VH, Valente HF, Casal SI, Marques AF, Moreira PA. Antioxidants do not prevent postexercise peroxidation and may delay muscle recovery. Med Sci Sports Exerc. 2009;41:1752. doi: 10.1249/MSS.0b013e31819fe8e3. –. [DOI] [PubMed] [Google Scholar]
- Tomas M, Elosua R, Senti M, Molina L, Vila J, Anglada R, Fito M, Covas MI, Marrugat J. Paraoxonase1-192 polymorphism modulates the effects of regular and acute exercise on paraoxonase1 activity. J Lipid Res. 2002;43:713. –. [PubMed] [Google Scholar]
- Valimaki IA, Vuorimaa T, Ahotupa M, Kekkonen R, Korpela R, Vasankari T. Decreased training volume and increased carbohydrate intake increases oxidized LDL levels. Int J Sports Med. 2012;33:291. doi: 10.1055/s-0031-1291223. –. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa AT, Nguyen J, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304. doi: 10.1038/ki.1996.186. –. [DOI] [PubMed] [Google Scholar]


