Abstract
Background and Aims
Diagnostic yield of Small Bowel Capsule Endoscopy (SBCE) for the assessment of small bowel (SB) lesions is higher than radiologic imaging techniques. However, magnetic resonance enterography (MRE) data are scarce and inconclusive. Colon Capsule Endoscopy (CCE) is a new capsule modality. The primary aim of our study was to compare MRE and capsule endoscopy (CE) for the assessment of Crohn’s disease (CD). The secondary objectives were to compare the diagnostic accuracy of both CE modalities and changes in Montreal classification after each examination.
Methods
We included 47 patients with established (n = 32) or suspected CD (n = 15). MRE was performed first to rule out strictures. In patients with a suspected stricture by MRE, an Agile Patency Capsule was performed. SB disease activity was measured by MaRIA score (MRE) and Lewis Index (CE).
Results
SB lesions were found in 36 of47 patients with CE and in 21 of47 patients with MRE (76.6% vs 44.7%, P = 0.001). Jejunal inflammation was detected by CE in 31.9% of patients and by MRE in 6.4% of patients (15/47 vs 3/47; P = 0.03); lesions in ileum were detected in 57.4% of patients by CE, and in 21.3% of patients by MRE (27/ 47 vs 10/ 47; P = 0.04). Finally, in terminal ileum, CE showed lesions in 68.1% (32/47) of patients, whereas MRE detected lesions in 38.3% (18/ 47 patients), (P = 0.001). The original Montreal classification was changed in 53.1% of patients (25/ 47) based on CE findings and in 12.7% of patients (6/47) based on MRE findings (P < 0.05).
Conclusions
In our cohort CE was significantly superior to MRE for detecting SB lesions, mainly superficial and proximal lesions. CE is useful for a appropriate patients’ classification according to Montreal classification.
Keywords: small bowel capsule endoscopy, colon capsule endoscopy, magnetic resonance enterography
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory bowel disease that can affect any segment of the gastrointestinal tract (GI). The small bowel (SB) is the most commonly affected location, and in 30% of cases represents the only segment affected by the disease. Diagnosing inflammatory lesions in the SB represents a challenge, especially those located in the upper GI track and research is hampered by the absence of an established gold standard.
Capsule Endoscopy (CE) is a noninvasive tool that allows evaluation of the entire SB and commonly is used for the assessment of disease activity in CD. CE examination provides positive findings, defined as demonstration of relevant lesions in a high proportion of patients with CD.1 Results of previous studies suggest that CE can assess the intestinal mucosa with similar or higher sensitivity than radiologic procedures. The relevance of the information provided by Small Bowel Capsule Endoscopy (SBCE) has been shown by its impact on disease management and associated outcomes. The main limitation of CE is that evaluation of stenosis is often incomplete2,3 and extraluminal penetrating complications cannot be assessed. In CD, the presence of lesions in the upper GI carries a higher risk of developing a stricturing phenotype and is associated with higher surgical requirements.4,5 By contrast, cross-sectional imaging such computed tomography enterography (CTE) or magnetic resonance enterography (MRE) allows evaluation of transmural lesions and provides an accurate assessment of stricturing and penetrating complications.However, superficial ulcerations can be missed because of the lower sensitivity of MRE to detect mild lesions (0.80 for mild lesions, 0.91 for moderate-severe lesions) and a suboptimal distension of upper GI track using enterography and not enteroclisis.6,7
Since 2006 colon CE (CCE) is available. This device has 2 side cameras and allows obtaining a greater number of pictures and would potentially leave less unexplored areas. To date there are no data comparing the diagnostic yield of this type of CE with the SBCE for the assessment of SB lesions. Furthermore the most effective approach for diagnosing SB-CD has not been definitively established.8
The primary aim of this study was to compare the diagnostic yield of MRE, assessed by an expert inflammatory bowel disease (IBD) radiologist, and CE, evaluated by experienced IBD endoscopists, for the assessment of CD in patients with suspected or known SB disease. Secondary objectives were to compare the diagnostic accuracy of both CE modalities and changes in Montreal classification after each examination was analyzed.
METHODS
Patients
Patients who were submitted to CE and MRE on clinical grounds from June 2011 to June 2013 were retrospectively reviewed. Those who met the following inclusion criteria were included in the study: (1) age ≥18 year-old and (2) established or suspected CD. Exclusion criteria were history of previous known intestinal strictures, fistulizing CD, intolerance or contraindication to MRE, any contraindication for CE, or severe comorbidities. All patients underwent MRE as the first examination to rule out strictures, and CE examination was performed thereafter. CE and MRE were performed within 3 months. During this period no changes in CD therapy were registered. CCE was used in 26 patients and SBCE in 21 patients. Patients with suspected CD, and SB lesions, underwent an endoscopic procedure (balloon enteroscopy) with biopsies to confirm the diagnoses. The study protocol was approved by the local ethical committee.
Magnetic Resonance Enterography
All examinations were performed using a 3.0-T MR unit (TrioTim; Siemens Medical Solutions, Erlangen, Germany). One liter of 4000 PEG (Polietilenglicol) water solution or 1 liter of 2.5% mannitol solution was administered 30 minutes before MRE and used as the intraluminal contrast agent. Intravenous scopolamine butylbromide 40 mg/mL (Buscapina, Boehringer Ingelheim, Spain) was given iv to reduce peristalsis and to prolong SB distension. MR protocol was described elsewhere and includes T2-weighted sequences in axial and coronal plane and T1-weighted sequences in coronal plane before and after gadolinium injection (Multihance at 0.2 mL/kg body weight dose and 2 ml/sec rate injection) (Supplementary Table 1).9
Patients with SB wall thickening (>3 mm), hyperenhancement, edema, comb sign (increased mesenteric vascularity adjacent to the inflamed intestinal loop), or presence of ulcers were considered signs of active CD by MRE criteria.7,10 Strictures were defined as a persisting luminal narrowing less than 10 mm in the presence of wall thickening. The presence of fistulas or abscesses also was evaluated. MRE interpretations were assessed by a single radiologist at that time with 7 years of experience in the evaluation of IBD lesions who was blinded to the results of CE.
To quantify the severity of the SB lesions, the MaRIA score of each SB segment (jejunum, proximal ileum, and terminal ileum) was calculated.9 Even though there is a lack of standardized accepted division of the SB on MRE, the following segmentation was used to define each SB segment: the terminal ileum was considered the distal 15 cm proximal to the ileocecal valve or ileocolonic junction; proximal ileum was considered the SB located on left lower inferior quadrant and upper right quadrant; whereas jejunum was considered the SB located on the left quadrant11 and base on connivant valves. Active disease was defined as MaRIA ≥ 7, whereas severe disease was defined as MaRIA ≥ 11.12,13
Capsule Endoscopy
CE was performed using the Pillcam SB2 (Given Imaging Inc, Yoqneam, Israel) or Pillcam Colon Capsule C2 (Given Imaging Inc, Yoqneam, Israel), which measured 11 × 26 mm and 11.6 × 31.5 mm, respectively. Pillcam SB2 (SBCE) has a single side camera and captures 2 frames per second. Colon Capsule C2 (CCE) has 2 side cameras and an adaptive frame rate between 4–35 frames per second according to the capsule movement.
To improve the visualization of the SB, patients were given 1 liter of 4000 PEG water solution the night before and 1 liter of PEG or 2.5% mannitol solution in the morning before the CE.
Preparation for CCE included additional prep consisting of a split dose of PEG before the capsule ingestion and 1 or 2 additional 500 ml boosters of 4000 PEG once the capsule had passed the pylorus. Ingestion of CE was performed with water and simethicone (80 mg) to reduce intestinal bubbles. Images were analyzed separately by 2 gastroenterologists (Cristina Romero, Begoña González-Suárez ) with experience in assessment of CE.
Capsule retention was defined as the failure of the passage of the capsule from theGI for more than 2weeks.
All images were analyzed by using the RAPID 7 software (Given Imaging). SB was divided into 3 segments: jejunum, ileum, and terminal ileum, using the 2–4 last minutes of images before CE reached the cecum and corresponding approximately to the distal 15 cm of terminal ileum. CE was considered normal if no lesions were found. The degree of inflammation was evaluated according to 2 previously described indices. The first is a qualitative index7,14: 0 (no lesions), (1) mild (≤3 aphthae, erythema, erosions, villous denudation, and ≤2 superficial ulcers),(2) moderate inflammation (>3 aphthae, >2 superficial, or deep ulcers), and 3 severe inflammation (strictures or very deep ulcers). The second index, the Lewis Score, is a quantitative index, described by Gralnek et al, and is based on 3 main CE variables in 3 tertiles15: villous appearance, ulcers, and stenosis. A total score is created as follows: maximum tertile score {[(Villous parameter × extent × descriptor) + (Ulcer parameter × extent × size)] for tertile 1 or [(Villous parameter × extent × descriptor) + (Ulcer parameter × extent × size)] for tertile 2 or [(Villous parameter × extent × descriptor) + (Ulcer parameter × extent × size)] for tertile 3} + (Stenosis number × ulcerated × traversed) A score lower than 135 was considered as normal or nonclinically significant mucosal inflammatory changes, a score between 135 and 790 was considered as mild, and a score ≥790 was defined as moderate to severe disease.
The quality of CE images was evaluated following a previously published methodology,16 according to the proportion of the SB mucosa visualized without debris, liquid, or bubbles. It was categorized as excellent (>90% of the mucosa can be visualized), good (≥75% of the mucosa can be observed), fair (50%–75% of the mucosa is evaluable), or poor (<50% of the mucosa can be evaluated). Patients with poor quality CE images were excluded from the study.
Statistical Analysis
Continuous variables are expressed as mean and corresponding standard deviation. Categorical data are expressed as frequencies and percentages. Chi-square test was used to evaluate the difference for categorical variables. Bivariate correlations were analyzed using Spearman’s correlation coefficient. A P value less than 0.05 was considered statistically significant. The analysis was performed using IBM SPSS statistic package (version 21.0).
RESULTS
From June 2011 to June 2013, a total of 55 patients were identified. Six patients were excluded for exceeding the period allowed between CE and MRE, and 2 patients due to nonevaluable MRE. Finally, 47 patients were included in the study. Demographic and clinical characteristics of patients are summarized in Table 1. In 32 patients the diagnosis of SB CD had been previously established and CE was performed with the purpose of assessing disease activity. In 15 additional patients the examination was performed due to a suspected diagnosis of CD after a normal ileocolonoscopy (n = 9), or with a previous diagnosis of Ulcerative Colitis (UC) (n = 6). CD diagnosis was confirmed in 66% of patients with diagnostic suspicion (6/9) and 33% (2/6) of patients primarily diagnosed with UC were changed to a diagnoses of CD. No complications related to CE or MRE were observed.
Table 1:
Clinical and Demographic Characteristics of Patients in the Study
| Female, no. | 30 |
|---|---|
| Median age ± SD (years) | 35.62 ± 11.9 |
| Capsule (CCE / SBCE), n | 21 / 26 |
| Agile Patency Capsule, n | 10 |
| CDAI score mean (range) | 170.07 (16–378) |
| CRP (mg/L), mean (range) | 0.91 (0.01–8.71) |
| CD / Suspected CD/ UC, no. | 32 / 9 / 6 |
| Montreal Classification (L1/L2/L3/L4), no. |
16/6/7/3 |
MRE Findings
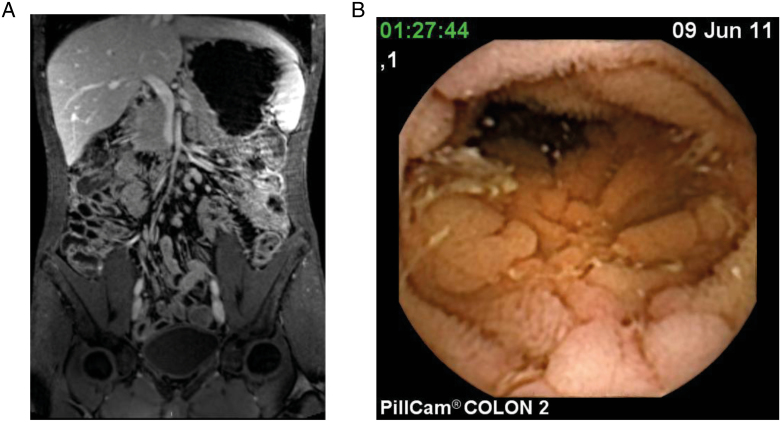
The MRE findings detected lesions in 44.7 % of patients (21/47) consisting of signs of inflammation with SB wall thickening, contrast enhancement, edema, or presence of ulcers suggesting active CD. Proximal SB MRE examination was considered suboptimal in 29.7% of the patients due to poor distension. In 3 patients with CE showing ulcers and aphthae in jejunum (Fig. 1), MRE did not detect characteristic lesions of active CD but noted in 2 cases (66.6%) thickening and edema of jejunal mucosal folds.
FIGURE 1.
A, Thickening and enlargement of jejunal mucosal folds in MRE. B, Same patient’s lesions seen by CE showing cobblestone pattern and ulcers.
The MaRIA index was calculated in jejunum, proximal, and terminal ileum. Nineteen patients had active disease (MaRIA ≥ 7) and 12 patients had severe disease (MaRIA ≥ 11). In 2 patients MaRIA index couldnot be calculated due to poor quality images at this level. No fistulas, abscesses or other extraintestinal lesions were detected by MRE
CE Findings
CCE was performed in 26 patients and SBCE in 21 patients. All patients could properly swallow the CE. Intestinal transit time was significantly shorter for the CCE compared with the SBCE (157.04 vs 295.83 minutes, P = 0.002).
Agile patency capsule was performed in 10 patients after MRE findings suggesting stenotic complications. In all cases, the Agile patency capsule was excreted intact, ruling out significant strictures. CE was performed thereafter without complications in any case (6 CCE and 4 SBCE).
All patients excreted the capsule spontaneously. The entire SB could be examined in 91% of examinations (43/47). In 4 patients, due to a delayed capsule excretion (after 10 hours), the terminal ileum could not be examined. No cases of CE retention were observed,
Regarding the quality of CE images, the overall examination was considered as good or excellent in 100% of patients explored by the CCE26 and in 98% of patients examined by the SBCE (20 /21). The remaining patient explored by the SBCE had fair preparation.
Lesions were detected by CE in 76.6% of patients (36/ 47). According to Capsule index of severity, 6 patients (12.8 %) had mild lesions, 25 (53.2%) had moderate lesions, and 5 patients (10.6%) had strictures-deep ulcers. The prevalence and type of lesions detected by CCE or SBCE was similar (Table 2).
Table 2:
Findings Detected in CE(SBCE or CCE) According to Inflammation Score (P = 0.1)
| SBCE No. = 21 |
CCE No. = 26 |
|
|---|---|---|
| No inflammation, no. | 3 | 8 |
| Mild lesions, no. | 5 | 1 |
| Moderate Lesions, no. | 11 | 14 |
| Strictures, no. | 2 | 3 |
Lewis index was graded as normal or clinically insignificant (LS < 135) in 11 patients (23.4%), mild disease (135 ≤ LS ≤ 790) in 8 patients (17%), and moderate to severe disease (LS > 790) in 28 patients (59.6%). There was a good correlation between Lewis score and Capsule Index of severity (r = 0.80, P < 0.01) Table 3.
Table 3:
Correlation Between Lewis Score and Capsule Inflammation Score (r = 0.9; P < 0.01)
| Lewis < 135 | Lewis 135–790 | Lewis > 790 | |
|---|---|---|---|
| No inflammation (0), no. | 11 | 0 | 0 |
| Mild (1), no. | 6 | 0 | |
| Moderate (2), no. | 0 | 2 | 23 |
| Strictures (3), no. | 0 | 0 | 5 |
CE AND MRE FINDINGS
CE and MRE findings agreed in 21 patients (44.7 %; Kappa index 0.3) and CE detected significantly more patients with lesions than MRE (76.6% vs 44.7%, respectively, P = 0.001).
When analyzing lesions at a segment level, jejunal active inflammation was detected by CE in 31.9% of patients and by MRE in 6.4% of patients (15/47 vs 3/47; P = 0.03); lesions in the ileum were detected in 57.4% of patients by CE, and in 21.3% of patients by MRE (27/ 47 vs 10/ 47; P = 0.04). Finally, in terminal ileum, CE showed lesions in 68.1% (32/47) of patients, whereas MRE detected lesions in 38.3% (18/ 47 , P = 0.002). Table 4.
Table 4:
Diagnostic Yield for CE and MRE: Patients With Lesions
| SB lesionsa | CE | MRE |
|---|---|---|
| Jejunum, no. (%) | 15 (31.9%) | 3 (6.4%) |
| Ileum, no. (%) | 27 (57.4%) | 10 (21.3%) |
| Terminal Ileum, no. (%) | 33 (68.1%) | 18 (38.3%) |
aSome patients have lesions in more than 1 segment.
MRE did not identify patients with mild lesions at CE (0/6), but detected 64 % of patients (16/25) with moderate lesions and 100% of patients with strictures—severe disease.
Correlation between MaRIA and Lewis index was moderate and significant in terminal ileum (r = 0.6, P = 0.002) but not in jejunum (r = -0.3, P = 0.6), or ileum (r = 0.01, P = 0.9). In Table 5 you can see MaRIA findings according to location.
Table 5:
MaRIA Findings According to Location
| Wall Thickening | Dilatation of Mesenteric Vasculature | Ulcers | Hyper- enhacement | Pseudopolyps | Enlarged Lymph Nodes | Strictures | Thickening and Enlargement of Mucosal Folds | |
|---|---|---|---|---|---|---|---|---|
| Jejunum | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 4 |
| Ileum | 7 | 5 | 4 | 8 | 1 | 5 | 1 | 0 |
| Terminal ileum | 18 | 8 | 8 | 17 | 3 | 8 | 4 | 0 |
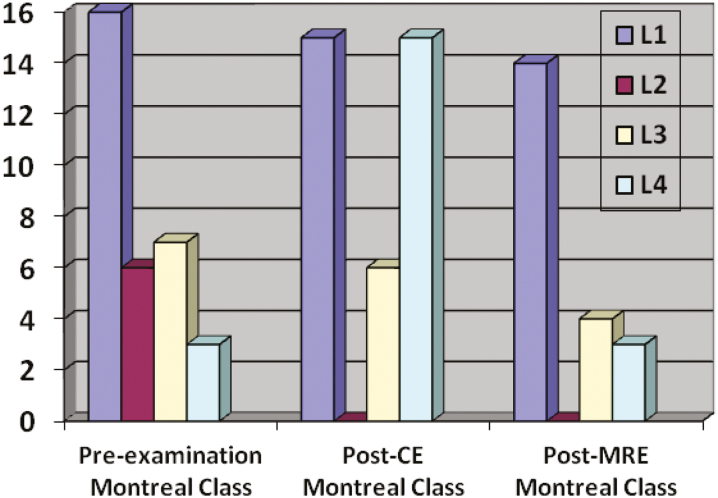
Figure 2 shows patients’ Montreal classification before and after the study according to the CE findings. The original Montreal classification was changed in 48.9% of patients (23/47) based on capsule findings and in 17% of patients (8/47) based on MRE findings (P < 0.05).
FIGURE 2.
Patients changes in Montreal classification after CE and MRE.
Colonic findings were not included. Patients with normal test are not shown.
We also analyzed the subpopulation of patients with confirmed CD separately (n = 32 patients, 68% women). In this setting, CE also detected more patients with lesions than MRE (87.5% vs 56.2%, respectively, P = 0.01). Results by segments were similar to the previous analysis: jejunal inflammation was detected by CE in 37.5% of patients and by MRE in 9.4% of patients (12/32 vs 3/32; P = 0.01); lesions in the ileum were detected in 68.7% of patients by CE, and in 28% of patients by MRE (22/ 32 vs 9/ 32; P = 0.01). Finally, in terminal ileum, CE showed lesions in 78.1% (25/32) of patients, whereas MRE detected lesions in 46.9% (15/32 patients), (P = 0.005). Regarding the Montreal classification, including only patients with an established final diagnosis of CD, the original classification was changed in 46.8% of patients (15/32) after CE and in 15.6% of patients (5/32) based on MRE findings (P < 0.05).
DISCUSSION
Our study compares the diagnostic yield of MRE and CE for the assessment of CD in patients with suspected or known SB involvement. The results of this study show a significantly higher sensitivity of CE for detecting proximal and distal disease in SB (jejunum and ileum) compared to MRE (76.6% vs 44.7% P = 0.001). Furthermore, regarding severity of lesions, MRE missed patients with mild (≤3 aphthae, erythema, erosions, villous denudation, and ≤2 superficial ulcers), or moderate lesions (>3 aphthae, >2 superficial, or deep ulcers) but detected all patients with strictures or deep ulcers.
MRE has proven to be superior to CE for detecting inflammatory changes beyond the intestinal wall but in our study no fistulas, abscesses, or other extraintestinal lesions were detected. This can be explained because we excluded patients with known strictures and fistulizing disease.
Greener et al have recently published the reclassification of CD following Montreal classification, based on CE or MRE findings.17 These techniques may provide additional data regarding disease extent and phenotype. They included 79 patients referred to MRE and CE and describe changes in disease classification in 62% of them due to the detection of previously unknown lesions. In our study CE detected upper SB disease in 15 patients compared with MRE that detected proximal lesions only in 5 patients, leading to changes in patients’ Montreal classification. This fact may influence the therapeutic management of CD, triggering an earlier introduction of immunomodulators and/or biological therapy.18,19 In our study MRE-detected lesions in proximal SB that had not been recognized as characteristic of CD in 3 patients (thickening and edema of jejunal mucosal folds). These changes were associated with the presence of ulcers and aphthae in CE examination in 2 of the 3 patients showing these changes. Although we have classified these findings as unspecific, they might be considered as inflammatory signs in the proximal SB of CD patients.
Although CE cannot replace endoscopy, it offers valuable information on the evaluation of IBD and has a significant impact on disease reclassification of patients with a previous diagnosis of UC or IBD unclassified/indeterminate colitis. In our study, CD diagnosis was confirmed in 66% of patients with diagnostic suspicion, and 33% of them primarily diagnosed with UC were changed to diagnoses of CD. MRE could have diagnosed only 50% and 16% of these patients, respectively.20,21
The main complication of CE is capsule retention that can occur in about 13% of patients with known CD and in 2% of patients with suspected CD.22 In our study MRE was performed as the first examination to exclude the presence of strictures. Patients in whom MRE identified stricturing lesions were examined with an Agile patency capsule before CE. All of these capsules were integrally excreted and conventional CE could be performed later despite a suspected stricture by MRE. As it has been previously published, radiological studies can underestimate or overestimate SB strictures.23–25 Capsule information allows a distinction between rigid fibrotic strictures and flexible ones.26
With CE the entire SB could be examined in most of the patients, providing a full assessment of disease extent, which is crucial for patients’ management in CD.27
Correlation between CE and MRE disease scores was only statistically significant in the terminal ileum but not in proximal segments. This outcome is probably due to a higher incidence of superficial mucosal lesions in jejunum and ileum for which CE has higher sensitivity.14 The main limitation of our study is that there is not a well- established, reliablegold standard.
Watanabe et al showed a high accuracy of MRE for the diagnosis of SB lesions when compared with Balloon Enteroscopy (BE). The authors described a higher sensitivity and specificity of MRE (82.4% and 87.6%) for the detection of SB inflammatory lesions. In this study they used the retrograde approach for BE, and jejunum was not visualized in 60% of patients. Proximal lesions could have been missed by both MRE and BE.28 Capsule endoscopy is a noninvasive technique compared with BE and should be preferred for nontherapeutic procedures.
CONCLUSIONS
In conclusion, in our cohort CE was significantly superior to MRE for detecting SB lesions, mainly superficial and proximal lesions. CE is useful for an appropriate Montreal classification, not only for patients with unclassified colitis but also for accurate assessment of disease extension in patients with an established diagnosis of CD. Future studies should be done to clarify the significance of these lesions identified by CE and missed by MRE.
SUPPLEMENTARY DATA
Supplementary data are available at Inflammatory Bowel Diseases online.
Supported by: Grants: NIH Spanish Radiology Society for Sonia Rodriguez (SR)
Conflicts of Interest: Jordi Rimola acts as central reader for Robarts Clinical Research and Genentech and as aconsultant for Abbvie, Robarts, Takeda, and Boehringer Ingelheim. Julia Panés acts a an advisor for Janssen, Celtrio, MSD, TiGenix, and Takeda.
REFERENCES
- 1. Long MD, Barnes E, Isaacs K et al. . Impact of capsule endoscopy on management of inflammatory bowel disease: a single tertiary care center experience. Inflamm Bowel Dis. 2011;17:1855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dionisio PM, Gurudu SR, Leighton JA et al. . Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240–8; quiz 1249. [DOI] [PubMed] [Google Scholar]
- 3. Jensen MD, Nathan T, Rafaelsen SR et al. . Diagnostic accuracy of capsule endoscopy for small bowel crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124–9. [DOI] [PubMed] [Google Scholar]
- 4. Wolters FL, Russel MG, Sijbrandij J et al. . Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut. 2006;55:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flamant M, Trang C, Maillard O et al. . The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1390–6. [DOI] [PubMed] [Google Scholar]
- 6. Albert JG, Martiny F, Krummenerl A et al. . Diagnosis of small bowel Crohn’s disease: a prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut. 2005;54:1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tillack C, Seiderer J, Brand S et al. . Correlation of magnetic resonance enteroclysis (MRE) and wireless capsule endoscopy (CE) in the diagnosis of small bowel lesions in Crohn’s disease. Inflamm Bowel Dis. 2008;14:1219–28. [DOI] [PubMed] [Google Scholar]
- 8. Böcker U, Dinter D, Litterer C et al. . Comparison of magnetic resonance imaging and video capsule enteroscopy in diagnosing small-bowel pathology: localization-dependent diagnostic yield. Scand J Gastroenterol. 2010;45:490–500. [DOI] [PubMed] [Google Scholar]
- 9. Rimola J, Ordás I, Rodriguez S et al. . Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17:1759–68. [DOI] [PubMed] [Google Scholar]
- 10. Ordás I, Rimola J, Rodríguez S et al. . Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology. 2014;146:374–82.e1. [DOI] [PubMed] [Google Scholar]
- 11. Coimbra AJ, Rimola J, O’Byrne S et al. . Magnetic resonance enterography is feasible and reliable in multicenter clinical trials in patients with Crohn’s disease, and may help select subjects with active inflammation. Aliment Pharmacol Ther. 2016;43:61–72. [DOI] [PubMed] [Google Scholar]
- 12. García-Bosch O, Ordás I, Aceituno M et al. . Comparison of diagnostic accuracy and impact of magnetic resonance imaging and colonoscopy for the management of Crohn’s disease. J Crohns Colitis. 2016;10:663–9. [DOI] [PubMed] [Google Scholar]
- 13. Rimola J, Rodriguez S, García-Bosch O et al. . Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–20. [DOI] [PubMed] [Google Scholar]
- 14. Gölder SK, Schreyer AG, Endlicher E et al. . Comparison of capsule endoscopy and magnetic resonance (MR) enteroclysis in suspected small bowel disease. Int J Colorectal Dis. 2006;21:97–104. [DOI] [PubMed] [Google Scholar]
- 15. Gralnek IM, Defranchis R, Seidman E et al. . Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146–54. [DOI] [PubMed] [Google Scholar]
- 16. Ben-Soussan E, Savoye G, Antonietti M et al. . Is a 2-liter PEG preparation useful before capsule endoscopy?J Clin Gastroenterol. 2005;39:381–4. [DOI] [PubMed] [Google Scholar]
- 17. Greener T, Klang E, Yablecovitch D et al. ; Israeli IBD Research Nucleus (IIRN) The impact of magnetic resonance enterography and capsule endoscopy on the re-classification of disease in patients with known Crohn’s disease: a prospective Israeli IBD research nucleus (IIRN) study. J Crohns Colitis. 2016;10:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cotter J, Dias de Castro F, Moreira MJ et al. . Tailoring Crohn’s disease treatment: the impact of small bowel capsule endoscopy. J Crohns Colitis. 2014;8:1610–5. [DOI] [PubMed] [Google Scholar]
- 19. Petruzziello C, Onali S, Calabrese E et al. . Wireless capsule endoscopy and proximal small bowel lesions in Crohn’s disease. World J Gastroenterol. 2010;16:3299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park SK, Yang SK, Park SH et al. . Long-term prognosis of the jejunal involvement of Crohn’s disease. J Clin Gastroenterol. 2013;47:400–8. [DOI] [PubMed] [Google Scholar]
- 21. Lopes S, Figueiredo P, Portela F et al. . Capsule endoscopy in inflammatory bowel disease type unclassified and indeterminate colitis serologically negative. Inflamm Bowel Dis. 2010;16:1663–8. [DOI] [PubMed] [Google Scholar]
- 22. Cheifetz AS, Kornbluth AA, Legnani P et al. . The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol. 2006;101:2218–22. [DOI] [PubMed] [Google Scholar]
- 23. Rozendorn N, Klang E, Lahat A et al. . Prediction of patency capsule retention in known Crohn’s disease patients by using magnetic resonance imaging. Gastrointest Endosc. 2016;83:182–7. [DOI] [PubMed] [Google Scholar]
- 24. Yadav A, Heigh RI, Hara AK et al. . Performance of the patency capsule compared with nonenteroclysis radiologic examinations in patients with known or suspected intestinal strictures. Gastrointest Endosc. 2011;74:834–9. [DOI] [PubMed] [Google Scholar]
- 25. Herrerias JM, Leighton JA, Costamagna G et al. . Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc. 2008;67:902–9. [DOI] [PubMed] [Google Scholar]
- 26. Spada C, Spera G, Riccioni M et al. . A novel diagnostic tool for detecting functional patency of the small bowel: the given patency capsule. Endoscopy. 2005;37:793–800. [DOI] [PubMed] [Google Scholar]
- 27. Karagiannis S, Faiss S, Mavrogiannis C. Capsule retention: a feared complication of wireless capsule endoscopy. Scand J Gastroenterol. 2009;44:1158–65. [DOI] [PubMed] [Google Scholar]
- 28. Takenaka K, Ohtsuka K, Kitazume Y et al. . Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn’s disease. Gastroenterology. 2014;147:334–42.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.